Abstract
Background/purpose
Conditioned media of cultured mesenchymal stem cells (MSCs) contain numerous kinds of secretomes such as cytokines and chemokines. We previously reported that conditioned media of bone marrow-derived MSCs (MSC-CM) promote bone formation. Recently, macrophage phenotype switching from the pro-inflammatory M1 type to the anti-inflammatory M2 type has been reported to be an important phenomenon during tissue regeneration. Some studies reported that this phenotype switching is regulated by secretomes. In this study, macrophage phenotype during bone formation by MSC-CM was investigated.
Materials and methods
Human MSCs (hMSCs) were cultured in serum-free medium and the collected medium was defined as MSC-CM. Macrophage-related gene expressions in hMSCs cultured with MSC-CM were evaluated by quantitative real-time polymerase chain reaction. MSC-CM was implanted and the evaluations by micro-CT and immunohistochemistry were performed using a rat the calvaria bone defect model.
Results
Two and four weeks after implantation, the MSC-CM group demonstrated enhanced bone regeneration. Gene expressions of C–C motif chemokine 2 (CCL2), colony-stimulating factor 2 (CSF2) and CD163 was significantly upregulated in cells exposed to MSC-CM. Immunohistochemical staining revealed that iNOS-positive M1 macrophages were reduced, while CD204-positive M2 macrophages were increased in the MSC-CM group at 72 h after implantation, and the M2/M1 ratio increased only in the MSC-CM group.
Conclusion
MSC-CM enhances macrophage migration and induces M1 to M2 type macrophage switching at an early stage of osteogenesis. Such phenotype switching provides a favorable environment for angiogenesis, cellular migration, and osteogenesis and contributes to MSC-CM-induced early bone formation.
Keywords: Bone regeneration, Conditioned media, Macrophage, Mesenchymal stem cell, Phenotype switching, Secretome
Introduction
Stem cell transplantation has been recognized as a promising strategy for regenerative medicine. Stem cell-based bone regenerative medicine has also been applied clinically, based on the observation that mesenchymal stem cells (MSCs) from multiple tissues can regenerate bones.1, 2, 3
Recent studies indicate that tissue regeneration is mediated by secretomes in part by activating the endogenous cellular behavior via paracrine mechanisms.4, 5, 6, 7 Secretomes include cytokines and chemokines that exert several biological effects.6,7 Thus, secretomes are expected to have clinical applications as a substitute for stem cell transplantation and provide novel strategies for regenerative medicine.8, 9, 10
We have reported that conditioned media (CM) of bone marrow-derived MSCs contain such secretomes and have great potential for bone and periodontal regeneration.8, 9, 10, 11, 12, 13 We have also performed the first-in-human study on CM of MSCs (MSC-CM) for alveolar bone regeneration. These studies suggested that MSC-CM could be a novel therapeutic strategy for bone and periodontal regeneration.9,10 In these studies, early bone formation with MSC migration and vessel formation was observed after MSC-CM implantation, indicating that MSC-CM enhanced the cellular migration, angiogenesis, and osteogenic differentiation of endogenous stem cells.8,13, 14, 15
Recently, MSC-induced immunomodulatory effects have been shown to play a role in macrophages’ effect on tissue regeneration.16, 17, 18 Macrophages can be categorized into phenotypes, and they can switch their phenotypes using cytokines and chemokines. M1 macrophages, which are known as “pro-inflammatory macrophages,” induce inflammation, whereas M2 macrophages, which are known as “anti-inflammatory macrophages,” contribute to tissue regeneration. However, the effects of macrophage switching on MSC-CM-induced osteogenesis are still unknown.
Here, we hypothesized that M2 macrophages contribute to MSC-CM-induced early osteogenesis. In this study, we investigated the macrophage status during MSC-CM-induced osteogenesis, including immunohistological analysis of the M2/M1 macrophage ratio using the rat calvaria bone defect model.
Materials and methods
Preparation of MSC-CM
Human MSCs (hMSCs) were purchased from Lonza Inc. (Walkersville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Rockville, MD, USA) with 10% fetal bovine serum (FBS; Biowest, Nuaillé, France). Cells were maintained at 37 °C in a humidified 5% CO2 incubator, and media were refreshed every 3 days. hMSCs from passages 3rd to 6th were used for experiments. When hMSCs reached about 70–80% confluence, the medium was replaced with serum-free DMEM (DMEM(−)). After an additional 48 h of incubation, CM was collected and filtered through a 0.22 μm filter sterilizer. The collected CM were defined as MSC-CM and stored at −80 °C until further use.
The rat calvaria bone defect model
All the animal experiments were performed in strict accordance with the protocols that were reviewed by the Animal Care and Use Committee of Niigata University (approval No. SA00456).
