Abstract
Background
Prehospital management of severe hemorrhage has evolved significantly in Norwegian medical emergency services in the last 10 years. Treatment algorithms for severe bleeding were previously focused on restoration of the blood volume by administration of crystalloids and colloids, but now the national trauma system guidelines recommend early balanced transfusion therapy according to remote damage control resuscitation principles.
Materials and Methods
This survey describes the implementation, utilization, and experience of the use of low titer group O whole blood (LTOWB) and blood components in air ambulance services in Norway. Medical directors from all air ambulance bases in Norway as well as the blood banks that support LTOWB were invited to participate.
Results
Medical directors from all 13 helicopter emergency medical services (HEMS) bases, the 7 search and rescue (SAR) helicopter bases, and the 4 blood banks that support HEMS with LTOWB responded to the survey. All HEMS and SAR helicopter services carry LTOWB or blood components. Four of 20 (20%) HEMS bases have implemented LTOWB. A majority of services (18/20, 90%) have a preference for LTOWB, primarily because LTOWB enables early balanced transfusion and has logistical benefits in time-critical emergencies and during prolonged evacuations.
Conclusion
HEMS services and blood banks report favorable experiences in the implementation and utilization of LTOWB. Prehospital balanced blood transfusion using whole blood is feasible in Norway.
Keywords: Prehospital blood transfusion, Whole blood, Air ambulance, Bleeding, Trauma
Introduction
Prehospital management of severe hemorrhage has evolved significantly in Norwegian medical emergency services in the last 10 years [1, 2, 3]. Treatment algorithms for severe bleeding were previously focused on restoration of the blood volume by administration of crystalloids and colloids, but today, the national trauma system guidelines recommend balanced transfusion therapy as early as possible [4, 5]. In Norway, military-civilian cooperation has been instrumental in facilitating this shift in policy. The initial step was the implementation of freeze-dried plasma (FDP), followed by red blood cell (RBC) concentrates and low titer group O whole blood (LTOWB) at some of the air ambulance bases. This paradigm shift is based on recent scientific progress in remote damage control resuscitation, which states that life-threatening hemorrhage requires immediate intervention and resuscitation with blood and blood products to reduce the impact of hemorrhagic shock, including subsequent coagulopathy and organ failure, to increase survival and reduce morbidity [6, 7, 8, 9, 10]. Evidence from military and civilian practice suggests that early administration of blood may improve survival to the hospital, decrease ongoing transfusion requirements, and decrease mortality [11]. Coagulopathy of trauma is a process that occurs in patients suffering from hemorrhagic shock after major trauma. It has become evident that the duration and severity of shock influence the degree of coagulopathy, the activation of inflammation and overall clinical status when the patient is admitted to the emergency room. Studies have demonstrated that platelet dysfunction and fibrinogen deficiency are of paramount importance in early trauma-induced coagulopathy [12, 13, 14, 15]. An early balanced transfusion strategy addresses both critical oxygen delivery due to the loss of volume, the ability to form clots at bleeding sites and the prevention of endotheliopathy [16, 17, 18, 19].
Current evidence supports the implementation of LTOWB in prehospital services. A major challenge is the limited availability of platelet concentrates, as most institutions utilize room temperature-stored platelets under constant agitation with a maximum holding time of 5–7 days. Therefore, a true balanced component-based transfusion strategy is difficult to achieve in the prehospital environment. In addition, whole blood-based transfusion therapy causes reduced hemodilution due to the relatively reduced amount of additive solution in LTOWB compared to a balanced component transfusion alternative [20]. Furthermore, the simplicity of the logistics involving LTOWB is advantageous because it is easier to prepare and transfuse single units of LTOWB compared to separate bags for plasma, red cells, and platelets during prehospital time-dependent critical emergencies.
The aims of this study were to describe Norwegian prehospital air ambulance blood transfusion programs, to describe the rationale for and the practical aspects of implementation of a prehospital LTOWB program, and to present the experience with LTOWB in Norway from the air ambulance and blood banking perspective.
Materials and Methods
Study Design and Ethics
This was a survey of the implementation, utilization, and experience with the use of blood products in the HEMS and SAR helicopter services in Norway with a focus on our LTOWB program. This survey was conducted and funded by the Department of Immunology and Transfusion Medicine and the Department of Anesthesia and Intensive Care, Haukeland University Hospital (Bergen, Norway). No information on individual patients were collected. The local data protection officer reviewed the survey.
