Abstract
Purpose
The aim of the study was to evaluate equivalence of growth rate and pathologic confirmation in small choroidal melanoma (SCM).
Design
This study is a case series.
Subjects, Participants, and Controls
A total of 61 patients with a choroidal melanocytic tumor of size 5.0–16.0 mm in the largest basal diameter and 1.0–2.5 mm in thickness were classified into the pathology-confirmed group (n = 19), growth-confirmed group (n = 30), and with combined observations (n = 12).
Methods
Distribution of clinical variables (age, gender, laterality, tumor dimensions, tumor location, and presence of orange pigment, subretinal fluid, drusen, and retinal pigment epithelial [RPE] atrophy) between the groups was analyzed. Patient and disease characteristics were summarized as the median and interquartile range for continuous variables and the frequency and percentage for categorical variables. Comparisons were made using the Wilcoxon rank sum test for continuous variables and either Fisher's exact test or the χ2 test for categorical variables with a p value threshold of 0.05 for statistical significance. Growth rate (change in basal dimension/12 months) diagnostic of SCM was quantified.
Main Outcome Measures
The primary aim of this study was to test the hypothesis that “growth” was diagnostic of SCM with the secondary aim of quantifying the malignant “growth rate” (growth rate of SCM).
Results
The clinical characteristics among all 3 groups were similar except more patients with symptoms (68 vs. 20 vs. 42%, p = 0.004) and juxtapapillary location (p = 0.03) were in the pathology group than in the growth-confirmed group. Those in the combined and growth-confirmed groups had more patients with drusen (11 vs. 60 vs. 50%, p = 0.003) and RPE atrophy (11 vs. 23 vs. 67%, p = 0.003), respectively, than in the pathology group. The median time to detect growth was 9 months (range 3–26 months). The mean growth rate in basal dimension was 1.8 mm/12 months (range, 0.0–7.4 mm; [95% CI: 1.32–2.28]).
Conclusions and Relevance
Choroidal melanocytic lesions exhibiting a defined growth rate can be clinically diagnosed as SCM without a need for biopsy.
Keywords: Small choroidal melanoma, Growth rate, Histopathology, Diagnosis
Introduction
Pathologic confirmation is the gold standard for diagnosis of malignancy prior to definitive therapy by surgery, radiation, or chemotherapy. Ophthalmic oncology seems to be the only exception to this standard particularly when it relates to management of uveal melanoma [1]. Even though hesitation for performing biopsy seems to be partially overcome with increasing acceptance of prognostic biopsy, diagnostic biopsy is not routinely performed [2]. Given inherent technical and functional challenges in biopsying small choroidal tumors that are predominantly located near vision critical structures, the current practice pattern of treating small choroidal melanoma (SCM) in the USA is of treatment without a diagnostic biopsy. Differentiation between choroidal nevus and choroidal melanoma can be challenging because of lack of clear boundaries for the size-based classification system [3]. The majority of tumors (69%, Kaplan-Meier estimate at 5 years) labeled as “SCM” within the COMS Small Tumor Study included tumors with clinical behavior that was compatible with the diagnosis of choroidal nevus [4, 5]. Therefore, growth over time is commonly used to differentiate SCM from choroidal nevus [6, 7, 8, 9].
Clinical “risk factors” predictive of growth provide indirect diagnostic evidence for SCM. Clinical “risk factors” such as size, symptoms, presence or absence of orange pigment, and drusen were initially studied almost 40 years ago by Gass [10]. Over the years, others have largely corroborated the concept of “risk factors” [3, 10, 11, 12, 13, 14, 15]. The estimated maximal predicted risk of growth is not >63% [13, 16] implying that presence or absence of “risk factors” is therefore only an approximate guide to the diagnosis of SCM. The underlying premise is that “rapid growth” is diagnostic of SCM.
However, there are scant data regarding the amount of growth (absolute or relative change in basal dimension) and/or height and the time period over which growth must be observed [6, 8]. Within the COMS Small Tumor Study, tumor growth was defined as an increase of 0.1 mm in the largest basal dimension or height to either medium- or large-size tumors [4]. Considering that choroidal nevi can also grow in absence of malignant transformation [17], definition of “growth” that differentiates between benign growth (of choroidal nevi) and malignant growth (of choroidal melanoma) remains to be quantified. Moreover, the validity of the conceptual equivalence of “growth” and pathologic confirmation of the diagnosis, although generally assumed to be true, has not been tested for SCM. In fact, we could find only limited number of case series where melanocytic choroidal tumors had been evaluated pathologically after documented growth [6, 18, 19, 20, 21].
Use of conservative methods for treatment such as thermotherapy and radiation precludes pathologic confirmation of diagnosis in most cases. Yet, in cases where visual potential is limited due to the location of the tumor or patient's preference for pathologic confirmation prior to conservative therapy or enucleation, availability of tissue offers unique opportunity for growth and pathologic correlation. We performed 3-way comparison between sets of patients in whom “growth” could be quantified prior to therapy (growth-confirmed group), those with pathologic confirmation (pathology-confirmed group), and a combined group (growth and pathology confirmed). The primary aim of this study was to test the hypothesis that “growth” was clinically diagnostic of SCM with the secondary aim of quantifying the “malignant” growth rate.
