Abstract
A variety of engineered materials have gained acceptance in orthopedic practice as substitutes for autologous bone grafts, although the regenerative efficacy of these engineered grafts is still limited compared with that of transplanted native tissues. For bone defects greater than 4–5 cm, however, common bone grafting procedures are insufficient and more complicated surgical interventions are required to repair and regenerate the damaged or missing bone. In this review, we describe current grafting materials and surgical techniques for the reconstruction of large bone defects, followed by tissue engineering (TE) efforts to develop improved therapies. Particular emphasis is placed on graft vascularization, because for both autologous bone and engineered alternatives, achieving adequate vascular development within the regenerating bone tissues remains a significant challenge in the context of large bone defects. To this end, TE and surgical strategies to induce development of a vasculature within bone grafts are discussed.
Impact statement
This review aims to present an accessible and thorough overview of current orthopedic surgical techniques as well as bone tissue engineering and vascularization strategies that might one day offer improvements to clinical therapies for the repair of large bone defects. We consider the lessons that clinical orthopedic reconstructive practices can contribute to the push toward engineered bone.
Keywords: bone graft, reconstructive surgery, bone tissue engineering, vascular tissue engineering
Introduction
Reconstructive surgery can restore skeletal function and aesthetics in patients who have experienced major bone loss. Current approaches to heal large-scale defects typically require use of a bone graft at some stage of the reconstructive process; and to this aim, multiple grafting materials have been developed. However, for bone defects larger than 4–5 cm, application of a bone graft alone is not sufficient to achieve healing, and specialized surgical procedures are required to enhance the bone's regenerative capabilities.
Tissue engineering (TE) seeks to create bone grafts with increased regenerative potential that will mature either in vitro or in vivo to form functional new bone tissue, but poor vascularization of large constructs remains an obstacle to their successful engraftment. We believe the strategies that enable current reconstructive techniques to heal very large bone defects can offer lessons to inform future TE therapies. In this review, we outline techniques used to reconstruct major bone defects and examine the features that promote their efficacy in large-scale contexts, followed by discussion of TE strategies to develop grafts that can restore the complex functionality and vascularity of native bone. Perspectives beyond the canonical TE framework of cells, signals, and scaffolds are considered, as understanding the practical requirements for a clinical graft can help guide TE research toward translatable solutions.
Bone Biology and Defects
Bone tissue provides structural support to the body through an impressive combination of lightweight, tough, and load bearing properties. These features arise from bone's highly organized hierarchical structure.1 At the nanoscale, calcium phosphate mineral crystals are embedded within a protein matrix consisting primarily of type I collagen.2,3 At the microscale, bone tissues are organized into two main architectural forms: cancellous bone and cortical bone.4 Cancellous bone has a spongy microstructure, consisting of anisotropically ordered struts called trabeculae with porous spaces between them. By contrast, cortical bone tissue is very dense and provides the hardness and compressive strength needed for load bearing. A dense vascular network permeates bone, with larger arteries branching into smaller arterioles and capillaries that pass through networks of pores and channels in the mineralized tissues to furnish cells with oxygen and nutrients.5–8
Bone cells carry out a dynamic process of resorption and deposition, which enables bones to remodel in response to microdamage and mechanical loads. Bone is one of the few tissues that can heal without scarring,9 and many bone defects mend with minor intervention. However, in cases of extreme bone loss owing to trauma or disease, surgery may be required for reconstruction. Defects that are unable to spontaneously heal despite appropriate stabilization are known as critical size defects.10 In humans, the threshold size of a critical defect depends on the nature of the injury and varies between patients, but typically defects >2 cm in length require surgical intervention to enable recovery.11 A variety of grafting materials and surgical techniques may be called upon to treat critical size defects, their suitability varying based on the defect size, and location in the body.12
Clinical Bone Graft Materials
Bone grafts are characterized in terms of their osteoconductivity, osteoinductivity, osteogenicity, and mechanical strength. An osteoconductive graft provides a scaffold for the deposition of new bone. Osteoinduction refers to the act of recruiting or inducing the differentiation of osteoprogenitor cells. A graft is deemed osteogenic if it provides cells capable of forming new bone. The mechanical strength of a graft material determines whether it can provide structural stability and support to the reconstructed bone, although no graft is able to provide immediate load bearing without additional stable fixation.13
