Abstract
We have analyzed possible qualitative and quantitative differences in antigen expression between Helicobacter pylori strains isolated from the antrum and different locations in the duodenum of 21 duodenal ulcer (DU) patients and 20 asymptomatic subjects (AS) by enzyme-linked immunosorbent assay (ELISA) and inhibition ELISA. Almost all antral and duodenal strains grown in vitro expressed the N-acetyl-neuroaminyllactose-binding hemagglutinin, flagellins (subunits FlaA and FlaB), urease, a 26-kDa protein, and a neutrophil-activating protein. In 75% of both the DU patients and the AS, antral H. pylori strains expressed either the blood group antigen Lewis y (Ley) alone or together with the Lex antigen. However, duodenal H. pylori strains of DU patients expressed Ley antigen more frequently than corresponding strains of AS (P < 0.05). Presence of Ley on H. pylori was related to the degree of active duodenitis (P < 0.05). Duodenal H. pylori strains isolated from AS were significantly more often Lewis nontypeable than duodenal strains of DU patients (P < 0.01). Presence of H. pylori blood group antigen-binding adhesin (BabA) was significantly higher on both antral and duodenal strains isolated from DU patients than on corresponding strains isolated from AS (P < 0.05). BabA-positive duodenal H. pylori strains isolated from DU patients were associated with active duodenitis more frequently than corresponding strains isolated from AS (P < 0.01). Infection with H. pylori strains positive for Ley and BabA in the duodenum is associated with development of duodenal ulcer formation.
Helicobacter pylori colonizes gastric-type epithelium in the stomach and areas of gastric metaplasia in the duodenum. Infection with H. pylori is strongly associated with the development of acute and chronic gastritis, peptic ulcers, and gastric cancer (8, 25, 30). Although most infected individuals remain asymptomatic, about 10 to 15% develop duodenal ulceration (36). Few studies have investigated if it is H. pylori infection in the duodenum itself that is associated with the development of duodenal ulcer (DU). An important question is whether it is possible to differentiate between any specific properties exposed on those H. pylori strains colonizing the duodenum and those colonizing the antrum.
We have previously reported that the presence of cytotoxin-associated gene A (cagA) is markedly increased in duodenal H. pylori strains in DU patients compared to in asymptomatic subjects (AS) (20). We also showed that the distribution of inflammatory cells in the duodenal bulb was related to the duodenal gastric metaplasia and found a positive correlation between the extent of duodenal gastric metaplasia and duodenal H. pylori density (20).
Other antigens in H. pylori that could be associated with increased virulence or induction of symptoms are urease and neutrophil-activating protein (NAP). Urease causes ammonium formation which protects the bacteria from lethal gastric acidity but which may also have a toxic effect on human epithelial cells (22). NAP induces the production of interleukin-8, thus mediating greater infiltration of neutrophils at the site of infection (17). Additional antigens proposed to be associated with virulence of H. pylori infection are the HpaA protein, which has been suggested to be a colonization factor promoting adherence of the bacteria to receptors on erythrocytes and gastric epithelium containing sialic acid (14), and the H. pylori blood group antigen-binding adhesin (BabA), which has been suggested to mediate adhesion to Lewis b (Leb) receptors on gastric epithelium (7, 21). There is also the flagellin of the flagellae providing motility-promoting colonization (23), as well as a 26-kDa protein that is a major protein of the bacteria (28). The lipopolysaccharide (LPS) O chains of H. pylori were recently shown to contain fucosylated N-acetyllactoseamino-glycan determinants, for example, the Lex antigen, the Ley antigen, or both, which are also present in human mucosa and are known as blood group antigens (34).
In this study, we sought to determine if any of a number of antigens may be associated with virulence and if these antigens are expressed in higher frequencies by H. pylori isolated from patients with symptoms, i.e., DU patients, than by strains from AS. Furthermore, it was investigated whether H. pylori strains isolated from the duodenum express different antigens than corresponding H. pylori strains in the antrum. Possible quantitative differences between various antigens of H. pylori isolated from DU patients and AS were also studied.
MATERIALS AND METHODS
Patients and AS.
Twenty-one patients suffering from active or inactive DU disease (15 males; mean age 54 years, range 26 to 84 years) and 20 AS (14 men; mean age 50 years, range 29 to 60 years) participated in this study. The DU patients were recruited from patients seeking care for gastroduodenal disease at the Sahlgrenska University Hospital in Göteborg, Sweden, and the AS were recruited from healthy, age-matched blood donors with no symptoms of dyspepsia at the blood bank of the Sahlgrenska University Hospital as previously described (20). Informed consent was obtained from all patients and AS who participated in the study, and the protocol was approved by the Ethical Committee of the Medical Faculty, Göteborg University, Sweden. All subjects were screened for the presence of H. pylori-specific antibodies in serum as described (19), and AS were recruited based on positive H. pylori serology. Infection was confirmed by culturing of biopsy specimens provided by gastroscopy (20).
