Abstract
Purpose:
We assessed 18-month cumulative mother-to-child HIV transmission (MTCT) risk and risk factors for no antiretroviral medication use during pregnancy among adolescent, young women, and adult mothers in Zimbabwe.
Methods:
We analyzed data from a prospective survey of 1,171 mother–infant pairs with HIV-exposed infants aged 4–12 weeks who were recruited from 151 immunization clinics from February to August 2013. HIV-exposed infants were followed until diagnosed with HIV, death, or age 18 months. Findings were weighted and adjusted for complex survey design and nonresponse.
Results:
The 18-month cumulative MTCT risk was highest among adolescent aged ≤19 years (12%) followed by young women aged 20–24 years (7.5%) and adult women aged ≥25 years (6.9%). Across these groups, more than 94% had ≥1 antenatal care visit by 21 weeks of gestation, more than 95% had ≥1 HIV test, and more than 98% knew their HIV status. Of known HIV-positive mothers, maternal antiretroviral medication coverage during pregnancy was 76.8% (95% confidence interval: 65.1–85.5), 83.8% (78.6–87.9), and 87.8% (84.6–90.4) among adolescent, young women, and adult mothers, respectively. Among HIV-positive mothers diagnosed prenatally, the adjusted odds ratio of no ARV use during pregnancy was increased among those who had no antenatal care attendance (adjusted odds ratio: 7.7 [3.7–16.0]), no HIV testing (7.3 [2.3–23.5]), no prepartum CD4 count testing (2.1 [1.3–3.4]), and maternal HIV identification during pregnancy (2.9 [1.8–4.8]). Age was not a risk factor.
Conclusions:
With similar coverage of prevention of MTCT services, the 18-month cumulative MTCT risk was higher among adolescents and young women, compared with adults. Additional research should examine the causes to develop targeted interventions.
Keywords: Mother-to-child HIV transmission (MTCT), Adolescents, Young women, Zimbabwe
Although adolescent girls and young women (aged 15–24 years) were only 10% of the population, they accounted for 26% of new HIV infections in 2016 in eastern and southern Africa [1]. Given their high HIV incidence, HIV-infected pregnant and breastfeeding adolescents and young women are likely to be at high risk for mother-to-child HIV transmission (MTCT) [2]. Sub-Saharan Africa has the world’s highest adolescent birth rates; approximately 25% of young women aged 20–24 years delivered a child before 18 years old between 1995 and 2011 [3]. Two studies from South Africa found adolescent mothers have significantly higher risk of early MTCT measured at 4–8 weeks postpartum compared with older mothers [2,4]. Prevention of MTCT (PMTCT) services during pregnancy, including the use of antiretroviral medications (ARV), have reduced MTCT significantly [5]. Although HIV-positive adolescents may contribute a small proportion (4% in Kenya and 7% in Zimbabwe) [6–8] to the overall HIV-prevalence among pregnant women, PMTCT programs must focus on this vulnerable group, and the young women who have aged into the next age category to achieve the global elimination of MTCT [9].
In this analysis, we report the observed cumulative risk of MTCT from the perinatal period through 18 months postdelivery among adolescents aged ≤19 years, young women aged 20–24 years, and adult women aged ≥25 years. We also describe the coverage of services along the perinatal PMTCT cascade among these age groups and potential risk factors associated with no ARV use during pregnancy among those whose HIV infection was diagnosed prenatally.
Methods
Case definitions
To define maternal and infant HIV status, we used a composite of maternal self-report, HIV test results on infant dried blood spot (iDBS) tests, and information extracted from antenatal care (ANC) and child health clinic (CHC) cards. We defined (1) HIV-infected mother if she reported HIV-positive diagnosis with supporting documentation in the ANC or CHC cards or her infant had a baseline iDBS that was positive by HIV enzyme immunoassay (EIA); (2) HIV-exposed infant (HEI) if infant born to an HIV-infected mother; and (3) HIV-infected infant if infant had 2 positive iDBS HIV DNA polymerase chain reaction (PCR) tests before 18 months of age or 2 reactive rapid HIV tests at 18 months of age.
Study design, study visits, and data collection
We conducted a secondary analysis of data from Zimbabwe’s 2013–2014 PMTCT Effectiveness Survey, a prospective cohort study of a nationally representative sample of mother–infant pairs with infants aged 4–12 weeks. Details of the study design were described elsewhere [10,11]. Briefly, the survey recruited participants from 151 immunization clinics using multistage stratified cluster sampling from all <10 provinces of Zimbabwe [12]. Facilities providing <100 first diphtheria, pertussis, and tetanus immunizations in 2011 (n = 535) were excluded from the sampling frame for logistical considerations. The remaining 1,007 facilities providing ≥100 first diphtheria, pertussis, and tetanus immunizations were divided into three strata based on the number of immunizations provided annually, and 15% of clinics were selected from each stratum using simple random selection without replacement method. All mother–infant pairs were recruited sequentially from each of the selected clinics over 6-week periods. Infants who were severely ill or whose caregivers refused consent were not eligible to participate in the study. From February to August 2013, the primary survey recruited 6,051 pairs of mother–infant aged 4–12 weeks, including 1,171 pairs with HIV-infected mothers and HEIs. These 1,171 pairs were included in the secondary analysis (Figure 1). As 99.7% of caregivers were biological mothers, we refer to all nonbiological caregivers as mothers in this report. After the baseline interview, the mother–infant pairs with HEIs were scheduled for additional visits at 6, 9, 12, 15, and 18 months postdelivery until the occurrence of one of the following infant events: HIV infection, death, or age 18 months. At all visits, a trained research assistant administered an oral questionnaire and collected the mothers’ responses on a handheld device. The questionnaire elicited maternal and infant characteristics and coverage of ANC and PMTCT services. When possible, information was confirmed with patient-held ANC and CHC cards and medical records.
