Highlights
-
•
The COVID-19 pandemic has significantly impacted gynecologic cancer care.
-
•
Referrals to gynecologic oncology decreased in the early months of the pandemic.
-
•
Referral time to gynecologic oncology evaluation was not impacted by the pandemic.
-
•
Time to cancer treatment initiation decreased significantly during the pandemic.
-
•
Disparities in time to cancer treatment initiation improving during the pandemic.
Keywords: COVID-19, Pandemic, Gynecologic oncology, Access to care
Abstract
Objective
To evaluate the impact of the COVID-19 pandemic on referral to and delivery of gynecologic oncology care at a National Cancer Institute-designated Comprehensive Cancer Center.
Methods
We conducted a retrospective cohort study of patients referred for evaluation by a gynecologic oncologist at Washington University in St. Louis from October 2019 – February 2020 (pre-COVID-19), and April - August 2020 (COVID-19). The primary outcome, time from referral to evaluation by a gynecologic oncologist, was compared between the two time periods. Secondary outcomes included time from initial evaluation to treatment and delays/interruptions in care due to the pandemic. Sub-group analyses were performed on patients with a cancer diagnosis to evaluate the impact of COVID-19 on treatment decision making.
Results
884 patients were referred during the study period. Total referrals fell by 32% (526 to 358 patients, p < 0.001) and referrals for cancer fell by 18% (228 to 188 patients, p = 0.049). The pandemic did not impact time from referral to initial gynecologic oncology appointment overall (pre-COVID-19: 19.1 vs. COVID-19: 17.4 days, p = 0.315) or among patients with cancer (14.4 vs. 13.9 days, p = 0.662). Time from initial appointment to cancer treatment decreased by 9 days (34 days to 25 days, p = 0.001).
Conclusion
Referrals to gynecologic oncology decreased significantly during the early months of COVID-19. Though time from referral to evaluation was not impacted by the pandemic, time to treatment initiation decreased despite institutional changes related to COVID-19.
1. Introduction
Disasters and pandemics burden the healthcare system, delay care for chronic diseases, and interrupt oncology services (Ford et al., 2006, Man et al., 2018). As the United States COVID-19 case count approaches 50 million and the death toll reaches 800,000, continued examination of the pandemics’ burden on cancer care is critical for evaluating access and equity of our healthcare system as well as planning for the future (COVID Data Tracker, 2021). During the COVID-19 pandemic the Society of Gynecologic Oncology (SGO) and the American College of Surgeons (ACS) developed recommendations for the care of cancer patients that attempted to balance patient risk of COVID-19 exposure against the risk of cancer progression (Fader et al., 2020). The goals of the recommendations were fair allocation of resources, minimization of virus exposure through reduction in unnecessary procedures, and minimal impact on cancer survivorship (Bogani et al., 2020).
The COVID-19 pandemic’s impact on cancer care has been multi-faceted. In addition to facing higher risk for COVID-19 complications, cancer patients may experience delays or disruptions in evaluation and treatment (Dai et al., 2020). Patients have reported concerns about seeking care during the pandemic, and fear of COVID-19 infection may have prompted patients with cancer symptoms to delay seeking care (Catania et al., 2020, Sutcuoglu et al., 2020). The pandemic has also reduced cancer screening, delayed referrals, and impacted treatment decisions in gynecology oncology (Bogani et al., 2020, Brugel et al., 2021, Pothuri et al., 2020). Furthermore, many gynecologic oncologists reported practice changes due to COVID-19 (Martinelli and Garbi, 2020). Such practice changes included alteration in treatment selection, delay in asymptomatic surveillance visits, and emphasis on early post-operative discharges. We will see the impacts of the pandemic for years to come, with current estimates predicting increased mortality and avoidable deaths among cancer patients, especially in those with more than one medical comorbidity (Lai et al., 2020, Maringe et al., 2020).
Sustained access to gynecologic oncology care can be challenging for patients with geographic, financial, and social barriers. These challenges translate to compromised survival and decreased adherence to standard of care treatment for Black and Hispanic patients as well as patients who are uninsured or covered by Medicaid insurance (Chatterjee, 2016, Ward et al., 2008, Bradley et al., 2004). The COVID-19 pandemic has exacerbated these pre-existing disparities. In a study by Schmidt et al, Black and Hispanic patients with cancer were more likely to develop COVID-19, and Hispanic patients were more likely to experience delays in care, compared to White patients (Schmidt et al., 2020). Understanding the impact of the pandemic on the care of historically underserved demographic groups will instruct health systems planning to mitigate existing disparities in the future.