The Norwegian System for Prehospital Critical Care
The Norwegian healthcare system is organized into four different government-funded regional health authorities that administer hospital systems in the regions. Prehospital critical care is the joint responsibility of municipal health services, regional ground ambulance services, the civilian National Air Ambulance Services of Norway, and military and civilian search and rescue (SAR) helicopters. In Norway, the combination of fjords, mountains, and scattered populations often results in long transport times to hospitals. Due to bad weather conditions, communities may also be isolated for days at a time.
The National Air Ambulance Services of Norway are responsible for helicopter emergency medical services (HEMS) and fixed wing air ambulances in Norway. This nationwide service operates 14 helicopters located at 13 bases and 9 fixed wing aircraft located at 7 bases [21] and responds to all types of medical emergencies 24 h a day every day. More than 20,000 patients are supported yearly by helicopter or a rapid response car. Although the primary role of HEMS is to respond to medical and trauma cases, it also handles pediatric and neonatal retrievals, interhospital transfers, and SAR missions. The service is government funded, but the regional health authorities administer the bases. Hence, there are some differences in the medical capabilities and standard operating protocols among the bases. The HEMS are staffed with a prehospital anesthesiologist at the consultant level, a HEMS rescue paramedic, and a pilot. The HEMS conduct missions in almost all weather conditions, including instrument flying conditions or visual flying conditions with night vision capabilities. The national dispatch strategy aims to reach 90% of the population within 45 min after dispatch.
The Norwegian SAR helicopters are largely operated by the Royal Norwegian Air Force. SAR is funded by the Ministry of Justice and Public Security and operates 6 bases along the coastline of Norway. A civilian contractor operates an additional base at Svalbard. The Norwegian SAR helicopter services area of responsibility is extensive and primarily focuses on the vast sea territories bordering Norway and the Arctic regions, but they also respond to ambulance missions when needed. Two pilots, two system operators, a rescue swimmer, and a prehospital anesthesiologist at the consultant level normally operate on SAR helicopters. The civilian SAR helicopter at Svalbard has the same medical capabilities as military SAR helicopters.
The Norwegian Blood Bank Structure
Norwegian blood banks are hospital based, and their activities include blood collection, component production, and storage in addition to in-hospital immunohematology and transfusion services. The blood banks adhere to EU regulations and standards [22]. All hospitals in Norway have their own blood banks, but many of the smaller blood banks do not provide platelet concentrates due to low demand and the risk of waste. Whole blood for transfusion is made available for in-hospital use by some blood banks, whereas the use of whole blood is under discussion in others. Because the blood bank system is decentralized, accessibility of LTOWB to the different air ambulance bases is dependent on local blood bank policies.
Data Collection
The medical directors of the 13 HEMS bases and 7 SAR helicopter bases were contacted by telephone and invited to participate in the survey. The aims of the survey were explained, and the telephone call was followed by an electronic questionnaire sent by email. In the case of missing responses, the medical directors were contacted again by phone. A similar approach was applied to the blood banks supporting HEMS bases with LTOWB.
Three sets of questions were used: one for the ambulance bases that had implemented LTOWB, one for bases that had not implemented LTOWB, and a separate survey for the blood banks that support prehospital LTOWB.
Statistical Analysis
Descriptive statistics were performed using Prism 9 v. 9.1.2 for Mac − GraphPad Software LLC. Results are shown as medians (IQR, min.–max.). No statistical comparisons were performed between the services providing LTOWB and those not providing LTOWB due to the low numbers.
Results
All medical directors from the 13 HEMS and 7 SAR bases and the 4 blood banks that supply LTOWB responded to the surveys, yielding a response rate of 100%. Only one response per service was received.
