Key Points
Question
Is ω-3–phospholipid/free fatty acid (ω-3–PL/FFA), a naturally derived krill oil with both free fatty acid and phospholipid ω-3s, an effective and safe treatment for severe hypertriglyceridemia?
Findings
In pooled data from 2 randomized clinical trials including 520 patients, blood triglyceride levels were reduced by 26.0% in the ω-3–PL/FFA group and 15.1% in the placebo group at 12 weeks, for a significant mean treatment difference that persisted at 26 weeks. ω-3–PL/FFA was well tolerated, with a safety profile similar to that of placebo.
Meaning
This study found that ω-3–PL/FFA reduced triglyceride levels and was safe and well tolerated in patients with severe hypertriglyceridemia.
This study of pooled data from 2 randomized clinical trials compares the efficacy and safety of a novel ω-3–phospholipid/free fatty acid to lower triglyceride levels in patients with hypertriglyceridemia.
Abstract
Importance
Intense interest exists in novel ω-3 formulations with high bioavailability to reduce blood triglyceride (TG) levels.
Objective
To determine the phase 3 efficacy and safety of a naturally derived krill oil with eicosapentaenoic acid and docosahexaenoic acid as both phospholipid esters (PLs) and free fatty acids (FFAs) (ω-3–PL/FFA [CaPre]), measured by fasting TG levels and other lipid parameters in severe hypertriglyceridemia.
Design, Setting, and Participants
This study pooled the results of 2 identical randomized, double-blind, placebo-controlled trials. TRILOGY 1 (Study of CaPre in Lowering Very High Triglycerides) enrolled participants at 71 US centers from January 23, 2018, to November 20, 2019; TRILOGY 2 enrolled participants at 93 US, Canadian, and Mexican centers from April 6, 2018, to January 9, 2020. Patients with fasting TG levels from 500 to 1500 mg/dL, with or without stable treatment with statins, fibrates, or other agents to lower cholesterol levels, were eligible to participate.
Interventions
Randomization (2.5:1.0) to ω-3–PL/FFA, 4 g/d, vs placebo (cornstarch) for 26 weeks.
Main Outcomes and Measures
The primary outcome was the mean percentage of change in TG levels at 12 weeks; persistence at 26 weeks was the key secondary outcome. Other prespecified secondary outcomes were effects on levels of non–high-density lipoprotein cholesterol (non–HDL-C), very-low-density lipoprotein cholesterol (VLDL-C), HDL-C, and low-density lipoprotein cholesterol (LDL-C); safety and tolerability; and TG level changes in prespecified subgroups.
Results
A total of 520 patients were randomized, with a mean (SD) age of 54.9 (11.2) years (339 men [65.2%]), mean (SD) body mass index of 31.5 (5.1), and baseline mean (SD) TG level of 701 (222) mg/dL. Two hundred fifty-six patients (49.2%) were of Hispanic or Latino ethnicity; 275 (52.9%) had diabetes; and 248 (47.7%) were receiving statins. In the intention-to-treat analysis, TG levels were reduced by 26.0% (95% CI, 20.5%-31.5%) in the ω-3–PL/FFA group and 15.1% (95% CI, 6.6%-23.5%) in the placebo group at 12 weeks (mean treatment difference, −10.9% [95% CI, −20.4% to −1.5%]; P = .02), with reductions persisting at 26 weeks (mean treatment difference, −12.7% [95% CI, −23.1% to −2.4%]; P = .02). Compared with placebo, ω-3–PL/FFA had no significant effect at 12 weeks on mean treatment differences for non–HDL-C (−3.2% [95% CI, −8.0% to 1.6%]; P = .18), VLDL-C (−3.8% [95% CI, −12.2% to 4.7%]; P = .38), HDL-C (0.7% [95% CI, −3.7% to 5.1%]; P = .77), or LDL-C (4.5% [95% CI, −5.9% to 14.8%]; P = .40) levels; corresponding differences at 26 weeks were −5.8% (95% CI, −11.3% to −0.3%; P = .04) for non–HDL-C levels, −9.1% (95% CI, −21.5% to 3.2%; P = .15) for VLDL-C levels, 1.9% (95% CI, −4.8% to 8.6%; P = .57) for HDL-C levels, and 6.3% (95% CI, −12.4% to 25.0%; P = .51) for LDL-C levels. Effects on the primary end point did not vary significantly by age, sex, race and ethnicity, country, qualifying TG level, diabetes, or fibrate use but tended to be larger among patients taking statins or cholesterol absorption inhibitors at baseline (mean treatment difference, −19.5% [95% CI, −34.5% to −4.6%]; P = .08 for interaction) and with lower (less than median) baseline blood eicosapentaenoic acid plus docosahexaenoic acid levels (−19.5% [95% CI, −33.8% to −5.3%]; P = .08 for interaction). ω-3–PL/FFA was well tolerated, with a safety profile similar to that of placebo.
Conclusions and Relevance
This study found that ω-3 –PL/FFA, a novel krill oil–derived ω-3 formulation, reduced TG levels and was safe and well tolerated in patients with severe hypertriglyceridemia.
Trial Registration
ClinicalTrials.gov Identifiers: NCT03398005 and NCT03361501
Introduction
ω-3 Fatty acids are of great interest as therapeutic agents for reducing blood triglyceride (TG) levels, especially novel formulations with higher bioavailability and lack of adverse effects on low-density lipoprotein cholesterol (LDL-C) or apolipoprotein B (ApoB). The incidence of obesity and type 2 diabetes, each of which is associated with hypertriglyceridemia, is rapidly rising, especially in low- and middle-income nations.1,2 In the US, half of adults now have diabetes or prediabetes, and one-third have elevated TG levels (≥150 mg/dL [to convert to mmol/L, multiply by 0.0113]), including approximately 36 million (16.2% of adults) with hypertriglyceridemia (TG levels of 200 to <500 mg/dL) and 4 million (nearly 2% of adults) with severe hypertriglyceridemia (TG levels of ≥500 mg/dL).3,4 Hypertriglyceridemia is often accompanied by nephrotic syndrome, chronic renal insufficiency, hypothyroidism, and fatty liver; and severe hypertriglyceridemia increases risk of pancreatitis, a life-threatening condition.5 Based on epidemiological, genetic, clinical, and mechanistic studies, elevated TG levels also increase atherosclerotic cardiovascular disease (ASCVD), which is related to atherogenic potential (particularly remnants of chylomicron very-low-density lipoprotein [VLDL] particles) and other metabolic abnormalities associated with hypertriglyceridemia.6 Safe and effective new ω-3 agents for reducing TG levels can add to the armamentarium for practicing cardiologists, lipidologists, and other clinicians.