The ABO blood group status of each subject was analyzed by a serological test in which equal volumes of serum and erythrocytes of known ABO blood groups were mixed for 10 min. Serum antibodies agglutinating specific blood groups were recorded, and the relationship between the ABO blood group status and the expression of BabA on the best-growing H. pylori strain in the antrum and the duodenum was analyzed for each subject.
Design of study.
Initial screening for the presence of specific H. pylori antigens was determined by enzyme-linked immunosorbent assay (ELISA) on H. pylori isolated from one of the two antral biopsy specimens (except for the BabA, which was analyzed on H. pylori isolated from each of the two antral biopsy specimens) and on all H. pylori isolates that were recovered from four to five different duodenal biopsy specimens collected from each subject. Some of the antigens positive in the qualitative analyses were also tested for quantitative analyses, i.e., the concentrations of the HpaA, flagellins, urease, and the 26-kDa protein were determined on H. pylori strains isolated from one of the two antral strains isolated from each subject by using inhibition ELISA. Possible relationships between the presence of specific antigens on H. pylori and active or chronic inflammatory score in the duodenum were based on analyses of the best-growing strain from either type of the four to five duodenal biopsy specimens from each subject.
Culture conditions for H. pylori and reference strains.
Each biopsy specimen was stored in 1 ml of saline for approximately 1 h after gastroscopy and was thereafter analyzed for growth of H. pylori by disintegrating the tissue with a homogenizer (Ultra-Turrax T25; IKA-Laboratechnik) at a rate of 8,000 to 9,500 rpm for 20 to 40 s. H. pylori strains were cultured on 8.5% horse blood Columbia agar plates and selective agar plates supplemented with 3 μg of vancomycin per ml, 5 μg of trimethoprim per ml, 10 μg of nalidixic acid per ml, and 10 μg of nystatin per ml and were incubated under microaerobic conditions at 37°C for 3 to 6 days and stored in a specific freeze-drying medium at −80°C.
The following reference strains positive and, in some instances, negative for the different antigens were included in the study: H. pylori CCUG (Culture Collection, Göteborg University) 17874, a spontaneous H. pylori urease-negative mutant (provided by G. I. Perez-Perez, Nashville, Tenn.), H. pylori strains G21 and G39 (provided by N. Figura, Siena, Italy), H. pylori strains P466 and MO19 (provided by J. Çelik, Karolinska Institute, Stockholm, Sweden), H. pylori E32, Helicobacter felis CCUG 28540, and Helicobacter nemestrinae CCUG 32350. Two Escherichia coli reference strains, E1392 and WM73494, were cultured on 5% horse blood agar plates and incubated at 37°C for 24 h under aerobic conditions.
Microbiological examination of H. pylori strains.
The typical rod-shaped morphology of H. pylori strains was identified by phase-contrast microscopy. The activities of urease (10), catalase (Merck, Darmstadt, Germany), and oxidase (Sigma-Aldrich Co., Tyresö, Sweden) were measured on all H. pylori strains. Growth of H. pylori was confirmed by using a specific monoclonal antibody (MAb) against HpaA in a dot blot method, as previously described (5).
H. pylori antigen preparations.
Whole membrane proteins were prepared from selected H. pylori strains, CCUG 17874 and Hel 305, by sonication followed by differential centrifugation as described previously (1). These strains were selected since they had previously been shown to be positive for most of the antigens analyzed by ELISA and in other immunological analyses. NAP (15, 17), HpaA (5, 14), and the 26-kDa proteins (28) were recombinantly produced in E. coli. E. coli cells were transformed with an expression vector containing the H. pylori napA gene under the control of the T7 promoter. Cells were disrupted, and the soluble proteins were applied to a Q-Sepharose column (Pharmacia BioTech, Uppsala, Sweden) at pH 8.0 in a buffer containing 25 mM Tris-HCl and 50 mM NaCl. The recombinant NAP (rNAP) was found in the nonbinding fraction. The fractions containing the rNAP were concentrated and purified by gel filtration on a Superdex 200 column (Pharmacia). The rNAP eluted as a single peak. The recombinant HpaA (rHpaA) protein was purified from E. coli cells transformed with an expression vector containing the H. pylori hpaA gene under the control of the T7 promoter. Cells were dissolved with 1.5% Triton X-114 (TX-114; Sigma) and were subjected to two-phase partitioning (6). The detergent phase was diluted to approximately 1% TX-114 and was applied to a Q-Sepharose column (Pharmacia) at pH 8.0 in a buffer containing 50 mM Tris, 2 mM EDTA, 50 mM NaCl, and 0.1% Triton X-100. The rHpaA was found in the nonbinding fraction. The fractions containing the rHpaA were pooled and applied to a new Q-Sepharose column (Pharmacia) at pH 8.6, and the rHpaA was collected by isocratic elution with a buffer containing 10 mM Tris, 2 mM EDTA, 1 M NaCl, and 0.1% Triton X-100, pH 8.6.