To evaluate MSC-CM induced bone formation, critical-sized defects in the rat calvaria bones were created according to the previously described method.19 Ten-week-old male Wistar rats (Japan SLC, Shizuoka, Japan) were anesthetized by an intraperitoneal injection of 8% chloral hydrate at 400 mg/kg and 1 ml of local injection of 2% lidocaine. After shaving the parietal region, a mucosal periosteal incision was made on the line connecting t the two ear bases to peel the skin and the tissue forward from the incision and expose the sagittal suture of the parietal bone. Two 5-mm defects were made at the calvaria bone using a trephine drill (Micro Tech. Corp., Tokyo, Japan) while avoiding damage to the dura mater and they were rinsed with saline to remove any bone debris. Atelocollagen sponges (Terudermis®, Olympus Terumo Biomaterials Corp., Tokyo, Japan) were used as scaffolds, soaked in MSC-CM or phosphate-buffered saline (PBS) and implanted into the defects. The flaps were sutured with a nylon thread. The experimental groups were the MSC-CM, PBS, and defect-only groups. Seventy-two hours, or 1, 2 or 4 weeks after implantation, the specimens were harvested and investigated. Four rats (8 bone defects) were used in every experimental group at each time points.
Micro computed tomography (micro-CT) analysis
The bone formation in the calvaria bone defects in all the groups was evaluated using micro-CT (CosmoScan Gx, Rigaku Co., Tokyo, Japan) 2 or 4 weeks after implantation. The rats were anesthetized by an intraperitoneal injection of 4% chloral hydrate. Three-dimensional (3D) images were reconstructed using the Analyze 12.0 software according to the manufacturer protocol (AnalyzeDirect Inc., Overland Park, KS, USA). The newly formed bone area was calculated as a percentage of the total bone defect.
RNA extraction and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR).
hMSCs were cultured in DMEM (−) with or without the addition of MSC-CM for 48 h. Total RNA was extracted using RNeasy Mini kit (QIAGEN N.V., Venlo, Netherlands), treated with RNase-free DNase set (QIAGEN N.V.) to remove potential genomic DNA contamination, and then reverse transcribed into cDNA using PrimeScript RT Master Mix (TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer's instructions. qRT-PCR analysis was performed using TB Green Premix Ex Taq II (TaKaRa Bio Inc.) in combination with Thermal Cycler Dice Real Time System III (TaKaRa Bio Inc.). The sequences of specific primers of macrophage chemoattractant [C–C motif chemokine 2 (CCL2) and colony-stimulating factor 2 (CSF2)], marker for M1 macrophage [CD86], and marker for M2 macrophage [CD163] are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR.
| Gene | Sequence | Accession no. |
|---|---|---|
| CCL2 | NM_002982.4 | |
| Forward | 5′-CTTCTGTGCCTGCTGCTCATA-3′ | |
| Reverse | 5′-CTTTGGGACACTTGCTGCTG-3′ | |
| CFS2 | NM_000758.4 | |
| Forward | 5′-CATGATGGCCAGCCACTACAA-3′ | |
| Reverse | 5′-ACTGGCTCCCAGCAGTCAAAG-3′ | |
| CD86 | NM_001206924.1 | |
| Forward | 5′-AAGCGGCCTCGCAACTCTTA-3′ | |
| Reverse | 5′-GTCGCATGAAGATGTCTTCGAACT-3′ | |
| CD163 | NM_004224.5 | |
| Forward | 5′-CAAGTGGCCTCTGTAATCTGCTC-3′ | |
| Reverse | 5′-TCTGGAATGGTAGGCCTTGTTG-3′ | |
| GAPDH | NM_017008.4 | |
| Forward | 5′-GGCACAGTCAAGGCTGAGAGAATG-3′ | |
| Reverse | 5′-ATGGTGGTGAAGACGCCAGTA-3′ |
qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; CCL2, C–C motif chemokine 2; CSF2, colony-stimulating factor 2; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Immunohistochemical analysis
Samples from all groups were harvested 72 h or 1, 2, or 4 weeks after implantation. The specimens were fixed in 10% neutral formalin and decalcified with 10% EDTA (pH 7.4) for 4 weeks for the immunohistochemical analysis. After fixation and decalcification, if necessary, the samples were embedded in paraffin and cut the coronal plane into 4-μm sections using a microtome (REM-710, YAMATO KOHKI Industrial Co., Ltd., Saitama, Japan). The sections were dewaxed, rehydrated, stained with hematoxylin-eosin (H&E), and analyzed using a light microscope (FX630, OLYMPUS Co., Tokyo, Japan) and the FLVFS-LS software according to the manufacturer's instructions (OLYMPUS Co.). The sections were immunohistochemically stained for CD68, inducible nitric oxide synthase (iNOS), and CD204. The sections were rehydrated, subjected to antigen retrieval using Tris–EDTA buffer (pH 9.0) for CD68 and citrate buffer (pH 6.0) for iNOS and CD204 for 15 min at 110 °C, blocked for endogenous peroxidase with 0.3% H2O2 in methanol, and incubated for 30 min. After washing with PBS, the sections were blocked against non-specific binding with 5% skim milk for 1 h, and then incubated with the primary antibody against CD68 (1:100; MCA341GA, Bio-Rad Laboratories, Inc., Hercules, CA, USA), inducible nitric oxide synthase (iNOS) (1:100; MAB9502, R&D Systems, Inc., Minneapolis, MN, USA), and CD204 (1:500; KT022, Trans Genic Inc., Fukuoka, Japan) overnight at 4 °C. Subsequently, the sections were reacted with EnVision Plus (Dako, California, CA, USA) for 1 h and developed with a 3,3′-diaminobenzidine (DAB) solution. Hematoxylin counterstaining was performed following the DAB reaction.
