Abstract
Background
Type 1 diabetes (T1D) is an autoimmune disease characterized by impaired immune tolerance to β-cell antigens and progressive destruction of insulin-producing β-cells. Animal models have provided valuable insights for understanding the etiology and pathogenesis of this disease, but they fall short of reflecting the extensive heterogeneity of the disease in humans, which is contributed by various combinations of risk gene alleles and unique environmental factors. Collectively, these factors have been used to define subgroups of patients, termed endotypes, with distinct predominating disease characteristics.
Scope of review
Here, we review the gaps filled by these models in understanding the intricate involvement and regulation of the immune system in human T1D pathogenesis. We describe the various models developed so far and the scientific questions that have been addressed using them. Finally, we discuss the limitations of these models, primarily ascribed to hosting a human immune system (HIS) in a xenogeneic recipient, and what remains to be done to improve their physiological relevance.
Major conclusions
To understand the role of genetic and environmental factors or evaluate immune-modifying therapies in humans, it is critical to develop and apply models in which human cells can be manipulated and their functions studied under conditions that recapitulate as closely as possible the physiological conditions of the human body. While microphysiological systems and living tissue slices provide some of these conditions, HIS mice enable more extensive analyses using in vivo systems.
Abbreviations: APC, antigen-presenting cell; BCR, B cell receptor; DC, dendritic cell; DP, double positive; HC, healthy control; HIS, human immune system; HLA, human leukocyte antigen; hPSC, human pluripotent stem cell; HSC, hematopoietic stem cell; iPSC, induced pluripotent stem cell; MHC, major histocompatibility; MPS, microphysiological system; mTEC, medullary thymic epithelial cell; NK, natural killer; NOD, non-obese diabetic; PBMC, peripheral blood mononuclear cell; PI, personalized immune; RBC, red blood cell; SNP, single nucleotide polymorphism; SP, single positive; TCR, T cell receptor; TOC, thymic organ culture; Treg, regulatory T cell; T1D, Type 1 diabetes; xGvHD, xenogeneic graft-versus-host disease
Keywords: Type 1 diabetes, Disease modeling, Autoimmunity, Beta cell destruction, In vitro models, Humanized mice
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease mediated by autoreactive T cells targeting β-cell antigens and resulting from defective immune processes. Genetic polymorphisms contribute substantially, mainly affecting immune processes, leading to loss of tolerance and the emergence of autoreactive responses [1]. The greatest risk to developing T1D is conferred by the human leukocyte antigen (HLA) haplotype, which influences antigen presentation to T cells, and additional polymorphisms at other gene loci increase that risk. The manifestation of these genetic predispositions is modulated by environmental factors that have contributed to an overall increase in T1D disease incidence [2]. A large proportion of T1D individuals become overtly diabetic at a young age from a rapidly progressing disease, while others have a slow progression with onset during adulthood. The T1D patient population is heterogeneous, and even people with similar age and genetic risk factors may be exposed to different environments and potential triggers, leading to different disease characteristics and immune profiles and enabling their stratification as “endotypes” [3,4]. Understanding how these combined factors influence immune processes, the development of disease and responsiveness to treatment requires personalized models. In this section, we first introduce the immune processes that are likely involved and that need to be modeled and then explain why the in vitro and in vivo personalized models described in this review are needed.
1.1. Defective immune processes leading to autoimmunity and T1D
1.1.1. Central tolerance
Autoreactive T-cell receptor (TCR) and B-cell receptors (BCR) are generated by random genomic recombination processes theoretically producing billions of unique receptors capable of recognizing a wide array of antigens, including self-antigens [5]. T cells acquire their TCR during the CD4-CD8 double-positive (DP) development stage in the thymic cortex. Negative selection is the process upon which the recognition of the antigen with sufficiently high avidity leads to death by apoptosis. This process may already occur during the DP stage in the cortex, but most of the exposure to self-antigens takes place at the corticomedullary junction, which is rich in dendritic cells (DCs), and in the thymic medulla as the T cells become single positive (SP) for CD4 or CD8. Deletion results both from the presentation of ubiquitous/hematopoietic cell antigens by DCs and from the presentation of a broad array of peripheral tissue self-antigens produced by medullary thymic epithelial cells (mTECs) whose expression is enabled by the function of transcriptional regulators, such as AIRE and FEZF2 [6] and that may also be presented by DCs. This process purges a large fraction of autoreactive cells from the developing T-cell pool. Human mTECs express several key β-cell antigens, including insulin and islet antigen 2 (IA-2) [7] but do not appear to express others, such as GAD65 [7] or ZNT8 [8]. However, self-antigens that are not ectopically expressed in the thymus might possibly be presented by peripheral DCs following acquisition in the periphery and migration to the thymus [9]. Reduced thymic expression of insulin is thought to be associated with polymorphism at the INS gene promoter [10,11] and this may contribute to the enhanced escape of insulin-reactive T cells. Diabetogenic T cells (autoreactive T cells causing the destruction of β-cells) that are found in the periphery may either originate from the defective negative selection (if their antigens are normally presented in the thymus) or may never undergo negative selection (if their antigens are not presented in the thymus), in which case, peripheral tolerance is critical to prevent their unwanted reactivity.
1.1.2. Peripheral tolerance
Presentation of self-antigens to CD4+ T cells in the thymus can also result in their selection as Foxp3+ regulatory T cells (Tregs) in lieu of apoptosis. These suppressive Tregs circulate throughout the body and operate at sites where their antigen is presented. For example, if an antigen-presenting cell (APC) presents multiple β-cell antigens in the pancreatic lymph nodes and Tregs recognize one of them, they can suppress the activation of any T cell recognizing any antigen presented by that APC. Tregs function by stripping APCs of their immunogenic potential, depleting the local environment of T-cell growth factors (IL-2), and/or causing the release of immunosuppressive molecules (IL-10, IL-35, TGF-β, adenosine) [12]. Patients and mice with FOXP3 or IL2RA mutations that lead to defective Tregs frequently develop T1D [13], and it appears that Tregs are functionally impaired in a large proportion of T1D patients [14,15], but the extent to which these defects contribute to the disease is not clear. Chronic peripheral antigen exposure may also cause autoreactive T cells to be deleted or, alternatively, to become functionally inactive due to anergy or exhaustion. The phenotype and fate of these T cells likely depend on the type of APC involved in chronic antigen presentation and/or the site of exposure, but little is known in humans.
1.1.3. B cells and autoantibodies
B cells develop in the bone marrow, and similar to T cells, some may randomly acquire an autoreactive BCR. Upon encountering their antigen during development, these B cells may be deleted or adopt an alternative BCR. In the periphery, continuous recognition of self-antigen causes them to become anergic. In T1D, autoreactive B cells likely receive help from activated diabetogenic CD4+ T cells and produce autoantibodies, most prominently against insulin, GAD65, IA-2, and ZNT8 [16]. While not thought to be pathogenic, these autoantibodies are useful as biomarkers to estimate the risk and imminence of disease onset and to obtain an idea of the patient's immune profile (based on the antigens targeted) [17]. Little is known about the contribution of autoreactive B cells in human T1D; insulin-reactive B cells lose their anergic phenotype [18], and B-cell depletion treatment can significantly delay the onset of disease [19]. Defects in the negative selection of developing B cells have been identified in T1D [20].
1.1.4. Antigen-presenting cells
There are a wide variety of APC types involved in presenting β-cell antigens in the thymus, bone marrow, and peripheral lymphoid tissues, each equipped with its unique set of signals that will dictate the outcome of the T-cell response. Presentation of self-antigens, when not performed by tolerogenic APCs, can lead to autoreactive T-cell stimulation, unless Tregs are simultaneously engaged. However, in addition to impaired Treg functions, there are numerous examples of human immune populations (DCs, monocytes, macrophages) exhibiting abnormal/excessive activation and/or hindered tolerogenic signals in the context of T1D [21]. Most observations on human APCs are phenotypic, and how these translate into functional impairments has been difficult to assess thus far.
1.1.5. Tissue compartmentalization and cell trafficking
Finally, the processes of tolerance and pathogenesis require the trafficking of the above immune populations between the immune compartments within tissues (e.g. from the thymic cortex to the medulla) or between tissues (e.g. migration of DCs from inflamed tissue to draining lymph nodes or of activated T cells from lymph nodes to the target tissue). These processes have been the most difficult to study for human immune cells because they cannot easily be recapitulated using in vitro systems.
1.2. Animal models and their limitations
The nonobese diabetic (NOD) mouse model has been the most widely used T1D model, and unlike many other autoimmune disease models [22,23], the disease is spontaneous. Importantly, disease risk is associated with numerous gene polymorphisms, many of them also found in T1D patients (MHC-II, PTPN22, PTPN2, CTLA4, IL10, CTSH, CD226, IL2, RGS1, TAGAP). The disease develops with high incidence in females, with an age of onset typically starting around 12 weeks of age but variable, as in humans, with mice developing disease much later [24,25]. The lower incidence in males is not reflected in the human T1D patient population, but it is worth noting that among human children, males have a significantly slower progression of disease than females [26]. NOD mice develop extensive islet immune infiltrates, consisting of the same immune populations as humans, although the latter have substantially more CD8+ T cells than CD4+ T cells. NOD mice produce autoantibodies only against insulin, which is known to be the driver antigen in this model [27]. B cells are also required for disease to develop in NOD mice [28], and the successful prevention of diabetes by depletion of B cells in NOD mice [29,30] was consistent with the significant effect of B-cell depletion in T1D patients [19]. The NOD model has been useful to document the pathogenesis of the disease and how it is influenced by candidate genes (using knockout and transgenic mice) and environmental factors including diet, microbiome, and infections. The NOD mouse also enabled the identification of a vast array of β-cell antigens targeted by T cells, most of which were later confirmed in T1D patients, and the isolation of β-cell antigen-reactive T- and B-cell clones, some of which were used to produce mice transgenic for their TCRs (e.g. BDC2.5, NY8.3) [31,32] or BCRs (e.g. VH125) [33]. These TCR-transgenic mice have been commonly used by the T1D research community, as they greatly facilitated the tracking and phenotyping of antigen-specific T- and B-cell clones that were otherwise very rare. Using adoptive transfer models, the trafficking of these lymphocytes between tissues can be easily assessed. The NOD.SCID mouse has been another popular model, which rapidly developed T1D upon the transfer of splenocytes from diabetic NOD mice or some of the aforementioned TCR-transgenic T cells [34,35].
Other animal models have been sparsely used because they involve larger animals that are costlier to maintain, more difficult to genetically modify, and for which research reagents are more limited. The BioBreeding rat develops T1D (and thyroiditis) spontaneously with high incidence. Genetic studies in this model revealed risk-conferring polymorphisms at several gene loci also implicated in humans (MHC-II, INS, PTPN22, PTPN2, IL27, SKAP2, and IL7R) [36]. The LEW.1WR1 rat strain also develops T1D, but at a low incidence, and it is susceptible to other autoimmune diseases [37]. Both rat strains develop T1D following environmental perturbation or infection with specific viruses [[37], [38], [39]]. Finally, T1D occurs in some breeds of dogs at a prevalence similar to humans, which allows the study of this disease in larger animals [40]. The pathology resembles that of human patients more closely, making the canine model valuable, particularly if inbred lines can be established for late preclinical studies.
The existing animal models have provided insightful knowledge on the contribution of specific genes and environmental factors on the initiation, progression, and severity of autoimmune diabetes, particularly on the development and function of β-cell antigen-reactive lymphocytes. However, the heterogeneity of the human disease is such that, even collectively, these inbred animal models, which represent only a single genotype, are not able to serve as reliable models for the entire T1D patient population. This is best evidenced by the fact that most disease-modifying therapies that prevented or reverted disease in NOD mice and other animal models were generally not successful when tested in the general human T1D populations, although some individuals have shown responsiveness. Moreover, significant differences in ontology, phenotype, and function exist between human immune populations and their rodent counterparts. Thus, modeling the disease with human cells is necessary to understand the complexity of the human disease and more reliably predict the response of patients to immunotherapy.
1.3. Studies using human tissues and their limitations
Peripheral blood has been widely used as a source of human immune cells from many T1D patients of all ages and backgrounds, and a variety of phenotypic, transcriptomic and functional alterations have been reported in T cells [15,41], B cells [42], NK cells [43], and monocytes/APCs [21]. These analyses have been useful to identify “signatures” and biomarkers of disease progression and responsiveness to therapy [44,45]. Blood samples have enabled longitudinal and retrospective studies but provided a narrow spatial window into the pathology of T1D. Moreover, the relevance of circulating antigen-specific T cells has sometimes been questioned, as these T cells may differ in specificity and phenotype from the most relevant (diabetogenic) T cells found in islets [8]. However, many β-cell antigen-specific T-cell clones first identified from the blood of T1D patients have demonstrated meaningful reactivity [[46], [47], [48], [49]]. Thus, while patient blood samples may potentially misrepresent processes taking place around islets, they remain the most abundant and accessible source of cells and circulating molecules to study the disease on a large scale across the heterogeneous patient population. The mechanisms underlying these alterations affecting the immune cells are still being investigated for the most part, often requiring more sophisticated and/or reductionist models to simplify the biology for clearer answers.
Over the last 15 years, tissue procurement programs, most prominently the Network for Pancreatic Organ Donors with Diabetes and the Human Pancreas Analysis Program complemented with existing collections from patients, have helped widen the spatial window by facilitating the acquisition and study of other tissues of relevance, primarily pancreas, islets, spleen, pancreatic lymph nodes, and other lymphoid tissues from deceased T1D patients and control donors [[50], [51], [52]]. These studies have contributed informative snapshots into the pathology of T1D and insights into how the disease manifests itself across various tissues, helping us establish important hallmarks of human T1D. Among them, lobular insulitis distribution across the whole pancreas with predominant CD8+ T-cell presence [[53], [54], [55]], reduced pancreatic weight [56], increased B-cell infiltration in younger T1D donors [57], and evidence of possible viral infections in many cases [58,59] are features that were not expected based on the NOD mouse model. Importantly, these tissues enabled not only the phenotypical characterization of the various immune cell populations that infiltrate islets and the surrounding exocrine tissue during T1D [53,60,61] but also the isolation of antigen-specific T-cell clones that infiltrate islets [[62], [63], [64]]. Ex vivo testing of such clones, combined with peptidome studies [52,65,66], helps us decipher the T-cell epitopes most commonly targeted [67] and the sequence of these TCRs.
Comparison of multiple tissues from the same donors has been useful to assess the distribution of relevant immune-cell populations across these tissues, but the lack of longitudinal analysis precludes inferences about migration and redistribution between tissues over time. This cannot be done by analyzing multiple donors across ages, because the disease progresses at a different rate in different individuals. Cells and tissues from a particular T1D donor come in limited amounts and are requested by many investigators. In addition, issues of cell recovery and viability inherent to the logistics of tissue distribution (drugs administered to the donor, transportation, cryopreservation) can potentially confound the results. Important disease-relevant information can be obtained from these donors (e.g., autoantibodies, genotype of risk alleles, including MHC haplotype), which can be correlated with specific immune phenotypes. However, understanding the contribution of specific genes, for example, has been challenging because of the limited quantity and quality of the tissues to perform functional assays.
1.4. The need for models of human T1D
The succession of events that lead to T1D remain poorly understood. Failure of central and/or peripheral tolerance, diabetogenic T-cell activation and migration, antigen presentation by permissive versus protective MHC molecules, and the effects of immune cells and their secreted products on β-cells are all key processes that need to be recapitulated using adequate models. Doing so in the context of individual patients or groups of patients (endotypes) is critical to understand the heterogeneity of the disease, evident in the rate of disease progression and in responsiveness to immunotherapy. The immense progress made in recent years in the generation of immune cells, tissue cells, and organoids from a common induced pluripotent stem cell (iPSC) has paved the way to creating individualized models. Assessing the role of specific gene polymorphisms in the disease process and responsiveness to therapy may require a large iPSC collection from patients who typically carry multiple combinations of risk alleles; however, this may now be facilitated by performing loss-of-function or gain-of-function mutations in iPSCs. Modeling the actual disease processes requires approaches that combine the appropriate cell types, context, and environment.
Models that recapitulate the complex human in vivo environment are needed to obtain accurate biological and pathological insights. To this end, in vitro models using microphysiological systems (MPS, aka ‘tissue-on-a-chip’) have improved significantly, adding endothelial or epithelial layers between the compartments, adjustable cell flow, and controlled environment (temperature, oxygenation, etc.). On the other hand, human immune system (HIS) mice provide an environment with some degree of physiological compartmentalization of immune cells and normal blood circulation. Both types of models allow the cells of interest to be visualized and/or sampled over time for analysis, which is critical to evaluate the dynamics of the immune processes outside of peripheral blood. Both in vitro and in vivo models allow genetic and pharmacological manipulation of biological processes, which would not otherwise be possible in human individuals. HIS mice are better suited to study the migration of cells between tissues (or between compartments within tissues), although there remains a species barrier that prevent some cell–cell interactions from taking place. Models in which the frequency of antigen-reactive T or B cells can be increased are also needed to facilitate the study of their development, trafficking, and reactivity to naturally present or administered autoantigens. Finally, models are needed to recreate (and manipulate) the conditions in which β-cell–immune cell interactions occur to better understand the contribution of specific immune-cell populations to β-cell death. As part of a series on the research conducted within the Human Islet Research Network [68], we review here in vitro and in vivo systems used to model and analyze the human immune processes related to T1D etiology, pathogenesis, and immunotherapy.
