Supplemental Digital Content is available in the text.
Keywords: invasive bacterial infection, children, COVID-19, pandemic, nonpharmaceutical interventions
Abstract
Background:
Invasive bacterial infection (IBI) remains a major burden of mortality and morbidity in children. As coronavirus disease 2019 (COVID-19) emerged, stringent nonpharmaceutical interventions (NPIs) were applied worldwide. This study aimed to evaluate the impact of NPIs on pediatric IBI in Korea.
Methods:
From January 2018 to December 2020, surveillance for pediatric IBIs caused by 9 pathogens (S. pneumoniae, H. influenzae, N. meningitidis, S. agalactiae, S. pyogenes, S. aureus, Salmonella species, L. monocytogenes and E. coli) was performed at 22 hospitals throughout Korea. Annual incidence rates were compared before and after the COVID-19 pandemic.
Results:
A total of 651 cases were identified and the annual incidence was 194.0 cases per 100,000 in-patients in 2018, 170.0 in 2019 and 172.4 in 2020. Most common pathogen by age group was S. agalactiae in infants < 3 months (n = 129, 46.7%), S. aureus in 3 to < 24 months (n = 35, 37.2%), Salmonella spp. in 24 to < 60 months (n = 24, 34.8%) and S. aureus in children ≥ 5 years (n = 128, 60.7%). Compared with 2018 to 2019, the incidence rate in 2020 decreased by 57% for invasive pneumococcal disease (26.6 vs. 11.5 per 100,000 in-patients, P = 0.014) and 59% for Salmonella spp. infection (22.8 vs. 9.4 per 100,000 in-patients, P = 0.018). In contrast, no significant changes were observed in invasive infections due to S. aureus, S. agalactiae and E. coli.
Conclusions:
The NPIs implemented during the COVID-19 pandemic reduced invasive diseases caused by S. pneumoniae and Salmonella spp. but not S. aureus, S. agalactiae and E. coli in children.
Invasive bacterial infections (IBIs) are major causes of morbidity and mortality in children. Tracking epidemiologic changes of serious IBIs is important for developing strategies to decrease the disease burden and proper management. Accordingly, multicenter, hospital-based surveillance systems for IBIs in Korean children have been maintained since 1996.1,2 The surveillance system can be used as an important tool to assess changes in epidemiology of IBIs during the coronavirus disease 2019 (COVID-19).
After the first COVID-19 case was diagnosed in January 2020 in South Korea, a subsequent regional, however, large-scale outbreak occurred in February 2020, which prompted the health authorities to implement rigorous public health measures from March of 2020.3,4 Following implementation of nonpharmaceutical interventions (NPIs) in attempt to control the COVID-19, a reduction in various infectious diseases, including influenza, other respiratory viruses and vaccine-preventable diseases, has also been seen in South Korea.5–7 Reports from other countries have also shown marked decline in acute respiratory viral infections including influenza and respiratory syncytial virus.8,9
Recent global studies found a significant reduction of IBIs due to S. pneumoniae, H. influenzae and N. meningitidis following the introduction of national COVID-19 containment in many countries.10,11 An interesting study in children has also shown a dramatic reduction of common infectious diseases in Massachusetts between 2019 and 2020.12 However, such reduction of infectious diseases can also be explained by decreased seeking of medical care in addition to implementation of NPIs.13 Given the severity of invasive infections in children, IBIs are less likely to be affected by healthcare utilization patterns. With this background, we investigated changes in the epidemiologic and etiologic distribution of IBIs among children before and during the implementation of NPIs to contain COVID-19.