Methods
Institutional review board approval was obtained. The study adhered to the Declaration of Helsinki. This retrospective study included 61 patients with a clinical diagnosis of SCM (Collaborative Ocular Melanoma Study, COMS criteria) [4] defined as a pigmented choroidal melanocytic lesion of size 5.0–16.0 mm in the largest basal diameter and 1.0–2.5 mm in thickness, who were treated with primary enucleation, plaque brachytherapy, or transpupillary thermotherapy from January 2010 to January 2019. For analysis of the institutional deidentified dataset, institutional review board approval was obtained.
Data Collection
All patients were evaluated using a standard slit lamp and fundus examination to make a clinical diagnosis of SCM. Detailed fundus drawing depicting the entire extent of the lesion along with color fundus photography was performed for all the patients. The clinical records were reviewed for the following variables at the initial examination: patient age and sex, laterality, visual symptoms, presenting best-corrected visual acuity as measured by logMar chart, quadratic distribution (superotemporal, superonasal, inferotemporal, inferonasal, juxtapapillary, or macular), posterior tumor margin in relation to the optic disc and foveola (<3 or ≥3 mm), and tumor dimensions. The largest tumor base diameter (BD) was estimated in millimeters by ophthalmoscopy, and the greatest tumor height in millimeters was measured by ultrasonography. Specific tumor features, such as the presence of subretinal fluid (SRF), surface orange pigment, drusen, and retinal pigment epithelial (RPE) atrophy, were also assessed by 90D ophthalmoscopic examination and supplemented by ancillary studies such as optical coherence tomography and autofluorescence. The record of each patient was reviewed to establish if there was documented evidence of growth at any time before treatment. Growth was judged by an increase in BD of at least 0.5 mm by meticulous comparison of serial fundus photographs or by an increase in thickness of 0.3 mm by serial ultrasonograms. The interval time between the last stable examination and the documentation of tumor growth was recorded.
Review of Literature
Studies documenting growth rate (mm/12 months) of SCM/nevus were identified, and relevant growth rates were tabulated as a mean change in BD (mm)/12 months for each study.
Study Groups
In this retrospective study, all included patients had been treated based upon clinical criteria. According to the available data, each patient with SCM was assigned to one of the 3 study groups. The first group included patients with pathology-confirmed diagnosis (histopathology, cytology, GEP 1B, or class 2). Diagnostic cytology showing spindle or mixed-type melanoma cells [1] was included in the pathology group as previous reported studies have shown 100% correlation between cytologic and histopathologic diagnosis of choroidal melanoma in enucleated eyes [1, 22]. Also known from previous reported studies is a prognostic 15-gene expression profile (15-GEP) test which predicts 5-year metastatic risk with class 1A, 1B, and 2 results indicating low-, intermediate-, and high-risk groups, respectively [23, 24, 25]. The second group included patients with documented growth prior to treatment (growth-confirmed group), and the third group comprised patients wherein both pathologic confirmation and documented growth were available (combined group).
Statistical Analysis
Patient and disease characteristics were summarized as the median and interquartile range for continuous variables and the frequency and percentage for categorical variables. Comparisons of patient and disease characteristics between groups were made using the Wilcoxon rank sum test for continuous variables and either Fisher's exact test or the χ2 test, as appropriate based on expected cell counts, for categorical variables. For the comparison across all 3 groups, we used a p value threshold of 0.05 for statistical significance. For pairwise comparisons, a Bonferroni correction was applied with a p value threshold of 0.017 for statistical significance to account for multiple testing. All statistical analyses were conducted using R software version 4.0 (R Core Development Team, Vienna, Austria).
Results
Overall Patient Characteristics
We identified 61 patients of SCM that could be classified into the pathology-confirmed group (n = 19), growth-confirmed group (n = 30), and with combined observations (n = 12) (Table 1). The median age of presentation was 61 years (range, 27–81 years) including 36 (59%) males and 25 (41%) females. Visual symptoms were present in 24 (39%) patients. The presenting best-corrected visual acuity was <20/20 in 56% of patients. Median tumor thickness was 2.1 mm (mean 2.0 mm, range 0.5–2.5 mm), and the largest BD was 9.0 mm (mean 9.0 mm, range 3.0–15.0 mm). The tumor was located <3 mm from the optic disc and foveola in 33 (54%) and 30 (49%) cases, respectively. Various risk factors for growth such as presence of SRF in 43 (70%) cases, orange pigment in 47 (77%) cases, drusen in 26 (43%) cases, and RPE atrophy in 17 (28%) cases were noted. The median follow-up period was 41 months (range, 1.1–104.2 months).
Table 1.
