Abstract
Choriocarcinoma (CC) is a very rare and aggressive neoplasm. The characteristic feature of this disease is a rapid hematogenous spread, mainly to the lungs and brain, which largely defines clinical signs of the disease and complicates the diagnosis. Gastrointestinal metastases are rare, and of those, only few cases with gastric location have been reported. There are publications describing choriocarcinoma syndrome (CCS). As a rule, it presents in patients with an advanced disease and is characterized by hemorrhage from metastatic foci, leading to hemoptysis and gastrointestinal bleeding. CCS development is associated with poor prognosis and high mortality. This article describes a case of testicular CC with rare few gastric metastases, complicated by CCS.
Keywords: Gastric metastases, Gastrointestinal bleeding, Pulmonary metastases, Testicular choriocarcinoma, Choriocarcinoma syndrome
Introduction
Choriocarcinoma (CC) is a very rare and aggressive neoplasm that occurs mainly in women [1]. Among young men, testicular cancer is one of the most common neoplasms, but testicular CC is diagnosed in <2% [2]. Testicular CC is a malignant and fast-growing tumor originating from trophoblasts, secreting large amounts of beta-human chorionic gonadotropin (β-HCG). Usually, CC is a component of a mixed testicular germ-cell tumor. Isolated CC is extremely rare, while the primary tumor can be very small or even regressive, and clinical signs depend on the symptoms of metastatic lesions in other organs [1]. Specific feature of CC is rapid hematogenous spread, mainly to the lungs and brain. Thus, common presenting symptoms are progressive respiratory failure, hemoptysis, and cerebral symptoms. In CC, gastrointestinal metastasis is very rare (about 5% of cases), and gastric impairment occurs in 1%. The main clinical signs of gastric metastases are melena and/or bloody vomit followed by anemia [2].
Choriocarcinoma syndrome was first described in 1982 by Logothetis [3]. This syndrome is characterized by bleeding from metastatic foci due to the invasion of CC cells into small blood vessels. Usually, this bleeding occurs immediately after the onset of chemotherapy; however, several cases with bleeding preceding treatment were reported [4]. Risk of CCS increases significantly in patients with extrapulmonary visceral metastases and very high level of HCG who belong to a 3d group with poor prognosis according to the International Germ-Cell Cancer Collaborative Group (IGCCCG) risk classification [5].
Case Presentation
Patient B, 30 years old, was admitted to the intensive care unit of the University Clinical Hospital No. 4 (UCH No. 4) with complaints of weakness, malaise, fever up to 38.5°C, aching pain in the epigastric area not triggered by food intake, chest pain on the left during breathing, shortness of breath, repeated episodes hemoptysis, and dragging pain in the left testicle.
Malaise appeared about 2 months ago with worsening of weakness and shortness of breath and progressive pain in the epigastrium; the initial discomfort in the left testicle developed into a constant aching pain. During the specified period, he lost about 10 kg (body weight decreased by 11.5%, BMI was 24.88 kg/m2, now 22.08 kg/m2). He started to take proton pump inhibitors and topical NSAIDs on his own with a temporary positive effect. In the last 2 weeks, his general condition progressively worsened. Severe weakness and shortness of breath made any physical activity impossible. The patient was hospitalized with low blood pressure of unknown etiology and hemoptysis. Family history is not significant for cancer. Social status is unremarkable. Previous diseases include rare respiratory viral infections.
Physical examination revealed normosthenic constitution, pale skin, with moderate cyanosis of the lips, and acrocyanosis. Heart tones had normal rhythm. HR was 104 bpm and BP 95/60 mm Hg. On lung auscultation, disseminated dry rales and fine moist rales in the lower segments of both lungs were noted. RR reached 30 per min and SpO289%. The liver protruded by 1 cm below the lower costal margin. Tender upper abdomen on palpation was noted. Severe pain in the left testicle and scrotal edema were noted.