Autologous bone represents the graft of choice for nonunion treatment. These grafts, called autografts, are sourced from a portion of the patient's own bone that is harvested and then transplanted at the site of need. Autograft is of particular value for healing bone defects owing to its composition: it contains osteogenic cells, osteoinductive growth factors, and an osteoconductive matrix. Graft characteristics vary depending on the donor site; dense cortical grafts provide greater initial mechanical support, whereas cancellous grafts are richer in regenerative cells and growth factors and their highly porous structure promotes rapid bone ingrowth.14 The most common site from which to harvest autograft is the iliac crest of the pelvis, although it can also be sourced from other bones such as the femur, tibia, fibula, and radius.15,16 Despite the regenerative potential of autograft, living tissue harvest is accompanied by significant shortcomings. The volume of available tissue that can be harvested is limited and the donor site often suffers from morbidity, injury, infection, and pain.17
Allogeneic bone tissue harvested from cadavers can be used as an alternative graft source that avoids the limited availability and complications associated with autograft harvest. Allografts come in several forms. Demineralized bone matrix presents a high surface area of collagen and osteoinductive growth factors18; morselized cancellous and corticocancellous chips possess excellent osteoconductive properties15; and osteochondral and whole-bone segments can provide structural stability, although integration with host bone is slow because of their relative inertness.13,15 In general, allogeneic transplants suffer from inconsistent mechanical and osteoinductive characteristics owing to differences between donors,13,15,16 and the processing and sterilization techniques used to prepare donor tissue can further reduce the mechanical strength and activity of osteoinductive growth factors.13,14 Allogeneic tissues also carry a small risk of disease transmission from the donor to the recipient.19
Ceramic-based materials can serve as alternatives to biological tissue grafts and have found clinical use as bone void fillers and autograft extenders. A number of ceramic materials have been applied clinically, including calcium phosphates,20 calcium sulfates,21 and bioglasses22 as well as composite grafts containing organic polymers.23 As they typically lack biological components, the main function of synthetic grafts is to serve as osteoconductive scaffolds for new bone formation. However, it has been contended that the release of ions from calcium phosphate grafts into the surrounding interstitial fluids can have an osteoinductive effect, increasing cellular deposition of bone tissue.24,25 Ceramic grafts are typically resorbable, with the capacity to gradually degrade and be remodeled by bone cells, enabling their eventual replacement by functional native bone tissue.26,27 However, the inherent brittleness of synthetic ceramics limits their use in load bearing applications.13,19 Ceramic grafts are often applied in the form of granules, spongy blocks, or cements.13 Self-setting cements provide the advantage of solidifying in situ after placement to better fill irregular defect volumes; however, these cements often lack the macropores ideal for bone and vascular ingrowth, slowing integration with the native bone tissue.28,29
Polymer-based grafts include those made from processed biological polymers and synthetic polymers. Collagen represents the major organic component of native bone–tissue matrix2 and several grafts based on processed collagen are currently applied in clinics.19 Collagen scaffolds contain binding sites that interact with cells and other proteins,30 enhancing scaffold bioactivity compared with the relatively inert synthetic polymers. Despite this, degradable synthetic polymers constitute an area of great research interest for developing bone grafts, as their tunable mechanical properties and processability enable robust and complex construct designs. Several scaffolds based on polylactic acid and poly(lactic-co-glycolic acid) have been approved for use as bone void fillers and autograft extenders.19 Integration with other graft materials can produce composites with improved regenerative properties,31 and their complete hydrolysis enables the body to repair the tissue without any residual foreign material.
Surgical Techniques for Large Bone Defect Reconstruction
Some defects are so large that most autografts alone cannot heal the wound because the bone graft is subject to uncontrollable necrosis and resorption.32–35 Bone defects 4–5 cm in length or greater generally require more complex surgical intervention to heal fully.36
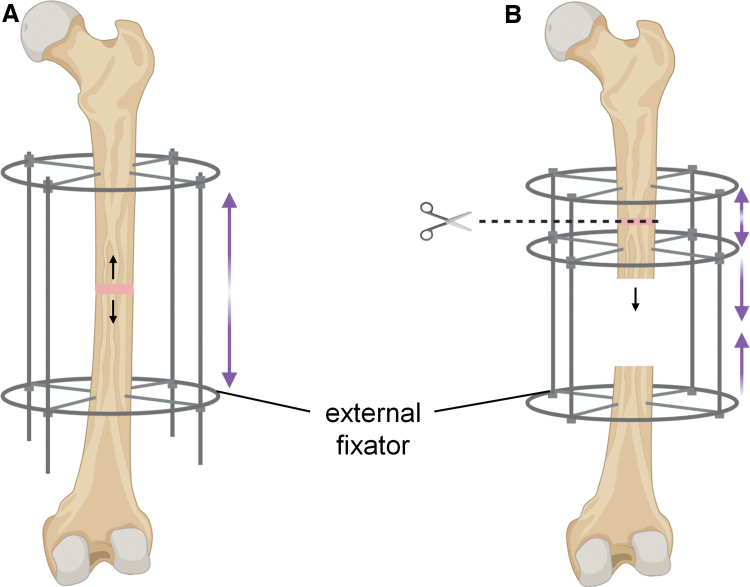
One method to regenerate almost unlimited lengths of bone is distraction osteogenesis, developed by Ilizarov in the 1950s.37 This surgical method involves placing the broken bone ends close together to initiate the knitting process (Fig. 1). Gradual separation of the ends at a rate of 0.5–1.5 mm per day extends the bone before closure of the gap can occur.37–40 As such, there is nearly no limit to the volume of bone that can be regenerated given sufficient time. However, the process is exceedingly cumbersome and painful, and patients might need to wear external braces for more than a year. These braces stabilize the unfused bone ends with strong pins that pass through the muscle and skin, which are highly prone to infection.37 A variant of distraction osteogenesis called bone transport enables bone undergoing reconstruction to remain at a fixed length throughout the procedure. To do this, a secondary break is created to produce a segment of bone that can then be slowly drawn from one end of the defect to the docking site. However, for most of the lengthening process, the docking surfaces are not held in close proximity and may become inactive owing to poor vascularization.41 To achieve union, a final surgery is typically required, often with application of a secondary autograft.42
FIG. 1.
Distraction osteogenesis takes advantage of bone's ability to generate new tissue under tension. (A) Monofocal bone lengthening. (B) Multifocal bone transport enables maintenance of limb length throughout the procedure. Color images are available online.