Flagellins (subunits FlaA and FlaB), kindly provided by I. Bölin, were purified from strain E32 as previously described (23). The flagellin fraction was further purified by flow pressure low chromatography fractionation on a Resource Q column (Pharmacia). Urease was purified from strain E32 by using a modified version of the method described by Dunn et al. (13) and Evans et al. (16). The positive fractions were pooled, dialyzed against phosphate-buffered saline (PBS), filtered through a 0.45-μm-pore-size membrane, and purified by flow pressure low chromatography.
The purity of the proteins was confirmed by Coomassie staining of material that had undergone sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by immunoblotting, using rabbit polyclonal antisera against whole H. pylori bacteria as well as MAbs reacting specifically with the respective protein (24).
Production of MAbs.
The properties of the manufactured and in-house MAbs used in the study are shown in Table 1. MAbs against Lewis antigens were purchased (Signet Laboratories, Inc., Amsterdam, The Netherlands) and also provided as a gift from B. Appelmelk, Amsterdam, The Netherlands. The MAbs specific to the 26-kDa protein, the HpaA, and the flagellin FlaA and FlaB subunits of H. pylori have previously been described (5, 24).
TABLE 1.
Characteristics of MAbs used to determine antigens on H. pyloria
| Specificity | Clone | Isotype | Ig concn (μg/ml) | Dilutions used in ELISA and inhibition ELISA | Reference |
|---|---|---|---|---|---|
| HpaA | 30:1:1:6 | IgG1 | 65 | 1/100 | 5 |
| Flagellins (FlaA+FlaB) | 48:13 | IgG1 | 40 | 1/100 | 24 |
| Urease 30-kDa subunit | 8:1 | IgG1 | 42 | 1/100 | I. Bölin and A.-M. Svennerholm, unpublished data |
| 26-kDa protein | 18:1 | IgG1 | 34 | 1/100 | 24 |
| NAP | 6:8 | IgG1 | 31 | 1/100 | D. G. Evans and A.-M. Svennerholm, unpublished data |
| Lea (type 1 chain) | T174 | IgG1 | 175 | 1/2,000 | 29, 35 |
| Leb (type 1 chain) | T218 | IgM | 810 | 1/2,000 | 29, 35 |
| Lex (type 2 chain) | P12 | IgM | 175 | 1/2,000 | 29, 35 |
| Sialyl-Lex | CSLEX1 | IgM | 50 | 1/2,000 | 29, 35 |
| Ley (type 2 chain) | F3 | IgM | 125 | 1/2,000 | 29, 35 |
Anti-Lewis MAbs were purchased from Signet Laboratories, Inc.
Qualitative determination of H. pylori antigens.
The presence of NAP, a 26-kDa protein, HpaA, urease, flagellins, and Lewis blood group antigens (Lex, Ley, Lea, Leb, and sialyl-Lex) on the different H. pylori isolates was determined by optimized whole-cell ELISAs as previously described (29). Briefly, H. pylori isolates were grown on 8.5% horse blood Columbia agar plates, resuspended in PBS at a final concentration of ∼2 × 109 bacteria/ml (the lower limit for detection of the antigens adjusted according to standard curves for the individual antigen). Thereafter, 100 μl of the bacterial suspensions was applied to each well of a 96-well polystyrene plate as a solid-phase antigen and was incubated at 4°C overnight. After blocking with 1% bovine serum albumin (BSA) in PBS at 37°C for 30 min and washing two times with PBS, the different MAbs described in Table 1 were diluted in 1% BSA–PBS–0.05% Tween to the lowest concentration giving clear cutoff absorbance values for positive and negative antigen expression, were added to the wells, and were incubated at 37°C for 90 min. After washing, the plates were developed with horseradish peroxidase-conjugated anti-mouse immunoglobulin M (IgM) and IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), followed by addition of H2O2 and o-phenylenediamine in sodium citrate buffer (pH 4.5), and were read at 450 nm. All H. pylori samples were run in duplicate on two different occasions. The same number of bacteria and concentration of MAb were used when testing samples and the negative and positive controls. Titers were determined as the reciprocal dilution giving an absorbance of 0.5 above background (i.e., slightly above the mean absorbance value plus three times the standard deviations of negative control samples).
Analyses of H. pylori BabA using Leb oligosaccharide probe.
Agar-grown H. pylori strains from the antrum and duodenum (H. pylori isolated from two antral biopsy specimens and one to three duodenal biopsy specimens) were directly transferred to nitrocellulose filters probed with biotinylated Leb oligosaccharides and incubated with streptavidin-alkaline phosphatases as previously described (9). The filters were developed by using the 5-bromo-4-chlor-3-indolylphosphate substrate and the nitroblue tetrazolium color reagent (BCIP/NBT tablets; Sigma). The intensity of the color reaction was observed by eye, and results were compared with those positive and negative H. pylori control strains, as well as E. coli controls.
Quantitative determination of H. pylori antigens by inhibition ELISA.
