Abstract
Background/purpose
Oral lichen planus (OLP) is a chronic inflammatory lesion of oral mucosal, and its pathogenesis involves immune cell-mediated aberrances. However, the findings conflict with each other. This research aimed to comprehensively detect the salivary cytokine profile of patients with OLP.
Materials and methods
The cohort included 60 OLP patients (30 reticular and 30 erosive), and 30 healthy controls, matched in age and sex. Human Cytokine/Chemokine Magnetic Bead Panel Kit (HCYTMAG-60K-PX41) was used to detect salivary inflammation-related cytokines. Rank sum test, group t-test, and ANOVA were used for data analysis in different groups. Moreover, Spearman's rank correlation analysis was used to analyze the correlation between salivary cytokine levels and OLP lesion severity.
Results
The levels of TNF-α, G-GSF, IL-1α, IL-1β and IL-8 were statistically significant higher in both erosive and reticular OLP patients than in the healthy group, while the IL-13 level was significantly lower. Particularly, the salivary TNF-α, GM-CSF, MIP-1α, MIP-1β, IL-1β, IL-6 and IL-8 levels were higher in erosive OLP group than other groups. Spearman's rank correlation analysis revealed that the salivary TNF-α, GM-CSF, MIP-1α, MIP-1β, IL-1β and IL-6 levels were positively correlated with OLP lesion severity.
Conclusion
Imbalance of the Th1/Th2-mediated immune response contributes to OLP. Certain salivary cytokines, such as MIP-1α, MIP-1β, GM-CSF, and IL-6, are positively correlated with OLP severity, and they have a high potential as biomarkers to diagnose and predict OLP prognosis.
Keywords: Oral lichen planus, Saliva, Cytokine profile, Cell-mediated immune response, Pathogenesis
Introduction
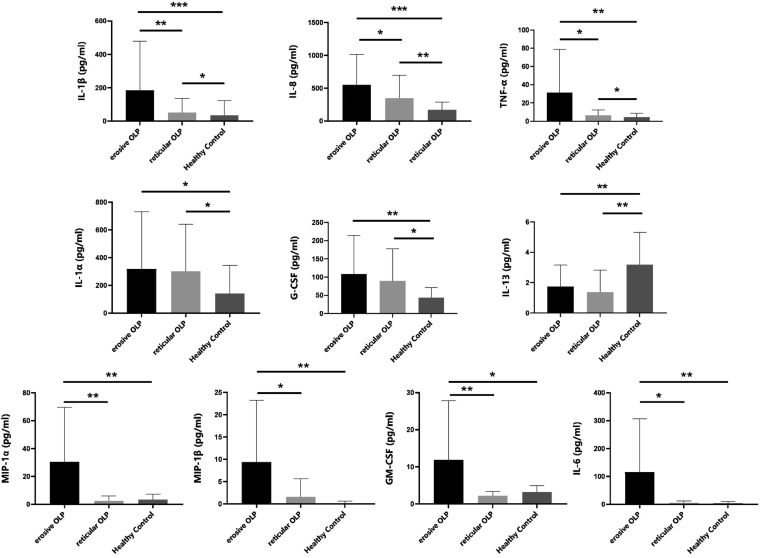
Oral lichen planus (OLP) is a common inflammatory oral mucosal disease with the prevalence of 1.5%, mainly occurring in middle and old aged women.1 In the clinical setting, there are three major subtypes of OLP lesion: atrophic, reticular, and erosive. The atrophic and erosive subtypes cancerate with a rate of 0.8–1.5%.2 This makes OLP a potential malignant disorder of the oral cavity, as defined by WHO. (see Fig. 1)
Figure 1.
Differential expression of salivary cytokines among erosive OLP group, reticular OLP group and healthy control group.