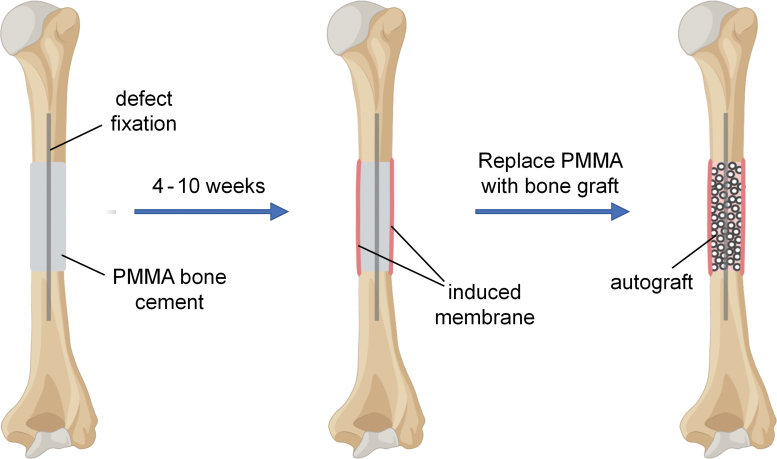
An alternative surgical method for reconstructing bone defects greater than 4–5 cm in length is the induced membrane technique, developed by Masquelet et al. in the 1980s.43 This technique is carried out in at least two stages (Fig. 2). First a poly(methyl methacrylate) cement spacer is placed within the defect. This enables any infection to be treated and allows the surrounding soft tissues to heal, while maintaining the defect cavity clear of unwanted tissue ingrowth. Critically, a vascularized fibrillar membrane forms around the polymer spacer as part of the immune system's foreign body reaction.44 This membrane is known as the “induced membrane.” After a period of 4–10 weeks, contingent on the successful healing of the soft tissues, the polymer spacer is removed and an autograft is implanted in its place within the cavity created by the induced membrane. Depending on the defect size and the available volume of autograft, augmentation with allograft or synthetic bone void fillers may be used.44 The presence of the induced membrane surrounding the bone graft creates a privileged environment for bone regeneration, although the mechanism of action is still unclear and may include growth factor secretion,45–47 high vascular density,45–47 stem cells,47 or serving as a barrier to prevent graft resorption and fibrous tissue invasion.36,48 The induced membrane technique for bone reconstruction works well but is lengthy, taking ∼9 months from start to finish, and potentially longer if complications arise.36,44 Formation of the membrane alone requires at least one surgery and 1–2 months of waiting before bone graft transfer may occur. Another downside is the need for large volumes of autograft, which may not be available and can cause secondary complications such as donor site morbidity.
FIG. 2.
In the induced membrane technique for bone reconstruction, the defect space is filled with a PMMA cement spacer, which induces formation of an enveloping biological membrane. The spacer is then removed, leaving the membrane in place, and autograft is placed in the cavity. PMMA, poly(methyl methacrylate). Color images are available online.
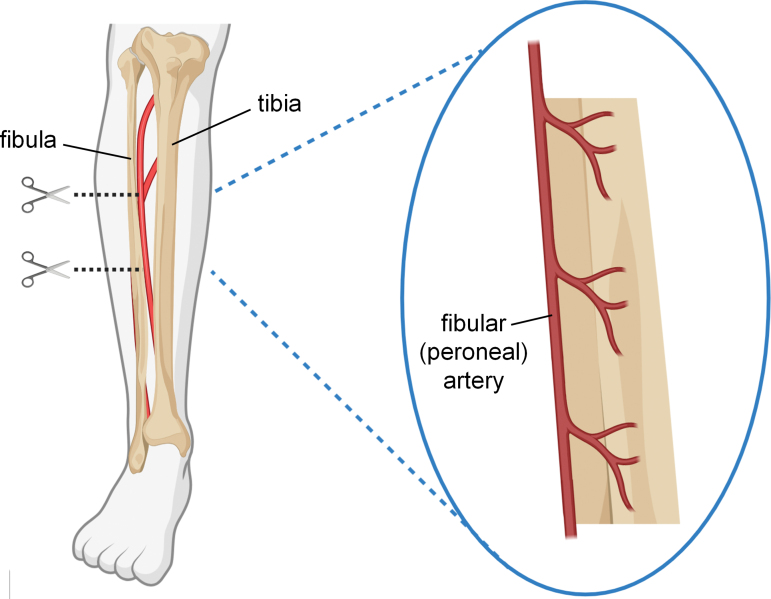
A third technique for reconstructing large-scale bone defects is vascularized cortical bone transfer (Fig. 3). There are several vascularized bone grafts to choose from, with the fibula being most common.7 The intact cortical strength of these grafts provides superior mechanical support during the 6–12 months after implantation,14 but the main advantage of these grafts is that they are harvested intact with their associated vascular beds. Where typical autograft fails to heal major defects owing to slow revascularization and subsequent graft necrosis, the rich network of blood vessels within the intact fibular bone segment is ideal for instantly reestablishing blood flow throughout the graft upon implantation.7 The procedure is challenging though, as it requires expertise not only in orthopedic reconstruction, but microvascular surgery, too, to reattach the graft blood vessels at the recipient site. In general, transplanted small diameter vessels often fail to sustain long-term circulation because of complications that lead to occlusion, however even maintaining blood flow for >1 month can provide sufficient time for new vessel development and spontaneous microvascular anastomosis between the graft and recipient site, enabling graft survival.49 The greater success of large-scale vascularized bone grafts over avascular autograft highlights the necessity of rapid vascularization in bone reconstruction.
FIG. 3.
Intact segments can be harvested from the fibula. The fibula is well furnished with blood by the fibular artery, which supplies arterial branches into the bone and surrounding periosteum. Color images are available online.
TE Bone Grafts
Tissue engineered bone holds promise to reproduce the regenerative benefits of autografts without the drawbacks associated with tissue harvest. However, despite some successes using synthetic bone void fillers to guide bone healing, discussed previously, existing synthetic bone constructs lack the osteoinductive and osteogenic potential of native bone. At present, much research focuses on finding ways to enhance the regenerative capabilities of engineered scaffolds to better serve as replacements for autologous bone. In addition, any construct's success in large-scale defects will be further determined by its ability to rapidly reestablish blood flow throughout the engineered tissue.
