Abstract
Tissue engineers often use biomaterials to provide structural support along with mechanical and chemical signals to modulate the wound healing process. Biomaterials that are implanted into the body interact with a heterogeneous and dynamic inflammatory environment that is present at the site of injury. Whether synthetically derived, naturally derived, or a combination of both, it is important to assess biomaterials for their ability to modulate inflammation to understand their potential clinical use. One important, but underexplored cell in the context of biomaterials is the mast cell (MC). MCs are granulocytic leukocytes that engage in a variety of events in both the innate and adaptive immune systems. Although highly recognized for their roles in allergic reactions, MCs play an important role in wound healing by recognizing antigens through pattern recognition receptors and the high-affinity immunoglobulin E receptor (FceRI) and releasing granules that affect cell recruitment, fibrosis, extracellular matrix deposition, angiogenesis, and vasculogenesis. MCs also mediate the foreign body response, contributing to the incorporation or rejection of implants. Studies of MC–biomaterial interactions can aid in the elucidation of MC roles during the host tissue response and tissue repair. This review is designed for those in the tissue engineering and biomaterial fields who are interested in exploring the role MCs may play in wound–biomaterial interactions and wound healing. With this review, we hope to inspire more research in the MC-biomaterial space to accelerate the design and construction of optimized implants.
Impact statement
Mast cells (MCs) are highly specialized inflammatory cells that have crucial, but not fully understood, roles in wound healing and tissue repair. Upon stimulation, they recognize foreign antigens and release granules that help orchestrate the inflammatory response after tissue damage or biomaterial implantation. This review summarizes the current use of MCs in biomaterial research along with literature from the past decade focusing on MC interactions with materials used for tissue repair and regeneration. Studying MC–biomaterial interactions will help (i) further understand the process of inflammation and (ii) design biomaterials and tissue-engineered constructs for optimal repair and regeneration.
Keywords: mast cells, biomaterials, inflammation, tissue repair, foreign body response, wound healing
Introduction
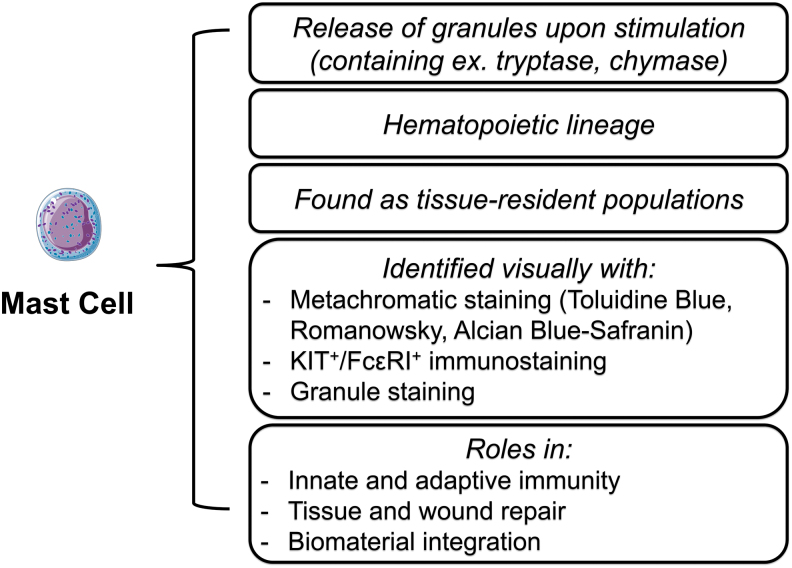
Mast cells (MCs) are immune cells that exist as tissue-resident populations when mature and perform important functions during normal wound healing and during the host tissue response toward biomaterials. A distinguishable characteristic of MCs is their diverse set of granular components that release when activated.1 These granules influence downstream inflammatory events that act against bacterial infection, toxin exposure, and other foreign substances such as biomaterials. The timing, amount, and type of granule release depend heavily on the extracellular environment and accompanying stimuli.2 While these mechanisms of activation and degranulation are still being investigated, MC activity has been shown to influence wound healing by orchestrating and participating in angiogenesis, scar formation, and reepithelialization.3–6 Characteristics of MCs, as well as their identifying criteria are summarized in Figure 1.
FIG. 1.
A list of MC characteristics as well as general identifying criteria. MC, mast cell. Color images are available online.
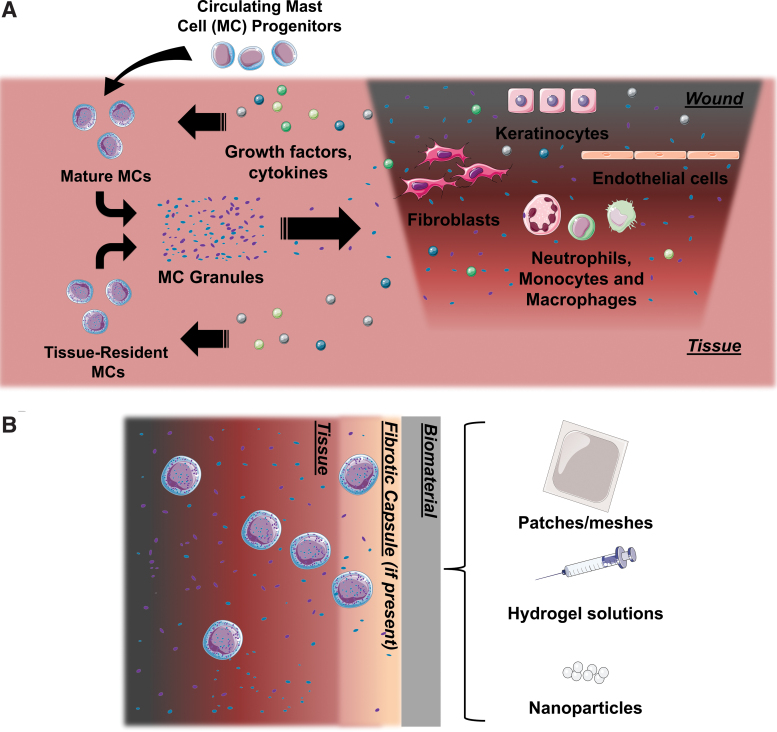
Biomaterials represent an important tool that can help in tissue repair and regeneration, delivering structural stability as well as chemical and physical cues to aid in tissue repair via cell–material interactions that can lead toward biomaterial integration or rejection.7–13 When implanted, biomaterials will interact with the dynamic cellular and chemical environment that is present during inflammation (Fig. 2A).14 As biomaterials may be considered foreign bodies by the immune system and can exhibit varying degrees of bioactivity, in vitro and in vivo testing is crucial to predict how they will perform and ultimately guide the appropriate tissue response. It is important to study MC responses to biomaterials to help elucidate the roles MCs take in tissue repair and other biological processes (Fig. 2B).
FIG. 2.
(A) A simplified summary of the players during the tissue repair process and their dynamic interactions. MC progenitors are recruited into the tissue and differentiate into mature, granulocytic MCs. Growth factors, cytokines, and other signaling molecules released by other players in the wound healing process stimulate MCs to release their granules. These granules help orchestrate the inflammatory process. (B) A schematic of a typical MC response to a biomaterial designed for implantation. Color images are available online.
In this review, we first explore MCs in the context of wound healing and tissue repair, including current methods of studying MCs as well as limitations and challenges. In the second half, we discuss recent research that studied MC responses toward both synthetically and naturally derived biomaterials, which are important in tissue engineering and regenerative medicine.
