Abstract
Although there are many hypotheses for the age-related difference in the severity of COVID-19, differences in innate, adaptive and heterologous immunity, together with differences in endothelial and clotting function, are the most likely mechanisms underlying the marked age gradient. Children have a faster and stronger innate immune response to SARS-CoV-2, especially in the nasal mucosa, which rapidly controls the virus. In contrast, adults can have an overactive, dysregulated and less effective innate response that leads to uncontrolled pro-inflammatory cytokine production and tissue injury. More recent exposure to other viruses and routine vaccines in children might be associated with protective cross-reactive antibodies and T cells against SARS-CoV-2.
There is less evidence to support other mechanisms that have been proposed to explain the age-related difference in outcome following SARS-CoV-2 infection, including pre-existing immunity from exposure to common circulating coronaviruses, differences in the distribution and expression of the entry receptors ACE2 and TMPRSS2, and difference in viral load.
Keywords: 2019 novel coronavirus, ACE2, antibodies, coagulation, endothelium, heterologous, immune system, outcome, pre-existing immunity, severity
Compared with adults, and in contrast to other respiratory viruses, children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), generally are asymptomatic or have mild disease with a significantly lower hospitalization rate and mortality.1–8
We have previously reviewed hypotheses for the age-related difference in the severity of coronavirus disease 2019 (COVID-19), separating them into factors that put adults at higher risk and those that protect children.8 Since then, more evidence has become available to support some of the hypotheses and make others less likely.
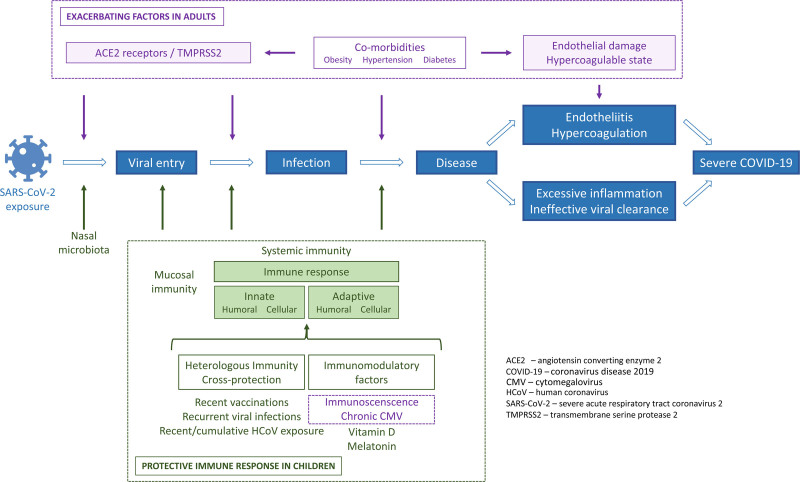
Here, we provide an updated review of the mechanisms that might explain the marked age gradient in the severity of COVID-19 (Table 1 and Figure 1).
TABLE 1.
Summary of Mechanisms Proposed to Contribute to the Age-Related Difference in the Severity of COVID-19
|
Mechanisms with the strongest supporting evidence to date 1. Differences in innate immunity In response to SARS-CoV-2, children have: • a stronger mucosal innate immune response, which helps clear the virus9,16,23,24 • lower levels of neutrophils, which have been associated with microangiopathy and thrombosis27,28,32 • differences in cytokines levels with a lower tendency to develop a cytokine storm26–29,33,35 2. Differences in adaptive immunity In response to SARS-CoV-2, children have: • higher lymphocyte counts with a higher proportion of naïve T cells, T regulatory cells and T follicular helper cells48–50 • lower T cell responses to spike and ORF1 proteins16,35,49,50 Results from studies are inconsistent in relation to: • T cell responses against nucleocapsid and membrane proteins; studies show both lower50 and higher16,35,49 levels in children • differences in SARS-CoV-2-specific mucosal and serum antibody levels27,28,32,35,37,38,44,58–62 3. Heterologous immunity and off-target effects of vaccines Children have: • more recently been vaccinated with BCG, MMR, Tdap and other vaccines which might offer indirect protection against COVID-1967,68,70,75–78,81–87,96 • more frequent recurrent or concurrent infections which might induce an enhanced state of activation of the immune system101 4. Differences in the endothelium and clotting function Children are: • less prone to endothelial damage and abnormal clotting110 Mechanisms with less supporting evidence to date 1. Pre-existing immunity from exposure to commonly circulating human coronaviruses • Although antibodies against HCoVs can be cross-reactive, they might not be cross-neutralizing16,35,49,58,118,135–139 • Role of cross-reactive T cells in relation to SARS-CoV-2 also remains unclear121,122,149 • There is conflicting evidence on whether children or adults have higher levels of antibodies and T cells cross-reactive between HCoVs and SARS-CoV-235,118,128-130 2. ACE2 and TMPRSS2 • There is conflicting evidence on whether children have lower numbers and different distribution of ACE2 and TMPRSS2 across body sites9,16,24,154,156–158 • ACE2-angiotensin system is affected by many factors other than age154–157,160,164–170 • ACE2-angiotensin system is complex and also involved in regulating immune responses171 3. Viral load • Children and adults have similar viral loads and shedding from the respiratory tract16,179–181 |
ACE2, angiotensin-converting enzyme 2; BCG, Bacillus Calmette-Guerin; COVID-19, coronavirus disease 2019; HCoVs, human coronaviruses; MMR, measles-mumps-rubella; ORF, open reading frame; SARS-CoV-2, severe acute respiratory tract coronavirus 2; Tdap, tetanus-diphtheria-pertussis; TMPRSS2, transmembrane serine protease 2.
FIGURE 1.
Mechanisms proposed to contribute to the age-related difference in the severity of COVID-19.
