Abstract
Omicron is a new variant of SARS-CoV-2, which is currently infecting people around the world. Spike glycoprotein, an important molecule in pathogenesis of infection has been modeled and the interaction of its Receptor Binding Domain with human ACE-receptor has been analysed by simulation studies. Structural analysis of Omicron spike glycoprotein shows the 30 mutations to be distributed over all domains of the trimeric protein, wherein the mutant residues are seen to be participating in higher number of intra-molecular interactions including two salt bridges emanating from mutant residues thereby stabilizing their conformation, as compared to wild type. Complex of Receptor Binding Domain (RBD) with human ACE-2 receptor shows seven mutations at interacting interface comprising of two ionic interactions, eight hydrogen bonds and seven Van der Waals interactions. The number and quality of these interactions along with other binding biophysical parameters suggests more potency of RBD domain to the receptor as compared to the wild type counterpart. Results of this study explains the high transmissibility of Omicron variant of SARS-CoV-2 that is currently observed across the world.
Abbreviations: ACE-2, Angiotensin Converting Enzyme-2; RBD, Receptor Binding Domain
Keywords: Omicron, Mutations, Spike protein, Human ACE-2 receptor, Interactions, Binding, Transmissibility
1. Introduction
Covid-19 is an ongoing global pandemic caused by SARS-CoV-2 that started in early 2020. It has so far affected more than 26 million people and resulted in more than 5 million deaths [1]. Over the last two years there have been a number mutations resulting in evolution of the virus and thereby a number of different variants. The severe disease-causing variants among these have been commonly known as ‘variants of concern’ and the rest have been designated as ‘variants of interest’ [2]. The delta variant is the most contagious and virulent strain, resulting in a high number of hospitalization and morbidity [3]. On 24th of November a new variant of the SARS-CoV-2 virus (B.1.1.529) was identified in South Africa which has now been named as the Omicron variant [4]. As of 14th December 2021, there have been more than 3000 cases affected from the omicron variant in more than 50 countries across the world [5].
The Omicron variant of SARS-CoV-2 has 30 mutations on its spike protein. The stability of this spike protein and its binding to human ACE-2 receptor is known to influence the transmissibility of SARS-CoV-2 [6]. In this study, a detailed modeling analysis of the Spike protein from Omicron has been done to show the effect of the mutations on the fold and its binding to human ACE-2 receptor. Data presented in this report provides a rationale for the clinical scenario currently seen due to Omicron.
2. Materials and methods
2.1. Generation of Omicron spike protein sequence
Sequence of the SARS-CoV-2 spike glycoprotein (P0DTC2) was retrieved from the UniProt protein sequence database. The residue changes reported by WHO for Omicron variant was manually incorporated in this sequence to obtain a 1211 residues of mutated spike glycoprotein with thirty mutations, six deletions and three insertions.
2.2. Homology modeling
The generated sequence was submitted to SWISS-MODEL, and Geno3D servers to obtain homology models of the spike glycoprotein of the omicron variant based on crystal structure of SARS-CoV-2 spike glycoprotein (PDB ID 7KRQ) [7,8]. Validation of these models was done based on Levitt-Gerstein (LG) score obtained from ProQ server; Ramachandran plot, 3D score and ERRAT from PROCHECK server [[9], [10], [11]]. The best validated model of the spike glycoprotein of the omicron variant was refined by energy minimization using Schrodinger and taken up for further analysis [12].
2.3. Generation of Omicron spike protein: human ACE-2 protein complex
Crystal structure of SARS-CoV-2 spike Receptor-Binding Domain (RBD) bound with human ACE2 (PDB ID 6M0J) was taken and, fifteen mutations was incorporated into the RBD using allowed rotamer configuration on Coot [13]. Model complex was then energy minimized and solvated using TIP3P water model in an orthorhombic box extending to 10 Å from protein atoms in each direction. The Solvated complex was neutralized by 12 Na+ ions and energy minimized with convergence threshold of 1 kcal/mol/Å. MD simulation was performed by Desmond simulation program with the help of force field parameters of OPLS_2005 [[14], [15], [16]]. The energy minimized solvated complex was equilibrated at 300 K and 1 atmospheric pressure for 200 ps under NTP condition with the help of Nose-Hoover thermostat and Martyna-Tobias-Klein barostat under periodic boundary condition. It was followed by recording the production trajectory for 100 ns under similar NTP condition and other run parameters [17]. Simulation was performed with step size of 2 fs in the presence of LINCS harmonic constrains and motion was integrated by RESPA dynamics integrator [14]. Long range electrostatic interactions were calculated by Particle Mesh Ewald (PME) algorithm. The same protocol was used for native wild type variant for the sake of comparison.