2. In vitro models for human immune processes
2.1. Thymic organ cultures to investigate the development of human T cells
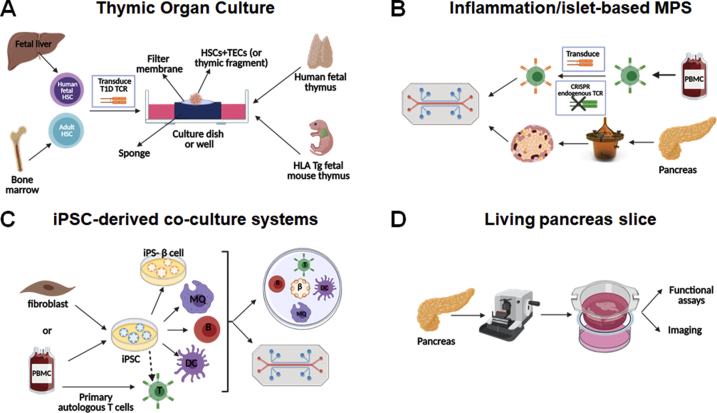
Due to the anatomical location and the age-related involution of the thymus, it is impractical to study the thymic development of human T cells in healthy and diseased individuals. To study this process in vitro, fetal thymic organ culture (TOC) assays were developed, in which a small fragment of human or mouse fetal thymus is cultured with pre-existing thymocyte progenitors, or the tissue is stripped of hematopoietic cells and reconstituted with hematopoietic stem cells (HSCs) [69,70] (Figure 1A). The thymic fragment containing these progenitor cells is cultured in vitro for several days and allows thymocyte development and selection to occur. TOC studies performed using thymus and HSCs from NOD mice demonstrated that the NOD background had deficient negative selection of insulin-reactive thymocytes along with anti-apoptotic and pro-survival gene expression changes [71] and that differences in thymic Treg diversion exist between the NOD and B6 backgrounds [72]. More in-depth fetal TOC studies demonstrated that lentivirally transduced human fetal and cord-blood HSCs and progenitors can differentiate into T cells using mouse fetal or human fetal and post-natal thymus tissue [69,70], allowing for the study of genetically altered thymocyte development to track the development of T cells with particular mutations or specific TCRs. Adult bone marrow-derived transduced HSCs cultured with fetal or adult human thymus can also be used in TOC assays, enabling further study of the impact of T1D genetic background on thymocyte development and selection by using HSCs and thymus tissue from T1D individuals. TOC studies focused on the thymic selection of a transduced insulin-reactive TCR in TOC using human fetal thymus and transduced HSCs from control or T1D donors demonstrated decreased negative selection and Treg diversion of insulin-reactive thymocytes in conditions with T1D HSCs compared to HC HSCs (R Madley et al., manuscript under review). These TOC systems present an alternative method of studying thymocyte development in T1D requiring less cells, tissue, and time than HIS mice.
Figure 1.
In vitro systems to study immune responses in T1D. Thymic organ culture systems (A) allow the study of the selection of T1D autoreactive TCRs in an in vitro system in human fetal thymus or HLA transgenic fetal mouse thymus using TCR-transduced HSCs from a fetal donor or T1D versus healthy control adult donors. The other three models presented, the inflammation- and/or islet-based microphysiological systems (B), iPSC-derived co-culture systems (C) and living pancreas slices (D), are suitable for functional assays to study the interactions of human β-cells and immune cells.
2.2. Microphysiological systems with primary and iPSC-derived cells
The development of environments that mimic conditions in the human tissues would be helpful to assess immune processes longitudinally. To achieve such environment, microfluidic devices have been designed to artificially create a normal flow of nutrients (and immune cells in some cases) and to facilitate the detection of any molecule that is expressed from the target tissue [52,73,74]. Microphysiological systems (MPS) enable the assessment of how the target tissue reacts to autoreactive immune cells in the body. MPS featuring pancreas [75,76], endocrine tissues [77] and pancreatic islets and liver spheroids [78] are of particular relevance to T1D research, and so are inflammation-based MPS, which allow the analysis of the activation of isolated neutrophils, monocytes, and lymphocytes in a proinflammatory environment affecting specific organ targets [73,74,[78], [79], [80], [81], [82]]. Altogether, inflammation- and islet-based MPS models (Figure 1B) could provide insight on how β-cell–immune cell interactions differ when using cells from T1D donors versus controls [52]. These interactions have been examined in vitro in culture wells using either β-cell lines or isolated islets co-cultured with “T-cell avatars” [83,84]. The islet-immune chip (iiChip) was subsequently developed as another system incorporating β-cells and autologous T1D autoantigen-specific T cells to allow the exploration of these interactions [85,86]. It includes various innate and adaptive immune cells implicated in T1D pathogenesis in a controlled environment.
The iiChip and other MPS also have the capability to use iPSCs from donors with or without T1D to generate endothelial/endocrine tissues along with immune cells. Importantly, iPSC-derived systems provide the most flexibility in producing isogenic systems with a variety of cell types (β-cells, endothelial cells, mesenchymal cells, monocytes, macrophages, DCs) from the same iPSC line [87] (Figure 1C). What makes iPSCs particularly attractive is the fact that they can be produced from any living donor with a desired genotype/endotype (preserving the genetics of the disease) without requiring invasive procedures or treatments, and they can be genetically engineered to dissect the role of specific genes. Using multiple isogenic cell types ensures that the interactions observed are devoid of confounding background such as allogeneic reactivity. When the use of primary T cells is needed, it is possible to use autologous cells (from the iPSC donor) or to selectively remove specific HLA molecules in order to prevent alloreactivity while retaining the HLA/peptide–TCR interaction of interest [[88], [89], [90]]. The possibility of using primary T cells is particularly important, as the differentiation of T cells from iPSCs is not yet possible until these T cells can undergo selection in the presence of TECs. Current systems of T-cell differentiation from iPSCs, which rely on Notch signaling for T lymphopoiesis [91,92], do not recapitulate full thymic selection, and efforts to develop TEC co-culture systems are underway. Major challenges thus remain as the function of these human differentiated cells is still not on par with their freshly isolated counterparts, and for some cell types (e.g. TECs), it has not yet been possible to differentiate them in vitro such that they sufficiently resemble the native cell phenotypically and functionally. Current in vitro studies using multiple iPSC-derived cell types are focusing on the interaction between iPSC-derived β-cells and primary T cells [93], although iPSC-derived myeloid cells have also been used [87]. The use of MPS and iPSCs to produce complex isogenic systems and its challenges will be the subject of a dedicated review in the series.
2.3. Studying immune processes in living tissue slices
Living pancreas slices are produced using fresh donor tissue blocks sectioned with a semiautomatic vibratome, which preserves the morphology and the activity of the various pancreatic cell types [[94], [95], [96]] (Figure 1D). They can be used to assess the responses of various pancreatic cell types to stimuli and to conduct multifluorescent 3D imaging [[94], [95], [96]]. Furthermore, the procedure avoids fixatives and other chemicals that could damage the islets [94], but it is not without challenges. The speed at which the tissue is processed after extraction from the donor is extremely important, as it determines the quality of the results [94,96]. The thickness of the slice is also critical, as it affects the level of oxygen and nutrient intake for the duration of the in vitro studies [94]. As these are not reductionist systems, the complex crosstalk between the cell type of interest and other pancreatic cell types may complicate data interpretation [94]. Immune cells in and around islets have been studied in the living pancreatic slice models. Infiltration, phenotype, and functionality of immune cells can be determined through imaging methods [95,96], for example to assess islet macrophages for function and calcium flux [97,98]. Thus, these models preserve the natural conformation and functionality of islets during processing and can be used to observe the behavior of infiltrating autoimmune cells during T1D pathogenesis.
Living tissue slices for T1D research have so far only involved pancreatic tissue. Other tissue slices made from human lymph nodes and tonsils [[99], [100], [101]] or thymus [102] have been used for studies in which preserving the tissue's structural organization and integrity is critical. They allowed the investigation of the regulation of T-cell responses by lymph node stroma [101] and the migration of thymocytes between the cortex and medulla [102]. Studies using thymic slices have been conducted primarily with tissue from young mice, while human studies, challenged by thymic involution, have focused on fetal and pediatric thymus [[103], [104], [105]]. In T1D, this approach could be useful to compare the behavior of TCR-transgenic T cells in pancreatic versus non-pancreatic lymph nodes or the migration of TCR-transgenic T cells, provided that the modified and/or labeled cells can be introduced into the tissue and monitored. The detection of antigen-specific T cells in thymic and lymph nodes is a major challenge that has not yet been achieved.
3. Models supporting human immune systems in vivo
3.1. Different types of recipient mice to host human immune systems
The most common immunodeficient recipient mice are built upon the NOD background, in which multiple genetic modifications have been applied throughout the years to improve human cell engraftment and minimize graft-versus-host reactivity (Table 1). Less frequently utilized are strains on the BALB/c and B6 backgrounds.
Table 1.
Mouse strains used to host human immune systems with alterations preventing host-vs-graft and graft-vs-host rejection. Sources: Jax: The Jackson Laboratory; Tac: Taconic; CRL: Charles River Laboratories; Inq: Inquire with original investigator for availability. Note: this is not an exhaustive list of suppliers for most common strains.
| Strain (and source) | Mutations | Advantages | Refs |
|---|---|---|---|
| NOD background | Reduced NK cells and C5a function, increased binding of NOD SIRPα to human CD47, which reduces macrophage phagocytosis | [[106], [107], [108]] | |
| NOD.scid (Jax 001303, Tac NODSC, CRL 394) | Prkdcnull (scid) | T- and B-cell deficiency | [106] |
| NOD.RagKO (Jax 003729) | Rag1null | ||
| NSG (Jax 005557) |
Prkdcnull (scid) Il2rgnull |
Eliminates any residual NK and lymphocyte activity | [109] |
| NOG (Tac NOG) | |||
| NCG (CRL 572) | |||
| NRG (Jax 007799) | Rag1nullIl2rgnull | [110] | |
| NRG-NFA2 (Jax 033127) |
Rag1nullIl2rgnull Flt3null |
Impaired murine myeloid cell development | [111] |
| NBSGW (Jax 026622) |
Prkdcnull (scid) Il2rgnullKitW41 |
Mutation in the mouse Kit gene limits hematopoiesis, providing an advantage to donor cells, and circumvents the need for irradiation | [112] |
| NSGW41 (Inq) | [113] | ||
| NSG.MHC-I/II−/- (Jax 030547) |
Prkdcnull (scid) Il2rgnullB2mnull(IA IE)null |
Prevention of xenogeneic reactivity of human immune cells | [114] |
| NOG.MHC-I/II−/- (Inq) | [115] | ||
| BALB/c background | |||
| BRG (Tac 11503) | Rag2nullIl2rgnull | Same as NSG, but less frequently used. | [116] |
| BRGS (GenOway) |
Rag2nullIl2rgnull SirpaNOD |
Same as BRG, with reduced murine macrophage phagocytosis | [117] |
| BRGSF (GenOway) |
Rag2nullIl2rgnull SirpaNODFlk2null |
Same as BRGS, with reduced myeloid cell compartment | [118] |
| C57BL/6 background | |||
| B6RGS (Inq) |
Rag2nullIl2rgnull SirpaNOD |
Same as BRG, with reduced murine macrophage phagocytosis | [119] |
| BRAGG47 (Jax 032088) |
Rag1nullIl2rgnull CD47null |
Phagocytes developing in this strain are more tolerant to transplanted human cells (B6 mice) | [120] |
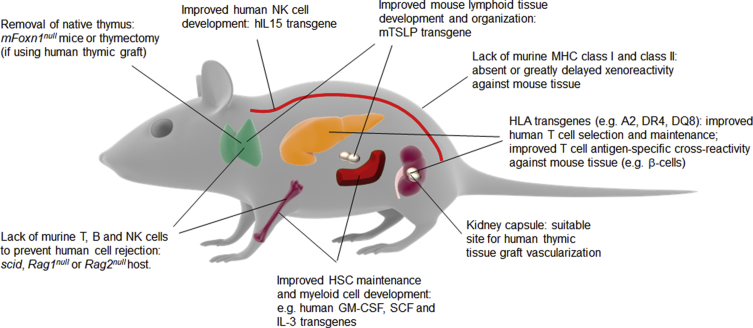
Further modifications have been made to improve the development of human immune cells from HSCs and their homeostasis. These typically consist of human cytokines expressed as transgenes to compensate for the lack of activity of their mouse counterparts on human cells or human HLA transgenes to improve the positive selection of human T cells (Table 2). Modifications of recipient mice for human immune systems are summarized in Figure 2. Additional strains are reported elsewhere [120].
Table 2.
Strains used to improve the development of human immune populations using cytokine- or HLA-expressing transgenes (listed are HLA haplotypes that are most commonly studied in T1D research). Sources: Jax: The Jackson Laboratory; Tac: Taconic; Inq: Inquire with original investigator for availability.
| Strain (and source) | Transgene products | Advantages | Refs |
|---|---|---|---|
| NOD | |||
| NSG.SGM3 (Jax 013062) | SCF, GM-CSF, IL-3 | Improvement of human myeloid cell development | [121] |
| NOG.EXL (Tac 13395) | GM-CSF, IL-3 | [122] | |
| NOG.hIL-6 (Tac 13686) | IL-6 | [123] | |
| NOG.hIL-15 (Tac 13683) | IL-15 | Improved human NK cell development | [124] |
| NSG.HLA-A2.HHD (Jax 014570) | H2-D and B2m (HLA-A2 haplotype) | Positive selection of human T cells restricted to human HLA; identification of T-cell clones reactive to specific HLA/peptide complexes; reactivity of human T cells against mouse tissues expressing specific antigens | [125] |
| NSG.HLA-DQ8 (Jax 026561) | HLA-DQA1 and DQB1 (HLA-DQ8 haplotype) | [126] | |
| NSG.Ab0.HLA-DR4 (Jax 017637) | HLA-DRB1 (HLA-DR4) haplotype), H2-Ab1null | [127] | |
| BALB/c | |||
| MISTRG (Jax 017712, discontinued) | Rag2null and Il2rgnull BALB/c with 5 human cytokines (SIRPA, M-CSF, GM-CSF, IL-3 and TPO). | Better differentiation of monocytes, macrophages, and NK cells | [128] |
| BRGST (Inq) | BRGS mice expressing mouse Tslp | Improved lymph node and thymic structures (correction of tissue disruptions caused by the pleiotropic effect of the Il2rgnull mutation), better immune responses | [129] |
Figure 2.
Mice as hosts of human immune systems. Engineering approaches and methodologies to make mice better hosts for human immune systems by improving human HSC and immune cell engraftment and facilitating the development of a diverse human immune system are shown.
3.2. Different models based on the type of donor human cells
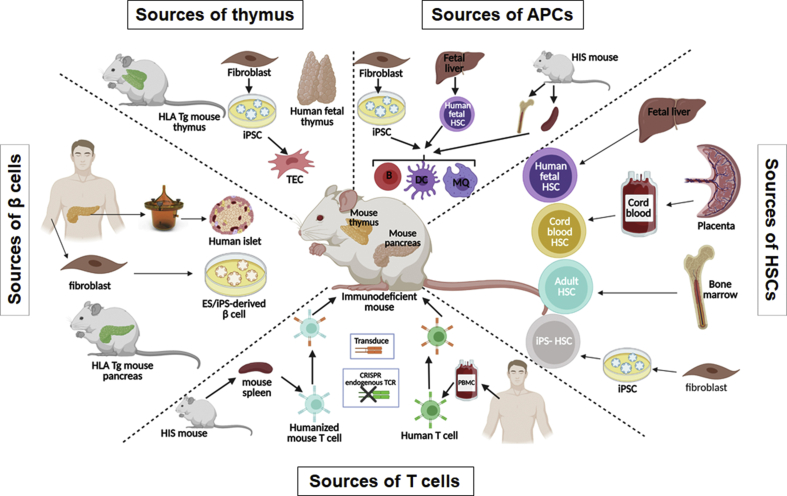
Multiple models exist based on the type of cells used to reconstitute recipient mice: peripheral blood mononuclear cells (PBMCs) or specific populations of antigen-specific T cells or APCs, or HSCs with or without human thymic tissue (Figure 3). For T1D studies, in addition to the use of specific TCRs or antigens, target cells such as β-cells from various sources may be incorporated into these models as well.
Figure 3.
Human immune system mouse models to study immune responses in T1D. Different sources of cells and tissues for generating HIS mice are shown. In order to study the interaction of islet β-cells with human immune cells, grafted human islets, grafted iPSC-derived β-cells or endogenous HLA transgenic (Tg) mouse pancreas can be used as the targets of the human immune system. Grafted human fetal thymus, grafted iPSC-derived thymic epithelial cells, or endogenous HLA Tg mouse thymus can support human thymopoiesis. HIS mice can be reconstituted with genetically-modified or non-modified HSCs from human fetal liver, cord blood, adult (e.g. T1D vs healthy controls) HSCs, and potential iPSC-derived HSCs. Autoreactive TCR-transduced T cells from human PBMCs or other HIS mice in the same cohort generated with autologous tissues can be adoptively transferred to recipient HIS mice to study the interaction of autoreactive T cells and β-cells. Adoptive transfer of different APCs (loaded or transduced with T1D autoantigens) from other HIS mice or differentiated from fetal liver HSCs and iPSC cell sources can be used to study the role of these cells in T1D development. Of note, iPSCs can themselves be generated from multiple sources, including not only fibroblasts but also PBMCs.
3.2.1. Models using human PBMCs
The Hu-PBMC-SCID model, in which human PBMCs are engrafted into immunodeficient C.B-17-scid mice, was first described in 1988 [130] and initially used to study HIV [131] but was soon recognized as a model of Epstein–Barr virus-induced human B-lymphoproliferative disease [132]. With the development of the NOD-scid mouse in 1995 [106], higher levels of human PBMC engraftment were achieved [133,134] and increased engraftment in human CD8+ T cells prevented the development of the Epstein–Barr virus-induced human B-lymphoproliferation disease, but at the expense of severe xenogeneic graft-versus-host disease (xGvHD) [135]. Using NSG mice as the recipient greatly improved PBMC engraftment but the mice rapidly developed an acute xGvHD, accelerated with low-dose irradiation [109]. Confirming the hypothesis that lethal xGvHD was directed against the murine MHC class I and class II molecules, human PBMCs engrafted into MHC class I/II-deficient NSG mice did not induce acute xGvHD [90,114,136] and the engrafted T cells retained their function and were able to reject primary and stem cell-derived islet allografts [90,114]. However, the development of acute xGvHD, even in the absence of murine MHC class I and class II molecules, occurs upon the administration of high levels of human IL-2, which indicates that murine MHC class I and II are not the only molecules controlling the reactivity of xenogeneic human T cells [114]. This model is highly accessible, as it can be implemented at a relatively low cost with minimum difficulty.