MECHANISMS WHICH ARE MORE LIKELY TO CONTRIBUTE TO THE AGE-RELATED DIFFERENCE IN SEVERITY OF COVID-19
Differences in Innate Immunity
Mucosal Innate Immunity
The control of SARS-CoV-2 requires an optimal early innate immune response. Children have a more robust innate immune response in their nasal mucosa when infected with SARS-CoV-2.9 Melanoma differentiation-associated protein 5 (MDA5) has been identified as the major pattern recognition receptor for SARS-CoV-2 on epithelial cells.10–12 Retinoic acid-inducible gene (RIG)-1 plays an additional minor role.10–12 Children have a stronger innate immune response to SARS-CoV-2 by way of a higher basal expression of MDA5 and RIG-I on nasal epithelial cells, macrophages and dendritic cells.9 The activation of MDA5 and RIG-1 leads to the activation of interferon regulatory factor (IRF) 3 which subsequently results in production of interferon (IFN)-alpha.13 Although the expression of these pattern recognition receptors is similar in children and adults after five days of infection, an early response is necessary to quickly control SARS-CoV-2.9
Interferon (IFN)-alpha and -gamma are important components of the early innate immune response against SARS-CoV-2.14,15 Children infected with SARS-CoV-2 have a higher expression of genes associated with IFN and NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome signaling in their nasal epithelial cells, particularly in ciliated cells.9,16 The expression of these genes is associated with strong antiviral activity against SARS-CoV-2.17,18 It has been hypothesized that, in contrast to infections with other respiratory viruses such as respiratory syncytial virus or influenza, there is a narrow window of opportunity for cells to express IFNs before SARS-CoV-2 shuts off the antiviral system.9,15,19–22
Compared with adults, healthy children also have much higher numbers of immune cells in their upper respiratory tract, especially cells of the innate immune system, such as neutrophils and natural killer cells.9,23 In adults, infection with SARS-CoV-2 leads to a large influx of immune cells to the upper respiratory tract that is not observed in children.9 However, SARS-CoV-2-infected children have a more pronounced activation of CCL3 and C-X-C chemokine receptor (CXCR) 1/2 expression in neutrophils in the upper respiratory tract.9 Furthermore, children have higher levels of certain cytokines and chemokines (specifically, IFN-alpha 2, IFN-gamma, C-X-C motif chemokine ligand 10 (CXCL10), interleukin (IL)-1beta, IL-8 and IL-17) in their nasal fluid.16,24
Systemic Innate Immunity
In comparison to children with other common respiratory viral infections, those with COVID-19 have a greater change in innate and T cell-mediated immune responses over time.25 Children with SARS-CoV-2 infection show a marked reduction in myeloid cells.25 They have low levels of dendritic cells, natural killer cells and classical ((CD14+CD16-), intermediate (CD14+CD16+) and non-classical (CD14-CD16+)) monocytes in blood.26 Adults with COVID-19, especially severe COVID-19, also have low levels of dendritic and natural killer cells, but only show a decrease of non-classical monocytes, while classical and intermediate monocytes, which contribute to the cytokine storm, increase.26–29 Non-classical monocytes have anti-inflammatory properties and are important for endothelial integrity.30 Interestingly, low numbers of non-classical monocytes are also found in children with pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS).31 Adults with COVID-19 have high numbers of neutrophils in blood, which may form extracellular traps that can activate the coagulation cascade and have been implicated in microangiopathy and thrombosis.27,28,32 The high neutrophil to lymphocyte ratio found in adults with severe COVID-19 is rarely observed in children.33,34
There are also age-related differences in serum levels of cytokines. Compared with adults, children with COVID-19 have higher levels of CXCL10, GM-CSF, IL-17A and IFN-gamma early in the disease, but not tumor necrosis factor (TNF) or IL-6.33,35,36 In addition, in children, genes associated with IFN responses have earlier expression in the blood, similar to the earlier and greater expression of these genes in nasal epithelial cells.9,16,36 One small study found similar levels of serum IFN-gamma and TNF-producing T cells, but lower IFN-gamma production in convalescent plasma of infants after mild COVID-19 compared with their parents.37 Compared with older children, infants with COVID-19 have lower levels of neutrophils, lymphocytes and complement C3c, but higher levels of lymphotoxin beta, IL-10, IL-6 and procalcitonin.38 Compared with children with mild COVID-19, those with severe COVID-19 have lower levels of T cells, natural killer cells and a limited IFN response, but higher levels of IL-6 and IL-10 in blood.16,39
In contrast to the increased NLRP3 activity in the nasal epithelium observed in children, increased NLRP3 activity is found in the blood in elderly adults and is associated with a predisposition to cytokine storm and increased COVID-19 severity.40–43 Early control of inflammation, as occurs in children, might limit disease severity.36
Differences in Adaptive Immunity
Mucosal Adaptive Immunity
SARS-CoV-2-specific immunoglobulin (Ig) A and IgG levels in nasal fluid have mostly been reported to be similar in children and adults.16 However, one study in adults reported that specific IgA levels in nasal fluid were inversely correlated with age.44 A small study showed that children can have IgA in their saliva without having had a positive respiratory SARS-CoV-2 polymerase chain reaction, which raises the possibility that a local immune response might prevent the establishment of an infection.45 One hypothesis for this is that individuals might be protected from SARS-CoV-2 due to pre-existing immunity to commonly circulating human coronaviruses (HCoVs).46 Adding to the evidence that mucosal immunity is important for controlling SARS-CoV-2 are the results from a study showing that adults who remain seronegative after mild COVID-19 have IgA antibodies with neutralizing activity in nasal fluid and saliva.44
Systemic Adaptive Immunity
In relation to adaptive immunity, rapid and coordinated appearance of SARS-CoV-2-specific CD4+ and CD8+ T cells in blood is associated with faster clearance of SARS-CoV-2 and milder COVID-19.47 Children with COVID-19 have higher lymphocyte counts, with a higher proportion of innate lymphoid and non-clonally expanded naïve T cells in the blood.48,49 Children also have higher numbers of T follicular helper cells, which are important for an early antibody response.50 Furthermore, they have lower T cell responses to S and ORF1 proteins and reduced CD4+ T cell effector memory.16,35,49,50 Results of T cell responses against N and membrane proteins are conflicting with some studies showing lower levels in children50 and others higher levels.16,35,49
Adults infected with SARS-CoV-2 typically have a decreased lymphocyte count, with reduced numbers of naïve CD4+ and CD8+, T regulatory and memory T cells.51–53 One study reported that acute and memory SARS-CoV-2-specific CD4+ cells increase with age.50 T cell exhaustion with impaired effector activity has been observed in adults with severe COVID-19.28,34 Poor and uncoordinated T cell responses, in addition to a scarcity of naïve T cells have been found in elderly adults and are associated with poor outcomes from COVID-19.28,54,55