Despite extensive studies on the topic, the precise pathogenesis of OLP remains unclear. Various factors, including genetic predisposition, psychological factors, and immune dysregulation, may contribute to the development of OLP disease.3,4 Basal cell hydropic degenerated and a band-like lymphocytes infiltrated (mainly CD8+ and CD4+ cells) in the lamina propria are two characteristic histologic findings of OLP. Hence, it is well-accepted that among the multiple risk factors, T-cell-mediated immunological aberration is of crucial importance.5, 6, 7 On antigen stimulation, CD4+ T cells differentiate into distinct subsets, each of which secrete certain cytokines.8 Previous studies indicate that the cytokine expression profile is altered in OLP patients, especially the expression of Th1-, Th2-, and Th17-associated cytokines;5, 6, 7 however, only a limited number of cytokines were implicated and the findings were inconsistent.9, 10, 11, 12
Several studies have been conducted to identify biomarkers involved in OLP pathogenesis, progression, diagnosis and prognosis, among which, cytokines are served as a potential tool. Cytokines in OLP patients have been assessed in the past in various samples such as tissue, serum, peripheral blood, saliva, and oral swabs. Whole saliva is a highly versatile biological fluid that contains local vasculature-derived blood constituents, serum, and desquamated epithelial cells. Furthermore, we can get it non-invasively. Therefore, it offers distinctive advantages in the diagnosis and monitoring of various systemic and local diseases.13 However, rare studies have been addressed to evaluate limited salivary cytokines in OLP, with controversial findings.14 The aim of the present study was to add to the current knowledge on the cytokine-related mechanisms of OLP by extensively investigating the expression of T-cell associated cytokines in patients with a diagnosis of OLP. To this end, Luminex liquid suspension chips were used to determine changes in a comprehensive array of cytokines in whole saliva samples from a cohort of patients with OLP.
Materials and methods
Ethical approval
The Peking University Institutional Review Board approved this study (PKUSSIRB-201840198). And all the methods followed relevant guidelines and regulations. Each participant signed the informed consent form before sample gathering.
Study participants
All 90 subjects who were referred to “the Department of Oral Medicine, Peking University School and Hospital of Stomatology, China”, from January 2019 to June 2019 were recruited, including 30 reticular OLP patients, 30 erosive OLP patients, and 30 healthy controls (who formed the healthy control or HC group). No statistic difference in age and sex between trial group and control group.
Inclusion and exclusion criteria
Inclusion criteria
The present cohort included patients who were informed thoroughly about the trial and were willing to participate, patients who were between 18 and 75 years of age who were not pregnant, and OLP patients diagnosed based on clinical and histological features according to the 2003 WHO criteria.15
Exclusion criteria
The study excluded patients with other diagnosed oral mucosa diseases and severe systemic diseases including low immune function, hematological diseases, diabetes mellitus, infectious diseases, extraoral premalignant diseases, and tumor; patients with an allergic constitution; patients who had received topical or systemic treatment 1 month prior to the study; and patients with moderate or severe periodontitis (clinical attachment loss ≥ 5 mm, probing depth ≥ 6 mm, and extension of bone loss to the apical portion of the root).
Clinical examination and scoring
General information, including age, gender, and disease duration, was collected from each subject. Oral hygiene status was recorded in terms of the number of decayed, missing, filling teeth and probing depth. The severity of OLP lesions was evaluated with the reticular/hyperkeratotic, erosive/erythematous, ulcerative (REU) scoring system, in accordance with a previous study.16 In brief, scores were assigned based on examination of reticular/hyperkeratotic lesions (0 = none, 1 = present) and erosive/erythematous (E) and/or ulcerative (U) lesions (0 = none, 1 = lesions smaller than 1 cm2 2 = lesions ranging in size from 1 to 3 cm2, 3 = lesions larger than 3 cm2), and the total REU score was a sum of the two lesion scores. All these clinical work was done by Hong Hua, Chunlei Li and Zhengda Zhu.