3.2.2. Models using fetal liver HSCs with or without grafted fetal thymus
The first HIS mouse model with durable T-cell development was developed by the McCune team through implantation of the human fetal thymus and liver fragments under the renal capsule in CB17-scid mice [137]. However, T cells were detected only in the thymus, with little to no peripheral immune reconstitution. Provision of human APCs in the periphery via intravenous injections of HSCs could greatly improve the human peripheral T-cell reconstitution and function in these animals [138,139]. This model was later termed the bone marrow–liver–thymus (BLT) model, despite the absence of any bone marrow in most versions of the model [140]. Later, the fetal liver fragment was found to be dispensable for sustained human hematopoiesis [141]. As NOD.scid mice have functional murine NK cells, engraftment of human HSCs and human immune cell reconstitution in these mice is lower compared with that in NSG mice that lack NK cells [142]. Moreover, NOD.scid mice (but not NSG mice) develop thymic lymphoma [143], which limits the experimental window. While the NSG mouse has its own drawbacks (most of them discussed further below), including the eventual development of xGvHD at later times [144], it remains the preferred recipient to date for engrafting human fetal tissues and cells.
This model is ideal for long-term studies, because as the human T cells develop in a grafted human thymus that is seeded by mouse APCs [145], they can be tolerized to many mouse antigens, thus limiting xGvH reactivity. Eventually, an autoimmune/GVH-like syndrome occurs in a timeframe that is dependent on multiple factors, including the degree to which the original fetal thymus graft was thymocyte-depleted. While this disease develops much later in animals with thymocyte-depleted human fetal thymus grafts than in animals with a non-depleted thymus or with a native mouse thymus, the eventual development of this syndrome under these “optimal” conditions may reflect the inability to tolerize to mouse tissue-restricted antigens that require mouse TECs for their production [146]. Because human donor-derived APCs are also present in the human thymic graft, the human T cells developing in this model are also tolerant to the human donor [145,147]. While hyporesponsiveness to human pluripotent stem cell (hPSC)-derived allografts has been reported in such animals [148], robust rejection of human and pig skin grafts [149] and of allogeneic hematopoietic cells is seen in these animals (HW Li and M Sykes, unpublished data).
3.2.3. Models using postnatal HSCs with or without a grafted thymus
The engraftment of human HSCs into immunodeficient mice was originally described by Dick and colleagues [150,151]. Because of the initial uncertainty of the identity of the human cells responsible for repopulating the hematopoietic system of the mouse, these were originally defined as “scid-repopulating cells” (SRC), which are now known to be CD34+ HSCs [152]. Human CD34+ HSCs can be engrafted into immunodeficient mice by various routes: intrahepatic or intracardiac (newborn), intrafemoral, or intravenous (adult) [153]. In contrast with PBMC-engrafted mice that predominantly maintain CD3+ T cells after injection, HSC-engrafted mice develop a complete human hematopoietic system, including B cells, myeloid cells, and other APCs, and the size of the T-cell compartment depends on the type of thymus available for their development. Although human granulocytes, platelets, and red blood cells (RBCs) are present in the bone marrow, there are only very low levels of these cell populations in the blood [120], due, in part, to macrophage-mediated destruction of RBCs and platelets and to cytokine incompatibilities [154,155]. The Hu-SRC-SCID model has been used extensively for the study of human hematopoiesis and cell-mediated immunity as well as infectious diseases [120,[156], [157], [158]].
Cord blood CD34+ HSCs have a similar or slightly lower immune reconstitution capacity compared with fetal HSCs, while their adult counterparts show substantially reduced immune reconstitution capacity [116,159,160]. As a major drawback, unlike fetal HSCs, these HSCs do not come with autologous thymic tissue. Of note, when mice are reconstituted with HSCs, regardless of the source (fetal, cord-blood, adult, iPSC), human thymopoiesis may be supported to a very limited extent by the NSG and NOG mouse thymus, despite its primitive and unorganized structure [144,156,161], and the resulting T cells are primarily restricted to mouse MHC [156]. The NeoThy model uses thymic tissue retrieved from neonatal cardiac surgeries, combined with cord blood from the same or unrelated (matched) donors [162]. However, neonatal thymic tissue grows significantly less than fetal thymic tissue after renal subcapsular grafting [162]. Whether it can support robust thymopoiesis would have to be demonstrated in the absence of the native mouse thymus [163]. For this reason, non-autologous fetal thymic tissue grafts, which have some of the HLA class I and II haplotypes shared with the donor HSCs, continue to be used to support the selection of human thymocytes [145,164]. Alternatively, fetal swine thymus can be used to support human thymopoiesis [147,165,166], although with subtle immunological defects related to the MHC disparity of TECs and human APCs in the periphery [149]. Transgenic pigs expressing common HLA alleles are being generated in an effort to overcome this disparity.
For modeling autoimmune disorders, reconstituting mice with HSCs from adult individuals confirmed as having developed the disease is preferable to ascertain the effects of genetic risk factors on these reconstituted immune systems. The only accessible tissue to evaluate the immune system of human patients is usually peripheral blood, which might not reflect immunity in the target organs. Personalized immune (PI) HIS mouse models overcome these limitations by allowing synchronized de novo development, in cohorts of immunodeficient mice, of functional immune systems from HSCs isolated from patients and healthy controls from bone marrow aspirations [145] or G–CSF–mobilized peripheral blood samples [159,167]. Genetic drivers of immune dysregulation affecting the hematopoietic cell lineage can be transferred to these mice by HSC transplantation [164]. A considerable limitation of the PI model is the reduced reconstitution levels of B cells and myeloid cells as compared to the model using fetal HSCs [164].
3.2.4. Models using iPSC-derived HSCs
Developing functional hPSC-derived HSCs is a goal for several groups. iPSC-derived HSCs would be especially valuable, as they allow the modeling of different diseases, including T1D, using patients’ own de novo-generated immune cells. Combined with the development of iPSC-derived thymus and pancreatic β-cells, this could be an ideal model for studying T1D pathogenesis. Two major approaches have been used to develop hPSC-derived HSCs: (1) directed differentiation by applying extrinsic factors to the culture media at different time points to mimic the different stages of HSC development [[168], [169], [170], [171]] and (2) forced differentiation by overexpressing transcription factors known to contribute to this process in pluripotent stem cells or partially differentiated intermediate cells, such as hemogenic endothelium [[172], [173], [174], [175], [176]]. Despite successes in generating iPSC-derived HSCs in vitro, it remains challenging to develop functional HSCs that can generate all hematopoietic lineages in vivo [177]. A forced differentiation based on the overexpression of seven transcription factors (ERG, HOXA5, HOXA9, HOXA10, LCOR, RUNX1, and SPI1) in embryonic stem cell- and iPSC-derived hemogenic endothelium led to the engraftment of myeloid, B, and T cells in primary and secondary NSG mouse recipients [174], but this approach is yet to be reproduced and adopted by other groups.
4. Applications of HIS models to study immune tolerance and autoimmune processes
4.1. Thymic development of autoreactive T cells
4.1.1. TCR-transgenic models for thymic selection
Insufficient presentation of specific autoantigens by mTECs or DCs in the thymus limits both the deletion of autoreactive thymocytes and the selection of Tregs. Depending on whether the corresponding antigens are presented or not in a ‘normal’ thymus (see section 1.1), it is expected that some T-cell clones never undergo negative selection and are therefore completely dependent on peripheral tolerance, whereas other T-cell clones may normally be deleted, but this process is hypothesized to fail in individuals with T1D. While T cells with specificity to several epitopes of β-cell antigens have been found in similar frequencies in the blood of T1D vs control [8,65,178], T1D individuals differ in their islet-infiltrating T cells [8], which may have higher TCR affinity and are allowed to develop due to defective negative selection, and those found in the blood may also have a different cytokine profile [179]. HIS mouse models with grafted human thymus can be leveraged to test whether islet-infiltrating T cells, from which an increasing number of TCRs have been sequenced, exist as a result of defective thymic selection or peripheral regulation. Specific TCRs can be introduced in a fraction of HSCs by lentiviral transduction to produce antigen-specific TCR-transgenic T cells at sufficiently high frequency to enable tracking and reliable analysis [47,180], as in mouse models. Once the HSC-derived cells start to express CD3, the TCR is expressed on the cell surface, allowing interaction with MHC/peptide complexes for selection. One study investigated the thymic selection of an insulin-reactive T-cell clone (clone 5, InsB9-23-reactive, HLA-DQ8-restricted) in HIS mice grafted with HLA-DQ8+ thymus and reported that this clone was indeed negatively selected starting at the double positive stage [47], as expected from the natural expression of insulin in mTECs. Interestingly, this negative selection was incomplete if only the grafted thymus (mTECs) and not the HSCs (DCs) were HLA-DQ8+, indicating a possible requirement for antigen transfer from mTECs to DCs to achieve efficient engagement of these insulin-specific developing thymocytes [47]. Another study illustrates the case of an autoreactive T-cell clone that does not undergo negative selection (clone DMF5, MART1-reactive, HLA-A2-restricted), due to lack of epitope presentation [181]. In one experimental group, the corresponding epitope was overexpressed in another fraction of HSCs, and this resulted in the efficient deletion of the specific T cells [180]. Comparison of groups with and without the transduced antigen revealed major phenotypic differences (e.g. PD-1 vs. CCR7) between developing thymocytes that encounter their cognate antigen and those that do not. Thus, HIS mouse models with grafted human thymus are useful to ascertain the development of diabetogenic T cells that contribute to human T1D. HIS mouse models without a human thymic graft but instead with HLA transgenes to facilitate positive selection in the mouse thymus can be used to produce TCR-transgenic T cells. However, the NSG mouse thymus structure remains abnormal, even if human HSCs are given early in life [144], making murine thymi unsuitable for the analysis of normal human T-cell negative selection.
4.1.2. Influence of the genetics of HSCs on thymocyte development
The PI mouse model can be a powerful tool for studying the thymic development of diabetogenic T cells of T1D individuals, as this can help us understand the initiation and pathogenesis of T1D. Studying the thymic development of T cells derived from HSCs from adult T1D and healthy control (HC) donors in PI mice revealed multiple phenotypic and functional differences that could lead to or contribute to T1D development (R Madley et al., manuscript under review). For example, multiple populations of thymocytes in ‘T1D mice’ have increased levels of negative selection and TCR signaling markers PD-1 and CD69 on the cell surface, yet CD4 SP thymocytes from PI mice derived from some T1D individuals in the study were more resistant to apoptosis than those from ‘HC mice’. Gene expression analysis comparing post-positive selection thymocytes from T1D and HC HSCs demonstrated decreased expression of T-cell signaling and apoptotic genes and increased expression of anti-apoptotic genes associated with T1D. This PI mouse model may be useful for identifying the abnormalities in T-cell thymic selection associated with T1D. To assess the selection of specific diabetogenic T cells, an insulin-reactive TCR (clone 5, see above) was introduced in the HSCs prior to transplantation into the mice. In this TCR-transgenic PI mouse model, the insulin-reactive T cells were less efficiently negatively selected or diverted to Tregs in mice with T1D HSCs than in mice with HC HSCs (R Madley et al., manuscript under review).
4.1.3. The role of thymic stromal cells in the selection of autoreactive TCRs
Genetic susceptibility to T1D can also manifest itself in the function of non-hematopoietic lineages that contribute to the shaping and regulating of immune cells, such as the thymic epithelium [10,11,[182], [183], [184]]. Our ability to model and understand the role of TECs in human T1D is, however, lacking. T-cell selection in current humanized mouse models relies on human fetal thymus due to the limited ability of postnatal tissue to grow and support thymopoiesis in these models [163]. These restrictions preclude the use of thymic tissue from T1D patients who are not diagnosed until later in life. In addition, primary TECs can be isolated from thymic tissue but they are not amenable to genetic modifications, thus impeding mechanistic studies.
To circumvent these issues, approaches focused on using hPSC-derived TECs to generate a functional thymus are being explored. Directed differentiation methods have been developed to produce TECs from human embryonic stem cells [[185], [186], [187], [188]] and iPSCs [189,190]. Transplantation of hPSC-derived TECs in nude [185,186,188,189] and humanized mice [186,188] have demonstrated that they can sustain low levels of thymopoiesis from mouse and human hematopoietic progenitors. However, the complex structure of the thymus has not yet been recapitulated, and there is no direct evidence of functional AIRE-expressing mTECs in these grafts, raising questions about their ability to mediate T-cell-negative selection. For further optimizing the function of hPSC-derived TECs, it will therefore be important to understand the microenvironment required for the proper development of different subsets of TECs. This microenvironment is likely to be complex, since TECs interact with many hematopoietic and stromal components, including mesenchyme, endothelial cells, and pericytes [191]. Incorporation of hPSC-derived TECs into porcine thymic tissue in humanized mice has produced promising results [192], making use of the supporting structure and stromal cells as well as crosstalk with human immune cells that are necessary for complete maturation of TECs, to allow hPSC-derived TECs to survive and function. Once the models demonstrating the ability of hPSC-derived TECs to fully support the development and the proper selection of human T cells through all stages have been achieved, it will become possible to test the mutations and polymorphisms that are suspected to affect the thymic epithelium in its ability to shape the T-cell repertoire and purge/redirect autoreactive T cells.
4.2. Regulatory T cells (in the thymus and periphery)
4.2.1. Thymic selection of Tregs
Apart from death by negative selection, autoreactive thymocytes can also undergo conversion to Treg cells during thymic selection. Studies in mice have demonstrated that the differentiation of a Treg from an autoreactive thymocyte depends on the pattern of antigen expression in the thymus and the affinity of the TCR for the cognate antigen. More specifically, self-antigens expressed in an mTEC-restricted fashion in the thymus, such as insulin, result in a combination of negative selection and Treg induction to induce tolerance to the antigen [193]. The innovative PI and TOC models discussed above have been helpful in studying the thymic development and selection of Tregs in T1D and HC immune systems. FOXP3+ HELIOS+ “natural” Tregs develop normally in human fetal thymic grafts [194]. Thymic Tregs in grafted human thymus have a highly diverse TCR repertoire [195]. PI mice produced from T1D or HC HSC donors produce similar percentages of Tregs among thymocytes [145]. However, the percentage of insulin-reactive TCR-transgenic thymic Tregs in T1D TOCs and some T1D PI mice was lower than that in HC, indicating a deficiency in antigen-specific Treg diversion in T1D (R Madley et al., manuscript under review). Once a sufficient number of HLA class II-restricted TCRs have been tested in TCR-transgenic models and compared for relative affinity to their respective epitopes, it will be possible to study and better understand the factors that control the decision of CD4+ T cells to undergo apoptosis or become a Treg (or neither) upon encountering their antigen in the thymus and determine how these factors may be influenced by genetic susceptibility. An alternative model would be to use HSCs transduced to express epitope variants (as described in 4.1.1.) that are recognized by a given TCR with varying affinities.
4.2.2. Treg functionality and stability
Deficiencies in Treg function and stability, investigated in NOD mice and patient samples, have not been easy to pinpoint due to biological variation and, in some cases, evidence of effector T cell resistance to regulation rather than Treg dysfunction [14,15]. Specific single nucleotide polymorphisms (SNPs) likely contribute to altered Treg functionality in some patients. Seminal studies in purified peripheral blood bulk CD4+ T cells and Tregs from T1D patients and healthy controls harboring mutations in the IL-2 signaling pathway (IL2RA, PTPN2) revealed that these mutations lead to lower STAT5 phosphorylation, lower FOXP3 levels, and poorer in vitro suppression by Tregs [[196], [197], [198]], indicating that, at least in a subset of T1D patients, there are some intrinsic deficiencies in Treg stability and function detectable in vitro. However, despite well-established molecular and functional assays to assess Treg suppressive function in vitro [199], in vivo models are required to assess survival, trafficking, and longer-term suppression by human Tregs.
The suppressive function of Tregs was demonstrated in several allogeneic islet rejection models (section 4.3.2) [200,201]. However, such models have limited utility to study the function of Tregs in a T1D patient's immune system and/or under normal autoimmune settings (i.e., outside the context of allo/xenoreactivity). These Tregs migrate to the peripheral organs and exhibit normal suppressive functions [194]. The suppressive functions of the peripheral Tregs are needed to prevent autoimmunity in HIS mice, as HIS mice reconstituted with HSCs from a patient with immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, associated with aberrant FOXP3 function, developed a lethal inflammatory disorder [202]. Similarly, in a HIS mouse model in which HSCs were stably knocked down or knocked out for the FOXP3 gene, effector T cells were expanded and showed signs of homeostatic proliferation, such as a significantly contracted TCR repertoire and a reduced naive compartment [203]. Conversely, the overexpression of FOXP3 in HSCs improved T-cell engraftment but reduced T-cell responsiveness overall [204]. A HIS model using adult HSCs without human thymus in an HLA-DQ8 transgenic NSG recipient was used to verify in vivo that blocking of miRNA181a or NFAT5 can increase Treg induction, consistent with the overstimulated miRNA181a-NFAT5 axis in children with T1D [205].