The primary objective of this study was to evaluate the impact of the COVID-19 pandemic on referral to and delivery of gynecologic oncology care at a National Cancer Institute-designated Comprehensive Cancer Center. Secondary objectives were to identify challenges in cancer care delivery, specifically delays and interruptions in evaluation and treatment. We were also interested in assessing the impact of social determinants of health (race and insurance status) among gynecologic oncology patients on cancer treatment initiation.
2. Methods
We conducted a single-center, retrospective cohort study of patients who were newly referred for evaluation by a gynecologic oncologist at the School of Medicine at Washington University in St. Louis from October 1, 2019 – February 29, 2020 (pre-COVID-19), and April 1, 2020 – August 31, 2020 (COVID-19). This study was approved by the University’s Institutional Review Board. Electronic medical records were reviewed to obtain patient demographic, pathologic, and treatment data. All data were entered into Research Electronic Data Capture, which is a Health Insurance Portability and Accountability Act compliant database.
In anticipation of the March 18, 2020 lockdown for the state of Missouri, many changes were implemented which impacted the comprehensive care of gynecologic oncology patients. Scheduling, administrative, and research staff were moved to remote locations, and some were furloughed. The division of Gynecologic Oncology maintained a stable workforce capacity of nine faculty and four fellows. Hospital access was restricted, and temperature and symptom screening were required. Masking was mandated for all hospital personnel, with face shields required for encounters with high-risk patients. On oncology units, visitation was eliminated, and enhanced disinfection was instituted.
Faculty and fellows reviewed clinic schedules. Patients with Medicaid insurance or uninsured status are seen in the fellow run clinic under faculty supervision while privately insured and Medicare patients are seen in the faculty clinics. Prior work has demonstrated that the care provided by the fellow run clinic results in similar survival as compared to the faculty clinics (Buchanan et al., 2020). Most surveillance appointments were rescheduled with a delay of 4 to 12 weeks and pre-chemotherapy visits were converted to telemedicine encounters. New and symptomatic established patients were still seen as scheduled in the office. Chemotherapy units remained open, but companions were prohibited.
Non-urgent surgical procedures were cancelled, but the impact on oncologic surgery was complex. Scheduled cancer surgeries were completed, but surgery for presumed benign masses and risk-reduction indications were delayed indefinitely. Risk of malignancy in an imaged mass was assessed and incorporated into the decision to schedule or defer surgery. Women considered at high risk for malignancy were treated like those with proven cancer. To preserve intensive care capacity, surgeons were initially instructed to hold cases when a delay of up to three weeks was considered unlikely to result in patient harm. Beginning in May 2020, surgical cases were categorized according to the tier system of the American College of Surgeons (Siddiqui, 2020). Cases qualifying as tiers 2 or 3 were allowed to proceed.
The comorbidities that were captured included those that the Center for Disease Control (CDC) listed as placing patients at high risk for severe illness from COVID-19 in September 2020 (Centers for Disease Control and Prevention, 2020).
The primary outcome was time from referral to evaluation by a gynecologic oncologist. Secondary outcomes included time from initial evaluation to treatment initiation (defined as time from initial gynecologic oncology evaluation to first cancer-directed therapy, including surgery, chemotherapy, immunotherapy, hormone therapy, or radiation), delays, or interruptions in care due to the pandemic, and evaluation of the impact of the COVID-19 pandemic on cancer treatment decision making.
All categorical variables were analyzed using chi-squared and Fisher's exact tests. Continuous parametric variables were analyzed with two sample t-tests. For non-parametric variables, a Wilcoxon rank sum test was used and subsequently validated using data log transformation. A p-value < 0.05 indicated a statistically significant difference for all comparisons of primary and secondary outcomes. Two-sided statistical assessment was used for all analyses.
3. Results
From October 1, 2019 through August 31, 2020, 884 patients were seen for new patient visits by faculty and fellows in the Division of Gynecologic Oncology. Patient characteristics are listed in Table 1. The mean age was 56.5 years, and the majority of patients were White (81%), non-Hispanic (96.5%), privately insured (52.5%), and seen in the faculty private clinics (88.7%). Overall, 526 (59.4%) patients were diagnosed with a gynecologic cancer or pre-cancer, 345 (39.0%) had non-cancer indications for referral, and 14 (1.6%) had non-gynecologic primary disease. Of those with a gynecologic cancer or pre-cancer, 264 (50.2%) had uterine disease, 96 (18.3%) had ovarian, fallopian tube, or primary peritoneal disease, and 108 (20.5%) had cervical disease.
Table 1.