Uncertainties and unanswered questions remain about the ω-3 class, especially effects of different ω-3 formulations on bioavailability, lowering of TG levels, increasing of LDL-C and ApoB levels, and other cardiometabolic risks. Two recent clinical trials showed differing effects of 2 ω-3 formulations on ASCVD among patients with hypertriglyceridemia while taking stable statin therapy, with risk reduction observed with 4 g/d of ω-3 icosapent ethyl compared with mineral oil,7,8 but not 4 g/d of ω-3 carboxylic acids compared with corn oil.9 This finding highlights the importance of continued evaluation and testing of different ω-3 formulations.
ω-3–Phospholipid/free fatty acid (ω-3–PL/FFA [CaPre]) is a new investigational drug containing a naturally derived, krill oil mixture with a novel formulation that includes ω-3s as FFAs and bound to PLs (approximate ratio of 50:50). Both FFA and PL ω-3s have high absorption and bioavailability,10,11 and PL ω-3s may also not increase LDL-C levels compared with other ω-3 formulations. In previous phase 2 trials,12,13 ω-3–PL/FFA lowered TG levels without increasing LDL-C levels in patients with high fasting TG levels (200 to <877 mg/dL). The TRILOGY (Study of CapRe in Lowering Very High Triglycerides) phase 3 program compared the efficacy and safety of ω-3–PL/FFA, 4 g/d, vs placebo on altering TG levels and other lipid risk factors in patients with severe hypertriglyceridemia (fasting TG level, 500 to 1500 mg/dL). This study reports the pooled results of the TRILOGY 1 and TRILOGY 2 phase 3 trials.
Methods
Study Design
TRILOGY 1 and 2 were double-blind, placebo-controlled phase 3 randomized clinical trials to test the efficacy and safety of ω-3–PL/FFA, 4 g/d, or matched placebo (cornstarch) in adults 18 years or older with fasting TG levels from 500 to 1500 mg/dL. The 2 trials had identical study designs, with TRILOGY 1 enrolling 242 participants at 71 US centers from January 23, 2018, to November 20, 2019, and TRILOGY 2 enrolling 278 participants at 93 US, Canadian, and Mexican centers from April 6, 2018, to January 9, 2020. Both trials enrolled participants and completed follow-up as designed. As reported by the sponsor, although TG levels were lowered in the active treatment group in both trials, these differences did not achieve statistical significance compared with placebo in either individual trial owing to larger than expected reductions in TG levels in the placebo groups. This analysis reports the pooled results, based on a statistical analysis plan finalized after both trials were completed but before any data were pooled for analysis. The final pooled statistical analysis plan and original trial protocols and statistical analysis plans are provided in Supplement 1 and the eMethods in Supplement 2, respectively. This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for randomized trials.
This study was conducted according to Good Clinical Practice Guidelines, the Declaration of Helsinki,14 and US Title 21 Code of Federal Regulations.15 The lead investigator at each site (Supplement 3) obtained clinical protocol approval from an appropriately constituted institutional review board or independent ethics committee. All patients provided informed written consent.
Screening and Eligibility
Adults 18 years or older with fasting TG levels from 500 to 1500 mg/dL were eligible. Participants could be taking stable doses of fibrates, statins, proprotein convertase subtilisin/kexin type 9 serine protease inhibitors (PCSK9I), cholesterol-absorption inhibitors (CAIs) such as ezetimibe, or any combination of these agents. Detailed inclusion and exclusion criteria are provided in the eMethods in Supplement 2.
At screening, potentially eligible patients entered a diet, lifestyle, and medication stabilization period, including information on and instructions to maintain the National Cholesterol Education Program Therapeutic Lifestyle Changes Diet16 throughout the study (eFigure 1 in Supplement 2 presents a flowchart of study visits). Patients were reevaluated after 4 weeks if taking a stable dose of permissible agents to alter lipid levels or none or at 6 weeks if doses of permissible agents had changed or if the patient and their clinician had discontinued prohibited lipid level–altering therapy (ie, ω-3 prescription agents or supplements, bile acid sequestrants, niacin supplement of >200 mg/d). After the stabilization period, patients were eligible to be randomized and enter the double-blind intervention period if mean fasting TG level at the 2 qualification visits ranged from 500 to 1500 mg/dL. If not, 1 additional optional TG level measurement was permitted 1 week later, and eligibility was based on the mean levels from the 2 most recent visits.
From 2099 patients screened at 164 study centers from January 2018 to April 2019, 520 were randomized and included in the intention-to-treat analysis for efficacy (eFigure 2 in Supplement 2). Race and ethnicity, classified by participants with options defined by the investigator, were assessed to inform generalizability and explore as effect modifiers.
Randomization and Treatments
Patients meeting eligibility criteria were randomized to receive ω-3–PL/FFA, 4 g/d (including 1.24 g/d of eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA] as FFAs or PLs and 2.4 g of total PLs) or matched placebo (cornstarch, selected to be relatively inert compared with lipid-based or mineral oil placebos). Patients were randomized in a 2.5:1.0 ratio (ω-3–PL/FFA to placebo) to increase the ability to assess safety and tolerability, stratified at study level by both qualifying (mean) TG level (≤750 vs >750 mg/dL) and use of statin, CAI, or PCSK9I alone or in combination (yes or no). After randomization, patients were seen at approximately 4, 11, 12, 18, and 26 weeks for physical examination, continued dietary counseling, fasting blood draw (≥9 h except water and medications), and urinalysis. Concomitant medications and any adverse events were recorded. Adherence was assessed using measured pill counts.
End Points
The primary end point for this pooled analysis was the same as in the 2 individual TRILOGY trials: the percentage of change from baseline in fasting TG level at 12 weeks, comparing ω-3–PL/FFA with placebo. This change at 26 weeks was defined as a key secondary end point. Other secondary end points were percentage changes in non–high-density lipoprotein cholesterol (non–HDL-C), VLDL-C (using ultracentrifugation), HDL-C, and LDL-C (using ultracentrifugation) levels at 12 and 26 weeks. Prespecified subgroup analyses evaluated heterogeneity in TG effects at 12 and 26 weeks; other secondary end points were assessed in subgroups for which the primary end point varied at α < .10.
Exploratory end points at 12 and 26 weeks included the proportion of patients achieving a fasting TG level of less than 500 mg/dL and percentage changes in concentrations of total cholesterol, ApoB, ApoC3, ApoA5, LDL particle, high-sensitivity C-reactive protein (hs-CRP), log hs-CRP, fasting glucose, and hemoglobin A1c (the latter 2 overall and among patients with diabetes only). Pharmacokinetic end points included absolute and percentage changes at 4, 12, 18, and 26 weeks in plasma phospholipid and total serum EPA, DHA, and EPA plus DHA concentrations (percentage of fatty acids). Change in TG levels according to tertiles of achieved EPA plus DHA, EPA, and DHA concentrations was also evaluated. Laboratory measures were performed using standardized methods (eMethods in Supplement 2) by personnel unaware of randomization assignments. Safety and tolerability were assessed by physical examination, adverse events, 12-lead electrocardiography, and clinical laboratory measures.