Modeling the function of immune populations in HIS mice requires a better understanding of the possible differences from the same populations freshly isolated from humans. In HIS mice in which human T cells develop in the disordered murine thymus [144], Tregs are functional and in fact have a more pronounced Treg-like phenotype with increased suppression compared with PBMC-derived Tregs [206], yet they fail to protect mice from autoimmune-like disease [144]. HIS mice can also be used to track engineered Tregs and document their stability in vivo to model what happens to these cells in cell-based immunotherapy. Finally, the issue of resistance to Treg suppression in human T1D is one that remains poorly understood [207] but may be in part associated with enhanced responsiveness to IL-6 [208]. Interestingly, in HIS mice made with PBMCs from multiple sclerosis patients, an exacerbated GvHD that was resistant to prevention by HC Tregs could be improved using IL-6 blockade [209], illustrating the possibility of also evaluating resistance to regulation in T1D in vivo models.
4.3. Peripheral reactivity of T cells against islets and β-cell antigens
4.3.1. Mouse immune systems with human components
Multiple studies in mice with human components, but with mouse immune systems, have been performed to study the interactions of human β-cell-reactive TCRs and HLAs presenting human islet autoantigens. In the YES mouse model, knocked out for mouse MHC class I and II and double transgenic for HLA-A2/DQ8, murine T cells that developed on transgenic HLAs showed a significant response to human insulin peptides after immunization [210]. In a more recent version, human insulin and B7.1 molecules were also expressed in β-cells, and the mice (both males and females) spontaneously developed diabetes between 10 and 30 weeks of age [211]. In another mouse model with transgenic expression of human GAD65 in murine pancreatic β-cells and HLA-DQ8 on murine APCs, the mouse immune system was able to destroy β-cells and induce spontaneous diabetes [212]. In an HLA-A2+ NOD-based model, dysfunction of one allele of the murine Ins2 gene, which is also expressed in the thymus, led to accelerated diabetes development and HLA-A2-restricted T-cell responses [213]. Finally, HLA-DR4 transgenic mice have been used to generate retrogenic mice with chimeric (mouse-human) TCR constructs to study the responses against GAD peptides presented on HLA-DR4 [214].
4.3.2. Human T-cell responses against allogeneic islet grafts
Studies investigating β-cell allograft rejection by human T cells in vivo have been helpful to understand islet transplant rejection in T1D patients and evaluate ways to prevent it. Several PBMC-based HIS mouse studies have been reported using allogenic islets [200,215,216] and hPSC-derived β-cells [217,218], demonstrating protection by ex vivo expanded human CD4+ Tregs [200], CTLA4-Ig and CD40L blockade [217], or PD-L1 overexpression [218]. Models based on human HSCs have also been used with variable results in the rejection rate and composition of the islet infiltrates, which were influenced by the recipient strain and HSC dose used [201,219,220].
4.3.3. Human T-cell responses against mouse islets
Other models focused on the rejection of mouse β-cells that can be targeted by human T cells by expressing HLA transgenes. In one such model, human CD4+ T cells from HLA-DR4+ T1D and HC individuals stimulated in vitro with HLA-DR4-restricted β-cell antigenic peptides were transferred into HLA-DR4 transgenic NSG mice, which then developed insulitis, but not overt diabetes, and T cells from T1D donors induced a more severe insulitis [221]. In a similar study, HLA-A2+ PBMCs from T1D donors induced insulitis more readily than those from HC when transferred into HLA-A2 transgenic NSG mice [222]. Although the contribution of xGvHD in such models cannot be excluded (use of mouse MHC class I and II deficient recipients might improve these models), the increased ability of T cells from T1D to induce insulitis in both models is noteworthy. In another study, adoptive transfer of a single clone of HLA-A2-restricted IGRP-specific CD8+ T cell derived from a T1D donor to HLA-A2 transgenic NSG mice led to destructive insulitis [48].
One method to generate HIS mice harboring antigen-specific T cells capable of attacking islet β-cells and induce diabetes without mounting a xenogeneic response is to conduct an adoptive transfer of TCR-transduced T cells wherein the donor T cells come from the same cohort of mice as the recipient mice. Since the transferred T cells went through selection in grafted human thymic tissue in the presence of both human and mouse APCs, they are largely tolerant to both species and do not easily induce acute GvHD after adoptive transfer. As an added benefit to this system, TCR transgenes that are otherwise susceptible to be negatively selected can be introduced in these T cells, because the T cells used have already undergone thymic education and are injected into the periphery. These human T cells can target mouse islet β-cells expressing the same HLA they are restricted to. In one such system, mature T cells harvested from one HIS mouse were transduced to express the InsB:9-23-specific Clone 5 TCR and then adoptively transferred into multiple recipient mice of the same cohort, all made with HLA-DQ8+ HSCs and thymic graft in HLA-DQ8 transgenic NSG mice, which enable both the positive selection of the above TCR and the presentation of this peptide on HLA-DQ8 by mouse β-cells (the sequence of mouse and human InsB:9–23 is identical) [223]. Upon treatment with low-dose streptozotocin to induce islet inflammation and immunization with InsB:9–23 peptide and adjuvant, these mice developed insulitis and hyperglycemia [223]. Mouse/human epitope cross-reactivity may limit the responses of other β-cell-reactive TCRs, and this could be improved by expressing human β-cell autoantigens in mouse β-cells [224,225]. Removal of endogenous TCR chains upon transduction might improve the response of TCR transgenic T cells, as transgenic TCR mispairing (more problematic when transducing mature T cells than HSCs) may hinder the autoimmune destruction of the host's β-cells.
4.3.4. Evaluation of T-cell responses to immunotherapies
HIS mouse models may also be used to evaluate the response of antigen-specific (e.g. TCR transgenic) T cells to antigen-specific immunotherapy, for example by tolerogenic APCs (see 4.4.2 below), and inform on the phenotype and fate of autoreactive T cells following antigen encounter. Assessment of the potential clinical benefits of such therapies will require the development of models that demonstrate robust and reproducible destruction of autologous human grafts or, by cross-reactivity, of mouse β-cells (i.e., not allogeneic or xenogeneic destruction). HIS mice have been used to evaluate immunotherapy in other autoimmune models [226]. HIS mouse models have tested the induction and expansion of human Tregs in vivo in several settings, including: induction of antigen-specific HLA-DQ8-restricted Tregs by insulin mimotopes [126]; induction of CD4+ and CD8+ Tregs by artificial APC nanoparticles [227]; and expansion of Tregs by low-dose IL-2 treatment in a model of colitis, resulting in reduced disease severity [228]. HIS mouse models were also leveraged to evaluate the persistence, stability, and efficacy of human chimeric antigen receptor (CAR) Tregs, first in the protection against xGvHD by HLA-A2+ PBMCs and against HLA-A2+ skin rejection using HLA-A2-specific CAR Tregs [[229], [230], [231]], and later in the protection of HLA-A2+ islet allografts [232]. Of note, CAR Tregs tested in the NOD model provided limited efficacy in blocking disease development [233,234].
Recently, T1D occurring as a result of immune checkpoint blockade-based cancer therapy [235,236] has generated interest among immunologists. Of note, HIS mice treated with an anti-CTLA-4 antibody used in the clinic (ipilimumab) developed infiltration of the liver, adrenals and salivary glands [237]. Although this could simply reflect accelerated GvHD in these mice, T1D-prone HIS models could potentially be useful in understanding the adverse effects of these treatments.
4.4. Other immune cells
4.4.1. Development of autoreactive B cells
Central B-cell tolerance has been shown to be impaired in T1D, as well as in other autoimmune patients [20], often associated with the presence of the human PTPN22 R620W allele variant, which correlates with diminished autoreactive B-cell deletion [238]. Establishment of central B tolerance is defective in HIS mice produced with HSCs from donors with PTPN22 T alleles, resulting in a large proportion of polyreactive B cells, including insulin-reactive B cells [239]. Thus, HIS mice represent a useful tool to investigate altered tolerogenic mechanisms, which is facilitated by the high frequency of human B cells generated in almost all HIS models and by the relatively easy access to key organs where tolerance is promoted (e.g. bone marrow). Despite the general low specific humoral response normally generated in HIS mice [240], two recent reports support the feasibility of reproducing some of the intrinsic defects associated with impaired B-cell tolerance. Consistent with the PTPN22 studies [239] and the fact that PTPN22 is the third highest risk allele in T1D, autoreactive and polyreactive human B cells were higher in PI mice produced with HSCs obtained from the bone marrow of T1D or rheumatoid arthritis patients compared with those from healthy donors [164]. Moreover, human B cells that developed in BRGS (BALB/c Rag2null Il2rgnull SirpaNOD) mice injected with HSCs isolated from the cord blood of newborns born from mothers with T1D, rheumatoid arthritis, or systemic lupus erythematosus showed altered expression of markers linked to B-cell tolerance [241]. The differential development of autoreactive (κ+) versus nonautoreactive (λ+) B cells was studied by the same group employing a humanized mouse model in which all the human κ+ B cells recognize a synthetic membrane-bound self-antigen (Hcκ) and therefore undergo central tolerance during their development in the mouse bone marrow [242]. Using a similar approach, it should be possible to further investigate and elucidate specific tolerance mechanisms as it pertains to T1D by transferring HSCs from T1D patients into immunodeficient recipient mice that have been genetically engineered to express human insulin [224,225]. Such models can help to elucidate how insulin-reactive B cells escape deletion/editing, the mechanisms involved in rendering them anergic in the periphery and the events that relieve them from this anergic state [42]. Finally, the role of insulin-reactive transgenic B cells might be further investigated in HIS mice by generating BCR-transgenic B cells, using a similar strategy to that described above for testing human TCR-transgenic T cells. This would allow easier characterization due to increased frequency (contingent on defective deletion), easier identification and tracing, and evaluation of their response to autoantigen and contribution to promoting T1D development.
4.4.2. Role of different APCs presenting T1D autoantigens in the development of T1D
DCs and macrophages are present in pancreatic islets, and some have been shown to present β-cell antigens directly to diabetogenic T cells, at least in animal models [243,244]. Other APC populations are present in pancreatic lymph nodes, some resident that acquire antigens draining from islets, others draining directly from islets loaded with antigens. Little is known about the contribution of these different APC subsets and their function as tolerogenic or immunogenic cells in steady-state conditions and in the context of T1D. Particular T1D-associated gene polymorphisms, environmental factors (microbiome in particular), and disease-related inflammation can cause alterations in the phenotype and function of these APCs, potentially playing a causative or exacerbating role in the disease process [21]. However, the mechanisms by which these APCs might play a role in the disease has not been deciphered, primarily due to the lack of suitable modeling systems. In vivo models may be utilized to assess the immunogenic potential or tolerogenic properties of various types of APCs, either for the purpose of dissecting the pathogenesis of T1D or to evaluate the tolerogenic strategies for treatment. Relevant antigens may be provided by engineering HSCs to express them [180] or may be ‘pulsed’ in the form of proteins or peptides ex vivo using autologous APCs. For the simplest models, PBMCs transferred into MHC-deficient NSG mice could be used, whereby some of the T cells can be modified to express transgenic TCRs while antigen-pulsed APCs (monocytes or monocyte-derived DCs or macrophages, or even B cells) from the same donor are subsequently transferred. For more sophisticated models, HIS mice capable of generating a full immune system can be produced, whereby some of the mice from the cohort can be used as donors for specific APCs of interest. Alternatively, APC populations may be differentiated in vitro from the same HSCs used to produce the HIS mice according to optimized differentiation protocols [245]. Comparing the function of APCs from control vs T1D donors may help ascertain whether APCs from T1D donors are inherently impaired in transmitting tolerogenic signals or more easily become immunogenic under specific conditions. These studies may also help to distinguish APCs that are functionally impaired from those that preserved their function, thereby informing on APC populations to preferentially target in antigen-specific immunotherapies.
5. Limitations of humanized mouse models and recent improvements
Current in vivo models address many of the shortcomings of in vitro models, including the production of a diverse and representative immune system capable of circulating between tissues. Most of the modifications have been made to these models with the purpose of improving HIS engraftment with minimal reactivity against the host (i.e., no host-vs-graft or graft–vs–host reaction). While challenges remain to be overcome, it is expected that mouse-based HIS models will remain the preferred in vivo system for years to come even though it will always be a chimeric system.
5.1. Immunodeficient status of the host
The common cytokine receptor γ chain (γc), encoded by Il2rg, constitutes a receptor signaling component shared by the IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 cytokine receptors [246]. The lack of γc in the NSG, NRG, NOG, and NCG strains (Il2rgnull mutation) causes a profound deficiency in many cell types that are dependent on these cytokines for their development, extending beyond the lymphocytes that need to be removed to prevent human cell rejection. For example, lymphoid tissue inducer (LTi) cells, a subset of innate lymphoid cells, depend on IL-7 normally produced by lymphoid tissue organizer (LTo) cells. In response, LTi cells provide ligands such as RANKL and lymphotoxin that stimulate the differentiation and organization of LTo cells to form the lymphoid tissue stroma and compartments during organogenesis, including germinal centers [247]. Therefore, models based on γc-deficient host strains have poorly developed lymphoid tissues and lymphatics [248], which can also limit the proper migration of T cells. It is unclear whether human HSCs can give rise to LTi cells capable of communicating with mouse LTo cells, but lymph nodes can develop, to some extent, several weeks after human HSC engraftment and accumulation of human T cells, although they remain abnormally small and disorganized. The absence of organized germinal centers limits the complete differentiation of human B cells, particularly in response to antigen (ineffective class switching), which may in part be due to the lack of proper interaction with follicular T-helper cells. Human T cells also do not elicit strong recall responses to vaccination [111] and adaptive responses to infection [249,250], possibly in part due to improper positioning of APCs and T cells, which is normally facilitated by the stroma.
In the developing thymus, TECs are an additional source of IL-7 required for the proliferation, survival, and maturation of thymocytes [251]. The lack of IL-7R signaling and LTi is also responsible for poor murine thymic tissue development, resulting in small size tissue and ill-defined corticomedullary junction [248]. Human T cells can develop to a limited extent through this vestigial tissue, and this may be facilitated by the expression of HLA transgenes that support human T-cell-positive selection [252]. However, using TCR-transduced HSCs with and without the presentation of cognate antigen for the TCR in APCs, it was shown that this lack of a well-organized thymic structure leads to impaired positive and negative selection processes, unlike in the grafted human thymus [144]. Moreover, in models in which human thymic tissue is implanted, this rudimentary mouse thymus may be unable to delete specific T-cell clones that the human thymus would otherwise purge, which may complicate data interpretation. In this case, the use of thymectomized [163] or athymic (Foxn1null) [120] mice would be required. Human thymic tissue, if not from fetal tissue, may be generated from iPSCs, but thymic epithelial populations and LTo cells are diverse, complex, and compartmentalized, and reconstituting a basic human thymus from iPSCs has been extremely challenging.
Interestingly, besides pairing with γc, the IL-7Rα chain can alternatively associate with the thymic stromal lymphopoietin receptor (TSLPR) chain to form a receptor for the cytokine TSLP, which has structural and functional similarity to IL-7 [253]. Transgenic TSLP expression in IL7−/− mice restores thymic cellularity and structure [254]. Based upon this work, the overexpression of TSLP under the Keratin 14 promoter in a BRGS HIS mouse model restores both lymph node and thymus size and structure [129]. Thus, the development of TSLP-transgenic NSG mice would likely improve the lymphoid tissue structure and at least partially overcome some of the aforementioned challenges.
Upon hematopoietic reconstitution, the HIS develops in a lymphopenic environment caused by the immunodeficient nature of the host strain and exacerbated by the irradiation process. This lymphopenia can cause human lymphocytes to undergo extensive proliferation to repopulate the host. During this process, naïve T cells can acquire effector and memory markers [255]. Of particular concern are the lymphocytes present in the fetal thymic tissue at the time of grafting and those potentially contaminating the injected HSCs, which are enriched for CD34+ cells but not pure. These lymphocytes do not get tolerized against mouse tissues and likely contribute to a T-cell-dependent autoimmune-like GvHD in NSG mice [144]. By generating HIS mice with fetal liver HSCs and transplanting allogeneic fresh fetal thymus, it was possible to show that pre-existing thymocytes can repopulate the periphery very quickly and constitute the dominant T-cell population in the periphery until the mice developed GvHD [144]. Thus, protocols had to be modified to deplete the pre-existing T cells, including freezing and thawing of fetal thymus fragments prior to transplantation, mechanical release of excess thymocytes, and treatment with a T-cell-depleting anti-human CD2 antibody to remove recent thymic emigrants and HSC-contaminating T cells. With these measures applied, the level of pre-existing grafted thymus-derived T cells significantly decreased, and the HSC-derived T cells constituted the dominant T-cell population in HIS mice [144].
5.2. Cross-species barriers
The ability of the murine host to support the development and homeostasis of human immune cells is limited by the ability of mouse cytokines and growth factors to signal through their counterpart human receptors. This limited cross-reactivity is responsible for the impaired development of a variety of human immune cell populations. Most notably, myeloid cells reconstitute poorly from HSCs and constitute 5–15% of circulating CD45+ cells in the blood (versus >50% in humans) [180,256]. Myeloid cells encompass a vast array of highly specialized cells that differentiate within specific niches in each tissue. The stromal cells that constitute this niche in HIS mice are of mouse origin and communication is for the most part broken. Hydrodynamic injection of plasmid DNA encoding human cytokines can improve the development of human DCs, monocytes, and macrophages in HIS mice [257]; however, the transient expression of cytokines, the high mortality rate of the injected mice, and the technical difficulties of this method has limited its utilization. The use of transgenes expressing human growth factors and cytokines (as in NSG-SGM3 and NOG-EXL mice; Table 2) support better myeloid cell development [256,258], but their expression is not restricted to specific niches and not regulated in level and in time, and excessive and/or continuous production of some of these cytokines can eventually lead to lethal adverse effects [258,259]. NSG-SGM3 mice are most susceptible, possibly because they express 25–100 times higher levels of transgenic cytokines than NOG-EXL mice [256,259]. Ideally, future modifications should attempt to replace mouse genes by human genes instead of relying on transgenes to achieve better spatial, temporal, and quantitative regulation of gene expression. In the absence of these transgenes, NSG mice humanized with human CD34+ HSCs are still capable of producing diverse populations of immune cells that commonly act as APCs (plasmacytoid and conventional DC subsets) albeit at low levels [180]. The use of HLA transgenes allows mouse APCs to participate in antigen presentation to human T cells; however, potential incompatibilities in the binding of costimulatory and adhesion molecules and in cytokine interactions (e.g. IFN-γ) can potentially limit the response [260].