The concentrations of HpaA, flagellins, urease, and the 26-kDa protein in different H. pylori isolates were measured by an optimized inhibition ELISA (24). The lower limit for detection of H. pylori antigens studied varied between 2 and 25 μg for individual antigens. Plates were coated with one of the following antigen preparations: whole membrane proteins of CCUG strain 17874 (25 μg/ml), 26-kDa protein (10 μg/ml), urease (2 μg/ml), or a crude flagellin preparation (2 μg/ml) and incubated at room temperature (RT) overnight. Suspensions of frozen H. pylori bacteria (∼2 × 1010 bacteria/ml) were serially diluted threefold in 0.1% BSA-PBS in noncoated plates. Thereafter, an equal volume of each of the specific MAbs, diluted in BSA-PBS to a concentration corresponding to 10 to 20 times the ELISA titer against the respective antigen, was added to each of the bacterial dilutions and incubated at RT for 1 h with moderate shaking. The mixtures were then transferred to the antigen-coated plates and were incubated at RT for 90 min. Plates were washed and developed as described for ELISA. The bacterial concentrations causing 50% inhibition of the binding of the respective MAb to the solid-phase antigen were determined (24). All samples and reference strains were tested in duplicate on two different occasions.
Histological examination of duodenal biopsy specimens.
In each biopsy specimen, the severity and activity of chronic inflammation (infiltration of lymphocytes and plasma cells) and active inflammation (infiltration of neutrophils) were graded on a scale of 0 to 3, which correspond to no inflammation, 0; mild inflammation, 1; moderate inflammation, 2; and severe mucosal inflammation (duodenitis), 3. The severity of active inflammation was expressed as the mean score of the total number of biopsies, whereas the activity of active inflammation was expressed as the highest score observed among the different duodenal biopsies from each subject.
RESULTS
Isolation of H. pylori from DU patients and AS.
Biopsy specimens were collected from the antrum and different locations in the duodenum from each of 21 DU patients and 20 AS. H. pylori was recovered from all antral biopsy specimens in each subject. H. pylori was positive by culture in one or more of four to five duodenal biopsy specimens collected from 20 out of 21 DU patients, that is in as many as 67 out of 94 (71%) duodenal biopsy specimens. H. pylori was cultured from one or more of four duodenal biopsy specimens collected from 16 AS, that is only in 41 out of 81 (51%) duodenal biopsy samples. H. pylori could not be cultured from any of the duodenal biopsy specimens from four AS. In some cases, duodenal strains could not be recultured after storage at −80°C.
Qualitative and quantitative analyses H. pylori antigens.
The presence of HpaA, urease, flagellins, NAP, and a 26-kDa protein was determined by different ELISAs. Almost all DU patients and AS had antral as well as at least one of the four to five duodenal strains, which expressed the different antigens studied (Table 2).
TABLE 2.
Number (%) of DU patients and AS with antral and duodenal H. pylori strains expressing indicated antigens
| Antigen | Antrum
|
Duodenum
|
||
|---|---|---|---|---|
| DU (n = 21) | AS (n = 20) | DU (n = 20) | AS (n = 16) | |
| HpaA | 21 (100) | 20 (100) | 20 (100)a | 14 (88)b |
| Flagellins | 21 (100) | 20 (100) | 20 (100) | 15 (94) |
| Urease | 20 (95) | 19 (95) | 20 (100) | 14 (88) |
| 26-kDa protein | 20 (95) | 20 (100) | 20 (100) | 14 (88) |
| NAP | 18 (86) | 18 (90) | 20 (100) | 16 (100) |
| BabA | 13 (67)c | 5 (25)c | 13 (65)c | 5 (31)cd |
At least one of the duodenal isolates from each subject was positive for the respective antigen. In most cases, all individual duodenal isolates from each subject gave similar results.
Two AS carriers had HpaA-negative duodenal strains determined by ELISA. However, these strains were positive for HpaA protein by a dot blot assay using a specific MAb (5).
In those subjects that were negative for BabA, all individual isolates from the same subject were negative.
P < 0.05 when comparing prevalence of BabA-positive strains in the antrum and the duodenum of DU patients as compared to in AS (Fisher's exact test).
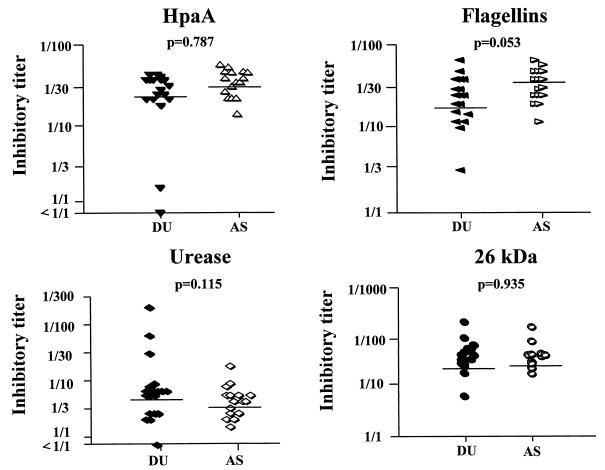
Antral H. pylori strains isolated from most of the DU patients and AS were tested for concentrations of HpaA, flagellins, urease, and the 26-kDa protein in inhibition ELISA (Fig. 1). The mean concentrations of HpaA, flagellins, urease, and the 26-kDa protein did not differ significantly between strains isolated from the DU and AS groups; however, the mean concentration of flagellins was somewhat higher on the H. pylori strains isolated from the AS (P = 0.053).