In children, during COVID-19, genes associated with B cell activation are expressed earlier.36 In relation to differences in levels of SARS-CoV-2-specific antibodies between children and adults, the evidence is conflicting. An early increase of IgA, IgM and, to a lesser extent IgG, is associated with asymptomatic and mild SARS-CoV-2 infection.27,56 An early rise in antibodies is observed in children.27,28 One study reported that infants have lower serum SARS-CoV-2-specific IgG levels compared with older children,38 another that children have lower serum SARS-CoV-2 neutralizing antibody levels compared with adults35 and one that adults with severe COVID-19 have higher levels of specific IgA antibodies.32 Another study reported children were less likely than adults to seroconvert to SARS-CoV-2.57 However, there are also studies which report higher levels of specific IgG in children compared with adults.58 One small study found higher levels of serum spike-specific IgG and IgA in infants after mild COVID-19 compared with their parents.37 Yet another study did not find differences in serum SARS-CoV-2-specific IgG levels between children and adults.59 When interpreting these results, it is also important to consider that antibody levels depend on both disease severity, with more severe disease leading to higher antibody levels, and the timing of measurement after the infection.44,60,61 A further possible explanation for the conflicting results is the target of the antibody measured. One study found that children have lower levels of IgG against the spike (S) and nucleocapsid (N) protein, but higher levels of antibodies against non-structural proteins (NSP) and open reading frames (ORF), which are known IFN antagonists.62
Heterologous Immunity and Off-Target Effects of Vaccines
Heterologous immunity describes immune responses generated by an antigen providing immunity against other (unrelated) pathogens.63–65 This includes innate and adaptive immune responses and can result from natural infection or vaccination.63,66
COVID-19 severity is reported to be lower in individuals who have been vaccinated with measles-mumps-rubella (MMR) or tetanus-diphtheria-pertussis (Tdap) vaccine.67 As children have generally been more recently vaccinated with these vaccines, this might contribute to the age-related difference in COVID-19 severity.68
MMR vaccines contain attenuated enveloped ribonucleic acid (RNA) viruses that have glycoprotein spikes, similar to SARS-CoV-2 and also share other sequence homologies.69,70 Cross-reactive epitopes have also been found between antigens included in Tdap and SARS-CoV-2.71 It has therefore been hypothesized that MMR and Tdap vaccination might lead to cross-protective antibodies and T cells that protect against COVID-19.72–74 In one small prospective study, MMR-vaccinated participants who later developed COVID-19, all had a mild course.75 Another, much larger case-control study, showed that MMR vaccination might have a protective effect against COVID-19 in males but not females.76 A case-control study in children also showed that children vaccinated with a measles-containing vaccine had lower infection rates with SARS-CoV-2 and, if infected, had milder symptoms.77 Another study found that the outcome of COVID-19 inversely correlated with levels of rubella-specific antibodies70 and another with levels of mumps-specific antibodies.78 An interim analysis of an ongoing randomized control trial (RCT) of MMR to reduce SARS-CoV-2 infection and severity in health care workers reported that MMR reduces the risk of symptomatic infection.79 A second RCT to investigate the influence of MMR on the severity of COVID-19 is also ongoing.80 In individuals after SARS-CoV-2 infection or after COVID-19 vaccination, enhanced in vitro T cell responses to components of the MMR and Tdap vaccine have been found.67 Identical T cell receptor clonotypes can be found on T cells activated by SARS-CoV-2, Tdap or MMR antigens, consistent with heterologous immunity.67 Another study reports that antibodies induced by inactivated poliovirus vaccination bind the RNA-dependent RNA polymerase of SARS-CoV-2 and inhibit infection of Vero cells in vitro.81
Another vaccine proposed to provide beneficial effects against COVID-19 is Bacillus Calmette-Guérin (BCG). Ecologic studies claim to identify associations between countries’ BCG vaccination policy and their COVID-19 rates and severity.82–87 But such studies are subject to confounding by timing of the study in relation to the epidemic in each country, lockdown and other mitigation measures, testing and reporting rates, and are also limited by inaccurate BCG vaccination status reporting.88–91 Furthermore, it is unlikely that the beneficial effects of BCG vaccination last for many years as they are likely abrogated by the impact of intervening vaccines and other factors that also modulate the immune system. Consistent with this, some ecologic studies92,93 and retrospective case-control studies94,95 have not found any protection against COVID-19 from BCG given many decades earlier. One RCT in the elderly reported that BCG reduced the incidence and severity of COVID-19.96 Larger RCTs of BCG to reduce the severity of COVID-19 are ongoing.97,98
Children infected with SARS-CoV-2 often have co-infections with other viruses (including commonly circulating HCoVs).7,99,100 Frequent recurrent or concurrent infections could induce an enhanced state of activation of the immune system, including epigenetic changes inducing trained immunity, making it more effective in clearing SARS-CoV-2.101
Differences in the Endothelium and Clotting Function
Widespread endothelial injury and coagulation activation by SARS-CoV-2 is a key feature of severe COVID-19.102 This is associated with thromboembolisms, such as deep vein thrombosis and pulmonary emboli, as well as arterial thrombosis or microvascular thrombosis.102–106 When SARS-CoV-2 binds to the ACE2 receptor, the expression of the receptor is downregulated, resulting in increased levels of angiotensin II, which is associated with inflammation, endothelial dysfunction and a procoagulant state.102 Endothelial damage leads to the release of plasminogen activator inhibitor 1 and to the activation of tissue factor, which leads to further inflammation and induction of the thrombotic cascade.107 This increases endothelial damage and platelet activation.108 Platelets express both ACE2 and TMPRSS2 and can therefore be directly activated by SARS-CoV-2.109
The endothelium in children is less “pre-damaged” compared with adults and the coagulation system also differs, which makes children less prone to abnormal clotting.110 In adults with COVID-19, the overall rate of venous thrombosis is approximately 14 to 20%, and of arterial thrombosis 2%.111,112 Higher rates are observed in adults admitted to intensive care.111,112 Thrombotic coagulopathy has been observed in SARS-CoV-2-infected children of all age groups, often occurring during hospitalization and despite thromboprophylaxis, and is associated with a high mortality of up to 28%.113,114 Although the incidence is less well described in children, it is much lower than in adults. One study reports rates of 2% in children with COVID-19 and 7% in children with PIMS-TS.114 Children above the age of 12 years, those with cancer or a central venous catheter are at higher risk for thromboembolic events.114 Of note, however, thromboembolic events have also been observed in children with asymptomatic SARS-CoV-2 infections.114
MECHANISMS WHICH ARE LESS LIKELY TO CONTRIBUTE TO THE AGE-RELATED DIFFERENCES IN SEVERITY OF COVID-19
Pre-existing Immunity from Commonly Circulating Human Coronaviruses
Commonly circulating human coronaviruses (HCoV-229E, -HKU1, -NL63 and -OC43) are responsible for approximately 6 to 8% of acute respiratory tract infections and most individuals develop immunity to HCoVs during childhood.2,115,116 Individuals who have not been infected with SARS-CoV-2 can therefore have cross-reactive, neutralizing and non-neutralizing antibodies, and T cells against the S protein (up to 5%), N protein (up to 24%) and ORF regions of SARS-CoV-2.117–125 Seroprevalence depends on geographical location.119