3. Results and discussion
3.1. Modeling and structural analysis of mutated spike protein in Omicron
A total of six homology models of the mutated Omicron glycoprotein were generated of which the one with best validation statistics was chosen for analysis (Table S1). The overall structure of Omicron spike protein is shown in Fig. S1. The 30 mutations, 6 deletions and 3 insertion are distributed across the entire fold of the protein. Each monomeric conformation is stabilized by an array of intra-molecular interactions. Of particular interest are two ionic interactions (Oδ1 Asp142 … NH1 Arg246 = 2.6 Å and NZ Lys764 … Oδ1 Asp737 = 2.7 Å) arising from the mutations Gly142Asp and Asn764Lys. In addition, there are a total of 18 hydrogen bonded interactions. In comparison, wild type variant is stabilized only by 13 hydrogen bonded interactions, and no ionic interactions. This clearly establishes that spike protein of Omicron variant has a more stable conformation.
3.2. Analysis of RBD of spike protein-ACE-2 receptor complex
Molecular dynamics simulations were performed for RBD of both mutant and native with ACE-2 receptor to understand the dynamic behavior of the complexes. The plot of temperature, pressure, volume and energy with respect to time during simulation shows that simulation system was found to be stable during simulation (Not shown). The r. m. s deviation of the backbone C-α RBD of spike protein and ACE-2 receptor indicates that complex converged after 60 ns of simulation (Fig. 2 A). The r. m. s fluctuation plot shows that flexible loop region and terminal portions of the proteins displayed larger fluctuations while rest of the complex were within the thermal vibration range which is about 1.5 Å (Fig. 2B). These plots clearly indicate that Omicron complex is more stable as compared to wild type.
Spike glycoprotein's RBD interaction with ACE-2 receptor is the foremost and most integral event for infection of mammalian host cell by the virus [18]. 15 out of 30 mutations reported for Omicron spike protein are on this RBD, which is both sequentially and structurally conserved in this variant as with its SARS-CoV-2 predecessor. RBD core comprises of five anti-parallel sheets connected by long loops and short helices. Binding surface of RBD presents a concave surface that is complementary to binding surface provided by the ACE-2 receptor (Fig. 1A). The interface of RBD that presents interacting residues with the ACE receptor comprises mainly of long loops with a anti-parallel β-wing in the middle. RBD that is recognized by the extracellular peptidase domain of ACE-2, has 12 polar residues in comparison to 11 polar residues on the wild variant. The enhanced polarity of the binding surface is the molecular basis for corona virus recognition and infection [19].
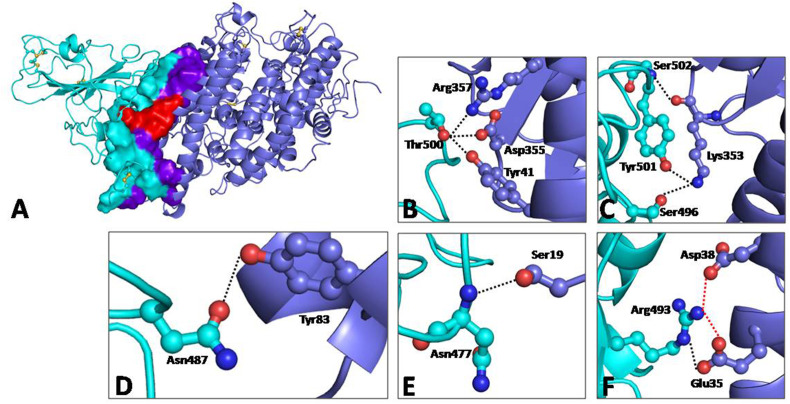
Fig. 1.