Statistical Analysis
For each individual trial, a sample size of 245 patients (randomized 2.5:1.0) was calculated to provide 90% power to detect a difference of 20 percentage points between the ω-3–PL/FFA and placebo groups in the primary end point, based on an estimated 15% dropout rate and 2-sided α = .05. After confirming that results of the primary and key secondary end points were similar in both individual trials (eTable 1 in Supplement 2), the findings were pooled at the individual patient level. All assessments of efficacy were performed using intention-to-treat analysis (all participants who provided informed consent and were randomized).
The primary end point, key secondary end point, and continuous efficacy parameters were assessed using analysis of covariance (ANCOVA) with main effects of treatment; qualifying TG category (≤750 or >750 mg/dL); use of statin, CAI, or PCSK9I alone or in combination at randomization (yes or no); and the baseline parameter value as covariates. Missing end point values were imputed using multiple imputation (eMethods in Supplement 2). To minimize the effects of usual within-person variation, the baseline values for TG, non–HDL-C, HDL-C, and total cholesterol concentrations were defined as the mean of the last 3 measurements obtained before randomization; and for VLDL-C and LDL-C (measured by ultracentrifugation), as the mean of the last 2 measurements. Similarly, the week 12 values for these end points were defined as the mean of as many as 2 measurements at weeks 11 and 12.
Prespecified subgroups for stratified analyses were by age (≤65 vs >65 years); race (minoritized racial group [American Indian or Alaska Native, Asian, Black or African American, or other] vs White); ethnicity (Hispanic or Latino vs non-Hispanic or Latino); sex (male or female); study (TRILOGY 1 or 2); country (US, Canada, or Mexico); qualifying TG level (≤750 vs >750 mg/dL); use of statin, CAI, and/or PCSK9I at randomization (yes or no); use of fibrate at randomization (yes or no); prevalent diabetes (yes or no); and baseline phospholipid EPA plus DHA level (median or less vs greater than median). Statistical significance of interaction was tested by including a main effect of subgroup and a treatment-by-subgroup interaction effect in the primary efficacy ANCOVA model. The proportion of participants with achieved fasting TG level of less than 500 mg/dL was assessed using the Cochran-Mantel-Haenszel test, controlling for qualifying TG category (≤750 vs >750 mg/dL) and the use of statin, CAI, or PCSK9I alone or in combination at randomization (yes or no). In sensitivity analyses, normality of the primary and secondary efficacy end points was investigated using the Shapiro-Wilk test for the residuals; if not normally distributed, these end points were assessed using a nonparametric rank-based ANCOVA, with the same covariates as the primary efficacy analysis.
Although this pooled investigation and analysis plan were prespecified before pooling, the pooling occurred after completion of each individual trial, and thus all efficacy analyses should be considered exploratory. Accordingly, no adjustment for multiplicity was performed, and as such, nominal P values are reported. Analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Study Population
Among the 520 patients who were randomized, the mean (SD) age was 54.9 (11.2) years (339 men [65.2%] and 181 women [34.8%]); mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared), 31.5 (5.1); and baseline mean (SD) TG level, 701 (222) mg/dL (with 168 [32.3%] >750 mg/dL) (Table 1). Two hundred fifty-six patients (49.2%) were Hispanic or Latino; 275 (52.9%) had diabetes; and 248 (47.7%) were receiving statins. Other agents to lower lipid levels included fibrates (50 [9.6%]) and CAI (14 [2.7%]). Baseline characteristics were comparable between treatment groups. Four hundred eighty-one participants (92.5%) in both treatment groups completed the 26-week double-blind treatment period (eFigure 2 in Supplement 2).
Table 1. Participant Characteristics at Baseline.
| Characteristic | Patient treatment groupa | ||
|---|---|---|---|
| All (n = 520) | Placebo (n = 148) | ω-3–PL/FFA, 4 g/d (n = 372) | |
| Country | |||
| US | 439 (84.4) | 127 (85.8) | 312 (83.9) |
| Canada | 26 (5.0) | 4 (2.7) | 22 (5.9) |
| Mexico | 55 (10.6) | 17 (11.5) | 38 (10.2) |
| Sex | |||
| Men | 339 (65.2) | 100 (67.6) | 239 (64.2) |
| Women | 181 (34.8) | 48 (32.4) | 133 (35.7) |
| Age, mean (SD), y | 54.9 (11.2) | 53.9 (11.8) | 55.3 (10.9) |
| Ethnicity | |||
| Hispanic or Latino | 256 (49.2) | 75 (50.7) | 181 (48.7) |
| Not Hispanic or Latino | 264 (50.8) | 73 (49.3) | 191 (51.3) |
| Race | |||
| American Indian or Alaska Native | 46 (8.8) | 13 (8.8) | 33 (8.9) |
| Asian | 26 (5.0) | 8 (5.4) | 18 (4.8) |
| Black or African American | 15 (2.9) | 3 (2.0) | 12 (3.2) |
| White | 426 (81.9) | 123 (83.1) | 303 (81.5) |
| Other,b unknown, or not reported | 7 (1.3) | 1 (0.7) | 6 (1.6) |
| BMI, mean (SD) | 31.5 (5.1) | 31.5 (5.5) | 31.5 (5.1) |
| Diabetesc | 275 (52.9) | 81 (54.7) | 194 (52.1) |
| Smoking | |||
| Never | 291 (56.0) | 92 (62.2) | 199 (53.5) |
| Former | 112 (21.5) | 27 (18.2) | 85 (22.8) |
| Current | 117 (22.5) | 29 (19.6) | 88 (23.7) |
| Alcohol use | |||
| Never | 247 (47.5) | 68 (45.9) | 179 (48.1) |
| Former | 20 (3.8) | 6 (4.1) | 14 (3.8) |
| Current | 253 (48.7) | 74 (50.0) | 179 (48.1) |
| Statin, CAI, and/or PCSK9I use | |||
| Any | 254 (48.8) | 73 (49.3) | 181 (48.7) |
| Statin | 248 (47.7) | 70 (47.3) | 178 (47.8) |
| CAI | 14 (2.7) | 7 (4.7) | 7 (1.9) |
| PCSK9I | 0 | 0 | 0 |
| Fibrate use | 50 (9.6) | 17 (11.5) | 33 (8.9) |
| Fasting TG level, mg/dL | |||
| Mean (SD) | 701 (222) | 706 (219) | 699 (223) |
| Median (IQR) | 641 (541-804) | 644 (551-807) | 637 (530-803) |
| TG category at qualification | |||
| ≤750 mg/dL | 352 (67.7) | 100 (67.6) | 252 (67.7) |
| >750 mg/dL | 168 (32.3) | 48 (32.4) | 120 (32.3) |
| Non–HDL-C level, mean (SD), mg/dL | 201.7 (47.6) | 202.9 (48.9) | 201.2 (47.2) |
| VLDL-C level, mean (SD), mg/dL | 117.9 (47.4) | 120.0 (46.1) | 117.0 (47.9) |
| HDL-C level, mean (SD), mg/dL | 31.6 (7.8) | 30.7 (8.1) | 31.9 (7.6) |
| LDL-C level, mean (SD), mg/dL | 87.0 (32.4) | 87.9 (33.8) | 86.6 (31.8) |
| HbA1c level, mean (SD), % | 6.7 (1.5) | 6.7 (1.7) | 6.6 (1.4) |
| HbA1c level among participants with diabetes only, mean (SD), % | 7.6 (1.5) | 7.9 (1.7) | 7.6 (1.4) |
| Phospholipid level, mean (SD), % fatty acids | |||
| EPA | 0.54 (0.37) | 0.58 (0.50) | 0.52 (0.30) |
| DHA | 1.89 (0.64) | 1.86 (0.61) | 1.90 (0.65) |
| EPA plus DHA | 2.42 (0.82) | 2.45 (0.86) | 2.41 (0.81) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAI, cholesterol-absorption inhibitor; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ω-3–PL/FFA, ω-3–phospholipid/free fatty acid; PCSK9I, proprotein convertase subtilisin/kexin type 9 serine protease inhibitors; TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
SI conversion factors: To convert HbA1c to proportion of total hemoglobin, multiply by 0.01; HDL-C, LDL-C, and VLDL-C to mmol/L, multiply by 0.0259; TG to mmol/L, multiply by 0.0113.