Sample collection
No drinking or eating for 2 h before saliva sampling. Using standard techniques, in the morning between 08:00 and 10:00, collect 5 ml of whole unstimulated saliva (WUS). The tube containing WUS was centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatant was collected and stored at −80 °C for further analysis.17
Measurement of salivary cytokine expression
The expression of salivary cytokines was detected with a liquid suspension chip, which was then detected by the platform - Luminex 200 system (Luminex, Austin, TX, USA). Briefly, the liquid suspension chip comprised fluorescence-encoded microspheres covalently cross-linked with monoclonal antibodies against the target molecules. After fluorescein-labeled antibody was added, the concentration of target molecules was determined by identifying a single microsphere via laser scanning of the fluorescence code and measurement of the fluorescence intensity.18 In our study, the human cytokine/chemokine magnetic bead panel kit - HCYTMAG-60K-PX41 (Millipore, Burlington, MA, USA) was used, and it contained antibodies against soluble “CD40-ligand (Scd40L), epidermal growth factor (EGF), eotaxin/CCL11, fibroblast growth factor (FGF-2, also known as FGF-basic), Fms-related tyrosine kinase 3 ligand (Flt-3 ligand), fractalkine, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), CXCL1/GRO, interferon-α2 (IFN-α2), IFN-γ, interleukin-1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8 (also known as CXCL8), IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A (also known as CTLA8), interferon-γ-produced protein-10 (IP-10, also known as CXCL10), monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), MCP-3 (also known as CCL7), macrophage-derived chemokine (MDC, also known as CCL22), macrophage inflammatory protein-1α (MIP-1α, also known as CCL3), MIP-1β (also known as CCL4), platelet-derived growth factor-AA (PDGF-AA), PDGF-AB/BB, regulated upon activation normal T cell expressed and presumably secreted chemokine (RANTES, also known as CCL5), transforming growth factor-α (TGF-α), tumor necrosis factor-α (TNF-α), TNF-β (also known as lymphotoxin-α [LTA]), and vascular endothelial growth factor (VEGF)”.
Statistical analysis
Data were analyzed using SPSS version 24.0. The normality and homogeneity of variance were assessed. The rank-sum test, group t-test, and ANOVA were used for data analysis in different groups. Spearman's rank correlation analysis was used to analyze correlation between salivary levels of cytokines and OLP lesions. P value < 0.05 was set as statistical significance.
Results
Demographic and clinical feature of the patients
No significant differences in the demographic and basic clinical characteristics were found among the reticular OLP, erosive OLP, and HC groups (Table 1).
Table 1.
Baseline characteristics of the study population.
| Item | Reticular OLP | Erosive OLP | Healthy control |
|---|---|---|---|
| Age (year) | 53.27 ± 9.35 | 54.73 ± 11.66 | 51.67 ± 12.17 |
| Female: Male | 22 : 8 | 23 : 7 | 22 : 8 |
| Disease duration (month) | 4.57 ± 2.73 | 5.43 ± 3.87 | 5.60 ± 3.17 |
| PD (mm) | 4.13 ± 1.01 | 4.53 ± 1.11 | 4.26 ± 0.98 |
| DMFT | 1.77 ± 0.68 | 2.03 ± 1.03 | 1.96 ± 0.85 |
Data are presented as mean ± standard deviation; OLP: oral lichen planus; PD: probing depth; DMFT: decayed, missing, filling teeth.
Differential expression of salivary cytokines among the erosive OLP, reticular OLP, and HC groups
Compared to the HC group, the salivary levels of IL-1β, IL-8, TNF-α, IL-1α, and G-CSF were significantly increased in the erosive and reticular OLP groups, while the IL-13 level was decreased. In particular, the salivary concentrations of IL-1β, IL-8, and TNF-α were significantly higher in the erosive OLP group than in the reticular OLP group.
In addition, the salivary levels of MIP-1α, MIP-1β, GM-CSF, and IL-6 were significantly higher in the erosive OLP group than in the reticular OLP group and HC group, while no significant differences were observed between the reticular OLP group and HC group.