(A) Structure complex of RBD domain of spike protein from Omicron with human ACE-2 receptor. The secondary structural elements of RBD domain of spike protein and human ACE-Receptor are shown in cyan and purple, respectively; (B-F) Details of the interactions between residues of RBD domain of spike protein from Omicron (cyan) and human ACE-2 receptor (purple). Red dotted lines indicate ionic interactions, and black dotted lines indicate hydrogen bonded interactions.
A total of 15 residues of RBD make an array of interactions with 14 residues of ACE-2 receptor at the interfacial binding surface. Of these, two are ionic interactions and six are hydrogen bonded interactions. (Fig. 1B–F). Interestingly, six of these interactions are from the side chains of four mutated residues. The presence of two ionic interactions Arg493 NH2 … Asp38 Oδ1 = 2.8 Å and Arg493 NH2 … Glu35 Oε1 = 2.9 Å at the very center of interacting surfaces seems to be crucial to receptor binding and in turn the potency of Omicron spike protein (Fig. 1A). Among the hydrophobic interactions, Aspargine at position 501 in the wild type is mutated to tyrosine which makes two hydrophobic interactions (Tyr501 Cζ … Lys353 Cδ = 3.5 Å and Tyr501 Cε2 … Tyr41 Cε1 = 3.7 Å). In order to further ascertain the potency of RBD of wild and mutant variants, relevant biophysical binding parameters were compared between its respective complexes with human ACE-2 receptors (Table 1 ). It can therefore be concluded that the mutant spike protein has a better binding affinity to ACE-receptor than wild type. Structural stability of Omicron spike protein and its enhanced potency to human ACE-2 receptor could therefore be a crucial factor dictating infectivity of this virus. Molecular modeling in this study places the high transmissibility of Omicron in the right perspective [20,21].
Table 1.
Biophysical parameters governing the binding of spike protein RBD to human ACE-2 Receptor.
| Binding parameters | Wild Type | Omicron |
|---|---|---|
| Binding Energy (kcal/mol) | −116 | −130 |
| Buried Surface Area (Å2) | 829 Å2 | 862 Å2 |
| Number of Ionic Bonds | 2 | 2 |
| Number of Hydrogen Bonds | 13 | 8 |
| Number of Van der Waals interactions | 6 | 7 |
4. Conclusion
Modeling analysis of spike glycoprotein from Omicron shows the 30 mutations to be distributed over all the domains of the trimer protein. Mutant residues are seen to be participating in higher number of intra-molecular interactions thereby providing more stability to individual monomeric conformations as compared to its wild type counterpart. Also, the increased quantity and quality of interactions between the RBD of Omicron spike protein and human ACE-2 receptor hints at a higher potency. This study therefore provides a biophysical basis and the reason for the high transmissibility of Omicron virus.
Additional information
-
1
Coordinates for the Omicron spike protein trimer model is available at: https://www.aiims.edu/aiims/departments_17_5_16/biophysics/Omicron%20Spike%20protein%20trimer%20model.pdb
-
2
Coordinates for the Omicron spike protein (RBD)_Human ACE-2 receptor complex model at 100ns MD is available at: https://www.aiims.edu/aiims/departments_17_5_16/biophysics/Omicron%20Spike%20protein%20(RBD)_Human%20ACE-2%20receptor%20complex%20model%20at%20100ns%20MD.pdb
Declaration of competing interest
Authors report no conflict of interest in this work.
Acknowledgements
The work was funded by All India Institute of Medical Sciences, New Delhi, India (Grant # A-COVID-2) to Gururao Hariprasad.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2021.12.082.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1 Overall structure of the Omicron spike protein model with mutations. Mutated residues are shown as stick and ball representation in pink. Different domains and number of mutations on them are: N-terminal domain (yellow): 4; Receptor binding domain (cyan): 15; fusion peptide (red): 2; heptad repeat 1 (blue): 3; central helix (orange), connector domain (grey), heptad repeat 2 (light blue), transmembrane domain (wheat); cytoplasmic tail (brown), unassigned regions (black) are shown.
Supplementary Fig. 2 Biophysical parameters for complexes of spike protein RBD with human ACE-2. A. RMSD; B. RMSF. The plots for complex of RBD of spike protein from wild type variant (SARS-CoV-2) with human ACE-2 receptor is shown in red and plots for complex of RBD of spike protein from Omicron with human ACE-2 receptor is shown in cyan.
