Abstract
We sought to compare oncologic and functional outcomes between thermal and nonthermal energy partial gland ablation (PGA) modalities. We conducted comprehensive, structured literature searches, and 39 papers, abstracts, and presentations met the inclusion criteria of pre-PGA magnetic resonance imaging, oncologic outcomes of at least 6 months, and systematic biopsies after PGA. Twenty-six studies used thermal ablation: high-intensity focused ultrasound (HIFU), cryotherapy, focal laser ablation, or radiofrequency ablation. In-field recurrence rates ranged from 0 to 36% for HIFU, 6 to 24% for cryotherapy, 4 to 50% for focal laser ablation, and 20 to 25% for radiofrequency ablation. Twelve studies used nonthermal technologies of focal brachytherapy, vascular-targeted photodynamic therapy, or irreversible electroporation. Focal brachytherapy had the lowest reported failure rate of 8%, vascular-targeted photodynamic therapy had >30% positive in-field biopsies, and irreversible electroporation had in-field recurrence rates of 12–35%. PGA was well tolerated, and nearly all patients returned to baseline urinary function 12 months later. Most modalities caused transient decreases in erectile function. Persistent erectile dysfunction was highest in patients who underwent HIFU. Although oncologic outcomes vary between treatment modalities, systematic review of existing data demonstrates that PGA is a safe treatment option for patients with localized prostate cancer.
Keywords: Focal therapy, Non-thermal, Partial gland ablation, Prostate cancer, Thermal
Highlights
-
•
Partial gland ablation (PGA) is an emerging prostate cancer treatment that targets the “index lesion,” defined as the largest lesion of the highest grade.
-
•
PGA methods can be broadly characterized by the type of energy used: thermal or nonthermal.
-
•
Patients treated with PGA have fewer treatment-related side effects than patients treated with radical therapies.
-
•
Long-term oncologic outcomes of patients with clinically significant disease are needed to verify the safety of PGA.
-
•
Clarity is needed regarding how to monitor these patients post-PGA, the need for repeat biopsies, and how to use PSA and MRI to monitor for disease progression.
1. Introduction
Advances in imaging and the emergence of new technology have enabled the development of novel, minimally invasive treatments for localized prostate cancer. Partial gland ablation (PGA), designed to be a tissue-preserving technique to treat prostate cancer, aims to provide satisfactory oncologic benefits while minimizing the genitourinary side effects associated with therapies such as radical prostatectomy (RP) or radiation. This approach has been adopted in the treatment of many other solid tumors, including those of the kidney, breast, thyroid, liver, and pancreas.1
Although whole-mount analysis of RP specimens has demonstrated that most cases have multiple foci of disease, prostate cancer is predominantly driven by the largest lesion with the highest grade, the index lesion.2,3 Therefore, treatment of the index lesion while sparing the remainder of the gland may be a viable approach in men with localized, low-volume disease. Advances in multiparametric magnetic resonance imaging (MRI) and targeted biopsies have not only improved the diagnosis of clinically significant prostate cancer (grade group [GG] ≥ 2)4 but have also facilitated their use in monitoring patients after PGA.
In recent years, PGA has emerged as a treatment option for localized prostate cancer. Although it is predominantly used in Europe, it has become increasingly adopted in the United States. PGA modalities can be categorized based on the type of energy used: high-intensity focused ultrasound (HIFU), cryotherapy, focal laser ablation (FLA), and radiofrequency ablation (RFA) use thermal energy; and focal brachytherapy, vascular-targeted photodynamic therapy (VTP), and irreversible electroporation (IRE) are nonthermal. In this systematic review, we aim to provide a comprehensive comparison of the efficacy and side effects of PGA modalities for the treatment of localized prostate cancer.
2. Methods
Comprehensive, structured literature searches for human research studies published in English were conducted in three databases (on August 27, 2018). MEDLINE (via PubMed), Embase, and the Cochrane Library were searched, results were combined in a bibliographic management tool (EndNote), and duplicates were eliminated both electronically and through manual review. Search results were then imported into the systematic review support tool Covidence for further reference management and review processes.
Controlled vocabularies and text words were used in the development of the search strategies for all databases. The search terminology included two major components that were linked together with “and”: (1) prostate cancer, including prostatic neoplasms/neoplasia; and (2) focal therapy, including high-frequency ultrasound, cryotherapy/cryosurgery, and focal IRE. To investigate the gray literature, comprehensive searches of all publication types, including conference proceedings, research, and other reports, and theses/dissertations were conducted in Embase. For a complete list of Medical Subject Headings (MeSH) and keyword terms used, please refer to the PubMed search strategy accompanying this review (Supplementary Fig. S1).