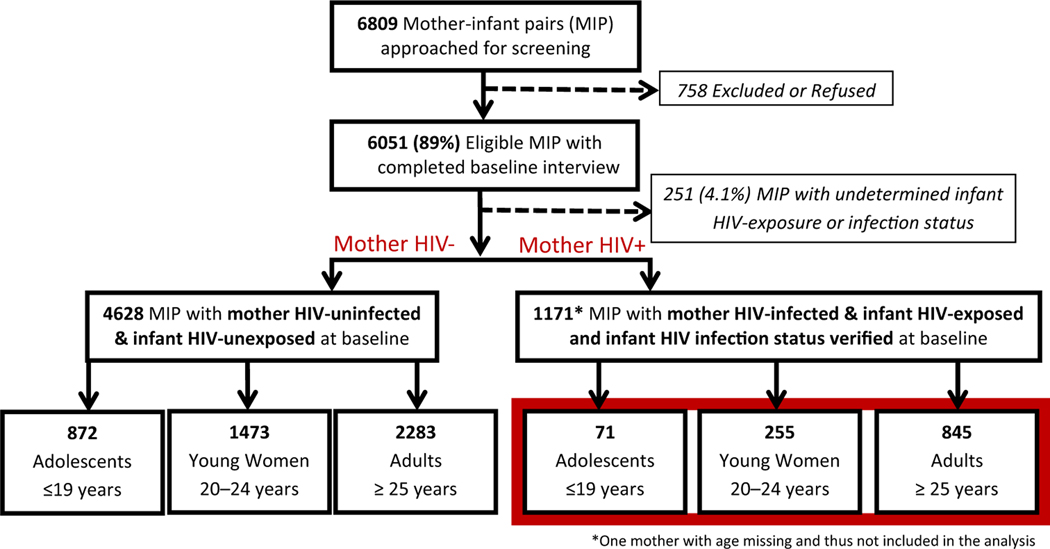
Figure 1.
Analytical population of HIV-infected mother-infant pairs, Zimbabwe PMTCT Effectiveness Survey, 2013–2014.
Infant dried blood spot samples were collected at the baseline for HIV testing for all enrolled infants (HIV antibodies and HIV DNA). HEIs had additional iDBS at 6, 9, 12, and 15 months for HIV DNA testing; and HIV rapid diagnostic testing was performed at 18 months, per national and World Health Organization (WHO) recommendations [13]. Clinic nurses returned infant HIV DNA test results to mothers within 4 weeks, and HIV rapid test results were returned to mothers immediately. Routine HIV-related care and treatment services were provided per the national guidelines. All mothers provided written consent at the baseline, and mothers with HEIs provided written consent for the 18-month follow-up at the 6-month visit. In addition, verbal consent was obtained before each blood specimen was collected for HIV testing.
Laboratory-based HIV testing
Infant dried blood spot samples were collected on Munktell-TFN 5-spot paper and sent to the National Microbiology Reference Laboratory in Harare for serology testing of HIV antibodies using an EIA (Ani labsystem HIV1/2 Ab EIA third generation; Labsystems Diagnostics Oy, Vantaa, Finland). All antibody-positive samples were retested using a second EIA (Enzygnost Anti-HIV1/2 Plus; Siemens Healthcare Diagnostics Products GmbH, Marburg/Germany). Baseline iDBS, which tested positive via EIAs and iDBS from infants born to known HIV-infected mothers, were tested for HIV DNA by PCR (COBAS Ampli Prep/COBAS TaqMan-CAP/CTM-Qualitative assay version 1.0 assay; Roche Diagnostics, Branchburg, NJ). If the first PCR result was positive or indeterminate, a second PCR (Amplicor HIV-1 DNA Test version 1.5; Roche Diagnostics) was performed to confirm the final result.
Statistical analysis
Survey analysis procedures were used to account for complex survey design, including stratification, weighting, clustering, and nonresponse. Domain analyses were performed on HIV-infected mothers, and the outcome variables were infant HIV infection and maternal ARV use during pregnancy. Statistical analyses were performed with Stata version 14.1 statistical software.
Competing risk analysis was used to estimate the cumulative risk of MTCT through 18 months postdelivery by maternal age group using the stcompet package in Stata [14]. We assessed MTCT longitudinally over the 18-month follow-up at 4–12 weeks and 6, 9, 12, 15, and 18 months after delivery. Cox regression analysis was used to test for differences in MTCT by maternal age group.
We compared demographic, reproductive, and HIV-status variables between age groups of HIV-infected mothers by computing frequencies, weighted proportions, and 95% confidence intervals (CIs) and Rao-Scott chi-square tests. Demographic and reproductive variables included education level, marital status, socioeconomic status (SES), number of pregnancies, planned pregnancy, HIV status of infant’s father, and disclosure of HIV status to family/friends. We defined the SES variable as a composite variable constructed using a clustering algorithm that considered home appliances, water, toilet, house material, income, and fuel quality and access [15].
We also described the coverage of the PMTCT cascade services during pregnancy and postpartum through study enrollment at 4–12 weeks. The PMTCT cascade includes ANC attendance, HIV testing, knowledge of HIV test result, CD4 count testing, maternal ARV use, and infant ARV prophylaxis.
We developed a multivariable model using purposeful selection to identify risk factors associated with no ARV use during pregnancy among mothers whose HIV infection was diagnosed before giving birth, using demographic, reproductive, and PMTCT service cascade variables, including time to clinic [16]. Although all mothers were HIV-positive during pregnancy, some were not diagnosed until 4–12 weeks postdelivery and could not start ARVs during pregnancy. Therefore, they were not included in the model. The algorithm began with a univariate screening of each independent variable and the outcome, and variables with p < .25 were included in the initial multivariable model. Then, variables with the largest p value were excluded one at a time, and a smaller model was refitted until all variables had p < .05. Maternal age was retained in the model regardless of the p value. Excluded variables were tested to see if their exclusion caused at least a 20% change in the regression coefficient of the remaining variables in the model, and if so, they were retained in the model. Interactions between biologically plausible variables were evaluated, including age group and timing of HIV diagnosis, and ANC attendance and HIV testing. The final model was used to estimate crude and adjusted odds ratios and associated 95% CI for predictors of interest.