Despite seroconversion at an early age, re-infections with HCoVs later in life are common.126,127 There is conflicting evidence on whether children or adults have higher levels of cross-reactive antibodies and T cells. Some studies report that levels of neutralizing and non-neutralizing cross-reactive antibodies, as well as cross-reactive T cells, increase with age,128,129 while other studies report higher levels of these antibodies in SARS-CoV-2-uninfected children and adolescents130 or no differences between age groups.35,118 One study found higher cross-reactive IgA and IgG levels in healthy elderly adults and higher IgM levels in healthy children, suggesting that children have a less-experienced but more polyreactive humoral immunity.131
Delayed production of neutralizing antibodies during a SARS-CoV-2 infection is associated with increased mortality.132 Antibodies against commonly circulating HCoVs are boosted during a SARS-CoV-2 infection.118,133,134 Importantly, however, although antibodies against HCoVs can be cross-reactive, they do not necessarily protect against SARS-CoV-2, as they are not necessarily cross-neutralizing.35,58,118,135–139 One study, however, reported that a recent documented history of a common cold caused by HCoV is associated with lower rate of admission to intensive care unit and lower mortality from COVID-19.140 Another study showed a correlation between pre-existing antibodies against HCoV-OC43 and COVID-19 severity, but not between antibodies against HCoV-NL63, -229E and -HKU1, indicating that cross-protection might differ between different HCoVs and SARS-CoV-2.141 Consistent with this, one study found higher antibody levels against HCoV-229E but not -OC43, and higher levels of cross-reactive antibodies in children compared with adults.142 SARS-CoV-2 and HCoV-NL63 both use ACE2 as an entry receptor.143 However, sequencing data shows SARS-CoV-2 is more closely related to HCoV-OC43 and -HKU1 than -NL63 and -229E.143 There is a region coding for 11 amino acids that is highly conserved between SARS-CoV-2 and all four HCoVs, which overlaps with the S2 fusion peptide in SARS-CoV-2.144 It has been suggested that cross-reactive antibodies against S2 might provide neutralizing activity and protection against SARS-CoV-2.133,134 One study reported that pre-existing S2-specific antibodies against HCoV-OC43 are associated with mild COVID-19.145 These antibodies are more frequently present in children and adolescents.130 Another study found cross-reactive antibodies against SARS-CoV-2 ORF-1 in pre-pandemic samples, but not against protein S or N. The presence of these antibodies was associated with milder COVID-19.144 As discussed above, higher antibody levels against ORF (IFN antagonists) are found in children with COVID-19 compared with adults.62 This could indicate that antibodies against ORF play a role in controlling SARS-CoV-2.
There is also a theoretical risk that higher levels of non-neutralizing cross-reactive HCoVs antibodies might lead to more severe COVID-19 through antibody-dependent enhancement (ADE).128 In ADE, pre-existing non-neutralizing antibodies can bind to virions, which can then more easily enter and replicate in macrophages and granulocytic cells, leading to higher viral loads.146,147 To date, there is scant evidence for ADE in COVID-19.
T cells that are cross-reactive between commonly circulating HCoVs and SARS-CoV-2 have been identified in a number of studies.120–125 In one study more than half of participants with no known exposure to SARS-CoV-2 had T cell activity against SARS-CoV-2.125 There is a correlation between levels of specific IgA and IgG and specific T cell responses.148–150 Few studies have compared T cell immunity against HCoVs in different age groups, but one study reported lower levels of T cells against HCoVs in older adults.124 However, as with cross-reactive antibodies, the role of cross-reactive T cells in relation to SARS-CoV-2 remains unclear.121,122,149 It has been suggested that the presence of cross-reactive CD8+ T cells is associated with milder COVID-19 disease,120,122,123,149,151,152 but also that pre-existing T cells with low avidity, which are usually present in higher numbers in the elderly, negatively impact T cell responses to SARS-CoV-2.129
ACE2 and TMPRSS2
Angiotensin-converting enzyme 2 (ACE2) receptor, present on multiple different cell types throughout the body, is the main receptor for the entry of SARS-CoV-2 into human cells.153–155 Transmembrane serine protease 2 (TMPRSS2), is the key entry-associated protease, cleaving and activating the SARS-CoV-2 S protein, which greatly facilitates entry of the virus into cells.
It has been postulated that different distribution of ACE2 and TMPRSS2 across body sites between children and adults, as well as lower affinity of ACE2 for SARS-CoV-2 in children, contribute to the age-related differences in the severity of COVID-19.154 However, the many studies on this topic report conflicting results. Some studies report lower expression of ACE2 and TMPRSS2 in the nasal epithelium in children compared with adults,154,156,157 while others did not find age-related differences.9,16,24,158 One study reported that the expression of neuropilin-1 (NRP1), a protein that promotes virus interaction with ACE2, is lower in the nasal epithelium of children.159 A study in adults showed that ACE2 and TMPRSS2 in the oral mucosa are higher in elderly compared with young adults.160 Conflicting results have also been reported for the expression of ACE2 and TMPRSS2 in lungs. Some studies report that the expression of ACE2 and TMPRSS2 in lungs increases with age,154,157,161,162 while others report a higher expression of ACE2 in lungs in children compared with elderly adults159 or no difference between the age groups.163
Intestinal expression of TMPRSS2 and NRP1 has been reported to be similar between children and adults, while intestinal ACE2 expression might be higher in children, which might explain the higher frequency of gastrointestinal symptoms in this age group.158
The conflicting results on the expression of ACE2 and TMPRSS2 reflect the fact that the ACE2-angiotensin system is complex. Apart from age, the ACE2-angiotensin system is affected by many other factors, including genetics, sex, smoking, diet, vitamin D, body-mass index, drugs and comorbidities including diabetes mellitus, chronic obstructive pulmonary disease and hypertension.154–157,160,164–170 ACE2 is not only an entry receptor for SARS-CoV-2 but also plays an important role in regulating immune responses, especially in the lungs. After SARS-CoV-2 enters cells, ACE2 receptors are down-regulated, which prevents them from converting angiotensin II to angiotensin-(1–7). The consequent excess of angiotensin II might be partly responsible for the organ injury in COVID-19,171 as serum levels of angiotensin II are significantly elevated in SARS-CoV-2-infected patients and there is a positive correlation with viral load and lung damage.172
Viral Load
There is little evidence to support the hypothesis that viral load is responsible for age-related differences in COVID-19 severity. Viral load in the respiratory tract has been associated with transmission risk, disease severity and mortality of COVID-19.173–178 Children and adults are mostly reported to have similar viral loads and shedding from the respiratory tract.16,179–181 However, one study found significantly greater viral loads in nasopharyngeal samples from children less than 5 years of age compared with older children or adults,182 and there are also studies which report lower viral loads in children compared with adults.24,183 An increased viral load in blood has also been associated with increased disease severity and increased cytokine storm.184–186
Conclusions
There are many hypotheses for the age-related difference in the severity of COVID-19, and it is likely that the explanation for the marked age gradient is multifactorial.8 The proposed mechanisms that relate specifically to the pathogenesis of SARS-CoV-2 seem more likely to be important than those that would also apply to other viral infections for which a similar age gradient is not seen. The latter include, for example, differences in vitamin D and melatonin levels, and chronic cytomegalovirus infection.8