Complete blood count showed signs of hypochromic, normocytic anemia (RBC 2.82 × 1012/L, hemoglobin 76 g/L, MCH 26.9 pg, and MCV 82.6 fL), leukocytosis (WBC 17.2 109/L), and significantly increased ESR (55 mm/h). The patient underwent sternal puncture; bone marrow smear showed hypercellularity, granulocytic lineage 39.2%, normoblastic erythropoiesis, and neutrophil maturation index 0.7; and erythroblast maturation index 0.7. Blood biochemistry showed slight increase in liver enzymes: ALT 141 U/L and AST 87 U/L; hypoalbuminemia 27.6 g/L; reduced serum iron concentration − 5.5 μmol/L; and increased level of C-reactive protein − 102 mg/L. Follow-up control did not show specific findings. Serological markers of viral hepatitis (HbsAg and anti-HCV), HIV, and syphilis screening test were negative.
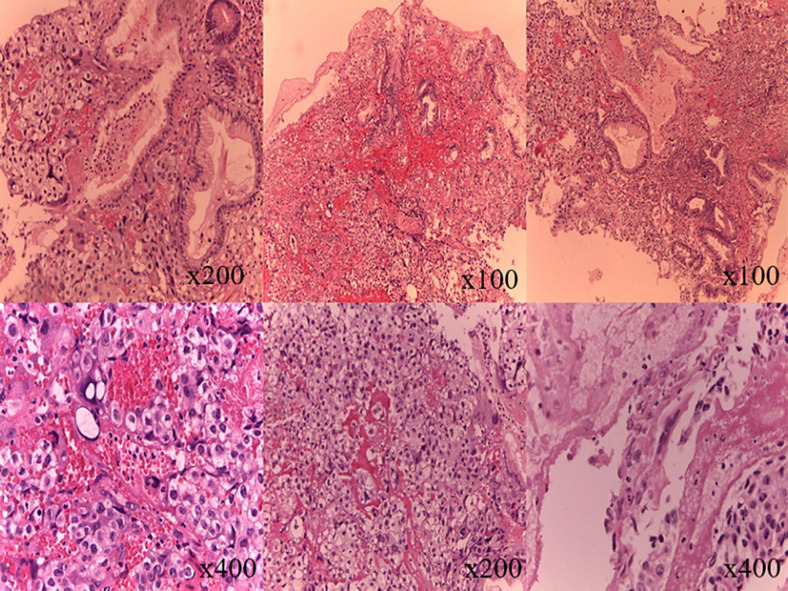
The abdominal ultrasound revealed focal changes in the liver and spleen, probably of metastatic origin, splenomegaly, and signs of retroperitoneal space-occupying lesion (most likely, a conglomerate of enlarged lymph nodes). Esophagogastroduodenoscopy revealed an 8 × 10 in diameter and 3- to 4-mm high tumor-like mass in the cardiac segment on the anterior wall. The lesion had irregular shape with an ulcerated uneven surface with significant hyperemia. A biopsy was performed. Three fragments of the gastric mucosa with fundus glands were examined (Fig. 1). Conclusion: taking into account clinical and diagnostic data, metastasis of testicular CC may be assumed.
Fig. 1.
Fragments of the gastric mucosa. The tumor consists of cytotrophoblasts (cells with optically transparent cytoplasm with 1 large nucleus and a well-differentiated nucleolus) and syncytiotrophoblasts (large polynucleated cells with large, moderately polymorphic nuclei). The morphological structure corresponds to choriocarcinoma.
Ultrasound investigation of the external genital organs showed a tumor in the left testicle: a 7 × 5 mm hyperdense lesion with unclear, uneven contours, as well as venous dilation of the aciniform plexus up to 4 mm (left varicocele). β-HCG level was 55,346 mIU/mL (ref. value: up to 2.5–5 mIU/mL). The patient was consulted by a urologist several times, with following conclusions: due to the severity of condition, orchiotomy is currently unreasonable. Chemotherapy was indicated.
CT scan showed round-shaped lesions in the lungs, probably of secondary metastatic origin, and interstitial changes in the lungs. Flexible bronchoscopy revealed chronic tracheobronchitis against the background of focal atrophy of the mucosa. Abdominal CT scan with contrast enhancement showed a para-aortic space-occupying lesion at the level of L1–L4 vertebrae on the left. The tumor had uneven, tuberous margins, with dimensions of 62 × 69 × 136 mm and a density of 19 N–46 N. Brain CT showed a 5 × 9 mm focal lesion in the right frontal lobe with a density of 51 N, probably of secondary origin. The patient was consulted by a neurosurgeon with following conclusion: taking into account the severity of patient's condition and multiple lesions, surgical treatment (resection of cerebral metastases) is not indicated.