PMTCT clinical guidelines during the study
At the time of study enrollment (February to August 2013), Zimbabwe national guidelines recommended Option A, in which pregnant and breastfeeding women with WHO stage III/IV or CD4 ≤350 cells/μL were initiated on lifelong antiretroviral therapy (ART), whereas others received zidovudine ARV prophylaxis [17]. Therefore, in this analysis, ARV initiation for PMTCT includes both ART and ARV prophylaxis. Option B+, in which all pregnant and breastfeeding women are started on lifelong ART, was implemented after study enrollment ended, beginning in November 2013 until it was implemented in all clinics by December 2014 [18,19].
The Medical Research Council of Zimbabwe, and the Center for Global Health Office of the Associate Director for Science at the U.S. Centers for Disease Control and Prevention approved the study protocol.
Results
Of the 6,809 mother–infant pairs approached, 6,051 were eligible and completed the baseline interview (Figure 1). We excluded 251 (4.1%) mother–infant pairs from the analysis because the infants’ HIV exposure or infection status could not be confirmed and excluded one mother–infant pair because the mother’s age was missing. Of the 1,171 HIV-infected mothers, 71 were adolescents (aged 10 to ≤19 years), 255 were young women (aged 20 to ≤24 years), and 845 were adults (aged ≥25 years).
Study population characteristics
Table 1 shows the weighted HIV prevalence among all adolescents, young women and adults included in the primary survey was 7.5% (95% CI: 6.1%–9.3%), 14.8% (95% CI: 12.8–16.9), and 27.2% (95% CI: 24.7–29.8), respectively (p < .01). Overall HIV prevalence among all adolescents and young women aged ≤24 years was 12.2% (95% CI: 10.8–13.7). Among HIV-infected mothers included in the secondary analysis, parity varied with age (p < .01); 19.1% (95% CI: 11.6–30.0) of adolescents, 69% (95% CI: 62.8–74.6) of young women, and 95% (95% CI: 93.5–96.3) of adults had ≥2 children. Adolescents and young women were more likely to be unsure of the HIV status of the infant’s father, compared with of adults (p = .05). Timing of maternal HIV identification also varied across the three age groups (p < .01); A lower proportion of adolescents were identified at HIV-infected preconception (23.0% [95% CI: 14.4–34.6]), compared with young women and adults (45.6% [95% CI: 38.3–53.1] and 60.5% [95% CI: 55.5–65.3]). A higher proportion of adolescents were identified as HIV-infected during pregnancy (61.9% [95% CI: 48.6–73.6]) compared with 47.6% (95% CI: 40.2–55.1) of young women and 35.4% (95% CI: 30.8–40.3) of adults. All age groups had similar distributions of education, marital status, SES, planned pregnancy, knowledge of HIV status of infant’s father, disclosure of HIV status to family/friends, and breastfeeding practices before study enrollment.
Table 1.
Demographic and reproductive variables of HIV-infected women at study enrollment at 4e12 weeks postdelivery by maternal age group, Zimbabwe, 2013–2014
| Observed n, Weighted HIV prevalence % (95% CI) | HIV-positive adolescents (<19 years), n = 71 (of 943 total), 7.5 [6.1—9.3] | HIV-positive young women (aged 20 to <24 years), n = 255 (of 1,728 total), 14.8 [12.8 — 16.9] | HIV-positive adults (>25 years), n = 845 (of 3,125 total), 27.2 [24.7—29.8] | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Variable | Observed n | Weighted Col% | Weighted 95% CI | Observed n | Weighted Col% | Weighted 95% CI | Observed n | Weighted Col% | Weighted 95% CI | |