The field of TE can be broadly defined as the application of principles from engineering and the life sciences to generate functional biological or biointegrative replacements for native tissues and organs. In regenerative medicine, the aim of TE therapies is to manipulate cells to produce healthy new tissue for defect repair or prevention. To achieve this, much current bone TE research focuses on the development of new stem cell sources, the delivery of chemical signals, and the fabrication of bioactive scaffolds to engineer constructs capable of regenerating functional bone tissue.
Current obstacles to the production of engineered bone include the intricacy needed to mimic the characteristics of native bone and the difficulty of ensuring cell survival throughout large-scale constructs owing to slow revascularization. Reproducing the complexity of living bone presents a formidable fabrication challenge: bone tissue includes intimately integrated minerals, structural biopolymers, growth factors, and cellular components, having vastly divergent mechanical properties. Additive manufacturing techniques capable of printing multiple material types and integrating various deposition modalities show promise to achieve complex integrated constructs with the desired spatial patterning of cells, supportive struts, and signaling molecules.50–52 However, currently the requirements of cytocompatible printing techniques impose a limit on the achievable printing resolution. In addition, as grafts become more complex with multiple cell types, determining the optimal coculture conditions for tissue regeneration becomes increasingly challenging. Fortunately, a TE strategy for regenerative medicine does not need to fully recreate the complex structure of bone, as the goal is to reproducibly guide a successful healing response. As such, it is perhaps not the structure of native bone that must be engineered as much as the processes involved in bone development and healing. Mineralized bone tissue is also not the only potential target: TE approaches to mimic the periosteum53 or the induced membrane54 have also been considered. Similarly, in the guided bone regeneration (GBR) technique, an acellular barrier membrane is applied over a defect site to block the invasion of soft tissue that could inhibit bone growth.55,56 GBR is a common procedure in periodontal bone augmentation and can be performed either with or without simultaneous implantation of a bone graft.
From the perspective of meeting medical standards, off-the-shelf acellular synthetic scaffolds are currently more reproducible than biologic products. And synthetic materials are more readily and economically manufactured at the volumes required for clinical application. Shelf-stable constructs that do not require specialized equipment are also more widely accessible for low-resource communities. These acellular scaffolds rely on the recruitment of local host cells to invade and deposit new tissues, which might be compromised in cases where a patient's bone healing capabilities are limited, such as after radiation or ablation therapy, infection, or tissue degeneration.57 On the contrary, cellularized constructs that require prolonged in vitro culture periods are costly and risk undesirable phenotypic changes that might prohibit clinical translation.58 Constant advances in device fabrication,52,59,60 stem cell biology,61 and in vitro bioreactor technologies62–64 are striving to one day resolve these concerns.
In vivo TE strategies can circumvent the risks associated with prolonged in vitro cell culture. Engineered constructs, with or without cells, may be implanted either directly into the defect site,57,65,66 or at a secondary location where ectopic bone is allowed to develop before transplantation to the defect site.57,67 An advantage of ectopic development is that it enables formation of a vascular network within the engineered construct before defect reconstruction, essentially yielding a vascularized autologous bone graft, which will be discussed in greater detail in a later section. Intraoperative procedures that can carry out cell harvest, rapid incorporation into a TE construct, and subsequent reimplantation can be used to include cells or harvested tissue components within in vivo engineered constructs with minimal ex vivo cell manipulation.
In determining construct design, the tissue engineer has a variety of building blocks to choose from. Cell sources include harvested primary differentiated cells and stem cells,68–70 induced pluripotent stem cells,71 cell aggregates or organoids,72,73 and cells with genetic modification74,75; scaffold materials include synthetic and biologically derived polymers,76,77 metals,78 ceramics,79 and composites77; and a range of native and engineered signaling molecules80 are available to regulate cellular activity. Applying mechanical,81,82 electrical,83,84 and magnetic85,86 forces can provide cells with additional developmental cues to promote bone regeneration.
The success of vascularized fibular autografts in large bone defect reconstruction, discussed previously, demonstrates that immediate blood perfusion through a graft significantly improves graft survival and enables healing of much larger defects than would be possible using avascular grafts. Nearly all cells in the human body are located within 100–200 μm of a blood vessel, a spatial limit imposed by the diffusion limit of oxygen.87 TE constructs need to either include a vascular system capable of being connected to the host system for immediate reperfusion like the vascularized fibular graft or else promote rapid revascularization in situ to ensure graft survival and tissue repair.
