Abstract
Agrobacterium radiobacter is a Gram-negative bacillus and a rare cause of endophthalmitis. An 85-year-male presented with late-onset endophthalmitis associated with exposure of an inferonasal Baerveldt tube. The patient was initially treated with anterior chamber paracentesis and intravitreal antibiotics. Aqueous humor culture revealed A. radiobacter resistant to cefazolin, ceftazidime, amikacin, tobramycin, and trimethoprim-sulfamethoxazole. Subsequently, the patient underwent explantation of the glaucoma drainage implant (GDI). After initial improvement, the patient had clinical worsening and was diagnosed with recurrence. Subsequent treatment involved explantation of the second GDI in addition to pars plana vitrectomy with silicone oil infusion, intraocular lens removal, and administration of intravitreal antibiotics. Visual acuity improved but remained at count fingers at 2 weeks. This is the first reported patient with A. radiobacter endophthalmitis associated with an exposed GDI. This report illustrates the resistant nature of this organism in addition to the efficacy of silicone oil administration and intraocular prosthesis explantation.
Keywords: Agrobacterium radiobacter, Endophthalmitis, Baerveldt tube
Introduction
Endophthalmitis is a rare but devastating complication of glaucoma drainage implant (GDI) surgery and can have a delayed onset [1]. Tube exposure is a major risk factor for development of endophthalmitis after GDI surgery [1]. Most cases of endophthalmitis after GDI surgery are caused by coagulase-negative Staphylococci, Haemophilus influenzae, and Streptococcus species [2, 3, 4].
Agrobacterium (Rhizobium) radiobacter is a Gram-negative bacillus commonly found in soil [5]. Endophthalmitis due to A. radiobacter is rare but has been reported after cataract surgery and intravitreal injection [6, 7]. In this report, we describe a pseudophakic patient with A. radiobacter endophthalmitis secondary to an exposed Baerveldt tube and discuss the treatment which involved pars plana vitrectomy (PPV) and removal of both implants.
Case Report/Case Presentation
An 85-year-old male with a history of chronic angle closure glaucoma presented to the emergency department at the Bascom Palmer Eye Institute complaining of left eye pain and decreased visual acuity for 4 days. The patient had a history of superotemporal and inferonasal Baerveldt tube insertion 8 years prior to presentation.
On the initial examination, visual acuity in the left eye was light perception, and intraocular pressure (IOP) was 16 mm Hg. Visual acuity and IOP in the right eye were 20/50 and 20 mm Hg, respectively. Examination of the left eye revealed 1+ conjunctival injection and exposure of the inferonasal Baerveldt tube. The anterior chamber (AC) was deep with 4+ cell and a 2-mm hypopyon. A posterior chamber intraocular lens (IOL) was in place, and there was 360° of posterior synechiae with poor pupillary dilation.
B-scan ultrasound demonstrated evidence of vitritis and posterior vitreous detachment. The patient was treated initially with AC paracentesis and injection of intravitreal vancomycin 1 mg and ceftazidime 2.25 mg. He was started on moxifloxacin 0.5% drops every 2 h. The following day, he underwent AC washout with explantation of the exposed inferonasal Baerveldt tube. The episcleral plate and an undiluted sample from the AC were collected and cultured on chocolate agar at that time. Vancomycin 1 mg and ceftazidime 2.25 mg were again administered both intravitreally and subconjunctivally.
On postoperative day 5, visual acuity improved to count fingers at 1 foot. Initial Gram stain of the episcleral plate and aqueous humor revealed Gram-negative bacilli, which were later identified as A. radiobacter. Susceptibility results demonstrated resistance to cefazolin, ceftazidime, amikacin, tobramycin, and trimethoprim-sulfamethoxazole. The Gram stain of the vitreous specimen demonstrated moderate leukocytes but no organisms.
On postoperative week 2, the patient returned with worsening pain and decreased vision to hand motion. Examination demonstrated a new hypopyon, fibrin in the AC, and an external plaque surrounding the superotemporal tube, which was unaffected on initial exam (Fig. 1). The patient subsequently underwent explantation of the second GDI in addition to PPV with silicone oil infusion, IOL removal, and administration of a half-dose (0.2 mg) of intravitreal gentamicin. Gram strain of the explanted GDI again demonstrated Gram-negative bacilli (Fig. 2). Visual acuity remained stable for 2 weeks at 3/200 using aphakic correction. The patient was subsequently lost to follow-up and ultimately passed away.
Fig. 1.