The final clinical diagnosis was as follows: CC of the left testicle with metastases to the stomach, lungs, brain, liver, and spleen; choriocarcinoma syndrome; and moderate iron-deficiency anemia. The patient was admitted in a critical condition with a disseminated metastatic process and died in a few days after hospitalization from progressive multiple organ failure. Due to the short course of hospital stay, we could not provide necessary treatment in full scope.
Discussion
Gastric metastases are extremely rare, <2% of all oncologic patients according to autopsy data. Usually, the primary tumor is breast cancer (27.9%), lung cancer (23.8%), esophageal cancer (19.1%), renal cell carcinoma (7.6%), and malignant melanoma (7.0%) [6]. Thus, the gastric metastasis occurs as a result of predominantly hematogenic spread from a distant primary tumor (lung cancer, breast cancer, or melanoma), or due to mixed genesis: hematogenic, lymphogenic, or contact − as a result of the tumor invasion from the adjacent tissue (esophageal cancer, pancreatic cancer, and gallbladder carcinoma) [7].
According to the published data, a majority of patients with CC already have metastases at the time of diagnosis. Hematogenic spread is characterized by predominant involvement of the lungs, brain, and occasionally liver and retroperitoneal lymph nodes [8]. There were individual cases of metastatic lesions of the skin [9] and pancreas [10]. Metastasis of CC into the stomach is a very rare phenomenon. Only single cases are described in publications [2, 7], wherein the development of a secondary lesion in the stomach may be accompanied by uncontrolled gastric bleeding, requiring urgent resection [7].
According to the International Germ-Cell Cancer Collaborative Group (IGCCCG), risk assessment depends on the primary tumor site; the presence or absence of extrapulmonary visceral metastases; and level of β-HGG, alpha-fetoprotein, and lactate dehydrogenase [5]. Our patient with an HCG level of >50,000 mIU/L and the metastases in the brain, liver, spleen, and stomach belonged to the group with the worst prognosis and the lowest overall survival. In addition, it should be noted that patients with very high HCG level are less sensitive to chemotherapy, which makes their outcome even worse [4].
According to the published data, patients with advanced disease may develop CCS, which is a potentially fatal complication. This syndrome is observed not only in CC but also in other nonseminomatous germ-cell tumors [11]. Its characteristic feature is bleeding from metastatic foci due to high vascularization of the neoplasm and CC invasion into small vessels. The most common clinical manifestation is hemoptysis [4]; however, pulmonary hemorrhage [12] and bleeding from a metastatic lesion in the liver [13] were also reported. It should be noted that in most cases, the triggering factor for the CCS is the prescription of chemotherapy, leading to massive lysis of tumor cells and, as a result, release in cytokines that aggravate cell damage. In some single cases, this syndrome develops before treatment [4]. Analyzing clinical, laboratory, and instrumental data, we came to the conclusion about the CCS development in our patient, which manifested before chemotherapy.
Unfortunately, the patient was admitted to the University Hospital No. 4 too late. Multiple metastatic lesions and development of CCS led to the fatal outcome.
The described case is typical in many respects: the patient's age and rapid disseminated spread to the lungs and brain, liver, lymph nodes; however, the secondary lesion of the stomach and the development of CCS make this case unique. Multisystemic metastatic lesions, and, as a consequence, a variety of clinical signs significantly complicate the diagnosis of testicular CC. One should remember that testicular CC is a very aggressive and malignant germ-cell tumor; therefore, prompt active treatment is required to improve the prognosis [14]. A clinician should be aware of the multiple signs of this disease, the possibility of atypical metastasis, and the importance of timely diagnosis for successful management.