Demographics and clinical characteristics of newly referred gynecologic oncology patients.
| Variable | Characteristics | Total | Pre-COVID-19 | COVID-19 | P value |
|---|---|---|---|---|---|
| N=884 | N=526 (60) | N=358 (40) | |||
| Age | Mean +/− SD | 56.5 (15.4) | 56.4 (15.7) | 56.6 (15.1) | 0.879 |
| Race | White | 717 (81.0) | 421 (79.9) | 296 (82.7) | 0.762 |
| Black | 143 (16.2) | 89 (16.9) | 54 (15.1) | ||
| Asian | 16 (1.8) | 10 (1.9) | 6 (1.7) | ||
| Other | 3 (0.3) | 2 (0.4) | 1 (0.3) | ||
| Unknown | 6 (0.7) | 4 (1.0) | 1 (0.3) | ||
| Ethnicity | Hispanic | 20 (2.3) | 15 (2.9) | 5 (1.4) | 0.251 |
| Non-Hispanic | 854 (96.5) | 504 (95.6) | 350 (97.8) | ||
| unknown | 11 (1.2) | 8 (1.5) | 3 (0.8) | ||
| Insurance | Private | 465 (52.5) | 290 (55.0) | 175 (48.9) | 0.400 |
| Medicare | 286 (32.3) | 164 (31.1) | 122 (34.1) | ||
| Medicaid | 106 (12.0) | 58 (11.0) | 48 (13.4) | ||
| Uninsured | 11 (1.2) | 5 (0.95) | 6 (1.7) | ||
| Other | 17 (1.9) | 10 (1.9) | 7 (2.0) | ||
| BMI | Mean +/− SD | 32.0 (10.0) | 31.6 (9.7) | 32.4 (10.4) | 0.268 |
| <30 | 426 (48.1) | 262 (49.7) | 164 (45.8) | 0.542 | |
| 30–39 | 287 (32.4) | 169 (32.1) | 118 (33.0) | ||
| 40–49 | 127 (14.4) | 73 (13.9) | 54 (15.1) | ||
| 50–59 | 27 (3.1) | 14 (2.7) | 13 (3.6) | ||
| 60–69 | 10 (1.1) | 6 (1.1) | 4 (1.1) | ||
| 70+ | 8 (0.9) | 3 (0.6) | 5 (1.4) | ||
| Comorbidities | Serious heart condition | 81 (9.2) | 54 (10.3) | 27 (7.5) | 0.192 |
| CKD | 68 (7.7) | 42 (8.0) | 26 (7.3) | 0.797 | |
| Diabetes | 156 (17.6) | 93 (17.7) | 63 (17.6) | 1.0 | |
| COPD | 46 (5.2) | 34 (6.5) | 12 (3.4) | 0.045 | |
| Sickle cell disease | 2 (0.2) | 0 | 2 (0.6) | 0.163 | |
| Immunocompromised | 6 (0.7) | 2 (0.4%) | 4 (1.1) | 0.229 | |
| Smoking status | Non-smoker | 734 (82.9) | 436 (82.7) | 298 (83.2) | 0.331 |
| Current smoker | 147 (16.6) | 87 (16.5) | 60 (16.8) | ||
| unknown | 4 (0.5) | 4 (0.8) | 0 | ||
| ECOG performance status | 0 | 415 (78.8) | 246 (80.4) | 169 (76.5) | 0.512 |
| 1 | 79 (15.0) | 45 (14.7) | 34 (15.4) | ||
| 2 | 25 (4.7) | 12 (3.9) | 13 (5.9) | ||
| 3 | 4 (0.8) | 1 (0.3) | 3 (1.4) | ||
| 4 | 4 (0.8) | 2 (0.7) | 2 (0.9) | ||
| Service location | Fellow Clinic | 100 (11.3) | 47 (8.9) | 53 (14.8) | 0.009 |
| Faculty Clinics | 785 (88.7) | 480 (91.1) | 305 (85.2) | ||
| Disease site | Uterus | 264 (29.8) | 155 (29.6) | 109 (30.5) | <0.001 |
| Ovary, fallopian tube, primary peritoneal | 96 (10.8) | 48 (9.1) | 48 (13.4) | ||
| Cervix | |||||
| Vulva | 108 (12.2) | 67 (12.7) | 41 (11.5) | ||
| Vagina | 47 (5.3) | 31 (5.9) | 16 (4.5) | ||
| Benign | 11 (1.2) | 5 (0.95) | 6 (1.7) | ||
| Other | 345 (39.0) | 210 (39.5) | 135 (36.9) | ||
| 14 (1.6) | 10 (2.3) | 4 (1.7) | |||
| FIGO stage | I | 233 (43.2) | 128 (40.3) | 105 (47.1) | <0.001 |
| II | 30 (5.6) | 9 (2.8) | 21 (9.4) | ||
| III | 79 (14.6) | 44 (13.9) | 35 (15.7) | ||
| IV | 49 (9.1) | 34 (10.7) | 15 (6.7) | ||
| Unstaged | 25 (4.6) | 13 (4.1) | 12 (5.4) | ||
| Pre-invasive | 124 (23.0) | 89 (28.1) | 35 (15.7) |
SD, standard deviation; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecologists and Obstetricians.
