This pooled analysis assesses whether an increased risk of serious adverse events is associated with radiation therapy given within 90 days prior to an immune checkpoint inhibitor.
Key Points
Question
Is there an increased risk of serious adverse events (AEs) if radiation therapy (RT) is given within 90 days prior to an immune checkpoint inhibitor?
Findings
In this pooled analysis of patient-level data from prospective trials in the US Food and Drug Administration databases that included 16 835 patients, patients receiving RT had generally similar rates of AEs overall with no difference seen in high-grade AEs vs those who did not receive RT.
Meaning
In this pooled analysis, the administration of an immune checkpoint inhibitor within 90 days following RT did not appear to be associated with an increased risk of serious AEs.
Abstract
Importance
Immune checkpoint inhibitors (ICIs) and radiation therapy (RT) are widely used to treat various cancers, but little data are available to guide clinicians on ICI use sequentially with RT.
Objective
To assess whether there is an increased risk of serious adverse events (AEs) associated with RT given within 90 days prior to an ICI.
Design, Setting, and Participants
Individual patient data were pooled from 68 prospective trials of ICIs submitted in initial or supplemental licensing applications in the US Food and Drug Administration (FDA) databases through December 2019. Two cohorts were generated: (1) patients who received RT within the 90 days prior to beginning ICI therapy and (2) those who did not receive RT within the 90 days prior to beginning ICI therapy, and AE frequencies were determined. A 1:1 propensity score–matched analysis was performed.
Interventions
All patients received an ICI (atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, or pembrolizumab); 1733 received RT within the 90 days prior to starting ICI therapy, and 13 956 did not.
Main Outcomes and Measures
The primary outcome was frequency and severity of AEs. Incidence of AEs was compared descriptively between participants who did vs did not receive RT in the propensity score–matched set. Because all analyses are exploratory (ie, not preplanned and no alpha allocated), assessment for statistical significance of the differences between groups was not considered appropriate.
Results
A total of 25 469 patients were identified; 8634 were excluded because they lacked comparators who had received RT (n = 976), did not receive an ICI (n = 4949), received RT outside of the target window (n = 2338), or had missing data in 1 or more variables used in the propensity analysis (n = 371), leaving 16 835 patients included in the analysis. The majority were younger than 65 years (9447 [56.1%]), male (10 459 [62.1%]), and White (13 422 [79.7%]). Patients receiving RT had generally similar rates of AEs overall to those patients who did not receive RT. The average absolute difference in rates across the AEs was 1.2%, and the difference ranged from 0% for neurologic AEs to 8% for fatigue. No difference in grade 3 to 4 AEs was observed between the 2 groups (absolute difference ranged from 0.01% to 2%). These findings persisted after propensity score matching.
Conclusions and Relevance
In this pooled analysis, administration of an ICI within 90 days following RT did not appear to be associated with an increased risk of serious AEs. Thus, it would appear to be safe to administer an ICI within 90 days of receiving RT. These findings should be confirmed in future prospective trials.
Introduction
Radiation therapy (RT) plays a vital role in both curative and palliative treatment of most types of cancer. It has been estimated that more than 50% of patients with cancer will receive RT.1 Whether it is safe to administer RT in proximity with novel systemic agents is not known, as most trials exclude patients who received RT within a prespecified time frame. Because it is generally a trial requirement to record additional nonprotocol cancer therapies received by study participants, the raw data necessary to assess the safety of delivering RT sequentially or concurrently with new anticancer agents may exist within clinical trial databases. These data in deidentified form are submitted to the US Food and Drug Administration (FDA) for analysis as part of the process of review of new drug applications (NDAs) and biologic licensing applications (BLAs). Thus, the opportunity to address the safety of RT in conjunction with newly approved antineoplastic agents may exist even if these concerns were not a specified trial end point.
Among the newer agents widely used in recent years are the immune checkpoint inhibitors (ICIs), which are approved by the FDA for the treatment of numerous advanced malignant neoplasms.2 The toxicity profiles of these agents are well characterized and include immune-mediated adverse events (AEs) affecting the lungs, liver, thyroid, gastrointestinal tract, and, less commonly, the heart and central nervous system, among other organs.3 Given emerging data suggesting systemic immune effects of radiation and redundancies in the mechanisms of ICI- and RT-induced toxic effects, there exists potential for enhanced toxicity when combining these agents.4,5
However, little data are available to guide clinicians on ICI use in conjunction with RT.6 To address this question, we performed a pooled analysis of patient-level data from prospective trials in the FDA databases.
Methods
Study Design and Population
All prospective trials of ICIs submitted to the FDA in initial or supplemental new drug applications or biologic licensing applications through December 2019 were included. Applications that were withdrawn or did not lead to approval were not included. A total of 68 trials were identified that met inclusion criteria (eTable 1 in the Supplement). The ICIs studied in these trials are atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, and pembrolizumab. The earliest trial opened in February 2003. These trials were integrated, with individual patient data pooled for demographic characteristics, concomitant medications, procedures, and AE domains. Data on age, sex, race and ethnicity, country, performance scores (Eastern Cooperative Oncology Group, Karnofsky, World Health Organization), prior lines of therapy, and cancer type were included for each patient when recorded, as these factors might influence whether a patient received RT. The study was exempted from institutional review board approval as necessary for protection of public health under the Common Rule, at 45 CFR 46.102(l)(2).