Differences in innate, adaptive and heterologous immunity, as well as differences in the endothelial and clotting function, are the most likely mechanisms to explain the observed age gradient in COVID-19. Children have a faster and stronger innate immune reaction to SARS-CoV-2, especially in the nasal mucosa, involving IFN signaling and the NLRP3 inflammasome, which is able to rapidly control the virus.9,16,23 In contrast, adults can have an overactive, dysregulated and ineffective innate response leading to uncontrolled pro-inflammatory cytokine production and tissue injury.27 Children also have a higher proportion of innate lymphoid and non-clonally expanded naïve T cells in the blood, while the elderly can have poor and uncoordinated T cell responses with additional scarcity of naïve T cells.48,54,55 More recent MMR and other vaccines might lead to protective cross-reactive antibodies and T cells against SARS-CoV-2.67,68,70,72–76,78 In addition to these differences in immune responses, immunosenescence187–192 and inflammaging,40,41,193,194 as well as co-morbidities might contribute to more severe COVID-19 in elderly, as described in our previous review.8 Another immunologic mechanism that might be important is the age-related increase in auto-antibodies against type I IFN.195
Mechanisms which are less likely to explain the age-related differences in COVID-19 are pre-existing immunity from commonly circulating HCoVs and differences in the expression of ACE2 and TMPRSS2, although evidence can be found both for and against these hypotheses. Studies investigating antibodies and T cells against HCoVs in children and adults also report conflicting results.35,118,128–130 Furthermore, it has not been proven that cross-reactivity between HCoVs and SARS-CoV-2 leads to cross-protection.35,118,135–138 Studies which have investigated the expression of ACE2 and TMPRSS2 in children and adults also report conflicting results.9,16,154,156–163 Moreover, the ACE2-angiotensin system is complex and influenced by many other internal and external factors other than age.154–157,160,164–170 Although viral load is associated with COVID-19 severity and mortality, viral load between children and adults is similar, meaning this is also unlikely to contribute to the age-related difference in severity of COVID-19.16,179–181
It is likely that the age gradient in severity of COVID-19 results from both factors that protect children and factors that make the elderly more susceptible. It is possible that following exposure to SARS-CoV-2, immunologic factors in children are important in preventing infection or controlling the virus after infection, and age-related differences in endothelial and clotting function are more important in putting the elderly at risk of the complications of COVID-19 that lead to higher mortality.
SARS-CoV-2 is constantly mutating with new variants becoming better at evading host defenses; understanding the mechanisms underlying the age-related difference in the severity of COVID-19 will provide key insights into its pathogenesis and opportunities for prevention and treatment.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
P.Z. drafted the initial manuscript. N.C. contributed to the writing and critical revision of the manuscript, and both authors approved the final manuscript as submitted.
REFERENCES
- 1.Götzinger F, Santiago-García B, Noguera-Julián A, et al.; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R, Votto M, Licari A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uka A, Buettcher M, Bernhard-Stirnemann S, et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study [published online ahead of print May 27, 2021]. Eur J Pediatr. doi: 10.21203/rs.3.rs-517119/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward JL, Harwood R, Smith C, et al. Risk factors for intensive care admission and death amongst children and young people admitted to hospital with COVID-19 and PIMS-TS in England during the first pandemic year. medRxiv. Preprint posted online July 5, 2021. doi: 10.1101/2021.07.01.21259785. [Google Scholar]
- 7.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of Epidemiologic and Clinical Features. Pediatr Infect Dis J. 2020;39:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections [published online ahead of print December 1, 2020]. Arch Dis Child. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 9.Loske J, Röhmel J, Lukassen S, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children [published online ahead of print August 18, 2021]. Nat Biotechnol. doi: 10.1038/s41587-021-01037-9. [DOI] [PubMed] [Google Scholar]
- 10.Rebendenne A, Valadão ALC, Tauziet M, et al. SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells J Virol. 2021;95:e02415–e02420.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin X, Riva L, Pu Y, et al. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 2021;34:108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne LG, Reuschl AK, Zuliani-Alvarez L, et al. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40:e107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio NG, Chauveau L, Hertzog J, et al. The RNA sensor MDA5 detects SARS-CoV-2 infection. Sci Rep. 2021;11:13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park A, Iwasaki A. Type I and Type III Interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YM, Shin EC. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021;53:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce CA, Sy S, Galen B, et al. Natural mucosal barriers and COVID-19 in children. JCI Insight. 2021;6:148694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaender S, Mar KB, Michailidis E, et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat Microbiol. 2020;5:1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Sancho L, Lewinski MK, Pache L, et al. Functional landscape of SARS-CoV-2 cellular restriction. Mol Cell. 2021;81:2656–2668.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H, Shi PY. Antagonism of Type I interferon by severe acute respiratory syndrome coronavirus 2. J Interferon Cytokine Res. 2020;40:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Ishida R, Strilets T, et al. SARS-CoV-2 nonstructural protein 1 inhibits the interferon response by causing depletion of key host signaling factors. J Virol. 2021;95:e0026621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amor S, Fernández Blanco L, Baker D. Innate immunity during SARS-CoV-2: evasion strategies and activation trigger hypoxia and vascular damage. Clin Exp Immunol. 2020;202:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkley K, Banerjee D, Bradley T, et al. Immune cell residency in the nasal mucosa may partially explain respiratory disease severity across the age range. Sci Rep. 2021;11:15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Chew KY, Karawita AC, et al. Pediatric nasal epithelial cells are less permissive to SARS-CoV-2 replication compared to adult cells. bioRxiv. Preprint posted online March 8, 2021. doi: 10.1101/2021.03.08.434300. [Google Scholar]
- 25.Neeland MR, Bannister S, Clifford V, et al. Children and adults in a household cohort study have robust longitudinal immune responses following SARS-CoV-2 infection or exposure. Front Immunol. 2021;12:741639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeland MR, Bannister S, Clifford V, et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat Commun. 2021;12:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carsetti R, Zaffina S, Piano Mortari E, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11:610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordallo B, Bellas M, Cortez AF, et al. Severe COVID-19: what have we learned with the immunopathogenesis? Adv Rheumatol. 2020;60:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordoni V, Sacchi A, Cimini E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2272–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narasimhan PB, Marcovecchio P, Hamers AAJ, et al. Nonclassical monocytes in health and disease. Annu Rev Immunol. 2019;37:439–456. [DOI] [PubMed] [Google Scholar]