Prehospital Blood Inventory in Norway
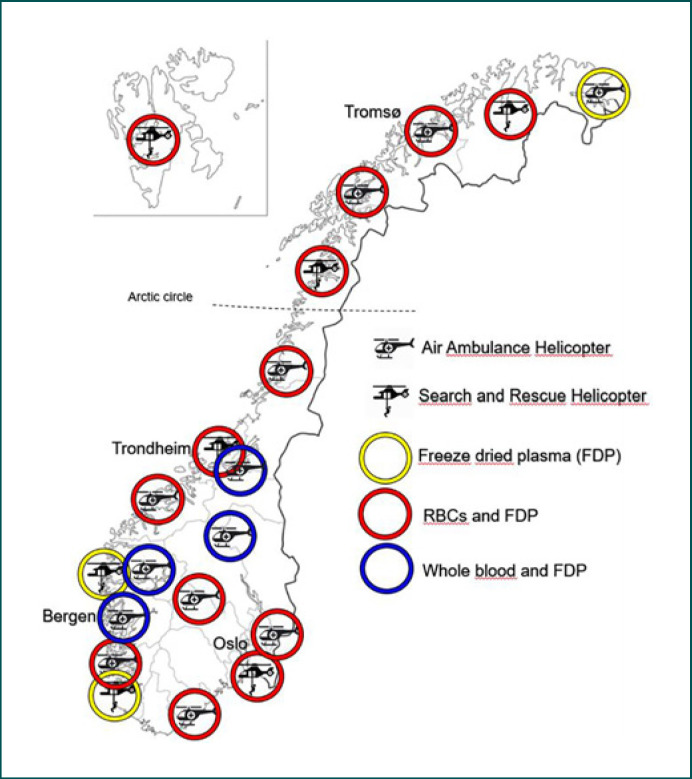
All 20 Norwegian HEMS and SAR services carry dried plasma. In addition, 13/20 (65%) services carry RBC concentrates, and 4/20 (20%) carry LTOWB (Table 1). The variation in the blood inventory is shown in Figure 1. All services carry tranexamic acid in addition to various hemostatic adjuncts, such as fibrinogen concentrate and prothrombin complex concentrate (Table 1). Calcium supplementation is included in the transfusion protocols for a majority of the services. No services carry frozen plasma, platelet concentrates, albumin, or desmopressin. With one exception, all services store the blood and blood products at the base, ready for emergency response. One HEMS service collects RBCs at the hospital but FDP is available at the base. HEMS implemented battery-powered blood warmers in 2021. SAR services have procured blood warmers, but some still await implementation.
Table 1.
Blood and hemostatic adjunct inventory in SAR and HEMS in Norway
| Inventory | Total (n = 20) | Components (n = | 16) WB (n = 4) |
|---|---|---|---|
| RBC | 13 (65%) | 13 (81%) | 0 (0%) |
| FDP | 20 (100%) | 16 (100%) | 4 (100%) |
| LTOWB | 4 (20%) | 0 (0%) | 4 (100%) |
| TXA | 20 (100%) | 16 (100%) | 4 (100%) |
| Calcium chloride/calcium gluconate | 16 (80%) | 13 (81%) | 3 (75%) |
| Fibrinogen concentrate | 6 (30%) | 5 (31%) | 1 (25%) |
| Prothrombin complex concentrate | 2 (10%) | 1 (6%) | 1 (25%) |
RBC, red blood cells; FDP, freeze-dried plasma; LTOWB, low titer group O whole blood; TXA, tranexamic acid.
Fig. 1.
The location and inventory of the HEMS and SAR helicopter bases in Norway.
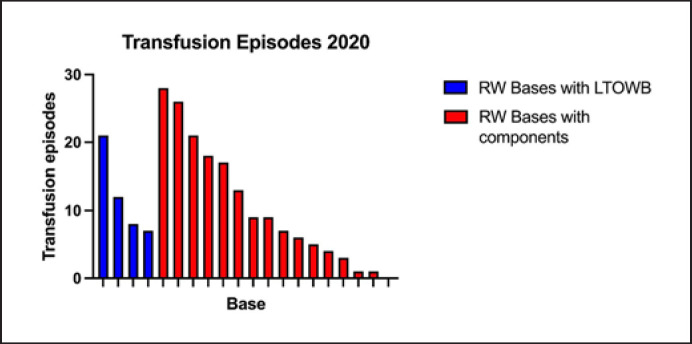
The number of transfusion episodes in 2020 in Norway is shown in Figure 2. In total, there were 216 prehospital transfusion events. The median number of transfusions per base was 8.5 (4.3–18, 0–28). The number of transfusions varied based on location and type of service. SAR services generally have the lowest number of transfusions.
Fig. 2.
The number of transfusion events at the individual HEMS and SAR helicopter base in 2020.