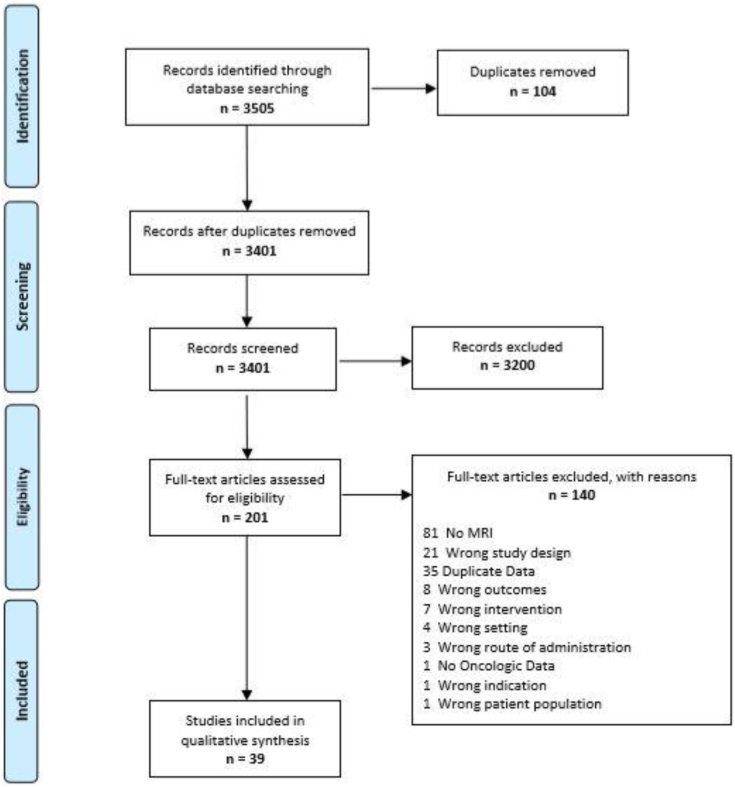
A total of 3401 abstracts were screened by two authors (J.S.F. and B.A.), of which 201 full texts were reviewed. Thirty-nine papers, abstracts, and presentations met the inclusion criteria of targeted PGA with preprocedure MRI, oncologic outcomes of at least 6 months, and systematic posttherapy biopsies (Fig. 1). One hundred sixty-two studies did not meet inclusion criteria or contained duplicate data. If the same group from a specific center published multiple studies, the most recent or largest was used. If updated data were published during manuscript preparation, the most up-to-date data were used. Any discrepancies were moderated by the senior author (B.E.).
Fig. 1.
PRISMA diagram.
3. Results
3.1. Thermal energy
Thermal energy modalities include HIFU, cryotherapy, FLA, and RFA, each of which uses a different method for producing apoptosis and tissue destruction. HIFU and cryotherapy are older technologies that are very well studied, whereas RFA and FLA were developed more recently and have fewer data available, particularly regarding new-onset incontinence and erectile dysfunction (ED) (Table 1).
Table 1.
Summary of outcomes after partial gland ablation
| Modality | Number of patients | Percentage of patients with clinically significant disease | Percentage of patients with negative follow-up biopsy | Failure-free survival (%) | Erectile function | Urinary function | Reference |
|---|---|---|---|---|---|---|---|
| Thermal | |||||||
| HIFU | 625 | 84 | 75 | 88 | 15% new ED | 83% pad-free at 3 y | Guillaumier et al, 2018 |
| 164 | 80 | N/A | 96 | 18% new ED | 0% new incontinence | Mistry et al, 2017 | |
| 149 | 89 | N/A | 83 | 14% new ED | 0.6% new incontinence | Hanna et al, 2018 | |
| 111 | 32 | 93 | 89 | 22% new ED | 3% new incontinence | Rischmann et al, 2017 | |
| Cryotherapy | 301 | 62 | N/A | 95 | No change | Improved flow rates | Bianco et al, 2018 |
| 122 | 90 | N/A | 91 | 16% new ED | No change | Shah et al, 2019 | |
| 107 | 24 | 48 | N/A | N/A | N/A | Barret et al, 2018 | |
| FLA | 98 | N/A | 69 | N/A | No change | No change | Feller et al, 2018 |
| 25 | 56 | 84 | N/A | No change | No change | Lepor et al, 2015 | |
| 18 | N/A | 28 | 66 | No change | N/A | Elkhoury et al, 2018 | |
| RFA | 21 | N/A | 76 | N/A | No change | No change | Taneja et al, 2018 |
| 20 | N/A | 80 | 90 | No change | No change | Orczyk et al, 2018 | |
| Nonthermal | |||||||
| VTP | 206 | 0 | 49 | 94 | No change | No change | Azzouzi et al, 2015 |
| 21 | 0 | 76 | 87 | No change | No change | Taneja et al, 2018 | |
| Brachytherapy | 354 | 17 | N/A | N/A | N/A | N/A | King et al, 2018 |
| IRE | 25 | 28 | 72 | 8 | No new ED | No change | Murray et al, 2016 |
| 63 | 86 | 76 | 89 | Mild decrease in scores (EPIC) | No change | van de Bos et al, 2018 | |
ED, erectile dysfunction; EPIC, Expanded Prostate Cancer Index Composite; FLA, focal laser ablation; HIFU, high-intensity focused ultrasound; IRE, irreversible electroporation; N/A, not available; RFA, radiofrequency ablation; VTP, vascular-targeted photodynamic therapy.