Microvascular Engineering
Several strategies have been explored to achieve prevascularization of large TE constructs. Perhaps most straightforward, endothelial cells (ECs) or their precursor cells embedded within 3D scaffolds can self-assemble to form capillary-like networks with a branching morphology similar to that found in native tissues.88,89 Co-culture with vascular mural cells (smooth muscle cells, pericytes, and mesenchymal stem cells) promotes maturation and stabilization of the newly formed vessel networks.89,90 Cell seeding can be carried out using single cells or spheroid aggregates. EC spheroids demonstrate considerable angiogenic potential meaning they can rapidly sprout to form capillary-like structures.91 Several fabrication strategies may be used to organize the microvasculature within constructs to ensure it is sufficiently extensive to supply all encapsulated cells with oxygen and nutrients. These methods include 3D printing,73,92 microchannel pattern molding,93 cell sheet stacking,53,94,95 and reseeding decellularized tissues.96 TE grafts prevascularized with capillary-like networks in vitro depend on fusion of the graft vessels with the host vasculature after implantation, which is a faster process than complete neovascularization through graft invasion by host vessels but slower than surgical anastomosis.97,98
An alternative approach is in vivo prevascularization, where a TE scaffold is implanted in the body, at a secondary implantation site, and the graft is vascularized by angiogenic ingrowth from the surrounding tissues. Scaffolds for in vivo prevascularization may be cellularized99 or acellular,100,101 although growth factor delivery might be necessary to ensure vascular invasion into thicker acellular grafts.98 In vivo vascularization often occurs over the course of several weeks to months, depending on the graft size, after which the graft may be moved to the site of the tissue defect. Connection of the host and graft vasculature may occur by surgical anastomosis or natural inosculation. A small number of case reports detail use of this technique to engineer vascularized autologous bone grafts for mandibular reconstruction in humans, with mixed results.67 Because of the length of time required for vascularization, there is a risk of necrosis at the center of large grafts,98 and relatively low levels of bone formation in ectopic sites can be an issue.102
Regardless of fabrication method, various developmental cues might also be applied to guide EC network formation within constructs such as the scaffold material stiffness and nanostructure and the application of shear stress.91 The delivery of biochemical signaling molecules such as growth factors is an additional powerful director of tissue regeneration if the correct types, doses, and delivery time windows can be determined. Effective growth factor use requires spatial and temporal control of delivery—chemical gradients and sequential release provide important cues to tissue development.100 Advanced biomaterial scaffolds need to be designed to include optimal biochemical, structural, and mechanical cues to their resident cells.
Major Vessel Inclusion
In the body, the vascular system exists as a hierarchical structure with arteries and veins carrying large volumes of blood to tissues and organs quickly and branching into smaller vessels and then capillaries for distribution at the microlevel. Most TE approaches to prevascularization focus on developing microvascular networks, but the inclusion of a large-scale blood conduit through a TE construct can supply greater volumes of blood across the graft immediately upon implantation. Strategies to incorporate major vessels within bone grafts include moving an artery to the defect site during reconstruction,65,103 developing TE constructs at a secondary in vivo location around a vascular bundle104 or within a created arteriovenous loop,105 and designing construct architectures that include an engineered channel or suturable vessel graft.6,51 Small diameter vascular grafts with an inner diameter <6 mm are prone to occlusive failure owing to thrombosis and intimal hyperplasia, leading to low long-term patency rates.106 However, for the purposes of inclusion within a TE bone graft, long-term patency may not be required, as vascularized fibular grafts demonstrate that providing flow for even 1 month may be sufficient to enable vascular ingrowth and remodeling within the graft.49 We propose that incorporation of a synthetic engineered vessel conduit within large-scale bone constructs could complement microvascular prevascularization techniques by supplying a large volume of blood throughout the regenerating defect site immediately upon implantation.
Outlook for the Future
TE approaches may one day offer improvements to current surgical techniques for large bone defect reconstruction. Tissue engineers face a daunting challenge if they seek to replicate the intricate complexity of biological tissues; however, therapies for regenerative medicine may not need to fully reproduce this complexity to guide a successful healing response. Greater understanding of the fundamental biological mechanisms behind bone formation and repair will help inform future regenerative therapies. Concurrently, advancements in tissue engineered construct design and fabrication technologies are required to develop bone scaffolds that can provide the necessary spatial and temporal delivery of developmental cues. Even then, large-scale bone constructs cannot maintain viability without an included vasculature. Engineered bone scaffolds will need to either come with a preexisting blood vessel system or else rapidly revascularize in situ. The challenge of vascularizing large reconstructed tissues is not limited to bone; success in this endeavor could greatly advance the development of a wide range of engineered tissues and organs.
Disclosure Statement
No competing financial interests exist.
Funding Information
This research was funded through the financial support of NIAMS grant R01AR057837, U01AR069395, R01AR072613, R01AR074458, DoD grant W81XWH-20-1-0343, and the Stanford Woods Institute for the Environment.
References