MCs in Wound Healing
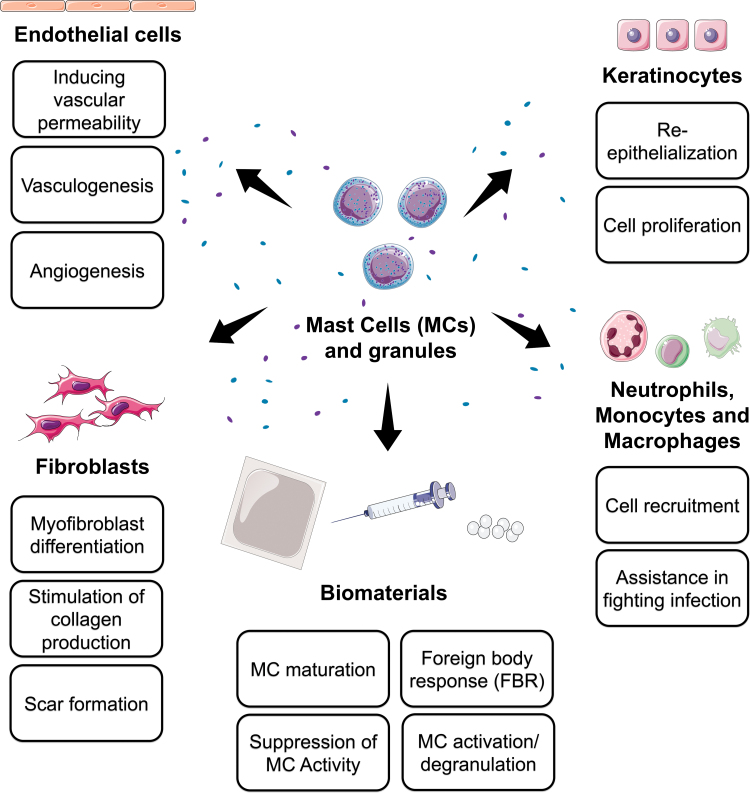
MCs have many roles in both the innate and adaptive immune systems. One important factor that differentiates MCs from other granulocytes is the expression of the high-affinity immunoglobulin E (IgE) receptor, FcɛRI. Activation of FcɛRI by a foreign antigen or allergen leads to the release of chemical and lipid mediators, cytokines, and chemokines through degranulation. This ability makes MCs a crucial cell type for many biological processes, including wound repair. In this section, we begin by summarizing some of the ways MCs contribute to the phases of wound healing (also summarized in Fig. 3), followed by discussions of some of the current challenges and techniques in studying MCs in this context.
FIG. 3.
A summary of the ways MCs affect cells of wound healing and their processes during wound healing, as well as their possible impact on biomaterials. Color images are available online.
The role of MCs in wound healing
MCs participate in all three of the classical stages of wound healing: inflammation, proliferation, and remodeling.5 MCs are directly activated by antigens and pathogens through FcɛRI, as well as other pattern recognition receptors such as toll-like, c-type lectin, NOD-like, and RIG-like receptors.15 MCs are also recruited to the site of injury and mature in tissues from mediators released by keratinocytes, platelets, and macrophages during the initial inflammatory response.16–19 In turn, MCs release signals to further recruit neutrophils, monocytes, and other immune cell types. Cellular access to the wound site is facilitated by molecules and enzymes, such as histamine and tryptase, to dilate vasculature and increase capillary permeability.20,21
MCs begin the proliferative stage by continuing to release mediators that influence fibrosis, angiogenesis, and neovascularization. MC-derived histamine, tryptase, and monocyte chemoattractant protein-1 activate fibroblasts, which in turn release stem cell factor (SCF) to continue regulating MCs through c-KIT (also known CD117). This is in addition to collagen deposition and extracellular matrix (ECM) secretion.22 MCs not only release products that lead to the development of fibrosis but also continue to recruit and activate monocytes.23 Growth factors secreted from endothelial cells associated with angiogenesis attract MCs, which release enzymes to clear damaged tissue and make room for new growth and vascularization.24 The role of MCs in wound healing then extends even further into the remodeling phase, as MCs continue to be present and accumulate in fibrotic capsules and during scar formation.25–28 However, there are conflicting reports on the exact mechanisms and influence of MCs, cultivating doubt of whether MCs play detrimental or ameliorative roles. This research is reviewed elsewhere.29
As such, more experiments are needed to clarify the mechanisms and roles of MCs in these processes. While this is a highly simplified summary of the role of MCs in the wound healing process, it conveys the necessity for an increase in MC studies in the context of inflammation and biomaterials research. However, there are limitations and challenges to studying MCs that makes elucidation difficult.
Limitations and challenges to studying MCs
Studying MCs can be particularly challenging for several reasons. The first of these challenges is during MC isolation. In vitro, MCs have extreme difficulties with maturation and do not possess the same phenotypic characteristics observed in vivo or clinically. Isolating primary MCs from their resident tissues can induce phenotypic changes and/or damage the cells.30–32 This could be due to the harshness of enzymatic and mechanical digestions required for cell isolation. In addition, extremely limited numbers of cells are isolated and are often not sufficient for MC studies. To overcome this limitation, cell lines have been established. However, these cell lines still differ from their in vivo expression patterns and phenotypes. Stem cells, such as CD34+ hematopoietic, embryonic stem cells, or induced pluripotent stem cells, offer a promising source of MCs,33–36 but how similar these derived cells are to in vivo or clinical phenotypes is yet to be fully established.
MCs differentiate from CD34+ hematopoietic stem cells in the bone marrow, circulate throughout the bloodstream as progenitors, but mature in tissues.37 As tissue-resident cells, they express a large amount of transcriptional and phenotypic heterogeneity across different tissues, as well as within the tissues themselves.32,38–40 This heterogeneity results in diverse degrees of maturation, expression of proteases and mediators, and proliferation rates. These differences also extend between species.41,42 As such, MC-associated diseases, such as MC tumors (MCTs), leukemias, MC activation syndromes, and mastocytosis, also exhibit a large amount of clinical diversity, making prognosis, identification, and treatment even more difficult.43–46
Because of these major limitations, MC-associated diseases and MCs themselves are understudied. In addition, the use of different cell lines, cell sources, and/or animal models present inconsistencies in MC research and poor conclusions may be drawn based on using an inappropriate cell type or model. However, despite the challenges, researchers are searching for novel ways to understand MC responses to implants and biomaterials. The second half of this review takes a look at the last 10 years of biomaterial research that incorporates MCs and measures their responses, but we will first look at commonly used techniques to study MCs.
Current approaches to MC study
As primary MCs are difficult to isolate for in vitro culture due to the reasons mentioned above, there are established MC lines available for some species. Examples of animal lines are the rat basophilic leukemia line RBL-2H3, derived from peripheral blood, murine P815 mastocytoma cells, and the canine HRMC line derived from a MCT.47,48 Human cell lines include HMC-1, derived from the blood of a patient with MC leukemia, and LAD2, derived from aspirates of bone marrow from a patient with MC leukemia.49,50 While they still can lack proper maturation and may not match in vivo profiles, these cell lines offer a relatively dependable source of MCs.
The in vitro study of MCs, regardless of MC origin, generally revolves around the cardinal functions of MCs in a specific physiological or pathophysiological setting. MC numbers are increased at the sites of allergic inflammation and increased MC recruitment and survival likely contribute to this feature.42 In addition, MCs release both preformed stored mediators and de novo synthesized mediators at the sites of inflammation. The activation of MC to release mediators can be achieved through both IgE-dependent and IgE-independent mechanisms, which is context dependent.
The ability for MCs to release granules is a hallmark of MC function, with mature MCs possessing more secretory granules compared to immature MCs.51 The contents of MC granules changes during maturation and differ according to MC location, but generally are high in proteases, particularly tryptase and chymase.52 MC maturation is often determined by granular composition and increases in histamine, tryptase, and chymase, which can be associated with higher responsiveness and increased intracellular [Ca2+] and β-hexosaminidase release.53–57 For further granule identification, MCs are detected by using toluidine blue histological staining.58 As this staining is metachromatic, this dye stains MCs in a range from red to deep purple, depending on the number of anionic sites present. This staining is notably strong in MC granules and analysis of stained MCs at high magnification can lead to the identification of MCs undergoing degranulation. The pH of the toluidine blue solution used can also help to differentiate between benign and malignant MC tissue.