High-Intensity Focused Ultrasound. HIFU uses sonic waves to precisely deliver thermal energy that destroys target tissue. Tissue destruction results from coagulative necrosis from temperatures ≥60 °C and internal cavitation from the negative pressure of the ultrasound wave.5,6 HIFU has been used in Europe for several years, and it attained Food and Drug Administration approval for prostate ablation in the United States in 2015. Although HIFU was originally developed as a minimally invasive alternative to whole gland treatment, randomized clinical trials comparing HIFU to RP or radiation are lacking. More recently, HIFU has gained popularity in PGA. Because no prospective studies have exclusively evaluated in-bore MRI-guided HIFU (performed by a radiologist in an MRI suite) at the time of this publication, all HIFU studies referenced in this article were ultrasound-guided.
The results of the largest study of PGA with HIFU were recently published.7 Prospective data on 625 consecutive patients with nonmetastatic prostate cancer undergoing HIFU between 2006 and 2015 in Europe were collected. Based on the D'Amico risk groups,8 13% of the men had low-risk disease, 81% had intermediate-risk disease, and 6% had high-risk disease. Median follow-up duration was 56 months. The primary outcome, failure-free survival (FFS), defined as freedom from radical or systemic therapy, was 99% at 1 year, 92% at 2 years, and 88% at 5 years. Metastasis-free survival at 5 years was 98%. At least one repeat HIFU treatment was needed in 121 (19%) patients. The most common side effects were urinary tract infection (8.5%) and epididymo-orchitis (1.9%). Two patients developed recto-urethral fistula; one was managed with urinary diversion via suprapubic tube, and the other required reconstructive surgery. At 1- and 3-year follow-up visits, pad-free continence rates were 97% and 98%, respectively. By multivariate analysis only pre-HIFU prostate-specific antigen (PSA) level and disease classified as T3 were significantly associated with treatment failure. A considerable strength of this study is that 87% of men had clinically significant disease. Although the authors used the Sexual Health Inventory for Men (SHIM), those data were unavailable at the time of publication, so ED rates for this cohort remain unknown.
In an abstract from 2018, Hanna et al reported on 149 men treated with HIFU at a single center over an 8-year period.9 Eighty-nine percent of the cohort had intermediate-/high-risk disease; 31% had high-volume GG 1, 55% had GG 2, and 13% had GG 3. Six percent of men underwent salvage therapy, and 83% of patients did not undergo any additional procedure, including repeat HIFU, for disease recurrence. The procedure was well tolerated, with only 0.6% of patients reporting new usage of incontinence pads, 1.3% reporting a urethral stricture, and 14% reporting new-onset ED.
The French Urological Association initiated a prospective, multi-institutional study to evaluate HIFU hemiablation as the primary treatment for clinically localized prostate cancer.10 Between 2009 and 2015, 111 patients (68% low risk and 32% intermediate risk) were treated with unilateral HIFU hemiablation. All patients underwent biopsy 1 year after HIFU, at which time clinically significant cancer was absent from 95% of treated lobes and 93% of contralateral lobes. The radical treatment-free survival rate was 89% at 2 years. Good functional outcomes were reported, with 97% of patients being continent and 78% of patients with preserved erectile function at 1 year. Although promising, this study is limited by the fact that over two-third of the patients were at low risk.