Statistical analysis
All the data were expressed as the mean ± standard deviation (SD). The experimental groups and control groups were compared using Tukey's honestly significant difference test. The differences with p < 0.05 were considered statistically significant.
Results
MSC-CM enhanced bone regeneration in vivo
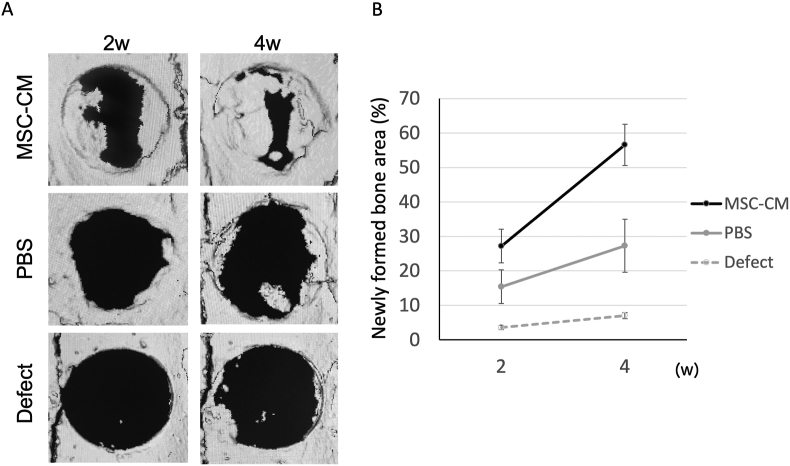
The newly formed bone area in each implantation group was evaluated by quantifying it as the percentage of the total defect area using three-dimensional micro-CT reconstructed images. Two weeks after implantation, the newly formed bone area in the MSC-CM group was 27.2% ± 4.9%, higher than those in the defect and PBS groups, at 3.6% ± 0.6% and 15.4% ± 4.5%, respectively. Four weeks after implantation, a significant portion of the defect area was replaced with the newly formed bone in the MSC-CM group at 56.6% ± 6.0%. In contrast, the newly formed bone areas were not sufficient in the defect and PBS groups, at 7.0% ± 0.9% and 27.3% ± 7.7%, respectively (Fig. 1A). The newly formed bone area in the MSC-CM group was significantly higher than that in the other groups (p < 0.01) (Fig. 1B).
Figure 1.
Micro-CT evaluation of bone formation following implantation of MSC-CM. (A) 3D reconstruction images of the calvaria with the indicated materials. In the PBS and MSC-CM groups, the materials were implanted as a mixture with atelocollagen, while in the Defect group, the defect was left unfilled (B) The newly formed bone area was measured by micro-CT, indicating that the MSC-CM increased bone formation. Data represent the mean ± SD.
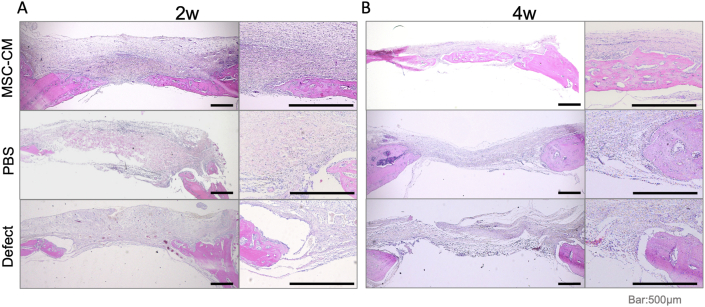
The H&E staining of the undecalcified specimens showed a larger area of newly formed bone in the MSC-CM group than in the defect and PBS groups at 2 and 4 weeks after implantation (Fig. 2A and B). Particularly, the bone bridge with several osteocytes was observed in the MSC-CM group 4 weeks after implantation, indicating that the newly formed bone was maturing. In addition, more vascular structures were observed around the newly formed bone in the MSC-CM group than the other groups (Fig. 2B). These histological data demonstrated the same trend as the results of the micro-CT analysis, further supporting the findings of bone regeneration in the MSC-CM group.
Figure 2.