Comparison of characteristics across growth-confirmed, pathology-confirmed, and both combined groups of melanoma patients
| Characteristic | Growth (N = 30) | Pathology (N = 19) | Combined (N = 12) | p value |
|---|---|---|---|---|
| Age at presentation, years | 59 (56, 68) | 64 (54, 70) | 60 (45, 66) | 0.5 |
| Male sex, n (%) | 16 (53) | 12 (63) | 8 (67) | 0.7 |
| Laterality, n (%) | ||||
| Left eye | 13 (43) | 11 (58) | 7 (58) | 0.5 |
| Right eye | 17 (57) | 8 (42) | 5 (42) | |
| Symptoms, n (%) | 6 (20) | 13 (68) | 5 (42) | 0.004 |
| Distance from the optic disc, n (%) | ||||
| <3 mm | 13 (43) | 15 (79) | 5 (42) | 0.032 |
| ≥3 mm | 17 (57) | 4 (21) | 7 (58) | |
| Distance from fovea, n (%) | ||||
| <3 mm | 14 (47) | 11 (58) | 5 (42) | 0.6 |
| ≥3 mm | 16 (53) | 8 (42) | 7 (58) | |
| LBD, mm | 8.00 (7.50, 9.00) | 9.00 (7.75, 11.50) | 9.50 (8.62, 10.62) | 0.068 |
| Height, mm | 2.00 (1.50, 2.20) | 2.30 (2.00, 2.50) | 2.05 (2.00, 2.32) | 0.12 |
| SRF, n (%) | 18 (60) | 17 (89) | 8 (67) | 0.081 |
| Orange pigment, n (%) | 20 (67) | 17 (89) | 10 (83) | 0.2 |
| Drusen, n (%) | 18 (60) | 2 (11) | 6 (50) | 0.003 |
| RPE atrophy, n (%) | 7 (23) | 2 (11) | 8 (67) | 0.003 |
RPE, retinal pigment epithelial; SRF, subretinal fluid; LBD, largest basal diameter. Significant p values are in bold.
Pathology-Confirmed Group (n = 19)
In this group, 8 patients had histopathological confirmation following primary enucleation (patient preference), 7 patients had cytologic confirmation, and 4 patients GEP 2/IB. In the enucleation group, all the 8 patients had the tumor location within 3 mm from the optic disc and foveola, and histopathology showed mixed cell melanoma. In the cytology group (n = 7), patients were subjected to diagnostic FNAB [1] prior to plaque brachytherapy as a part of the prospective adjuvant systemic chemotherapy trial [26, 27]. Four patients were included in the pathology-confirmed group on the basis of prognostic biopsy (GEP class 2 in 3 patients and class 1B in 1 patient), which confers metastatic risk indicative of the tumor being a melanoma rather than a nevus.
Growth-Confirmed Group (n = 30)
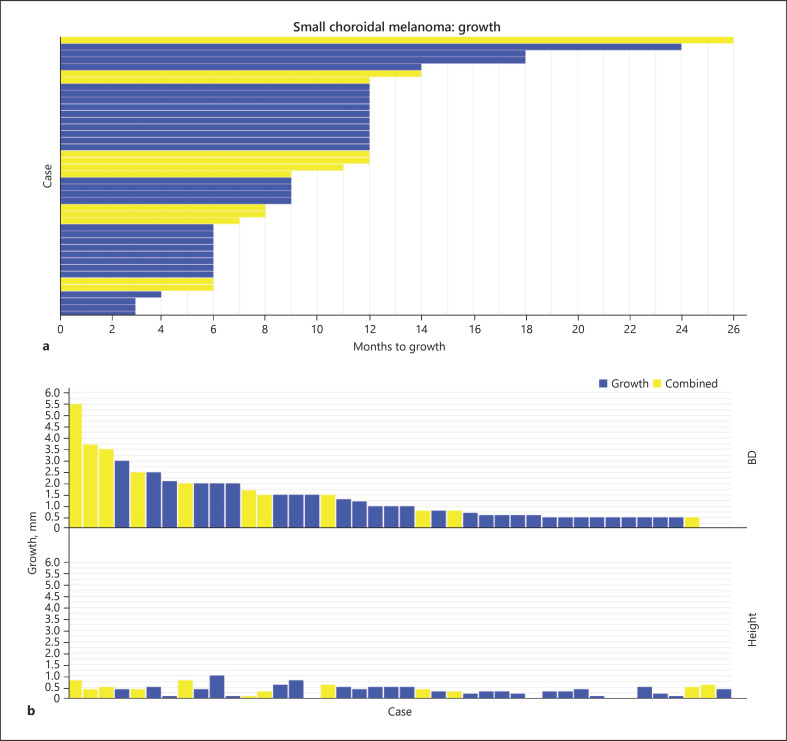
The growth group had 30 patients with a small pigmented choroidal melanocytic lesion which over a certain period had documented growth with an increase in either BD or thickness. The median time to growth was 9 months (mean 9.7 months, range 3–24 months) (Fig. 1a). The median change in BD and height was 0.75 (range, 0–3 mm) and 0.3 mm (range, 0–1 mm), respectively (Fig. 1b). Out of 30 patients, 25 patients received primary plaque brachytherapy, and 5 patients received transpupillary thermotherapy. Prognostic fine-needle aspiration biopsy showed GEP class 1A in 14 (47%) patients.
Fig. 1.
Small choroidal melanoma. a Swimmer plot showing time to growth (months) for all cases with documented growth. b Waterfall plot showing absolute change in BD and height for all cases with documented growth. BD, basal diameter; SCM, small choroidal melanoma.
Combined Group (n = 12)
These 12 patients with confirmed growth also had pathologic confirmation either by diagnostic FNAB (4 patients, mixed type [3 patients] and spindle type [1 patient]) or adverse-grade prognostic biopsy (8 patients, GEP class 2 [3 patients] and class 1B [5 patients]). The FNAB had been performed after growth had been documented and prior to ocular treatment. In other words, ocular treatment was agnostic to biopsy results. The median time to growth for all 12 patients was 10 months (mean 11.0 months, range 6–26 months), and the median change in BD and height was 1.6 (range, 0–5.5 mm) and 0.4 mm (range, 0.1–0.8 mm), respectively (Fig. 1). In 6 patients, the tumor location was within 3 mm from the optic disc and foveola.