A study from the UK in 2017 reported on 164 men with localized prostate cancer who were treated with partial gland HIFU.11 This cohort was more balanced, with 20% of patients with low-risk disease, 71% with intermediate-risk, and 9% with high-risk. Median follow-up duration was 50 months. Seven (4%) patients progressed to radical therapy, and one patient developed metastases and required systemic therapy. Metastasis-free and overall survival at 5 years were 99.4% and 100%, respectively. All men maintained pad-free continence, and 82% of patients who had satisfactory preoperative erections maintained good erectile function postoperatively.
Nine other studies met inclusion criteria, each with <100 patients.12, 13, 14, 15, 16, 17, 18, 19, 20 Oncologically, most studies reported treatment failure rates <25% (range 0–26.5%). Functionally, there were similar outcomes: minimal, if any, decrease in continence, and a variable but significant percentage of patients who reported new-onset ED (11–48%). One study found that HIFU was associated with significantly lower rates of ED than RP (20 vs. 44%, P = 0.03).20
Overall, HIFU is effective at tumor ablation, with reported 5-year FFS rates of approximately 88%.7,10 As with many PGA modalities, repeat treatment is not uncommon, typically around 10–20%.7,9 Incontinence rates post-HIFU appear low, with larger series reporting 0–3% de novo incontinence.7,9,10 HIFU does appear to have higher rates of ED than other forms of PGA, which may be due to its wider use among community urologists as a result of its Food and Drug Administration approval.
Cryotherapy. Cryotherapy (cryoablation) uses alternating cycles of tissue freezing and thawing to make cellular membranes permeable and induce apoptosis and cell death. Technically, needles that deliver bursts of cold gas to produce freeze-thaw cycles are placed transperineally. The initial use of cryotherapy as a whole gland treatment resulted in many sexual and urinary side effects,21 but partial gland cryotherapy has been shown to minimize these side effects.22 As a result, the use of cryotherapy for PGA has been increasing. The Cryo On-Line Database (COLD) Registry is an online retrospective database of patients with prostate cancer treated with cryotherapy. However, owing to the heterogenous nature of the studies in the registry, it did not meet the inclusion criteria of our review.
The largest study that met inclusion criteria was reported in an abstract from the article by Bianco et al from 2018 in which 301 men with prostate cancer underwent cryotherapy between 2013 and 2017.23 Thirty-eight percent of patients had GG 1 disease, 35% had GG 2, 18% had GG 3, and 9% had either GG 4 or 5. At a median follow-up of 2 years, 15% of patients required retreatment with cryotherapy, and 5% progressed to radical treatment. The median time to return to baseline erectile status was 33 days, although the overall de novo rate of ED is not indicated. Similarly, a urinary flow rate improvement of 72% compared to baseline is reported, but overall rates of de novo urinary incontinence are not. The strength of this study is the percentage of patients with clinically significant disease who were treated, as 62% of patients were GG 2 or higher.
Shah et al recently reported on a series of 122 patients treated with focal cryotherapy in five centers in the UK between 2013 and 2016.24 Based on National Comprehensive Cancer Network (NCCN) classification, 28.7% of patients had high-risk disease, and 71.3% had intermediate-risk disease. The primary outcome was FFS, defined as freedom from progression to radical treatment, metastasis, or death. Overall, 3-year FFS was 90.5%; when stratified by NCCN risk category, the FFS was 84.7% for patients with high-risk disease and 93.3% for those with intermediate risk. No patients reported urinary incontinence, and 16.1% reported new-onset ED. However, the rates of incontinence and ED may be underestimated because although the focus of this study was medium-term outcomes, only 45–55% of patients had medium-term data on these outcomes.
Barret et al published a series of 107 patients with unilateral disease treated with focal cryotherapy at a single high-volume center in France.25 Seventy-seven percent of patients had Gleason 6, and 23% had Gleason 7 (3 + 4). All patients underwent MRI and transperineal mapping biopsy before cryotherapy. Mean follow-up duration was 64 months. Prostate cancer was detected within the treatment zone in 35% of patients and outside the treatment zone in 28% of patients during follow-up. Only Gleason score was reported as a predictor of recurrence by multivariate regression. This study is limited by the high percentage of patients with low-risk disease and the relatively high rate of in-field recurrence.
In summary, cryotherapy is another viable thermal energy PGA modality. As with HIFU, it was initially used as whole gland therapy, but it is now used for PGA. It provides adequate cancer control, with studies reporting FFS >90% at 3-year follow-up.23,24 Incontinence rates in the aforementioned studies are low, but data are scarce. Cryotherapy appears to lead to transient ED in approximately 10–20% of patients.23,24 Further studies with full, long-term follow-up data are needed to better determine the side-effect profile of cryotherapy.