Like T cells, B cells are not maintained for long term, regardless of whether they are produced from adult HSCs [145] or from PBMCs [114]. B cells derived from fetal liver cells persist longer but are eventually diluted by increasing T-cell populations in HIS mice with human fetal thymus grafts, resulting in reduced B cells as a percentage of human leukocytes over time. T-cell reconstitution is needed to support maximal human B-cell and myeloid reconstitution in such animals [163], indicating cross-talk resulting from human T-cell–APC interactions. Moreover, human B cells have impaired maturation, although this is less severe when T cells are present [261]. However, the improved long-term engraftment of peripheral T cells did not seem to help with the maintenance of PBMC-derived B cells [114]. Moreover, the replacement of mouse BAFF by human BAFF failed to improve B-cell maturation [262]. This underscores the need to better understand the minimum ligands required to support the differentiation, maturation, and maintenance of human immune populations and the cross-reactivity potential of the corresponding mouse ligands.
Also limited is the development of human NK cells, which has in large part been overcome by the transgenic expression of IL-15 [124]. Although granulocytes, erythrocytes, and platelets develop from HSCs in the bone marrow, very few are present in the circulation [263], in large part due to the previously mentioned macrophage activity. Very high levels of human chimerism may be detrimental, because recipient mice depend on sufficient amounts of mouse bone marrow surviving irradiation to produce the circulating erythrocytes they need to survive. NSG mice also lack C5a and are unable to support hemolytic complement activity, although this has been recently remedied by the development of NSG mice with an intact C5a [264]. Human IL-15 knock-in HIS mice support the development and functional maturation of circulating and tissue-resident human NK cells that elicit antitumor responses [[265], [266], [267]]. Recently, the Flavell group has introduced a model based on the MISTRG background, in which combined human liver and cytokine humanization confer enhanced human erythropoiesis and RBC survival in the circulation [268].
5.3. Practical limitations
Long-term maintenance of human immune cells in mice requires the self-renewal capacity of HSCs, yet these early progenitors are rare and difficult to obtain in large quantities. Cord blood is advantageous, as it does not require human subject direct intervention. Isolation of HSCs following blood mobilization is expensive and laborious and has potential toxicity for the donor. Bone marrow aspiration is momentarily painful but relatively harmless, and it requires an appropriate team to consent patients and perform the procedure. However, these are currently the only options when donors with known active disease or disease predisposition are needed (e.g. T1D patient or first-degree relatives). Fetal liver HSCs have the advantage of allowing reconstitution from an autologous fetal thymus, which is superior at supporting T-cell development compared with post-natal thymus [162,163]. Fetal tissue, however, is fraught with ethical/political controversies. When immune systems are reconstituted de novo from HSCs, they may not recapitulate the age of disease onset of the donor. Progress in the differentiation of patient-derived iPSCs into HSCs and TECs will be critical in overcoming these limitations as this approach can provide greater quantities of cells that can be better characterized and modified for experimental purposes. Similarly, iPSC-derived β-cells or islet organoids will have to be sufficiently similar to native β-cells to be killed by activated T cells and not aberrantly tolerogenic.
As recipient mice are increasingly genetically modified to improve the engraftment, differentiation, and function of human immune cells, they may be more difficult to breed, particularly when different lines of mice are crossed and a large number of mutations and transgenes have to be genotyped as a result. As new modifications created by different groups and centers demonstrate substantial improvement of models, issues of intellectual property can complicate and delay the use of multiple modifications in combination.
6. Future development and applications
Mice lacking γc have been essential for enabling the engraftment of human immune cells by blocking host–vs–graft reactions. However, when modeling autoimmune processes such as those leading to T1D, it is crucial to prevent xenogeneic GvH reactions by the human immune cells against mouse tissues to not only extend the timeline of studies before mice develop GvHD but also exclude tissue damage and inflammation that is not a direct result of and could mask autoimmune reactions. Mice in which MHC class I and II molecules are replaced by common HLA haplotypes may prove ideal HIS recipients. Transgenic expression of mouse TSLP could compensate for some of the detrimental effects of γc deficiency by supporting better lymphoid structure development. Addition of transgenes expressing human cytokines and ligands will continue to improve the engraftment, differentiation, and function of human immune cell populations; however, better control of their expression is important to minimize adverse effects and achieve more physiological frequencies of these cells. In particular, more work is needed to promote or sustain other human cell populations such as macrophages, eosinophils, and megakaryocytes. With major strides recently been made in cell engineering fueled by the CRISPR/Cas9 technology, it will become easier and more common in the future to humanize mouse genes instead of relying on transgenes to achieve a quasi-normal spatiotemporal regulation of the expression of the desired genes.
While the above improvements will produce more reliable in vivo models to study human immune processes in general, better understanding of autoimmune diseases and how to cure them will come from personalized HIS models [269]. The PI mouse was the first model to enable the comparison of control and diseased patients; however, it is difficult to implement it on a large scale because of high costs and invasive procedures involved. Models based on iPSC-derived cells are expected to transform the whole biomedical field for the study of a broad range of diseases. While deriving multiple cell types and organoids from iPSCs remains a lengthy, onerous, and expensive process, these iPSCs constitute an almost unlimited source of cells, which in some cases can be produced from unique and interesting cases. For in vitro models to be physiologically relevant, multiple cell types will have to be generated, combined together in the right proportions and maintained under appropriate conditions for the duration of the experiment. For example, modeling infiltrated islets in T1D will require islet organoids containing not only β-cells but other endocrine cells, macrophages, endothelial cells, fibroblasts, T and B lymphocytes, and possibly other populations such as neural cells [87]. For in vivo models, on the other hand, improved humanized recipient mice will provide the stromal infrastructure and many of the signals required for human immune cells to develop, and in this case, most efforts should be focused on producing iPSC-derived HSCs with comparable differentiation potential as bona fide HSCs. In addition, these HIS models will not be complete without TECs derived from the same iPSCs, which may be introduced into a ‘reconstituted’ thymus produced on a scaffold with other supporting cells, into neonatal thymic tissue or into swine fetal thymus tissue. Only this kind of system will ensure that the human lymphocytes will be tolerant to their host and should be favored over attempts to differentiate T and B cells in vitro and transfer them in vivo. However, certain types of iPSC-derived organoids remain infiltrated (and rejected) by autologous T cells, while other types are well tolerated [270].
Once such a model is established with demonstrated physiological relevance and lack of teratoma formation, incorporating mutations into iPSCs, to either disable a particular gene or introduce a SNP of interest (to be compared with the original unmodified iPSC line), can follow at a larger scale to assess the mechanisms and responses. For example, TECs from T1D patient-derived iPSCs might reveal the thymic epithelium-intrinsic defects that allow the escape of autoreactive T cells from negative selection in T1D individuals. Alternatively, the expression of specific genes such as AIRE or insulin could be modified to study their role in shaping the human (auto)immune TCR repertoire. Another ideal feature of such models is the ability to observe the killing of HLA-matched transplanted iPSC-derived islets or mouse β-cells (in mice transgenic for HLA and human antigens) by human immune cells, whereby any xeno- or alloreactivity can be ruled out. If spontaneous insulitis and β-cell destruction can be observed, it would be interesting to identify the factors that influence the aggressiveness of the disease and whether this correlates with the age of onset of the donor. Box 1 lists the major fundamental and mechanistic questions that could be addressed with HIS models to better understand human T1D.
Box 1. Potential applications of models to understand the immune processes leading to human T1D.
-
1)
Role of specific gene polymorphisms in altering the normal thymic selection of autoreactive T cells, resulting in skewed T-cell repertoires; increased numbers of T cells reactive to specific autoantigens; and reduced numbers or functionality of thymic-derived T cells. Influence of high-risk versus protective HLA haplotypes on T-cell repertoire and T-cell differentiation (conventional vs regulatory T cell).
-
2)Role of gene polymorphisms (or specific environmental factors) in:
-
a)altering the function of peripheral APCs, their acquisition and presentation of β-cell antigens and their phenotype.
-
b)altering the threshold of activation of T cells, their resistance to regulation, their ability or not to undergo apoptosis, anergy, or senescence.
-
c)affecting the function of Tregs, their plasticity, and their ability to co-localize with their target effector T cells.
-
d)the selection of autoreactive B cells, their stimulation to secrete autoantibodies against specific β-cell antigens, and the resulting autoantibody profile.
-
a)
-
3)
Recapitulation of the unique disease characteristics (endotype) of a particular donor in the corresponding in vivo model (e.g. same predominant autoantibodies, comparable T-cell repertoire, circulating antigen-specific T cells, etc.).
-
4)
Achieving spontaneous insulitis and β-cell killing without resorting to β-cell-damaging treatments and/or immunizations and determining whether this requires the coordinated action of multiple immune cell subsets?
-
5)
Determining the factors that control the immunogenicity of β-cells and the relative contribution of infection (role of specific enteroviruses (e.g. Coxsackie) or other viruses), different types of stress, and local inflammation.
Another major application that is increasingly sought after in both academic and industrial research settings is the evaluation of immune responses to treatments. Beyond establishing the mode of action of drugs on human cells, one may leverage personalized models to understand the differences in responsiveness between groups of patients to a specific treatment. These models will allow more in-depth mechanistic studies to pinpoint the cells or pathways that control responsiveness and identify new and reliable biomarkers that will facilitate the selection of suitable patients to achieve a greater rate of success using precision medicine. Box 2 lists the potential applications of in vitro or in vivo models to evaluate the efficacy and safety of therapies as an additional preclinical stage to reduce risk and potentially avert costly clinical trial failures.
Box 2. Potential applications of models to evaluate treatments for human T1D.
-
1)
Testing of islet encapsulation devices not only for the persistence of β-cell numbers and function but also for their resistance to autoimmune attacks or immune-mediated factors.
-
2)
Testing of iPSC modifications conferring certain immune evasion capabilities to iPSC-derived β-cells for long-term in vivo persistence.
-
3)
Testing the effect of new biologicals on human immune cells in vivo by assessing the phenotype of targeted cells across multiple key tissues, not just blood.
-
4)
Determining the mode of action of tolerogenic antigen-specific immunotherapies by following the fate of antigen-specific T cells in multiple key tissues.
-
5)
Testing the potential of linked and bystander suppression by induced Tregs when using a limited number of antigens/peptides.
-
6)
Testing the efficacy and specificity of cellular therapies such as CAR Tregs as well as determining the biodistribution of those cells and their persistence in vivo.
-
7)
Development of optimized and streamlined in vitro systems allowing the high-throughput generation of multiple cell types from multiple patients with subsequent testing of multiple treatments to assess the suitability for specific types of treatments. If the cost of such a system can be reduced sufficiently, it can subsequently save money on implementing expensive therapies that are doomed to fail.
While the incidence of T1D continues to increase in most parts of the world and many promising therapeutic approaches are in development or being clinically evaluated, there is still no single treatment that efficiently prevents or reverses disease. Recognizing and documenting the vast heterogeneity of T1D patients has considerably changed our way of mechanistically deciphering and treating this disease. Both in vitro and in vivo models presented here will benefit from the efforts of creating personalized systems. One must not forget similar efforts to model other autoimmune diseases with commonalities with T1D, from which useful insights may be gained as far as immune processes are concerned. Conversely, knowledge gained on the defective mechanisms of tolerance with T1D models may be applicable to other autoimmune diseases. While focus is expected to be on making iPSC-derived human immune systems as physiologically relevant and accurate as possible, efforts are equally needed to make these models more accessible and affordable, so that once outstanding scientific questions are addressed, these models can be used on a larger scale for evaluating and accelerating the development of multiple drugs and therapies that will be most suitable to groups of patients.
Funding and acknowledgments
This work was written using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID: SCR_014393; https://hirnetwork.org). The following grants currently support (or have supported) the development and use of in vitro and/or in vivo models by the authors to study human T1D-associated autoimmune processes: NIH grants TL1 TR001875 and F31 DK121377 to RM; R01 AI132963 to MAB; R24 OD0426640 and U01 DK104218 to MAB and DLG; U01 DK123559 to AVP, MSA, and MS; R01 DK107383 to MSA; R01 DK103585, UC4 DK104207, and R21 AI146828 to MS; R01 AI142428 to RJC; Human Islet Research Network Emerging Leader in Type 1 Diabetes awards (U24 DK104162) to LMRF and RCS; Juvenile Diabetes Research Foundation grant 5-COE-2020-967-M-N to MAB and DLG; and American Diabetes Association Postdoctoral Fellowship 1-18-PDF-074, Russell Berrie Foundation Fellowship, and Nelson Faculty Development Award to MKM.
Authors' contributions
MKM and RJC conceived and drafted the manuscript and generated the figures. All other authors contributed sections of the manuscript. MKM, RM, CB, LMRF, RCS, MAB, DLG, AVP, MSA, MS, and RJC edited the manuscript. MKM, MSA, MS, and RJC revised the manuscript. All co-authors have approved the submitted version, agreed to be personally accountable for his/her contributions, and will ensure that any questions regarding accuracy or integrity are investigated and resolved.
Conflict of interest
All co-authors are members of the HIRN. DLG and MAB receive research support from and are consultants for The Jackson Laboratory. The authors declare no other competing interests.