Experimentally, MCs can be both positively and negatively affected by exogenously applied compounds. Cromolyn, also known as disodium cromoglycate, acts as an MC suppressor by inhibiting histamine or other inflammatory chemical releases.59 Although cromolyn inhibits MCs in some species, the mechanism and selectivity are not fully understood, and there are doubts whether it is effective in mice, both in vitro and in vivo.60 Compound 48/80 (C48/80) has the opposite effect, stimulating histamine release and promoting MC activity.61 Both of these compounds are commonly used in studying MC response to foreign bodies and can be used in vitro and in vivo. However, the receptors for C48/80 as well as other charged compounds have only been recently identified. As such, FcɛRI activation and signaling have been the most studied in MC degranulation, with C48/80 used as an IgE-independent control. Identification of the receptors for C48/80 has increased recent interest in the role of these receptors in MC biology. Thus, MC activation through either IgE-dependent or IgE-independent pathways remains at the forefront. Dinitrophenyl IgE and other IgE antibodies can be used experimentally to activate MC in an IgE-dependent manner through FcɛRI.62
Other factors of MC biology that are studied regulate processes not associated with degranulation, but are likely equally important in allergic inflammation. MC numbers are increased at the site of allergic inflammatory diseases,63 or at the site of a wound healing response.64 Therefore, mediators and receptors that regulate migration, survival, and proliferation are often studied. One example is SCF, a strong chemoattractant and adhesion factor that is critical for MC survival and proliferation, and its receptor KIT.
In vivo models of studying MCs include MC-deficient animals, especially mice with mutations with the receptor tyrosine kinase KIT (also known as CD117).65 Some examples of these mice are the KitW/W-v and KitW-sh/W-sh (“sash”) strains, which have different levels of MC producibility: KitW/W-v with some ability to produce MCs under inflammation and KitW-sh/W-sh with zero ability.66,67 Other strains have been bred with mutations to elucidate other MC-related functions.32,68 Due to these mutations, however, all of these strains can have secondary issues caused by their deficiencies and data garnered from experiments with these strains can be controversial. More recent gene-targeted mice strains, such as Cre mice, have become state-of-the-art tools for studying genes and pathways with MCs. As such, there is a plethora of data associated with older MC-deficient strains which may need to be corrected or updated with the newer gene-targeted mice.
MC–Biomaterial Interactions
Biomaterials encompass a variety of materials, including synthetically made, naturally derived, and/or a combination of both. Any implanted biomaterial will interact with the heterogeneous inflammatory environment present at the site of injury and these interactions can differ depending on cell type.69 Many biomaterials are tested for their ability to modulate immune cells such as neutrophils, macrophages, and T cells.70 However, studies of MC–biomaterial interactions are lacking. As MCs have crucial roles in the innate immune system and the foreign body response (FBR), it is important to measure how biomaterials may modulate these cells as well.
MCs, in addition to many other cells of the immune system, are significantly affected by biomaterial architecture, surface topography, and chemical composition. In vivo, MCs accumulate in fibrotic capsules with evidence that they contribute to the capsule formation themselves.29,71 As it may be difficult for MCs to attach directly to the biomaterials, some researchers have bypassed this limitation by coating the biomaterial, culturing with factors and cytokines to enhance MC activation, or adding binding peptides to the biomaterial to encourage attachment.56,72–74 In the following section, we have organized a discussion of the recent in vitro, in vivo, and ex vivo studies that have focused on the MC response to biomaterials by their different sources.
Synthetic biomaterials
Synthetic biomaterials allow for the control of material and mechanical properties during the manufacturing process. In this review, a synthetic biomaterial is defined as material from manmade sources that are designed to interact with the body. Some examples of these are manmade polymers and plastics in the forms of meshes, nanoparticles and fibers. Degradation rates are also tunable properties that could be of value when selecting a biomaterial.75 The ability to control the physical and topographical surface of biomaterials can have an effect on MC behavior, including adhesion, morphology, and activation. These studies have been summarized in Table 1.
Table 1.
Summary of the Studies Featured in This Review Using Synthetic Biomaterials
| Biomaterial | MC type | Use of biomaterial | Outcome |
|---|---|---|---|
| Electrospun PDO, PCL, silk fibroin scaffolds76 | Murine: BMMCs | BMMCs, nonactivated and activated by IgE and IgE with dinitrophenol were seeded in vitro onto electrospun scaffolds. Cell adhesion, proliferation, and cytokine secretion were measured. | Nonactivated BMMCs were able to adhere to electrospun scaffolds other than silk. Activation increased adhesion, proliferation, and secretion of TNF-α, macrophage inflammatory protein-1α, and IL-13. |
| PP mesh77 | Rat: in vivo | PP mesh was implanted on the surface of the thorax for up to 30 days. Quantities of cNOS+ and iNOS+ MCs were measured to quantify activation. | cNOS+ MC count peaked at day 1 while iNOS+ MC count was highest in day 5. On day 30, MC count was at control levels. |
| Conjugated nanofiber matrix56 | Human: LAD2 cell line and ex vivo skin | The PAMP-12 motif, which is known to activate MCs, was conjugated to nanofiber matrices. These matrices were cultured with MCs in vitro and on human dermis ex vivo. | Matrices with PAMP-12 motif upregulated β-hexosaminidase release in a dose-dependent manner in LAD2s. On human ex vivo skin, tryptase mRNA transcripts were higher in dermis treated with matrices with PAMP-12 motif than nonconjugated matrices. |
| pPE78 | Murine: in vivo | pPE was coated with murine microvascular fragments derived from adipose tissue or platelet-rich plasma. | While coated pPE promoted a prohealing phenotype in macrophages, there was no difference to uncoated controls in terms of MC number and adhesion. |
| Nanosilver particles79 | Rat: in vivo | Commercial orthodontic brackets were coated with nanosilver particles and implanted subcutaneously for up to 60 days. | No difference to controls in terms of MC count, except at day 7 where MC count was lower in coated brackets than control brackets. |
| Polystyrene film (honeycomb-like)80 | Murine: NCL-2 cell line | MCs were cultured in vitro on honeycomb structures made from polystyrene films. | More clustering and formation of multinucleated cells occurred on structures with larger pores. |
| Ti discs81 | Rat: RBL-2H3 cell line | MCs were cultured in vitro on Ti discs with and without nanotopography. | Ti promoted faster adhesion than glass. However, there were no differences in β-hexosaminidase release and vinculin expression. MCs cultured on Ti discs with nanotopography exhibited increased growth and migration. |
| PDO73 | Murine: BMMCs | PDO was electrospun into scaffolds with varying pore size and diameter. MCs were cultured on these scaffolds. | Stimulated MCs on PDO scaffolds of larger pore and fibers demonstrated downregulation of IL-6 and TNF-α, and upregulation of VEGF. |
| Polyester fibers coated with masitinib-releasing poly(lactic-co-glycolic acid) microspheres82 | Murine: in vivo (MC-deficient model) | Masitinib-releasing fibers were subcutaneously implanted for up to 28 days in MC-deficient mice. Masitinib has been shown to inhibit MC proliferation. | There was no significant difference in FBR capsule formation of coated vs. noncoated implants in MC-deficient mice. However, MC-deficient mice experienced thicker fibrotic capsules around the coated implants than wild type mice. |
| Commercial PP, PP with poliglecaprone, and polyester meshes83 | Murine: in vivo | Meshes were implanted subcutaneously for 14 days with or without daily cromolyn treatment (cromolyn is a known MC suppressant). | Inflammation and signs of FBR were seen in all 4 meshes, however, cromolyn treatment downregulated the FBR and inflammatory response. One exception was PP with poliglecaprone, where cromolyn treatment did not significantly downregulate inflammation compared to the saline control. |
| ZnO nanoparticles55 | Rat: RBL-2H3 cell line, and Murine: BMMCs | NPs and bulk ZnO (particulates) were cultured in vitro with MCs. MC activation was assessed through histamine and β-hexosaminidase. | Histamine and β-hexosaminidase release were inhibited in a dose-dependent manner in response to ZnO NPs vs. bulk ZnO. |
BMMCs, bone marrow-derived MCs; cNOS, constitutive NOS; FBR, foreign body response; IgE, immunoglobulin E; IL-13, interleukin 13; iNOS, inducible NOS; MC, mast cell; NOS, nitric oxide synthase; NPs, nanoparticles; PCL, poly-ɛ-caprolactone; PDO, polydioxanone; PP, polypropylene; pPE, porous polyethylene; Ti, titanium; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor; ZnO, zinc oxide.