Focal Laser Ablation. FLA uses laser fiber probes inserted transperineally or transrectally into the visible prostate tumor to deliver heat, thereby causing coagulative necrosis and cell death. Although many phase 1 trials have established the safety of FLA, there are few high-quality series with long-term data.
The largest series comes from Feller et al, in which 98 men with 138 distinct cancer foci were treated with FLA and monitored by MRI-guided biopsy.26 At 6 months, 23% of patients had an in-field recurrence, and 7% had cancer outside of the ablated region. There were no serious adverse events, and International Prostate System Score (IPSS) and SHIM scores remained unchanged at 12 months. This phase 2 study has limitations, mainly that the preprocedure Gleason scores and percentage of men with clinically significant disease were not reported.
Elkhoury et al recently presented data from UCLA on 18 patients treated with FLA.27 Eight patients were treated in-bore (by a radiologist in an MRI suite), and 10 were treated out-of-bore (by a urologist in an outpatient clinic).27 MRI-ultrasound fusion biopsy at a median of 12 months follow-up showed 5 patients had no evidence of disease and 4 had improved Gleason score or lower tumor volume. The reported recurrence rate was 50%, with 9 patients (4 in-bore and 5 out-of-bore) experiencing treatment failure, for which 8 patients sought further treatment.
In 2013, Lindner et al published a series of 38 men treated with FLA in an outpatient setting in Canada.28 Sixty-four percent of men had GG 1 disease, and 36% had GG 2. Median follow-up duration was 538 days. Thirty-four men had evaluable biopsies, of which 16 (47%) were completely negative. Nine men (26%) had a negative biopsy in the treatment lobe but a positive biopsy outside the treatment area. The remaining 9 (26%) patients experienced recurrence within the treatment zone. Only one patient required medication for new-onset ED, and no patients reported long-term urinary incontinence. This study reported a relatively high (26%) treatment failure rate and is limited by the fact that two-third of the patients had low-risk disease.
In 2015, Lepor et al published a series of 25 men with Gleason <8 prostate cancer treated with in-bore FLA.29 There was no residual tumor in 96% of MRI-guided biopsies performed 3 months after FLA. In addition, there was no change in the American Urological Association Symptom Score or SHIM score at 3 months. Longer term data are needed on this cohort.
Finally, Mehralivand et al presented data on 15 patients with GG ≤ 2 disease who underwent FLA.30 Nine (60%) patients had GG 2, and 6 (40%) had GG 1.30 Median follow-up time was 40.5 months. Follow-up targeted biopsy revealed no evidence of disease in 10 (66.7%) patients. Of the 5 patients with recurrence, 3 underwent repeat FLA, and 2 underwent RP. There were no differences in pretreatment and posttreatment SHIM scores and IPSS.
In summary, FLA is a promising method for PGA. Although one study reported >95% tumor destruction at short-interval biopsy,29 others have treatment failure rates of approximately 25–35%.26,28,30 In studies that report tolerability, the rates of ED and urinary incontinence are very low.
Radiofrequency Ablation. RFA uses a transperineally placed probe to deliver heat via medium-frequency alternating current. It is the least studied of all PGA modalities.
Taneja et al presented a series of 21 patients treated with bipolar RFA between 2014 and 2015. Fifteen men had one area treated, and 6 men had two areas treated.31 MRI performed seven days postprocedure showed complete ablation in 19 (90%) men. Six-month biopsy showed no tumor in the treatment zone of 16 (76%) men, with 5 (24%) men having an in-field recurrence. SHIM score and IPSS before and after RFA remained unchanged.
Orczyk et al reported a UK series of 20 patients with MRI-visible prostate lesions treated with RFA.32 Eighteen (90%) patients had GG 2 or GG 3 disease. At 12-month biopsy, no significant tumor was found in 16 (80%) patients. Of the 4 patients experiencing treatment failure, 2 were maintained on active surveillance (AS), and 2 underwent repeat RFA. One patient developed a urethral stricture requiring dilation with subsequent incontinence pads; all other patients remained continent. There was no change in IPSS at 12-month follow-up.
Similar to FLA, RFA is a promising technology for PGA. In the two studies included in this review, follow-up biopsy at 6–12 months postprocedure showed no tumor in 76–80% of men.31,32 It also appears well tolerated, with no change in IPSS and minimal new incontinence requiring pads.
