Abstract
Two rapid human immunodeficiency virus (HIV) screening assays, HIV TRI-DOT and HIV-SPOT were compared with standard enzyme-linked immunosorbent assays according to a testing algorithm. Sensitivities and specificities in the real-time evaluation were 99.5 and 99.9% for TRI-DOT and 98.2 and 99.7% for HIV-SPOT, respectively. These two tests are suitable for use where facilities and laboratory expertise are limited.
Infection with human immunodeficiency virus (HIV), which causes AIDS, has become a worldwide epidemic since its first documentation in 1981, and it is a major public health concern for all countries (3, 6, 12, 16). Diagnosis of HIV infection is important for prevention and patient management (9, 17). Several different assays are presently available for the detection of specific antibodies to diagnose HIV type 1 (HIV-1), HIV-2 or combined infection (13). The disadvantages of the enzyme-linked immunosorbent assay (ELISA) are the need for well-trained technical manpower, appropriate equipment, and batch testing (18). In a developing country such as India, technical support is not available in most of the peripheral hospitals and blood banks. The number of samples screened per day is usually small, and facilities for ELISA are not cost-effective. There is also a need to establish voluntary counseling and testing (VCT) facilities as part of the HIV infection prevention strategy. In these situations, tests need to be simple and rapid (1, 2, 4, 7). We estimated the accuracy indices of two rapid HIV tests (HIV TRI-DOT and HIV-SPOT).
Blood samples were received in our laboratory from patients who were to undergo emergency high-risk procedures or from the delivery room of the Obstetrics Department at the Christian Medical College Hospital (a tertiary-care hospital) at Vellore in India. The HIV antibody testing was done with the sole purpose of ensuring better patient handling; the required medical or surgical treatment was never withheld from any patient. In our hospital, a general consent is obtained for all investigations, including blood tests. Hospital policy is to refer HIV-positive individuals to the infectious-disease clinic, where counseling services are offered.
A total of 11,702 routine hospital-based samples were received for rapid HIV antibody testing from September 1997 through November 1998. The HIV TRI-DOT kit (J. Mitra & Co. Ltd., New Delhi, India) was used for testing 9,312 samples, and the remaining 2,390 samples were tested by HIV-SPOT (Gene Labs Diagnostics, Singapore). The two kits used are immunodot assays and detect antibodies to both HIV-1 and HIV-2. Kit protocols were strictly followed in carrying out the tests, and the technicians who performed these tests were adequately trained. The results were available in 10 min.
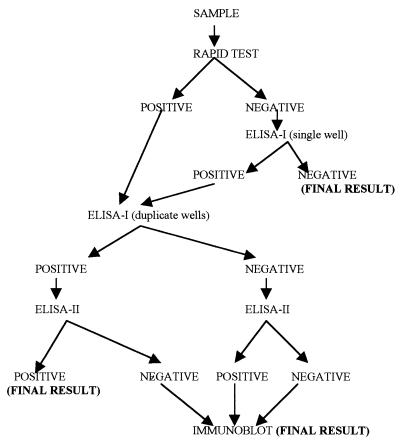
The algorithm used for specimen testing is shown in Fig. 1. One of three World Health Organization (WHO)/Joint United Nations Progamme on HIV/AIDS (UNAIDS-approved ELISA kits of equivalent performance, DETECT HIV (BioChem ImmunoSystems Inc., Montreal, Canada), INNOTEST (Innogenetics N.V., Zwijnaarde, Belgium), or UBI (United Biomedical, Inc., Hauppauge, N.Y.), was used as the first microwell ELISA (ELISA-1). All the rapid-test-negative samples were tested by ELISA-1 in a single well; singleton testing is recommended for serum screening by these kits. All rapid-test-reactive samples were tested in duplicate wells by ELISA-1. All rapid-test- or ELISA-1-positive samples were tested by a supplementary third-generation EIA (ELISA-2) (Abbott Laboratories, North Chicago, Ill.). When there was a discrepancy between results of a rapid test and ELISAs or between ELISA-1 and ELISA-2 results, a WHO-UNAIDS-approved immunoblot kit (HIV Blot 2.2 [Gene Labs Diagnostics] or Line Immuno Assay [Innogenetics N.V.]) was used, and this result was taken as the final result for categorizing the sample.
FIG. 1.
Testing algorithm followed during the study, adapted from WHO/UNAIDS recommendations.
No attempt was made to discriminate between HIV-1 and HIV-2 infections. Since this was a real-time evaluation, comparisons were performed with the ELISA kits used at the laboratory at the time the rapid assay was performed. During this period, three different ELISA-1 kits were used at different times. The “gold standard” (infected or uninfected status) was determined by concordant results between ELISA-1 and ELISA-2. In the case of discordance between the rapid tests and/or ELISAs, the immunoblot results were taken.