The pooled trials were used to determine whether an individual patient was recorded as having received RT within 90 days (referred to as RT≤90) or more than 90 days prior to the start of ICI therapy (referred to as RT>90). The former time frame (≤90 days) classically represents the interval for manifestation of acute radiation-related injuries and may be a period of increased risk for AEs from additional systemic therapies even though the trials generally required AEs from prior anticancer therapies be no worse than grade 1 at the time of enrollment. The latter time frame (>90 days) corresponds to the period during which late radiation-related injuries would manifest but acute radiation-related injuries would have resolved. These patients might be less likely to demonstrate an increased risk of developing AEs after administration of ICIs. The reason for receipt of RT was reviewed, and patients for whom it was determined that RT was not delivered for the purpose of treating cancer were excluded. If no reason was given, it was assumed that RT was delivered for cancer treatment, and these patients were included. Because details regarding radiation were generally limited, it was not possible to determine radiation dose/fractionation schedules, treatment field location or sizes, or dose/volume relationships for normal tissues or tumors. Adverse events were identified using Medical Dictionary for Regulatory Activity terminology. System organ class and all-level terms were used to search the pooled data. Preferred terms with overlapping toxic effect connotations were combined into a grouped preferred term (eTable 2 in the Supplement). An individual AE was counted only once per patient, and the highest grade recorded was used in the analysis. For both patient cohorts, the period for toxicity evaluation began with the beginning of ICI treatment. To avoid immortal time bias, a time-from-treatment-to-AE analysis was not performed.
Statistical Analysis
Propensity score–matched analysis7 was performed using SAS, version 9.4 (SAS Institute) to assess the outcome of potential selection bias owing to an imbalance in some factors that could influence the decision to deliver RT. These factors include performance status, number of prior lines of therapy, cancer type, type of ICI-based therapy, age, sex, race and ethnicity, and country. The propensity score was calculated by using these variables in a multivariable logistic regression model. Using the propensity scores, patients who had received RT were matched 1:1 to patients who had not receive RT. Nearest-neighbor matching was performed with a caliper width of 0.5 units of the pooled estimate of the common SD of the logits of the propensity scores. This caliper specifies that for a match to be made, the difference in the logits of the propensity scores for pairs of individuals from the 2 groups must be less than or equal to 0.5 times the pooled estimate of the common SD of the logits of the propensity scores.
Incidence of AEs was compared descriptively between participants who had not received RT vs the RT≤90 and the RT>90 cohorts in the propensity score–matched set. Because all analyses are exploratory, assessment for statistical significance of the differences between groups was not considered appropriate. See eMethods in the Supplement for additional analysis details.
Results
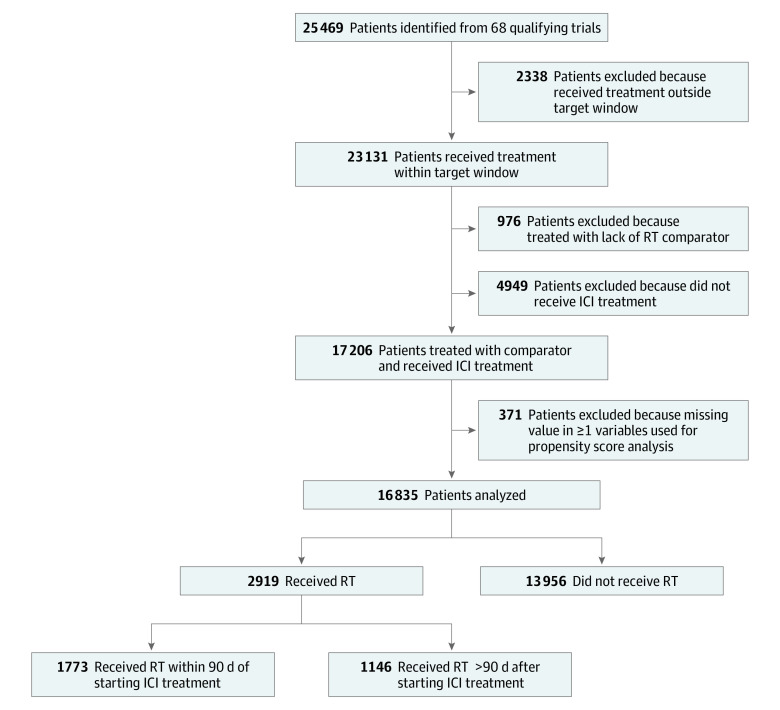
A total of 25 469 patients were identified (Figure). Excluded from the analysis were 8634 patients (34%) owing to lack of a comparator who had received RT (n = 976), enrollment in a trial arm that did not include an ICI (n = 4949), receiving RT outside the target window (n = 2338), or missing data in 1 or more variables used in the analysis (n = 371). This left 16 835 patients for analysis. Of these 16 835 patients, 13 956 (83%) did not receive RT, 1773 (11%) were RT≤90, and 1146 (6%) were RT>90. The demographic characteristics of this patient population are summarized in Table 1. The majority were younger than 65 years (9447 [56.1%]), male (10 459 [62.1%]), and White (13 422 [79.7%]). Approximately 40% were from the US. Nearly all had Eastern Cooperative Oncology Group performance status of 0 or 1. The most common primary tumors were lung, melanoma, kidney, head and neck, and bladder. Prior to propensity matching, there were imbalances in the number of lines of therapy, age, and tumor type. After matching, the populations were better balanced.