- 31.Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell. 2020;183:982–995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartsch YC, Wang C, Zohar T, et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat Med. 2021;27:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Chen K, Liu M, et al. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect. 2020;81:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacques FH, Apedaile E. Immunopathogenesis of COVID-19: summary and possible interventions. Front Immunol. 2020;11:564925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vono M, Huttner A, Lemeille S, et al. Robust innate responses to SARS-CoV-2 in children resolve faster than in adults without compromising adaptive immunity. Cell Rep. 2021;37:109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goenka A, Halliday A, Gregorova M, et al. Young infants exhibit robust functional antibody responses and restrained IFN-γ production to SARS-CoV-2. Cell Rep Med. 2021;2:100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji SQ, Zhang M, Zhang Y, et al. Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China. World J Pediatr. 2021;17:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu W, Yang L, Li X, et al. Early immune responses and prognostic factors in children with COVID-19: a single-center retrospective analysis. BMC Pediatr. 2021;21:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. [DOI] [PubMed] [Google Scholar]
- 41.Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan P, Shen M, Yu Z, et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12:4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. 2020;11:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147:545–557.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tosif S, Neeland MR, Sutton P, et al. Immune responses to SARS-CoV-2 in three children of parents with symptomatic COVID-19. Nat Commun. 2020;11:5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sealy RE, Hurwitz JL. Cross-reactive immune responses toward the common cold human Coronaviruses and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Mini-Review and a Murine Study. Microorganisms. 2021;9:1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida M, Worlock KB, Huang N, et al. The local and systemic response to SARS-CoV-2 infection in children and adults. medRxiv. Preprint posted online March 15, 2021. doi: 10.1101/2021.03.09.21253012. [Google Scholar]
- 49.Fazolo T, Lima K, Fontoura JC, et al. Strong anti-viral responses in pediatric COVID-19 patients in South Brazil. medRxiv. Preprint posted online April 16, 2021. doi: 10.1101/2021.04.13.21255139. [Google Scholar]
- 50.Cohen CA, Li APY, Hachim A, et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat Commun. 2021;12:4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. [DOI] [PubMed] [Google Scholar]
- 53.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi T, Ellingson MK, Wong P, et al.; Yale IMPACT Research Team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sananez I, Raiden SC, Algieri SC, et al. A poor and delayed anti-SARS-CoV2 IgG response is associated to severe COVID-19 in children. EBioMedicine. 2021;72:103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toh ZQ, Anderson J, Mazarakis N, et al. Reduced seroconversion in children compared to adults with mild COVID-19. medRxiv. Preprint posted onine October 18, 2021. doi: 10.1101/2021.10.17.21265121. [Google Scholar]
- 58.Renk H, Dulovic A, Becker M, et al. Typically asymptomatic but with robust antibody formation: Children’s unique humoral immune response to SARS-CoV-2. medRxiv. Preprint posted online July 22, 2021. doi: 10.1101/2021.07.20.21260863. [Google Scholar]
- 59.Márquez-González H, López-Martínez B, Parra-Ortega I, et al. Analysis of the behaviour of Immunoglobulin G antibodies in children and adults convalescing from severe acute respiratory syndrome-Coronavirus-2 infection. Front Pediatr. 2021;9:671831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–88.e11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130:5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hachim A, Gu H, Kavian O, et al. The SARS-CoV-2 antibody landscape is lower in magnitude for structural proteins, diversified for accessory proteins and stable long-term in children. medRxiv. Preprint posted online January 4, 2021. doi: 10.1101/2021.01.03.21249180. [Google Scholar]
- 63.Messina NL, Zimmermann P, Curtis N. The impact of vaccines on heterologous adaptive immunity. Clin Microbiol Infect. 2019;25:1484–1493. [DOI] [PubMed] [Google Scholar]
- 64.Balz K, Trassl L, Härtel V, et al. Virus-induced T cell-mediated heterologous immunity and vaccine development. Front Immunol. 2020;11:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil A, Kenney LL, Mishra R, et al. Vaccination and heterologous immunity: educating the immune system. Trans R Soc Trop Med Hyg. 2015;109:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. 2017;102:1077–1081. [DOI] [PubMed] [Google Scholar]
- 67.Mysore V, Cullere X, Settles ML, et al. Protective heterologous T cell immunity in COVID-19 induced by the trivalent MMR and Tdap vaccine antigens. Med (N Y). 2021;2:1050–1071.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marakasova E, Baranova A. MMR vaccine and COVID-19: measles protein homology may contribute to cross-reactivity or to complement activation protection. mBio. 2021;12:e03447–e03420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franklin R, Young A, Neumann B, et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv. Preprint posted online April 10, 2020. doi: 10.1101/2020.04.10.20053207. [Google Scholar]
- 71.Reche PA. Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Front Immunol. 2020;11:586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fidel PL, Jr, Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to Dampen septic inflammation associated with COVID-19 infection? mBio. 2020;11:e00907–e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam N, Khunti K, Majeed A. COVID-19, seasonal influenza and measles: potential triple burden and the role of flu and MMR vaccines. J R Soc Med. 2020;113:485–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashford JW, Gold JE, Huenergardt MA, et al. MMR vaccination: a potential strategy to reduce severity and mortality of COVID-19 illness. Am J Med. 2021;134:153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larenas-Linnemann DE, Rodríguez-Monroy F. Thirty-six COVID-19 cases preventively vaccinated with mumps-measles-rubella vaccine: all mild course. Allergy. 2021;76:910–914. [DOI] [PubMed] [Google Scholar]
- 76.Lundberg L, Bygdell M, Stukat von Feilitzen G, et al. Recent MMR vaccination in health care workers and Covid-19: a test negative case-control study. Vaccine. 2021;39:4414–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gujar N, Tambe M, Parande M, et al. A case control study to assess effectiveness of measles containing vaccines in preventing Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in children Hum Vaccin Immunother. 202117:3316–3321.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gold JE, Baumgartl WH, Okyay RA, et al. Analysis of Measles-Mumps-Rubella (MMR) titers of recovered COVID-19 patients. mBio. 2020;11:e02628–e02620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fedrizzi EN, Girondi JBR, Sakae TM, et al. Efficacy of the Measles-Mumps-Rubella (MMR) vaccine in the reducing the severity of COVID-19: an interim analysis of a Randomised Controlled Clinical Trial. medRxiv. Preprint posted online September 20, 2021. doi: 10.1101/2021.09.14.21263598. [Google Scholar]
- 80.Avidan M. CROWN CORONATION: COVID-19 Research Outcomes Worldwide Network for CORONAvirus prevenTION (CROWN CORONA). Available at: https://clinicaltrials.gov/ct2/show/NCT04333732. Accessed August, 27, 2021. [Google Scholar]