Effect of synthetic material properties and concentration on MC response
Synthetic polymers have, in some cases, been shown to be more conducive to MC growth when compared to natural polymers. The cell response of bone marrow-derived murine MCs was measured on electrospun polydioxanone (PDO), poly-ɛ-caprolactone (PCL), silk scaffolds, and tissue culture plastic cultured with or without activation by interleukin (IL)-3, SCF, IgE, and IgE with dinitrophenol.76 While it was previously thought that MCs only exhibited adhesion and cytokine secretion when activated, the adhesion of nonactivated MCs was reported. The silk scaffold did not support MC adhesion or cytokine secretion; however, activation by IgE and IgE with dinitrophenol significantly enhanced adhesion, proliferation, migration, and secretion for the synthetic scaffolds. This study demonstrates the complexity of MC adhesion to biomaterials and further supports that MCs do respond differently to varying biomaterial properties.
More recent research has started to explore the role that MCs play in association with currently used synthetically derived medical implants. One study investigated the role of MCs in biomaterial integration after implanting polypropylene (PP) mesh into rats, a mesh commonly used in reconstructive surgery.77 The authors observed the activities of constitutive nitric oxide synthase (cNOS) and inducible NO synthase (iNOS) to measure cell activation. MCs were categorized as iNOS+ MCs or cNOS+ MCs. A peak in cNOS+ MCs was seen first, followed by a peak in iNOS+ MCs a few days later. It appeared that the activity of iNOS+ MCs and cNOS+ MCs were connected, eliciting a NO-mediated response. Production of cytokines and growth factors by MCs also appeared to lead to the angiogenesis processes and mobilize the fibroblasts to the zone of injury.
MC response was also found to be dependent on the biomaterial concentration.56 One group prepared a nanofiber matrix designed to activate MCs through a nonselective MC cell membrane receptor, MRGPRX2. By conjugating the self-assembling peptide (RADA)4 ([Ac]-RADARADARADARADA-CONH2) with the PAMP-12 motif (FRKKWNKWALSR), known as a ligand for Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2),64 they synthesized (RADA)4-GG-(PAMP-12) ([Ac]-RADARADARADARADA-GG-FRKKWNKWALSR-CONH2, where GG is a glycine spacer). Matrices with >20% (RADA)4-GG-(PAMP-12) induced degranulation in LAD2s. This activation was found to be dose-dependent, with matrices of higher (RADA)4-GG-(PAMP-12) percentage resulting in higher amounts of degranulation.
A difference in MC response has not always been seen between different implanted synthetic biomaterials. When porous polyethylene (pPE) was coated in murine microvascular fragments derived from adipose tissue and/or platelet-rich plasma, there was no significant difference observed in the accumulation of MCs between the uncoated and coated implants in the dorsal skinfold chamber and flank models.78 The same was seen for nanosilver particles, previously shown to have antibacterial and antifungal properties, and used in the medical/dental field to prevent infection.79 No significant difference was found between the MC population in orthodontic brackets coated with nanosilver particles and control groups when implanted subcutaneously in the dorsal region of rats up to 60 days, except at 7 days where MC count was lower in coated brackets. These two studies further contribute to the complexity of MC–biomaterial interactions by demonstrating that MCs can show similar responses between diverse materials.
Effect of topography/porosity of biomaterial on MC response
The use of synthetic biomaterials allows for increased control over physical structure and topography. When a honeycomb-like structure made out of polystyrene film was used to culture bone marrow-derived mouse MCs (NCL-2 cells), attachment and proliferation were found to increase with honeycomb hole size.80 This increase in proliferation was not observed when the cells were cultured on a flat film. The authors observed the clustering and multinuclear formation of MCs inside the holes; however, very few multinucleated cells were found on flat films or structures with small hole sizes. This study indicated that MCs alter their shape depending on their material environment.
Nanotopography on surfaces has also provoked changes in MC adhesion and proliferation.81 Titanium (Ti) was shown to promote faster adhesion of rat MC line, RBL-2H3, than glass controls. Nanotopographical etching on Ti via H2SO4 and H2O2 enhanced cell migration and cell growth versus titanium discs without nanotopographical changes. While these properties were affected, β-hexosaminidase release and vinculin expression were not significantly different than glass or to each other. These results indicate that nanotopography may be a good strategy to influence MC proliferation and migration when implanted.
The physical structure of the synthetic biomaterials has also been shown to lead to a chemical response from MCs.73 Different pore and fiber diameters on a PDO scaffold were used to determine how these changes affected murine MCs. MCs stimulated with proinflammatory molecules, lipopolysaccharide, and IL-33 expressed less IL-6 and tumor necrosis factor-alpha (TNF-α), which are both markers of inflammation, on scaffolds of larger pore and fiber sizes when compared to scaffolds of small pore and fiber sizes. Larger pore sizes were also observed to elicit a greater response mediated by vascular endothelial growth factor, which is known to be important in angiogenesis and vasculogenesis. This study, along with the previous studies mentioned in this section, demonstrates how the physical structure of materials can influence the MC response.
Blocking MC activation and/or maturation with synthetic scaffolds
Additional research has focused on blocking MC activity using synthetic biomaterials. It was hypothesized that blocking MC activity around implants could affect the host FBR and lengthen the lifetime of sensing implantable devices.82 Implants modeling these sensors were made of polyester fibers and coated with a rapidly dissolving polymer containing drug (mastinib)-releasing degradable microspheres and implanted subcutaneously in MC-deficient mice. FBR was determined based on the chronic inflammation and fibrous capsule formation around the location of the implantation. No significant differences were seen in capsule formation between the control and drug-releasing implants. However, when these sensors were implanted in wild-type mice, the fibrous encapsulation around the drug-releasing implant was found to be significantly lower. This indicated that when the mouse is MC-deficient, the body finds alternative pathways.
Another study incorporated cromolyn treatment while studying the effects of material pore size on MC response.83 Meshes were made of either PP, PP with poliglecaprone, or polyester, all with varying porosities, and implanted subcutaneously in mice. All of these conditions were administered with and without cromolyn treatment. Interfiber inflammation was seen to decrease as pore size increased and MC accumulation was apparent at the edges of mesh-induced fibrosis and neovascularization. Overall, blocking MC degranulation using cromolyn was shown to decrease early inflammation and fibrosis in the publication. As noted earlier in this review, there are doubts about the efficacy of cromolyn treatment in mice,60 so further studies of cromolyn optimization in mice, as well as in other species, are needed before solidifying cromolyn as a solution for host-biomaterial integration.
Synthetic biomaterial nanoparticles have also shown the ability to inhibit MC response.55 The effect of size and dispersion/concentration of zinc oxide (ZnO) NPs compared to bulk (particulate) ZnO on MC degranulation and viability was investigated. The exposure of cells to ZnO NPs was found to inhibit both histamine and β-hexosaminidase release. This effect was determined to be inversely proportional to NP size and dispersion, leading authors to conclude that ZnO NPs may have greater potential than bulk ZnO in the inhibition of MC-mediated allergic responses.
Using MC response to understand biological processes
Biomaterials have been used as tools to understand mechanisms of some biological processes that involve MCs. Studies have shown a strong connection between MC activation and the presence of fibrin. In a study by Tsai et al., local inflammation was induced in mice by implanting titanium dioxide, silicon dioxide, or polylactic acid.84 Mice were then subcutaneously injected with MC activating C48/80. By engineering GPRPPGGSKGC peptide probes that bind to fibrin, the authors found increased probe accumulation at implant sites with C48/80 stimulation, indicating that MC activation led to increased fibrin. Conversely, a decrease in probe accumulation was seen when implanted in MC-deficient mice.
Another study used poly-l-glycolic acid films in both wild-type and MC-deficient murine models.71 Some of these films incorporated cromolyn and C48/80. Films treated with C48/80 resulted in increased thickness of the fibrotic capsule, a higher cell density and a higher amount of collagen I, than controls. Films treated with cromolyn resulted in thinner fibrotic capsules, lower cell densities and a lower amount of collagen I, than controls. MC-deficient mice also had thinner fibrotic capsules, lower cell density and lower collagen I, when compared to wild type. These two studies used biomaterials as a conduit to study the role MCs play in the recruitment of fibrocytes in fibrotic responses.
Naturally derived biomaterials
While the mechanical, material, and manufacturing properties of synthetic materials can generally be controlled, there are advantages to using materials derived from natural sources. Natural biomaterials exhibit bioactivity that can modulate processes such as inflammation.85,86 They also tend to exhibit some degree of degradation and/or reabsorption.87,88 There has been a body of research that have shown how the use of natural biomaterials promotes maturation and/or activation of MCs, but as with synthetic materials, that is not always the case. Some of the studies have been summarized in Table 2.