FIG. 1.
Quantitative expression of different H. pylori antigens as determined by inhibition ELISA. Strains (one per subject) isolated from antral biopsy specimens from 16 to 18 of the DU patients (filled symbols) and 11 to 16 of the AS (open symbols) were, after adjustment to similar bacterial concentration (∼2 × 1010 bacteria/ml), analyzed for 50% inhibitory titers in inhibition ELISA by using specific MAbs. Horizontal lines indicate geometric mean titer for each group, and P values refer to comparisons of geometric mean values for DU patients and AS (Student's t test).
H. pylori isolates were analyzed for the expression of the different Lewis blood group antigens, that is, Lex, sialyl-Lex, Ley, Lea, and Leb, by ELISAs (Table 3). On the antral isolates from DU patients as well as AS, Ley alone or together with Lex was detected in 75% of the cases. When analyzing duodenal isolates, on the other hand, as many as 18 of 20 (90%) of the DU patients but only 7 of 16 (42%) of the AS had H. pylori strains which expressed any Lewis antigen (P < 0.01). None of the strains isolated from the antrum or the duodenum were sialyl-Lex positive in either group. Duodenal H. pylori strains that only expressed the Ley antigen were found in six (30%) of the DU patients but in none of the AS. In 64% of the DU patients and AS, the antral isolates differed from the corresponding duodenal isolates with regard to Lewis antigen profile. In some cases, 3 of 20 (15%) of the DU patients and 1 of 16 (6%) of the AS, two different Lewis phenotypes were detected on the different duodenal strains from the same subject. The results, using commercially available MAbs (Signet Laboratories), were confirmed by using MAbs provided by B. J. Appelmelk.
TABLE 3.
Number (%) of DU patients and AS with antral and duodenal H. pylori strains expressing different Lewis antigens
| Lewis antigen | Antrum
|
Duodenum
|
||||
|---|---|---|---|---|---|---|
| DU (n = 21) | AS (n = 20) | P values | DU (n = 20) | AS (n = 16) | P values | |
| Lex | 0 (0) | 0 (0) | NSf | 1 (5)a | 1 (6) | NS |
| Ley | 4 (19)b | 7 (35) | NS | 6 (30) | 0 (0) | <0.05d |
| Lexy | 12 (57)c | 8 (40) | NS | 7 (35) | 3 (19) | NS |
| Lea | 0 (0) | 0 (0) | NS | 2 (10) | 0 (0) | NS |
| Leb | 1 (5) | 2 (10) | NS | 1 (5) | 3 (19) | NS |
| Leab | 1 (5) | 0 (0) | NS | 1 (5) | 0 (0) | NS |
| Le negative (nontypeable) | 3 (14) | 3 (15) | NS | 2 (10) | 9 (58) | <0.01e |
At least one of the duodenal isolates from each subject was positive for the respective antigen. In most cases, all individuals duodenal isolates from each subject gave similar results.
Two of four patients had antral H. pylori strains coexpressing the Leab antigens.
Two of 12 patients had antral H. pylori strains coexpressing the Lea or the Leb antigens.
P < 0.05 when comparing the higher presence of Ley-positive strains in the duodenum of DU patients to that in AS (Fisher's exact test).
P < 0.01 when comparing the higher frequency of Le-negative (nontypeable) strains in the duodenum of AS to that in DU patients (Fisher's exact test).
NS, not significant.
No major changes in the Lewis antigens of the different H. pylori strains were seen even after eight subcultures, although a few, 5 of 108 (5%) of the H. pylori strains tested for Lewis antigens, converted from being Lewis antigen negative to Lewis antigen (mostly Lex) positive after several subcultures.
The presence of BabA was assessed on H. pylori isolated from both antral biopsy specimens and from one to three of the duodenal biopsy specimens of each subject. The results were almost consistent in all subjects, that is, the two antral and all the different duodenal isolates were either BabA positive or negative. In the DU patients, 13 of 21 (67%) had BabA-positive H. pylori strains in the antrum, and 13 of 20 (65%) had BabA-positive H. pylori strains in the duodenum (Table 2). Both antral and duodenal strains isolated from AS were significantly less often positive for BabA (P < 0.05). Thus, only 5 of 20 (25%) of the AS had BabA-positive strains in the antrum, and 5 out of 16 (31%) had BabA-positive strains in the duodenum.
Correlations of H. pylori BabA and Lewis antigens with human ABO blood group status and inflammation in the duodenum.
BabA expression in strains isolated from either antrum or duodenum did not correlate with the blood group of the host. However, AS with blood group O were more often colonized by duodenal H. pylori strains that lacked BabA than by BabA-positive strains.