Figure. Study and Patient Enrollment Diagram.
ICI indicates immune checkpoint inhibitor; RT, radiation therapy.
Table 1. Demographic Characteristics Before and After Propensity Score Matching.
| Characteristic | Patients, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Before matchinga | After matchinga | ||||||
| No RT (n = 13 956) | RT≤90 d (n = 1733) | RT>90 d (n = 1146) | No RT (n = 1662) | RT≤90 d (n = 1662) | No RT (n = 1145) | RT>90 d (n = 1145) | |
| Age, y | |||||||
| <65 | 7665 (54.9) | 1079 (62.3) | 703 (61.3) | 1060 (63.8) | 1036 (62.2) | 730 (63.8) | 702 (61.3) |
| ≥65 | 6291 (45.1) | 654 (37.7) | 443 (38.7) | 602 (36.2) | 626 (37.8) | 415 (36.2) | 443 (38.7) |
| Sex | |||||||
| Female | 5394 (38.7) | 555 (32.0) | 427 (37.3) | 554 (33.3) | 554 (33.3) | 427 (37.3) | 427 (37.3) |
| Male | 8562 (61.3) | 1178 (68.0) | 719 (62.7) | 1108 (66.7) | 1108 (66.7) | 718 (62.7) | 718 (62.7) |
| Race | |||||||
| Asian | 1667 (11.9) | 169 (9.8) | 166 (14.5) | 143 (8.9) | 155 (9.3) | 157 (13.7) | 165 (14.4) |
| White | 11 046 (79.1) | 1475 (85.1) | 901 (78.6) | 1449 (87.2) | 1420 (85.6) | 934 (81.6) | 901 (78.7) |
| Otherb | 1243 (8.9) | 89 (5.1) | 79 (6.9) | 70 (4.7) | 87 (5.2) | 54 (4.7) | 79 (6.9) |
| Country | |||||||
| US | 6199 (44.4) | 666 (38.4) | 441 (38.5) | 665 (40.0) | 665 (40.0) | 441 (38.5) | 441 (38.5) |
| Non-US | 7757 (55.6) | 1067 (61.6) | 705 (61.5) | 997 (60.0) | 997 (60.0) | 704 (61.5) | 704 (61.5) |
| ECOG PS | |||||||
| 0-1 | 13 594 (97.4) | 1721 (99.3) | 1126 (98.2) | 1642 (98.8) | 1650 (99.3) | 1127 (98.4) | 1125 (98.3) |
| 2-4 | 232 (1.7) | 11 (0.6) | 17 (1.5) | 19 (1.1) | 11 (0.7) | 15 (1.3) | 17 (1.5) |
| Unknown | 130 (0.9) | 1 (0.1) | 3 (0.3) | 1 (0.1) | 1 (0.1) | 3 (0.3) | 3 (0.3) |
| Prior lines of therapy | |||||||
| 0-1 | 7196 (51.6) | 408 (23.5) | 731 (35.2) | 1236 (23.3) | 1236 (24.4) | 729 (35.5) | 730 (35.3) |
| ≥2 | 5595 (40.1) | 1295 (74.7) | 404 (63.8) | 392 (74.4) | 406 (73.8) | 406 (63.7) | 404 (63.8) |
| Unknown | 1165 (8.3) | 30 (1.7) | 11 (1.0) | 34 (2.0) | 30 (1.8) | 10 (0.9) | 11 (1.0) |
| Cancer type | |||||||
| Bladder | 2580 (18.5) | 69 (4.0) | 152 (13.3) | 69 (4.2) | 69 (4.2) | 152 (13.3) | 152 (13.3) |
| Head and neck | 213 (1.5) | 193 (11.1) | 44 (3.8) | 187 (11.3) | 187 (11.3) | 44 (3.8) | 44 (3.8) |
| Lung | 4236 (30.4) | 618 (35.7) | 617 (53.8) | 553 (33.3) | 553 (33.3) | 616 (53.8) | 616 (53.8) |
| Melanoma | 2904 (20.8) | 417 (24.1) | 159 (13.9) | 417 (25.1) | 417 (25.1) | 159 (13.9) | 159 (13.9) |
| Kidney | 1067 (7.6) | 117 (6.8) | 19 (1.7) | 117 (7.0) | 117 (7.0) | 19 (1.7) | 19 (1.7) |
| Otherc | 2956 (21.2) | 319 (18.4) | 155 (13.5) | 319 (19.2) | 319 (19.2) | 155 (13.5) | 155 (13.5) |
| Treatment type | |||||||
| Anti–CTLA-4 | 559 (4.0) | 39 (2.3) | 16 (1.4) | 46 (2.8) | 39 (2.3) | 17 (1.5) | 16 (1.4) |
| Anti–PD-1 | 7567 (54.2) | 1110 (64.1) | 676 (59.0) | 1151 (69.3) | 1087 (65.4) | 790 (69.1) | 676 (59.1) |
| Anti–PD-1 + TKI | 688 (4.9) | 9 (0.5) | 7 (0.6) | 12 (0.7) | 9 (0.5) | 9 (0.8) | 7 (0.6) |
| Anti–PD-1 + anti-CTLA-4 | 911 (6.5) | 197 (11.4) | 15 (1.3) | 189 (11.4) | 197 (11.9) | 13 (1.1) | 15 (1.3) |
| Anti–PD-1 + chemotherapy | 454 (3.3) | 4 (0.2) | 38 (3.3) | 4 (0.2) | 4 (0.2) | 34 (3.1) | 38 (3.3) |