Methods
Multicenter Surveillance for IBIs
This multicenter surveillance for IBIs has been maintained in South Korea since 1996.1,2 From January 2018 to December 2020, 22 university-affiliated hospitals participated in this study: 13 hospitals located in the national capital and suburban region (Seoul St. Mary’s Hospital, Seoul National University Children’s Hospital, Severance Children’s Hospital, Samsung Medical Center, Seoul Asan Medical Center, Nowon Eulji Medical Center, Ewha Womans University Medical Center, Inha University Hospital, Gachon University Gil Medical Center, Inje University Ilsan Paik Hospital, Korea University Ansan Hospital, Seoul National University Bundang Hospital and CHA Bundang Medical Center), and 9 hospitals located in the regional central cities of provinces (Gangwon-do: Wonju Christian Hospital, Chungcheongbuk-do: Chungbuk National University Hospital, Chungcheongnam-do: Chungnam National University Hospital, Jeollabuk-do: Jeonbuk National Univeristy Hospital, Gwangju/Jeollanam-do: Chonnam National University Hospital, Daegu/Gyeongsangbuk-do: Keimyung University Dongsan Medical Center, Busan/Gyeongsangnam-do: Pusan National University Yangsan Hospital, Kosin University Gospel Hospital and Jeju-do: Jeju National University Hospital).
Data Collection
IBI was defined as the isolation of bacterial organisms from a normally sterile site by using culture, antigen detection or polymerase chain reaction. IBIs caused by nine bacterial organisms were collected; S. pneumoniae, H. influenzae, N. meningitidis, S. agalactiae, S. pyogenes, S. aureus, Salmonella species, L. monocytogenes and E. coli. Infants over 37 weeks of gestation to adolescents under 19 years of age were included. We excluded E. coli infections in children > 3 months of age to mainly collect community acquired infection rather than repetitive infections due to urogenital anomalies or immunocompromised status. S. aureus infection in those with central catheters or immunocompromised conditions was also excluded. Cases reported for invasive bacteria were retrospectively obtained on the annual basis (from January to December each year) for 3 years. All investigators submitted case information using a standard case report form that included age, sex, underlying medical conditions, isolated organism and date of isolation.
Statistical Analysis
The distribution of bacterial organisms was analyzed according to age and time. Age groups were stratified as follows: < 3 months of age, 3 to 23 months of age, 24 to 59 months of age, and ≥ 5 years of age. Pearson’s χ2 was used to test annual difference of the proportion of age group and etiologic agents. The weekly number of cases for each IBI was assessed in 2020 versus 2018 to 2019. The annual incidence of IBIs except S. agalactiae and E. coli were calculated as the number of 100,000 new in-patients in the corresponding year. The annual incidence of IBIs due to S. agalactiae and E. coli were calculated per 10,000 newborns. The difference in annual cumulative incidences of IBIs by organism was compared by using a Poisson test. We performed statistical analyses using R version 4.0.5, and P value < 0.05 was considered statistically significant.
RESULTS
Annual Distribution of the Causative Organisms of IBIs
During the study period, a total of 362,581 new in-patients were identified in 22 hospitals; 134,544 cases in 2018, 132,330 in 2019 and 95,707 in 2020, respectively, and the annual number of births was 20,110 babies in 2018, 20,067 in 2019 and 18,151 in 2020, respectively. The male to female ratio was 1.63:1 and the median age was 10.0 months (range, 0–226 months).
Among these, 651 cases with IBIs were identified in the participating hospitals; 261 cases in 2018, 225 in 2019 and 165 in 2020, respectively (Table 1). Overall, S. aureus was the most frequent cause of IBIs (n = 206, 31.6%), followed by S. agalactiae (n = 139, 21.4%), E. coli (n = 117, 18.0%), S. pneumoniae (n = 82, 12.6%) and Salmonella spp. (n = 70, 10.8%). There were few cases of S. pyogenes (n = 18, 2.8%) and H. influenzae (n = 15, 2.3%). In 2018 to 2019, more than 10% of IBIs were attributed to S. pneumoniae (13.0% in 2018 and 16.4% in 2019) and Salmonella spp. (13.8% in 2018 and 11.1% in 2019); however, only 6.7% and 5.5% were caused by S. pneumoniae and Salmonella spp. in 2020. Meanwhile, the proportion of S. aureus, S. agalactiae and E. coli infection did not show significant differences during the study period. The number of IBIs caused by S. pyogenes and H. influenzae in 2020 decreased by 75.0% and 87.5%, respectively, compared with the previous years. Case of IBI due to N. meningitidis and L. monocytogenes was not observed in 2020.
TABLE 1.