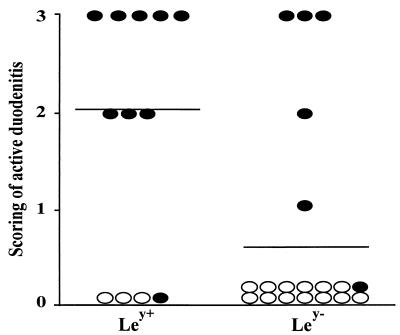
We have previously reported that DU patients had significantly higher (P < 0.001) infiltration of neutrophils, lymphocytes, and plasma cells in the duodenum than AS, as shown by histopathological analysis (20). When comparing the Lewis antigen type of the H. pylori strain in the duodenum with the extent of active inflammation, we found that Ley positivity was related to increased numbers of neutrophils in the duodenum (P < 0.05) (Fig. 2). However, there was no correlation between the expression of Ley positivity on the duodenal H. pylori strains and the number of chronic inflammatory cells in the duodenum.
FIG. 2.
The relationship between Ley expression on H. pylori strains and inflammation in the duodenum. H. pylori strains (the best growing strain from each DU patient) isolated from the duodenum of DU patients (filled symbols) and AS (open symbols). Five patients with Ley-positive H. pylori strains who had been treated with antiulcer medication (from 1 week to 2 months prior to gastroscopy) were excluded from the analysis, since such treatments have been shown to lower the activity of chronic gastritis as well as to significantly decrease the accumulation of inflammatory infiltrates and release of toxic products in the antrum of DU patients and AS (31). The highest score of duodenitis of several duodenal biopsy specimens collected from the duodenum in the same subject is shown on the vertical axis. Lines indicate average values for each group. Ley type of the H. pylori strain in the duodenum was related to increased numbers of neutrophils in the duodenum (P < 0.05, Fisher's exact test).
There was also a significant correlation between active inflammation in the duodenum and presence of strains possessing BabA in DU patients as compared to AS (P < 0.01), whereas chronic inflammation in the duodenum was not related to infection with strains expressing BabA.
DISCUSSION
Few studies have demonstrated a correlation between specific antigens on H. pylori and the development of DU in humans. However, in animal models, specific surface-exposed and/or other antigens on H. pylori strains isolated from the antrum (12), e.g., urease, cagA, and the vacuolating cytotoxin, have been suggested to be associated with the development of ulcer (18). We have been focused on studying if there are any antigens exposed on H. pylori isolated from both the antrum and the duodenum of patients with DU that differ from those exposed on strains isolated from AS.
Even if the genome of H. pylori seems to be rather heterogeneous (33), we found that most of the antigens studied, i.e., HpaA, urease, flagellins (subunit FlaA and FlaB), NAP, and a 26-kDa protein, remained constant to a high degree in almost all H. pylori strains, irrespective of whether they had been isolated from the antrum or the duodenum from DU patients or AS. Even though these antigens do not seem to be linked to the severity of inflammation, they are important for survival and colonization of H. pylori in the host (12, 14, 16, 17, 23, 28).
The biological function of Lewis antigens expressed by H. pylori is still unknown. We found a correlation between Ley-positive strains and active inflammatory score (neutrophils) in the duodenum (P < 0.05) (Fig. 2). It is therefore possible that strains colonizing the duodenum of ulcer patients may survive and evade inflammatory responses due to specific expression of Ley H. pylori antigen mimicking the Ley antigen expressed on the duodenal epithelium (data obtained from immunohistochemical staining of duodenal tissue sections [unpublished data]). A potential inflammatory effect of the Ley antigen has also been suggested by Muguruma et al. (27), who showed a relationship between increased Ley antigen expression on the epithelial cells and chronic inflammatory liver diseases, and also that the plasma levels of Ley increased significantly with the severity of inflammation. McGowan et al. (26) also recently showed that growth of H. pylori in acidity may result in induction of certain LPS determinants on bacteria, since an isogenic strain lacking wbcJ (a gene with predicted homology to known bacterial O antigen biosynthesis involved in the conversion of GDP-mannose to GDP-fucose) failed to express either O antigen or Lex and Ley antigens. Thus, H. pylori growing in the duodenum may survive the hostile environment by developing special features, e.g., certain Lewis antigens to withstand the increased exposure to gastric acidity, including the alteration of its LPS structures. We also show that H. pylori strains isolated from the duodenum of DU patients, who have reduced bicarbonate secretion and an increased gastric acid output in the duodenum compared to AS, more frequently express Lewis antigens on the LPS, that is Lex and Ley, than corresponding strains from AS (P < 0.01). Thus, duodenal strains from AS that often are nontypeable for Lewis antigens may be more sensitive to the higher acid load in the duodenum, which may explain the significantly lower numbers of H. pylori in AS than in corresponding regions of DU patients that we have previously reported (20).