| Anti–PD-L1 | 3777 (27.1) | 374 (21.6) | 394 (34.4) | 260 (15.6) | 326 (19.6) | 282 (24.6) | 393 (34.3) |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte associated protein 4; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; PD-L1, programmed cell death 1 ligand 1; PD-1, programmed cell death 1; RT, radiation therapy; TKI, tyrosine kinase inhibitor.
Time intervals refer to the time from the completion of RT to initiation of ICI treatment.
Other category included patients whose race was coded as Black and patients with unknown race.
Breakdown of other cancer types prior to matching (no RT, RT): blood (0.5%, 7.1%), cervical (0%, 0.5%), colorectal (0.6%, 1.5%), endometrial (1.6%, 0%), gastric (3.3%, 0.9%), liver (1.6%, 2.3%), lymphoma (1.1%, 2.3%), Merkel cell (0.5%, 0%), ovarian (0.2%, 1.2%), and solid tumors not otherwise specified (10.2%, 2.1%).
The distribution of AEs is shown in Table 2. The results of the propensity score analysis for this patient population also are shown in Table 2. The most common AEs of all grades were fatigue, diarrhea, endocrinopathies, hematologic AEs, and pneumonitis.
Table 2. Adverse Events (AEs) and Results of Propensity Match Analysis.
| AE | Patients, No (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1-5 AEs | Grade 3-4 AEs | 1:1 Propensity score matching, all gradesa,b | ||||||||
| No RT | RT≤90 d | RT>90 d | No RT | RT≤90 d | RT>90 d | No RT | RT≤90 dc | No RT | RT>90 dd | |
| No. | 13 956 | 1733 | 1146 | 13 956 | 1733 | 1146 | 1662 | 1662 | 1145 | 1145 |
| Neutropenia | 329 (2.4) | 42 (2.4) | 23 (2.0) | 181 (1.3) | 20 (1.2) | 14 (1.2) | 41 (2.5) | 41 (2.5) | 25 (2.2) | 23 (2.0) |
| Thrombocytopenia | 361 (2.6) | 57 (3.3) | 25 (2.2) | 101 (0.7) | 16 (0.9) | 7 (0.6) | 35 (2.1) | 57 (3.4) | 23 (2.0) | 25 (2.2) |
| Pneumonitis | 535 (3.8) | 118 (6.8) | 41 (3.6) | 158 (1.1) | 33 (1.9) | 14 (1.2) | 77 (4.6) | 113 (6.8) | 45 (3.9) | 41 (3.6) |
| Colitis | 469 (3.4) | 49 (2.8) | 29 (2.5) | 273 (2) | 29 (1.7) | 19 (1.7) | 51 (3.1) | 49 (2.9) | 22 (1.9) | 29 (2.5) |
| Hepatitis | 145 (1.0) | 17 (1.0) | 7 (0.6) | 110 (0.8) | 12 (0.7) | 5 (0.4) | 18 (1.1) | 17 (1.0) | 7 (0.6) | 7 (0.6) |
| Myocarditis | 11 (<0.1) | 1 (0.1) | 0 | 7 (<0.1) | 0 | 0 | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Neurologic AEs | 32 (0.2) | 4 (0.2) | 2 (0.2) | 20 (0.1) | 3 (0.2) | 2 (0.2) | 4 (0.2) | 4 (0.2) | 0 | 2 (0.2) |
| Skin AEs | 28 (0.2) | 2 (0.1) | 2 (0.2) | 7 (0.1) | 1 (0.1) | 0 | 5 (0.3) | 2 (0.1) | 4 (0.3) | 2 (0.2) |
| Kidney AEs | 38 (0.3) | 7 (0.4) | 1 (0.1) | 25 (0.2) | 3 (0.2) | 0 | 3 (0.2) | 7 (0.4) | 0 | 1 (0.1) |
| Endocrine AEs | 1873 (13.4) | 256 (14.8) | 114 (9.9) | 140 (1) | 17 (1) | 5 (0.4) | 246 (14.8) | 249 (15) | 134 (11.7) | 114 (10.0) |
| Fatigue | 6351 (45.5) | 921 (53.1) | 475 (41.4) | 668 (4.8) | 84 (4.8) | 32 (2.8) | 850 (51.1) | 890 (53.5) | 490 (42.8) | 475 (41.5) |
| Diarrhea | 3775 (27.0) | 470 (27.1) | 224 (19.5) | 607 (4.3) | 68 (3.9) | 35 (3.1) | 470 (28.3) | 456 (27.4) | 230 (20.1) | 224 (19.6) |
| Musculoskeletal AEs | 202 (1.4) | 25 (1.4) | 10 (0.9) | 25 (0.2) | 2 (0.1) | 2 (0.2) | 25 (1.5) | 25 (1.5) | 10 (0.9) | 10 (0.9) |
Abbreviation: RT, radiation therapy.