Fourteen of the 16 (88%) services that did not carry LTOWB stated that they wished to implement LTOWB in the future. In total, 18 of the 20 (90%) Norwegian HEMS and SAR services preferred LTOWB in the prehospital service, and only 2 of the 20 (10%) reported that they had not decided on a future strategy concerning LTOWB. None answered that they did not wish LTOWB. One of the services that only carries FDP stated a goal to implement RBCs in the future.
The medical directors who had requested LTOWB from their local blood banks reported the following challenges in the potential implementation of LTOWB: (1) LTOWB is not currently available at their supporting blood bank; (2) there is a need for evidence on efficacy and safety in the implementation of LTOWB in HEMS and SAR services to present when discussing with the blood bank; (3) negative cost-benefit analysis due to few potential transfusions annually, including concerns with respect to the potential for waste; and (4) concerns expressed by the local blood bank with respect to a lack of LTOWB donors.
Prehospital LTOWB in Norway
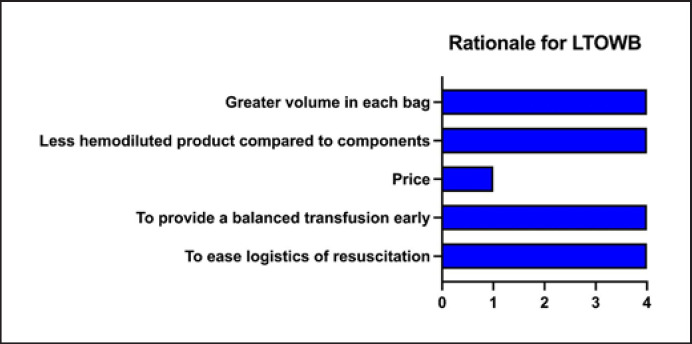
Four of the 20 (20%) services have implemented LTOWB, all of which are HEMS bases. The first service initiated their LTOWB program in 2015, the second in 2017, and the last two in 2019 and 2021. Prior to the implementation of LTOWB, the services had RBCs and FDP available. Figure 3 shows the rationales given for implementing LTOWB. The four (20%) bases all store two units of LTOWB in temperature-monitored thermal containers (Credo Duracube HD with Golden Hour inner container, Pelican BioThermal) within a refrigerator at the base. The storage time is 7 days at two of the bases and 14 days at the other two. There were no reports on breaches in cold chain storage.
Fig. 3.
The rationale for implementation of LTOWB at the four HEMS bases.
Only one base reported that LTOWB was carried on every mission. Others reported that the individual crew decided whether blood was to be included depending on the nature of the emergency dispatch. The reasons for not including blood on every mission were the limited space and weight restraints for the helicopter and the risk of breaking the cold chain if the blood was forgotten in the cabin of the helicopter. One of the directors commented that a powered, fixed cooler suitable for the helicopter would be beneficial.
All bases reported that the doctor on call decides whether to initiate a whole blood transfusion. Three of the four bases stated that they have a supporting transfusion protocol but that the transfusion triggers are broad with the decision relying on the clinical judgment and experience of the crew. No respondents reported any suspected transfusion reactions since the initiation of the LTOWB programs.
When the respondents were asked to subjectively assess the logistical burden of the LTOWB program from a numeric scale (1–10) where 1 is minimal and 10 is severe, two bases reported 2 and two bases reported 1.
Information from Blood Banks Supporting LTOWB for Prehospital Transfusion
All four blood banks supporting HEMS with LTOWB provide Rhesus D-negative whole blood. The definitions of low titers implied are as follows: IgM anti-A and anti-B <250 and IgG anti-A and anti-B <500 for three of the blood banks, whereas the fourth blood bank defines low titers as IgM and IgG anti-A and anti-B <256. Different column/gel agglutination techniques are applied for the measurement of titer. Two blood banks use male donors only, while two include both male and female donors. No additional testing is required for female donors. Three blood banks stated that they had sufficient LTOWB donors, while one stated that they had challenges from time to time with supply.
All blood banks provide LTOWB with citrate phosphate dextrose, which is leukoreduced with a platelet-sparing filter (Terumo Imuflex® WB-SP). If whole blood is used, two of the blood banks resupply the HEMS base with RBCs between the preplanned whole blood exchange dates, while one blood bank answered that they are usually able to substitute one unit of LTOWB. The fourth blood bank uses LTOWB in the hospital massive transfusion packages with a minimum inventory of 8 LTOWB units at any time and is therefore able to resupply HEMS with LTOWB when needed.