Data are expressed as number (%) for categorical variables (percentages have been rounded and may not total 100) and mean (SD) for continuous variables; median (IQR) values are also shown for TG. Detailed methods for measurement of each of the variables herein are provided in eMethods in Supplement 2.
Patients self-identified as “other” if they did not identify as any of the other races or ethnicities.
Defined by history of diabetes diagnosis, current use of antidiabetic medication, or HbA1c level of at least 6.5% at baseline.
Primary and Key Secondary End Point
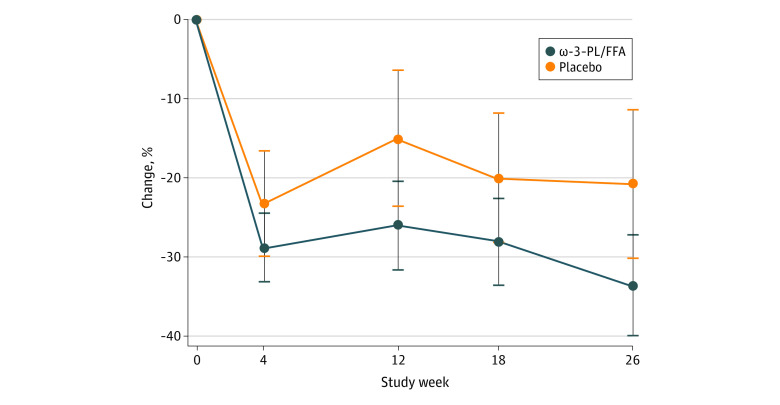
The mean percentage changes in TG levels during the 26 weeks treatment period are in Figure 1. At 12 weeks (primary end point), TG levels were reduced by 26.0% (95% CI, 20.5%-31.5%) in the ω-3–PL/FFA group and 15.1% (95% CI, 6.6%-23.5%) in the placebo group (mean treatment difference, −10.9% [95% CI, −20.4% to −1.5%]; P = .02). The treatment difference persisted at 26 weeks, with TG level reduced by 33.5% (95% CI, 27.2%-39.8%) in the ω-3–PL/FFA group and 20.8% (95% CI, 11.5%-30.1%) in the placebo group (mean treatment difference, −12.7% [95% CI, −23.1% to −2.4%]; P = .02). Absolute changes in TG levels are given in eFigure 3 in Supplement 2.
Figure 1. Percentage Changes in Fasting Triglyceride (TG) Levels Between Baseline and Week 26 (N = 520).
The 520 participants received ω-3–phospholipid/free fatty acid (ω-3–PL/FFA), 4 g/d, or placebo. Values are least-square mean differences from baseline, with 95% CIs (error bars) from analysis of covariance, including the main effects of treatment; qualifying TG category (≤750 vs >750 mg/dL); use of statins, cholesterol-absorption inhibitors, and/or proprotein convertase subtilisin/kexin type 9 serine protease inhibitors (yes or no); and baseline TG value as covariates.
Other Secondary End Points
Compared with placebo, ω-3–PL/FFA had no significant effect at 12 weeks on mean treatment differences in non–HDL-C (−3.2% [95% CI, −8.0% to 1.6%]; P = .18), VLDL-C (−3.8% [95% CI, −12.2% to 4.7%]; P = .38), HDL-C (0.7% [95% CI, −3.7% to 5.1%]; P = .77), or LDL-C (4.5% [95% CI, −5.9% to 14.8%]; P = .40) levels (Table 2). Corresponding differences at 26 weeks were −5.8% (95% CI, −11.3% to −0.3%; P = .04) for non–HDL-C, −9.1% (95% CI, −21.5% to 3.2%; P = .15) for VLDL-C, 1.9% (95% CI, −4.8% to 8.6%; P = .57) for HDL-C, and 6.3% (95% CI, −12.4% to 25.0%; P = .51) for LDL-C levels.
Table 2. Changes in Primary, Key Secondary, and Other Secondary End Points in the Intention-to-Treat Analysisa.
| End point | Change by treatment group (95% CI), % | Treatment difference (95% CI), % | P value | |
|---|---|---|---|---|
| Placebo (n = 148) | ω-3–PL/FFA (n = 372) | |||
| TG level, mg/dL | ||||
| Week 12 | −15.1 (−23.5 to −6.6) | −26.0 (−31.5 to −20.5) | −10.9 (−20.4 to −1.5) | .02 |
| Week 26 | −20.8 (−30.1 to −11.5) | −33.5 (−39.8 to −27.2) | −12.7 (−23.1 to −2.4) | .02 |
| Non–HDL-C level, mg/dL | ||||
| Week 12 | −4.6 (−8.8 to −0.5) | −7.8 (−10.5 to −5.1) | −3.2 (−8.0 to 1.6) | .18 |
| Week 26 | −3.6 (−8.3 to 1.2) | −9.4 (−12.7 to −6.1) | −5.8 (−11.3 to −0.3) | .04 |
| VLDL-C level, mg/dL | ||||
| Week 12 | −9.4 (−16.9 to −1.9) | −13.2 (−18.0 to −8.3) | −3.8 (−12.2 to 4.7) | .38 |
| Week 26 | −10.7 (−22.0 to 0.6) | −19.8 (−27.7 to −12.0) | −9.1 (−21.5 to 3.2) | .15 |
| HDL-C level, mg/dL | ||||
| Week 12 | 8.8 (4.9 to 12.6) | 9.4 (7.0 to 11.9) | 0.7 (−3.7 to 5.1) | .77 |
| Week 26 | 10.7 (5.0 to 16.4) | 12.6 (8.7 to 16.5) | 1.9 (−4.8 to 8.6) | .57 |
| LDL-C level, mg/dL | ||||
| Week 12 | 8.1 (−1.9 to 18.1) | 12.6 (8.0 to 17.2) | 4.5 (−5.9 to 14.8) | .40 |
| Week 26 | 14.9 (−4.5 to 34.4) | 21.2 (12.4 to 30.1) | 6.3 (−12.4 to 25.0) | .51 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ω-3–PL/FFA, ω-3–phospholipid/free fatty acid; TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
SI conversion factors: To convert HDL-C, LDL-C, and VLDL-C levels to mmol/L, multiply by 0.0259; TG to mmol/L, multiply by 0.0113.