Model variables: age, sex, race, country, Eastern Cooperative Oncology Group performance status, prior lines of treatment, cancer type, and treatment category.
Adverse events based on all patients, patients with complete data, and 1:1 propensity score matching.
A total of 71 patients in the RT cohort did not have matched patients in the no-RT cohort (control).
One RT patient did not have a matched no-RT patient (control).
Considering the RT and no-RT groups as a whole prior to propensity matching, most AEs were grade 1 to 2, and the AE profiles were similar between the 2 groups. The RT≤90 patients had slightly numerically higher rates of fatigue, endocrinopathies, and pneumonitis vs the no-RT group. These differences were primarily due to low-grade (grade 1-2) AEs. Ratios of incidence frequencies for less common AEs were comparable between the 2 groups. Ratios of incidence of less common AEs were comparable between the 2 groups. In general, AEs were numerically slightly more common in RT≤90 patients vs the RT>90 cohort. These differences were predominantly due to low-grade AEs.
The propensity score analysis is presented in Table 2. After propensity score matching, the findings were slightly different than those noted previously for the overall analysis population. There was a numerically slightly higher incidence of pneumonitis, thrombocytopenia, and fatigue, but not endocrinopathies, in the RT≤90 patients vs the no-RT group. There was also a slightly higher incidence of kidney AEs, but a slightly lower incidence of dermatologic AEs, in the RT group.
To account for potential differences in follow-up or censoring, we analyzed AE rates for the matched RT≤90 patients vs the corresponding matched no-RT patients, adjusted for length of time that each patient was on study. As with the original analysis of AE frequency unadjusted for follow-up/censoring, RT≤90 patients had a slightly numerically higher rate of pneumonitis, thrombocytopenia, endocrine AEs, and fatigue (eTable 3 in the Supplement).
Table 3 compares frequency of treatment discontinuation due to AEs in patients who had or had not received RT. The RT≤90 patients were slightly more likely to discontinue treatment because of pneumonitis vs no-RT patients. This difference was not seen when comparing RT>90 patients vs the no-RT group. In general, fewer RT>90 patients had AEs leading to treatment discontinuation than either the no-RT or the RT≤90 group.
Table 3. Summary of Treatment Discontinuation Because of Adverse Events (AEs).
| AE | Patients, No. (%) | ||
|---|---|---|---|
| No RT | RT≤90 d | RT>90 d | |
| No. | 13 956 | 1733 | 1146 |
| Neutropenia | 13 (0.1) | 1 (0.1) | 2 (0.2) |
| Thrombocytopenia | 9 (<0.1) | 0 | 0 |
| Pneumonitis | 192 (1.4) | 46 (2.7) | 13 (1.1) |
| Colitis | 184 (1.3) | 20 (1.2) | 7 (0.6) |
| Hepatitis | 68 (0.5) | 9 (0.5) | 3 (0.3) |
| Myocarditis | 8 (0.1) | 1 (0.1) | 0 |
| Neurologic AEs | 15 (0.1) | 3 (0.2) | 1 (0.1) |
| Skin AEs | 3 (<0.1) | 1 (0.1) | 0 |
| Kidney AEs | 15 (0.1) | 1 (0.1) | 0 |
| Endocrine AEs | 44 (0.3) | 6 (0.3) | 1 (0.1) |
| Fatigue | 64 (0.5) | 13 (0.8) | 0 |
| Diarrhea | 311 (2.2) | 37 (2.1) | 10 (0.9) |
| Musculoskeletal AEs | 24 (0.2) | 5 (0.3) | 1 (0.1) |
Abbreviation: RT, radiation therapy.