Distribution of Causative Organisms for Invasive Bacterial Infection in Children by Year (2018–2020)
| Species of Bacteria | No. of Isolates by Year (%) | Total (%) | P Value* | ||
|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | |||
| S. aureus | 82 (31.4) | 63 (28.0) | 61 (37.0) | 206 (31.6) | 0.169 |
| S. agalactiae | 48 (18.4) | 47 (20.9) | 44 (26.7) | 139 (21.4) | 0.125 |
| E. coli | 44 (16.9) | 36 (16.0) | 37 (22.4) | 117 (18.0) | 0.220 |
| S. pneumoniae | 34 (13.0) | 37 (16.4) | 11 (6.7) | 82 (12.6) | 0.015 |
| Salmonella spp. | 36 (13.8) | 25 (11.1) | 9 (5.5) | 70 (10.8) | 0.025 |
| S. pyogenes | 8 (3.1) | 8 (3.6) | 2 (1.2) | 18 (2.8) | — |
| H. influenzae | 7 (2.7) | 7 (3.1) | 1 (0.6) | 15 (2.3) | — |
| L. monocytogenes | 2 (0.8) | 2 (0.9) | (0.0) | 4 (0.6) | — |
| N. meningitidis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | — |
| Total | 261 (100) | 225 (100) | 165 (100) | 651 (100) | |
Comparison among 2018, 2019 and 2020.
Age Proportion of IBIs by Year
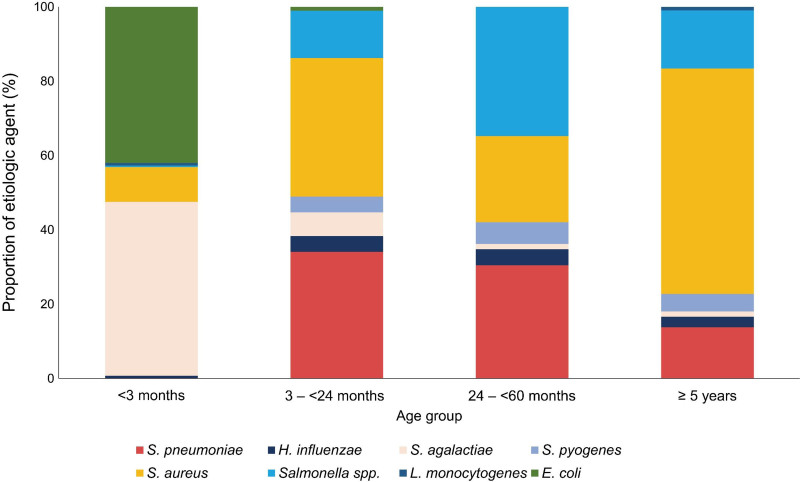
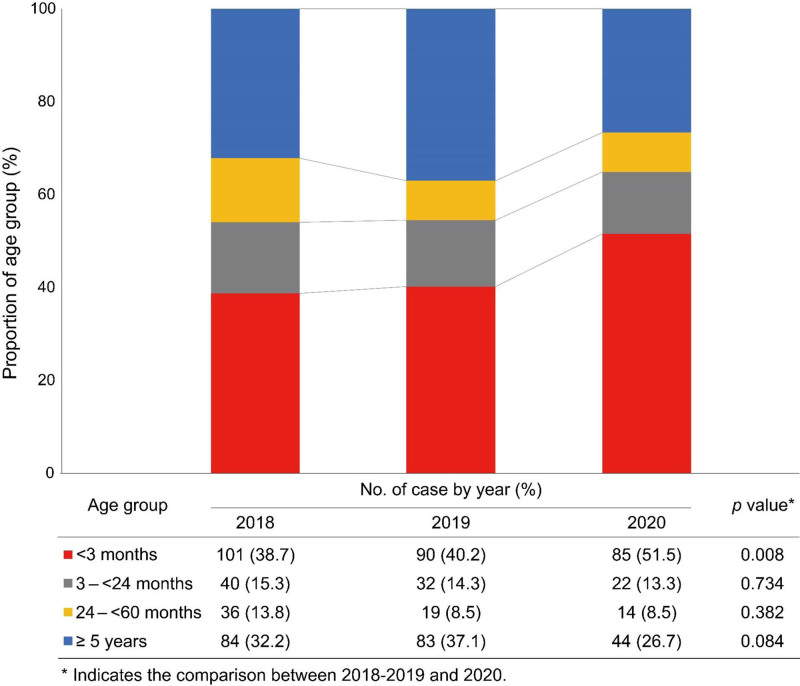
S. agalactiae was most predominant in infants younger than 3 months of age (n = 129, 46.7%), S. aureus in 3 to <24 months of age (n = 35, 37.2%), Salmonella spp. in 24 to <60 months of age (n = 24, 34.8%), S. aureus in children ≥ 5 years of age (n = 128, 60.7%) (Fig. 1). During the 3 years, the frequency of IBIs was highest in the infants under 3 months of age group (42.5%), followed by 32.5% in 5 to 18 years old, 14.5% in 3 to 23 months old and 10.6% in 24 to 59 months old. The age proportion of IBIs changed during the study period (Fig. 2). In 2020, the proportion of IBIs in infants younger than 3 months of age was significantly greater than in the previous 2 years (38.7% in 2018 and 40.2% 2019 vs. 51.5% in 2020, P = 0.008) and the proportion of children 5 to 18 years of age tended to decrease (32.2% in 2018 and 37.1% in 2019 vs. 26.7% in 2020, P = 0.084).