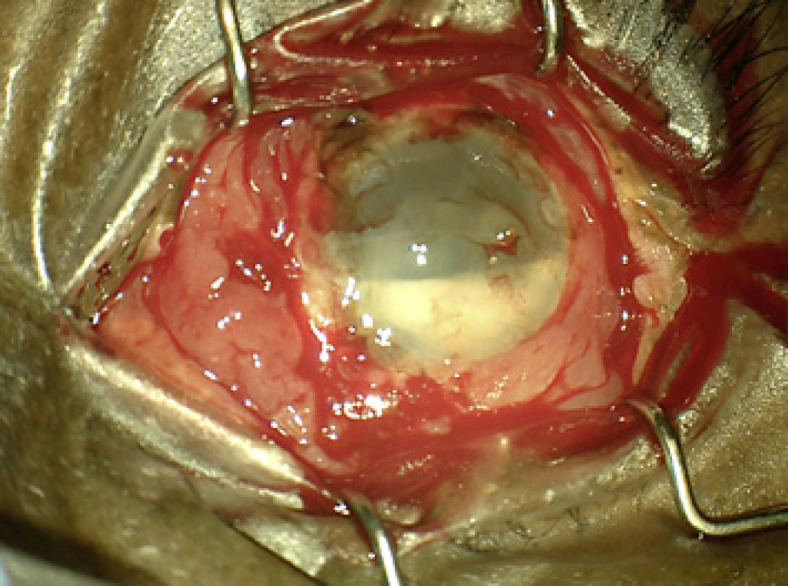
Intraoperative photograph demonstrating the presence of a new hypopyon with fibrin in the anterior chamber and a plaque surrounding the superotemporal Baerveldt tube.
Fig. 2.
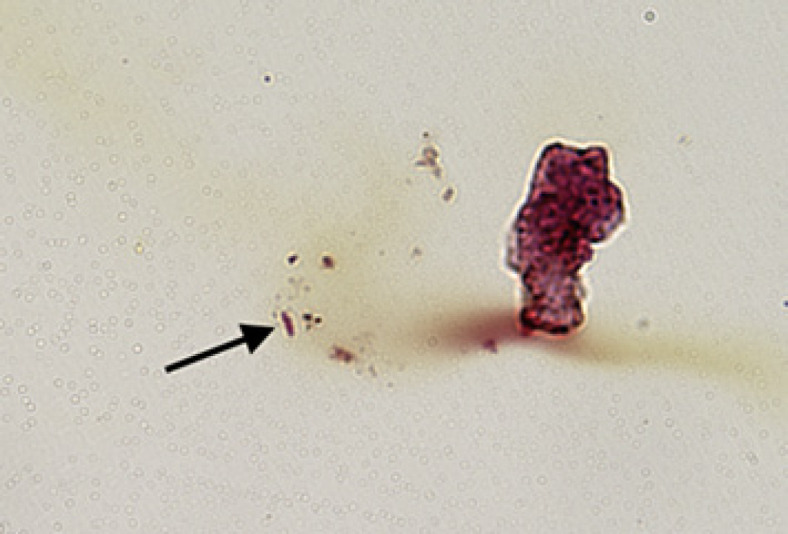
Gram-negative bacilli were present in a portion of the Baerveldt tube fragment (Gram stain; original magnification, ×600).
Discussion
A. radiobacter is a soil-dwelling organism that rarely causes endophthalmitis and has not been previously reported in association with GDI surgery [5]. Endophthalmitis secondary to A. radiobacter presents similarly to other causes of endophthalmitis, with decreased visual acuity, pain, and hypopyon as common symptoms [6, 7, 8, 9, 10, 11, 12, 13]. The organism can be isolated after 2–3 days on MacConkey agar or blood-enriched media and is frequently resistant to empiric intravitreal antibiotics such as vancomycin, ceftazidime, and amikacin, possibly due to its coexistence with antibiotic-producing organisms in soil [5, 9, 14, 15, 16]. Most cases of A. radiobacter endophthalmitis have occurred after cataract surgery (Table 1) [6, 8, 9, 10], which some have attributed to its propensity to adhere to and form biofilms on silicone substances such as IOL implants [9, 17].
Table 1.