Correlation between salivary cytokine levels and REU scores
Spearman's rank correlation analysis revealed that the salivary levels of IL-1β, IL-6, TNF-α, MIP-1α, MIP-1β, and GM-CSF were positively correlated with the OLP oral lesion score (or the REU score) (Table 2).
Table 2.
Correlation analysis between cytokine levels (pg/ml) and oral lesion.
| Cytokine | REU score |
|
|---|---|---|
| r-value | P-value∗ | |
| IL-1β | 0.322 | 0.026 |
| IL-6 | 0.330 | 0.022 |
| IL-8 | 0.200 | 0.172 |
| TNF-α | 0.361 | 0.012 |
| MIP-1α | 0.421 | 0.003 |
| MIP-1β | 0.343 | 0.017 |
| GM-CSF | 0.339 | 0.018 |
∗Spearman's rank correlation analysis; statistical significance: P < 0.05.
REU score: reticular, erosive and ulcerative scoring system for measuring OLP lesion; IL: interleukin; TNF: tumor necrosis factor; MIP: macrophage inflammatory protein; GM-CSF: granulocyte macrophage colony stimulating factor.
Discussion
In the present study, the comprehensive cytokine expression profile of the saliva was investigated to shed light on the pathogenesis of OLP.
The results indicate that the levels of pro-inflammatory cytokines (IL-1β, IL-8, TNF-α, IL-1α, and G-CSF), which are mainly Th1-type cytokines, were significantly increased, while the level of IL-13, a Th2-type cytokine, was decreased in both the erosive and reticular OLP group. Some of the findings were consistent with previous studies.11,19 It is generally accepted that Th1/Th2 immune imbalance plays a crucial role in inflammatory and/or immunologic disorders; thus, based on these findings, imbalance in the Th1/Th2 immune response might participate in the pathogenesis of OLP. Interestingly, over half of the cytokines with significantly different expression, including IL-1α, IL-1β, TNF-α, and IL-8, were associated with nuclear factor kappa B (NF-κB). Similar findings have also been reported previously.20,21 Thus, we assumed that the NF-κB pathway might be of crucial importance in the development of OLP. However, the mechanisms involved need to be further explored.
According to the definitions proposed by WHO, OLP is a potential malignant disorder of the oral cavity; in particular, erosive and atrophic lesions could greatly increase the risk of malignancy.1 In our study, the salivary levels of MIP-1α, MIP-1β, GM-CSF, and IL-6 were specifically upregulated in the erosive OLP group, as this was not observed in the HC group or the reticular OLP group. MIP-1α and MIP-1β are members of the CC chemokine family, and they exert their chemotactic effects on T cells by binding to surface receptor.22 An in vivo experiment showed that if activated, CD4+ T cells produced MIP-1α and MIP-1β and upregulated CCR5 via native CD8+ T cells; in turn, cognate MIP-1α and MIP-1β accumulated CD8+ T cells to dendritic cell sites and induced CD4+ T-cell interaction via CCR5.23 We assumed that upregulated MIP-1α and MIP-1β related to the accumulation of CD8+ T cells in the OLP sites; furthermore, cytotoxic (activated CD8+) T cells induced intensive basal cell rupture and keratinocyte apoptosis, resulting in erosive lesions. This hypothesis might be supported by the observed positive relationship between MIP-1α/β and OLP severity, as indicated by the REU score in the present study. It is noteworthy that the generation of MIP-1α/β is related to Toll-like receptor 2 and/or 4, which has been shown to initiate the NF-κB pathway.24 This is another indication of the role of the NF-κB pathway in the pathogenesis of OLP.
IL-6, which is an NF-κB-associated cytokine, is critical in perpetuating chronic inflammation and autoimmunity, and is recognized as a key mediator in the development of chronic inflammation into cancer.25 Upregulated IL-6 expression has been observed in chronic inflammatory disorders, such as inflammatory bowel disease, rheumatoid arthritis, and lung cancer.26 In addition, higher levels of IL-6 were found in oral premalignant (oral leukoplakia) and malignant lesions.27, 28, 29 With regard to OLP, the findings are inconsistent.30 In our study, salivary IL-6 concentration was obviously high detected in the erosive OLP group and positively associated with lesion severity. This supports its diagnostic and prognostic utility as a biomarker in OLP, too.