Table 2.
Summary of the Studies Featured in This Review Using Natural and Hybrid Biomaterials
| Biomaterial | MC type | Use of biomaterial | Outcome |
|---|---|---|---|
| Chitosan coating on TCP and sponges89 | Rat: RBL-2H3 cell line and in vivo | MCs were plated on chitosan-coated TCP. Chitosan sponges, treated with and without acid, were implanted subcutaneously into the lumbar regions of rats in vivo for 7 days. | In vitro, chitosan increased β-hexosaminidase release. In vivo, histological staining showed MC markers such as Kit and NASDCE found in throughout the fibrous capsules that developed in response to implanted chitosan. |
| Chitosan and chitosan/alginate NPs90 | Human: HMC-1 cell line | NPs prepared from chitosan and chitosan/alginate were loaded with C48/80 and cultured in vitro with MCs. | In vitro β-hexosaminidase release on chitosan NPs with and without C48/80 was increased vs. chitosan/alginate NPs and C48/80 alone. |
| Chitosan and chitosan/alginate nanoparticles91 | Human: HMC-1 cell line | NPs prepared from chitosan and chitosan/alginate and cultured in vitro with HMC-1 cells. | β-Hexosaminidase release was increased in MCs cultured with chitosan only NPs and C48/80 alone vs. chitosan/alginate NPs. Toluidine blue staining gave evidence of degranulation. |
| COS92 | Rat: RBL-2H3 cell line | COS were cultured in vitro for 1 h with MCs, which were sensitized via dinitrophenyl-specific IgE overnight then stimulated via dinitrophenyl-bovine serum albumin for 1 h. | Histamine and β-hexosaminidase release as well as intracellular [Ca2+] were downregulated when in culture with COS. Protein and mRNA expression of all three chains (α, β and γ) of FcɛRI, were also downregulated. |
| Collagen and fibrinogen connective tissue model54 | Human: CD133+ progenitors from peripheral blood | MC progenitors cocultured with fibroblasts in a collagen matrix for 6 weeks. A collagen/fibronectin coating was applied on top at the seventh week and endothelial cells were seeded a week later. This model represented connective tissue with a capillary wall (CTEM). | MC progenitors in the CTEM demonstrated signs of maturation through CD117(KIT), FcɛRI, chymase and tryptase expression. These now matured MCs also released histamine in response to Dermatophagoides pteronyssinus allergen. |
| Hyaluronan and gelatin93 | Rat: RBL-2H3 cell line | Hyaluronan and gelatin coatings were used to culture MCs on glass or TCP. MCs were also antigen-stimulated before measuring intracellular [Ca2+], β-hexosaminidase release, and paxillin and FAK phosphorylation. | MCs cultured on hydrogel-coated glass exhibit a round morphology compared to spreading out on glass only. Depending on the type of antigen stimulation, degranulation and intracellular [Ca2+] were suppressed in MCs on hydrogel coating. FAK phosphorylation was also suppressed on hydrogel coatings. |
| PCL/chitosan NPs57 | Human: HMC-1 cell line; murine: in vivo | NPs were cultured with MCs in vitro for 45 min. NPs with a hepatitis B surface antigen (HBsAG) were subcutaneously injected into mice to test whether they would cause an elevation in IgE. | MCs cultured with NPs increased in vitro β-hexosaminidase release in a dose-dependent manner. IgE levels in mice injected with HBsAg-NPs remained low, while rising IgG titers to levels comparable to commercial levels. |
| poly(DL-lactide-co-glycolide/chitosan NPs96 | Rat: RBL-2H3 cell line; murine: PMCs and in vivo (anaphylaxis model) | NPs made with and without chitosan were cultures in vitro with MCs. | NPs made with chitosan had higher uptake levels than NPs without chitosan. NPs, both with and without chitosan suppressed histamine release. Serum histamine levels in vivo were decreased with NPs with chitosan vs. NPs alone. |
| Decellularized dermis ECM hydrogels100 | Human: LAD2 cell line | LAD2 cells were cultured in vitro in porcine-derived dermis ECM hydrogels for up to 7 days. | Upregulated metabolic activity and gene expression of all three chains of FcɛRI was found at 7 days when compared to a commercial collagen control. |
C48/80, compound 48/80; COS, chitosan oligosaccharides; CTEM, connective tissue-equivalent matrix; ECM, extracellular matrix; FAK, focal adhesion kinase; HBsAg, hepatitis B surface antigen; TCP, tissue culture plastic; NASDCE, naphthol AS-d chloroacetate esterase; PMCs, peritoneal mast cells.
MC activation and/or maturation with naturally derived biomaterials
Chitosan has been used extensively as a naturally derived biomaterial that can modulate MC activation. In vitro culture of chitosan with the RBL-2H3 line has demonstrated a steady increase of β-hexosaminidase release for 38–44 min after exposure.89 Chitosan sponges, prepared with and without acid to produce globular and fibrillar shapes, were implanted into the lumbar regions of rats. The Kit receptor was detected sporadically in the fibrous capsule only in globular chitosan, while Kit was expressed throughout both the implant and the fibrous capsule in fibrillar chitosan. The presence of naphthol AS-d chloroacetate esterase (NASDCE), which is present in granulocytic cells and a sign of MC activation, was higher in chitosan than in the controls, and was located primarily in the implant rather than the fibrous capsule.
Chitosan NPs have also been shown to promote activation.90 When chitosan NPs loaded with MC activator C48/80 were cultured with HMC-1 cells, β-hexosaminidase release was increased when compared with NPs made by combined chitosan and alginate, with values slightly higher on NPs loaded with C48/80. This finding is further corroborated with the findings in Soares et al.91 Chitosan NPs in concentrations of 500 or 100 μg/mL stimulated β-hexosaminidase release in HMC-1 cells at the same levels of MC activator C48/80, while chitosan NPs coated with alginate did not stimulate similar levels. Toluidine blue staining also revealed degranulation in HMC-1s treated with C48/80 and chitosan NPs. These results confirmed the activation of MCs. However, chitosan oligosaccharides do not report the same findings.92 RBL-2H3 cells treated with chitosan oligosaccharides that were then sensitized with IgE antibody showed decreases in histamine release, β-hexosaminidase release, and intracellular [Ca2+]. In addition, there was a decrease in protein and mRNA expression of all three chains (α, β, and γ) of FcɛRI in the treated cells.
Collagen and fibrin hydrogels have been shown to enhance the maturation of MC progenitors. Derakhshan et al. developed a connective tissue-equivalent model by culturing MC progenitors (CD133+) and dermal fibroblasts in type I collagen gels for 6 weeks, layering the top with collagen type IV and fibronectin at the seventh week and then seeding human umbilical vein endothelial cells.54 The expression of FcɛRI, determined by flow cytometry, was increased. Histamine release was also observed when the cells were stimulated via Dermatophagoides pteronyssinus allergen. Cells also expressed increased levels of intracellular tryptase and chymase. While expression of these proteases is not necessarily an indicator of MC activation, increased intracellular content can suggest increased granularity.
Not all naturally derived biomaterials have the ability to promote maturation or activation of MC responses. One example of this is commercial hyaluronan and gelatin hydrogel coatings on glass used to culture the rat MC line RBL-2H3.93 MCs spread out on hydrogel-coated glass, maintaining a round and spherical morphology. Attributing this morphology to possibly interrupted focal adhesion assembly and cytoskeleton formation, the authors further observed histological staining for α-vinculin and α-tubulin, two cytoskeleton adaptor proteins. They additionally found that α-vinculin was not distributed throughout the cytoplasm and was pooled at the bottom of the cells. After antigen stimulation, phosphorylation of focal adhesion kinase was decreased in MCs on hydrogels, but not paxillin, which is found in focal adhesions. Stimulated degranulation in MCs was inhibited on the hydrogel coating by showing a decrease in β-hexosaminidase release. These results indicate that biomaterial composition can affect MC focal adhesion formation.