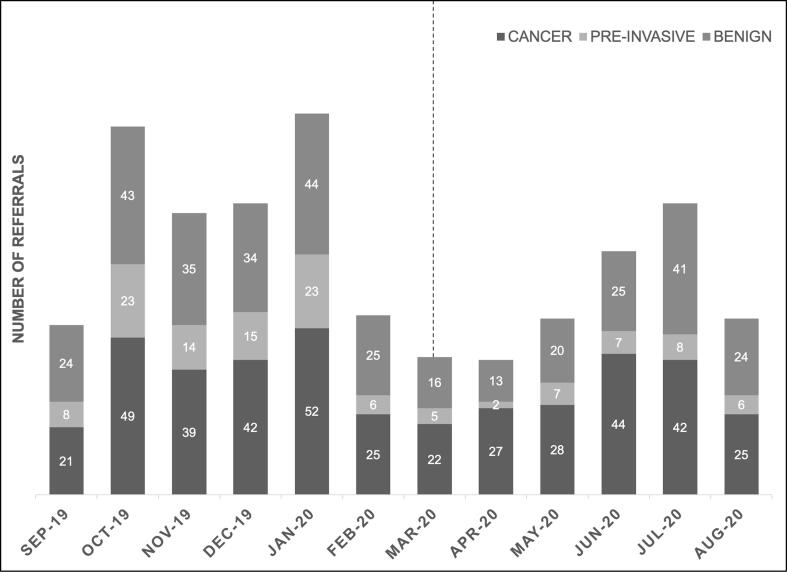
The pandemic resulted in a large reduction in the number of patients presenting for care. New referrals fell from 526 during the 5 months prior to the pandemic to 358 in the first five months of the pandemic, a 32% decline (p < 0.001) (Fig. 1). This decline was consistent across racial/ethnic groups, modes of insurance, and service locations. We observed a 17.5% decrease in referrals for patients with cancer (p = 0.049), a 60.7% decrease in referrals for pre-invasive disease (p = 0.017), and a 36.5% decrease in referrals for benign pathology (p < 0.001) (Table 1). Referral for ovarian disease was stable between the pre-COVID-19 and COVID-19 cohorts, but referrals for uterine and cervical disease decreased by 30.1% and 38.8% respectively (Table 1). Referrals for stage I cancer decreased by 18.9%, stage II increased by 33.3%, stage III decreased by 20.5%, and stage IV decreased by 55.9% (p = 0.0003) (Table 1). During the pandemic a higher proportion of new patient referrals were seen in the fellows’ clinic, which serves patients without insurance or insured only by Medicaid (pre-COVID-19: 8.9% vs. COVID-19: 14.8%, p = 0.009).
Fig. 1.
Referrals to Gynecologic Oncology from September 2019 – August 2020 Referrals to gynecologic oncology for cancer, pre-invasive disease, and benign disease decreased in the early months of the pandemic. The vertical dotted line indicates the beginning of the COVID-19 pandemic.
3.1. Referral times to see a gynecologic oncologist during the COVID-19 pandemic
The COVID-19 pandemic did not impact median time from referral to initial gynecologic oncology appointment (pre-COVID-19: 19.1 days vs. COVID-19: 17.4 days, p = 0.334) and did not differ among patients with a cancer diagnosis (14.4 days vs. 13.9 days, p = 0.716) or those with benign/pre-invasive disease (22.3 days vs 18.4 days, p = 0.423) (Table 2).
Table 2.