- 1. McKee, M., Addison, W., and Kaartinen, M.. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs 181, 176, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Viguet-Carrin, S., Garnero, P., and Delmas, P.. The role of collagen in bone strength. Osteoporosis Int 17, 319, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Turunen, M.J., Kaspersen, J.D., Olsson, U., et al. . Bone mineral crystal size and organization vary across mature rat bone cortex. J Struct Biol 195, 337, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Ott, S.M. Cortical or trabecular bone: what's the difference? Am J Nephrol 47, 373, 2018. [DOI] [PubMed] [Google Scholar]
- 5. van der Linden, J.C., and Weinans, H.. Effects of microarchitecture on bone strength. Curr Osteoporosis Rep 5, 56, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Mercado-Pagán, Á.E., Stahl, A.M., Shanjani, Y., and Yang, Y.. Vascularization in bone tissue engineering constructs. Ann Biomed Eng 43, 718, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bumbasirevic, M., Stevanovic, M., Bumbasirevic, V., Lesic, A., and Atkinson, H.D.. Free vascularised fibular grafts in orthopaedics. Int Orthop 38, 1277, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grüneboom, A., Hawwari, I., Weidner, D., et al. . A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab 1, 236, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marsell, R., and Einhorn, T.A.. The biology of fracture healing. Injury 42, 551, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitz, J.P., and Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 299, 1986. [PubMed]
- 11. Keating, J., Simpson, A., and Robinson, C.. The management of fractures with bone loss. J Bone Joint Surg Br Vol 87, 142, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Schemitsch, E.H. Size matters: defining critical in bone defect size! J Orthop Trauma 31, S20, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84, 454, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Pape, H.C., Evans, A., and Kobbe, P.. Autologous bone graft: properties and techniques. J Orthop Trauma 24, S36, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin, P., Li, D.J., Auston, D.A., Mir, H.S., Yoon, R.S., and Koval, K.J.. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma 33, 203, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Nauth, A., McKee, M.D., Einhorn, T.A., Watson, J.T., Li, R., and Schemitsch, E.H.. Managing bone defects. J Orthop Trauma 25, 462, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Amini, A.R., Laurencin, C.T., and Nukavarapu, S.P.. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40, 363, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang, H., Yang, L., Yang, X.g., et al. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg 11, 725, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laurencin, C., Khan, Y., and El-Amin, S.F.. Bone graft substitutes. Expert Rev Med Devices 3, 49, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Saikia, K., Bhattacharya, T., Bhuyan, S., Talukdar, D., Saikia, S., and Jitesh, P.. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J Orthop 42, 169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar, Y., Nalini, K., Menon, J., Patro, D.K., and Banerji, B.. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J Clin Diagn Res 7, 2926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hench, L.L. Bioactive glass bone grafts: history and clinical applications. In: Antoniac I.V., ed. Handbook of Bioceramics and Biocomposites. Cham: Springer International Publishing, 2016, pp. 23–33. [Google Scholar]
- 23. Campana, V., Milano, G., Pagano, E., et al. . Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25, 2445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang, Z., Li, X., Tan, Y., Fan, H., and Zhang, X.. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen Biomater 5, 43, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chai, Y.C., Carlier, A., Bolander, J., et al. . Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater 8, 3876, 2012. [DOI] [PubMed] [Google Scholar]
- 26. Schilling, A.F., Linhart, W., Filke, S., et al. . Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials 25, 3963, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Rolvien, T., Barbeck, M., Wenisch, S., Amling, M., and Krause, M.. Cellular mechanisms responsible for success and failure of bone substitute materials. Int J Mol Sci 19, 2893, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang, J., Liu, W., Gauthier, O., et al. . A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater 31, 326, 2016. [DOI] [PubMed] [Google Scholar]
- 29. Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J Appl Biomater Funct Mater 17, 2280800019872594, 2019. [DOI] [PubMed] [Google Scholar]
- 30. Hoop, C.L., Zhu, J., Nunes, A.M., Case, D.A., and Baum, J.. Revealing accessibility of cryptic protein binding sites within the functional collagen fibril. Biomolecules 7, 76, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruyas, A., Lou, F., Stahl, A.M., et al. . Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: influence of composition and porosity. J Mater Res 33, 1948, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mauffrey, C., Barlow, B.T., and Smith, W.. Management of segmental bone defects. J Am Acad Orthop Surg 23, 143, 2015. [DOI] [PubMed] [Google Scholar]
- 33. Hertel, R., Gerber, A., Schlegel, U., Cordey, J., Rüegsegger, P., and Rahn, B.. Cancellous bone graft for skeletal reconstruction muscular versus periosteal bed—preliminary report. Injury 25, SA59, 1994. [DOI] [PubMed] [Google Scholar]
- 34. Wong, T.M., Lau, T.W., Li, X., Fang, C., Yeung, K., and Leung, F.. Masquelet technique for treatment of posttraumatic bone defects. ScientificWorldJournal 2014, 710302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiland, A.J., Phillips, T.W., and Randolph, M.A.. Bone grafts: a radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg 74, 368, 1984. [PubMed] [Google Scholar]
- 36. Masquelet, A.C., and Begue, T.. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 41, 27, 2010. [DOI] [PubMed] [Google Scholar]
- 37. Spiegelberg, B., Parratt, T., Dheerendra, S., Khan, W., Jennings, R., and Marsh, D.. Ilizarov principles of deformity correction. Ann R Coll Surg Engl 92, 101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hariri, F., Chin, S.Y., Rengarajoo, J., Foo, Q.C., Abidin, S.N.N.Z., and Badruddin, A.F.A.. Distraction osteogenesis in oral and craniomaxillofacial reconstructive surgery. In: Yang, H.,ed. Osteogenesis and Bone Regeneration. London, UK: IntechOpen, 2018, pp. 1–19. [Google Scholar]