FIGURE 1.
Proportion of etiologic organism by age group.
FIGURE 2.
Age proportion of invasive bacterial infection by year (2018–2020).
The etiologic organisms of IBIs differed according to the age group (Fig. 1). S. agalactiae (129/276, 46.7%) and E. coli (116/276, 42.0%) were responsible for the majority of IBIs in infants < 3 months of age. In children 3 months of age or older, S. aureus was the leading pathogen (47.9%, 179/374) followed by S. pneumoniae (21.9%, 82/374) and Salmonella spp. (18.5%, 69/374). In children 3 to <60 months of age, S. aureus infection was the most common pathogen in 2020 (50.0%), while invasive pneumococcal disease was most prevalent in 2018 to 2019 (32.9% in 2018 and 39.2% in 2019). The proportion of Salmonella spp. infection in children under 2 years of age was 3.8% in 2018 to 2019 and 2.8% in 2020; however, in children 2 to 18 years of age, the proportion decreased from 23.0% in 2018 to 2019 to 10.3% in 2020 (Figure, Supplemental Digital Content 1; http://links.lww.com/INF/E599).
Annual Trend of Cumulative Cases and Incidence of Each Causative Organism of IBIs
The annual trend in the number of weekly count for IBIs by each organism was shown in Fig. 3A. There were significant reductions in the cumulative cases of S. pneumoniae and Salmonella spp. in 2020 compared with 2018 to 2019. The cumulative case number of S. pneumoniae was similar to that of the previous 2 years until March 2020, while thereafter, the case number did not rise. A sharp rising pattern in late autumn and early winter detected in 2018 to 2019 was not seen in 2020. Among Salmonella spp. infection, 21 and 17 cases were detected from June to September in 2018 and 2019, respectively, while 6 cases were identified during the same period in 2020. In contrast, the cumulative curve patterns of S. agalactiae, S. aureus and E. coli were similar throughout the study period. Figure 3B showed the annual incidence of IBIs by each organism from 2018 to 2020. Consistent with the results of the cumulative weekly count, a substantial reduction in the incidence of S. pneumoniae and Salmonella spp. infection in 2020 was observed, compared with 2018 to 2019.
FIGURE 3.
Annual trend in the number of weekly count for invasive bacterial infection by etiologic agent. A, Annual cumulative isolate count of invasive bacterial infection by etiologic organism from 2018 to 2020. B, Annual cumulative incidence of invasive bacterial infection in children by etiologic organism from 2018 to 2020.
In 2020, there was a 57% reduction in the incidence rate of invasive pneumococcal disease compared with that of 2018 to 2019 (26.6 per 100,000 in-patients in 2018–2019 vs. 11.5 per 100,000 in-patients in 2020, rate ratio of 0.43; 95% confidence interval, 0.22–0.86; P = 0.014, Table 2). Likewise, the incidence rate of invasive disease due to Salmonella spp. in 2020 showed 59% decrease compared with the average incidence over the past 2 years (22.8 per 100,000 in-patients in 2018–2019 vs. 9.4 per 100,000 in-patients in 2020, rate ratio of 0.41; 95% confidence interval, 0.19–0.88; P = 0.018, Table 2). The cumulative incidences of S. aureus, S. agalactiae and E. coli infection did not change significantly over 3 years (Table 2).