|
| ||||||||||
| Highest education | .3 | |||||||||
| Elementary school | 30 | 42.8 | 31.1–55.3 | 84 | 32.8 | 27.0–39.2 | 307 | 36.5 | 32.5–40.7 | |
| Grades 8+ | 41 | 57.2 | 44.9–68.7 | 171 | 67.2 | 60.8–73.0 | 538 | 63.5 | 59.3–67.5 | |
| Missing values (%) | 0 | - | 0 | - | 0 | - | ||||
| Marital status | .2 | |||||||||
| Single/divorced/widowed | 13 | 18.6 | 10.2–31.5 | 38 | 14.9 | 11.1 –19.6 | 98 | 11.6 | 9.2–14.6 | |
| Married/co-habitating | 58 | 81.4 | 68.5–89.9 | 217 | 85.1 | 80.4–88.9 | 747 | 88.4 | 85.4–90.8 | |
| Missing values (%) | 0 | - | 0 | - | 0 | - | ||||
| SES quintile | .2 | |||||||||
| Lowest | 18 | 25.8 | 17.0–37.0 | 37 | 14.4 | 10.2–19.9 | 147 | 17.4 | 14.4–20.9 | |
| Second | 14 | 20.2 | 12.7–30.5 | 41 | 16.4 | 12.1–21.8 | 174 | 20.9 | 17.8–24.3 | |
| Middle | 14 | 19.9 | 11.9–31.3 | 62 | 24.3 | 18.3–31.5 | 172 | 20.6 | 17.4–24.3 | |
| Fourth | 13 | 17.5 | 9.0–31.4 | 70 | 27.6 | 21.2–35.1 | 192 | 22.7 | 19.1–26.7 | |
| Highest | 12 | 16.7 | 10.4–25.7 | 45 | 17.3 | 12.8–23.0 | 160 | 18.4 | 14.1 –23.6 | |
| Missing values (%) | 0 | - | 0 | - | 0 | - | ||||
| Parity | .0 | |||||||||
| 1 | 57 | 80.9 | 70.0–88.4 | 78 | 31 | 25.4–37.2 | 42 | 5 | 3.7–6.5 | |
| >2 | 13 | 19.1 | 11.6–30.0 | 175 | 69 | 62.8–74.6 | 799 | 95 | 93.5–96.3 | |
| Missing values (%) | 1 | 1.4 | 2 | .7 | 4 | .5 | ||||
| Planned pregnancy | .2 | |||||||||
| Yes | 40 | 57.4 | 44.1 –69.7 | 162 | 63.9 | 56.6–70.6 | 484 | 57.3 | 53.3–61.1 | |
| No | 30 | 42.6 | 30.3–55.9 | 91 | 36.1 | 29.4–43.4 | 357 | 42.7 | 38.9–46.7 | |
| Missing values (%) | 1 | 1.4 | 2 | .7 | 4 | .5 | ||||
| Know HIV status of infanťs father | .05 | |||||||||
| Positive | 23 | 33.1 | 22.1–46.3 | 86 | 34.3 | 27.6–41.9 | 380 | 45.1 | 41.0–49.3 | |
| Negative | 11 | 15.4 | 8.2–27.2 | 42 | 16.3 | 12.0–21.8 | 117 | 13.9 | 11.7–16.5 | |
| Refused/unknown | 36 | 51.5 | 39.3–63.4 | 125 | 49.3 | 42.2–56.3 | 344 | 40.9 | 36.8–45.2 | |
| Missing values (%) | 1 | 1.4 | 2 | .7 | 4 | .5 | ||||
| Disclosed HIV status to family/friends | .1 | |||||||||
| Yes | 39 | 63.6 | 51.4–74.3 | 152 | 66 | 58.8–72.6 | 578 | 72.2 | 67.5–76.5 | |
| No | 22 | 36.4 | 25.7–48.6 | 79 | 34 | 27.4–41.2 | 221 | 27.8 | 23.5–32.5 | |
| Missing values (%) | 10 | 14.1 | 24 | 9.4 | 46 | 5.4 | ||||
| Timing of maternal HIV identification | .0 | |||||||||
| Identified preconception | 16 | 23.0 | 14.4–34.6 | 116 | 45.6 | 38.3–53.1 | 509 | 60.5 | 55.5–65.3 | |
| Identified during pregnancy | 44 | 61.9 | 48.6–73.6 | 122 | 47.6 | 40.2–55.1 | 301 | 35.4 | 30.8–40.3 | |
| Identified postpartum | 11 | 15.1 | 8.0–26.9 | 17 | 6.8 | 4.1–11.0 | 35 | 4.1 | 2.8–6.0 | |
| Missing values (%) | 0 | - | 0 | - | 0 | - | ||||
| Breastfeeding in past 7 days | .6 | |||||||||
| Exclusive | 48 | 69.6 | 56.0–80.5 | 173 | 68.4 | 61.6–74.5 | 590 | 70.6 | 65.8–75.0 | |
| Mixed | 21 | 30.4 | 19.5–44.0 | 71 | 28.5 | 22.2–35.8 | 227 | 27.4 | 23.0–32.3 | |
| None | 0 | - | 8 | 3.1 | 1.6–5.8 | 17 | 2.0 | 1.3–3.1 | ||
| Missing values (%) | 2 | - | 3 | 1.1 | 11 | 1.3 | ||||
CI = confidence interval; SES = socioeconomic status.
MTCT by maternal age group
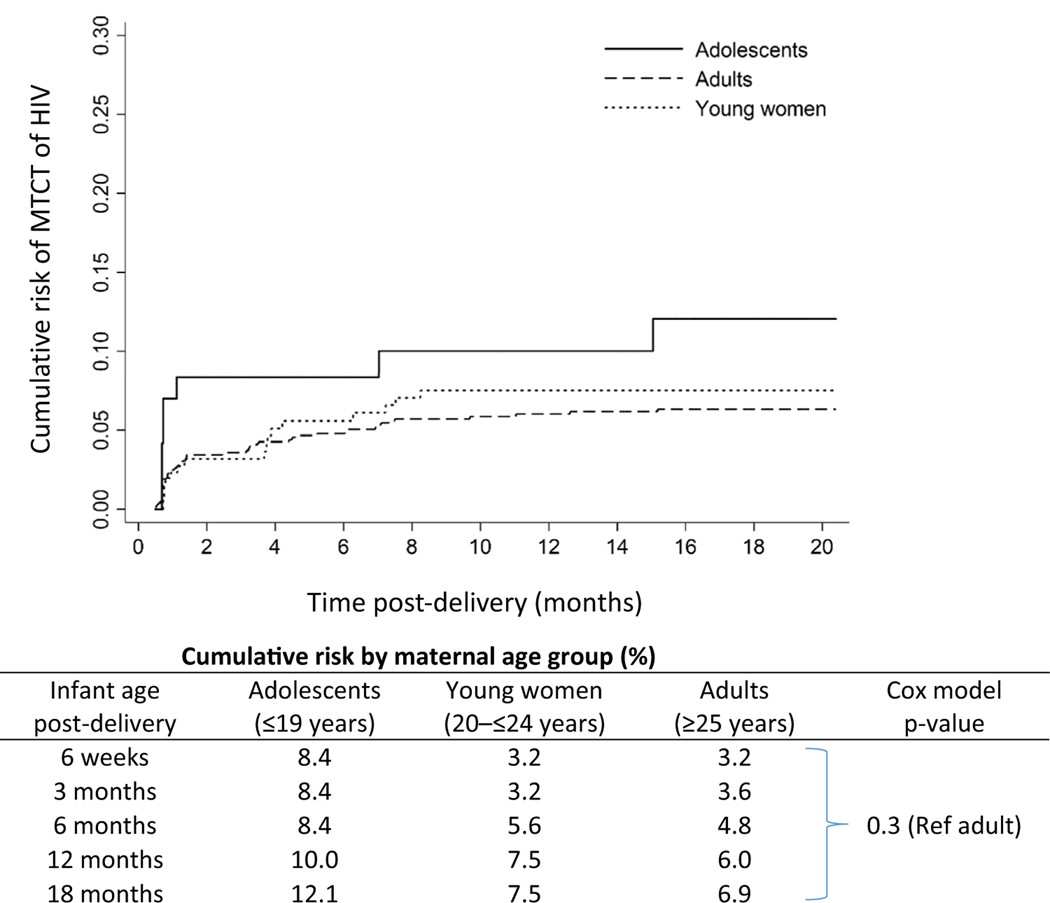
Although not statistically significant, the cumulative MTCT risk at 18 months was observed to be highest among adolescents followed by young women and adult mothers (12.1%, 7.5%, and 6.9%, respectively; p = .3; Figure 2). The observed MTCT risk measured at 6-week postdelivery was 8.4% for adolescents, 3.2% for young women, and 3.2% for adults.