- 39. Amir, L.R., Becking, A.G., Jovanovic, A., Perdijk, F.B., Everts, V., and Bronckers, A.L.. Vertical distraction osteogenesis in the human mandible: a prospective morphometric study. Clin Oral Implants Res 17, 417, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Iacobellis, C., Berizzi, A., and Aldegheri, R.. Bone transport using the Ilizarov method: a review of complications in 100 consecutive cases. Strategies Trauma Limb Reconstr 5, 17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fürmetz, J., Soo, C., Behrendt, W., et al. . Bone transport for limb reconstruction following severe tibial fractures. Orthop Rev (Pavia) 8, 6384, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rigal, S., Merloz, P., Le Nen, D., Mathevon, H., and Masquelet, A.-C.. Bone transport techniques in posttraumatic bone defects. Orthop Traumatol Surg Res 98, 103, 2012. [DOI] [PubMed] [Google Scholar]
- 43. Masquelet, A., Fitoussi, F., Begue, T., and Muller, G.. Reconstruction of the long bones by the induced membrane and spongy autograft [in French]. Ann Chir Plast Esthet 45, 346, 2000. [PubMed] [Google Scholar]
- 44. Masquelet, A., Kanakaris, N.K., Obert, L., Stafford, P., and Giannoudis, P.V.. Bone repair using the masquelet technique. J Bone Joint Surg Am 101, 1024, 2019. [DOI] [PubMed] [Google Scholar]
- 45. Aho, O.-M., Lehenkari, P., Ristiniemi, J., Lehtonen, S., Risteli, J., and Leskelä, H.-V.. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am 95, 597, 2013. [DOI] [PubMed] [Google Scholar]
- 46. Pelissier, P., Masquelet, A., Bareille, R., Pelissier, S.M., and Amedee, J.. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res 22, 73, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Gruber, H., Ode, G., Hoelscher, G., Ingram, J., Bethea, S., and Bosse, M.. Osteogenic, stem cell and molecular characterisation of the human induced membrane from extremity bone defects. Bone Joint Res 5, 106, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McBride-Gagyi, S., Toth, Z., Kim, D., et al. . Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J Orthop Res 36, 2228, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoon, A.P., and Jones, N.F.. Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: a review of the literature. Microsurgery 36, 604, 2016. [DOI] [PubMed] [Google Scholar]
- 50. Shanjani, Y., Siebert, S.M., Ker, D.F.E., Mercado-Pagan, A., and Yang, Y.P.. Acoustic patterning of growth factor for 3D tissue engineering. Tissue Eng 26, 602, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elomaa, L., and Yang, Y.P.. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng Part B Rev 23, 436, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koons, G.L., Diba, M., and Mikos, A.G.. Materials design for bone-tissue engineering. Nat Rev Mater 5, 584, 2020. [Google Scholar]
- 53. Kang, Y., Ren, L., and Yang, Y.. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl Mater Interfaces 6, 9622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeBaun, M.R., Stahl, A.M., Daoud, A.I., et al. . Preclinical induced membrane model to evaluate synthetic implants for healing critical bone defects without autograft. J Orthop Res 37, 60, 2019. [DOI] [PubMed] [Google Scholar]
- 55. Elgali, I., Omar, O., Dahlin, C., and Thomsen, P.. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci 125, 315, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dimitriou, R., Mataliotakis, G.I., Calori, G.M., and Giannoudis, P.V.. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med 10, 81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCullen, S.D., Chow, A.G., and Stevens, M.M.. In vivo tissue engineering of musculoskeletal tissues. Curr Opin Biotechnol 22, 715, 2011. [DOI] [PubMed] [Google Scholar]
- 58. Webber, M.J., Khan, O.F., Sydlik, S.A., Tang, B.C., and Langer, R.. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng 43, 641, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCormack, A., Highley, C.B., Leslie, N.R., and Melchels, F.P.. 3D Printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol 38, 584, 2020. [DOI] [PubMed] [Google Scholar]
- 60. Ouyang, L., Armstrong, J.P., Salmeron-Sanchez, M., and Stevens, M.M.. Assembling living building blocks to engineer complex tissues. Adv Funct Mater 30, 1909009, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kimbrel, E.A., and Lanza, R.. Next-generation stem cells—ushering in a new era of cell-based therapies. Nat Rev Drug Discov 19, 463, 2020. [DOI] [PubMed] [Google Scholar]
- 62. Lovecchio, J., Pannella, M., Giardino, L., Calzà, L., and Giordano, E.. A dynamic culture platform enhances the efficiency of the 3D HUVEC-based tube formation assay. Biotechnol Bioeng 117, 789, 2020. [DOI] [PubMed] [Google Scholar]
- 63. Hupfeld, J., Gorr, I.H., Schwald, C., et al. . Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng 111, 2290, 2014. [DOI] [PubMed] [Google Scholar]
- 64. Gaspar, D.A., Gomide, V., and Monteiro, F.J.. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2, 167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vidal, L., Kampleitner, C., Krissian, S., et al. . Regeneration of segmental defects in metatarsus of sheep with vascularized and customized 3D-printed calcium phosphate scaffolds. Sci Rep 10, 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwartz, N., and Hicks, B.. Segmental bone defects treated using recombinant human bone morphogenetic protein. J Orthop 3, e2, 2006. [PubMed] [Google Scholar]
- 67. Tatara, A., Wong, M., and Mikos, A.. In vivo bioreactors for mandibular reconstruction. J Dent Res 93, 1196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang, J., Chen, Z., Sun, M., et al. . Characterization and therapeutic applications of mesenchymal stem cells for regenerative medicine. Tissue Cell 64, 101330, 2020. [DOI] [PubMed] [Google Scholar]
- 69. Derkus, B., Okesola, B.O., Barrett, D.W., et al. . Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater 109, 82, 2020. [DOI] [PubMed] [Google Scholar]
- 70. Borciani, G., Montalbano, G., Baldini, N., Cerqueni, G., Vitale-Brovarone, C., and Ciapetti, G.. Co-culture systems of osteoblasts and osteoclasts: simulating in vitro bone remodeling in regenerative approaches. Acta Biomater 108, 22, 2020. [DOI] [PubMed] [Google Scholar]
- 71. Rana, D., Kumar, S., Webster, T.J., and Ramalingam, M.. Impact of induced pluripotent stem cells in bone repair and regeneration. Curr Osteoporos Rep 17, 226, 2019. [DOI] [PubMed] [Google Scholar]