References
- 1.Bakay M., Pandey R., Grant S.F.A., Hakonarson H. The genetic contribution to type 1 diabetes. Current Diabetes Reports. 2019;19(11):116. doi: 10.1007/s11892-019-1235-1. [DOI] [PubMed] [Google Scholar]
- 2.Rewers M., Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilonen J., Lempainen J., Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nature Reviews Endocrinology. 2019;15(11):635–650. doi: 10.1038/s41574-019-0254-y. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia M., Ahmed S., Anderson M.S., Atkinson M.A., Becker D., Bingley P.J., et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lythe G., Callard R.E., Hoare R.L., Molina-Paris C. How many TCR clonotypes does a body maintain? Journal of Theoretical Biology. 2016;389:214–224. doi: 10.1016/j.jtbi.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng M., Anderson M.S. Thymic tolerance as a key brake on autoimmunity. Nature Immunology. 2018;19(7):659–664. doi: 10.1038/s41590-018-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotter J., Brors B., Hergenhahn M., Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. Journal of Experimental Medicine. 2004;199(2):155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culina S., Lalanne A.I., Afonso G., Cerosaletti K., Pinto S., Sebastiani G., et al. Islet-reactive CD8(+) T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Science Immunology. 2018;3(20) doi: 10.1126/sciimmunol.aao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes N., Serge A., Ferrier P., Irla M. Thymic crosstalk coordinates medulla organization and T-cell tolerance induction. Frontiers in Immunology. 2015;6:365. doi: 10.3389/fimmu.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugliese A., Zeller M., Fernandez A., Jr., Zalcberg L.J., Bartlett R.J., Ricordi C., et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature Genetics. 1997;15(3):293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 11.Vafiadis P., Bennett S.T., Todd J.A., Nadeau J., Grabs R., Goodyer C.G., et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nature Genetics. 1997;15(3):289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 12.Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildin R.S., Ramsdell F., Peake J., Faravelli F., Casanova J.L., Buist N., et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature Genetics. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 14.Hull C.M., Peakman M., Tree T.I.M. Regulatory T cell dysfunction in type 1 diabetes: what's broken and how can we fix it? Diabetologia. 2017;60(10):1839–1850. doi: 10.1007/s00125-017-4377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettini M., Bettini M.L. Diabetes; 2021. Function, failure, and the future potential of Tregs in type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vehik K., Beam C.A., Mahon J.L., Schatz D.A., Haller M.J., Sosenko J.M., et al. Development of autoantibodies in the TrialNet natural history study. Diabetes Care. 2011;34(9):1897–1901. doi: 10.2337/dc11-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fousteri G., Ippolito E., Ahmed R., Hamad A.R.A. Beta-cell specific autoantibodies: are they just an indicator of type 1 diabetes? Current Diabetes Reviews. 2017;13(3):322–329. doi: 10.2174/1573399812666160427104157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M.J., Rihanek M., Wasserfall C., Mathews C.E., Atkinson M.A., Gottlieb P.A., et al. Loss of B-cell anergy in type 1 diabetes is associated with high-risk HLA and non-HLA disease susceptibility alleles. Diabetes. 2018;67(4):697–703. doi: 10.2337/db17-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pescovitz M.D., Greenbaum C.J., Krause-Steinrauf H., Becker D.J., Gitelman S.E., Goland R., et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. New England Journal of Medicine. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meffre E., O'Connor K.C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunological Reviews. 2019;292(1):90–101. doi: 10.1111/imr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creusot R.J., Postigo-Fernandez J., Teteloshvili N. Altered function of antigen-presenting cells in type 1 diabetes: a challenge for antigen-specific immunotherapy? Diabetes. 2018;67(8):1481–1494. doi: 10.2337/db17-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X., Huang Q., Petersen F. History and milestones of mouse models of autoimmune diseases. Current Pharmaceutical Design. 2015;21(18):2308–2319. doi: 10.2174/1381612821666150316115412. [DOI] [PubMed] [Google Scholar]
- 23.Yu X., Petersen F. A methodological review of induced animal models of autoimmune diseases. Autoimmunity Reviews. 2018;17(5):473–479. doi: 10.1016/j.autrev.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Anderson M., Bluestone J. The NOD mouse: a model of immune dysregulation. Annual Review of Immunology. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 25.Mathews C.E., Xue S., Posgai A., Lightfoot Y.L., Li X., Lin A., et al. Acute versus progressive onset of diabetes in NOD mice: potential implications for therapeutic interventions in type 1 diabetes. Diabetes. 2015;64(11):3885–3890. doi: 10.2337/db15-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krischer J.P., Liu X., Lernmark A., Hagopian W.A., Rewers M.J., She J.X., et al. The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes. 2017;66(12):3122–3129. doi: 10.2337/db17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama M. Insulin as a key autoantigen in the development of type 1 diabetes. Diabetes/Metabolism Research and Reviews. 2011;27(8):773–777. doi: 10.1002/dmrr.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serreze D.V., Chapman H.D., Varnum D.S., Hanson M.S., Reifsnyder P.C., Richard S.D., et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new "speed congenic" stock of NOD.Ig mu null mice. Journal of Experimental Medicine. 1996;184(5):2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C.Y., Rodriguez-Pinto D., Du W., Ahuja A., Henegariu O., Wong F.S., et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. Journal of Clinical Investigation. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiu Y., Wong C.P., Bouaziz J.D., Hamaguchi Y., Wang Y., Pop S.M., et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. The Journal of Immunology. 2008;180(5):2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 31.Katz J.D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 32.Verdaguer J., Schmidt D., Amrani A., Anderson B., Averill N., Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. Journal of Experimental Medicine. 1997;186(10):1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulbert C., Riseili B., Rojas M., Thomas J.W. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. The Journal of Immunology. 2001;167(10):5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 34.Gerling I.C., Serreze D.V., Christianson S.W., Leiter E.H. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992;41(12):1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 35.Balasa B., Van Gunst K., Sarvetnick N. The microbial product lipopolysaccharide confers diabetogenic potential on the T cell repertoire of BDC2.5/NOD mice: implications for the etiology of autoimmune diabetes. Clinical Immunology. 2000;95(2):93–98. doi: 10.1006/clim.2000.4855. [DOI] [PubMed] [Google Scholar]
- 36.Wallis R.H., Wang K., Marandi L., Hsieh E., Ning T., Chao G.Y., et al. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes. 2009;58(4):1007–1017. doi: 10.2337/db08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mordes J.P., Guberski D.L., Leif J.H., Woda B.A., Flanagan J.F., Greiner D.L., et al. LEW.1WR1 rats develop autoimmune diabetes spontaneously and in response to environmental perturbation. Diabetes. 2005;54(9):2727–2733. doi: 10.2337/diabetes.54.9.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirabassi R.S., Guberski D.L., Blankenhorn E.P., Leif J.H., Woda B.A., Liu Z., et al. Infection with viruses from several families triggers autoimmune diabetes in LEW∗1WR1 rats: prevention of diabetes by maternal immunization. Diabetes. 2010;59(1):110–118. doi: 10.2337/db09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guberski D.L., Thomas V.A., Shek W.R., Like A.A., Handler E.S., Rossini A.A., et al. Induction of type I diabetes by Kilham's rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254(5034):1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 40.O'Kell A.L., Wasserfall C., Catchpole B., Davison L.J., Hess R.S., Kushner J.A., et al. Comparative pathogenesis of autoimmune diabetes in humans, NOD mice, and canines: has a valuable animal model of type 1 diabetes been overlooked? Diabetes. 2017;66(6):1443–1452. doi: 10.2337/db16-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Tourino I., Arif S., Eichmann M., Peakman M. T cells in type 1 diabetes: instructors, regulators and effectors: a comprehensive review. Journal of Autoimmunity. 2016;66:7–16. doi: 10.1016/j.jaut.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Smith M.J., Cambier J.C., Gottlieb P.A. Endotypes in T1D: B lymphocytes and early onset. Current Opinion in Endocrinology Diabetes and Obesity. 2020;27(4):225–230. doi: 10.1097/MED.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marca V., Gianchecchi E., Fierabracci A. Type 1 diabetes and its multi-factorial pathogenesis: the putative role of NK cells. International Journal of Molecular Sciences. 2018;19(3) doi: 10.3390/ijms19030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrera S.M., Chen Y.G., Hagopian W.A., Hessner M.J. Blood-based signatures in type 1 diabetes. Diabetologia. 2016;59(3):414–425. doi: 10.1007/s00125-015-3843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed S., Cerosaletti K., James E., Long S.A., Mannering S., Speake C., et al. Standardizing T-cell biomarkers in type 1 diabetes: challenges and recent advances. Diabetes. 2019;68(7):1366–1379. doi: 10.2337/db19-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roep B.O., Arden S.D., de Vries R.R., Hutton J.C. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990;345(6276):632–634. doi: 10.1038/345632a0. [DOI] [PubMed] [Google Scholar]
- 47.Madley R., Nauman G., Danzl N., Borsotti C., Khosravi Maharlooei M., Li H.W., et al. Negative selection of human T cells recognizing a naturally-expressed tissue-restricted antigen in the human thymus. Journal of Translational Autoimmunity. 2020;3:100061. doi: 10.1016/j.jtauto.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unger W.W., Pearson T., Abreu J.R., Laban S., van der Slik A.R., der Kracht S.M., et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skowera A., Ellis R.J., Varela-Calvino R., Arif S., Huang G.C., Van-Krinks C., et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. Journal of Clinical Investigation. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell-Thompson M., Wasserfall C., Kaddis J., Albanese-O'Neill A., Staeva T., Nierras C., et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes/Metabolism Research and Reviews. 2012;28(7):608–617. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaestner K.H., Powers A.C., Naji A., Consortium H., Atkinson M.A. NIH initiative to improve understanding of the pancreas, islet, and autoimmunity in type 1 diabetes: the human pancreas analysis program (HPAP) Diabetes. 2019;68(7):1394–1402. doi: 10.2337/db19-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters L., Posgai A., Brusko T.M. Islet-immune interactions in type 1 diabetes: the nexus of beta cell destruction. Clinical and Experimental Immunology. 2019;198(3):326–340. doi: 10.1111/cei.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell-Thompson M., Fu A., Kaddis J.S., Wasserfall C., Schatz D.A., Pugliese A., et al. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson S.J., Rodriguez-Calvo T., Gerling I.C., Mathews C.E., Kaddis J.S., Russell M.A., et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59(11):2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coppieters K.T., Dotta F., Amirian N., Campbell P.D., Kay T.W., Atkinson M.A., et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. Journal of Experimental Medicine. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell-Thompson M.L., Kaddis J.S., Wasserfall C., Haller M.J., Pugliese A., Schatz D.A., et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–221. doi: 10.1007/s00125-015-3752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arif S., Leete P., Nguyen V., Marks K., Nor N.M., Estorninho M., et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63(11):3835–3845. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson S.J., Leete P., Bone A.J., Foulis A.K., Morgan N.G. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56(1):185–193. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 59.Krogvold L., Edwin B., Buanes T., Frisk G., Skog O., Anagandula M., et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64(5):1682–1687. doi: 10.2337/db14-1370. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Calvo T., Ekwall O., Amirian N., Zapardiel-Gonzalo J., von Herrath M.G. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63(11):3880–3890. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bender C., Rajendran S., von Herrath M.G. New insights into the role of autoreactive CD8 T cells and cytokines in human type 1 diabetes. Frontiers in Endocrinology. 2020;11:606434. doi: 10.3389/fendo.2020.606434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michels A.W., Landry L.G., McDaniel K.A., Yu L., Campbell-Thompson M., Kwok W.W., et al. Islet-derived CD4 T-cells targeting proinsulin in human autoimmune diabetes. Diabetes. 2016 doi: 10.2337/db16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pathiraja V., Kuehlich J.P., Campbell P.D., Krishnamurthy B., Loudovaris T., Coates P.T., et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64(1):172–182. doi: 10.2337/db14-0858. [DOI] [PubMed] [Google Scholar]
- 64.Babon J.A., DeNicola M.E., Blodgett D.M., Crevecoeur I., Buttrick T.S., Maehr R., et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nature Medicine. 2016;22(12):1482–1487. doi: 10.1038/nm.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez-Duque S., Azoury M.E., Colli M.L., Afonso G., Turatsinze J.V., Nigi L., et al. Conventional and neo-antigenic peptides presented by beta cells are targeted by circulating naive CD8+ T cells in type 1 diabetic and healthy donors. Cell Metabolism. 2018;28(6):946–960 e946. doi: 10.1016/j.cmet.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Russell M.A., Redick S.D., Blodgett D.M., Richardson S.J., Leete P., Krogvold L., et al. HLA class II antigen processing and presentation pathway components demonstrated by transcriptome and protein analyses of islet beta-cells from donors with type 1 diabetes. Diabetes. 2019;68(5):988–1001. doi: 10.2337/db18-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.James E.A., Mallone R., Kent S.C., DiLorenzo T.P. T-cell epitopes and neo-epitopes in type 1 diabetes: a comprehensive update and reappraisal. Diabetes. 2020;69(7):1311–1335. doi: 10.2337/dbi19-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaddis J.S., Rouse L., Parent A.V., Saunders D.C., Shalev A., Stabler C.L., et al. From type 1 diabetes biology to therapy: the human islet research Network. Molecular Metabolism. 2021:101283. doi: 10.1016/j.molmet.2021.101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verhasselt B., De Smedt M., Verhelst R., Naessens E., Plum J. Retrovirally transduced CD34++ human cord blood cells generate T cells expressing high levels of the retroviral encoded green fluorescent protein marker in vitro. Blood. 1998;91(2):431–440. [PubMed] [Google Scholar]
- 70.Evans J.T., Okamoto Y., Douek D.C., McFarland R.D., Gatlin J., Koup R.A., et al. Thymocyte differentiation from lentivirus-marked CD34(+) cells in infant and adult human thymus. Journal of Immunological Methods. 2000;245(1–2):31–43. doi: 10.1016/s0022-1759(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 71.Zucchelli S., Holler P., Yamagata T., Roy M., Benoist C., Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22(3):385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 72.Feuerer M., Jiang W., Holler P.D., Satpathy A., Campbell C., Bogue M., et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogal J., Zbinden A., Schenke-Layland K., Loskill P. Stem-cell based organ-on-a-chip models for diabetes research. Advanced Drug Delivery Reviews. 2019;140:101–128. doi: 10.1016/j.addr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Morsink M.A.J., Willemen N.G.A., Leijten J., Bansal R., Shin S.R. Immune organs and immune cells on a chip: an overview of biomedical applications. Micromachines. 2020;11(9) doi: 10.3390/mi11090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammed J.S., Wang Y., Harvat T.A., Oberholzer J., Eddington D.T. Microfluidic device for multimodal characterization of pancreatic islets. Lab on a Chip. 2009;9(1):97–106. doi: 10.1039/b809590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., Brooks J.C., Hu J., Ford K.I., Easley C.J. 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling. Lab on a Chip. 2017;17(2):341–349. doi: 10.1039/c6lc01201a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen D.T., van Noort D., Jeong I.K., Park S. Endocrine system on chip for a diabetes treatment model. Biofabrication. 2017;9(1) doi: 10.1088/1758-5090/aa5cc9. [DOI] [PubMed] [Google Scholar]
- 78.Bauer S., Wennberg Huldt C., Kanebratt K.P., Durieux I., Gunne D., Andersson S., et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Scientific Reports. 2017;7(1):14620. doi: 10.1038/s41598-017-14815-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han S., Yan J.J., Shin Y., Jeon J.J., Won J., Jeong H.E., et al. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab on a Chip. 2012;12(20):3861–3865. doi: 10.1039/c2lc40445a. [DOI] [PubMed] [Google Scholar]
- 80.Hamza B., Irimia D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab on a Chip. 2015;15(12):2625–2633. doi: 10.1039/c5lc00245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pavesi A., Tan A.T., Koh S., Chia A., Colombo M., Antonecchia E., et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight. 2017;2(12) doi: 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irimia D., Wang X. Inflammation-on-a-Chip: probing the immune system ex vivo. Trends in Biotechnology. 2018;36(9):923–937. doi: 10.1016/j.tibtech.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newby B.N., Brusko T.M., Zou B., Atkinson M.A., Clare-Salzler M., Mathews C.E. Type 1 interferons potentiate human CD8(+) T-cell cytotoxicity through a STAT4- and granzyme B-dependent pathway. Diabetes. 2017;66(12):3061–3071. doi: 10.2337/db17-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dean J.W., Peters L.D., Fuhrman C.A., Seay H.R., Posgai A.L., Stimpson S.E., et al. Innate inflammation drives NK cell activation to impair Treg activity. Journal of Autoimmunity. 2020;108:102417. doi: 10.1016/j.jaut.2020.102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y., Frei A.W., Yang E.Y., Labrada-Miravet I., Sun C., Rong Y., et al. In vitro platform establishes antigen-specific CD8(+) T cell cytotoxicity to encapsulated cells via indirect antigen recognition. Biomaterials. 2020;256:120182. doi: 10.1016/j.biomaterials.2020.120182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel S.N., Ishahak M., Chaimov D., Velraj A., LaShoto D., Hagan D.W., et al. Organoid microphysiological system preserves pancreatic islet function within 3D matrix. Science Advances. 2021;7(7) doi: 10.1126/sciadv.aba5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joshi K., Cameron F., Tiwari S., Mannering S.I., Elefanty A.G., Stanley E.G. Modeling type 1 diabetes using pluripotent stem cell technology. Frontiers in Endocrinology. 2021;12:635662. doi: 10.3389/fendo.2021.635662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu H., Wang B., Ono M., Kagita A., Fujii K., Sasakawa N., et al. Targeted disruption of HLA genes via CRISPR-cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24(4):566–578 e567. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Han X., Wang M., Duan S., Franco P.J., Kenty J.H., Hedrick P., et al. Generation of hypoimmunogenic human pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(21):10441–10446. doi: 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parent A.V., Faleo G., Chavez J., Saxton M., Berrios D.I., Kerper N.R., et al. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Reports. 2021;36(7):109538. doi: 10.1016/j.celrep.2021.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohtashami M., Shah D.K., Nakase H., Kianizad K., Petrie H.T., Zuniga-Pflucker J.C. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. The Journal of Immunology. 2010;185(2):867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 92.Trotman-Grant A.C., Mohtashami M., De Sousa Casal J., Martinez E.C., Lee D., Teichman S., et al. DL4-mubeads induce T cell lineage differentiation from stem cells in a stromal cell-free system. Nature Communications. 2021;12(1):5023. doi: 10.1038/s41467-021-25245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leite N.C., Sintov E., Meissner T.B., Brehm M.A., Greiner D.L., Harlan D.M., et al. Modeling type 1 diabetes in vitro using human pluripotent stem cells. Cell Reports. 2020;32(2):107894. doi: 10.1016/j.celrep.2020.107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marciniak A., Cohrs C.M., Tsata V., Chouinard J.A., Selck C., Stertmann J., et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nature Protocols. 2014;9(12):2809–2822. doi: 10.1038/nprot.2014.195. [DOI] [PubMed] [Google Scholar]