The frequency of AEs with or without RT in the 3 largest ICI treatment subgroups are compared in Table 4. In other treatment groups, there were too few patients who had received RT for a meaningful analysis. Several differences emerge. Colitis was more common in patients receiving anti–programmed cell death 1 (anti–PD-1) plus anti–cytotoxic T-lymphocyte associated protein 4 (anti–CTLA-4) without prior RT vs those receiving the same ICI combination following prior RT. Fatigue was more common in previously irradiated patients in all 3 ICI groups. Pneumonitis was more common only in previously irradiated patients who also received an anti–PD-1 ligand 1 (anti–PD-L1) agent. Endocrinopathies were more common in patients receiving an anti–PD-L1 agent after RT vs without prior RT but less common in patients who received anti–PD-1 plus anti–CTLA-4 without RT vs following prior RT.
Table 4. Adverse Events (AEs) by Immune Checkpoint Inhibitor Treatment and Radiation Therapy (RT).
| AE | Treatment group | Patients, No. (%) | ||
|---|---|---|---|---|
| Anti–PD-1a | Anti–PD-1 + anti–CTLA-4b | Anti–PD-L1c | ||
| Colitis | RT≤90 d | 27 (2.4) | 12 (6.1) | 4 (1.1) |
| RT>90 d | 17 (2.5) | NAd | 4 (1.0) | |
| No RT | 182 (2.4) | 100 (11.0) | 52 (1.4) | |
| Diarrhea | RT≤90 d | 269 (24.2) | 93 (47.2) | 86 (23.0) |
| RT>90 d | 145 (21.4) | NAd | 48 (12.2) | |
| No RT | 1915 (25.3) | 453 (49.7) | 515 (13.6) | |
| Endocrine AEs | RT≤90 d | 139 (12.5) | 51 (25.9) | 61 (16.3) |
| RT>90 d | 73 (10.8) | NAd | 32 (8.1) | |
| No RT | 957 (12.6) | 273 (30) | 207 (5.5) | |
| Fatigue | RT≤90 d | 592 (53.3) | 147 (74.6) | 143 (38.2) |
| RT>90 d | 298 (44.1) | NAd | 133 (33.8) | |
| No RT | 3576 (47.3) | 583 (64.0) | 1141 (30.2) | |
| Hepatitis | RT≤90 d | 7 (0.6) | 7 (3.6) | 2 (0.5) |
| RT>90 d | 6 (0.9) | NAd | 1 (0.3) | |
| No RT | 62 (0.8) | 37 (4.1) | 10 (0.3) | |
| Musculoskeletal AEs | RT≤90 d | 13 (1.2) | 4 (2.0) | 7 (1.9) |
| RT>90 d | 8 (1.2) | NAd | 2 (0.5) | |
| No RT | 121 (1.6) | 25 (2.7) | 24 (0.6) | |
| Myocarditis | RT≤90 d | 0 | 1 (0.5) | 0 |
| RT>90 d | 0 | NAd | 0 | |
| No RT | 6 (0.1) | 2 (0.2) | 1 (<0.1) | |
| Neurologic | RT≤90 d | 3 (0.3) | 1 (0.5) | 0 |
| RT>90 d | 2 (0.3) | NAd | 0 | |
| No RT | 14 (0.2) | 2 (0.2) | 5 (0.1) | |
| Neutropenia | RT≤90 d | 21 (1.9) | 5 (2.5) | 10 (2.7) |
| RT>90 d | 5 (0.7) | NAd | 9 (2.3) | |
| No RT | 121 (1.6) | 15 (1.6) | 29 (0.8) | |
| Pneumonitis | RT≤90 d | 47 (4.2) | 19 (9.6) | 51 (13.6) |
| RT>90 d | 22 (3.3) | NAd | 18 (4.6) | |
| No RT | 326 (4.3) | 66 (7.2) | 73 (1.9) | |
| Kidney | RT≤90 d | 3 (0.3) | 2 (1.0) | 1 (0.3) |
| RT>90 d | 0 | NAd | 0 | |
| No RT | 20 (0.3) | 4 (0.4) | 3 (0.1) | |
| Skin AEs | RT≤90 d | 1 (0.1) | 0 | 1 (0.3) |
| RT>90 d | 1 (0.1) | NAd | 1 (0.3) | |
| No RT | 15 (0.2) | 1 (0.1) | 8 (0.2) | |
| Thrombocytopenia | RT≤90 d | 46 (4.1) | 4 (2.0) | 5 (1.3) |
| RT>90 d | 15 (2.2) | NAd | 3 (0.8) | |
| No RT | 163 (2.2) | 29 (3.2) | 47 (1.2) | |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte associated protein 4; NA, not applicable; PD-L1, programmed cell death 1 ligand 1; PD-1, programmed cell death 1.
RT≤90 days: n = 1110; RT>90 days: n = 676; no RT: n = 7567.
RT≤90 days: n = 197; RT>90 days: n = 15; no RT: n = 911.
RT≤90 days: n = 374; RT>90 days: n = 394; no RT: n = 3777.
The number of patients in this group was too small for meaningful comparison (n = 15).