Lewis antigen conversion in H. pylori strains has been reported and may be due to different levels of glycosyltransferase expression and also due to the fact that H. pylori strains may turn glycosyltransferase genes on or off, depending on host and/or growth factors during the infection (3), which may enable the bacteria to survive for a long time in gastric-like epithelium (4). We found that Lewis antigens on H. pylori were fairly stable even after several subcultures of the bacteria and repeated analyses. However, 5% of all H. pylori isolates tested converted from being Lewis antigen negative to Lewis antigen positive after several subcultures on agar plates. Our results from genetic analyses confirm that in these instances when H. pylori strains from the antrum and duodenum express different Lewis antigens, they also differ with regard to their DNA fingerprints (32). This suggests that the same patient may be colonized by a combination of H. pylori strains.
It has been claimed that the expression of Lex and Ley by H. pylori isolates is related to cagA status (35). However, we were unable to find any relationship between any of the secreted antigens or surface antigens studied, including Lex and Ley, with the previously demonstrated cagA genotype of the respective strain (20). This is puzzling, since we have previously reported that H. pylori density in the duodenum is associated with cagA+ genotype as well as with active duodenitis (20). This paradox may be explained by the fact that duodenal cagA+ strains cause active inflammation. However, colonization is not dependent on cagA status, since Ley-positive strains seem to survive irrespective of whether they are cagA+ or cagA.
In this study, we show that significantly higher numbers of both the antral and duodenal strains isolated from DU patients had BabA adhesin than corresponding strains from the AS, supporting the notion that colonization by BabA-positive strains is associated with ulcer formation (21). A previous study by Borén et al. (7) showed that strains with BabA preferentially bound to Leb receptors, which are more often expressed in hosts with blood group O than in individuals with other blood groups. We did not find any such relationship between blood group and BabA-positive H. pylori infecting strains. Presence of BabA on the duodenal strains from the DU patients was related to both presence of cagA on the strain and to amount of neutrophils in the duodenal mucosa. A correlation between cagA on both duodenal and antral H. pylori strains and the bacterial binding to Leb receptors on freshly collected gastric tissues from blood group O individuals has also been shown (21). However, the relationship between cagA and BabA was shown to not have any biological significance, since deletion of the entire cag pathogenicity island did not affect binding capacity.
In summary, our study shows that there seem to be antigens on H. pylori that differ between strains isolated from DU patients and AS. For example, Ley was significantly more often detected in duodenal strains isolated from DU patients than from AS, and a relationship between Ley and active inflammation in the duodenum was found. We have previously demonstrated an association between presence of cagA and duodenitis (20). In this study, we found a correlation between presence of BabA and active duodenitis. Thus, Ley and BabA may be relevant markers to predict DU development following H. pylori infection.
ACKNOWLEDGMENTS
This study was financially supported by a grant from AstraZeneca, Mölṅḋal, Sweden, as well as by the Swedish Medical Research Council (grants 17X-760 and 16X-09085).
The skillful technical assistance of Kerstin Andersson is gratefully acknowledged. We acknowledge Ben Appelmelk, Vrije University, Amsterdam, The Netherlands, for providing MAbs against H. pylori Lewis blood group antigens.
REFERENCES
- 1.Achtman M, Schwuchow S, Helmuth R, Morelli G, Manning P A. Cell-cell interaction in conjugating Escherichia coli: Con− mutants and stabilization of mating aggregates. Mol Gen Genet. 1978;164:171–183. [Google Scholar]
- 2.Appelmelk B J, Negrini R. Helicobacter pylori and gastric autoimmunity. Curr Opin Gastroenterol. 1997;13:31–34. [Google Scholar]
- 3.Appelmelk B J, Shiberu B, Trinks C, Tapsi N, Zheng P Y, Verboom T, Maaskant J, Hokke C H, Schiphorst W E C M, Blanchard D, Simoons-Smit I M, van den Eijnden D H, Vandenbroucke-Grauls C M J E. Phase variation in Helicobacter pylori lipopolysaccharide. Infect Immun. 1998;66:70–76. doi: 10.1128/iai.66.1.70-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg D E, Hoffman P S, Appelmelk B J, Kuster J G. The Helicobacter pylori genome sequence: genetic factors for long life in the gastric mucosa. Trends Microbiol. 1997;5:468–474. doi: 10.1016/s0966-842x(97)01164-5. [DOI] [PubMed] [Google Scholar]
- 5.Bölin I, Lönroth H, Svennerholm A-M. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J Clin Microbiol. 1995;33:381–384. doi: 10.1128/jcm.33.2.381-384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 7.Borén T, Falk P, Roth K A, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 8.Carrick J, Lee A, Hazell S, Ralston M, Daskalopoulos G. Campylobacter pylori, duodenal ulcer, and gastric metaplasia: possible role of functional heterotopic tissue in ulcerogenesis. Gut. 1989;30:790–797. doi: 10.1136/gut.30.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Çelik J, Su B, Tirén U, Finkel Y, Thoreson A-C, Engstrand L, Sandstedt B, Bernander S, Normark S. Virulence and colonization-associated properties of Helicobacter pylori isolated from children and adolescents. J Infect Dis. 1998;177:247–252. doi: 10.1086/517365. [DOI] [PubMed] [Google Scholar]