Correlation between Groups
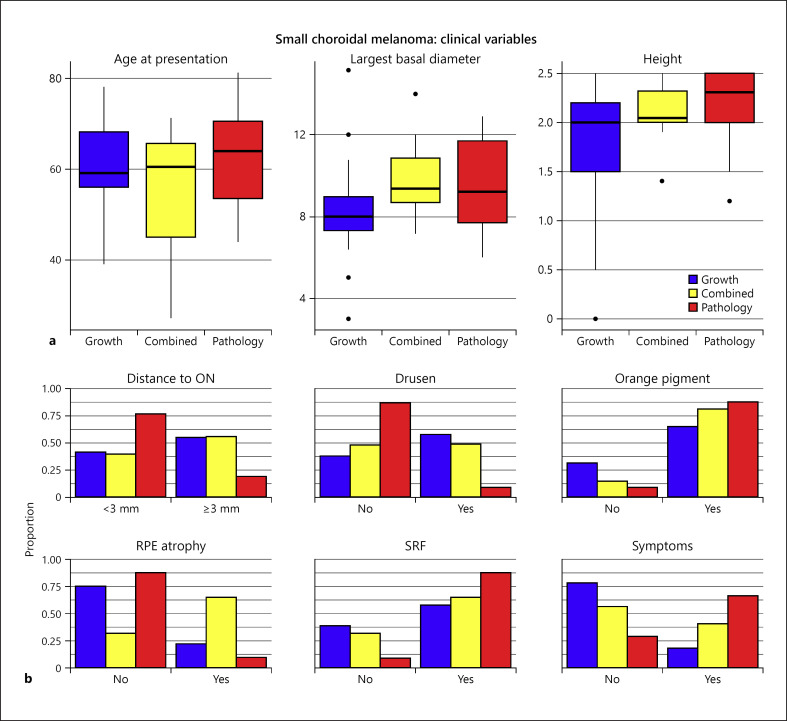
The comparison of disease characteristics (12 variables) between growth-confirmed, pathology-confirmed, and combined groups showed similar distribution of age, BD, and tumor height (Fig. 2a). Additionally, other clinical variables such as gender, laterality, and presence of surface orange pigment and SRF also showed similar distribution among the 3 groups. The only difference noted was in the pathology group, wherein patients were more frequently presented with symptoms (68% pathology vs. 20% growth vs. 42% combined, p = 0.004) and had juxtapapillary location (<3 mm from the optic disc, p = 0.03). Also, the clinical features suggestive of chronicity such as drusen (11 vs. 60 vs. 50%, p = 0.003) and RPE atrophy were more frequently observed in the growth-confirmed and combined groups (11% pathology vs. 23% growth vs. 67% combined, p = 0.003) (Table 1). Pairwise comparison of clinical characteristics between the growth-confirmed group and the pathology-confirmed group showed that the pathology-confirmed group more frequently presented with symptoms while the growth-confirmed group more frequently had drusen. Similarly, comparison between pathology-confirmed and combined groups as well as between growth-confirmed and combined groups showed that RPE atrophy was more frequently associated with the combined group (Fig. 2b).
Fig. 2.
Small choroidal melanoma. a Box plots of age at presentation (years), LBD (mm), and tumor height (mm) in growth-confirmed, pathology-confirmed, and combined groups. b Distribution of clinical variables in growth-confirmed, pathology-confirmed, and combined groups. RPE, retinal pigment epithelial; SRF, subretinal fluid; SCM, small choroidal melanoma; LBD, largest basal diameter.
Growth and Growth Rate
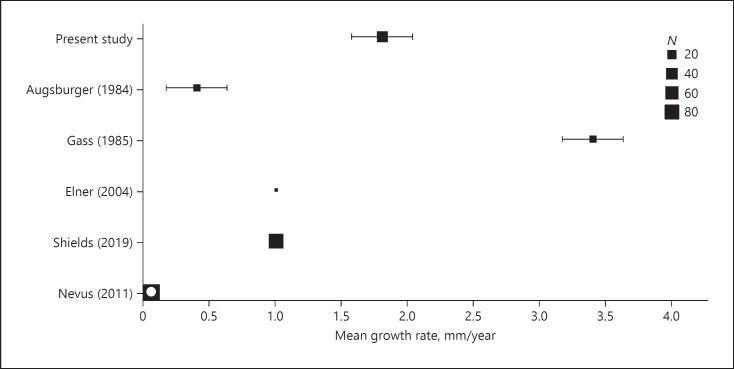
In our study, we observed growth as change in BD (with or without change in height) in 40 cases and height only in 2 cases. The mean growth rate (BD) was 1.8 mm/12 months (range, 0–7.4 mm; [95% CI: 1.32–2.28]). Literature review revealed only a few studies [6, 14, 19, 28] wherein the growth rate of SCM has been reported or could be calculated (with 95% CI) by the available data (Fig. 3). The mean growth rate of enlargement in BD for each study ranged from 0.4 mm/12 months [28] to 3.4 mm/12 months [19]. For comparison, the growth rate of choroidal nevi (0.06 mm/12 months) could be retrieved only from 1 study [29].