Discussion
To our knowledge, this is the largest reported analysis of AE risk associated with the use of RT prior to ICIs. Patients receiving RT prior to an ICI generally had similar rates of AEs overall compared with those who did not receive prior RT. There were no meaningful differences in the incidence of higher-grade AEs between the RT vs no-RT groups. The RT≤90 patients had slightly numerically higher rates of fatigue, endocrinopathies, and pneumonitis. These differences were mostly due to low-grade (grade 1-2) AEs. In general, RT≤90 patients had numerically slightly higher rates of AEs than RT>90 patients. This difference also was attributed to low-grade AEs.
The risk of specific AEs associated with prior use of RT may vary depending on the type of ICI used. Without knowing the details of RT treatments, such as the body region treated, the reasons for these apparent differences in toxicity risks cannot be explained definitively. However, the fact that AEs occurred with a slightly higher numeric frequency when ICIs were administered within 90 days vs more than 90 days of RT suggests a possible interaction between the treatments. Focal RT has been shown to have both local and systemic inflammatory and immunologic effects that may persist for prolonged periods of time and affect the risk of AEs associated with ICIs.4,6,8,9,10,11,12,13,14 Nevertheless, this finding will require prospective confirmation.
Immune checkpoint inhibitors produce durable responses in a substantial minority of patients with advanced or metastatic cancers. Despite this success, most patients with metastatic cancers will still die of their disease. Thus, there remains an important unmet need to develop additional approaches to treat these patients. As a result of the demonstrated efficacy of ICI therapies, however, interest in combining them with other agents that might favorably modify the immunologic environment, including RT, has blossomed.6,14,15
A recent review of studies describing the safety of concurrent or sequential RT and ICIs found that the available data were generally from small, mostly retrospective trials with short follow-up.16 In total, these trials concluded that there was no good evidence that concurrent or sequential RT plus ICIs increased the risk of high-grade toxic effects in any organ. Other studies indicate that a small increase in the risk of certain toxic effects, such as brain, pulmonary, and hematologic AEs, in patients receiving RT in proximity to ICI treatment cannot be ruled out.17,18,19,20,21,22,23
When an RT-related toxic effect occurs in conjunction with ICI treatment, it may be more likely to occur within the organ receiving radiation, suggesting that the risk may be due to local rather than systemic effects of the combination therapy. Luke et al24 enrolled 79 patients in a study of stereotactic body RT delivered to various sites followed by pembrolizumab. Radiation therapy was given to stimulate an immune response. Pembrolizumab, 200 mg every 3 weeks, was initiated within 7 days of RT. Six patients experienced grade 3 treatment-related toxic effects. In all cases, these toxic effects occurred in the region that was irradiated.
Taken together, these data do not demonstrate a large increase in risk of AEs when RT and ICIs are used in combination. The results in the literature also support the findings of the present study. While we did find a very slight increase in the risk of pneumonitis, thrombocytopenia, kidney AEs, and fatigue in RT≤90 patients and a similarly small increased risk of pneumonitis in RT>90 patients, the absolute magnitude of the risk increase was very small, and most AEs were grade 1 to 2. Hematologic AEs, particularly lymphopenia, might adversely influence the immunologic response to ICIs.25,26 This would suggest the need to minimize radiation to bone marrow in patients who will receive ICIs. Future studies also should evaluate whether all low-grade AEs are truly AEs. A low-grade inflammatory response may indicate an enhanced antitumor immune effect, similar to a graft-vs-host reaction, which may portend a better prognosis.27 Though beyond the scope of the present analysis, this question deserves further study.
Limitations
Our study has several limitations. Because most trials included in this analysis were not designed to study the outcomes of combining RT with an ICI, important details regarding the radiation treatments received were lacking. In future trials, these details, such as treatment site, should be available to better address the risks of integrating RT with ICIs. These RT-related factors may be associated with the desired immune response and the risk of normal tissue injury. For example, a multifraction treatment with large fields targeting a locally advanced tumor with curative intent may create an immunosuppressive/profibrotic response. Meanwhile, a palliative regimen using a small treatment field targeting only tumor with 1 to 2 fractions would be more likely to alter the tumor microenvironment to facilitate neoantigen release to stimulate an immune response in a phenotypically cold tumor with less likelihood of normal tissue damage. Given limitations in the available data, future studies combining these therapies should pay careful attention to commonly accepted normal tissue dose-volume guidelines used in RT. This caution may be especially applicable when irradiating the organs known to be both sensitive to radiation and susceptible to immune-mediated AEs, such as the lung, liver, and bowel.
Despite the prospective nature of the studies in the FDA database, there were considerable amounts of missing data pertaining to important factors, such as numbers of prior lines of therapy, which may affect the risk of AEs from either therapy. Fortunately, the database included a large enough sample size to permit an exploratory analysis using propensity score matching in more than 5000 patients. This provides some reassurance that an important safety signal was not missed. However, this preliminary suggestion that combinations of RT and ICIs may be safe to use will require prospective confirmation, and such trials are under way.
In addition, the time frame during which AEs were collected was relatively short because most patients enrolled in these trials had late-stage metastatic disease. This precludes drawing conclusions about the long-term safety of sequential RT plus ICIs. Longer-term follow-up in future studies will be required to address this issue.