TABLE 2.
Comparison of Cumulative Incidence Between 2018 to 2019 and 2020
| Species of Bacteria | Cumulative Incidence (per 100,000 in-Patients/Year)* | Rate Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| 2018-2019 | 2020 | |||
| S. aureus | 54.3 | 63.7 | 1.17 (0.82–1.68) | 0.387 |
| S. agalactiae | 23.6 | 24.2 | 1.03 (0.58–1.81) | 0.931 |
| S. pneumoniae | 26.6 | 11.5 | 0.43 (0.22–0.86) | 0.014 |
| Salmonella spp. | 22.8 | 9.4 | 0.41 (0.19–0.88) | 0.018 |
| S. pyogenes | 6.0 | 2.1 | 0.35 (0.07–1.68) | 0.171 |
| H. influenzae | 5.2 | 1.0 | 0.19 (0.23–1.63) | 0.092 |
| E. coli | 19.9 | 20.4 | 1.03 (0.55–1.90) | 0.937 |
| Total | 182.0 | 172.4 | 0.95 (0.77–1.17) | 0.610 |
For E. coli and S. agalactiae, incidence rates were calculated with annual birth number and divided by 10,000.
CI indicates confidence interval.
DISCUSSION
In this nationwide multicenter retrospective study, we demonstrated a significant reduction in invasive bacterial disease due to S. pneumoniae and Salmonella spp. during the COVID-19 outbreak, in comparison with the previous 2 years of 2018 to 2019. However, there was no significant difference in the incidence of invasive disease due to S. aureus, S. agalactiae and E. coli during the same period.
Most noteworthy was the observation that IBIs caused by S. pneumoniae and Salmonella spp. markedly decreased. Although there was no statistical significance due to the small number, S. pyogenes and H. influenzae also declined. The most possible explanation for the differential impact of COVID-19 NPIs on the epidemiology of pediatric IBIs is the difference in the transmission route by organism. S. pneumoniae, S. pyogenes and H. influenzae reside in the respiratory tracts and are mainly transmitted person-to-person through the inhalation of respiratory droplets or close contact.14,15 Day care attendance and crowding are well-known risk factors for invasive pneumococcal disease in children by facilitating the person-to-person transmission and nasopharyngeal carriage.16,17 It is likely that school closure, avoiding attendance to daycare center, mandatory use of facial masks and enhanced personal hygiene applied during the COVID-19 pandemic led to substantial decrease in pneumococcal transmission. Studies from England described the association of reduction in invasive pneumococcal disease with lockdown during the COVID-19 pandemic.18,19 A report from a single center found significant declines in 2020 compared with 2018 to 2019 in invasive infections due to S. pyogenes and S. pneumoniae but not S. aureus.20 Brueggemann et al10 reported worldwide reduction in invasive disease caused by S. pneumoniae and H. influenzae in early 2020 along with the implementation of public large-scale containment measures.
Another remarkable finding was a reduction in the incidence of Salmonella spp. infection in 2020. Salmonella infection is a food-borne illness and it peaks during the summer months on north hemisphere, which is probably associated with rapid replication in warm temperature and frequent eating out in summer.21 Approximately 95% of salmonellosis in humans occurs through contaminated food, in particular, eggs, raw meat, dairy products and fresh vegetables.22 International travel is also a major source in Salmonella infection, comprising 4% to 9% of traveler’s diarrhea.23 In a study for imported infectious diseases in South Korea, salmonellosis was associated with international travel during summer season, and imported cases comprised of 15.3% of salmonellosis with increasing trend.24 Hand hygiene procedures are already known as an effective tool to lower the incidence of Salmonella infection.25 The mandatory 14-day quarantine policy for all overseas travelers introduced from April 2020 in South Korea and worldwide lockdown led to decrease in domestic and international travel. Enhanced public hygiene and food handling processes, changes in eating behavior and decreased international travel may have contributed to a reduction in Salmonella infection.