LTOWB that is not transfused during the predefined storage time at the HEMS base is returned to the blood bank. Two blood banks utilize LTOWB that is not used at HEMS for in-hospital patients, whereas for the other two blood banks, the units are outdated and discarded. The waste rates were 79.6%, 73.2%, 77.0%, and 26.4% for the four different blood banks, with the lowest waste rate observed at the blood bank that regularly utilizes whole blood for in-hospital massive transfusion packages.
Two blood banks followed every prehospital transfusion with registration in a local quality registry and/or posttransfusion hemolysis panel. Two blood banks did not have follow-up protocols after transfusion. All respondents stated that their overall experience in providing LTOWB for HEMS was favorable.
Discussion
This survey provides an overview of the Norwegian HEMS and SAR helicopter service capabilities, experiences, and future visions for prehospital blood transfusion. There has been a significant shift in strategy since 2012, when no service had blood readily available at the base. Some of the services had an option to pick up blood at the blood bank, but crystalloid and colloid therapy was the backbone of fluid resuscitation. Recent evidence has highlighted the importance of early aggressive hemostatic resuscitation of hemorrhagic shock both in civilian and military settings. Shackelford et al. [23] found that prehospital blood product transfusion in the military setting was associated with increased 24-h and 30-day survival. In a civilian setting, studies of early prehospital transfusion have shown a significant reduction in mortality in patients with bleeding without a significant increase in harm [24, 25]. With a population of only 5.4 million across an area of 385,000 km2, Norway has a demanding topography consisting of mountains and fjords, which may make ground ambulance transport of critical trauma patients challenging. Furthermore, the long distances between Norwegian Level 1 trauma centers (approximately 600 km) support the use of air transport and demand that HEMS use a remote damage control resuscitation approach for critical patients [3].
In Norway, experienced prehospital anesthesiologists at consultant level staff the HEMS and SAR helicopter services. Although most services utilize transfusion protocols with evaluation of common transfusion triggers (mechanism of injury compatible with hemorrhage, blood pressure <100 mm Hg, pulse rate >90 bpm, lactate >4 mmol/L, and impaired mentation), the services emphasize the overall clinical judgment of the doctor on call. The low overall number of transfusion episodes reported in 2020 likely reflects the relatively small trauma population in Norway.
The differences in inventory and strategy at the SAR and HEMS services in Norway are explained by differences in local policies and the availability of LTOWB at the blood banks supporting the different services. However, the national trauma guidelines that state that services should be able to provide an early balanced transfusion strategy have had an impact, as all HEMS and SAR helicopter services now carry LTOWB or blood components.
Civilian-military cooperation has been of instrumental importance for the implementation of LTOWB in Norway [26, 27, 28]. Initial protocols for cold-stored LTOWB were initiated to support the Norwegian Armed Forces with trauma resuscitation in international operations in 2013. This development seems to be consistent with the increasing implementation of cold-stored LTOWB in civilian systems in other countries [29, 30].
Almost all HEMS and SAR services prefer LTOWB in the prehospital service. The predominant reasons seem to be linked to the logistical benefits of LTOWB transfusions for provision of a balanced transfusion in time-dependent critical emergencies where there often is lack of personnel, a goal to reduce on-scene time to a minimum, and constrained working spaces during the flight. This is consistent with experience from the HEMS in London, UK. To mitigate these challenges, they are performing a 2-year feasibility study on the utilization of a combined red cell and plasma component for forward resuscitation to reduce the workload and time spent on the scene [31]. The responders also highlight the ability to provide a balanced transfusion to bleeding patients, which is in accordance with Norwegian national and international guidelines [4, 32]. In-hospital studies suggest that RBCs, plasma, and platelets should be given together in a 1:1:1 ratio [33, 34]. Furthermore, LTOWB may be of benefit since platelets may be more effective if given early [35]. Finally, the respondents emphasize the impact of larger volumes in each bag, further highlighting the importance of reducing the workload during resuscitation.
In a previous study, we evaluated the safety and hemostatic properties of LTOWB stored at the air ambulance base [36]. The study concluded that forward storage of LTOWB is feasible, easy, and safe and that the product quality is within EU regulation standards during storage. Platelet function seems to deteriorate after 8 days of storage but is still clinically useful for up to 21 days [36]. Three of the blood banks that supply HEMS with LTOWB report relatively high waste levels (>70%). In Norway, with a relatively small population and a small number of trauma cases, it seems that hospitals need to accept high waste levels of LTOWB to implement its use in the HEMS system. On the other hand, as demonstrated by one of the respondents, if the blood bank facilitates in-hospital transfusions of LTOWB, waste might be considerably mitigated. The blood bank that utilizes LTOWB for massive transfusion in the hospital rotates unused LTOWB units at the HEMS to the hospital and reports a waste level of approximately 26%.