Histological evaluation of bone formation following implantation of MSC-CM. Hematoxylin and eosin staining of the specimen from the calvaria 2 and 4 weeks after implantation of the indicated materials. (A) Immature trabecular bones were formed along with the osteoid 2 weeks after implantation in the MSC-CM group (B) Four weeks after implantation, the bone bridge was clearly observed in the MSC-CM group. In addition, vascular structures were also observed around the newly formed bone. Bars = 500 μm.
MSC-CM regulates expression of macrophage chemoattractant and marker for M2 macrophage
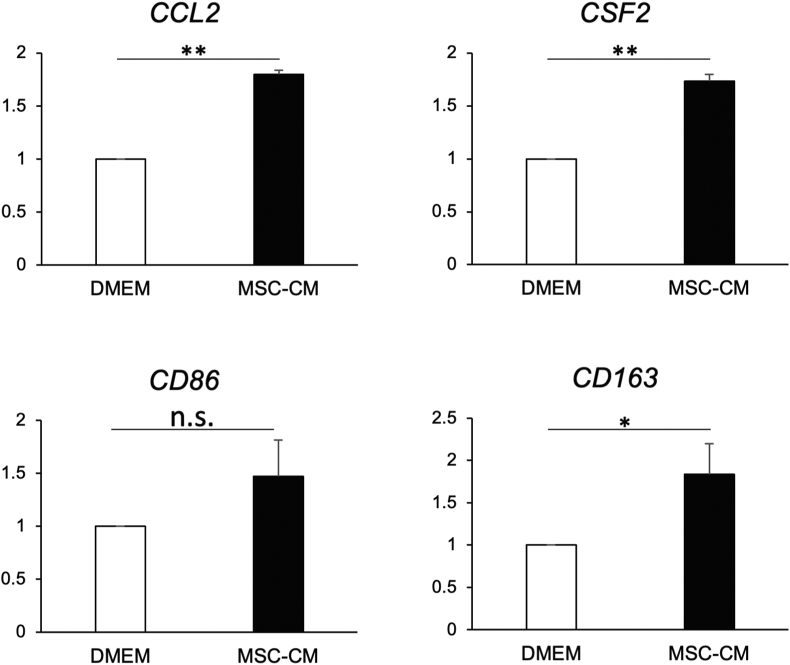
Expression of CCL2, CSF2 and CD163 was significantly and comparably upregulated in cells exposed to MSC-CM compared to cells cultured in basal DMEM; whereas the expression of CD86 showed no statistical differences between both groups (Fig. 3).
Figure 3.
Effects of MSC-CM on hMSCs gene expression. Relative expression levels of CCL2, CSF2, CD86 and CD163 in hMSCs cultured with MSC-CM were analyzed. Expression of CCL2, CSF2 and CD163 was significantly and comparably upregulated in cells exposed to MSC-CM than in cells cultured in basal DMEM, although expression of CD86 showed no statistical difference between two groups (∗p < 0.05, ns: not significant).