Impact of the COVID-19 pandemic on initiation of Gynecologic Oncology care.
| Variable | Pre-COVID-19 | COVID-19 | P value | |
|---|---|---|---|---|
| N=527 (60) | N=358 (40) | |||
| Time from referral to initial appointment | Overall cohort | 19.1 (32.7) | 17.4 (20.4) | 0.334 |
| (days, mean +/− SD) | Cancer patients | 14.4 (12.5) | 13.9 (14.2) | 0.716 |
| Benign + pre-invasive | 22.3 (48.3) | 18.4 (23.7) | 0.243 | |
| Referral source | Emergency room | 14 (2.7) | 11 (3.1) | 0.023 |
| Inpatient hospital | 6 (1.1) | 7 (2.0) | ||
| Gynecologist | 375 (71.2) | 284 (79.3) | ||
| Outpatient PCP | 47 (8.9) | 25 (7.0) | ||
| Self | 24 (4.6) | 9 (2.5) | ||
| other | 61 (11.6) | 22 (6.2) | ||
| Appointment attendance | Attended | 444 (84.3) | 318 (88.8) | 0.060 |
| Rescheduled (cancel or no show) | 83 (15.8) | 40 (11.2) | ||
| Reason for appointment cancellation | Patient cancel, COVID-19 | 0 | 8 (21.1) | 0.042 |
| Patient cancel, unspecified | 57 (89.1) | 18 (47.4) | ||
| Provider cancelled | 4 (6.3) | 11 (28.9) | ||
| Other | 3 (4.7) | 1 (2.6) | ||
The majority of patients were referred to gynecologic oncology by gynecologists (71% pre-COVID-19 vs. 79% COVID-19) followed by other non-gynecologic subspecialists (GI, medical oncology, colorectal surgery, etc.) or primary care providers. Absolute numbers of referrals from gynecologists, primary care providers, and other referral sources decreased during the pandemic, whereas the number of referrals from the emergency room or inpatient remained stable (Table 2).
Though COVID-19 did not impair clinic attendance, reasons for cancellation changed. During the pandemic, 21% of cancellations were patient-initiated due to fear related to COVID-19. Provider-initiated cancellation increased from 6.0% to 28.9% of all cancellations, and patient-initiated cancellations fell from 89% to 47% of all cancellations (Table 2).
3.2. Challenges of delivering gynecologic cancer care during the COVID-19 pandemic
The following results were restricted to patients with a confirmed diagnosis of a gynecologic malignancy (n = 416, Table 3). Compared to the pre-COVID-19 period, during the pandemic, time from initial appointment to cancer treatment initiation decreased from 33.5 days to 24.7 days (p = 0.001). Within this enriched cancer population, factors associated with shorter time to treatment during the COVID-19 pandemic included White race, absence of comorbid conditions, BMI ≥ 30, non-smokers, faculty clinic provider (vs. fellows’ clinic), and uterine or vaginal disease (Table 3).
Table 3.
Impact of COVID-19 pandemic on time to treatment initiation among cancer patients.
| Variable | Pre-COVID 19 | COVID-19 | P value | |
|---|---|---|---|---|
| (N=228) | (N=188) | |||
| Time from initial visit to treatment initiation | 33.5 (31.5) | 24.9 (17.4) | 0.001 | |
| (days, mean +/− SD) | ||||
| race | White | 31.6 (28.0) | 25.1 (16.8) | 0.012 |
| Black | 43.5 (45.1) | 26.1 (22.8) | 0.062 | |
| Asian | 30.0 (18.5) | 22.0 (3.5) | 0.803 | |
| insurance | Private | 31.1 (30.3) | 23.9 (15.8) | 0.050 |
| Medicare | 33.4 (30.7) | 26.9 (17.6) | 0.107 | |
| Medicaid | 44.0 (42.1) | 26.1 (23.5) | 0.102 | |
| Uninsured | 45.3 (28.4) | 20.0 (14.4) | 0.238 | |
| Number of comorbidities | 0 | 31.5 (21.7) | 24.5 (18.8) | 0.007 |
| 1 | 33.0 (38.3) | 25.3 (15.1) | 0.168 | |
| 2 | 47.9 (64.1) | 24.4 (12.9) | 0.200 | |
| >2 | 46.6 (26.8) | 35.3 (9.4) | 0.511 | |
| BMI | <25 | 32.5 (41.3) | 28.2 (20.0) | 0.554 |
| 25–29.9 | 29.6 (22.1) | 30.5 (22.6) | 0.846 | |
| 30–39.9 | 28.5 (16.8) | 20.6 (13.1) | 0.004 | |
| ≥4# | 49.0 (45.0) | 22.6 (12.4) | 0.001 | |
| Smoking status | Non-smoker | 33.5 (31.5) | 24.4 (16.5) | 0.001 |
| Current smoker | 33.4 (32.1) | 26.9 (21.0) | 0.346 | |
| Service location | Fellow clinic | 39.3 (32.8) | 25.8 (21.6) | 0.120 |
| Faculty clinics | 32.9 (31.4) | 26.7 (25.9) | 0.002 | |
| Disease site | Uterus | 36.2 (35.4) | 23.6 (14.3) | <0.001 |
| Ovary, fallopian tube, primary peritoneal | 24.8 (17.9) | 24.8 (20.0) | 1.0 | |
| Cervix | ||||
| Vulva | 44.8 (32.6) | 33.3 (24.8) | 0.204 | |
| Vagina | 32.0 (23.3) | 21.1 (10.9) | 0.222 | |
| 10.3 (8.5) | 34.3 (15.3) | 0.034 | ||
Pre-COVID-19, cancer treatment for Black patients was delayed by more than 10 days compared to White patients (Black: 43.5 days vs. White: 31.6 days, p = 0.033), but this disparity disappeared during the pandemic (Black: 26.1 days vs. White: 25.1, p = 0.759) (Table 4). Similarly, before the pandemic, patients with Medicaid faced significant delays compared to those with private insurance (private: 31.1 days vs. Medicaid: 44.0 days, p = 0.006), but these disparities also resolved within the first five months of the pandemic (private: 23.9 days vs. Medicaid: 26.1 days, p = 0.541) (Table 3).