- 72. Hasani-Sadrabadi, M.M., Sarrion, P., Pouraghaei, S., et al. . An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med 12, eaay6853, 2020. [DOI] [PubMed] [Google Scholar]
- 73. Anada, T., Pan, C.-C., Stahl, A.M., et al. . Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci 20, 1096, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nie, W.-B., Zhang, D., and Wang, L.-S.. Growth factor gene-modified mesenchymal stem cells in tissue regeneration. Drug Des Dev Ther 14, 1241, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goodman, S.B., Pajarinen, J., Yao, Z., and Lin, T.D.. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol 7, 230, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Amiryaghoubi, N., Fathi, M., Pesyan, N.N., Samiei, M., Barar, J., and Omidi, Y.. Bioactive polymeric scaffolds for osteogenic repair and bone regenerative medicine. Med Res Rev 40, 1833, 2020. [DOI] [PubMed] [Google Scholar]
- 77. Kashte, S., Jaiswal, A.K., and Kadam, S.. Artificial bone via bone tissue engineering: current scenario and challenges. Tissue Eng Regen Med 14, 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Putra, N., Mirzaali, M., Apachitei, I., Zhou, J., and Zadpoor, A.. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater 109, 1, 2020. [DOI] [PubMed] [Google Scholar]
- 79. Ribas, R.G., Schatkoski, V.M., do Amaral Montanheiro, T.L., et al. . Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: a review. Ceram Int 45, 21051, 2019. [Google Scholar]
- 80. Dang, M., Saunders, L., Niu, X., Fan, Y., and Ma, P.X.. Biomimetic delivery of signals for bone tissue engineering. Bone Res 6, 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McDermott, A.M., Herberg, S., Mason, D.E., et al. . Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci Transl Med 11, eaav7756, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boerckel, J.D., Kolambkar, Y.M., Stevens, H.Y., Lin, A.S., Dupont, K.M., and Guldberg, R.E.. Effects of in vivo mechanical loading on large bone defect regeneration. J Orthop Res 30, 1067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Damaraju, S.M., Shen, Y., Elele, E., et al. . Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 149, 51, 2017. [DOI] [PubMed] [Google Scholar]
- 84. Meng, S., Rouabhia, M., and Zhang, Z.. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 34, 189, 2013. [DOI] [PubMed] [Google Scholar]
- 85. Yun, H.-M., Ahn, S.-J., Park, K.-R., et al. . Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 85, 88, 2016. [DOI] [PubMed] [Google Scholar]
- 86. Fini, M., Cadossi, R., Canè, V., et al. . The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: a morphologic and microstructural in vivo study. J Orthop Res 20, 756, 2002. [DOI] [PubMed] [Google Scholar]
- 87. Carmeliet, P., and Jain, R.K.. Angiogenesis in cancer and other diseases. Nature 407, 249, 2000. [DOI] [PubMed] [Google Scholar]
- 88. Chen, W., Thein-Han, W., Weir, M.D., Chen, Q., and Xu, H.H.. Prevascularization of biofunctional calcium phosphate cement for dental and craniofacial repairs. Dent Mater 30, 535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koike, N., Fukumura, D., Gralla, O., Au, P., Schechner, J.S., and Jain, R.K.. Creation of long-lasting blood vessels. Nature 428, 138, 2004. [DOI] [PubMed] [Google Scholar]
- 90. Kim, J., Chung, M., Kim, S., Jo, D.H., Kim, J.H., and Jeon, N.L.. Engineering of a biomimetic pericyte-covered 3D microvascular network. PLoS One 10, e0133880, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang, G., Mahadik, B., Choi, J.Y., and Fisher, J.P.. Vascularization in tissue engineering: fundamentals and state-of-art. Progr Biomed Eng 2, 012002, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kolesky, D.B., Truby, R.L., Gladman, A.S., Busbee, T.A., Homan, K.A., and Lewis, J.A.. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26, 3124, 2014. [DOI] [PubMed] [Google Scholar]
- 93. Hasan, A., Paul, A., Vrana, N.E., et al. . Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 35, 7308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Asakawa, N., Shimizu, T., Tsuda, Y., et al. . Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials 31, 3903, 2010. [DOI] [PubMed] [Google Scholar]
- 95. Sakaguchi, K., Shimizu, T., and Okano, T.. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release 205, 83, 2015. [DOI] [PubMed] [Google Scholar]
- 96. Wu, Q., Li, Y., Wang, Y., et al. . The effect of heparinized decellularized scaffolds on angiogenic capability. J Biomed Mater Res A 104, 3021, 2016. [DOI] [PubMed] [Google Scholar]
- 97. Tremblay, P.L., Hudon, V., Berthod, F., Germain, L., and Auger, F.A.. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant 5, 1002, 2005. [DOI] [PubMed] [Google Scholar]
- 98. Rademakers, T., Horvath, J.M., van Blitterswijk, C.A., and LaPointe, V.L.. Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J Tissue Eng Regen Med 13, 1815, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zou, D., Zhang, Z., He, J., et al. . Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1α mediated BMSCs. Biomaterials 33, 2097, 2012. [DOI] [PubMed] [Google Scholar]
- 100. Chen, R.R., Silva, E.A., Yuen, W.W., and Mooney, D.J.. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24, 258, 2007. [DOI] [PubMed] [Google Scholar]
- 101. Wagner, E.R., Parry, J., Dadsetan, M., et al. . VEGF-mediated angiogenesis and vascularization of a fumarate-crosslinked polycaprolactone (PCLF) scaffold. Connect Tissue Res 59, 542, 2018. [DOI] [PubMed] [Google Scholar]
- 102. Heliotis, M., Lavery, K., Ripamonti, U., Tsiridis, E., and Di Silvio, L.. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg 35, 265, 2006. [DOI] [PubMed] [Google Scholar]
- 103. Horch, R.E., Beier, J.P., Kneser, U., and Arkudas, A.. Successful human long-term application of in situ bone tissue engineering. J Cell Mol Med 18, 1478, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li, B., Ruan, C., Ma, Y., et al. . Fabrication of vascularized bone flaps with sustained release of recombinant human bone morphogenetic protein-2 and arteriovenous bundle. Tissue Eng Part A 24, 1413, 2018. [DOI] [PubMed] [Google Scholar]
- 105. Weigand, A., Horch, R.E., Boos, A.M., Beier, J.P., and Arkudas, A.. The arteriovenous loop: engineering of axially vascularized tissue. Eur Surg Res 59, 286, 2018. [DOI] [PubMed] [Google Scholar]
- 106. Carrabba, M., and Madeddu, P.. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front Bioeng Biotechnol 6, 41, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]