Figure 2.
Weighted cumulative risk of MTCT from pregnancy to 18 months postdelivery among HIV-infected mothers by age group, Zimbabwe PMTCT Effectiveness Survey, 2013–2014.
Coverage of PMTCT cascade services by age group
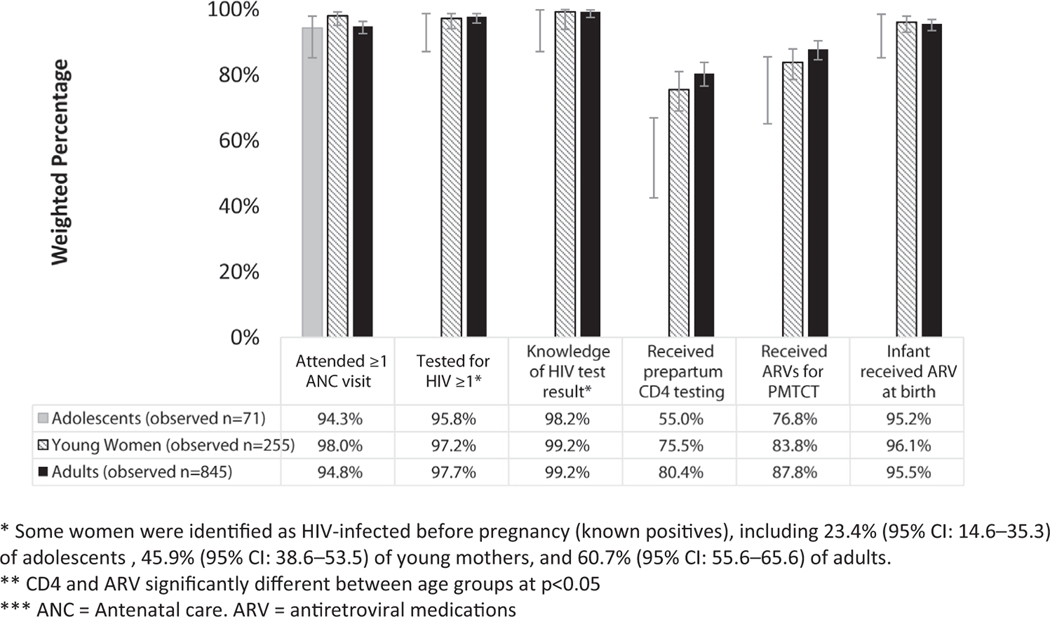
Figure 3 shows that having ≥1 ANC visit, ≥1 HIV test, and knowledge of the HIV test result was high for all HIV-infected mothers. Greater than 94% of women in all age groups had ≥1 ANC visit, and the mean total number of visits was 4.7 (95% CI: 4.1–5.2), 4.2 (95% CI: 3.9–4.5), and 4.2 (95% CI: 4.1–4.5) for adolescents, young women, and adults, respectively. Gestational age at first ANC visit was also similar between adolescents, young women, and adults (20.9 weeks [95% CI: 19.0–22.9], 21.3 weeks [95% CI: 20.0–22.5], and 20.8 weeks [95% CI: 20.1–21.4], respectively). Approximately 70% of mothers in all age groups reported being tested for HIV only once during pregnancy. Of women who initially tested negative, 30% of adolescents and young women were retested during pregnancy, compared with 21% of adults. Greater than 98% of mothers across all groups received HIV test results during pregnancy.
Figure 3.
Coverage of services along the perinatal prevention of mother-to-child transmission (PMTCT) cascade in HIV-infected women by age group, Zimbabwe PMTCT Effectiveness Survey, 2013–2014.
CD4 count testing varied by age (p ≤ .01). A lower proportion of adolescents received prepartum CD4 count testing (55.0% [95% CI: 42.5–66.9] vs. young women [75.5% (95% CI: 69.0–81.0)] and adults [80.4% (95% CI: 76.6–83.8)]). Use of ARVs for PMTCT varied by maternal age group (p = .02); maternal ARV coverage during pregnancy was 76.8% (95% CI: 65.1–85.5) among adolescents, 83.8% (95% CI: 78.6–87.9) among young women, and 87.8% (95% CI: 84.6–90.4) among adults. Similar proportions of women in all age groups who were identified as HIV-infected preconception were not on ARVs; 5.8% (95% CI: .7–34.0) of adolescents, 6.1% (95% CI: 3.5–10.5) of young women, and 4.2% (95% CI: 2.7–6.4) of adults. Our data show a comparable trend of ARV use across all three age groups of mothers whose HIV status were diagnosed postpartum (adolescents 90.5 [78.7–96.1], young women 89.9 [85.8–92.9], adults 91.6 [89.1–93.6]).
Infant ARV prophylaxis was ≥95% for all age groups.