Table 4.
Delay and disruption in care due to the COVID-19 pandemic.
| Variable | Total | |
|---|---|---|
| Delay/disruption in care due to COVID 19 pandemic | 115 (13.0) | |
| How was care impacted? | Delay in chemo initiation | 0 |
| Delay in radiation initiation | 6 (5.2) | |
| Delay in surgery | 21 (18.3) | |
| Treatment interruption | 4 (3.5) | |
| Treatment alteration | 4 (3.5) | |
| Rescheduled outpatient visits | 36 (31.3) | |
| Delay in initial visit | 21 (18.2) | |
| Delay due to personal COVID 19 diagnosis | 6 (5.2) | |
| Lost to follow up | 13 (11.3) | |
| Other | 4 (3.5) | |
Thirteen percent of all gynecologic oncology patients experienced a treatment delay or disruption due to the COVID-19 pandemic. The most cited delays were rescheduled outpatient visits (31.3%) and delay in surgery (18.3%) (Table 4). In total, 36 (10%) cancer patients seen during the pandemic were diagnosed with COVID-19. Of those patients, 6 (16.7%) experienced a delay or interruption in care due to their COVID-19 diagnosis.
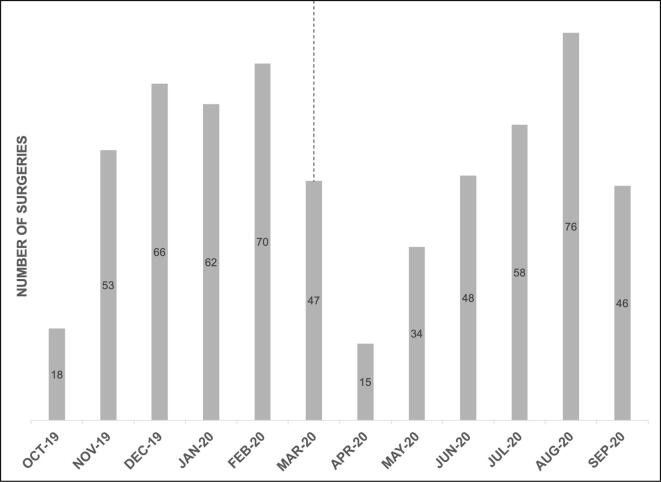
A decrease in surgical volume was observed in March 2020 and began to recover to pre-pandemic levels in July 2020 (Supplementary Fig. 1). Despite this shift in surgical volume, similar rates of primary surgical management for uterine, ovarian, cervical, and vulvar disease were observed (Table 5a). Likewise, there were similar rates of interval cytoreductive surgeries after neo-adjuvant chemotherapy for patients with advanced stage ovarian cancer (Table 5b).
Table 5a.
Impact of COVID-19 pandemic on treatment management among cancer patients.
| Variable | Total N = 416 |
Pre-COVID-19 N = 228 (54.8) |
COVID-19 N = 188 (45.2) |
P value | |
|---|---|---|---|---|---|
| Recommendation for primary surgical management | Uterus (n = 265) | 209 (78.9) | 126 (80.8) | 83 (76.2) | 0.365 |
| Ovary (n = 96) | 65 (67.7) | 30 (62.5) | 35 (72.9) | 0.275 | |
| Cervix (n = 108) | 41 (38.0) | 30 (44.8) | 11 (26.8) | 0.070 | |
| Vulva (n = 47) | 32 (68.1) | 22 (71.0) | 10 (62.5) | 0.742 | |
| Same day discharge (n = 324) | 91 (28.1) | 24 (13.9) | 67 (44.4) | <0.001 | |
| Readmission within 30 days of surgery | 20 (6.2) | 10 (5.8) | 10 (6.6) | 0.819 | |
Table 5b.