- 81.Comunale BA, Engineer L, Jiang Y, et al. Poliovirus vaccination induces a humoral immune response that cross reacts with SARS-CoV-2. Front Med (Lausanne). 2021;8:710010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller A, Reandelar MJ, Fasciglione K, et al. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. Preprint posted online March 28, 2020. doi: 10.1101/2020.03.24.20042937. [Google Scholar]
- 83.Shet A, Ray D, Malavige N, et al. Differential COVID-19-attributable mortality and BCG vaccine use in countries. medRxiv. Preprint posted online April 6, 2020. doi: 10.1101/2020.04.01.20049478. [Google Scholar]
- 84.Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Nat Acad Sci. 2020117:17720–17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brooks NA, Puri A, Garg S, et al. The association of Coronavirus Disease-19 mortality and prior bacille Calmette-Guerin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohamed Hussein AAR, Salem MR, Salman S, et al. Correlation between COVID-19 case fatality rate and percentage of BCG vaccination: is it true the vaccine is protective? Egypt J Bronchol. 2020;14:25. [Google Scholar]
- 87.Berg MK, Yu Q, Salvador CE, et al. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020;6:eabc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Curtis N, Sparrow A, Ghebreyesus TA, et al. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395:1545–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riccò M, Gualerzi G, Ranzieri S, et al. Stop playing with data: there is no sound evidence that Bacille Calmette-Guérin may avoid SARS-CoV-2 infection (for now). Acta Biomed. 2020;91:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asahara M. The effect of BCG vaccination on COVID-19 examined by a statistical approach: no positive results from the Diamond Princess and cross-national differences previously reported by world-wide comparisons are flawed in several ways. medRxiv. Preprint posted online May 22, 2020. doi: 10.1101/2020.04.17.20068601. [Google Scholar]
- 91.Sayed A, Challa KT, Arja S. Epidemiological differences of COVID-19 over the world. Cureus. 2020;12:e10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukui M, Kawaguchi K, Matsuura H. Does TB vaccination reduce COVID-19 infection? no evidence from a regression discontinuity analysis. medRxiv. Preprint posted online April 22, 2020. doi: 10.1101/2020.04.13.20064287. [Google Scholar]
- 93.Hensel J, McAndrews KM, McGrail DJ, et al. Exercising caution in correlating COVID-19 incidence and mortality rates with BCG vaccination policies due to variable rates of SARS CoV-2 testing. medRxiv. Preprint posted online April 11, 2020. doi: 10.1101/2020.04.08.20056051. [Google Scholar]
- 94.Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323:2340–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pépin J, Labbé AC, Carignan A, et al. Does BCG provide long-term protection against SARS-CoV-2 infection? A case-control study in Quebec, Canada [published online ahead of print August 11, 2021]. Vaccine. doi: 10.1016/j.vaccine.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsilika M, Taks E, Dolianitis K, et al. Activate-2: a double-blind randomized trial of BCG vaccination against COVID19 in individuals at risk. medRxiv. Preprint posted online May 24, 2021. doi: 10.1101/2021.05.20.21257520. [Google Scholar]
- 97.Pittet LF, Messina NL, Gardiner K, et al.; BRACE trial Consortium Group. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: Protocol for a randomised controlled trial (BRACE trial). BMJ Open. 2021;11:e052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ClinicalTrials.gov. BCG and COVID. Available at https://clinicaltrials.gov/ct2/results?cond=BCG+COVID. Accessed November 28, 2021.
- 99.Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146:e20200961. [DOI] [PubMed] [Google Scholar]
- 100.Kim D, Quinn J, Pinsky B, et al. Rates of Co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canzano P, Brambilla M, Porro B, et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. 2021;6:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lippi G, Sanchis-Gomar F, Favaloro EJ, et al. Coronavirus Disease 2019-associated coagulopathy. Mayo Clin Proc. 2021;96:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ignjatovic V, Mertyn E, Monagle P. The coagulation system in children: developmental and pathophysiological considerations. Semin Thromb Hemost. 2011;37:723–729. [DOI] [PubMed] [Google Scholar]
- 111.Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nopp S, Moik F, Jilma B, et al. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4:1178–1191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitchell WB, Davila J, Keenan J, et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021;68:e28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whitworth H, Sartain SE, Kumar R, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dijkman R, Jebbink MF, El Idrissi NB, et al. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46:2368–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaye HS, Marsh HB, Dowdle WR. Seroepidemiologic survey of coronavirus (strain OC 43) related infections in a children’s population. Am J Epidemiol. 1971;94:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mveang Nzoghe A, Essone PN, Leboueny M, et al. Evidence and implications of pre-existing humoral cross-reactive immunity to SARS-CoV-2. Immun Inflamm Dis. 2021;9:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anderson EM, Goodwin EC, Verma A, et al.; UPenn COVID Processing Unit. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tso FY, Lidenge SJ, Peña PB, et al. High prevalence of pre-existing serological cross-reactivity against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int J Infect Dis. 2021;102:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. [DOI] [PubMed] [Google Scholar]
- 123.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. [DOI] [PubMed] [Google Scholar]
- 124.Saletti G, Gerlach T, Jansen JM, et al. Older adults lack SARS CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. Sci Rep. 2020;10:21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meckiff BJ, Ramírez-Suástegui C, Fajardo V, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell. 2020;183:1340–1353.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Isaacs D, Flowers D, Clarke JR, et al. Epidemiology of coronavirus respiratory infections. Arch Dis Child. 1983;58:500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Monto AS, Lim SK. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol. 2020;92:512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bacher P, Rosati E, Esser D, et al. Pre-existing T cell memory as a risk factor for severe 1 COVID-19 in the elderly. medRxiv. Preprint posted online September 18, 2020. doi: 10.1101/2020.09.15.20188896. [Google Scholar]
- 130.Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Selva KJ, van de Sandt CE, Lemke MM, et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat Commun. 2021;12:2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucas C, Klein J, Sundaram ME, et al.; Yale IMPACT Research Team. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med. 2021;27:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen-Contant P, Embong AK, Kanagaiah P, et al. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio. 2020;11:e01991–e01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song G, He WT, Callaghan S, et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat Commun. 2021;12:2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lv H, Wu NC, Tsang OT, et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31:107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miyara M, Sterlin D, Anna F, et al. Pre-COVID-19 humoral immunity to common coronaviruses does not confer cross-protection against SARS-CoV-2. medRxiv. Preprint posted online August 15, 2020. doi: 10.1101/2020.08.14.20173393. [Google Scholar]