The primary end point was the treatment difference in TG levels at 12 weeks; the key secondary end point, the treatment difference in TG levels at 26 weeks. Values are least-square mean differences from baseline, with P value from analysis of covariance including main effects of treatment; qualifying TG category (≤750 vs >750 mg/dL); use of statin, calcium-absorption inhibitors, and/or proprotein convertase subtilisin/kexin type 9 serine protease inhibitors (yes or no); and baseline TG value as covariates.
Exploratory End Points
At 12 weeks, 210 of 372 patients (56.5%) in the ω-3–PL/FFA group and 68 of 148 (45.90%) in the placebo group had achieved TG levels of less than 500 mg/dL (treatment difference, 10.5% [95% CI, 1.0% to 20.1%]; P = .02); corresponding proportions at 26 weeks were 234 of 372 (62.9%) and 76 of 148 (51.4%) (treatment difference, 11.5% [95% CI, 2.1% to 21.0%]; P = .01) (eTable 2 in Supplement 2). Changes in concentrations of total cholesterol, ApoB, ApoC3, ApoA5, LDL particle, hs-CRP, log hs-CRP, fasting glucose, and hemoglobin A1c are also shown in eTable 2 in Supplement 2. For example, mean treatment differences in ApoB were −2.07% (95% CI, −6.39% to 2.24%; P = .35) at 12 weeks and −0.89% (95% CI, −5.30% to 3.51%; P = .69) at 26 weeks. Concentrations of ApoC3 were reduced by ω-3–PL/FFA at 26 weeks (treatment difference, −21.99% [95% CI, −42.01% to −1.77%]; P = .03). Changes in other exploratory end points were not statistically significant.
Pharmacokinetic End Points
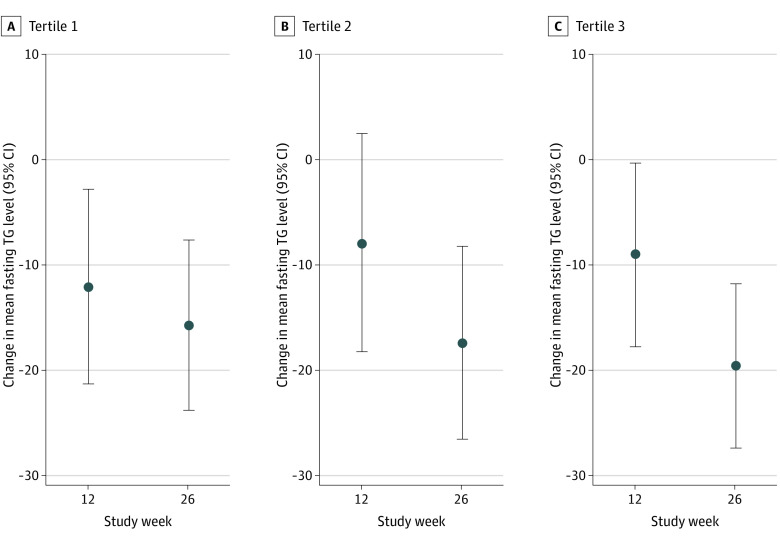
Treatment with ω-3–PL/FFA significantly increased levels of EPA, DHA, and EPA plus DHA compared with placebo at all study visits (eFigure 4 in Supplement 2). Proportional increases were larger for EPA compared with DHA; absolute increases were more similar. Changes in TG levels according to tertiles of achieved plasma PL EPA plus DHA are presented in Figure 2. At 26 weeks in all participants combined, TG levels were reduced by 15.6% (95% CI, −23.7% to −7.6%) in tertile 1, 17.3% (95% CI, −26.4% to −8.2%) in tertile 2, and 19.5% (95% CI, −27.2% to −11.7%) in tertile 3 of achieved EPA plus DHA concentrations; these reductions were not statistically significantly different from one another.
Figure 2. Percentage Change From Baseline in Fasting Triglyceride Levels According to Tertiles of Achieved Concentrations of Plasma Phospholipid Eicosapentaenoic Acid and Docosahexaenoic Acid.
Values are least-square mean differences from baseline (circles), with 95% CIs (error bars) from the analysis of covariance, including main effects of treatment; qualifying triglyceride (TG) category (≤750 vs >750 mg/dL); use of statin, cholesterol-absorption inhibitors, and/or proprotein convertase subtilisin/kexin type 9 serine protease inhibitors (yes or no); and baseline TG value as covariates. The ranges of achieved eicosapentaenoic acid plus docosahexaenoic acid concentrations in each tertile, assessed as percent of fatty acids, are as follows: T1, 1.2 to 2.4 (12 weeks) and 1.1 to 2.3 (26 weeks); T2, 2.5 to 3.6 (12 weeks) and 2.4 to 3.5 (26 weeks); T3, 3.7 to 9.0 (12 weeks) and 3.6 to 11.3 (26 weeks).
Efficacy Across Subgroups
Changes in the primary end point were not significantly different by age, sex, race and ethnicity, country, qualifying TG level, diabetes, or fibrate use (Figure 3). Patients taking statins or CAI (mean treatment difference, −19.5% [95% CI, −34.5% to −4.6%]; P = .01; P = .08 for interaction) and with lower baseline blood EPA plus DHA levels (−19.5% [95% CI, −33.8% to −5.3%]; P = .007; P = .08 for interaction) had larger effects than those in comparison groups, but the interaction was not statistically significant. Changes in secondary end points in these 2 subgroups are presented in eTables 3 and 4 in Supplement 2. Patients taking baseline statins or CAI, when compared with patients not taking these medications, were older (mean [SD] age, 56.2 [10.1] vs 53.7 [11.9] years), had higher mean (SD) body mass index (32.2 [5.0] vs 30.8 [5.2]) and hemoglobin A1c levels (6.9% [1.6%] vs 6.4% [1.5%]), and were more commonly taking fibrates (36 patients [14.6%] vs 14 patients [5.1%]).
Figure 3. Percentage Changes in Fasting Triglyceride (TG) Levels at 12 Weeks Across Prespecified Subgroups.