Studying MC–biomaterial interactions to understand biological processes
In addition to the response of MCs to biomaterials, in vivo models with biomaterials have been used to elucidate the roles of MCs in tissue repair. In an effort to assess the formation of vascular networks and find the best approach for restoring local vascularization, bioengineered tissues, composed of chitosan scaffolds, were created and enriched with MCs and/or platelet-rich plasma in vivo.94 Generally, ischemia causes a reduction in capillary density and the number of large vessels while increasing the number of small vessels. Also, a reduced gastrocnemius muscle fiber diameter and capillary-to-muscle fiber ratio are generally observed. The incorporation of MCs in the chitosan was seen to significantly increase capillary density as well as increase the mean number of large blood vessels when compared to the other treatment groups. This chitosan and MC treatment significantly increased muscle fiber diameter and capillary-to-muscle fiber ratio in gastrocnemius muscles when compared with the other treatments. Overall, the authors concluded that incorporating MCs within a chitosan scaffold for bioengineered tissues could be very important and applicable in therapeutic angiogenesis.
Another ischemic in vivo model was used with alginate/gelatin three-dimensional scaffolds. The goal was to find a proper method for improvement of the ischemic condition in the rat hind limb and also observe the effectiveness of cell engraftment with alginate/gelatin three-dimensional scaffolds.95 Three different groups were used. The first was the ischemia group where the femoral artery was removed after ligation. The second was a scaffold group where a hydrogel scaffold was added to the site of the transected femoral artery, and in the third group, MCs were added in addition to the hydrogel scaffold. The average number of blood capillaries and the average number of medium and large blood vessels was significantly higher in the MC group than in the other conditions. The application of MCs can be seen as a new approach for therapeutic angiogenesis under ischemic conditions. A partial possible explanation is the effect of the chemical mediators secreted from MCs, which may encourage vascular development.
Hybrid biomaterials
Bioengineers have also combined both synthetic materials with naturally derived materials to capitalize on the strengths of both types. Just as chitosan is used on its own, it has also been combined with synthetic materials to create a hybrid. One example is combined PCL with chitosan, making MC-activating nanoparticles.57 The goal of this in vitro study was to combine both materials to make an adjuvant for the hepatitis B surface antigen. These PCL/chitosan nanoparticles promoted MC activation through β-hexosaminidase release and when administered through the subcutaneous route, these NPs could induce high specific IgG antibody titers without increasing IgE titers. The specific antibody titers induced were found to be dose-dependent allowing for control of intensity of immune response. Chitosan has also been combined with poly(DL-lactide-co-glycolide).96 These NPs were cocultured with rat RBL-2H3 cells and murine peritoneal MCs. The NPs modified with chitosan were more readily taken up by the cells, but once the NPs became saturated, they were exocytosed. At this stage, β-hexosaminidase and histamine release decreased. Serum histamine levels found in vivo were decreased in mice treated with NPs with chitosan than poly(DL-lactide-co-glycolide) NPs alone. These studies demonstrate the use of hybrid materials to modulate MCs and more studies are needed to further explore the potential of hybrid biomaterials to modulate MC responses.
Future Directions
In addition to studying MC responses to biomaterials, there is a need to incorporate MCs in tissue engineering models to determine how MCs can influence biological processes. Currently, there are limited studies that use tissue-engineered models and therapeutics with MCs. Mammalian inflammation is a dynamic response characterized by an intricate network of cells, cytokines, and signaling pathways. Modeling some, let alone all, of these aspects, is extremely difficult and represents a significant limitation in the use of immune cells in tissue-engineered models. However, including MCs in tissue-engineered models can help elucidate some aspects of inflammation and replace less accurate models.
There are also other immunomodulatory biomaterials that are important to consider. One of these is the ECM derived from porcine tissues that have been decellularized and have little to no cellular content. They also possess many of the advantages of naturally derived biomaterials. These biomaterials have been extensively tested using macrophages and T cells and how these cells are influenced to promote healing, regeneration, and a shift toward less inflammatory environment.97–99 Our group has recently looked at MC viability and maturation in vitro with porcine dermis-derived ECM hydrogels.100 It was found that the LAD2 cell line exhibits good cell viability, higher rates of metabolism, and upregulation of cKIT and IgE receptor chains of FcɛRI at 7 days. As studies looking at MC modulation are currently limited, future experiments can look at other markers of cell viability and maturation, enhancing our understanding of these unique biomaterials and helping us further harness local inflammatory environments.
Conclusions
In this review, we have highlighted the complex role that MCs play during the physiological response to biomaterials used for tissue repair and wound healing. We have also reviewed the interactions that these MCs have with biomaterials, both synthetically and naturally derived. While more optimization and studies of MCs are necessary to fully understand the inflammatory mechanisms, it is worthwhile to use biomaterials as a way to elucidate these processes and predict clinical MC responses.
Acknowledgment
The figures in this publication were made, in part, with Servier Medical ART (SMART).
Disclosure Statement
Dr. Cruse has research support from Hoth Therapeutics for a project not directly related to the research reported in this publication and also serves on their Scientific Advisory Board. The research findings included in this publication were not funded by Hoth Therapeutics and may not necessarily be related to the interests of Hoth Therapeutics. The terms of this arrangement have been reviewed and approved by NC State University in accordance with its policy on objectivity in research. The remaining authors declare no competing financial interests.
Funding Information
We thank our funding sources: The National Institutes of Health (NIH)/National Institute on Deafness and Other Communication Disorders (R01DC017139, R01DC017743), NIH National Institute of Allergy and Infectious Diseases (NIAID), Award Number (R01AI143985), The American Heart Association Predoctoral Fellowship (19PRE34380006) to E.W.O., the UNC-CH/NCSU Joint Department of Biomedical Engineering and its Abrams Scholar Program, the NCSU Office of Undergraduate Research (OUR), NCSU Center for Human Health and the Environment (Grant P30ES025128), and the Comparative Medicine Institute (CMI) at NCSU, especially the CMIs Summer Interdisciplinary Research Initiative (SIRI) and Young Scholar Program (YSP).
References
- 1. Wernersson, S., and Pejler, G.. Mast cell secretory granules: armed for battle. Nat Rev Immunol 14, 478, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Moon, T.C., Befus, A.D., and Kulka, M.. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 5, 569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wulff, B.C., and Wilgus, T.A.. Mast cell activity in the healing wound: more than meets the eye?. Exp Dermatol 22, 507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishida, K., Hasegawa, A., Yamasaki, S., et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci Rep 9, 10842, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Komi, D.E.A., Khomtchouk, K., and Santa Maria, P.L.. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol 58, 298, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Weller, K., Foitzik, K., Paus, R., Syska, W., and Maurer, M.. Mast cells are required for normal healing of skin wounds in mice. FASEB J 20, 2366, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Yao, X., Peng, R., and Ding, J.. Cell-material interactions revealed via material techniques of surface patterning. Adv Mater 25, 5257, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Peng, Y., Liu, Q.J., He, T., Ye, K., Yao, X., and Ding, J.. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials 178, 467, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Liu, Q., Zheng, S., Ye, K., et al. Cell migration regulated by RGD nanospacing and enhanced under moderate cell adhesion on biomaterials. Biomaterials 263, 120327, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Lin, J., Zhou, W., Han, S., et al. Cell-material interactions in tendon tissue engineering. Acta Biomater 70, 1, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Martino, S., D'Angelo, F., Armentano, I., Kenny, J.M., and Orlacchio, A.. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol Adv 30, 338, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Othman, Z., Cillero Pastor, B., van Rijt, S., and Habibovic, P.. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 167, 191, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Witherel, C.E., Abebayehu, D., Barker, T.H., and Spiller, K.L.. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater 8, e1801451, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klopfleisch, R., and Jung, F.. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A 105, 927, 2017. [DOI] [PubMed] [Google Scholar]