- 95.Panzer J.K., Hiller H., Cohrs C.M., Almaca J., Enos S.J., Beery M., et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight. 2020;5(8) doi: 10.1172/jci.insight.134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huber M.K., Drotar D.M., Hiller H., Beery M.L., Joseph P., Kusmartseva I., et al. Observing islet function and islet-immune cell interactions in live pancreatic tissue slices. Journal of Visualized Experiments. 2021;170 doi: 10.3791/62207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weitz J.R., Makhmutova M., Almaca J., Stertmann J., Aamodt K., Brissova M., et al. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia. 2018;61(1):182–192. doi: 10.1007/s00125-017-4416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weitz J.R., Jacques-Silva C., Qadir M.M.F., Umland O., Pereira E., Qureshi F., et al. Secretory functions of macrophages in the human pancreatic islet are regulated by endogenous purinergic signaling. Diabetes. 2020;69(6):1206–1218. doi: 10.2337/db19-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giger B., Bonanomi A., Odermatt B., Ladell K., Speck R.F., Kojic D., et al. Human tonsillar tissue block cultures differ from autologous tonsillar cell suspension cultures in lymphocyte subset activation and cytokine gene expression. Journal of Immunological Methods. 2004;289(1–2):179–190. doi: 10.1016/j.jim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Grivel J.C., Margolis L. Use of human tissue explants to study human infectious agents. Nature Protocols. 2009;4(2):256–269. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knoblich K., Cruz Migoni S., Siew S.M., Jinks E., Kaul B., Jeffery H.C., et al. The human lymph node microenvironment unilaterally regulates T-cell activation and differentiation. PLoS Biology. 2018;16(9) doi: 10.1371/journal.pbio.2005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halkias J., Melichar H.J., Taylor K.T., Ross J.O., Yen B., Cooper S.B., et al. Opposing chemokine gradients control human thymocyte migration in situ. Journal of Clinical Investigation. 2013;123(5):2131–2142. doi: 10.1172/JCI67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross J.O., Melichar H.J., Halkias J., Robey E.A. Studying T cell development in thymic slices. Methods in Molecular Biology. 2016;1323:131–140. doi: 10.1007/978-1-4939-2809-5_11. [DOI] [PubMed] [Google Scholar]
- 104.Sood A., Dong M., Melichar H.J. Preparation and applications of organotypic thymic slice cultures. Journal of Visualized Experiments. 2016;114 doi: 10.3791/54355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lancaster J.N., Ehrlich L.I. Analysis of thymocyte migration, cellular interactions, and activation by multiphoton fluorescence microscopy of live thymic slices. Methods in Molecular Biology. 2017;1591:9–25. doi: 10.1007/978-1-4939-6931-9_2. [DOI] [PubMed] [Google Scholar]
- 106.Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B., et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. The Journal of Immunology. 1995;154(1):180–191. [PubMed] [Google Scholar]
- 107.Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I., et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nature Immunology. 2007;8(12):1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 108.Sharp R.C., Brown M.E., Shapiro M.R., Posgai A.L., Brusko T.M. The immunoregulatory role of the signal regulatory protein family and CD47 signaling pathway in type 1 diabetes. Frontiers in Immunology. 2021 doi: 10.3389/fimmu.2021.739048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 110.Pearson T., Shultz L.D., Miller D., King M., Laning J., Fodor W., et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clinical and Experimental Immunology. 2008;154(2):270–284. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Douam F., Ziegler C.G.K., Hrebikova G., Fant B., Leach R., Parsons L., et al. Selective expansion of myeloid and NK cells in humanized mice yields human-like vaccine responses. Nature Communications. 2018;9(1):5031. doi: 10.1038/s41467-018-07478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McIntosh B.E., Brown M.E., Duffin B.M., Maufort J.P., Vereide D.T., Slukvin, et al. Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports. 2015;4(2):171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rahmig S., Kronstein-Wiedemann R., Fohgrub J., Kronstein N., Nevmerzhitskaya A., Bornhauser M., et al. Improved human erythropoiesis and platelet formation in humanized NSGW41 mice. Stem Cell Reports. 2016;7(4):591–601. doi: 10.1016/j.stemcr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brehm M.A., Kenney L.L., Wiles M.V., Low B.E., Tisch R.M., Burzenski L., et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. The FASEB Journal. 2019;33(3):3137–3151. doi: 10.1096/fj.201800636R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yaguchi T., Kobayashi A., Inozume T., Morii K., Nagumo H., Nishio H., et al. Human PBMC-transferred murine MHC class I/II-deficient NOG mice enable long-term evaluation of human immune responses. Cellular and Molecular Immunology. 2018;15(11):953–962. doi: 10.1038/cmi.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A., et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 117.Legrand N., Huntington N.D., Nagasawa M., Bakker A.Q., Schotte R., Strick-Marchand H., et al. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13224–13229. doi: 10.1073/pnas.1101398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y., Mention J.J., Court N., Masse-Ranson G., Toubert A., Spits H., et al. A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. European Journal of Immunology. 2016;46(5):1291–1299. doi: 10.1002/eji.201546132. [DOI] [PubMed] [Google Scholar]
- 119.Yamauchi T., Takenaka K., Urata S., Shima T., Kikushige Y., Tokuyama T., et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121(8):1316–1325. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
- 120.Shultz L.D., Keck J., Burzenski L., Jangalwe S., Vaidya S., Greiner D.L., et al. Humanized mouse models of immunological diseases and precision medicine. Mammalian Genome. 2019;30(5–6):123–142. doi: 10.1007/s00335-019-09796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wunderlich M., Chou F.S., Link K.A., Mizukawa B., Perry R.L., Carroll M., et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24(10):1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ito R., Takahashi T., Katano I., Kawai K., Kamisako T., Ogura T., et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. The Journal of Immunology. 2013;191(6):2890–2899. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]
- 123.Hanazawa A., Ito R., Katano I., Kawai K., Goto M., Suemizu H., et al. Generation of human immunosuppressive myeloid cell populations in human interleukin-6 transgenic NOG mice. Frontiers in Immunology. 2018;9:152. doi: 10.3389/fimmu.2018.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katano I., Nishime C., Ito R., Kamisako T., Mizusawa T., Ka Y., et al. Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15- transgenic NOG mouse. Scientific Reports. 2017;7(1):17230. doi: 10.1038/s41598-017-17442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shultz L.D., Saito Y., Najima Y., Tanaka S., Ochi T., Tomizawa M., et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Serr I., Furst R.W., Achenbach P., Scherm M.G., Gokmen F., Haupt F., et al. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nature Communications. 2016;7:10991. doi: 10.1038/ncomms10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Covassin L., Laning J., Abdi R., Langevin D.L., Phillips N.E., Shultz L.D., et al. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease. Clinical and Experimental Immunology. 2011;166(2):269–280. doi: 10.1111/j.1365-2249.2011.04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S.V., Teichmann L.L., et al. Development and function of human innate immune cells in a humanized mouse model. Nature Biotechnology. 2014;32(4):364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y., Masse-Ranson G., Garcia Z., Bruel T., Kok A., Strick-Marchand H., et al. A human immune system mouse model with robust lymph node development. Nature Methods. 2018;15(8):623–630. doi: 10.1038/s41592-018-0071-6. [DOI] [PubMed] [Google Scholar]
- 130.Mosier D.E., Gulizia R.J., Baird S.M., Wilson D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 131.Mosier D.E., Gulizia R.J., Baird S.M., Spector S., Spector D., Kipps T.J., et al. Studies of HIV infection and the development of Epstein-Barr virus-related B cell lymphomas following transfer of human lymphocytes to mice with severe combined immunodeficiency. Current Topics in Microbiology and Immunology. 1989;152:195–199. doi: 10.1007/978-3-642-74974-2_23. [DOI] [PubMed] [Google Scholar]
- 132.Nakamine H., Okano M., Taguchi Y., Pirruccello S.J., Davis J.R., Beisel K.W., et al. Hematopathologic features of Epstein-Barr virus-induced human B-lymphoproliferation in mice with severe combined immunodeficiency. A model of lymphoproliferative diseases in immunocompromised patients. Laboratory Investigation. 1991;65(4):389–399. [PubMed] [Google Scholar]
- 133.Hesselton R.M., Greiner D.L., Mordes J.P., Rajan T.V., Sullivan J.L., Shultz L.D. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. The Journal of Infectious Diseases. 1995;172(4):974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 134.Greiner D.L., Shultz L.D., Yates J., Appel M.C., Perdrizet G., Hesselton R.M., et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. American Journal of Pathology. 1995;146(4):888–902. [PMC free article] [PubMed] [Google Scholar]
- 135.Wagar E.J., Cromwell M.A., Shultz L.D., Woda B.A., Sullivan J.L., Hesselton R.M., et al. Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. The Journal of Immunology. 2000;165(1):518–527. doi: 10.4049/jimmunol.165.1.518. [DOI] [PubMed] [Google Scholar]
- 136.King M.A., Covassin L., Brehm M.A., Racki W., Pearson T., Leif J., et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clinical and Experimental Immunology. 2009;157(1):104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Namikawa R., Weilbaecher K.N., Kaneshima H., Yee E.J., McCune J.M. Long-term human hematopoiesis in the SCID-hu mouse. Journal of Experimental Medicine. 1990;172(4):1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lan P., Wang L., Diouf B., Eguchi H., Su H., Bronson R., et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 139.Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 140.Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A., et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature Medicine. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 141.Hu Z., Yang Y.G. Human lymphohematopoietic reconstitution and immune function in immunodeficient mice receiving cotransplantation of human thymic tissue and CD34(+) cells. Cellular and Molecular Immunology. 2012;9(3):232–236. doi: 10.1038/cmi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wunderlich M., Chou F.S., Sexton C., Presicce P., Chougnet C.A., Aliberti J., et al. Improved multilineage human hematopoietic reconstitution and function in NSGS mice. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prochazka M., Gaskins H.R., Shultz L.D., Leiter E.H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khosravi-Maharlooei M., Li H., Hoelzl M., Zhao G., Ruiz A., Misra A., et al. Role of the thymus in spontaneous development of a multi-organ autoimmune disease in human immune system mice. Journal of Autoimmunity. 2021;119:102612. doi: 10.1016/j.jaut.2021.102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalscheuer H., Danzl N., Onoe T., Faust T., Winchester R., Goland R., et al. A model for personalized in vivo analysis of human immune responsiveness. Science Translational Medicine. 2012;4(125):125ra130. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fudaba Y., Onoe T., Chittenden M., Shimizu A., Shaffer J.M., Bronson R., et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. The Journal of Immunology. 2008;181(11):7649–7659. doi: 10.4049/jimmunol.181.11.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nikolic B., Gardner J.P., Scadden D.T., Arn J.S., Sachs D.H., Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. The Journal of Immunology. 1999;162(6):3402–3407. [PubMed] [Google Scholar]
- 148.Kooreman N.G., de Almeida P.E., Stack J.P., Nelakanti R.V., Diecke S., Shao N.Y., et al. Alloimmune responses of humanized mice to human pluripotent stem cell therapeutics. Cell Reports. 2017;20(8):1978–1990. doi: 10.1016/j.celrep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kalscheuer H., Onoe T., Dahmani A., Li H.W., Holzl M., Yamada K., et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. The Journal of Immunology. 2014;192(7):3442–3450. doi: 10.4049/jimmunol.1302886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kamel-Reid S., Dick J.E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 151.Lapidot T., Pflumio F., Doedens M., Murdoch B., Williams D.E., Dick J.E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255(5048):1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 152.Bhatia M., Bonnet D., Murdoch B., Gan O.I., Dick J.E. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nature Medicine. 1998;4(9):1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 153.Pearson T., Greiner D.L., Shultz L.D. Creation of "humanized" mice to study human immunity. Current Protocols in Immunology. 2008;15 doi: 10.1002/0471142735.im1521s81. Unit 15 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu Z., Van Rooijen N., Yang Y.G. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118(22):5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu Z., Yang Y.G. Full reconstitution of human platelets in humanized mice after macrophage depletion. Blood. 2012;120(8):1713–1716. doi: 10.1182/blood-2012-01-407890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Watanabe Y., Takahashi T., Okajima A., Shiokawa M., Ishii N., Katano I., et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) International Immunology. 2009;21(7):843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 157.Theocharides A.P., Rongvaux A., Fritsch K., Flavell R.A., Manz M.G. Humanized hemato-lymphoid system mice. Haematologica. 2016;101(1):5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ito R., Takahashi T., Ito M. Humanized mouse models: application to human diseases. Journal of Cellular Physiology. 2018;233(5):3723–3728. doi: 10.1002/jcp.26045. [DOI] [PubMed] [Google Scholar]
- 159.Hess N.J., Lindner P.N., Vazquez J., Grindel S., Hudson A.W., Stanic A.K., et al. Different human immune lineage compositions are generated in non-conditioned NBSGW mice depending on HSPC source. Frontiers in Immunology. 2020;11:573406. doi: 10.3389/fimmu.2020.573406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Han A.R., Lee J.E., Lee M.J., Ko S.Y., Shin H.S., Lee J.Y., et al. Distinct repopulation activity in hu-mice between CB- and LPB-CD34+ cells by enrichment of transcription factors. International Journal of Stem Cells. 2021;14(2):203–211. doi: 10.15283/ijsc21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sonntag K., Eckert F., Welker C., Muller H., Muller F., Zips D., et al. Chronic graft-versus-host-disease in CD34(+)-humanized NSG mice is associated with human susceptibility HLA haplotypes for autoimmune disease. Journal of Autoimmunity. 2015;62:55–66. doi: 10.1016/j.jaut.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 162.Brown M.E., Zhou Y., McIntosh B.E., Norman I.G., Lou H.E., Biermann M., et al. A humanized mouse model generated using surplus neonatal tissue. Stem Cell Reports. 2018;10(4):1175–1183. doi: 10.1016/j.stemcr.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khosravi-Maharlooei M., Hoelzl M., Li H.W., Madley R.C., Waffarn E.E., Danzl N.M., et al. Rapid thymectomy of NSG mice to analyze the role of native and grafted thymi in humanized mice. European Journal of Immunology. 2020;50(1):138–141. doi: 10.1002/eji.201948205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Borsotti C., Danzl N.M., Nauman G., Holzl M.A., French C., Chavez E., et al. HSC extrinsic sex-related and intrinsic autoimmune disease-related human B-cell variation is recapitulated in humanized mice. Blood Advances. 2017;1(23):2007–2018. doi: 10.1182/bloodadvances.2017006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimizu I., Fudaba Y., Shimizu A., Yang Y.G., Sykes M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation. 2008;86(4):601–610. doi: 10.1097/TP.0b013e318182d47a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Habiro K., Sykes M., Yang Y.G. Induction of human T-cell tolerance to pig xenoantigens via thymus transplantation in mice with an established human immune system. American Journal of Transplantation. 2009;9(6):1324–1329. doi: 10.1111/j.1600-6143.2009.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S., et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. The Journal of Immunology. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 168.Ledran M.H., Krassowska A., Armstrong L., Dimmick I., Renstrom J., Lang R., et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3(1):85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 169.Choi K.D., Vodyanik M., Slukvin Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nature Protocols. 2011;6(3):296–313. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sturgeon C.M., Ditadi A., Awong G., Kennedy M., Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nature Biotechnology. 2014;32(6):554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ditadi A., Sturgeon C.M. Directed differentiation of definitive hemogenic endothelium and hematopoietic progenitors from human pluripotent stem cells. Methods. 2016;101:65–72. doi: 10.1016/j.ymeth.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 172.Elcheva I., Brok-Volchanskaya V., Kumar A., Liu P., Lee J.H., Tong L., et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nature Communications. 2014;5:4372. doi: 10.1038/ncomms5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Doulatov S., Vo L.T., Chou S.S., Kim P.G., Arora N., Li H., et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13(4):459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sugimura R., Jha D.K., Han A., Soria-Valles C., da Rocha E.L., Lu Y.F., et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545(7655):432–438. doi: 10.1038/nature22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park M.A., Kumar A., Jung H.S., Uenishi G., Moskvin O.V., Thomson J.A., et al. Activation of the arterial program drives development of definitive hemogenic endothelium with lymphoid potential. Cell Reports. 2018;23(8):2467–2481. doi: 10.1016/j.celrep.2018.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lange L., Hoffmann D., Schwarzer A., Ha T.C., Philipp F., Lenz D., et al. Inducible forward programming of human pluripotent stem cells to hemato-endothelial progenitor cells with hematopoietic progenitor potential. Stem Cell Reports. 2020;14(1):122–137. doi: 10.1016/j.stemcr.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lange L., Morgan M., Schambach A. The hemogenic endothelium: a critical source for the generation of PSC-derived hematopoietic stem and progenitor cells. Cellular and Molecular Life Sciences. 2021;78(9):4143–4160. doi: 10.1007/s00018-021-03777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Azoury M.E., Tarayrah M., Afonso G., Pais A., Colli M.L., Maillard C., et al. Peptides derived from insulin granule proteins are targeted by CD8(+) T cells across MHC class I restrictions in humans and NOD mice. Diabetes. 2020;69(12):2678–2690. doi: 10.2337/db20-0013. [DOI] [PubMed] [Google Scholar]
- 179.Nakayama M., McDaniel K., Fitzgerald-Miller L., Kiekhaefer C., Snell-Bergeon J.K., Davidson H.W., et al. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(14):4429–4434. doi: 10.1073/pnas.1502967112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Y., Teteloshvili N., Tan S., Rao S., Han A., Yang Y.G., et al. Humanized mice reveal new insights into the thymic selection of human autoreactive CD8(+) T cells. Frontiers in Immunology. 2019;10:63. doi: 10.3389/fimmu.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pinto S., Sommermeyer D., Michel C., Wilde S., Schendel D., Uckert W., et al. Misinitiation of intrathymic MART-1 transcription and biased TCR usage explain the high frequency of MART-1-specific T cells. European Journal of Immunology. 2014;44(9):2811–2821. doi: 10.1002/eji.201444499. [DOI] [PubMed] [Google Scholar]
- 182.Vafiadis P., Ounissi-Benkalha H., Palumbo M., Grabs R., Rousseau M., Goodyer C.G., et al. Class III alleles of the variable number of tandem repeat insulin polymorphism associated with silencing of thymic insulin predispose to type 1 diabetes. Journal of Clinical Endocrinology & Metabolism. 2001;86(8):3705–3710. doi: 10.1210/jcem.86.8.7733. [DOI] [PubMed] [Google Scholar]