Of the 9,312 samples tested by TRI-DOT, 210 were reactive. Among the reactive samples, 194 were concordant with the results of the two ELISAs and 16 were discordant. Twenty TRI-DOT-negative samples showed discrepant results in the ELISA. Among the 2,390 samples tested by HIV-SPOT, 64 were reactive. Fifty-four reactive samples gave concordant results in the two ELISAs; the remaining 10 were discordant. Among the samples negative by HIV-SPOT, two were discordant with ELISAs. Details of samples that gave discordant results are shown in Table 1.
TABLE 1.
Results of discordant samples in rapid tests, ELISA-1, ELISA-2, and immunoblot analysis
| Rapid test and result (n) | No. of resultsa by:
|
||||||
|---|---|---|---|---|---|---|---|
| ELISA-1
|
ELISA-2b
|
Immunoblotc
|
|||||
| Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Indeterminate | |
| TRI-DOT | |||||||
| Reactive (16) | 0 | 16 | 1 | 15 | 0 | 13 | 1 |
| Negative (20) | 18 | 2d | 5 | 14 | 1 | 15 | 3 |
| HIV-SPOT | |||||||
| Reactive (10) | 0 | 10 | 1 | 9 | 0 | 7 | 3 |
| Negative (2) | 1 | 1d | 2 | 0 | 1 | 1 | 0 |
Pos., positive; Neg., negative.
One sample was not tested by ELISA-2.
Three samples were not tested by immunoblot.
These samples showed visible color in the assay although results were below the cutoff, so they were tested by ELISA-2.
The accuracy indices of TRI-DOT and HIV-SPOT results are given in Table 2. Seven samples with indeterminate results by the immunoblot test and discordant ELISA results were removed from the analysis. There were no ELISA-1- and -2-discordant but immunoblot-positive samples. One each of the TRI-DOT- and HIV-SPOT-negative samples was reactive by both ELISAs and was found to be positive for HIV-1 by immunoblotting. The sample that was negative by HIV-SPOT was positive by TRI-DOT, while the sample that was negative by TRI-DOT was negative by HIV-SPOT as well. The difference in reactivity between these two rapid tests may be due to the difference in the epitopes of the recombinant antigen used in the formulation of these tests.
TABLE 2.
Comparison of the rapid tests with final results
| Rapid test and result | No. of true positive results | No. of true negative results | Sensitivity (%)a | Specificity (%)a | PPV (%)ab | NPV (%)ac |
|---|---|---|---|---|---|---|
| TRI-DOT | 99.5 (96.7–100) | 99.9 (99.7–99.9) | 93.7 (89.3–96.5) | 100 (99.9–100) | ||
| Positive | 194d | 13e | ||||
| Negative | 1f | 9,097g | ||||
| HIV-SPOT | 98.2 (89.0–99.9) | 99.7 (99.4–99.9) | 88.5 (77.2–94.9) | 100 (99.7–100) | ||
| Positive | 54d | 7e | ||||
| Negative | 1f | 2,325g |
Values in parentheses are 95% confidence limits.
PPV, positive predictive value.
NPV, negative predictive value.
Concordant with two ELISAs.
Discordant between rapid test and ELISA-1 and/or ELISA-2, immunoblot negative.
Immunoblot-proven ELISA-1- and ELISA-2-reactive sample.
Concordant with ELISA-1.
The accuracy indices of these two rapid tests were found to be satisfactory in comparison to concordant test results with ELISAs and/or immunoblotting in cases of discordant samples. The rapid tests have advantages such as ease of use, minimal training required for the user, easy interpretation, and a long shelf life. These tests can be done with a short turnaround time, avoiding the delay incurred in batching. Thus, rapid tests can be used as an alternative to ELISAs in small peripheral hospitals, blood banks, and VCT centers which lack facilities and skilled technicians. Our study has helped in the real-time evaluation of these rapid tests in an area of moderate prevalence (1.7%) of HIV infection (14). There are several reports of evaluations of certain rapid assays, often with a small panel of samples; only a few are field studies. The accuracy indices of all those kits were close to 100% (5, 10, 11, 15, 19). However, there are only a very few reports on real-time evaluations with hospital-based samples (8). WHO previously evaluated the two kits used in the study reported here only on serum panels. To our knowledge, there is no report of such an evaluation of these kits on a large sample from any area. This study adds the valuable perspective of a user, especially in light of the WHO/UNAIDS recommendations (18) for the use of simple, rapid tests to facilitate the expansion of VCT centers towards strengthening strategies for prevention of HIV infection.
Acknowledgments
We thank the National AIDS Control Organization (India) for supplying some of the kits used in this study.