Fig. 3.
Growth rate of SCM. Published studies wherein the growth rate has been reported or could be calculated (with 95% CI) with the available data. For comparison, the growth rate of choroidal nevi (0.06 mm/12 months) is plotted (hollow box). SCM, small choroidal melanoma.
Discussion
There is a significant disconnect in the literature wherein pathology-confirmed cases [18, 20, 21, 28, 30, 31, 32] or tumors with metastatic potential [33, 34, 35] do not have associated reported growth rates (with a few exceptions [Table 2]) [6, 19], and the studies with growth rates do not include tumors with pathologic confirmation (without exception) [3, 4, 11, 14]. Therefore, we could extract data regarding growth rates only in 2 pathologic studies [6, 19]. In a series of 2 cases reported by Elner [6], “growth” was observed prior to enucleation in both cases, one case was confirmed as melanoma and the other was interpreted as nevus. Subsequently, others doubted the pathologic interpretation as a nevus and argued that the second case was also a melanoma [7]. An additional case of nevus with “growth” on a careful review appears to represent a case of a choroidal nevus that after documented stability of almost 6 years had transformed into melanoma [36].
Table 2.
Growth rate of histopathology-confirmed SCM: published reports
| Authors | Small melanoma/total cases | Pathology |
Growth rate,* mm/12 months | ||
|---|---|---|---|---|---|
| histopathology | cytology | bad prognosis | |||
| Char and Hogan [21] | 11/11 | 9 | − | − | Data unavailable |
| Mims and Shields [20] | 7/7 | 7 | − | − | Data unavailable |
| Gass [18] | 28/35 | 28 | − | − | Data unavailable |
| Augsburger et al. [19] | 9/17 | 9 | − | − | 3.4 |
| Shields et al. [32] | Unknown/54 | 38 | 54 | − | Data unavailable |
| Augsburger et al. [31] | 34/34 | 4 | 23# | − | Data unavailable |
| Singh et al. [44] | 6/10 | 6 | − | − | Data unavailable |
| Elner et al. [6] | 1/2 | 1 | − | 1.0 | |
| Singh et al. [26] | 15/106 | − | 5 | 2^ | Data unavailable |
| Shields et al. [33] | 55/55 | − | − | 25$ | Data unavailable |
| Harbour et al. [34] | 207/207 | − | − | 84 | Data unavailable |
| Binkley et al. [35] Present study | 25/215 | − | − | 8 | Data unavailable |
SCM, small choroidal melanoma.
Change in basal diameter.
Includes 4 cases with histopathology.
Ascertained by fluorescent in situ hybridization.
Ascertained by SNP array.
Such compartmentalization of data is not surprising, given the current practice patterns of treating SCM without diagnostic biopsy with conservative methods that preclude pathologic confirmation of diagnosis in most cases. Careful search of patients from various databases of the Department of Ophthalmic Oncology, Cole Eye Institute, led to the identification of unique sets of patients with SCM wherein growth rates could be calculated (change in BD/12 months) in pathology-confirmed cases (combined group). Two additional sets of patients were identified in whom growth rate could be quantified prior to therapy (growth-confirmed group) and those with pathologic confirmation (pathology-confirmed group) allowing 3-way comparison of clinical variables between the sets.
The primary aim of this study was to test the hypothesis that “growth” is clinically diagnostic of SCM. We could prove the hypothesis to be true by demonstrating that all cases with confirmed growth (n = 12, 100%) were indeed pathologically confirmed to be choroidal melanoma in the combined group. The pathologic confirmation of the clinical diagnosis was done by one of the 3 criteria: standard histopathology (postenucleation [n = 8, 26%]), cytopathology (n = 11, 35%), and GEP class (n = 12, 39%). As published previously by us and others, an experienced cytopathologist can achieve a high accuracy of diagnosis with a concordance of 96–100% between cytologic and histopathologic diagnosis [22, 26, 32], thereby highlighting the role of cytology in diagnosis of SCM. This is not to say that small melanoma are likely to yield negative diagnostic FNAB due to insufficient biopsy material (9–35%) [26, 31]. We only included those SCM that were positive for melanoma having undergone diagnostic FNAB as part of the adjuvant therapy trial [26]. We did not observe any case in any our databases that had been clinically diagnosed as SCM and was cytologically interpreted to be choroidal nevus because we do not perform diagnostic FNAB to differentiate nevus from melanoma. Augsburger et al. [31] also reported very low percentage (2%) of cytologically benign choroidal nevus among patients who underwent diagnostic FNAB for suspected melanoma. Also, reports on histopathology of choroidal nevi are very sparse [6, 36, 37] considering biopsy of such lesions is not recommended. The pathology-confirmed group also included 4 patients with GEP class 1B and 2, as tumors with such profile have metastatic potential [38, 39], an important but not exclusive hall mark of malignancy [34]. In the present analysis, we did not include cases with class 1A GEP as choroidal nevi may share the same GEP profile [34, 40].