Increasing numbers of hospitals, especially in the USA, have included LTOWB in their in-house emergency massive transfusion protocols. Data suggest that transfusion of uncrossmatched LTOWB for catastrophic hemorrhage in the hospital is safe and that there is no significant difference in survival rates between nongroup O patients receiving incompatible plasma and group O patients receiving compatible plasma [37, 38, 39, 40]. The available literature supports the feasibility and safety of LTOWB used in the treatment of civilian patients, but the evidence regarding clinical outcomes, particularly with direct comparison to balanced-ratio transfusions with components, is limited [29, 41]. Hence, with respect to hard endpoints, there is not enough evidence to support or refute the clinical superiority of LTOWB compared to component therapy. However, there are three ongoing randomized controlled trials for early trauma resuscitation with whole blood for severely bleeding patients [42, 43, 44].
Respondents to the survey indicated several challenges in the potential implementation of LTOWB. Most commonly, there was a lack of availability of LTOWB at the local blood bank and a lack of evidence for efficacy and safety when arguing their case to blood providers. The results from ongoing studies and clinical experience from current users are essential for the future use of LTOWB in prehospital emergency care services.
The strengths of this study include the high response rate from survey participants (100%) and the level of detail in the information given. Limitations include a potential uncertainty regarding the exact number of transfusion episodes given from 7 of the 20 bases due to underreporting of data in their electronic patient journal systems.
In this survey, we described the use of LTOWB in prehospital air ambulance services in Norway. HEMS and blood banks report favorable experiences in the implementation and utilization of LTOWB. We conclude that LTOWB is feasible both from the air ambulance and blood banking perspectives and that LTOWB is preferred by a majority of the HEMS and SAR bases. Our findings support further efforts to enable the implementation of prehospital LTOWB programs.
Statement of Ethics
This was a survey on the implementation, utilization, and experience with the use of blood products in the HEMS and SAR helicopter services in Norway with a focus on our LTOWB program. The survey was conducted and funded by the Department of Immunology and Transfusion Medicine and the Department of Anesthesia and Intensive Care, Haukeland University Hospital (Bergen, Norway). No information on individual patients was collected. The local data protection officer reviewed the survey.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Transfusion Medicine and Hemotherapy. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Norwegian Armed Forces Medical Services.
Funding Sources
This study was funded by the Department of Immunology and Transfusion Medicine and the Department of Anesthesia and Intensive Care at Haukeland University Hospital, Bergen, Norway.
Author Contributions
C.K.B. and T.O.A. designed the survey, performed the data collection, and drafted the manuscript. G.S. contributed in the design of the survey. G.S., T.H., and G.A.S. revised the manuscript critically and contributed to the writing of the manuscript. All authors approved the final version of the manuscript for publication.
Acknowledgments
The authors thank the medical directors at the Norwegian HEMS and SAR helicopter bases for participating in the survey. Likewise, we thank the blood banks at Innlandet Hospital, Haukeland University Hospital, St. Olavs Hospital, and Førde Hospital for participating in the survey.