Reported cases of endophthalmitis caused by Agrobacterium radiobacter
| Authors | Age/gender | Setting | BCVA at last follow-up |
|---|---|---|---|
| Miller et al. [8] | 70/M | Cataract surgery | Not reported |
| Namdari et al. [9] | 62/M | Cataract surgery | Not reported |
| Pierre-Filho et al. [10] | 57/M | Cataract surgery | Hand motions |
| Pierre-Filho et al. [10] | 49/F | Cataract surgery | Counting fingers |
| Joshi et al. [11] | 78/F | Intravitreal ranibizumab | 20/100 |
| Moreau-Gaudry et al. [6] | 81/F | Cataract surgery | 20/25 |
| Moreau-Gaudry et al. [6] | 75/M | Cataract surgery | 20/125 |
| Moreau-Gaudry et al. [6] | 84/F | Cataract surgery | 20/32 |
| Al-Abdullah et al. [12] | 29/M | Phakic IOL implantation | 20/50 |
| Rohowetz et al. [7] | 79/M | Intravitreal aflibercept | 20/80 |
| Parlak et al. [13] | 47–89/M, F | Intravitreal ranibizumab | Not reported |
| Current | 85/M | GDI | Counting fingers |
BCVA, best-corrected visual acuity; GDI, glaucoma drainage implant; IOL, intraocular lens.
The incidence of acute-onset endophthalmitis after GDI surgery is low, occurring in 0.00074–6.4% of patients [4, 18]. The most significant risk factor for developing GDI-associated endophthalmitis is tube exposure, with infection occurring in about 16% of exposed implants [1, 19, 20]. Inferiorly located implants are associated with the greatest risk of exposure, as was the case in our patient, possibly due to increased mechanical disruption from the lower eyelid and increased tear lake exposure [19]. Exposed inferior implants are also more likely to progress to endophthalmitis, as the inferior tube may serve as a better conduit for bacteria-rich tear film to pass into the eye [3, 19]. Outcomes of endophthalmitis associated with GDIs are generally poor secondary to pre-existing glaucomatous damage and fluctuating IOP [1, 2, 3].
Treatment of GDI-associated endophthalmitis is similar to other causes of endophthalmitis and involves administration of intravitreal antibiotics with or without PPV, although recommendations regarding explantation of the GDI are controversial [21, 22]. While there have been reports of successful treatment without removal of the IOL [23, 24], many recommend IOL and capsular bag removal to eradicate the source of infection [1, 3]. This patient presented with frank endophthalmitis with a significant decrease in visual acuity and signs of bacterial seeding of the inferonasal GDI, leading to the decision to remove the exposed implant. Given the recurrence of infection, it is plausible that the bacteria had seeded the superotemporal GDI and formed a biofilm. As a result of the outcome in this case, with need for a subsequent surgery to explant the IOL and remaining GDI, it may be advised to remove all intraocular prostheses in cases of A. radiobacter endophthalmitis.
In addition to the removal of infectious sources and the administration of intravitreal antibiotics, the use of silicone oil may be indicated in the treatment of severe endophthalmitis or endophthalmitis with concurrent retinal detachment, as silicone oil has demonstrated antimicrobial activity against a wide variety of microorganisms, including those of multidrug resistance [25, 26]. The antimicrobial effect of silicone oil has been attributed to the bactericidal and fungistatic properties of its individual components in addition to the oil's ability to act as a physical barrier, preventing the spread and proliferation of microorganisms [26, 27]. When used with silicone oil, the dose of intravitreal antibiotics should be reduced to one-quarter to one-half of the standard dose, as traditional concentrations have been demonstrated to cause retinal toxicity [28]. This toxic effect may be explained by a reduction in preretinal space due to the presence of the oil bubble and a corresponding increase in drug elimination time [28].
A notable limitation to the current report is the abbreviated follow-up period. While this patient's visual acuity remained stable at 2 weeks, more prolonged observation would have yielded valuable information regarding the patient's long-term outcome. Indeed, future investigation should seek to evaluate the extended efficacy of various management approaches in the treatment of patients with similar presentations.
Conclusion
This is the first report of GDI-associated endophthalmitis due to A. radiobacter. This case highlights the polymicrobial resistance of this organism in addition to its propensity to seed intraocular prostheses. In this patient, removal of intraocular implants, vitrectomy with silicone oil, and repeated intravitreal antibiotics were used to resolve this complex infection.
Statement of Ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The manuscript conforms to the tenets of the Declaration of Helsinki. Ethical approval was not required on the basis of the University of Miami Human Subjects Research Office/Institutional Review Board Written Policies and Procedures for the Protection of Human Subject in Research.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The Department of Ophthalmology receives Grant support from the NIH Center Core Grant P30EY014801 (Bethesda, MD) and the Research to Prevent Blindness Unrestricted Grant to U.M.