The ancillary proof supporting the hypothesis that “growth” is clinically diagnostic of SCM comes from 3-way comparison of clinical variables between groups: growth-confirmed, pathology-confirmed, and combined (growth and pathology confirmed) groups. Of the 12 clinical variables that could be assessed, the majority (8, 67%) of them were similar (absence of statistical significance difference). Age, tumor dimensions (BD and height), presence of SRF, and surface orange pigment which have been identified as important predictors of the lesions growing in the future and hence being melanoma [3, 10, 11, 12, 13, 14, 15, 34] were similar among all 3 groups. Only statistical difference between the growth-confirmed and pathology-confirmed group was of symptoms (68 vs. 20%) and tumor location (<3 mm from ON) (79 vs. 43%), respectively, which were primary indications for such eyes to be enucleated (due to guarded visual prognosis with radiation therapy). The growth group and the combined group had more patients with drusen (60%) and RPE atrophy (67%) perhaps due to pre-existing nevus that underwent malignant transformation. Our comparative analysis provides indirect validation that the observed variables (high-risk factors) are indeed relevant in prediction of future growth of SCM [3, 10, 11, 12, 13, 14, 15] as they were consistently present in growing tumors. Similar distribution of observed clinical variables between pathology-confirmed and growth-confirmed groups further supports our hypothesis that “growth” is a diagnostic surrogate of SCM.
Quantification of malignant growth is a prerequisite to validate conceptual equivalence of growth and pathologic confirmation. Given the lack of quantification of malignant growth [6, 8], we explored it as the secondary aim of the present study. Growth of a small melanocytic choroidal lesion (nevus and melanoma) can be determined by clinic-based growth parameters such as absolute change in BD and height or percentage change measured over a specified period. Growth rate calculated as tumor doubling times has also been reported [19, 28, 41]. We used absolute change in BD for the purpose of this study because tumor growth was observed predominantly in BD (with or without height) in 40 cases (Fig. 1). The tumors may demonstrate asymmetric growth, and hence percentage change may not be an accurate representation of the change. Tumor doubling time estimates may also be misleading as they assume uniform shape (ellipsoidal) for all tumors [42]. We observed growth only in height without change in BD in the minority of cases (2, 5%) consistent with published reports [14, 28]. Considering the margin of error of ±0.2 mm while measuring tumor height by ultrasonography [43], absolute change in BD only was considered while comparing our data with the limited number of reported studies [6, 14, 19, 28]. Optical coherence tomography-based height measurements may be incorporated in future studies.
In our study, the mean growth rate of change in BD of 1.8 mm/12 months (range, 0–7.4 mm; [95% CI: 1.32–2.28]) is within the range of previously reported studies (0.4–3.4 mm/12 months). In the study by Gass (mean, 3.4 mm) [28] and Augsburger (mean, 0.4 mm) [19], the diameter measurements were estimated by indirect ophthalmoscopy which might explain some of the differences in growth rates. However, our results of mean growth rate of 1.8 mm/12 months are comparable to a recent contemporaneous report by Shields et al. [14] (1.0 mm/12 months) with comparable methodology of documenting tumor growth by serial fundus photography.
An important aspect to be considered while reporting growth rate is the denominator (time). In our study, the median time to observe significant growth in all the cases of melanoma was 9 months (range, 3–26 months) which is comparable to that reported in the literature [3, 4, 14, 19]. The denominator used to calculate the growth rate represents time between clinic visits that are not necessarily scheduled or planned unless the patients are part of a prospective observational study [4, 18]. Such a discrepancy in recorded time is not likely to influence the estimates of rates of benign growth rate (choroidal nevus growth). However, the malignant growth rates (choroidal melanoma) are likely to be underestimated unless the patient was observed at defined periodic intervals [6].
As emphasized in the study by Harbour et al. [34], the growth rate, that is, growth observed over a defined time, rather than growth (absolute or percentage) seems to be an important diagnostic parameter when differentiating nevus from SCM with melanoma exhibiting 10–20 faster growth rate than that of nevus. With a growth rate of 1.8 mm/12 months (95% CI: 1.32–2.28), one can observe growth in suspected choroidal melanoma over an interval of 3–6 months (0.3 mm–0.6 mm) by careful ophthalmoscopy and confirmed by serial photography. With current clinical available methods, growth of nevus will not be observable at such a short interval (0.015–0.03 mm) thereby differentiating nevus from a melanoma over a short period of careful observation. In our own clinical experience, we have not observed detectable growth in basal dimensions of a choroidal nevus over a period of 12 months or less (unpublished data).
Limitations
Our interpretation is limited based upon a small number of patients who were selected specifically for this comparative study. Due to selection bias, the growth rate estimates may not be representative of the entire population of SCM. Therefore, our results can only be considered as preliminary that need to be validated by larger datasets or a prospective study using standardized documentation and follow-up clinical protocols. In conclusion, choroidal melanocytic lesions exhibiting a defined growth rate can be clinically diagnosed as SCM without a need for biopsy.
Statement of Ethics
The study protocol (17-397) was approved by the Cleveland Clinic Institutional Review Board. Patient consent was not required as the study was based on review of medical records. The research complied with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
Arun D. Singh, Editor-in-Chief of Ocular Oncology and Pathology, reported financial activities outside the submitted work: Aura Biosciences (stock options), IsoAid LLC (consultancy), Immunocore (consultancy), Isoaid (consultancy), and Eckert and Zeigler (consultancy). Other authors have no conflicts of interest to declare.