Impact of COVID-19 pandemic on administration of neo-adjuvant chemotherapy in patients with ovarian cancer.
| Variable | Total N = 96 |
Pre-COVID-19 N = 48 |
COVID-19 N = 48 |
P value |
|---|---|---|---|---|
| Patients who received neo-adjuvant chemotherapy | 20 (20.8) | 11 (22.9) | 9 (18.8) | 0.615 |
| Number of cycles of neo-adjuvant chemotherapy | 3.5 (1.0) | 3.8 (1.2) | 3.1 (0.6) | 0.111 |
In response to the pandemic, we adapted perioperative management to minimize bed utilization. As such, patients were more likely to be discharged on the day of surgery during the pandemic (pre-COVID-19: 14% vs. COVID-19: 44%, p < 0.001). Rates of readmission within 30 days of surgery remained stable (5.8% vs. 6.6%, p = 0.819) (Table 5a).
4. Discussion
Our study found that during the first five months of the COVID-19 pandemic, referrals to gynecologic oncology for any indication decreased by 32% while referrals for patients with cancer decreased by 18%. We believe this observation is most likely due to patients deferring evaluation for symptoms/screening, rather than the referring providers delaying referral for diagnosed oncologic problems. This is supported by our finding that referrals for patients with pre-invasive disease decreased by 61%, likely because of a decrease in cervical cancer screening. Two other large studies that investigated the pandemic effects on cervical cancer screening found reductions during 2020 ranging from 67% to 80% (Miller et al., 2021, Mast and Munoz del Rio, 2020). Decreases in cancer diagnoses were observed for cancers with (e.g., breast, colon and lung cancers) and without (e.g., pancreatic, gastric and esophageal cancers) established screening tests (Kaufman et al., 2020). These findings highlight the need for expanded screening and the prioritization of high-risk patients.
Going forward, delayed screening and deferred evaluation imply that patients will be presenting with more advanced disease requiring more intensive therapy with worse prognosis. There is currently no organized and validated strategy to quantify and eliminate the screening and undiagnosed cancer deficits created by the pandemic. One important step is re-engaging patients in the healthcare system. As COVID-19 vaccination rates continue to increase and patients are more comfortable entering a healthcare setting, hospital systems need to identify patients who are overdue for their cervical cancer screening. We will rely on our generalist and primary care colleagues to assist with screening volume, while gynecologic oncology efforts can be focused on high-risk patients.
Despite many practice changes made at our institution and within our division in response to the pandemic, our service maintained expeditious care for patients who were referred with minimal treatment delays or disruptions. In addition, COVID-19 did not have a significant impact on treatment decision-making as evidenced by similar rates of primary surgical management for uterine, ovarian, cervical, and vulvar disease, and unchanged frequency of neo-adjuvant chemotherapy for ovarian cancer. In addition to increased operating room availability due to postponement of non-urgent procedures, the availability of support services at our institution including social workers, case managers, clinical psychologists, and counselors, as well as financial and transportation support were instrumental in sustaining efficient delivery of cancer care. These factors likely helped mitigate some of the barriers to care that many patients endured.
We acknowledge that our single institution experience may limit generalizability of results and, in fact, contrasts with other published institutional data. Though disproportionately burdened by COVID-19 in the early months of the pandemic, one-third of patients with gynecologic cancer at three New York City hospitals experienced treatment delays, modifications, or cancellations (Frey et al., 2020). Another study found that during the first wave of the COVID-19 pandemic, gynecologic oncology patients treated at a publicly funded Canadian hospital were nine times more likely to have a surgical treatment modification and two times more likely to have a surgical delay compared to an equal volume but privately funded hospital in the United States (Piedimonte et al., 2021). These studies, in addition to our own data, suggest that major factors that influenced delivery of gynecologic cancer care during the early months of the pandemic included: COVID-19 burden within the community, prioritization of operating room resources, inpatient bed allocation, and institutional policies related to the pandemic.
Other limitations of our study include its retrospective nature which is associated with inherent biases. Additionally, sub-group analyses were limited by small numbers of patients in multiple groups which made the detection of statistically meaningful differences more challenging. We also were not able to give a definitive explanation for the observed decrease in referrals across the study period. Possible explanations including patient deference of evaluation of symptoms and screening versus delayed referral by referring providers for diagnosed oncologic problems. Similarly, though we observed that time to treatment initiation decreased during the pandemic, we are unable to determine correlation with fewer number of cancer referrals during this time.