- 10.Christensen W B. Urea decomposition as a means of differentiating Proteus and paracolon cultures from each other and from Salmonella and Shigella types. J Bacteriol. 1946;52:461–466. doi: 10.1128/jb.52.4.461-466.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree J E, Peichl P, Wyatt J, Stachl U, Lidley I J D. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:67–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 12.Doig P, Trust T J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect Immun. 1994;62:4526–4533. doi: 10.1128/iai.62.10.4526-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn B E, Cambell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Bacteriol. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 14.Evans D G, Karjalainen T K, Jr, Evans D J, Graham D Y, Lee C-H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans D J, Jr, Evans D G, Lampert H C, Nakano H. Identification of four new prokaryotic bacterioferritins, from Helicobacter pylori, Anabaena variabilis, Bacillus subtilis and Treponema pallidum, by analysis of gene sequences. Gene. 1995;153:123–127. doi: 10.1016/0378-1119(94)00774-m. [DOI] [PubMed] [Google Scholar]
- 16.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 17.Evans D J, Jr, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P R. Characterization of the Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiara P, Marchetti M, Blaser M J, Tummuru M K R, Cover T L, Segal E D, Tompkins L S, Rappuoli R. Role of Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamlet A, Erlandsson K I M, Olbe L, Svennerholm A-M, Backman V E M, Pettersson A B. A simple and rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1058–1063. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 20.Hamlet A, Thoreson A-C E, Nilsson O, Svennerholm A-M, Olbe L. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology. 1999;116:259–269. doi: 10.1016/s0016-5085(99)70121-6. [DOI] [PubMed] [Google Scholar]
- 21.Ilver D, Arnqvist A, Ögren J, Frick I-M, Kersulyte D, Incecik E T, Berg D E, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–376. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 22.Ito S L, Kohli Y, Kato T, Murakita H, Ohotaki Y, Hirai M, Azuma T, Kuriyama M. Differences in urease activity in live Helicobacter pylori cultured from patients with gastroduodenal diseases. Eur J Gastroenterol Hepatol Health Dis. 1995;7(Suppl. 1):S83–S88. [PubMed] [Google Scholar]
- 23.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindholm C, Osek J, Svennerholm A-M. Quantification of conserved antigens in Helicobacter pylori during different culture conditions. Infect Immun. 1997;65:5376–5380. doi: 10.1128/iai.65.12.5376-5380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall B J, Armstrong J A, McGechie D B, Clancy R J. Attempt to fulfill Koch's postulates for pyloric Campylobacter. Med J Aus. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 26.McGowan C C, Necheva A, Thompson S A, Cover T L, Blaser M. Acid-induced expression of an LPS-associated gene in Helicobacter pylori. Mol Microbiol. 1998;30:19–31. doi: 10.1046/j.1365-2958.1998.t01-1-01079.x. [DOI] [PubMed] [Google Scholar]
- 27.Muguruma M, Okada Y, Tsuji T. Hepatic neoexpression and increased plasma levels of Lewis y, a carbohydrate antigen, in chronic inflammatory liver diseases. Am J Clin Pathol. 1994;102:176–181. doi: 10.1093/ajcp/102.2.176. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole P W, Logan S M, Kostrzynska M, Wadström T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoons-Smith I M, Appelmelk B J, Verboom T, Negrini R, Penner J L, Aspinall G O, Moran A P, Fei Fei S, Bi-Shan S, Rudnica W, Savio A, de Graaff J. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34:2196–2200. doi: 10.1128/jcm.34.9.2196-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipponen P, Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol. 1993;28(Suppl. 196):3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Nakamura M, Mori M, Miura S, Tsuchiya M, Ishii H. Lansoprazole inhibits oxygen-derived free radical production from neutrophils activated by Helicobacter pylori. J Clin Gastroenterol. 1995;20:S93–S96. doi: 10.1097/00004836-199506002-00025. [DOI] [PubMed] [Google Scholar]
- 32.Thoreson A-C E, Hosseini N, Svennerholm A-M, Bölin I. Different Helicobacter pylori strains colonize the antral and duodenal mucosa of duodenal ulcer patients. Helicobacter. 2000;5:69–78. doi: 10.1046/j.1523-5378.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 33.Van der Ende A, Rauws E A J, Faller M, Mulder C J J, Tytgat G N J, Dankert J. Heterogenous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]
- 34.Wirth H-P E, Manqiao M, Peek R M, Jr, Tham K T, Blaser M J. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology. 1997;113:1091–1098. doi: 10.1053/gast.1997.v113.pm9322503. [DOI] [PubMed] [Google Scholar]
- 35.Wirth H P, Yang M, Karita M, Blaser M J. Expression of the human cell surface glycoconjugates Lewis X and Lewis Y by Helicobacter pylori isolates is related to cagA status. Infect Immun. 1996;64:4598–4605. doi: 10.1128/iai.64.11.4598-4605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyatt J I, Rathbone B J, Dixon M F, Heatley R V. Campylobacter pyloridis and induced gastric epithelium in the pathogenesis of duodenitis. J Clin Pathol. 1987;40:841–848. doi: 10.1136/jcp.40.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]