3.2. Nonthermal energy
There are three nonthermal energy PGA modalities: focal brachytherapy, VTP, and IRE. These use radiation, the production of reactive oxygen species, or electricity to damage target tissue, leading to apoptosis and tumor cell destruction. By not heating and/or freezing the target tissue, there is theoretically less damage to surrounding tissue, and the collagen matrix around important structures is preserved, allowing for more physiologic healing.33
Brachytherapy. Brachytherapy, the placement of radioactive seeds into the prostate, has long been used to treat prostate cancer. The local effects of radiation eradicate the tumor. This technique is easily adaptable to PGA by placing seeds only into a specific lesion rather than the entire gland. Three studies of brachytherapy met the inclusion criteria for this review.
The largest study of focal brachytherapy was reported in an abstract in 2018.34 This study included 348 patients who had MRI-guided focal brachytherapy seeds placed between 1997 and 2008. Of these, 295 (83%) patients had low-risk disease, and 59 (17%) had intermediate-risk disease as per the NCCN guidelines. Sixty-seven (19%) patients also received supplemental external beam radiotherapy, but whether these patients had low-, medium-, or high-risk disease is unknown. During the 8.6-year follow-up, low-risk patients had a 23.5% rate of biochemical progression based on the Phoenix criteria, 6.4% rate of local recurrence, and 2.0% rate of metastasis. Intermediate-risk patients fared worse, with a 51.2% rate of biochemical progression, 21.2% rate of local recurrence, and 6.6% rate of metastasis during the study period. Prostate cancer–specific death was 5.4% in the intermediate-risk group. On multivariate analysis, the only factor that predicted metastasis was biopsy-proven local recurrence. This study had a large cohort and long-term follow-up, and the investigators concluded that these long-term oncologic outcomes were suboptimal.
In addition, two smaller studies examined functional outcomes after focal brachytherapy. The first study, which is currently underway, included 21 patients and found that SHIM scores returned to baseline at the 12-month follow-up.35 This study has not yet reported any oncologic outcomes. The second included 16 patients (9 low risk and 7 intermediate risk) treated with focal brachytherapy and reported that IPSS and SHIM scores returned to baseline by 12 months.36 No patients had a biochemical recurrence during the median 9-month follow-up. However, longer and more robust follow-up is needed to put this oncologic data into clinical perspective.
Although standard brachytherapy has long been viewed as oncologically acceptable, it appears that the efficacy of focal brachytherapy remains unproven. Biochemical progression rates of 50% for intermediate-risk disease are high34; this corresponds to a 6.6% risk of metastasis at 8 years postprocedure. It does appear well tolerated, with low rates of incontinence and ED. With better oncologic outcomes and similar side effect profile, other PGA modalities appear to be superior to focal brachytherapy.
Vascular-Targeted Photodynamic Therapy. VTP uses a light-activated intravenous agent to generate radical oxygen species, causing vascular necrosis and tumor destruction in the illuminated area.
The results of a study (PCM301) comparing VTP to AS in patients with low-risk prostate cancer have been reported by Azzouzi et al37 (2-year follow-up) and Gill et al38 (4-year follow-up). All men had GG 1 disease and were eligible for AS; 207 patients received VTP, and 206 were placed on AS. Biopsies performed at 2 years were negative throughout the prostate in 50% of the patients treated with VTP and 14% of the patients on AS, and there were no differences in SHIM score or IPSS between the VTP and AS arms. The rate of adverse events was higher in the patients who underwent VTP than that in patients who were on AS. The most common adverse events were urinary tract infections, which occurred in 19 (10%) patients on VTP and 7 (3%) patients on AS, and perineal pain, which occurred in 30 (14%) patients on VTP and 1 (<1%) patient on AS. Compared with AS, VTP was associated with a significantly lower rate of cancer progression (hazard ratio 0.42, P < 0.001) and a lower rate of conversion to radical therapy (53% vs. 24%; hazard ratio 0.31, P < 0.001) at 4 years. Although this study provides a metric for demonstrating the benefit of PGA—delay in time to radical therapy—it includes only low-risk patients, and a high percentage of the AS patients (53%) went on to receive radical therapy.
VTP is promising and has been shown to be safe and effective in low-risk patients, with lower rates of disease progression than AS. The side effect profile is comparable to other focal energy modalities. More robust data are needed in patients with clinically significant prostate cancer.
Irreversible Electroporation. IRE uses electrical pulses between electrodes to create pores in the cell membrane, leading to apoptosis and cell death. Using transrectal ultrasound guidance, probes outlining the area of desired destruction are placed transperineally. Because the energy remains between the leads, there should be no tissue destruction or damage beyond the outlined area.