The major strength of our study is the high-volume nature of our institution which allowed for the inclusion of 884 patients in the analysis. Due to the large cohort size, we were able to make clinically significant observations about gynecologic cancer care during the COVID-19 pandemic.
In summary, our study found that though referrals to gynecologic oncology decreased over the study period, time from referral to initial evaluation by a gynecologic oncologist remained unchanged. Furthermore, time from initial evaluation to cancer treatment initiation decreased significantly during the early months of the pandemic. Additional studies are necessary to examine the consequences of delayed screening and evaluation on cancer diagnosis and treatment trends in gynecologic oncology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.100928.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
References
- Bogani G., et al. Gynecologic oncology at the time of COVID-19 outbreak. J. Gynecol. Oncol. 2020;31(4):e72. doi: 10.3802/jgo.2020.31.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogani G., et al. Impact of COVID-19 in gynecologic oncology: a Nationwide Italian Survey of the SIGO and MITO groups. J. Gynecol. Oncol. 2020;31(6):e92. doi: 10.3802/jgo.2020.31.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C.J., Given C.W., Roberts C. Health care disparities and cervical cancer. Am J Public Health. 2004;94(12):2098–2103. doi: 10.2105/ajph.94.12.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugel M., et al. Dramatic Changes in Oncology Care Pathways During the COVID-19 Pandemic: The French ONCOCARE-COV Study. Oncologist. 2021;26(2):e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T.R., et al. A fellow-run clinic achieves similar patient outcomes as faculty clinics: A safe and feasible model for gynecologic oncology fellow education. Gynecol. Oncol. 2020;159(1):209–213. doi: 10.1016/j.ygyno.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania C., et al. Fears and Perception of the Impact of COVID-19 on Patients With Lung Cancer: A Mono-Institutional Survey. Front. Oncol. 2020;10:584612. doi: 10.3389/fonc.2020.584612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. 2020 May 13, 2021 September 1, 2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- Chatterjee S., et al. Disparities in Gynecological Malignancies. Front. Oncol. 2016;6:36. doi: 10.3389/fonc.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID Data Tracker. 12/13/2021 [cited 2021 December 14] , Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- Dai M., et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader A.N., et al. When to Operate, Hesitate and Reintegrate: Society of Gynecologic Oncology Surgical Considerations during the COVID-19 Pandemic. Gynecol. Oncol. 2020;158(2):236–243. doi: 10.1016/j.ygyno.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E.S., et al. Chronic disease in health emergencies: in the eye of the hurricane. Prev. Chronic. Dis. 2006;3(2):A46. [PMC free article] [PubMed] [Google Scholar]
- Frey M.K., et al. Gynecologic oncology care during the COVID-19 pandemic at three affiliated New York City hospitals. Gynecol. Oncol. 2020;159(2):470–475. doi: 10.1016/j.ygyno.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman H.W., et al. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw. Open. 2020;3(8):e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A.G., et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man R.-G., Lack D.A., Wyatt C.E., Murray V. The effect of natural disasters on cancer care: a systematic review. Lancet Oncol. 2018;19(9):e482–e499. doi: 10.1016/S1470-2045(18)30412-1. [DOI] [PubMed] [Google Scholar]
- Maringe C., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli F., Garbi A. Change in practice in gynecologic oncology during the COVID-19 pandemic: a social media survey. Int. J. Gynecol. Cancer. 2020;30(8):1101–1107. doi: 10.1136/ijgc-2020-001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast C., Munoz A., del Rio Delayed cancer screenings - A second look. Epic Health Research Network. 2020 [Google Scholar]
- Miller M.J., et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedimonte S., et al. Gynecologic oncology treatment modifications or delays in response to the COVID-19 pandemic in a publicly funded versus privately funded North American tertiary cancer center. Gynecol. Oncol. 2021;162(1):12–17. doi: 10.1016/j.ygyno.2021.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuri B., et al. Anti-cancer therapy and clinical trial considerations for gynecologic oncology patients during the COVID-19 pandemic crisis. Gynecol. Oncol. 2020;158(1):16–24. doi: 10.1016/j.ygyno.2020.04.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A.L., et al. Cancer Care Disparities during the COVID-19 Pandemic: COVID-19 and Cancer Outcomes Study. Cancer Cell. 2020;38(6):769–770. doi: 10.1016/j.ccell.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, S., 2020. COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. 2020 March 17, 2020 [cited 2021 June 17]; Available from: https://www.facs.org/covid-19/clinical-guidance/triage.
- Sutcuoglu O., et al. Harmful consequences of COVID-19 fear in patients with cancer. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2020-002628. [DOI] [PubMed] [Google Scholar]
- Ward E., et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J. Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]