Funding Sources
This work was supported in part by an unrestricted grant from Research to Prevent Blindness to the Cole Eye Institute. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
All authors contributed to writing and editing of the manuscript.
References
- 1.Medina CA, Biscotti CV, Singh N, Singh AD. Diagnostic cytologic features of uveal melanoma. Ophthalmology. 2015 Aug;122((8)):1580–4. doi: 10.1016/j.ophtha.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Davanzo JM, Binkley EM, Bena JF, Singh AD. Risk-stratified systemic surveillance in uveal melanoma. Br J Ophthalmol. 2019 Dec;103((12)):1868–71. doi: 10.1136/bjophthalmol-2018-313569. [DOI] [PubMed] [Google Scholar]
- 3.Singh AD, Mokashi AA, Bena JF, Jacques R, Rundle PA, Rennie IG. Small choroidal melanocytic lesions: features predictive of growth. Ophthalmology. 2006 Jun;113((6)):1032–9. doi: 10.1016/j.ophtha.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 4.The Collaborative Ocular Melanoma Study Group Factors predictive of growth and treatment of small choroidal melanoma: COMS report No. 5. Arch Ophthalmol. 1997;115((12)):1537–44. doi: 10.1001/archopht.1997.01100160707007. [DOI] [PubMed] [Google Scholar]
- 5.Murray TG. Small choroidal melanoma. Arch Ophthalmol. 1997;115((12)):1577–8. doi: 10.1001/archopht.1997.01100160747013. [DOI] [PubMed] [Google Scholar]
- 6.Elner VM, Flint A, Vine AK. Histopathology of documented growth in small melanocytic choroidal tumors. Arch Ophthalmol. 2004 Dec;122((12)):1876–8. doi: 10.1001/archopht.122.12.1876. [DOI] [PubMed] [Google Scholar]
- 7.Kivela T. Observed growth in small melanocytic choroidal tumors. Arch Ophthalmol. 2006 Apr;124((4)):607–8. doi: 10.1001/archopht.124.4.607-b. [DOI] [PubMed] [Google Scholar]
- 8.Kivela T. Diagnosis of uveal melanoma. Dev Ophthalmol. 2012;49:1–15. doi: 10.1159/000330613. [DOI] [PubMed] [Google Scholar]
- 9.Materin M, Singh AD. Benign melanocytic tumors of the uvea. Heidelberg: Springer Nature; 2019. [Google Scholar]
- 10.Gass JD. Problems in the differential diagnosis of choroidal nevi and malignant melanoma. XXXIII Edward Jackson Memorial lecture. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977 Jan–Feb;83((1)):19–48. [PubMed] [Google Scholar]
- 11.Augsburger JJ, Schroeder RP, Territo C, Gamel JW, Shields JA. Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol. 1989 Nov;73((11)):911–7. doi: 10.1136/bjo.73.11.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995 Sep;102((9)):1351–61. [PubMed] [Google Scholar]
- 13.Shields CL, Cater J, Shields JA, Singh AD, Santos MC, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000 Mar;118((3)):360–4. doi: 10.1001/archopht.118.3.360. [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Dalvin LA, Yu MD, Ancona-Lezama D, Di Nicola M, Williams BK, et al. Choroidal nevus transformation into melanoma per millimeter increment in thickness using multimodal imaging in 2355 cases: the 2019 wendell L. Hughes lecture. Retina. 2019 Oct;39((10)):1852–60. doi: 10.1097/IAE.0000000000002508. [DOI] [PubMed] [Google Scholar]
- 15.Butler P, Char DH, Zarbin M, Kroll S. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology. 1994 Apr;101((4)):710–7. doi: 10.1016/s0161-6420(94)31274-7. [DOI] [PubMed] [Google Scholar]
- 16.Singh AD, Schachat AP, Diener-West M, Reynolds SM. Small choroidal melanoma. Ophthalmology. 2008 Dec;115((12)):2319–e3. doi: 10.1016/j.ophtha.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Abramson DH. Growing melanocytic tumor is not always cancer. Arch Ophthalmol. 2005 Oct;123((10)):1457–8. doi: 10.1001/archopht.123.10.1457-b. [DOI] [PubMed] [Google Scholar]
- 18.Gass JD. Observation of suspected choroidal and ciliary body melanomas for evidence of growth prior to enucleation. Retina. 1980 Jun;23((6)):523–8. [PubMed] [Google Scholar]
- 19.Augsburger JJ, Gonder JR, Amsel J, Shields JA, Donoso LA. Growth rates and doubling times of posterior uveal melanomas. Ophthalmology. 1984 Dec;91((12)):1709–15. doi: 10.1016/s0161-6420(84)34088-x. [DOI] [PubMed] [Google Scholar]
- 20.Mims JL, 3rd, Shields JA. Follow-up studies of suspicious choroidal nevi. Ophthalmology. 1978 Sep;85((9)):929–43. doi: 10.1016/s0161-6420(78)35597-4. [DOI] [PubMed] [Google Scholar]
- 21.Char DH, Hogan MJ. Management of small elevated pigmented choroidal lesions. Br J Ophthalmol. 1977 Jan;61((1)):54–8. doi: 10.1136/bjo.61.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Augsburger JJ, Shields JA, Folberg R, Lang W, O'Hara BJ, Claricci JD. Fine needle aspiration biopsy in the diagnosis of intraocular cancer. Cytologic-histologic correlations. Ophthalmology. 1985 Jan;92((1)):39–49. doi: 10.1016/s0161-6420(85)34068-x. [DOI] [PubMed] [Google Scholar]