References
- 1.Espinosa A, Dybvik B, Medby C, Vangberg G. Implementation of a protocol for prehospital transfusion of low-titer, leukocyte-depleted whole blood for civilian bleeding patients. Transfus Apher Sci. 2019;58:212–5. doi: 10.1016/j.transci.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Sunde GA, Vikenes B, Strandenes G, Flo KC, Hervig TA, Kristoffersen EK, et al. Freeze dried plasma and fresh red blood cells for civilian prehospital hemorrhagic shock resuscitation. J Trauma Acute Care Surg. 2015;78:S26–30. doi: 10.1097/TA.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 3.Zielinski MD, Stubbs JR, Berns KS, Glassberg E, Murdock AD, Shinar E, et al. Prehospital blood transfusion programs: Capabilities and lessons learned. J Trauma Acute Care Surg. 2017;82:S70–S8. doi: 10.1097/TA.0000000000001427. [DOI] [PubMed] [Google Scholar]
- 4.Traumeplan Norge [monograph on the internet] Available from: https://traumeplan.no/
- 5.Cantle PM, Cotton BA. Balanced resuscitation in trauma management. Surg Clin North Am. 2017;97:999–1014. doi: 10.1016/j.suc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Butler FK, Holcomb JB, Schreiber MA, Kotwal RS, Jenkins DA, Champion HR, et al. Fluid resuscitation for hemorrhagic shock in tactical combat casualty care: TCCC guidelines change 14-01 − 2 June 2014. J Spec Oper Med. 2014;14:13–38. doi: 10.55460/DPOC-JWIY. [DOI] [PubMed] [Google Scholar]
- 7.Cap AP, Pidcoke HF, Spinella P, Strandenes G, Borgman MA, Schreiber M, et al. Damage Control Resuscitation. Mil Med. 2018;183:36–43. doi: 10.1093/milmed/usy112. [DOI] [PubMed] [Google Scholar]
- 8.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–6. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 10.Spinella PC, Doctor A. Role of transfused red blood cells for shock and coagulopathy within remote damage control resuscitation. Shock. 2014;41((Suppl 1)):30–4. doi: 10.1097/SHK.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 11.Powell EK, Hinckley WR, Gottula A, Hart KW, Lindsell CJ, McMullan JT. Shorter times to packed red blood cell transfusion are associated with decreased risk of death in traumatically injured patients. J Trauma Acute Care Surg. 2016;81:458–62. doi: 10.1097/TA.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 12.Brown LM, Call MS, Margaret Knudson M, Cohen MJ, Holcomb JB, Holcomb JB, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71:S337–42. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frith D, Brohi K. The pathophysiology of trauma-induced coagulopathy. Curr Opin Crit Care. 2012;18:631–6. doi: 10.1097/MCC.0b013e3283599ab9. [DOI] [PubMed] [Google Scholar]
- 14.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73:13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey MT, Fabian TC, Shahan CP, Sharpe JP, Mabry SE, Weinberg JA, et al. A prospective study of platelet function in trauma patients. J Trauma Acute Care Surg. 2016;80:726–3. doi: 10.1097/TA.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 16.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 17.Brohi K, Cole E, Hoffman K. Improving outcomes in the early phases after major trauma. Curr Opin Crit Care. 2011;17:515–9. doi: 10.1097/MCC.0b013e32834a9353. [DOI] [PubMed] [Google Scholar]
- 18.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 19.Bjerkvig CK, Strandenes G, Eliassen HS, Spinella PC, Fosse TK, Cap AP, et al. Blood failure” time to view blood as an organ: how oxygen debt contributes to blood failure and its implications for remote damage control resuscitation. Transfusion. 2016;56((Suppl 2)):S182–9. doi: 10.1111/trf.13500. [DOI] [PubMed] [Google Scholar]
- 20.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–31. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 21.Norwegian Air Ambulance [monograph on the internet] Available from: http://www.luftambulanse.no/about-national-air-ambulance-services-norway.
- 22.The Guide to the preparation, use and quality assurance of blood components [monograph on the internet] 2017. Available from: https://www.edqm.eu/en/blood-transfusion-guide.