Additional plausible explanation for decrease in IBIs may be related to decrease in various respiratory viral infections affected by NPIs. Secondary bacterial infections are commonly seen after respiratory infection.26 Respiratory viral infections have an effect on mucosal/epithelial barrier, immunologic dysregulation and microbiota residing on both respiratory and gastrointestinal tract, which might increase a susceptibility to secondary bacterial infections.27,28 During the COVID-19 pandemic, a remarkable reduction in respiratory viral infections have been reported in many countries including South Korea.29,30 Decline in viral respiratory tract infections may have affected a decrease in pneumococcal infection. However, a decline in Salmonella spp. infection, a non-respiratory pathogen, is more likely a direct result of NPIs itself through reinforcement of personal hygiene and interruption of movement and person-to-person contact.
Meanwhile, there was no significant difference in the incidences of S. agalactiae and E. coli over the 3 years. In line with earlier studies,1,2 S. agalactiae and E. coli remained main contributors to IBIs of the infants group. Since S. agalactiae and E. coli are usually acquired and colonizes during the perinatal period, it is assumed that these organisms were minimally affected by NPIs in 2020.31 This implicates S. agalactiae and E. coli are unlikely to be preventable by NPIs, especially in neonates and young infants. S. aureus remained the leading cause of pediatric IBI without a significant difference in incidence throughout the study period, especially in children ≥ 5 years of age. This may be due to the fact that S. aureus is a human commensal residing on the skin and anterior nares.32
In this study, an increase in the proportion of cases under 3 months of age was observed in concordance with the reduction of cases among preschool and school-age children in 2020. Taking into consideration of the major pathogens of this age group (S. agalactiae and E. coli) and route of acquisition of these pathogens this may be explained. The relative increase in proportion of infants under 3 months of age is also related to the decrease of infections in older children, who were more physically and socially active before COVID-19. Low adherence to personal hygiene and limitations in mask use might have a role in the preserved proportion of children under 5 years old.
There are several limitations in our study. First, the decline of IBI cases might result from reduced healthcare-seeking behaviors during the COVID-19 pandemic. To avoid the underestimation of IBI, we calculated the incidence with annual number of pediatric admission and birth number. Since most of IBIs manifest with a fever and lethargic feature, it is less likely to be missed. In fact, the number of infections caused by S. agalactiae and E. coli in young infants did not decrease, demonstrating that this surveillance system had not been disrupted by the COVID-19. Second, it is not possible to determine whether the decrease in IBIs is the result of reduced bacterial transmission and acquisition or the reduction in various viral respiratory tract infections. Changes in antibiotic prescriptions can have affected the epidemiology of IBIs.33 Lastly, serotypes were not determined for H. influenzae and S. pneumoniae in this surveillance.
Despite these limitations, through comparison of incidence rate of IBIs due to nine bacterial pathogens before and after stringent implementation of various NPIs, we found distinct differences in changes in epidemiology according to pathogen and different age groups in children. These findings give insights into factors associated with the development of IBIs in children.
Conclusions
In summary, among 9 major pathogens of IBIs in children, the incidences of S. pneumoniae and Salmonella spp. decreased significantly in 2020, when NPIs came into effect, compared with in 2018 and 2019. This finding suggests that public health measures may attribute to prevention of some IBIs in the pediatric population.
Supplementary Material
Footnotes
This study was supported by the Korea Disease Control and Prevention Agency (2018E240600).
The authors have no conflicts of interest to disclose.