- 15. Agier, J., and Pastwinska, J., Brzezinska-Blaszczyk, E.. An overview of mast cell pattern recognition receptors. Inflamm Res 67, 737, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collington, S.J., Hallgren, J., Pease, J.E., et al. The role of the CCL2/CCR2 axis in mouse mast cell migration in vitro and in vivo. J Immunol 184, 6114, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Cassia Campos, M.R., Toso, V.D., de Souza Jr., D.A., et al. Differential effects of chemoattractants on mast cell recruitment in vivo. Cell Immunol 289, 86, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Karhausen, J., Choi, H.W., and Maddipati, K.R., et al. Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci Adv 6, eaay6314, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kajiwara, N., Sasaki, T., Bradding, P., et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol 125, 1137.e6, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Ashina, K., Tsubosaka, Y., Nakamura, T., et al. Histamine induces vascular hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS One 10, e0132367, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunder, C.A., St John, A.L., and Abraham, S.N.. Mast cell modulation of the vascular and lymphatic endothelium. Blood 118, 5383, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cruse, G., Metcalfe, D.D., and Olivera, A.. Functional deregulation of KIT: link to mast cell proliferative diseases and other neoplasms. Immunol Allergy Clin North Am 34, 219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conti, P., Caraffa, A., Mastrangelo, F., et al. Critical role of inflammatory mast cell in fibrosis: potential therapeutic effect of IL-37. Cell Prolif 51, e12475, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Souza Junior, D.A., Borges, A.C., Santana, A.C., Oliver, C., and Jamur, M.C.. Mast cell proteases 6 and 7 stimulate angiogenesis by inducing endothelial cells to release angiogenic factors. PLoS One 10, e0144081, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wulff, B.C., Parent, A.E., Meleski, M.A., DiPietro, L.A., Schrementi, M.E., and Wilgus, T.A.. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 132, 458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen, L., Schrementi, M.E., Ranzer, M.J., Wilgus, T.A., and DiPietro, L.A.. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One 9, e85226, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiskirchen, R., Meurer, S.K., Liedtke, C., and Huber, M.. Mast cells in liver fibrogenesis. Cells 8, 1429, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vangansewinkel, T., Lemmens, S., Geurts, N., et al. Mouse mast cell protease 4 suppresses scar formation after traumatic spinal cord injury. Sci Rep 9, 3715, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradding, P., and Pejler, G.. The controversial role of mast cells in fibrosis. Immunol Rev 282, 198, 2018. [DOI] [PubMed] [Google Scholar]
- 30. Guhl, S., Neou, A., Artuc, M., Zuberbier, T., and Babina, M.. Skin mast cells develop non-synchronized changes in typical lineage characteristics upon culture. Exp Dermatol 23, 933, 2014. [DOI] [PubMed] [Google Scholar]
- 31. Motakis, E., Guhl, S., Ishizu, Y., et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 123, e58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moon, T.C., St Laurent, C.D., Morris, K.E., et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol 3, 111, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Kovarova, M., Latour, A.M., Chason, K.D., Tilley, S.L., and Koller, B.H.. Human embryonic stem cells: a source of mast cells for the study of allergic and inflammatory diseases. Blood 115, 3695, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikuno, T., Ito, S., and Inoue, T.. Human induced pluripotent stem cell-derived mast cells useful for in vitro mast cell activation assay exhibiting phenotypes and morphological characteristics of human mast cells. J Toxicol Sci 44, 789, 2019. [DOI] [PubMed] [Google Scholar]
- 35. Igarashi, A., Ebihara, Y., Kumagai, T., Hirai, H., Nagata, K., and Tsuji, K.. Mast cells derived from human induced pluripotent stem cells are useful for allergen tests. Allergol Int 67, 234, 2018. [DOI] [PubMed] [Google Scholar]
- 36. Schmetzer, O., Valentin, P., Smorodchenko, A., et al. A novel method to generate and culture human mast cells: peripheral CD34+ stem cell-derived mast cells (PSCMCs). J Immunol Methods 413, 62, 2014. [DOI] [PubMed] [Google Scholar]
- 37. Grootens, J., Ungerstedt, J.S., Nilsson, G., and Dahlin, J.S.. Deciphering the differentiation trajectory from hematopoietic stem cells to mast cells. Blood Adv 2, 2273, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dwyer, D.F., Barrett, N.A., Austen, K.F.; Immunological Genome Project Consortium.. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 17, 878, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xing, W., Austen, K.F., Gurish, M.F., and Jones, T.G.. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A 108, 14210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akula, S., Paivandy, A., Fu, Z., Thorpe, M., Pejler, G., and Hellman, L.. Quantitative in-depth analysis of the mouse mast cell transcriptome reveals organ-specific mast cell heterogeneity. Cells 9, 211, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dillahunt, S.E., Sargent, J.L., Suzuki, R., et al. Usage of sphingosine kinase isoforms in mast cells is species and/or cell type determined. J Immunol 190, 2058, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braddling, P., and Cruse, G.. Mast cells: biological properties and role in health and allergic diseases. In: Kay, A.B., Bousquet, J., and Holt, P. eds., Allergy and Allergic Diseases. Hoboken, NJ: Wiley-Blackwell, 2008. [Google Scholar]
- 43. Horta, R.S., Lavalle, G.E., Monteiro, L.N., Souza, M.C.C., Cassali, G.D., and Araujo, R.B.. Assessment of canine mast cell tumor mortality risk based on clinical, histologic, immunohistochemical, and molecular features. Vet Pathol 55, 212, 2018. [DOI] [PubMed] [Google Scholar]
- 44. Jawhar, M., Schwaab, J., Meggendorfer, M., et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica 102, 1035, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akin, C. Mast cell activation syndromes. J Allergy Clin Immunol 140, 349, 2017. [DOI] [PubMed] [Google Scholar]
- 46. Metcalfe, D.D., and Akin, C.. Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk Res 25, 577, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Falcone, F.H., Wan, D., Barwary, N., and Sagi-Eisenberg, R.. RBL cells as models for in vitro studies of mast cells and basophils. Immunol Rev 282, 47, 2018. [DOI] [PubMed] [Google Scholar]
- 48. Ohmori, K., Kawarai, S., Yasuda, N., et al. Identification of c-kit mutations-independent neoplastic cell proliferation of canine mast cells. Vet Immunol Immunopathol 126, 43, 2008. [DOI] [PubMed] [Google Scholar]
- 49. Kirshenbaum, A.S., Akin, C., Wu, Y., et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 27, 677, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Kirshenbaum, A.S., Petrik, A., Walsh, R.. et al. A ten-year retrospective analysis of the distribution, use and phenotypic characteristics of the LAD2 human mast cell line. Int Arch Allergy Immunol 164, 265, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Souza Jr., D.A., Toso, V.D., Campos, M.R., Lara, V.S., Oliver, C., and Jamur, M.C.. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS One 7, e40790, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hallgren, J., and Gurish, M.F.. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol 716, 14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fukuishi, N., Murakami, S., Ohno, A., et al. Does beta-hexosaminidase function only as a degranulation indicator in mast cells? The primary role of beta-hexosaminidase in mast cell granules. J Immunol 193, 1886, 2014. [DOI] [PubMed] [Google Scholar]
- 54. Derakhshan, T., Bhowmick, R., Meinkoth, J.H., Ritchey, J.W., and Gappa-Fahlenkamp, H.. Human mast cell development from hematopoietic stem cells in a connective tissue-equivalent model. Tissue Eng Part A 25, 1564, 2019. [DOI] [PubMed] [Google Scholar]