Author Contributions
Landon J. Rohowetz contributed to data analysis and interpretation, manuscript drafting, and literature search. Nimesh A. Patel, Ann V. Quan, Kenneth C. Fan, Nicolas A. Yannuzzi, Daniela P. Reyes Capó, Diana Laura, Zubair A. Ansari, Umangi Patel, and Sander R. Dubovy contributed to patient assessment, data collection, and manuscript revisions. Harry W. Flynn Jr. contributed to manuscript conception, design, revision, and final approval.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
This manuscript does not include any nonauthor contributors to acknowledge.
References
- 1.Gedde SJ, Scott IU, Tabandeh H, Luu KK, Budenz DL, Greenfield DS, et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001 Jul;108((7)):1323–7. doi: 10.1016/s0161-6420(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005 Apr;89((4)):454–8. doi: 10.1136/bjo.2004.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng CX, Moster MR, Khan MA, Chiang A, Garg SJ, Dai Y, et al. Infectious endophthalmitis after glaucoma drainage implant surgery: clinical features, microbial spectrum, and outcomes. Retina. 2017 Jun;37((6)):1160–7. doi: 10.1097/IAE.0000000000001329. [DOI] [PubMed] [Google Scholar]
- 4.Al Rashaed S, Arevalo F, Al Sulaiman S, Masoud J, Rushood A, Asghar N, et al. Endophthalmitis trends and outcomes following glaucoma surgery at a tertiary eye care hospital in Saudi Arabia. J Glaucoma. 2016 Feb;25((2)):e70–5. doi: 10.1097/IJG.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 5.Lai CC, Teng LJ, Hsueh PR, Yuan A, Tsai KC, Tang JL, et al. Clinical and microbiological characteristics of Rhizobium radiobacter infections. Clin Infect Dis. 2004 Jan 1;38((1)):149–53. doi: 10.1086/380463. [DOI] [PubMed] [Google Scholar]
- 6.Moreau-Gaudry V, Chiquet C, Boisset S, Croize J, Benito Y, Cornut PL, et al. Three cases of post-cataract surgery endophthalmitis due to Rhizobium (Agrobacterium) radiobacter. J Clin Microbiol. 2012 Apr;50((4)):1487–90. doi: 10.1128/JCM.06106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohowetz LJ, Yannuzzi NA, Gupta S, Patel NA, Miller D, Flynn HW., Jr Endophthalmitis caused by Agrobacterium radiobacter following intravitreal aflibercept for diabetic retinopathy. Case Rep Ophthalmol. 2020 Jan–Apr;11((1)):22–7. doi: 10.1159/000505227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JM, Novy C, Hiott M. Case of bacterial endophthalmitis caused by an Agrobacterium radiobacter-like organism. J Clin Microbiol. 1996 Dec;34((12)):3212–3. doi: 10.1128/jcm.34.12.3212-3213.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namdari H, Hamzavi S, Peairs RR. Rhizobium (Agrobacterium) radiobacter identified as a cause of chronic endophthalmitis subsequent to cataract extraction. J Clin Microbiol. 2003 Aug;41((8)):3998–4000. doi: 10.1128/JCM.41.8.3998-4000.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierre-Filho PT, Ribeiro AP, Passos ED, Torigoe M, de Vasconcellos JP. Endophthalmitis caused by Agrobacterium radiobacter. Scand J Infect Dis. 2003;35((6–7)):410–1. doi: 10.1080/00365540310012253. [DOI] [PubMed] [Google Scholar]
- 11.Joshi L, Morarji J, Tomkins-Netzer O, Lightman S, Taylor SR. Rhizobium radiobacter endophthalmitis following intravitreal ranibizumab injection. Case Rep Ophthalmol. 2012 Sep;3((3)):283–5. doi: 10.1159/000342693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Abdullah AA, Al-Falah M, Al-Rashaed S, Khandekar R, Arevalo JF. Endophthalmitis caused by Rhizobium radiobacter after posterior chamber phakic intraocular lens implantation to correct myopia. J Refract Surg. 2015 Aug;31((8)):561–3. doi: 10.3928/1081597X-20150728-02. [DOI] [PubMed] [Google Scholar]
- 13.Parlak M, Batur M, Ölmez S, Güdücüoğlu H, Otlu B. [The microbiological analysis of a Rhizobium radiobacter outbreak after intravitreal injection] Mikrobiyol Bul. 2020 Apr;54((2)):235–45. doi: 10.5578/mb.69286. [DOI] [PubMed] [Google Scholar]