Y.K.K. coordinated data collection, carried out the initial analyses, and drafted the initial manuscript. E.H.C. conceptualized and designed the study, supervised data collection, and reviewed and revised the manuscript. H.L. and Y.-J.K. collected data and revised the manuscript. Y.Y.C., E.S.S., J.G.A., S.E.P., T.L., H.-K.C., J.L., D.S.J., H.M.K., J.K.L., C.S.K., D.H.K., H.M.K., J.H.C., B.W.E., N.H.K., E.Y.C., Y.-K.K., C.E.O, and K.-H.K. collected data and reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Lee JH, Cho HK, Kim KH, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (1996-2005): a retrospective multicenter study. J Korean Med Sci. 2011;26:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhie K, Choi EH, Cho EY, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (2006-2010): a retrospective multicenter study. J Korean Med Sci. 2018;33:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu S, Ali ST, Jang C, et al. Effect of nonpharmaceutical interventions on transmission of severe acute respiratory syndrome coronavirus 2, South Korea, 2020. Emerg Infect Dis. 2020;26:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis. 2021;73:e132–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun HE, Ryu BY, Choe YJ. Impact of social distancing on incidence of vaccine-preventable diseases, South Korea. J Med Virol. 2021;93:1814–1816. [DOI] [PubMed] [Google Scholar]
- 7.Huh K, Jung J, Hong J, et al. Impact of non-pharmaceutical interventions on the incidence of respiratory infections during the COVID-19 outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2020;72:e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2020;72:2199–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang QS, Wood T, Jelley L, et al. ; NPIsImpactOnFlu Consortium. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taha MK, Deghmane AE. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res Notes. 2020;13:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatoun J, Correa ET, Donahue SMA, et al. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146:e2020006460. [DOI] [PubMed] [Google Scholar]
- 13.Kimberlin DW, Bjornstad EC. COVID-19 and the law of unforeseen consequences. Pediatrics. 2020;146:e2020019232. [DOI] [PubMed] [Google Scholar]
- 14.Sá-Leão R, Nunes S, Brito-Avô A, et al. High rates of transmission of and colonization by Streptococcus pneumoniae and Haemophilus influenzae within a day care center revealed in a longitudinal study. J Clin Microbiol. 2008;46:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockerill FR, 3rd, MacDonald KL, Thompson RL, et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- 16.Koliou MG, Andreou K, Lamnisos D, et al. Risk factors for carriage of Streptococcus pneumoniae in children. BMC Pediatr. 2018;18:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takala AK, Jero J, Kela E, et al. Risk factors for primary invasive pneumococcal disease among children in Finland. JAMA. 1995;273:859–864. [PubMed] [Google Scholar]
- 18.Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis. 2021;72:e65–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbarao S, Campbell H, Ribeiro S, et al. Invasive meningococcal disease, 2011-2020, and impact of the COVID-19 pandemic, England. Emerg Infect Dis. 2021;27:2495–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JC, Flores AR, Kaplan SL, et al. The indirect impact of the SARS-CoV-2 pandemic on invasive group a Streptococcus, Streptococcus pneumoniae and Staphylococcus aureus infections in Houston area children. Pediatr Infect Dis J. 2021;40:e313–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akil L, Ahmad HA, Reddy RS. Effects of climate change on Salmonella infections. Foodborne Pathog Dis. 2014;11:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scallan E. Activities, achievements, and lessons learned during the first 10 years of the Foodborne Diseases Active Surveillance Network: 1996-2005. Clin Infect Dis. 2007;44:718–725. [DOI] [PubMed] [Google Scholar]
- 23.Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–614. [PubMed] [Google Scholar]
- 24.Choe YJ, Choe SA, Cho SI. Importation of travel-related infectious diseases is increasing in South Korea: an analysis of salmonellosis, shigellosis, malaria, and dengue surveillance data. Travel Med Infect Dis. 2017;19:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloomfield SF, Aiello AE, Cookson B, et al. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am J Infect Control. 2007;35:S27–S64. [Google Scholar]
- 26.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–282. [DOI] [PubMed] [Google Scholar]
- 27.Hanada S, Pirzadeh M, Carver KY, et al. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hament JM, Kimpen JL, Fleer A, et al. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26:189–195. [DOI] [PubMed] [Google Scholar]
- 29.Yum S, Hong K, Sohn S, et al. Trends in viral respiratory infections during COVID-19 pandemic, South Korea. Emerg Infect Dis. 2021;27:1685–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan WY, Thoon KC, Loo LH, et al. Trends in respiratory virus infections during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. 2021;4:e2115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll BJ, Hansen NI, Sánchez PJ, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. [DOI] [PubMed] [Google Scholar]
- 33.Ryu S, Hwang Y, Ali ST, et al. Decreased use of broad-spectrum antibiotics during COVID-19 epidemic in South Korea. J Infect Dis. 2021;224:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.