Factors associated with no ARV use during pregnancy
In multivariable analysis (Table 2), no ANC attendance (adjusted odds ratio: 7.7 [95% CI: 3.7–16.0]), no HIV testing (7.3 [95% CI: 2.3–23.5]), no prepartum CD4 count testing (2.1 [95% CI: 1.3–3.4]), and maternal HIV identification during pregnancy (2.9 [95% CI: 1.8–4.8]) were associated with increased odds of no ARV use during pregnancy among women whose HIV infection was diagnosed prenatally. Age was not an independent risk factor. Socioeconomic status, time to clinic, parity, education, marital status, knowledge of HIV status of infant’s father, and planned pregnancy were not significant.
Table 2.
Factors associated with no maternal antiretroviral medication use during pregnancy among women whose HIV infection was diagnosed before giving birth, Zimbabwe, 2013–2014
| Variables | Unadjusted analysis | Adjusted analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Unadjusted odds ratio | 95% confidence interval | p-value | Adjusted odds ratio | 95% confidence interval | p value | |
|
| ||||||
| Maternal age groupa | ||||||
| Adults | Ref | - | - | Ref | - | - |
| Adolescents | 1.2 | .5–3.2 | .7 | .8 | .3–2.4 | .7 |
| Young women | 1.3 | .8–2.0 | .4 | 1.1 | .7–2.0 | .6 |
| SES quintile | ||||||
| Lowest | Ref | - | - | - | - | - |
| Second | 1.1 | .5–2.5 | .8 | - | - | - |
| Middle | 1.1 | .5–2.4 | .8 | - | - | - |
| Fourth | 1.2 | .6–2.5 | .6 | - | - | - |
| Fifth | 1.4 | .7–2.7 | .4 | - | - | - |
| Time to clinic (minutes) | 1.0 | .99–1.00 | .08 | 1.0 | .99–1.0 | .4 |
| Parity | ||||||
| 1 | Ref | - | - | Ref | - | - |
| >2 | .8 | .5–1.2 | .2 | .9 | .5–1.5 | .6 |
| Highest education | ||||||
| Elementary school | Ref | - | - | - | - | - |
| Grades 8+ | 1.1 | .6–1.8 | .8 | - | - | - |
| Marital status | ||||||
| Single/divorced/widowed | Ref | - | - | - | - | - |
| Married/cohabitating | .9 | .4–1.8 | .7 | - | - | - |
| Know HIV status of infanťs father | ||||||
| Negative | Ref | - | - | - | - | - |
| Positive | .7 | .4–1.4 | .3 | - | - | - |
| Refused/unknown | 1.6 | .9–3.2 | .1 | - | - | - |
| Planned pregnancy | ||||||
| Yes | Ref | - | - | Ref | - | - |
| No | 1.4 | 1.0–2.1 | .08 | 1.3 | .9–2.1 | .2 |
| Attend >1 ANC clinic | ||||||
| Yes | Ref | - | - | Ref | - | - |
| No | 14.3 | 8.4–24.3 | .0 | 7.7 | 3.7–16.0 | .0 |
| Tested for HIV >1 test during ANC | ||||||
| Yes | Ref | - | - | Ref | - | - |
| No | 49.6 | 15.6–157.6 | .0 | 7.3 | 2.3–23.5 | .0 |
| Received prepartum CD4 testing | ||||||
| Yes | Ref | - | - | Ref | - | - |
| No | 2.4 | 1.6–3.8 | .0 | 2.1 | 1.3–3.4 | .0 |
| Timing of maternal HIV identificationb | ||||||
| Identified preconception | Ref | - | - | Ref | - | - |
| Identified during pregnancy | 3.4 | 2.2–5.4 | .0 | 2.9 | 1.8–4.8 | .0 |
Bold values were statistically significant.
ANC = antenatal care; SES = socioeconomic status.
N is 1,108 (11 adolescents, 17 young women, and 35 adults).
This model excludes women who acquired HIV during pregnancy but whose HIV infection was not diagnosed until the baseline visit at 4–12 weeks postpartum
because none of them were able to start ARVs during pregnancy because of the timing of their diagnosis.
Discussion
Compared with the national risk of early MTCT measured at 4–12 weeks (3.6%) and cumulative MTCT at 18 months postdelivery (7.0%) reported by Dinh et al. [11], our analysis showed that both early and cumulative MTCT risk was higher in adolescents (8.4% and 12.1%) and young women (3.2% and 7.5%) than in adults mothers (3.2% and 6.9%). Although the observed MTCT risk in our analysis did not differ statistically across the three groups of mothers, the elevated MTCT risk among adolescent mothers is consistent with our finding of more HIV diagnosis during pregnancy as opposed to preconception and lower ARV use during pregnancy among this same group. Horwood et al. [2] found higher MTCT at 4–8 weeks of age among adolescent mothers compared with adult mothers, 10.8% versus 6.1%, respectively, in a 2008–2009 study in South Africa. Similarly, Ramraj et al. [4] found adolescents in three consecutive surveys conducted in South Africa had higher MTCT risk measured at 4–8 weeks postdelivery (5.8%–7.2%) than adults (2.4%–3.2%) from 2010 to 2013. Fatti et al. [20] found that the MTCT risk measured at 6 weeks was 3.4% across all ages in a study from South Africa in 2009–2012. However, Fatti et al. [20] reported in the same paper that adolescents and young women had increased risks of MTCT compared with adults with adjusted risk ratios of 4.5 and 2.8, respectively. We are unaware of other studies that have looked at the cumulative risk of MTCT at 18 months in adolescents and young women.