Murray et al published a pilot study in 2016 of 35 patients with Gleason ≤7 prostate cancer treated with partial gland IRE.39 Because 8 patients were salvage after radiation therapy and 2 did not have 6-month follow-up data, there were 25 patients with functional and oncologic data. Median follow-up time was 10.9 months for these patients. Median ablation time was 14 minutes, and median total voltage delivered was 2,340 V/cm. Oncologically, there were 7 patients with positive biopsies, 4 of which occurred in the treatment area. Three of these patients went on to receive definitive surgical management. Quality-of-life outcomes did not change significantly during the study. No patient needed to take new medication for ED, and at 12 months, only 1 patient reported a decrease in urinary score from baseline.
More recently, van den Bos et al reported on 63 patients with high-volume Gleason 6 or any Gleason 7 prostate cancer who underwent partial gland IRE between 2013 and 2016.40 The majority (85%) of patients had either GG 2 or GG 3 disease. There were no high-grade adverse events. One patient required an indwelling catheter for management of urinary retention, and 24% of patients reported lower urinary tract symptoms. By 12 months, the quality-of-life questionnaire showed no difference from baseline in the urinary, bowel, or mental domains; there was a small decline in the sexual domain from baseline. The average reduction in PSA was 70%. Of the 45 patients who had follow-up biopsies, 34 had no significant cancer, 7 had significant in-field disease, and 4 had significant out-of-field disease. Of the 11 patients with positive biopsies, 4 were actively monitored, 4 underwent repeat IRE, 1 underwent RP, and 2 received radiotherapy.
A study of 19 patients treated with partial gland IRE in the UK followed up patients for 12 months. Of the 16 (84%) patients available for follow-up at 12 months, all had pad-free/leak-free continence, and only one patient reported new-onset ED. On follow-up biopsy, 11 (69%) men were disease-free, 1 had clinically insignificant disease, and 5 had clinically significant disease.41
IRE provides a side effect profile and adequate oncologic outcomes that are comparable to other PGA modalities. Recurrence rates appear to be 20–25%.39,40 Similar to other modalities, larger studies including only patients with clinically significant disease are needed.
4. Conclusion
Although prostate cancer is frequently multifocal, data suggest that ablation of the index lesion can lead to adequate cancer control. PGA targets the index lesion using either thermal or nonthermal energy and is emerging as a viable technique in the treatment of localized prostate cancer. In this review, we have demonstrated that most PGA procedures are well tolerated with significantly fewer side effects, particularly pertaining to erectile function and urinary continence, than whole gland therapy. We did not see durable differences in functional outcomes between thermal and nonthermal PGA.
In order for PGA to become more widely used, long-term oncologic outcomes of patients with clinically significant disease are needed. In addition, a clearer definition of how to monitor these patients after treatment with regard to both PSA and MRI surveillance must be clarified. Clarity must also be given regarding the need for posttreatment biopsy and oncologic outcomes reported for both in-field and out-of-field recurrences. Many studies with longer term follow-up include patients with GG 1 disease who would be recommended for AS today. As the cohorts that only include patients with clinically significant disease mature, we will be able to compare the long-term outcomes of PGA to established radical therapies; delay in radical treatment appears to be a good surrogate for efficacy. Should oncologic outcomes remain comparable, PGA will become a standard-of-care option for a subset of patients with MRI-visible dominant lesions who wish to avoid the side effects of whole gland treatment.
Funding
None.
Contributions
All authors contributed to researching data for article. Jonathan Fainberg, Bashir Al Hussein Al Awamleh, Gregory T. Chesnut, Jonathan A. Coleman, Taehyoung Lee, and Behfar Ehdaie made substantial contribution to discussion of content and reviewing and editing the article. Jonathan Fainberg, Bashir Al Hussein Al Awamleh, and Behfar Ehdaie contributed to writing the article.