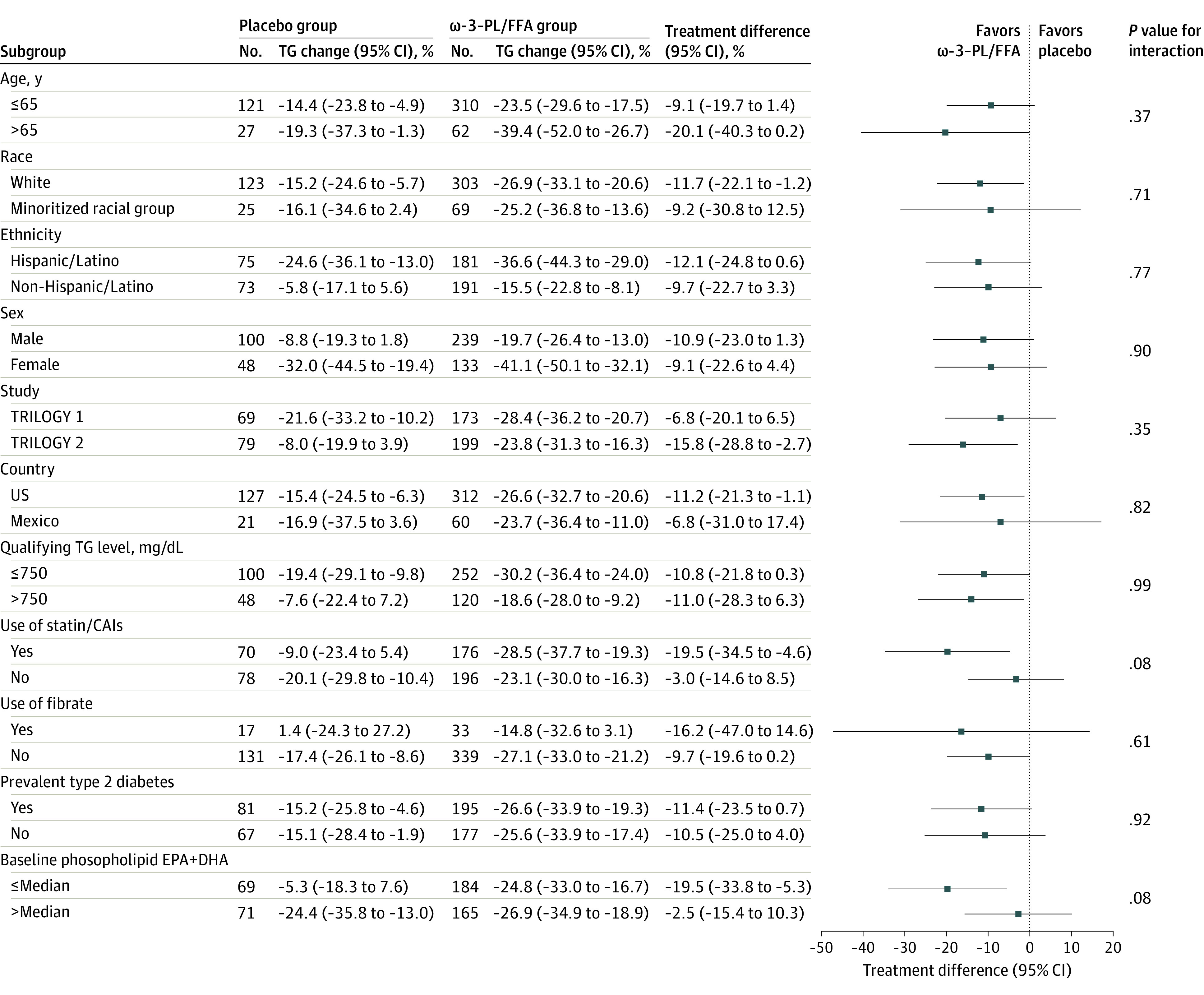
Comparisons were between ω-3–phospholipid/free fatty acid (ω-3–PL/FFA), 4 g/d, or placebo. Values are least-square mean differences from baseline, with P values based on analysis of covariance (ANCOVA), including main effects of treatment; qualifying TG category (≤750 vs >750 mg/dL); use of statin, cholesterol-absorption inhibitors (CAIs), and/or proprotein convertase subtilisin/kexin type 9 serine protease inhibitors; and baseline value as covariates. P value for interaction was tested by including a main effect of subgroup and a treatment-by-subgroup interaction effect within the same ANCOVA model. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid.
Sensitivity Analyses
Sensitivity analyses assessing the median percentage change in TG levels, using a nonparametric rank-based ANCOVA, were generally similar to the primary efficacy analysis, although with a modestly smaller median value and attenuated statistical significance compared with mean treatment differences (eTable 5 in Supplement 2). For example, comparing ω-3–PL/FFA with placebo at 26 weeks, the median percentage difference in TG levels was 10.1% (95% CI, −21.4% to 1.2%; P = .11), compared with a mean treatment difference of −12.7% (95% CI, −23.1% to −2.4%; P = .02).
Safety and Tolerability
The occurrence of any adverse event, serious adverse events, severe adverse events, and adverse events leading to treatment discontinuation was generally similar across the treatment groups (eTable 6 and eTable 7 in Supplement 2). Most adverse events (placebo group: 240 of 247 adverse events [97.2%] among 88 patients experiencing ≥1 adverse event; ω-3–PL/FFA group: 586 of 608 adverse events [96.4%] among 215 patients experiencing ≥1 adverse event) were mild to moderate in severity and were considered unrelated to the study drug, as assessed by study site investigators blinded to treatment assignment. The most common were headache (placebo, 9 of 148 [6.1%]; ω-3–PL/FFA, 27 of 372 [7.3%]), diabetes (placebo, 12 of 148 [8.1%]; ω-3–PL/FFA, 20 of 372 [5.4%]), and nasopharyngitis (placebo, 6 of 148 [4.1%]; ω-3–PL/FFA, 20 of 372 [5.4%]). No deaths occurred. Twenty-two patients experienced a serious adverse effect (placebo, 7 of 148 [4.7%]; ω-3–PL/FFA, 15 of 372 [4.0%]), and 20 discontinued treatment or participation because of an emergent adverse effect (placebo, 5 of 148 [3.4%]; ω-3–PL/FFA, 15 of 372 [4.0%]). One serious adverse effect of atrial fibrillation was reported in the ω-3–PL/FFA group, and none in the placebo group. ω-3–PL/FFA produced no significant changes in either trial in vital signs, electrocardiographic parameters, liver enzymes, or creatinine kinase values. Occurrence of bleeding adverse events (hemorrhages, grouping adverse events as Standardized MedDRA Query terms) was similar in both groups (5 of 148 [3.4%] vs 10 of 372 [2.7%]), as were gastrointestinal disorders (24 of 148 [16.2%] vs 68 of 372 [18.3%]).
Discussion
The TRILOGY program is the largest randomized, controlled investigation to date of an ω-3 formulation in severe hypertriglyceridemia. In pooled results of 2 multicenter trials, 4 g/d of ω-3–PL/FFA reduced TG levels by 10.9% at 12 weeks and 12.7% at 26 weeks, relative to placebo. At both 12 and 26 weeks, approximately 1 in 9 patients in the ω-3–PL/FFA group achieved TG levels of less than 500 mg/dL, providing a number needed to treat 11 patients for 1 more patient to achieve this goal. ω-3–PL/FFA was generally well tolerated, with a safety profile similar to that of placebo.