- 55. Feltis, B.N., Elbaz, A., Wright, P.F., Mackay, G.A., Turney, T.W., and Lopata, A.L.. Characterizing the inhibitory action of zinc oxide nanoparticles on allergic-type mast cell activation. Mol Immunol 66, 139, 2015. [DOI] [PubMed] [Google Scholar]
- 56. Lu, L., Parmar, M.B., Kulka, M., Kwan, P., and Unsworth, L.D.. Self-assembling peptide nanoscaffold that activates human mast cells. ACS Appl Mater Interfaces 10, 6107, 2018. [DOI] [PubMed] [Google Scholar]
- 57. Jesus, S., Soares, E., Borchard, G., and Borges, O.. Adjuvant activity of poly-epsilon-caprolactone/chitosan nanoparticles characterized by mast cell activation and IFN-gamma and IL-17 production. Mol Pharm 15, 72, 2018. [DOI] [PubMed] [Google Scholar]
- 58. Ribatti, D. The staining of mast cells: a historical overview. Int Arch Allergy Immunol 176, 55, 2018. [DOI] [PubMed] [Google Scholar]
- 59. Shin, H.Y., Kim, J.S., An, N.H., Park, R.K., and Kim, H.M.. Effect of disodium cromoglycate on mast cell-mediated immediate-type allergic reactions. Life Sci 74, 2877, 2004. [DOI] [PubMed] [Google Scholar]
- 60. Oka, T., Kalesnikoff, J., Starkl, P., Tsai, M., and Galli, S.J.. Evidence questioning cromolyn's effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Invest 92, 1472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rothschild, A.M. Mechanisms of histamine release by compound 48-80. Br J Pharmacol 38, 253, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawakami, T., and Kitaura, J.. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J Immunol 175, 4167, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tatemoto, K., Nozaki, Y., Tsuda, R., et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349, 1322, 2006. [DOI] [PubMed] [Google Scholar]
- 64. McNeil, B.D., Pundir, P., Meeker, S., et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13, 362, 2013. [DOI] [PubMed] [Google Scholar]
- 66. Galli, S.J., and Kitamura, Y.. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol 127, 191, 1987. [PMC free article] [PubMed] [Google Scholar]
- 67. Grimbaldeston, M.A., Chen, C.C., Piliponsky, A.M., Tsai, M., Tam, S.Y., and Galli, S.J.. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167, 835, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lei, Y., Gregory, J.A., Nilsson, G.P., and Adner, M.. Insights into mast cell functions in asthma using mouse models. Pulm Pharmacol Ther 26, 532, 2013. [DOI] [PubMed] [Google Scholar]
- 69. Sadtler, K., Wolf, M.T., Ganguly, S., et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 192, 405, 2019. [DOI] [PubMed] [Google Scholar]
- 70. Chung, L., Maestas, D.R.Jr., Housseau, F., and Elisseeff, J.H.. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev 114, 184, 2017. [DOI] [PubMed] [Google Scholar]
- 71. Thevenot, P.T., Baker, D.W., Weng, H., Sun, M.W., and Tang, L.. The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials. Biomaterials 32, 8394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vliagoftis, H. Thrombin induces mast cell adhesion to fibronectin: evidence for involvement of protease-activated receptor-1. J Immunol 169, 4551, 2002. [DOI] [PubMed] [Google Scholar]
- 73. Abebayehu, D., Spence, A.J., McClure, M.J., Haque, T.T., Rivera, K.O., and Ryan, J.J.. Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses. J Biomed Mater Res A 107, 884, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dastych, J., and Metcalfe, D.D.. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol 152, 213, 1994. [PubMed] [Google Scholar]
- 75. Lutolf, M.P., and Hubbell, J.A.. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23, 47, 2005. [DOI] [PubMed] [Google Scholar]
- 76. Garg, K., Ryan, J.J., and Bowlin, G.L.. Modulation of mast cell adhesion, proliferation, and cytokine secretion on electrospun bioresorbable vascular grafts. J Biomed Mater Res A 97, 405, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grigoryuk, A.A., Belov, S.A., and Kotsyuba, A.E.. Reaction of mast cells in the zone of polypropylene mesh implantation. Bull Exp Biol Med 167, 694, 2019. [DOI] [PubMed] [Google Scholar]
- 78. Spater, T., Tobias, A.L., Menger, M.M., Nickels, R.M., Menger, M.D., and Laschke, M.W.. Biological coating with platelet-rich plasma and adipose tissue-derived microvascular fragments improves the vascularization, biocompatibility and tissue incorporation of porous polyethylene. Acta Biomater 108, 194, 2020. [DOI] [PubMed] [Google Scholar]
- 79. Metin-Gursoy, G., Taner, L., and Baris, E.. Biocompatibility of nanosilver-coated orthodontic brackets: an in vivo study. Prog Orthod 17, 39, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Choi, H., Tanaka, M., Hiragun, T., Hide, M., and Sugimoto, K.. Non-tumor mast cells cultured in vitro on a honeycomb-like structured film proliferate with multinucleated formation. Nanomedicine 10, 313, 2014. [DOI] [PubMed] [Google Scholar]
- 81. Marcatti Amaru Maximiano, W., Marino Mazucato, V., Tambasco de Oliveira, P., Celia Jamur, M., and Oliver, C.. Nanotextured titanium surfaces stimulate spreading, migration, and growth of rat mast cells. J Biomed Mater Res A 105, 2150, 2017. [DOI] [PubMed] [Google Scholar]
- 82. Avula, M.N., Rao, A.N., McGill, L.D., Grainger, D.W., and Solzbacher, F.. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model. Acta Biomater 10, 1856, 2014. [DOI] [PubMed] [Google Scholar]
- 83. Orenstein, S.B., Saberski, E.R., Klueh, U., Kreutzer, D.L., and Novitsky, Y.W.. Effects of mast cell modulation on early host response to implanted synthetic meshes. Hernia 14, 511, 2010. [DOI] [PubMed] [Google Scholar]
- 84. Tsai, Y.T., Zhou, J., Weng, H., Tang, E.N., Baker, D.W., and Tang, L.. Optical imaging of fibrin deposition to elucidate participation of mast cells in foreign body responses. Biomaterials 35, 2089, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hortensius, R.A., and Harley, B.A.. Naturally derived biomaterials for addressing inflammation in tissue regeneration. Exp Biol Med (Maywood) 241, 1015, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morris, A.H., Stamer, D.K., and Kyriakides, T.R.. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol 29, 72, 2017. [DOI] [PubMed] [Google Scholar]
- 87. Costa, A., Naranjo, J.D., and Turner, N.J., et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 108, 81, 2016. [DOI] [PubMed] [Google Scholar]
- 88. Allen, A.B., Priddy, L.B., Li, M.T., and Guldberg, R.E.. Functional augmentation of naturally-derived materials for tissue regeneration. Ann Biomed Eng 43, 555, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Farrugia, B.L., Whitelock, J.M., and Jung, M., et al. The localisation of inflammatory cells and expression of associated proteoglycans in response to implanted chitosan. Biomaterials 35, 1462, 2014. [DOI] [PubMed] [Google Scholar]
- 90. Bento, D., Staats, H.F., Goncalves, T., and Borges, O.. Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity. Eur J Pharm Biopharm 93, 149, 2015. [DOI] [PubMed] [Google Scholar]
- 91. Soares, E., Jesus, S., and Borges, O.. Oral hepatitis B vaccine: chitosan or glucan based delivery systems for efficient HBsAg immunization following subcutaneous priming. Int J Pharm 535, 261, 2018. [DOI] [PubMed] [Google Scholar]
- 92. Vo, T.S., Kim, J., Ngo, D.H., Kong, C.S., and Kim, S.K.. Protective effect of chitosan oligosaccharides against FceRI-mediated RBL-2H3 mast cell activation. Process Biochem 47, 327, 2012. [Google Scholar]
- 93. Shiki, A., Inoh, Y., Yokawa, S., and Furuno, T.. Inhibition of degranulation in mast cells attached to a hydrogel through defective microtubule tracts. Exp Cell Res 381, 248, 2019. [DOI] [PubMed] [Google Scholar]
- 94. Karimi, A., Shahrooz, R., Hobbenagh, R., et al. Histological evidence for therapeutic induction of angiogenesis using mast cells and platelet-rich plasma within a bioengineered scaffold following rat hindlimb ischemia. Cell J 21, 391, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amani, S., Shahrooz, R., Mortaz, E., Hobbenaghi, R., Mohammadi, R., and Baradar Khoshfetrat, A.. Histomorphometric and immunohistochemical evaluation of angiogenesis in ischemia by tissue engineering in rats: role of mast cells. Vet Res Forum 10, 23, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tahara, K., Tadokoro, S., Yamamoto, H., Kawashima, Y., and Hirashima, N.. The suppression of IgE-mediated histamine release from mast cells following exocytic exclusion of biodegradable polymeric nanoparticles. Biomaterials 33, 343, 2012. [DOI] [PubMed] [Google Scholar]
- 97. Sadtler, K., Estrellas, K., Allen, B.W., et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Huleihel, L., Dziki, J.L., Bartolacci, J.G., et al. Macrophage phenotype in response to ECM bioscaffolds. Semin Immunol 292, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dziki, J.L., Wang, D.S., Pineda, C., Sicari, B.M., Rausch, T., and Badylak, S.F., Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J Biomed Mater Res A 105, 138, 2017. [DOI] [PubMed] [Google Scholar]
- 100. Ozpinar, E.W., Frey, A.L., Arthur, G.K., et al. Dermal extracellular matrix-derived hydrogels as an in vitro substrate to study mast cell maturation. Tissue Eng Part A 27, 1008, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]