Our study showed a much higher HIV prevalence among women aged ≤24 years than other studies in Zimbabwe (12.2% for all women aged ≤24 years), and HIV prevalence of 7.5% for adolescents and 14.8% for young women. Before widespread ARV availability, Gonese et al. [21] showed HIV prevalence for women aged 15–24 years was between 11.0% and 13.3% in Zimbabwe. More recently, the 2015–2016 Zimbabwe Population-based HIV Impact Assessment survey estimated an HIV prevalence of 5.9% among all females; 2.8% for ages 10–14 years, 3.9% for ages 15–19 years, and 8.1% for ages 20–24 years [22,23]. We would expect our prevalence to be higher than the national prevalence since the adolescents and young women included in our analysis have all been pregnant, and thus sexually active and at higher risk of HIV-infection. In our study, 69.0% of young women had two children or more, compared with 19.1% of adolescents. In addition, we expect prevalence to be higher in young women than adolescents because both adolescents and young women are living longer because of high ART coverage and early ART initiation.
We found that HIV-infected women of all ages had similar and very high coverage of ≥1 ANC visit (>94%), ≥1 HIV test during ANC (>95%), and knowledge of HIV test results during ANC (>98%). In Sub-Saharan Africa, Wong et al. found only one in five HIV-infected adolescent girls knew their HIV status (20%), and Fatti et al. reported in 2014 that 75.3% of adolescent women were unaware of their HIV status in Eastern Cape, South Africa [20,24]. Although these studies are among all adolescents, our high percentages of HIV test result knowledge during pregnancy may indicate that the national PMTCT program in Zimbabwe was in an advanced stage compared with other countries in the region.
Despite similar coverage for the first three steps in the PMTCT cascade, our analysis revealed that the coverage of CD4 count testing and maternal ARV use in pregnancy differs by age and is lower in adolescents. In contrast, Horwood et al. [2] found adolescents and adults had similar coverage of ARV or ART in 2008–2009, but more adult mothers received the full-recommended PMTCT regimen than adolescent mothers. Musarandega et al. [7] also found similar ARV uptake among adolescents and adults who knew their status in Zimbabwe in 2011–2013; however, our study differs in that coverage of ARVs in the perinatal PMTCT cascade includes mothers who were identified as HIV-infected postpartum. Because a higher proportion of women identified postpartum were adolescents and could not start ARVs during pregnancy without an HIV diagnosis, they may contribute to the observed difference in ARV use by age.
Although more than 95% of mothers in all three populations in our analysis were tested for HIV at the first ANC, only 30% of HIV-negative adolescents and young women were retested during pregnancy, compared with 21% of adults. These findings are similar to a 2012 study in Zambia that found only 24.5% of women were tested twice or more during pregnancy [25]. The WHO guidelines recommend that HIV testing be offered at 32 weeks of gestation to all pregnant women who had HIV-negative status at the first ANC visit to detect women with HIV seroconversion [26]. This is critical because many adolescents and young women do not know the HIV status of their sex partners. Our data showed that only 50% of both adolescents and young women, compared with 60% of adults, knew the HIV status of the infant’s father. If this guideline is not followed, and a mother has acquired HIV during pregnancy or seroconverted after the first HIV test, then ARVs would not be initiated, and MTCT would be more likely to occur [26–28].
Our multivariable analysis found that no ARV use during pregnancy is associated with no ANC attendance, HIV testing or CD4 count testing, and maternal HIV identification during pregnancy, compared with diagnosis preconception. Diagnosis during pregnancy allows less time to initiate ARVs before delivery, particularly since the median gestational age at first ANC was 21 weeks. Also, prior studies have shown that HIV diagnosis preconception is associated with early ARV initiation [26,29]. Of note, women diagnosed with HIV postpartum could not be included in the multivariable analysis because they were not eligible for ARVs during pregnancy as they had not yet been identified as HIV infected. This entire group of women identified as HIV-infected postpartum were not on ARVs during pregnancy and thus at high risk of MTCT. Importantly, a higher proportion of adolescents were diagnosed postpartum (15.1%), compared with young women (6.8%) and adults (4.1%). As all age groups had similar rates of HIV testing during pregnancy, the higher proportion of postpartum diagnosis in adolescents may suggest an increased risk of HIV acquisition during pregnancy, and therefore elevated MTCT risk for the adolescent population.
This study has several limitations. The study was not designed to measure the risk of MTCT among adolescents and young women. Although the small number of HIV-infected infants among adolescents and young women is a testament to the overall success of the PMTCT program, research is needed with larger adolescent and young women sample sizes to further understand MTCT rates and risk factors in these age groups. Although some self-reported data on HIV testing and ARV uptake were verified by reviewing ANC and CHC cards, our findings may have been influenced by maternal recall bias. Also, data on timing of HIV status knowledge is not available and may contribute to observed differences. In addition, although immunization rates are about 95% in Zimbabwe, recruitment from immunization clinics would have missed infants who died before age 4–12 weeks or who did not present for immunization. Although the national guidelines now recommended all HIV-infected patients are elegible for ART at the time of diagnosis, similar barriers to uptake still exist. Therefore, our findings are still relevant as they contribute to the knowledge of ARV/ART use in these populations.
To achieve the global target of elimination of MTCT, attention may be directed toward women who are not initiated on ARVs because of late identification of HIV infection during or after pregnancy. Maternal HIV infection identified during and after pregnancy highlights the importance of repeat HIV testing throughout the pregnancy and breastfeeding period in high-burden settings. In addition, targeted approaches to increase repeat testing and ARV use among pregnant and breastfeeding adolescents and young women, such as youth-friendly services and support groups, may reduce MTCT in these vulnerable age groups and help countries reach MTCT elimination. Furture qualitative studies are needed to understand how to better approach these populations including why some women identified as HIV-infected preconception were not on ARVs.
IMPLICATIONS AND CONTRIBUTION.
Maternal HIV infection identified during pregnancy highlights the importance of repeat HIV testing throughout pregnancy and breastfeeding in settings with high HIV burden. Targeted approaches to increase testing among pregnant and breastfeeding adolescents and young women may reduce MTCT in these vulnerable age groups and help countries reach MTCT elimination.