- 23.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010 Jul;12((4)):461–8. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012 Aug;119((8)):1596–603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaberg TM, Covington KR, Tsai T, Shildkrot Y, Plasseraud KM, Alsina KM, et al. Gene expression profiling in uveal melanoma: five-year prospective outcomes and meta-analysis. Ocul Oncol Pathol. 2020 Oct;6((5)):360–7. doi: 10.1159/000508382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AD, Medina CA, Singh N, Aronow ME, Biscotti CV, Triozzi PL. Fine-needle aspiration biopsy of uveal melanoma: outcomes and complications. Br J Ophthalmol. 2016 Apr;100((4)):456–62. doi: 10.1136/bjophthalmol-2015-306921. [DOI] [PubMed] [Google Scholar]
- 27.Binkley E, Triozzi PL, Rybicki L, Achberger S, Aldrich W, Singh A. A prospective trial of adjuvant therapy for high-risk uveal melanoma: assessing 5-year survival outcomes. Br J Ophthalmol. 2020 Apr;104((4)):524–8. doi: 10.1136/bjophthalmol-2019-314461. [DOI] [PubMed] [Google Scholar]
- 28.Gass JD. Comparison of uveal melanoma growth rates with mitotic index and mortality. Arch Ophthalmol. 1985 Jul;103((7)):924–31. doi: 10.1001/archopht.1985.01050070050028. [DOI] [PubMed] [Google Scholar]
- 29.Mashayekhi A, Siu S, Shields CL, Shields JA. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology. 2011 Feb;118((2)):382–8. doi: 10.1016/j.ophtha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Singh AD, Bena JF, Mokashi AA, Jacques R, Rundle PA, Rennie IG. Growth of small tumors. Ophthalmology. 2006 Jun;113((6)):1061–4. doi: 10.1016/j.ophtha.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Augsburger JJ, Corrêa ZM, Schneider S, Yassin RS, Robinson-Smith T, Ehya H, et al. Diagnostic transvitreal fine-needle aspiration biopsy of small melanocytic choroidal tumors in nevus versus melanoma category. Trans Am Ophthalmol Soc. 2002;100:225–4. discussion 32–4. [PMC free article] [PubMed] [Google Scholar]
- 32.Shields JA, Shields CL, Ehya H, Eagle RC, Jr, De Potter P. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993 Nov;100((11)):1677–84. doi: 10.1016/s0161-6420(93)31418-1. [DOI] [PubMed] [Google Scholar]
- 33.Shields CL, Pefkianaki M, Mashayekhi A, Shields JA, Ganguly A. Cytogenetic results of choroidal nevus growth into melanoma in 55 consecutive cases. Saudi J Ophthalmol. 2018 Jan–Mar;32((1)):28–32. doi: 10.1016/j.sjopt.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbour JW, Paez-Escamilla M, Cai L, Walter SD, Augsburger JJ, Correa ZM. Are risk factors for growth of choroidal nevi associated with malignant transformation? Assessment with a validated genomic biomarker. Am J Ophthalmol. 2019 Jan;197:168–79. doi: 10.1016/j.ajo.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binkley EM, Bena JF, Davanzo JM, Hinz C, Boldt HC, Singh AD. Gene expression profiling prognostication of posterior uveal melanoma: does size matter? Ophthalmol Retina. 2020 Jun;4((6)):620–9. doi: 10.1016/j.oret.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 36.MacIlwaine WA, Anderson B, Jr, Klintworth GK. Enlargement of a histologically documented choroidal nevus. Am J Ophthalmol. 1979 Apr;87((4)):480–6. doi: 10.1016/0002-9394(79)90234-4. [DOI] [PubMed] [Google Scholar]
- 37.Madigan Mc., Vader M, Ton H, Van der Velden P, Jager MJ. Human choroidal nevi histopathology revisited. Acta Ophthalmol. 2015;93((S255)) [Google Scholar]
- 38.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004 Oct 15;64((20)):7205–9. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, et al. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin Cancer Res. 2016 Mar 1;22((5)):1234–42. doi: 10.1158/1078-0432.CCR-15-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006 May 1;66((9)):4602–9. doi: 10.1158/0008-5472.CAN-05-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kivela T, Eskelin S. Transformation of nevus to melanoma. Ophthalmology. 2006 May;113((5)):887–8.e1. doi: 10.1016/j.ophtha.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 42.Juster RP, Char DH. Uveal melanoma: doubling time and mitotic index. Arch Ophthalmol. 1986 Feb;104((2)):174–5. doi: 10.1001/archopht.1986.01050140026002. [DOI] [PubMed] [Google Scholar]
- 43.Roelofs KA, O'Day R, Al Harby L, Hay G, Arora AK, Cohen VML, et al. Detecting progression of melanocytic choroidal tumors by sequential imaging: is ultrasonography necessary? Cancers. 2020 Jul 10;12((7)) doi: 10.3390/cancers12071856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh AD, Eagle RC, Jr, Shields CL, Shields JA. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121((3)):397–400. doi: 10.1001/archopht.121.3.397. [DOI] [PubMed] [Google Scholar]