References
- 1.https://covid19.who.int/
- 2.Parums D.V. Revised World Health Organization (WHO) terminology for variants of concern and variants of interest of SARS-CoV-2. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res.: International Medical Journal of Experimental and Clinical Research. 2021;27:933622–933631. doi: 10.12659/MSM.933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiehzadegan S., Alaghemand N., Fox M., Venketaraman V. Analysis of the delta variant B. 1.617. 2 COVID-19. Clin. Pract. 2021;11:778–784. doi: 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.who.int/news/item/28-11-2021-update-on-omicron
- 5.https://www.gisaid.org/hcov19-variants/
- 6.Kumar V., Singh J., Hasnain S.E., Sundar D. Possible link between higher transmissibility of alpha, kappa and delta variants of SARS-CoV-2 and increased structural stability of its spike protein and hACE2 affinity. Int. J. Mol. Sci. 2021;22:9131. doi: 10.3390/ijms22179131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:296–303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combet C., Jambon M., Deleage G., Geourjon C. Geno3D: automatic comparative molecular modelling of protein. Bioinformatics. 2002;18:213–214. doi: 10.1093/bioinformatics/18.1.213. [DOI] [PubMed] [Google Scholar]
- 9.Levitt M., Gerstein M. A unified statistical framework for sequence comparison and structure comparison. Proc. Natl. Acad. Sci. Unit. States Am. 1998;95:5913–5920. doi: 10.1073/pnas.95.11.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colovos C., Yeates T.O. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskowski R.A., MacArthur M.W., Moss D.S., Thornton J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 12.Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013;27(3):221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 13.Casañal A., Lohkamp B., Emsley P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 2020;29:1055–1064. doi: 10.1002/pro.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowers K.J., Chow D.E., Xu H., Dror R.O., Eastwood M.P., Gregersen B.A., et al. InSC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing. IEEE; 2006. Scalable algorithms for molecular dynamics simulations on commodity clusters; p. 43. [Google Scholar]
- 15.Jorgensen W.L., Maxwell D.S., Tirado-Rives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996;11:11225–11236. doi: 10.1021/ja9621760. [DOI] [Google Scholar]
- 16.Banks J.L., Beard H.S., Cao Y., Cho A.E., Damm W., Farid R., et al. Integrated modeling program, applied chemical theory (IMPACT) J. Comput. Chem. 2005;26:1752–1780. doi: 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar M., Dahiya S., Sharma P., Sharma S., Singh T.P., Kapil A., et al. Structure based in silico analysis of quinolone resistance in clinical isolates of Salmonella Typhi from India. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koley T., Madaan S., Chowdhury S.R., Kumar M., Kaur P., Singh T.P., et al. Structural analysis of COVID-19 spike protein in recognizing the ACE2 receptor of different mammalian species and its susceptibility to viral infection. 3 Biotech. 2021;11:1–6. doi: 10.1007/s13205-020-02599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katella K. Yale Medicine News; 2021. 5 Things to Know about the Delta Variant. [Google Scholar]
- 21.Kollmeyer B. International news; 2021. Japan Study Confirms Omicron Variant's High Transmissibility.https://www.marketwatch.com/story/japan-study-confirms-omicron-variants-high-transmissibility-report-2021-12-09 Report. accessed on 14 December. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Overall structure of the Omicron spike protein model with mutations. Mutated residues are shown as stick and ball representation in pink. Different domains and number of mutations on them are: N-terminal domain (yellow): 4; Receptor binding domain (cyan): 15; fusion peptide (red): 2; heptad repeat 1 (blue): 3; central helix (orange), connector domain (grey), heptad repeat 2 (light blue), transmembrane domain (wheat); cytoplasmic tail (brown), unassigned regions (black) are shown.
Supplementary Fig. 2 Biophysical parameters for complexes of spike protein RBD with human ACE-2. A. RMSD; B. RMSF. The plots for complex of RBD of spike protein from wild type variant (SARS-CoV-2) with human ACE-2 receptor is shown in red and plots for complex of RBD of spike protein from Omicron with human ACE-2 receptor is shown in cyan.