Other prescription ω-3 formulations investigated in their phase 3 trials for severe hypertriglyceridemia lowered TG levels from between a 12% to 39% reduction (eTable 8 in Supplement 2).17,18,19,20,21,22 These prior trials were generally much smaller (total often <50 patients and always <200 patients vs 520 patients in TRILOGY) and of shorter duration (typically 6-12 weeks vs 26 weeks in TRILOGY). Compared with these other formulations, ω-3–PL/FFA contains less EPA plus DHA per 4 g (1.24 vs 3.0-3.5 g) but has a unique and potentially more bioavailable formulation with about half of ω-3s as FFAs and half bound to PLs. Distinct from ethyl ester formulations, ω-3–PL/FFA bioavailability is also less dependent on coconsumption with higher-fat meals,10,11 which is relevant because low-fat diets are recommended for severe hypertriglyceridemia.
Treatment with ω-3–PL/FFA did not significantly raise LDL-C or ApoB levels. Other ω-3 formulations, such as ω-3 ethyl esters and ω-3 carboxylic acids, raise LDL-C levels, with usual increases of approximately 15% to 30% in patients with severe hypertriglyceridemia.23 In TRILOGY, nonsignificant numerical changes in LDL-C levels (4.5% at 12 weeks and 6.3% at 26 weeks) were not accompanied by any increase in ApoB level (−2.1% at 12 weeks and −0.9% at 26 weeks). This suggests potentially increased LDL particle size with ω-3–PL/FFA—a phenotype moving away from atherogenic dyslipidemia—rather than increased particle number. Prior ω-3–PL/FFA trials12,13 in patients with TG levels of at least 200 and less than 877 mg/dL also identified lowering of TG levels without raising LDL-C levels. The phospholipid-bound EPA and DHA in ω-3–PL/FFA may explain, at least partly, such an effect by interfering with intestinal cholesterol absorption24,25 and/or modulating expression and activity of transcriptional factors and enzymes involved in hepatic lipoprotein metabolism.26,27
Compared with baseline, TG levels in the ω-3–PL/FFA group decreased by 26.0% at 12 weeks and 33.5% at 26 weeks. However, reductions in TG levels in the placebo group were also larger than typically seen in such patients, declining 20.8% at 26 weeks vs baseline. In other ω-3 trials for severe hypertriglyceridemia, reductions in TG levels in the placebo group were much smaller, typically ranging from reductions of 4.6% to increases of 21.7% (eFigure 5 in Supplement 2).17,18,19,20,21,22 TRILOGY’s cornstarch placebo was unlikely to have meaningful TG-lowering effects: the dose herein of this widely used food ingredient was approximately 16 kcal/d. Fasting glucose and hemoglobin A1c levels generally did not significantly change (eTable 2 in Supplement 2), making it unlikely that better diabetes control explains the findings. We found that participants in the placebo group who experienced larger TG level reductions after randomization were also more likely to show a large increase in TG after the initial screening visit but before randomization. This suggests that the placebo-group reduction in TG levels may in part be associated with regression toward the mean among patients who had less stable or less severe hypertriglyceridemia at baseline.
The TG level–lowering effects of ω-3–PL/FFA appeared possibly stronger among patients already taking stable statin treatment (<3% took a CAI and none took PCSK9I), in whom the mean treatment difference was approximately 20%. The larger efficacy in this subgroup could be due to an unknown pharmacological interaction between ω-3–PL/FFA and statins or reflect statin use at study entry as a marker of patients with more persistent, severe hypertriglyceridemia status (ie, less prone to regression of episodically elevated TG levels to the mean). Exploration of TG changes in this subgroup, compared with patients not taking statins or CAI, showed both smaller reductions in the placebo group and larger reductions in the ω-3–PL/FFA group. Patients taking baseline statins or CAI were older and had higher BMI, had higher hemoglobin A1c levels, and were more commonly taking fibrates, each suggesting a higher-risk phenotype. This exploratory finding requires confirmation in future trials.
Effects of ω-3–PL/FFA on TG levels also appeared possibly stronger among patients with lower baseline circulating EPA plus DHA levels (mean treatment difference, 19.5%). This finding could be due to chance but is also consistent with potentially greater biological benefits of ω-3s when baseline exposure is lower.28 In a large ω-3 primary prevention trial,29 supplementation reduced the composite primary CVD end point in the prespecified subgroup of participants with lower baseline fish consumption. The exploratory result with ω-3–PL/FFA and baseline EPA plus DHA levels also requires confirmation in future studies.
Strengths and Limitations
This study has several strengths. A large number of participants increased statistical power for both efficacy and safety outcomes. Compliance and follow-up were high, minimizing bias. An extended 26-week intervention was performed, compared with 6 to 12 weeks in most prior ω-3 trials in severe hypertriglyceridemia.17,18,19,20,21,22 A range of important lipid, lipoprotein, and pharmacokinetic end points was assessed, adding to the knowledge base on effects on ω-3 therapy on these outcomes. Enrolled patients had considerable ethnic diversity, with 49.2% being Hispanic, increasing generalizability of findings to this important and growing demographic group.
This study also has some limitations. The placebo group experienced a large reduction in TG levels, reducing the statistical ability to detect a difference and requiring pooling of the 2 trials. Statistical significance was attenuated when using nonparametric analyses, although the observed TG reductions were similar numerically. Stronger findings in the prespecified subgroups of statin or CAI users and patients with lower baseline EPA plus DHA levels should be considered exploratory and warrant future investigation.
Conclusions
The pooled results of 2 large trials among patients with severe hypertriglyceridemia show that ω-3–PL/FFA, a novel, krill oil–derived mixture of ω-3, reduced TG levels at 12 and 26 weeks and increased the proportion of patients with TG levels of less than 500 mg/dL. The treatment was safe and well tolerated.