To sum up, consistent with previous findings, local immune dysfunction, especially imbalance of the Th1/Th2 immune response, may contribute to the pathogenesis of OLP. Certain salivary cytokines, such as MIP-1α, MIP-1β, GM-CSF, and IL-6, were positively associated with OLP severity; therefore, they might have potential as biomarkers in the diagnosis and prognosis of OLP. Additionally, the findings indicate that the NF-κB pathway might play a key role in the development of OLP, but the precise mechanism needs further exploration. With the nature of non-invasive collection, high-sensitive detection and good clinic relevance, salivary cytokines could be served as the satisfied biomarkers in diagnosis, prognosis, and monitoring treatment response of OLP.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
Funding. “National Natural Science Foundation of China” (grant numbers 81730030); and “Young People Fund of Peking University School and Hospital of Stomatology” (grant numbers PKUSS20180104) support this project.
References
- 1.Ioannides D., Vakirlis E., Kemeny L., et al. European s1 guidelines on the management of lichen planus: a cooperation of the European dermatology forum with the European academy of dermatology and venereology. J Eur Acad Dermatol Venereol. 2020;34:1403–1414. doi: 10.1111/jdv.16464. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Moles M.A., Ruiz-Avila I., Gonzalez-Ruiz L., Ayen A., Gil-Montoya J.A., Ramos-Garcia P. Malignant transformation risk of oral lichen planus: a systematic review and comprehensive meta-analysis. Oral Oncol. 2019;96:121–130. doi: 10.1016/j.oraloncology.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Cerqueira J.D.M., Moura J.R., Arsati F., Lima-Arsati Y.B.O., Bittencourt R.A., Freitas V.S. Psychological disorders and oral lichen planus: a systematic review. J Investig Clin Dent. 2018;9:e12363. doi: 10.1111/jicd.12363. [DOI] [PubMed] [Google Scholar]
- 4.Olson M.A., Rogers R.S., 3rd, Bruce A.J. Oral lichen planus. Clin Dermatol. 2016;34:495–504. doi: 10.1016/j.clindermatol.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Kurago Z.B. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72–80. doi: 10.1016/j.oooo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira P.A., Carneiro S., Ramos-E-Silva M. Oral lichen planus: an update on its pathogenesis. Int J Dermatol. 2015;54:1005–1010. doi: 10.1111/ijd.12918. [DOI] [PubMed] [Google Scholar]
- 7.Shen J., Yin C., Jiang X., Wang X., Yang S., Song G. Aberrant histone modification and inflammatory cytokine production of peripheral CD4+ T cells in patients with oral lichen planus. J Oral Pathol Med. 2019;48:136–142. doi: 10.1111/jop.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Zhang D., Han Q., et al. Role of distinct CD4(+) T helper subset in pathogenesis of oral lichen planus. J Oral Pathol Med. 2016;45:385–393. doi: 10.1111/jop.12405. [DOI] [PubMed] [Google Scholar]
- 9.Malekzadeh H., Robati M., Yousefimanesh H., Ghafourian Boroujerdnia M., Nadripour R. Salivary interferon gamma and interleukin-4 levels in patients suffering from oral lichen planus. Cell J. 2015;17:554–558. doi: 10.22074/cellj.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhou J., Fu S., Wang C., Zhou B. A study of association between oral lichen planus and immune balance of Th1/Th2 cells. Inflammation. 2015;38:1874–1879. doi: 10.1007/s10753-015-0167-4. [DOI] [PubMed] [Google Scholar]