- 137.To KK, Cheng VC, Cai JP, et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1:e111–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khan S, Nakajima R, Jain A, et al. Analysis of serologic cross-reactivity between common human coronaviruses and SARS-CoV-2 using Coronavirus antigen microarray. bioRxiv. Preprint posted online March 25, 2020. doi: 10.1101/2020.03.24.006544. [Google Scholar]
- 140.Sagar M, Reifler K, Rossi M, et al. Recent endemic coronavirus infection is associated with less-severe COVID-19. J Clin Invest. 2021;131:143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo L, Wang Y, Kang L, et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect. 2021;10:664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tamminen K, Salminen M, Blazevic V. Seroprevalence and SARS-CoV-2 cross-reactivity of endemic coronavirus OC43 and 229E antibodies in Finnish children and adults. Clin Immunol. 2021;229:108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gussow AB, Auslander N, Faure G, et al. Genomic determinants of pathogenicity in SARS-CoV-2 and other human coronaviruses. Proc Natl Acad Sci USA. 2020;117:15193–15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shrock E, Fujimura E, Kula T, et al.; MGH COVID-19 Collection & Processing Team. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020;370:eabd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaplonek P, Wang C, Bartsch Y, et al. Early cross-coronavirus reactive signatures of protective humoral immunity against COVID-19. bioRxiv. Preprint posted online May 12, 2021. doi: 10.1101/2021.05.11.443609. [Google Scholar]
- 146.Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. [DOI] [PubMed] [Google Scholar]
- 147.Beretta A, Cranage M, Zipeto D. Is cross-reactive immunity triggering COVID-19 immunopathogenesis? Front Immunol. 2020;11:567710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng Y, Mentzer AJ, Liu G, et al.; Oxford Immunology Network Covid-19 Response T cell Consortium; ISARIC4C Investigators. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schulien I, Kemming J, Oberhardt V, et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat Med. 2021;27:78–85. [DOI] [PubMed] [Google Scholar]
- 152.Nelde A, Bilich T, Heitmann JS, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol. 2021;22:74–85. [DOI] [PubMed] [Google Scholar]
- 153.Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Muus C, Luecken MD, Eraslan G, et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. Biorxiv. Preprint posted online April, 2020. doi: 10.1101/2020.04.19.049254. [Google Scholar]
- 155.Li Y, Zhou W, Yang L, et al. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berni Canani R, Comegna M, Paparo L, et al. Age-related differences in the expression of most relevant mediators of SARS-CoV-2 infection in human respiratory and gastrointestinal tract. Front Pediatr. 2021;9:697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Z, Guo L, Huang L, et al. Distinct disease severity between children and older adults with COVID-19: impacts of ACE2 expression, distribution, and lung progenitor cells [published online ahead of print January 3, 2021]. Clin Infect Dis. doi: 10.1093/cid/ciaa1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng J, Sun J, Zhao J, et al. Age and gender differences in ACE2 and TMPRSS2 expressions in oral epithelial cells. J Transl Med. 2021;19:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang A, Chiou J, Poirion OB, et al.; NHLBI LungMap Consortium. Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of SARS-CoV2 host genes. Elife. 2020;9:e62522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schuler BA, Habermann AC, Plosa EJ, et al.; Vanderbilt COVID-19 Consortium Cohort; Human Cell Atlas Biological Network. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin Invest. 2021;131:140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tao Y, Yang R, Wen C, et al. Preliminary analysis of scRNA sequencing of children’s lung tissues excludes the expression of SARS-CoV-2 entry related genes as the key reason for the milder syndromes of COVID-19 in children. medRxiv. Preprint posted online September 29, 2020. doi: 10.1101/2020.05.25.20110890. [Google Scholar]
- 164.Wooster L, Nicholson CJ, Sigurslid HH, et al. Polymorphisms in the ACE2 locus associate with severity of COVID-19 infection. medRxiv. Preprint posted online June 22, 2020. doi: 10.1101/2020.06.18.20135152. [Google Scholar]
- 165.Luo Y, Liu C, Guan T, et al. Association of ACE2 genetic polymorphisms with hypertension-related target organ damages in south Xinjiang. Hypertens Res. 2019;42:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu D, Chen Y, Zhang P, et al. Association between circulating levels of ACE2-Ang-(1-7)-MAS axis and ACE2 gene polymorphisms in hypertensive patients. Medicine (Baltimore). 2016;95:e3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Q, Cong M, Wang N, et al. Association of angiotensin-converting enzyme 2 gene polymorphism and enzymatic activity with essential hypertension in different gender: a case-control study. Medicine (Baltimore). 2018;97:e12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–540.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021;21:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen PZ, Bobrovitz N, Premji Z, et al. Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. Elife. 2021;10:e65774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Magleby R, Westblade LF, Trzebucki A, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with Coronavirus Disease 2019 [published online ahead of print June 30, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miller EH, Zucker J, Castor D, et al. Pretest symptom duration and cycle threshold values for Severe Acute Respiratory Syndrome Coronavirus 2 reverse-transcription polymerase chain reaction predict Coronavirus Disease 2019 mortality. Open Forum Infect Dis. 2021;8:ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen PZ, Bobrovitz N, Premji ZA, et al. SARS-CoV-2 shedding dynamics across the respiratory tract, sex, and disease severity for adult and pediatric COVID-19. Elife. 2021;10:e70458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jones TC, Mühlemann B, Veith T, et al. An analysis of SARS-CoV-2 viral load by patient age. medRxiv. Preprint posted online June 9, 2020. doi: 10.1101/2020.06.08.20125484. [Google Scholar]
- 181.Baggio S, L’Huillier AG, Yerly S, et al. SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin Infect Dis. 2021;73:148–150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heald-Sargent T, Muller WJ, Zheng X, et al. Age-related differences in nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate Coronavirus Disease 2019 (COVID-19). JAMA Pediatr. 2020;174:902–903.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Euser S, Aronson S, Manders I, et al. SARS-CoV-2 viral load distribution reveals that viral loads increase with age: a retrospective cross-sectional cohort study. medRxiv. Preprint posted online January 22, 2021. doi: 10.1101/2021.01.15.21249691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome Coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated Interleukin 6 level in critically ill patients with Coronavirus Disease 2019. Clin Infect Dis. 2020;71:1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brasen CL, Christensen H, Olsen DA, et al. Daily monitoring of viral load measured as SARS-CoV-2 antigen and RNA in blood, IL-6, CRP and complement C3d predicts outcome in patients hospitalized with COVID-19. Clin Chem Lab Med. 2021;59:1988–1997. [DOI] [PubMed] [Google Scholar]
- 187.Shaw AC, Joshi S, Greenwood H, et al. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ongrádi J, Kövesdi V. Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Caruso C, Buffa S, Candore G, et al. Mechanisms of immunosenescence. Immun Ageing. 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. [DOI] [PubMed] [Google Scholar]
- 193.Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590.. [DOI] [PubMed] [Google Scholar]
- 194.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 195.Bastard P, Rosen LB, Zhang Q, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]