Statement of Ethics
The published manuscript complies with the guidelines for human research and includes evidence that the research was conducted in an ethical manner in accordance with the World Medical Association's Declaration of Helsinki. Written informed consent was obtained from the patient's relatives for the publication of this case report and any accompanying images. This study protocol was reviewed and approved by the Local Ethics Committee of Sechenov University; Approval No. 10–21 dated June 17, 2021.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by Sechenov University.
Author Contributions
All authors participated in the examination and treatment of this patient, and the results were obtained, discussed, and analyzed. A.E. Pokrovskaya, A.I. Tarzimanova, and M.V. Vetluzhskaya helped to compose the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article and/or its online supplementary material files (for all online suppl. material, see www.karger.com/doi/10.1159/000519814). Further enquiries can be directed to the corresponding author.
Acknowledgment
The authors express their gratitude to Sechenov University for its support in publishing the article.
References
- 1.Bishop BN, Edemekong PF. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Jan, Choriocarcinoma. [Updated 2020 Jul 14]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535434/ [PubMed] [Google Scholar]
- 2.Chaar A, Mouabbi JA, Alrajjal A, Barawi M. Metastatic testicular choriocarcinoma: an unusual cause of upper gastrointestinal bleed. Cureus. 2019;11((7)):e5243. doi: 10.7759/cureus.5243. Published 2019 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logothetis CJ. Bulletin no. 19. Adv Eng Softw. 1984;6((2)):118–20. [Google Scholar]
- 4.Rejlekova K, Cursano MC, De Giorgi U, Mego M. Severe complications in testicular germ cell tumors: the choriocarcinoma syndrome. Front Endocrinol. 2019;10:218. doi: 10.3389/fendo.2019.00218. Published 2019 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer J. Prognostic factors in metastatic germ-cell cancer. Andrology. 2019;7:475–8. doi: 10.1111/andr.12615. [DOI] [PubMed] [Google Scholar]
- 6.Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today. 2014;44:1392–9. doi: 10.1007/s00595-013-0671-9. [DOI] [PubMed] [Google Scholar]
- 7.Kanthan R, Sharanowski K, Senger JL, Fesser J, Chibbar R, Kanthan SC. Uncommon mucosal metastases to the stomach. World J Surg Oncol. 2009 Aug 3;7:62. doi: 10.1186/1477-7819-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemtsova MV, Andreeva YY. Testicular germ cell tumors: molecular genetic and clinicomorphological aspects. Onkourologiya. 2015;11((1)):12–9. [Google Scholar]
- 9.Shabani S, Pritchard N, Padhya TA, Mifsud M. Head and neck cutaneous metastasis of testicular choriocarcinoma. BMJ Case Rep. 2020;13:e233337. doi: 10.1136/bcr-2019-233337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockton L, Green E, Kaur B, De Winton E. Non-gestational choriocarcinoma with widespread metastases presenting with type 1 respiratory failure in a 39-year-old female: case report and review of the literature. Case Rep Oncol. 2018 Mar 14;11((1)):151–8. doi: 10.1159/000486639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar-Mejía CE, García-Gutiérrez ME, Contreras-Salcido MI, Rodríguez-Álvarez CJ, Wimer-Castillo BO, Lara-Campos JG, et al. Choriocarcinoma syndrome as an initial presentation of testicular cancer. Case Rep Oncol Med. 2018 Nov 8;2018:8065615. doi: 10.1155/2018/8065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeitjian VS, Arslan W, Borja-Alvarez A, Amar S. Choriocarcinoma syndrome: a potentially fatal complication of testicular cancer. Case Rep Oncol Med. 2019 Feb 24;2019:4092941. doi: 10.1155/2019/4092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina A, Ramos M, Amenedo M, París L. Choriocarcinoma syndrome. Arch Esp Urol. 2014 Oct;67((8)):711–4. English, Spanish. [PubMed] [Google Scholar]
- 14.Reilley MJ, Pagliaro LC. Testicular choriocarcinoma: a rare variant that requires a unique treatment approach. Curr Oncol Rep. 2015;17((2)):2. doi: 10.1007/s11912-014-0430-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and/or its online supplementary material files (for all online suppl. material, see www.karger.com/doi/10.1159/000519814). Further enquiries can be directed to the corresponding author.