- 14.Martinez JL, Martinez-Suarez J, Culebras E, Perez-Diaz JC, Baquero F. Antibiotic inactivating enzymes from a clinical isolate of Agrobacterium radiobacter. J Antimicrob Chemother. 1989 Feb;23((2)):283–4. doi: 10.1093/jac/23.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Edmond MB, Riddler SA, Baxter CM, Wicklund BM, Pasculle AW. Agrobacterium radiobacter: a recently recognized opportunistic pathogen. Clin Infect Dis. 1993 Mar;16((3)):388–91. doi: 10.1093/clind/16.3.388. [DOI] [PubMed] [Google Scholar]
- 16.Paphitou NI, Rolston KV. Catheter-related bacteremia caused by Agrobacterium radiobacter in a cancer patient: case report and literature review. Infection. 2003 Dec;31((6)):421–4. doi: 10.1007/s15010-003-3175-5. [DOI] [PubMed] [Google Scholar]
- 17.Alnor D, Frimodt-Møller N, Espersen F, Frederiksen W. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin Infect Dis. 1994 Jun;18((6)):914–20. doi: 10.1093/clinids/18.6.914. [DOI] [PubMed] [Google Scholar]
- 18.Stein JD, Ruiz D, Jr, Belsky D, Lee PP, Sloan FA. Longitudinal rates of postoperative adverse outcomes after glaucoma surgery among medicare beneficiaries 1994 to 2005. Ophthalmology. 2008 Jul;115((7)):1109–16.e7. doi: 10.1016/j.ophtha.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levinson JD, Giangiacomo AL, Beck AD, Pruett PB, Superak HM, Lynn MJ, et al. Glaucoma drainage devices: risk of exposure and infection. Am J Ophthalmol. 2015 Sep;160((3)):516–21.e2. doi: 10.1016/j.ajo.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang GS, Varga Z, Shaarawy T. Postoperative infection in penetrating versus non-penetrating glaucoma surgery. Br J Ophthalmol. 2010 Dec;94((12)):1571–6. doi: 10.1136/bjo.2009.163923. [DOI] [PubMed] [Google Scholar]
- 21.Li A, Conti TF, Singh RP, Challa P. Infectious and sterile endophthalmitis in eyes with glaucoma drainage device from two large ophthalmic institutions. Ophthalmol Glaucoma. 2021;4((2)):193–200. doi: 10.1016/j.ogla.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wentzloff JN, Grosskreutz CL, Pasquale LR, Walton DS, Chen TC. Endophthalmitis after glaucoma drainage implant surgery. Int Ophthalmol Clin. Spring 2007;47((2)):109–15. doi: 10.1097/IIO.0b013e318037766a. [DOI] [PubMed] [Google Scholar]
- 23.Krebs DB, Liebmann JM, Ritch R, Speaker M. Late infectious endophthalmitis from exposed glaucoma setons. Arch Ophthalmol. 1992 Feb;110((2)):174–5. doi: 10.1001/archopht.1992.01080140024014. [DOI] [PubMed] [Google Scholar]
- 24.Ellis BD, Varley GA, Kalenak JW, Meisler DM, Huang SS. Bacterial endophthalmitis following cataract surgery in an eye with a preexisting Molteno implant. Ophthalmic Surg. 1993 Feb;24((2)):117–8. [PubMed] [Google Scholar]
- 25.Dave VP, Pathengay A, Relhan N, Sharma P, Jalali S, Pappuru RR, et al. Endophthalmitis and concurrent or delayed-onset rhegmatogenous retinal detachment managed with pars plana vitrectomy, intravitreal antibiotics, and silicone oil. Ophthalmic Surg Lasers Imaging Retina. 2017 Jul 1;48((7)):546–51. doi: 10.3928/23258160-20170630-05. [DOI] [PubMed] [Google Scholar]
- 26.Dave VP, Joseph J, Jayabhasker P, Pappuru RR, Pathengay A, Das T. Does ophthalmic-grade silicone oil possess antimicrobial properties? J Ophthalmic Inflamm Infect. 2019 Nov 1;9((1)):20. doi: 10.1186/s12348-019-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung EH, Stout JT. Antibiotics and antifungals in silicone oil. Int J Retina Vitreous. 2019;5:50. doi: 10.1186/s40942-019-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegazy HM, Kivilcim M, Peyman GA, Unal MH, Liang C, Molinari LC, et al. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina. 1999;19((6)):553–7. doi: 10.1097/00006982-199911000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