MSC-CM improved the M2/M1 ratio at an early phase of bone formation
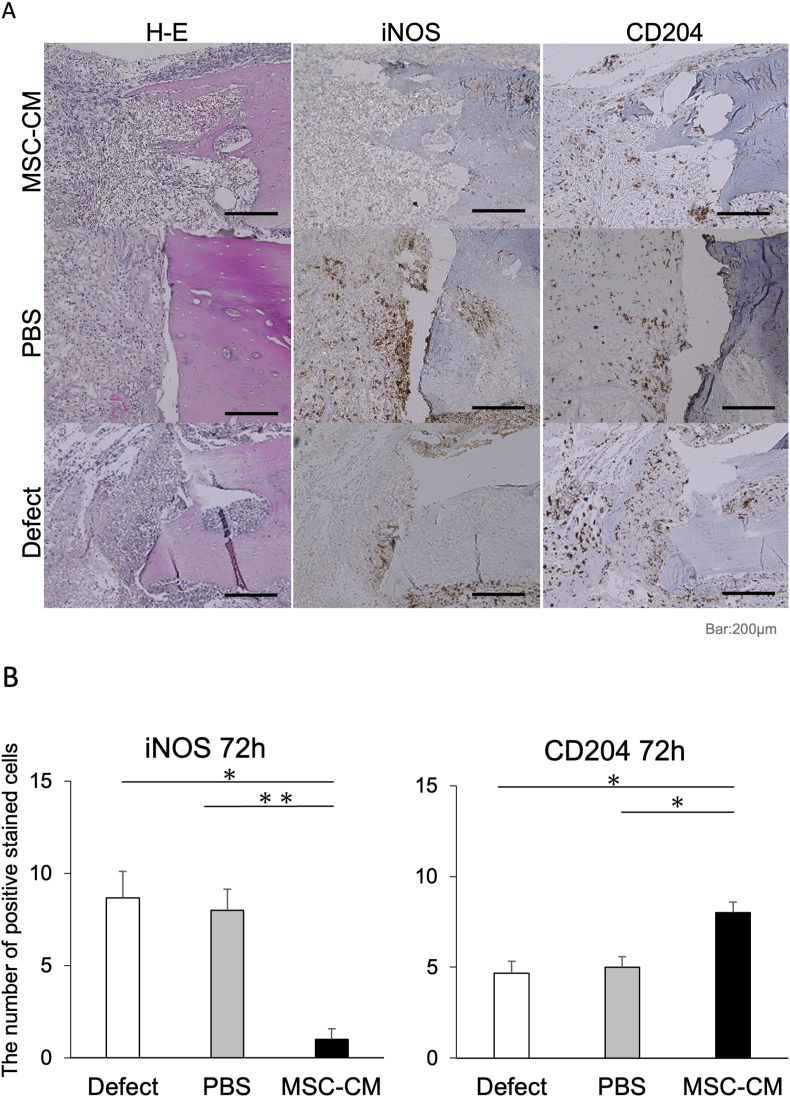
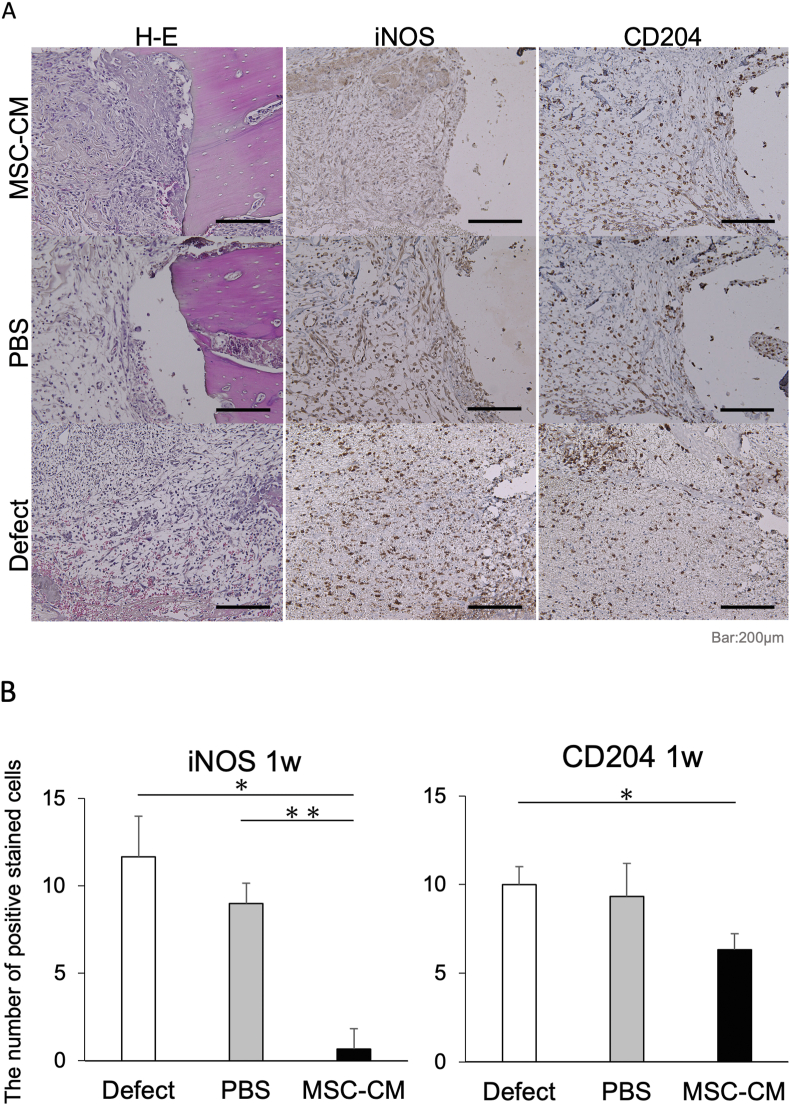
We investigated the migration and polarization of macrophages at an early stage of osteogenesis by examining the production level of iNOS and CD204 in each group 72 h and 1 week after implantation. Both iNOS-positive M1 macrophages and CD204-positive M2 macrophages were observed around the bone defect margin in the defect and PBS groups at each time point. The number of iNOS-positive cells observed around the bone defect margin was 8.7 ± 1.5 and 8.0 ± 1.2 for the defect and PBS groups, respectively, at 72 h after implantation; however, the number of iNOS-positive cells was lower in the MSC-CM group at 1.0 ± 0.6. On the other hand, the number of CD204-positive cells was 4.6 ± 0.7 and 5.0 ± 0.6 for the defect and PBS groups, respectively, but significantly higher in the MSC-CM group at 8.0 ± 0.6 at 72 h (Fig. 4A and B).
Figure 4.