Statistical Analysis Plan and Trial Protocol
eMethods. Trial Operations, Inclusion and Exclusion Criteria, Laboratory Measurements, Assignment to Treatment Groups, and Statistical Analysis
eFigure 1. Design of the TRILOGY Trials
eFigure 2. Flow Diagram of Participants
eFigure 3. Changes in Fasting Triglyceride (TG) Levels in the 4-g/d ω-3–PL/FFA and Placebo Groups Between Baseline and Week 26 (n = 520)
eFigure 4. Absolute and Percentage Changes in Plasma Phospholipid EPA, DHA, and EPA Plus DHA (% Fatty Acids) During the 26-Week Double-Blind Period
eFigure 5. Comparison of Triglyceride Lowering Between Placebo and ω-3 Drugs in Severe Hypertriglyceridemia
eTable 1. Percentage Changes in Primary, Key Secondary, and Other Secondary End Points in Intention-to-Treat Analysis for TRILOGY 1 and 2
eTable 2. Changes in Exploratory End Points in Intention-to-Treat Analysis
eTable 3. Changes in Primary, Key Secondary, and Other Secondary End Points After 12 and 26 Weeks in Intention-to-Treat Analysis, Subgroup—Use of Statin, CAI and/or PCSK9
eTable 4. Changes in Primary, Key Secondary, and Other Secondary End Points After 12 and 26 Weeks in Intention-to-Treat Analysis, Subgroup—Baseline Phospholipid EPA Plus DHA
eTable 5. Changes in Triglycerides After 12 and 26 Weeks in Intention-to-Treat Analysis (Sensitivity Analyses)
eTable 6. Summary of Adverse Events
eTable 7. Detailed Summary of Severe Adverse Events
eTable 8. Comparison of TG-Lowering Effect of ω-3 Drugs at 4 g/d in Severe Hypertriglyceridemia
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10 113):2627-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10 027):1513-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller M, Stone NJ, Ballantyne C, et al. ; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- 4.Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426. doi: 10.1016/j.jacl.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Berglund L, Brunzell J, Sacks FM. Patient guide to the assessment and treatment of hypertriglyceridemia (high triglycerides). J Clin Endocrinol Metab. 2012;97(9):31A-32A. doi: 10.1210/jcem.97.9.zeg31a [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547-563. doi: 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791-2802. doi: 10.1016/j.jacc.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268-2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapointe JF, Harvey L, Aziz S, Jordan H, Hegele RA, Lemieux P. A single-dose, comparative bioavailability study of a formulation containing OM3 as phospholipid and free fatty acid to an ethyl ester formulation in the fasting and fed states. Clin Ther. 2019;41(3):426-444. doi: 10.1016/j.clinthera.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 11.Lapointe JF, Harvey L, Aziz S, Hegele RA, Lemieux P. Evaluation of OM3-PL/FFA pharmacokinetics after single and multiple oral doses in healthy volunteers. Clin Ther. 2019;41(12):2500-2516. doi: 10.1016/j.clinthera.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov . TRIal For Efficacy of Capre on hyperTriglyceridemiA (TRIFECTA). NCT01455844. Accessed October 8, 2021. https://clinicaltrials.gov/ct2/show/NCT01455844
- 13.ClinicalTrials.gov . Assess the Safety and Efficacy of NKPL66 (CaPre™) in the Treatment of Mild-to-high Hypertriglyceridemia. NCT01516151. Accessed October 8, 2021. https://clinicaltrials.gov/ct2/show/NCT01516151
- 14.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration . Code of Federal Regulations—Title 21: Food and Drugs. Accessed November 30, 2021. https://www.fda.gov/medical-devices/medical-device-databases/code-federal-regulations-title-21-food-and-drugs
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 17.Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4(5-6):385-391. doi: 10.1177/174182679700400511 [DOI] [PubMed] [Google Scholar]
- 18.Abe Y, El-Masri B, Kimball KT, et al. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol. 1998;18(5):723-731. doi: 10.1161/01.ATV.18.5.723 [DOI] [PubMed] [Google Scholar]
- 19.Pownall HJ, Brauchi D, Kilinç C, et al. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143(2):285-297. doi: 10.1016/S0021-9150(98)00301-3 [DOI] [PubMed] [Google Scholar]
- 20.US Food & Drug Administration Center for Drug Evaluation and Research, Office of Translational Sciences, Office of Biostatistics . Application No. 202057. Statistical Review(s). Accessed November 30, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202057Orig1s000StatR.pdf
- 21.US Food & Drug Administration Center for Drug Evaluation and Research, Office of Translational Sciences, Office of Biostatistics . Application No. 205060. Statistical Review(s). Accessed November 30, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205060Orig1s000StatR.pdf
- 22.US Food & Drug Administration Center for Drug Evaluation and Research, Office of Translational Sciences, Office of Biostatistics . Application No. 204977. Statistical Review(s). Accessed November 30, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204977Orig1s000StatR.pdf
- 23.Skulas-Ray AC, Wilson PWF, Harris WS, et al. ; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140(12):e673-e691. doi: 10.1161/CIR.0000000000000709 [DOI] [PubMed] [Google Scholar]
- 24.Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2010;2(2):116-127. doi: 10.3390/nu2020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahebkar A. Fat lowers fat: purified phospholipids as emerging therapies for dyslipidemia. Biochim Biophys Acta. 2013;1831(4):887-893. doi: 10.1016/j.bbalip.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 26.Burri L, Berge K, Wibrand K, Berge RK, Barger JL. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front Genet. 2011;2:45. doi: 10.3389/fgene.2011.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossmeisl M, Medrikova D, van Schothorst EM, et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim Biophys Acta. 2014;1841(2):267-278. doi: 10.1016/j.bbalip.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D. Fish, cardiovascular disease, and mortality—what is the global evidence? JAMA Intern Med. 2021;181(5):649-651. doi: 10.1001/jamainternmed.2021.0045 [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Analysis Plan and Trial Protocol
eMethods. Trial Operations, Inclusion and Exclusion Criteria, Laboratory Measurements, Assignment to Treatment Groups, and Statistical Analysis
eFigure 1. Design of the TRILOGY Trials
eFigure 2. Flow Diagram of Participants
eFigure 3. Changes in Fasting Triglyceride (TG) Levels in the 4-g/d ω-3–PL/FFA and Placebo Groups Between Baseline and Week 26 (n = 520)
eFigure 4. Absolute and Percentage Changes in Plasma Phospholipid EPA, DHA, and EPA Plus DHA (% Fatty Acids) During the 26-Week Double-Blind Period
eFigure 5. Comparison of Triglyceride Lowering Between Placebo and ω-3 Drugs in Severe Hypertriglyceridemia
eTable 1. Percentage Changes in Primary, Key Secondary, and Other Secondary End Points in Intention-to-Treat Analysis for TRILOGY 1 and 2
eTable 2. Changes in Exploratory End Points in Intention-to-Treat Analysis
eTable 3. Changes in Primary, Key Secondary, and Other Secondary End Points After 12 and 26 Weeks in Intention-to-Treat Analysis, Subgroup—Use of Statin, CAI and/or PCSK9
eTable 4. Changes in Primary, Key Secondary, and Other Secondary End Points After 12 and 26 Weeks in Intention-to-Treat Analysis, Subgroup—Baseline Phospholipid EPA Plus DHA
eTable 5. Changes in Triglycerides After 12 and 26 Weeks in Intention-to-Treat Analysis (Sensitivity Analyses)
eTable 6. Summary of Adverse Events
eTable 7. Detailed Summary of Severe Adverse Events
eTable 8. Comparison of TG-Lowering Effect of ω-3 Drugs at 4 g/d in Severe Hypertriglyceridemia
eReferences
Nonauthor Collaborators
Data Sharing Statement