REFERENCES
- 1.Albritton W L, Vittinghoff E, Padian N S. Human immunodeficiency virus testing for patient-based and population-based diagnosis. J Infect Dis. 1996;174(Suppl. 2):S176–S181. doi: 10.1093/infdis/174.supplement_2.s176. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Update: HIV counseling and testing using rapid tests. United States, 1995. Morbid Mortal Wkly Rep. 1998;47:211–215. [PubMed] [Google Scholar]
- 3.Das Gupta R P, Jain M K, John T J. Government response to HIV/AIDS in India. AIDS. 1994;8(Suppl. 2):S83–S90. [PubMed] [Google Scholar]
- 4.Downing R G, Otten R A, Marum E, Biryahwaho B, Alwano-Edyegu M G, Sempala S D, Fridlund C A, Dondero T J, Campbell C, Rayfield M A. Optimizing the delivery of HIV counseling and testing services: the Uganda experience using rapid HIV antibody test algorithms. J Acqir Immune Defic Syndr Hum Retrovirol. 1998;18:384–388. doi: 10.1097/00042560-199808010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Giles R E, Perry K R, Parry J V. Simple/rapid devices for anti-HIV screening: do they come up to the mark? J Med Virol. 1999;59:104–109. [PubMed] [Google Scholar]
- 6.Jain M K, John T J, Keusch G T. Epidemiology of HIV and AIDS in India. AIDS. 1994;8(Suppl. 2):S61–S75. [PubMed] [Google Scholar]
- 7.Kassler W J, Alwano-Edyegu M G, Marum E, Biryahwaho B, Kataaha P, Dillon B. Rapid HIV testing with same-day results: a field trial in Uganda. Int J STD AIDS. 1998;9:134–138. doi: 10.1258/0956462981921882. [DOI] [PubMed] [Google Scholar]
- 8.Kelen G D, Shahan J B, Quinn T C. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33:147–155. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 9.Lackiritz E M. Prevention of HIV transmission by blood transfusion in the developing world: achievements and continuing challenges. AIDS. 1998;12(Suppl. A):S81–S86. [PubMed] [Google Scholar]
- 10.Lien T X, Tien N T, Chanpong G F, Cuc C T, Yen V T, Soderquist R, Laras K, Corwin A. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2000;62:301–309. doi: 10.4269/ajtmh.2000.62.301. [DOI] [PubMed] [Google Scholar]
- 11.Meda N, Gautier-Charpentier L, Soudre R B, Dahourou H, Ouedraogo-Traore R, Ouangre A, Bambara A, Kpozehouen A, Sanou H, Vallea D, Ky F, Cartoux M, Brain F, Van de Perre P. Serological diagnosis of human immunodeficiency virus in Burkina Faso: reliable, practical strategies using less expensive commercial test kits. Bull W H O. 1999;77:731–739. [PMC free article] [PubMed] [Google Scholar]
- 12.Mertens T, Piot P. Global aspects of human immunodeficiency virus epidemiology: general considerations. In: Devita V T Jr, Hellman S, Rosenberg S A, editors. AIDS: biology, diagnosis, treatment and prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 103–118. [Google Scholar]
- 13.Metcalf J A, Davey R T, Jr, Lane H C. Acquired immunodeficiency syndrome: serologic and virologic tests. In: Devita V T Jr, Hellman S, Rosenberg S A, editors. AIDS: biology, diagnosis, treatment and prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 177–195. [Google Scholar]
- 14.National AIDS Control Organization. National AIDS Control Programme India, country scenario. An update: December 1996. New Delhi, India: National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India; 1996. [Google Scholar]
- 15.Palmer C J, Dubon J M, Koenig E, Perez E, Ager A, Jayaweera D, Cuadrado R R, Rivera A, Rubido A, Palmer D A. Field evaluation of the Determine rapid human immunodeficiency virus diagnostic test in Honduras and the Dominican Republic. J Clin Microbiol. 1999;37:3698–3700. doi: 10.1128/jcm.37.11.3698-3700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Global AIDS surveillance. Part I. Wkly Epidemiol Rec. 1998;48:373–376. [PubMed] [Google Scholar]
- 17.World Health Organization. Recommendations on the safe and effective use of short-course ZDV for prevention of mother-to-child transmission of HIV. Wkly Epidemiol Rec. 1998;41:313–320. [PubMed] [Google Scholar]
- 18.World Health Organization. The importance of simple/rapid assays in HIV testing. WHO/UNAIDS recommendations. Wkly Epidemiol Rec. 1998;42:321–326. [Google Scholar]
- 19.Zaw M, Frerichs R R, Oo K Y, Eskes N. Local evaluation of a rapid HIV assay for use in developing countries. Trop Med Int Health. 1999;4:216–221. doi: 10.1046/j.1365-3156.1999.43383.x. [DOI] [PubMed] [Google Scholar]