Immunohistochemical staining of the newly formed bone 72 h after implantation. (A) Hematoxylin and eosin (H–E) staining indicates the orientation of the specimens, showing the bone edge of the defect where bone formation will start. In the Defect and PBS groups, there were many iNOS-positive cells and fewer CD204-positive cells around the bone edge. However, in the MSC-CM group, fewer iNOS-positive cells and numerous CD204-positive cells were observed around the bone edge. Bars = 200 μm (B) The number of iNOS-positive cells was significantly reduced in the MSC-CM group 72 h after implantation, and the number of CD204-positive cells in the MSC-CM group was significantly increased after 72 h after implantation.
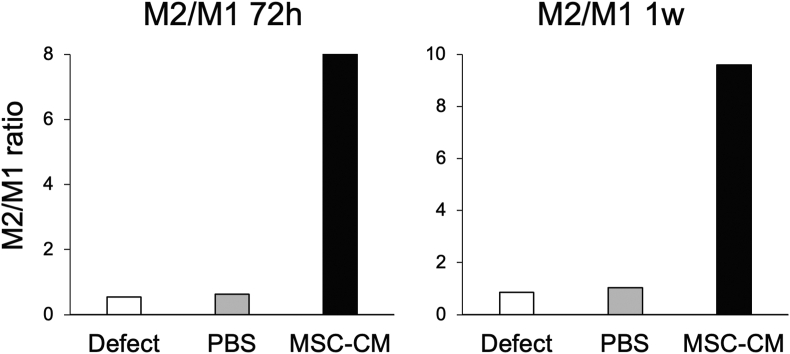
At 1 week after implantation, the number of CD204-positive cells was 11.7 ± 2.3, 9.0 ± 1.2, and 0.6 ± 1.2 for the defect, PBS, and MSC-CM groups (Fig. 5A and B). Interestingly, the total number of macrophages (M1 + M2) in the MSC-CM group was lower than that in the other groups 1 week after implantation. These results suggest that MSC-CM induces the M1-to-M2 macrophage phenotype switching within 72 h after MSC-CM implantation, and the M2/M1 ratio increases after 72 h and 1 week (Fig. 6).
Figure 5.
Immunohistochemical staining of the newly formed bone one week after implantation. (A) In the Defect and PBS groups, CD204-positive cells gradually increased compared with the specimens after 72 h. In the MSC-CM group, fewer iNos-positive cells and many CD204-positive cells were observed around the bone edge. Bars = 200 μm (B) The number of iNOS-positive cells was significantly reduced in the MSC-CM group 1 week after implantation, but the number of CD204-positive cells in the MSC-CM group was relatively decreased 1 week after implantation.
Figure 6.
The ratio of iNOS- and CD204-positive stained cells 72 h and 1 week after implantation. M2/M1 ratio around the bone formation area. The MSC-CM group showed a remarkable increase in the ratio both 72 h and 1 week after implantation. In the defect and PBS groups, the ratios were less than one because of the abundant iNOS-positive M1 macrophages.
MSC-CM reduced the infiltration of macrophages during osteogenesis
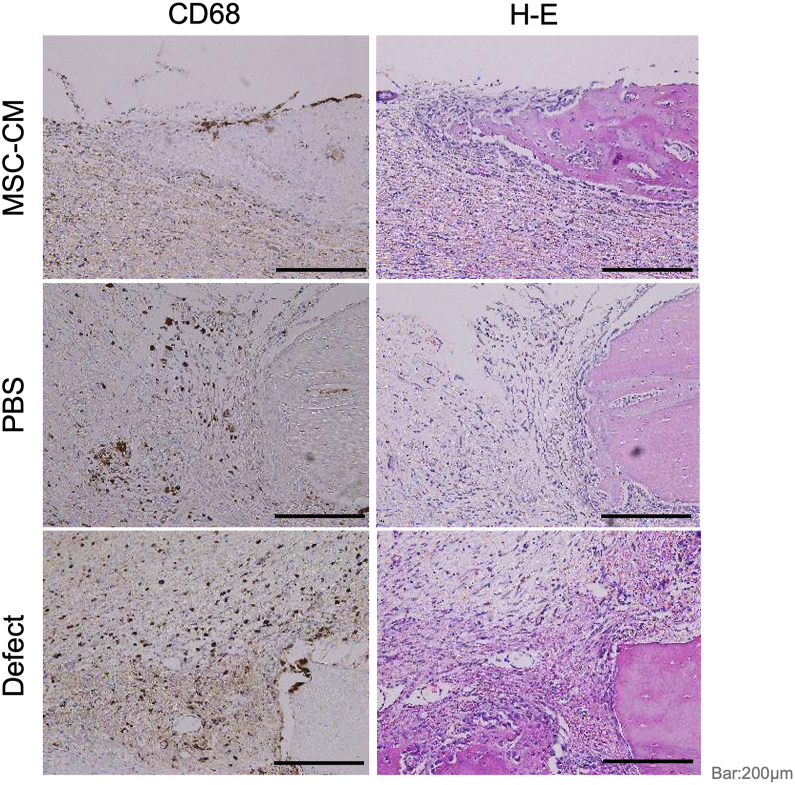
CD68 is one of the glycoproteins produced in monocytes and macrophages. Two weeks after implantation, the migration of CD68-positive cells to the bone defects was examined in each group by immunohistochemistry. Interestingly, the migration of CD68-positive cells was observed in the defect and PBS groups but reduced in the MSC-CM group (Fig. 7).