Acknowledgments
Centers for Disease Control and Prevention assisted with protocol development, managing the study, data analysis, and article writing.
Funding Sources
This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) under a Cooperative Agreement between Centers for Disease Control and Prevention (CDC) and the University of Zimbabwe through Department of Community Medicine (U2GGH00315-01).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
References
- [1].Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2017. Available at: http://www.unaids.org/en/resources/documents/2017/2017_data_book. Accessed February 12, 2018.
- [2].Horwood C, Butler LM, Haskins L, et al. HIV-infected adolescent mothers and their infants: Low coverage of HIV services and high risk of HIV transmission in KwaZulu-Natal, South Africa . PLoS One 2013;8:e74568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].United Nations Population Fund. Adolescent pregnancy: A review of the evidence. Available at: https://www.unfpa.org/sites/default/files/pub-pdf/ADOLESCENT%20PREGNANCY_UNFPA.pdf. Accessed February 12, 2018.
- [4].Ramraj T, Jackson D, Dinh T-H, et al. Adolescent access to care and risk of early mother-to-child HIV transmission. J Adolesc Health 2018;62:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Joint United Nations Programme on HIV/AIDS, ed. Countdown to Zero: Global Plan towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive, 2011–2015. Geneva, Switzerland: UNAIDS; 2011. [Google Scholar]
- [6].Ronen K, McGrath CJ, Langat AC, et al. Gaps in adolescent engagement in antenatal care and prevention of mother-to-child HIV transmission services in Kenya. J Acquir Immune Defic Syndr 2017;74:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Musarandega R, Machekano R, Chideme M, et al. PMTCT service uptake among adolescents and adult women attending antenatal care in selected health facilities in Zimbabwe. J Acquir Immune Defic Syndr 2017; 75:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].World Health Organization (WHO). Elimination of mother-to-child transmission (EMTCT) of HIV and syphilis. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- [9].Mustapha M, Musiime V, Bakeera-Kitaka S, et al. Utilization of “prevention of mother-to-child transmission” of HIV services by adolescent and young mothers in Mulago Hospital, Uganda. BMC Infect Dis 2018;18:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wiegert K, Dinh T-H, Mushavi A, et al. Integration of prevention of mother-to-child transmission of HIV (PMTCT) postpartum services with other HIV care and treatment services within the maternal and child health setting in Zimbabwe, 2012. PLoS One 2014;9:e98236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dinh T-H, Mushavi A, Shiraishi RW, et al. Impact of timing of antiretroviral treatment and birth weight on mother-to-child human immunodeficiency virus transmission: Findings from an 18-month prospective cohort of a nationally representative sample of mother-infant pairs during the transition from option A to option B+ in Zimbabwe. Clin Infect Dis 2018;66: 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].WHO. A short guide on methods: Measuring the impact of national PMTCT programmes : Towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- [13].WHO, Department of HIV/AIDS. WHO recommendations on the diagnosis of HIV infection in infants and children. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- [14].Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J Promoting Commun Stat Stata 2004;4:103–12. [Google Scholar]
- [15].The DHS program - Wealth Index construction. Available at: https://www.dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm. Accessed April 16, 2019.
- [16].Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied logistic regression: Hosmer/applied logistic regression. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2013. [Google Scholar]
- [17].WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Recommendations for a public health approach. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- [18].WHO | Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Available at: https://www.who.int/hiv/pub/mtct/programmatic_update2012/en/. Accessed April 16, 2019.
- [19].Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- [20].Fatti G, Shaikh N, Eley B, et al. Adolescent and young pregnant women at increased risk of mother-to-child transmission of HIV and poorer maternal and infant health outcomes: A cohort study at public facilities in the Nelson Mandela Bay Metropolitan district, eastern Cape, South Africa. S Afr Med J 2014;104:874–80. [DOI] [PubMed] [Google Scholar]
- [21].Gonese E, Dzangare J, Gregson S, et al. Comparison of HIV prevalence estimates for Zimbabwe from antenatal clinic surveillance (2006) and the 2005–06 Zimbabwe Demographic and Health Survey. PLoS One 2010;5:e13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brown K, Williams DB, Kinchen S, et al. Status of HIV epidemic Control among adolescent girls and young women aged 15–24 years - Seven African countries, 2015–2017. MMWR Morb Mortal Wkly Rep 2018;67:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ministry of Health and Child Care (MOHCC) Zimbabwe. Zimbabwe population-based HIV Impact Assessment (ZIMPHIA) 2015–2016: First report. Available at: https://phia.icap.columbia.edu/wp-content/uploads/2017/11/ZIMPHIA_First_Report_FINAL.pdf. Accessed May 8, 2018.
- [24].Wong VJ, Murray KR, Phelps BR, et al. Adolescents, young people, and the 90–90-90 goals: A call to improve HIV testing and linkage to treatment. AIDS 2017;31:S191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heemelaar S, Habets N, Makukula Z, et al. Repeat HIV testing during pregnancy and delivery: Missed opportunities in a rural district hospital in Zambia. Trop Med Int Health 2015;20:277–83. [DOI] [PubMed] [Google Scholar]
- [26].WHO. Consolidated guidelines on HIV testing services: 5Cs: Consent, confidentiality, counselling, correct results, and connection. Geneva, Switzerland: WHO; 2015. [PubMed] [Google Scholar]
- [27].Drake AL, Wagner A, Richardson B, et al. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: A systematic review and meta-analysis. Plos Med 2014;11: e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dinh T-H, Delaney KP, Goga A, et al. Impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 Weeks postpartum in South Africa 2011–2012: A national population-based evaluation. PLoS One 2015;10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyers K, Qian H, Wu Y, et al. Early initiation of ARV during pregnancy to Move towards Virtual elimination of mother-to-child-transmission of HIV-1 in Yunnan, China. PLoS One 2015;10:e0138104. [DOI] [PMC free article] [PubMed] [Google Scholar]