- 183.Chentoufi A.A., Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51(5):1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 184.Fan Y., Rudert W.A., Grupillo M., He J., Sisino G., Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. The EMBO Journal. 2009;28(18):2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parent A.V., Russ H.A., Khan I.S., LaFlam T.N., Metzger T.C., Anderson M.S., et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13(2):219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun X., Xu J., Lu H., Liu W., Miao Z., Sui X., et al. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell. 2013;13(2):230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 187.Soh C.L., Giudice A., Jenny R.A., Elliott D.A., Hatzistavrou T., Micallef S.J., et al. FOXN1 (GFP/w) reporter hESCs enable identification of integrin-beta4, HLA-DR, and EpCAM as markers of human PSC-derived FOXN1(+) thymic epithelial progenitors. Stem Cell Reports. 2014;2(6):925–937. doi: 10.1016/j.stemcr.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Su M., Hu R., Jin J., Yan Y., Song Y., Sullivan R., et al. Efficient in vitro generation of functional thymic epithelial progenitors from human embryonic stem cells. Scientific Reports. 2015;5:9882. doi: 10.1038/srep09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chhatta A.R., Cordes M., Hanegraaf M.A.J., Vloemans S., Cupedo T., Cornelissen J.J., et al. De novo generation of a functional human thymus from induced pluripotent stem cells. The Journal of Allergy and Clinical Immunology. 2019;144(5):1416–1419 e1417. doi: 10.1016/j.jaci.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 190.Ramos S.A., Morton J.J., Yadav P., Reed B., Alizadeh S.I., Shilleh A.H., et al. Generation of functional human thymic cells from induced pluripotent stem cells. The Journal of Allergy and Clinical Immunology. 2021 doi: 10.1016/j.jaci.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bautista J.L., Cramer N.T., Miller C.N., Chavez J., Berrios D.I., Byrnes L.E., et al. Single-cell transcriptional profiling of human thymic stroma uncovers novel cellular heterogeneity in the thymic medulla. Nature Communications. 2021;12(1):1096. doi: 10.1038/s41467-021-21346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gras-Pena R., Danzl N.M., Khosravi-Maharlooei M., Campbell S.R., Ruiz A.E., Parks C.A., et al. Human stem cell-derived thymic epithelial cells enhance human T cell development in a xenogeneic thymus. The Journal of Allergy and Clinical Immunology. 2021 doi: 10.1016/j.jaci.2021.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malhotra D., Linehan J.L., Dileepan T., Lee Y.J., Purtha W.E., Lu J.V., et al. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nature Immunology. 2016;17(2):187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Onoe T., Kalscheuer H., Danzl N., Chittenden M., Zhao G., Yang Y.G., et al. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. The Journal of Immunology. 2011;187(7):3895–3903. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Khosravi-Maharlooei M., Obradovic A., Misra A., Motwani K., Holzl M., Seay H.R., et al. Crossreactive public TCR sequences undergo positive selection in the human thymic repertoire. Journal of Clinical Investigation. 2019;129(6):2446–2462. doi: 10.1172/JCI124358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Long S.A., Cerosaletti K., Wan J.Y., Ho J.C., Tatum M., Wei S., et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes and Immunity. 2011;12(2):116–125. doi: 10.1038/gene.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garg G., Tyler J.R., Yang J.H., Cutler A.J., Downes K., Pekalski M., et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. The Journal of Immunology. 2012;188(9):4644–4653. doi: 10.4049/jimmunol.1100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang J.H., Cutler A.J., Ferreira R.C., Reading J.L., Cooper N.J., Wallace C., et al. Natural variation in interleukin-2 sensitivity influences regulatory T-cell frequency and function in individuals with long-standing type 1 diabetes. Diabetes. 2015;64(11):3891–3902. doi: 10.2337/db15-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Collison L.W., Vignali D.A. In vitro Treg suppression assays. Methods in Molecular Biology. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu D.C., Hester J., Nadig S.N., Zhang W., Trzonkowski P., Gray D., et al. Ex vivo expanded human regulatory T cells can prolong survival of a human islet allograft in a humanized mouse model. Transplantation. 2013;96(8):707–716. doi: 10.1097/TP.0b013e31829fa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xiao F., Ma L., Zhao M., Huang G., Mirenda V., Dorling A., et al. Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rgammanull mice. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Goettel J.A., Biswas S., Lexmond W.S., Yeste A., Passerini L., Patel B., et al. Fatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood. 2015;125(25):3886–3895. doi: 10.1182/blood-2014-12-618363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Santoni de Sio F.R., Passerini L., Restelli S., Valente M.M., Pramov A., Maccari M.E., et al. Role of human forkhead box P3 in early thymic maturation and peripheral T-cell homeostasis. The Journal of Allergy and Clinical Immunology. 2018;142(6):1909–1921 e1909. doi: 10.1016/j.jaci.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 204.Santoni de Sio F.R., Passerini L., Valente M.M., Russo F., Naldini L., Roncarolo M.G., et al. Ectopic FOXP3 expression preserves primitive features of human hematopoietic stem cells while impairing functional T cell differentiation. Scientific Reports. 2017;7(1):15820. doi: 10.1038/s41598-017-15689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Serr I., Scherm M.G., Zahm A.M., Schug J., Flynn V.K., Hippich M., et al. A miRNA181a/NFAT5 axis links impaired T cell tolerance induction with autoimmune type 1 diabetes. Science Translational Medicine. 2018;10(422) doi: 10.1126/scitranslmed.aag1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takahashi H., Tsuboi H., Abe S., Honda F., Kondo Y., Matsumoto I., et al. Humanized NOD/SCID/IL2rgamma(null) mice exhibit functionally augmented human regulatory T cells associated with enzymatic up-regulation of H3K27me3 in comparison with humans. Clinical and Experimental Immunology. 2021;204(2):239–250. doi: 10.1111/cei.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schneider A., Rieck M., Sanda S., Pihoker C., Greenbaum C., Buckner J.H. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. The Journal of Immunology. 2008;181(10):7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hundhausen C., Roth A., Whalen E., Chen J., Schneider A., Long S.A., et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Science Translational Medicine. 2016;8(356):356ra119. doi: 10.1126/scitranslmed.aad9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Trinschek B., Luessi F., Gross C.C., Wiendl H., Jonuleit H. Interferon-Beta therapy of multiple sclerosis patients improves the responsiveness of T cells for immune suppression by regulatory T cells. International Journal of Molecular Sciences. 2015;16(7):16330–16346. doi: 10.3390/ijms160716330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Luce S., Guinoiseau S., Gadault A., Letourneur F., Blondeau B., Nitschke P., et al. Humanized mouse model to study type 1 diabetes. Diabetes. 2018;67(9):1816–1829. doi: 10.2337/db18-0202. [DOI] [PubMed] [Google Scholar]
- 211.Luce S., Guinoiseau S., Gadault A., Letourneur F., Nitschke P., Bras M., et al. A humanized mouse strain that develops spontaneously immune-mediated diabetes. Frontiers in Immunology. 2021;12:748679. doi: 10.3389/fimmu.2021.748679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Imam S., Prathibha R., Dar P., Almotah K., Al-Khudhair A., Hasan S.A., et al. eIF5A inhibition influences T cell dynamics in the pancreatic microenvironment of the humanized mouse model of Type 1 Diabetes. Scientific Reports. 2019;9(1):1533. doi: 10.1038/s41598-018-38341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Babad J., Ali R., Schloss J., DiLorenzo T.P. An HLA-transgenic mouse model of type 1 diabetes that incorporates the reduced but not abolished thymic insulin expression seen in patients. Journal of Diabetes Research. 2016;2016:7959060. doi: 10.1155/2016/7959060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Boddul S.V., Sharma R.K., Dubnovitsky A., Raposo B., Gerstner C., Shen Y., et al. In vitro and ex vitro functional characterization of human HLA-DRB1 ∗04 restricted T cell receptors. Journal of Translational Autoimmunity. 2021;4:100087. doi: 10.1016/j.jtauto.2021.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kenney L.L., Shultz L.D., Greiner D.L., Brehm M.A. Humanized mouse models for transplant immunology. American Journal of Transplantation. 2016;16(2):389–397. doi: 10.1111/ajt.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.King M., Pearson T., Shultz L.D., Leif J., Bottino R., Trucco M., et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clinical Immunology. 2008;126(3):303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 217.Szot G.L., Yadav M., Lang J., Kroon E., Kerr J., Kadoya K., et al. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell Stem Cell. 2015;16(2):148–157. doi: 10.1016/j.stem.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 218.Yoshihara E., O'Connor C., Gasser E., Wei Z., Oh T.G., Tseng T.W., et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020;586(7830):606–611. doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jacobson S., Heuts F., Juarez J., Hultcrantz M., Korsgren O., Svensson M., et al. Alloreactivity but failure to reject human islet transplants by humanized Balb/c/Rag2gc mice. Scandinavian Journal of Immunology. 2010;71(2):83–90. doi: 10.1111/j.1365-3083.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 220.Brehm M.A., Bortell R., Diiorio P., Leif J., Laning J., Cuthbert A., et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgammanull Ins2Akita mice. Diabetes. 2010;59(9):2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Viehmann Milam A.A., Maher S.E., Gibson J.A., Lebastchi J., Wen L., Ruddle N.H., et al. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63(5):1712–1724. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Whitfield-Larry F., Young E.F., Talmage G., Fudge E., Azam A., Patel S., et al. HLA-A2-matched peripheral blood mononuclear cells from type 1 diabetic patients, but not nondiabetic donors, transfer insulitis to NOD-scid/gammac(null)/HLA-A2 transgenic mice concurrent with the expansion of islet-specific CD8+ T cells. Diabetes. 2011;60(6):1726–1733. doi: 10.2337/db10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tan S., Li Y., Xia J., Jin C.H., Hu Z., Duinkerken G., et al. Type 1 diabetes induction in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2017 doi: 10.1073/pnas.1710415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Elso C.M., Scott N.A., Mariana L., Masterman E.I., Sutherland A.P.R., Thomas H.E., et al. Replacing murine insulin 1 with human insulin protects NOD mice from diabetes. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0225021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Amdare N.P., Shultz L.D., Greiner D.L., DiLorenzo T.P. Characterization and use of human insulin transgenic mouse models for type 1 diabetes. The Journal of Immunology. 2020;201:114. Abstract 142. [Google Scholar]
- 226.Schinnerling K., Rosas C., Soto L., Thomas R., Aguillon J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Frontiers in Immunology. 2019;10:203. doi: 10.3389/fimmu.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Giang S., Horwitz D.A., Bickerton S., La Cava A. Nanoparticles engineered as artificial antigen-presenting cells induce human CD4(+) and CD8(+) Tregs that are functional in humanized mice. Frontiers in Immunology. 2021;12:628059. doi: 10.3389/fimmu.2021.628059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tyagi R.K., Jacobse J., Li J., Allaman M.M., Otipoby K.L., Sampson E.R., et al. HLA-restriction of human Treg cells is not required for therapeutic efficacy of low-dose IL-2 in humanized mice. Frontiers in Immunology. 2021;12:630204. doi: 10.3389/fimmu.2021.630204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. Journal of Clinical Investigation. 2016;126(4):1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Boardman D.A., Philippeos C., Fruhwirth G.O., Ibrahim M.A., Hannen R.F., Cooper D., et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. American Journal of Transplantation. 2017;17(4):931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 231.Noyan F., Zimmermann K., Hardtke-Wolenski M., Knoefel A., Schulde E., Geffers R., et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. American Journal of Transplantation. 2017;17(4):917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 232.Muller Y.D., Ferreira L.M.R., Ronin E., Ho P., Nguyen V., Faleo G., et al. Precision engineering of an anti-HLA-A2 chimeric antigen receptor in regulatory T cells for transplant immune tolerance. Frontiers in Immunology. 2021;12:686439. doi: 10.3389/fimmu.2021.686439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang L., Sosinowski T., Cox A.R., Cepeda J.R., Sekhar N.S., Hartig S.M., et al. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. Journal of Autoimmunity. 2019;96:50–58. doi: 10.1016/j.jaut.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tenspolde M., Zimmermann K., Weber L.C., Hapke M., Lieber M., Dywicki J., et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. Journal of Autoimmunity. 2019;103:102289. doi: 10.1016/j.jaut.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 235.Stamatouli A.M., Quandt Z., Perdigoto A.L., Clark P.L., Kluger H., Weiss S.A., et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471–1480. doi: 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lo Preiato V., Salvagni S., Ricci C., Ardizzoni A., Pagotto U., Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Reviews in Endocrine & Metabolic Disorders. 2021;22(2):337–349. doi: 10.1007/s11154-020-09618-w. [DOI] [PubMed] [Google Scholar]
- 237.Vudattu N.K., Waldron-Lynch F., Truman L.A., Deng S., Preston-Hurlburt P., Torres R., et al. Humanized mice as a model for aberrant responses in human T cell immunotherapy. The Journal of Immunology. 2014;193(2):587–596. doi: 10.4049/jimmunol.1302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Menard L., Saadoun D., Isnardi I., Ng Y.S., Meyers G., Massad C., et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. Journal of Clinical Investigation. 2011;121(9):3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schickel J.N., Kuhny M., Baldo A., Bannock J.M., Massad C., Wang H., et al. PTPN22 inhibition resets defective human central B cell tolerance. Science Immunology. 2016;1(1) doi: 10.1126/sciimmunol.aaf7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Seung E., Tager A.M. Humoral immunity in humanized mice: a work in progress. The Journal of Infectious Diseases. 2013;208(Suppl 2):S155–S159. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Alves da Costa T., Peterson J.N., Lang J., Shulman J., Liang X., Freed B.M., et al. Central human B cell tolerance manifests with a distinctive cell phenotype and is enforced via CXCR4 signaling in hu-mice. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(16) doi: 10.1073/pnas.2021570118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lang J., Ota T., Kelly M., Strauch P., Freed B.M., Torres R.M., et al. Receptor editing and genetic variability in human autoreactive B cells. Journal of Experimental Medicine. 2016;213(1):93–108. doi: 10.1084/jem.20151039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zirpel H., Roep B.O. Islet-resident dendritic cells and macrophages in type 1 diabetes: in search of bigfoot's print. Frontiers in Endocrinology. 2021;12:666795. doi: 10.3389/fendo.2021.666795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Unanue E.R., Ferris S.T., Carrero J.A. The role of islet antigen presenting cells and the presentation of insulin in the initiation of autoimmune diabetes in the NOD mouse. Immunological Reviews. 2016;272(1):183–201. doi: 10.1111/imr.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Monkley S., Krishnaswamy J.K., Goransson M., Clausen M., Meuller J., Thorn K., et al. Optimised generation of iPSC-derived macrophages and dendritic cells that are functionally and transcriptionally similar to their primary counterparts. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sugamura K., Asao H., Kondo M., Tanaka N., Ishii N., Ohbo K., et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annual Review of Immunology. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 247.Krishnamurty A.T., Turley S.J. Lymph node stromal cells: cartographers of the immune system. Nature Immunology. 2020;21(4):369–380. doi: 10.1038/s41590-020-0635-3. [DOI] [PubMed] [Google Scholar]
- 248.Cao X., Shores E.W., Hu-Li J., Anver M.R., Kelsall B.L., Russell S.M., et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 249.Strowig T., Gurer C., Ploss A., Liu Y.F., Arrey F., Sashihara J., et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. Journal of Experimental Medicine. 2009;206(6):1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Billerbeck E., Horwitz J.A., Labitt R.N., Donovan B.M., Vega K., Budell W.C., et al. Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. The Journal of Immunology. 2013;191(4):1753–1764. doi: 10.4049/jimmunol.1201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shitara S., Hara T., Liang B., Wagatsuma K., Zuklys S., Hollander G.A., et al. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta+ intraepithelial lymphocytes. The Journal of Immunology. 2013;190(12):6173–6179. doi: 10.4049/jimmunol.1202573. [DOI] [PubMed] [Google Scholar]
- 252.Giannoni F., Hardee C.L., Wherley J., Gschweng E., Senadheera S., Kaufman M.L., et al. Allelic exclusion and peripheral reconstitution by TCR transgenic T cells arising from transduced human hematopoietic stem/progenitor cells. Molecular Therapy. 2013;21(5):1044–1054. doi: 10.1038/mt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pandey A., Ozaki K., Baumann H., Levin S.D., Puel A., Farr A.G., et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nature Immunology. 2000;1(1):59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 254.Chappaz S., Flueck L., Farr A.G., Rolink A.G., Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110(12):3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 255.Onoe T., Kalscheuer H., Chittenden M., Zhao G., Yang Y.G., Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. The Journal of Immunology. 2010;184(12):6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Maser I.P., Hoves S., Bayer C., Heidkamp G., Nimmerjahn F., Eckmann J., et al. The tumor milieu promotes functional human tumor-resident plasmacytoid dendritic cells in humanized mouse models. Frontiers in Immunology. 2020;11:2082. doi: 10.3389/fimmu.2020.02082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yong K.S.M., Her Z., Chen Q. Humanized mice as unique tools for human-specific studies. Archivum Immunologiae et Therapiae Experimentalis. 2018;66(4):245–266. doi: 10.1007/s00005-018-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yoshihara S., Li Y., Xia J., Danzl N., Sykes M., Yang Y.G. Posttransplant hemophagocytic lymphohistiocytosis driven by myeloid cytokines and vicious cycles of T-cell and macrophage activation in humanized mice. Frontiers in Immunology. 2019;10:186. doi: 10.3389/fimmu.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tarrant J.C., Binder Z.A., Bugatti M., Vermi W., van den Oord J., Ranieri B., et al. Pathology of macrophage activation syndrome in humanized NSGS mice. Research in Veterinary Science. 2021;134:137–146. doi: 10.1016/j.rvsc.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 260.Rongvaux A., Takizawa H., Strowig T., Willinger T., Eynon E.E., Flavell R.A., et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annual Review of Immunology. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lang J., Kelly M., Freed B.M., McCarter M.D., Kedl R.M., Torres R.M., et al. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. The Journal of Immunology. 2013;190(5):2090–2101. doi: 10.4049/jimmunol.1202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lang J., Zhang B., Kelly M., Peterson J.N., Barbee J., Freed B.M., et al. Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood Advances. 2017;1(27):2729–2741. doi: 10.1182/bloodadvances.2017010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Martinov T., McKenna K.M., Tan W.H., Collins E.J., Kehret A.R., Linton J.D., et al. Building the next generation of humanized hemato-lymphoid system mice. Frontiers in Immunology. 2021;12:643852. doi: 10.3389/fimmu.2021.643852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Verma M.K., Clemens J., Burzenski L., Sampson S.B., Brehm M.A., Greiner D.L., et al. A novel hemolytic complement-sufficient NSG mouse model supports studies of complement-mediated antitumor activity in vivo. Journal of Immunological Methods. 2017;446:47–53. doi: 10.1016/j.jim.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Herndler-Brandstetter D., Shan L., Yao Y., Stecher C., Plajer V., Lietzenmayer M., et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(45):E9626–E9634. doi: 10.1073/pnas.1705301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Matsuda M., Ono R., Iyoda T., Endo T., Iwasaki M., Tomizawa-Murasawa M., et al. Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Sciences and Alliance. 2019;2(2) doi: 10.26508/lsa.201800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brehm M.A., Aryee K.E., Bruzenksi L., Greiner D.L., Shultz L.D., Keck J. Transgenic expression of human IL15 in NOD-scid IL2rgnull (NSG) mice enhances the development and survival of functional human NK cells. The Journal of Immunology. 2018;200:120. Abstract 103. [Google Scholar]
- 268.Song Y., Shan L., Gbyli R., Liu W., Strowig T., Patel A., et al. Combined liver-cytokine humanization comes to the rescue of circulating human red blood cells. Science. 2021;371(6533):1019–1025. doi: 10.1126/science.abe2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Serr I., Kral M., Scherm M.G., Daniel C. Advances in human immune system mouse models for personalized treg-based immunotherapies. Frontiers in Immunology. 2021;12:643544. doi: 10.3389/fimmu.2021.643544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhao T., Zhang Z.N., Westenskow P.D., Todorova D., Hu Z., Lin T., et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 2015;17(3):353–359. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]