Figure 7.
Immunohistochemical staining for CD68-positive non-specific macrophages 2 weeks after implantation. Immunohistochemistry for CD68-positive cells. CD68 is one of the glycoproteins expressed in the monocytes and macrophages. Two weeks after implantation of MSC-CM, CD68-positive macrophages were not apparent in the MSC-CM group. Bar = 200 μm.
Discussion
We have previously reported that MSC-CM has great potential for bone and periodontal regeneration.8, 9, 10, 11, 12, 13 In particular, with MSC-CM, a newly formed bone was seen at an earlier stage of osteogenesis than the control groups. According to our series of studies, MSC-CM accelerates cellular migration, angiogenesis, osteogenic differentiation and thereby promotes bone formation.
On the other hand, MSCs are known to have immunomodulatory effects, and we hypothesized that MSC-CM might exert these effects and contribute to earlier osteogenesis.
Recently, the immunomodulatory effects of macrophages have been shown to enhance tissue regeneration, and these effects are induced by secretomes of MSCs.16, 17, 18,20 Macrophages can be categorized into some phenotypes, and they can switch their phenotypes using cytokines and chemokines included in the secretomes of MSCs. Among the phenotypes of macrophages, M1 macrophages are known to induce inflammation, whereas M2 macrophages contribute to tissue regeneration.
The results of the present study indicated that macrophages migrated within 72 h of MSC-CM implantation in vivo. Previously, MSC-CM has been shown, via cytokine antibody array analysis, to contain several cytokines that may act as macrophage chemoattractant, such as monocyte chemotactic protein (MCP)-1.15 The qRT-PCR analysis in our study revealed that the expression of CCL2 (cording gene of MCP-1) and CSF2 (cording gene of granulocyte macrophage colony-stimulating factor (GM-CSF)), which are known to induce and activate macrophages, were statistically upregulated in MSCs during cell culture with MSC-CM for 48 h (Fig. 3). Furthermore, CD163 (one of the markers for M2 macrophages) was also statistically upregulated although CD86 (one of the markers for M1 macrophages) was not statistically upregulated in MSCs cultured with MSC-CM (Fig. 3). These results indicate that MSC-CM has the potential to induce migration and M1 to M2 phenotype switching of macrophages. In particular, the results of the present study indicated that macrophages migrated within 72 h of MSC-CM implantation in vivo.
Using cardiosphere-derived cells (CDCs), Hasan et al. reported that macrophages cultured with CDCs-conditioned medium showed significantly enhanced expression of CD206 (a marker for M2 macrophages) but decreased expression of CD86 (a marker for M1 macrophages) 3 days after culture.21
We found that the phenotype switching of macrophages also occurred before osteogenesis within 72 h of MSC-CM implantation in vivo. Our previous study using in vivo imaging analysis and immunohistochemistry indicated that the migration of MSCs to the bone defects, where the MSC-CM were implanted, peaked between 72 h and 1 week after implantation.15 These findings indicate that macrophage migration and phenotype switching occur before the migration of MSCs to the bone defect.
Also, many studies suggest that the paracrine factors derived from MSCs regulate the immune system via interaction with host endogenous immune cells. Heo et al. demonstrated the increased expression of arginase-1 and CD206, markers of M2 macrophage activation, in MSC-induced macrophages. Also, MSC-secreted factors were found to promote M2 macrophage polarization and inhibit the growth of activated T cells.22 Gao et al. reported that MSCs or treatment with MSC-CM reduced the expression of the tumor necrosis factor (TNF)-α gene while inducing the expression of the IL-10 and arginase-1 genes in lipopolysaccharide (LPS)-stimulated mouse macrophage RAW264.7 cells and splenic CD11b+ cells. MSC-CM treatment increased the expression of CD206, a marker of alternatively activated M2 macrophages, in RAW264.7 cells. MSC-CM treatment activated signal transducer and activator of transcription (STAT) 3 but inhibited nuclear factor (NF)–κB pathways in LPS-stimulated RAW264.7 cells.23
Here, we have demonstrated that MSC-CM improves the M2/M1 ratio at an early phase of bone formation. In another study, using in vitro cell co-culture of mouse macrophages and MSCs, Gong et al. demonstrated that M2 macrophages enhanced MSC osteoblast differentiation, whereas M1 macrophages impaired osteoblast differentiation. They suggested that the mechanisms might be due to pro-regenerative cytokines, such as TGF-β, VEGF, and IGF-1, produced by M2 macrophages and detrimental inflammatory cytokines, such as interleukin (IL)-6, IL-12, and TNF-α, produced by M1 macrophages.24
Thus, M1-to-M2 macrophage switching by secretomes of MSCs may not only provide better anti-inflammatory and immunomodulatory conditions for osteogenesis but also accelerate osteogenesis itself. However, in our study, CD68-positive macrophages were decreased 2 weeks after implantation of MSC-CM, indicating a limited role of M2 macrophages in osteogenesis. The immunomodulatory effects of secretomes of MSCs are still complex and remain to be investigated further.