- 11.Wei W., Sun Q., Deng Y., et al. Mixed and inhomogeneous expression profile of Th1/Th2 related cytokines detected by cytometric bead array in the saliva of patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:142–151. doi: 10.1016/j.oooo.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hu J.Y., Zhang J., Ma J.Z., et al. MicroRNA-155-IFN-γ feedback loop in CD4(+)T cells of erosive type oral lichen planus. Sci Rep. 2015;5:16935. doi: 10.1038/srep16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melguizo-Rodriguez L., Costela-Ruiz V.J., Manzano-Moreno F.J., Ruiz C., Illescas-Montes R. Salivary biomarkers and their application in the diagnosis and monitoring of the most common oral pathologies. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21145173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humberto J.S.M., Pavanin J.V., Rocha M., Motta A.C.F. Cytokines, cortisol, and nitric oxide as salivary biomarkers in oral lichen planus: a systematic review. Braz Oral Res. 2018;32:e82. doi: 10.1590/1807-3107bor-2018.vol32.0082. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Meij E.H., Van Der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32:507–512. doi: 10.1034/j.1600-0714.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 16.Park H.K., Hurwitz S., Woo S.B. Oral lichen planus: REU scoring system correlates with pain. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:75–82. doi: 10.1016/j.oooo.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Pekiner F.N., Demirel G.Y., Borahan M.O., Ozbayrak S. Cytokine profiles in serum of patients with oral lichen planus. Cytokine. 2012;60:701–706. doi: 10.1016/j.cyto.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Qian J., Li J., Zhu C. Clinical significance of serum chemokines in esophageal cancer. Med Sci Mon Int Med J Exp Clin Res. 2019;25:5850–5855. doi: 10.12659/MSM.916846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffari H.R., Sharifi R., Mirbahari S., Montazerian S., Sadeghi M., Rostami S. A systematic review and meta-analysis study of salivary and serum interleukin-8 levels in oral lichen planus. Postepy Dermatol Alergol. 2018;35:599–604. doi: 10.5114/ada.2018.77611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodus N.L., Cheng B., Myers S., Bowles W., Ho V., Ondrey F. A comparison of the pro-inflammatory, NF-kappaB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clin Immunol. 2005;114:278–283. doi: 10.1016/j.clim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Lin M., Zhang S., et al. NF-kappaB-dependent cytokines in saliva and serum from patients with oral lichen planus: a study in an ethnic Chinese population. Cytokine. 2008;41:144–149. doi: 10.1016/j.cyto.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Menten P., Wuyts A., Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 23.Castellino F., Huang A.Y., Altan-Bonnet G., Stoll S., Scheinecker C., Germain R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 24.Kochumon S., Wilson A., Chandy B., et al. Palmitate activates CCL4 expression in human monocytic cells via TLR4/MyD88 dependent activation of NF-kappaB/mapk/pi3k signaling systems. Cell Physiol Biochem. 2018;46:953–964. doi: 10.1159/000488824. [DOI] [PubMed] [Google Scholar]
- 25.Unver N., Mcallister F. IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018;41:10–17. doi: 10.1016/j.cytogfr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rath T., Billmeier U., Waldner M.J., Atreya R., Neurath M.F. From physiology to disease and targeted therapy: interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch Toxicol. 2015;89:541–554. doi: 10.1007/s00204-015-1461-5. [DOI] [PubMed] [Google Scholar]
- 27.Juretić M., Cerović R., Belušić-Gobić M., et al. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol (Praha) 2013;59:99–102. doi: 10.14712/fb2013059020099. [DOI] [PubMed] [Google Scholar]
- 28.Jinno T., Kawano S., Maruse Y., et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep. 2015;33:2161–2168. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brailo V., Vucićević-Boras V., Cekić-Arambasin A., Alajbeg I.Z., Milenović A., Lukac J. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006;42:370–373. doi: 10.1016/j.oraloncology.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Shi Q., Yang S., Wang Q., Xu J., Guo B. The relationship between levels of salivary and serum interleukin-6 and oral lichen planus: a systematic review and meta-analysis. J Am Dent Assoc. 2017;148:743–749. doi: 10.1016/j.adaj.2017.05.007. [DOI] [PubMed] [Google Scholar]