We did not address the question of variable duration of follow-up or censoring and its potential outcome on the frequency of AEs reported herein through a time-to-event analysis. The primary goal of the study was to analyze aggregated patient AE rates consistent with FDA safety review practices. In the process of reviewing these applications for approval, the FDA performs its own evaluation of the sponsor’s submitted safety (and efficacy) data, requesting additional information as needed to ensure that all available data are reviewed. Based on the rigor of this review process, it was judged unlikely that many AEs are unaccounted for owing to lack of follow-up or censoring, as nonnegligible amounts of missing data could be grounds for disapproval, and that did not occur for these trials. In addition, few patients experienced treatment discontinuation due to AEs, and the findings were similar when analyzed by person-years on study to account for follow-up. Therefore, we assessed that the database adequately captured the occurrence of AEs in both the RT and no-RT groups, and that differential follow-up/censoring did not pose a major risk for bias.
Finally, the analysis can be considered only exploratory because of its retrospective nature. However, the database draws from a worldwide population, and the findings might be more broadly applicable for future trial designs.
Conclusions
In this pooled analysis of data from 68 prospective trials, results showed no meaningful increase in serious AEs in patients receiving an ICI within 90 days following RT vs those who had not received prior RT. Patients who had received RT had slightly numerically higher rates of fatigue, endocrinopathies, and pneumonitis. These differences were due to low-grade (grade 1-2) AEs. These findings may help clinicians in the design of future trials testing concurrent or sequential combinations of ICIs and RT in the treatment of patients with advanced cancers.
eMethods. Statistical Analysis Details
eTable 1. List of Trials Included in Analysis
eTable 2. Grouping of Adverse Event Terms
eTable 3. Analysis of AE Rates for the Matched Patients Who Received RT Within 90 Days Before Initiating an ICI vs the Corresponding Matched Patients Who Did Not Receive RT Adjusted for the Length of Time Each Patient Was on Study
References
- 1.Jaffray DA, Gospodarowicz MK. Radiation therapy for cancer. In: Gelband H, Jha P, Sankaranarayanan R, et al. (eds): Disease Control Priorities: Cancer. The World Bank; 2015: 239-248. [PubMed] [Google Scholar]
- 2.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020;12(3):12. doi: 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504-515. doi: 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- 4.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373-377. doi: 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations—perspectives and challenges. Mol Oncol. 2020;14(7):1529-1537. doi: 10.1002/1878-0261.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anscher MS, Kong FM, Murase T, Jirtle RL. Short communication: normal tissue injury after cancer therapy is a local response exacerbated by an endocrine effect of TGF beta. Br J Radiol. 1995;68(807):331-333. doi: 10.1259/0007-1285-68-807-331 [DOI] [PubMed] [Google Scholar]
- 9.Hart JP, Broadwater G, Rabbani Z, et al. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2005;63(5):1448-1454. doi: 10.1016/j.ijrobp.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 10.Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001;50(4):899-908. doi: 10.1016/S0360-3016(01)01524-3 [DOI] [PubMed] [Google Scholar]
- 11.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728-734. [PubMed] [Google Scholar]
- 12.Lhuillier C, Vanpouille-Box C, Galluzzi L, Formenti SC, Demaria S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers. Semin Cancer Biol. 2018;52(Pt 2):125-134. doi: 10.1016/j.semcancer.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitroda SP, Chmura SJ, Weichselbaum RR. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019;20(8):e434-e442. doi: 10.1016/S1470-2045(19)30157-3 [DOI] [PubMed] [Google Scholar]
- 14.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498-e509. doi: 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 15.Kwon ED, Drake CG, Scher HI, et al. ; CA184-043 Investigators . Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700-712. doi: 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma V, Cushman TR, Tang C, Welsh JW. Toxicity of radiation and immunotherapy combinations. Adv Radiat Oncol. 2018;3(4):506-511. doi: 10.1016/j.adro.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4(8):1123-1124. doi: 10.1001/jamaoncol.2017.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15(8):477-494. doi: 10.1038/s41571-018-0046-7 [DOI] [PubMed] [Google Scholar]
- 19.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276-1282. doi: 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903. doi: 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6(6):848-855. doi: 10.1001/jamaoncol.2019.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase i results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23(6):1388-1396. doi: 10.1158/1078-0432.CCR-16-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 24.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611-1618. doi: 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084-1091. doi: 10.1016/j.ijrobp.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Patel RR, Verma V, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 2020;150:114-120. doi: 10.1016/j.radonc.2020.05.051 [DOI] [PubMed] [Google Scholar]
- 27.Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730-2737. doi: 10.1200/JCO.19.00318 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Statistical Analysis Details
eTable 1. List of Trials Included in Analysis
eTable 2. Grouping of Adverse Event Terms
eTable 3. Analysis of AE Rates for the Matched Patients Who Received RT Within 90 Days Before Initiating an ICI vs the Corresponding Matched Patients Who Did Not Receive RT Adjusted for the Length of Time Each Patient Was on Study