In conclusion, we have demonstrated that MSC-CM promotes the migration of macrophages and improves the M2/M1 ratio, namely the M1-to-M2 macrophage phenotype switching, before osteogenesis. M2 macrophage switching occurred within 72 h of MSC-CM implantation before MSC migration, although the direct contribution of M2 macrophage to MSC-CM induced early osteogenesis is not fully demonstrated from the results of this study.
The mechanism of direct contribution of M2 macrophage to MSC-CM induced early osteogenesis is to be demonstrated in our subsequent studies.
Declaration of competing interest
The authors declare that they have no competing interests relevant to this study.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (C) from the Ministry of Health, Labor and Welfare of Japan (Nos. 15K112123, 18K09785, and 20K10113). The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.Pelegrine A.A., da Costa C.E.S., Correa M.E.P., Marques J.F.C., Jr. Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin Oral Implants Res. 2010;21:535–542. doi: 10.1111/j.1600-0501.2009.01891.x. [DOI] [PubMed] [Google Scholar]
- 2.Rickert D., Sauerbier S., Nagursky H., Menne A., Vissink A., Raghhoebar G.M. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011;22:251–258. doi: 10.1111/j.1600-0501.2010.01981.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaigler D., Pagni G., Park C.H., et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 2013;22:767–777. doi: 10.3727/096368912X652968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samsonraj R.M., Raghunath M., Nurcombe V., Hui J.H., van Wijnen A.J., Cool S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadaran P., Rajendran R.L., Lee H.W., et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Contr Release. 2017;264:112–126. doi: 10.1016/j.jconrel.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Guiducci S., Manetti M., Romano E., et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis. 2011;70:2011–2021. doi: 10.1136/ard.2011.150607. [DOI] [PubMed] [Google Scholar]
- 7.Linero I., Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PloS One. 2014;9 doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osugi M., Katagiri W., Yoshimi R., Inukai T., Hibi H., Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. 2012;18:1479–1489. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katagiri W., Watanabe J., Toyama N., Osugi M., Sakaguchi K., Hibi H. Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant Dent. 2017;26:607–612. doi: 10.1097/ID.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri W., Osugi M., Kawai K., Hibi H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchyamal stem cells. Head Face Med. 2016;12:5. doi: 10.1186/s13005-016-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katagiri W., Osugi M., Kinoshita K., Hibi H. Conditioned medium from mesenchymal stem cells enhances early bone regeneration after maxillary sinus floor elevation in rabbits. Implant Dent. 2015;24:657–663. doi: 10.1097/ID.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 12.Inukai T., Katagiri W., Yoshimi R., et al. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430:763–768. doi: 10.1016/j.bbrc.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T., Katagiri W., Osugi M., Sugimura Y., Hibi H., Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369–381. doi: 10.1016/j.jcyt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri W., Kawai T., Osugi M., et al. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac Plast Reconstr Surg. 2017;39:8. doi: 10.1186/s40902-017-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogata K., Osugi M., Kawai K., et al. Secretomes of mesenchymal stem cells induce early bone regeneration by accelerating migration of stem cells. J Oral Maxillofac Surg Med Pathol. 2018;30:445–451. [Google Scholar]
- 16.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodeling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 17.Chang M.K., Raggatt L.J., Alexander K.A., et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q.Z., Su W.R., Shi S.H., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cell. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K.S., Lee J.Y., Kang Y.M., et al. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells. Biomaterials. 2010;31:1104–1113. doi: 10.1016/j.biomaterials.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 21.Hasan A.S., Luo L., Yan C., et al. Cardiosphere-derived cells facilitate heart repair by modulating M1/M2 macrophage polarization and neutrophil recruitment. PloS One. 2016;11 doi: 10.1371/journal.pone.0165255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo J.S., Choi Y., Kim H.O. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cell Int. 2019;2019:7921760. doi: 10.1155/2019/7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S., Mao F., Zhang B., et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med. 2014;239:366–375. doi: 10.1177/1535370213518169. [DOI] [PubMed] [Google Scholar]
- 24.Gong L., Zhao Y., Zhang Y., et al. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46:65–71. [PubMed] [Google Scholar]