- 23.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318:1581–91. doi: 10.1001/jama.2017.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pusateri AE, Moore EE, Moore HB, Le TD, Guyette FX, Chapman MP, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2020;155:e195085. doi: 10.1001/jamasurg.2019.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 26.Gerhardt RT, Strandenes G, Cap AP, Rentas FJ, Glassberg E, Mott J, et al. Remote damage control resuscitation and the Solstrand Conference: defining the need, the language, and a way forward. Transfusion. 2013;53((Suppl 1)):9S–16S. doi: 10.1111/trf.12030. [DOI] [PubMed] [Google Scholar]
- 27.Spinella PC, Strandenes G, Rein EB, Seghatchian J, Hervig T. Symposium on fresh whole blood for severe hemorrhagic shock: from in-hospital to far forward resuscitations. Transfus Apher Sci. 2012;46:113–7. doi: 10.1016/j.transci.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. 2014;41((Suppl 1)):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson B, Murphy C, Fontaine MJ. Current state of whole blood transfusion for civilian trauma resuscitation. Transfusion. 2020;60((Suppl 3)):S45–52. doi: 10.1111/trf.15703. [DOI] [PubMed] [Google Scholar]
- 30.Yazer MH, Spinella PC, Anto V, Dunbar NM. Survey of group A plasma and low-titer group O whole blood use in trauma resuscitation at adult civilian level 1 trauma centers in the US. Transfusion. 2021;61((6)):1757–63. doi: 10.1111/trf.16394. [DOI] [PubMed] [Google Scholar]
- 31.Transfusion Strategies in the Pre-Hospital Setting: Evaluating the Logistical Benefits of Pre-Hospital Whole Blood Transfusion, and a National Survey of Pre-Hospital Blood Transfusion [monograph on the internet] Available from: https://www.researchgate.net/publication/348614500_Transfusion_Strategies_in_the_Pre-Hospital_Setting_Evaluating_the_Logistical_Benefits_of_Pre-Hospital_Whole_Blood_Transfusion_and_a_National_Survey_of_Pre-Hospital_Blood_Transfusion/fulltext/600787b1a6fdccdcb8689b34/Transfusion-Strategies-in-the-Pre-Hospital-Setting-Evaluating-the-Logistical-Benefits-of-Pre-Hospital-Whole-Blood-Transfusion-and-a-National-Survey-of-Pre-Hospital-Blood-Transfusion.pdf.
- 32.The Joint Trauma System Tactical Combat Casualty Care (TCCC) Guidelines. 2020. [cited 13 Jul 2021]. Available from: https://www.deployedmedicine.com/content/40.
- 33.Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170:788–803. doi: 10.1111/bjh.13580. [DOI] [PubMed] [Google Scholar]
- 34.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, Group PS et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardenas JC, Zhang X, Fox EE, Cotton BA, Hess JR, Schreiber MA, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2:1696–704. doi: 10.1182/bloodadvances.2018017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjerkvig C, Sivertsen J, Braathen H, Lunde THF, Strandenes G, Assmus J, et al. Cold-stored whole blood in a Norwegian emergency helicopter service: an observational study on storage conditions and product quality. Transfusion. 2020;60:1544–51. doi: 10.1111/trf.15802. [DOI] [PubMed] [Google Scholar]
- 37.Yazer MH, Spinella PC. An international survey on the use of low titer group O whole blood for the resuscitation of civilian trauma patients in 2020. Transfusion. 2020;60((Suppl 3)):S176–S9. doi: 10.1111/trf.15601. [DOI] [PubMed] [Google Scholar]
- 38.Seheult JN, Anto V, Alarcon LH, Sperry JL, Triulzi DJ, Yazer MH. Clinical outcomes among low-titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018;58:1838–45. doi: 10.1111/trf.14779. [DOI] [PubMed] [Google Scholar]
- 39.Yazer MH, Freeman A, Harrold IM, Anto V, Neal MD, Triulzi DJ, et al. Injured recipients of low-titer group O whole blood have similar clinical outcomes compared to recipients of conventional component therapy: A single-center, retrospective study. Transfusion. 2021;61((6)):1710–20. doi: 10.1111/trf.16390. [DOI] [PubMed] [Google Scholar]
- 40.Hagen KG, Strandenes G, Kristoffersen E, Braathen H, Sivertsen J, Bjerkvig CK, et al. A whole blood based resuscitation strategy in civilian medical services; experience from a Norwegian hospital in the period 2017–2020. Transfusion. 2021. in press. [DOI] [PubMed]
- 41.Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81:21–6. doi: 10.1097/TA.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 42.Sperry J. Pragmatic Prehospital Group O Whole Blood Early Resuscitation (PPOWER) Trial: A Prospective, Interventional, Randomized, 3 Year, Pilot Clinical trial. Bethesda, MD: National Heart, Lung, and Blood Institute (NHLBI); 2018. [Google Scholar]
- 43.Sperry JLEB, Buck M, Silfies L. Shock, whole blood and assessment of TBI (SWAT) Pittsburgh, PA: University of Pittsburgh; 2018. [Google Scholar]
- 44.Martinaud C, Tiberghien P, Bégué S, Sailliol A, Gross S, Pouget T, et al. Rational and design of the T-STORHM Study: A prospective randomized trial comparing fresh whole blood to blood components for acutely bleeding trauma patients. Transfus Clin Biol. 2019;26:198–201. doi: 10.1016/j.tracli.2019.09.004. [DOI] [PubMed] [Google Scholar]