Conflict of interest
There are no conflicts of interest from the authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2021.04.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Valerio M., et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014;66:732–751. doi: 10.1016/j.eururo.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W., et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algaba F., Montironi R. Impact of prostate cancer multifocality on its biology and treatment. J Endourol. 2010;24:799–804. doi: 10.1089/end.2009.0462. [DOI] [PubMed] [Google Scholar]
- 4.Futterer J.J., et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol. 2015;68:1045–1053. doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Valerio M., et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;71:17–34. doi: 10.1016/j.eururo.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Chaussy C.G., Thuroff S. High-intensity focused ultrasound for the treatment of prostate cancer: a review. J Endourol. 2017;31:S30–S37. doi: 10.1089/end.2016.0548. [DOI] [PubMed] [Google Scholar]
- 7.Guillaumier S., et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74:422–429. doi: 10.1016/j.eururo.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Amico A.V., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 9.Hanna M., et al. A single centre experience in treating localised prostate cancer with focal HIFU ablation over 8 years. J Clin Urol. 2018;11:61. [Google Scholar]
- 10.Rischmann P., et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71:267–273. doi: 10.1016/j.eururo.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Mistry K., Reddy U., Bott S., Emara A., Hindley R. Medium term outcomes following focal HIFU for the treatment of localised prostate cancer: a single centre experience. J Urol. 2017;197:e939. [Google Scholar]
- 12.Branchi A., et al. Focal therapy of localized prostate cancer with "fusion" integrated path and high-intensity focused ultrasound (HIFU): initial experience. Anticancer Res. 2017;37:2139. [Google Scholar]
- 13.Feijoo E.R., et al. Focal high-intensity focused ultrasound targeted hemiablation for unilateral prostate cancer: a prospective evaluation of oncologic and functional outcomes. Eur Urol. 2016;69:214–220. doi: 10.1016/j.eururo.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Ganzer R., et al. Prospective multicenter phase ii study on focal therapy (hemiablation) of the prostate with high intensity focused ultrasound. J Urol. 2018;199:983–989. doi: 10.1016/j.juro.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Ghai S., et al. Real-time MRI-guided focused ultrasound for focal therapy of locally confined low-risk prostate cancer: feasibility and preliminary outcomes. AJR Am J Roentgenol. 2015;205:W177–W184. doi: 10.2214/AJR.14.13098. [DOI] [PubMed] [Google Scholar]
- 16.Haddad N., Anidjar M., Loutochin O., Bladou F. Focal high-intensity focused ultrasound for localized prostate cancer: a single centre experience. CUAJ Can Urol Assoc J. 2016;10:S73. [Google Scholar]
- 17.Hoquetis L., et al. MRI evaluation following partial HIFU therapy for localized prostate cancer: a single-center study. Prog Urol. 2016;26:517–523. doi: 10.1016/j.purol.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Tay K.J., et al. Focal therapy for prostate cancer with in-bore MR-guided focused ultrasound: two-year follow-up of a phase I trial-complications and functional outcomes. Radiology. 2017;285:620–628. doi: 10.1148/radiol.2017161650. [DOI] [PubMed] [Google Scholar]
- 19.Yuh B., Liu A., Beatty R., Jung A., Wong J.Y.C. Focal therapy using magnetic resonance image-guided focused ultrasound in patients with localized prostate cancer. J Ther Ultrasound. 2016;4:8. doi: 10.1186/s40349-016-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albisinni S., et al. Comparing high-intensity focal ultrasound hemiablation to robotic radical prostatectomy in the management of unilateral prostate cancer: a matched-pair analysis. J Endourol. 2017;31:14–19. doi: 10.1089/end.2016.0702. [DOI] [PubMed] [Google Scholar]
- 21.Wong W.S., et al. Cryosurgery as a treatment for prostate carcinoma. Cancer. 1997;79:963–974. [PubMed] [Google Scholar]
- 22.Onik G., Narayan P., Vaughan D., Dineen M., Brunelle R. Focal "nerve-sparing" cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology. 2002;60:109–114. doi: 10.1016/s0090-4295(02)01643-6. [DOI] [PubMed] [Google Scholar]
- 23.Bianco F., et al. Office-based MRI/US fusion target prostate cancer cryoablation under local anaesthesia: 301 patients. Eur Urol. 2018;17 [Google Scholar]
- 24.Shah T.T., et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol. 2019 doi: 10.1016/j.eururo.2018.1012.1030. [DOI] [PubMed] [Google Scholar]
- 25.Barret E., et al. Oncological outcomes of focal cryoablation in localized prostate cancer. Eur Urol. 2018;17 [Google Scholar]
- 26.Feller J., Greenwood B., Jones W., Toth R. Transrectally delivered, outpatient MRI-guided laser focal therapy of prostate cancer: seven year interim results of NCT #02243033. J Urol. 2018;199:e374–e375. [Google Scholar]
- 27.Elkhoury F., et al. MRI-guided biopsy following focal laser ablation of prostate cancer: subsequence outcomes of 2 clinical trials. J Urol. 2018;199:e375. [Google Scholar]
- 28.Lindner U., Davidson S.R., Fleshner N.E., Finelli A., Zlotta A.R. Initial results of MR-guided laser focal therapy for prostate cancer. J Urol. 2013;189:e227–e228. [Google Scholar]
- 29.Lepor H., Llukani E., Sperling D., Futterer J.J. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol. 2015;68:924–926. doi: 10.1016/j.eururo.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Mehralivand S., George A., Hoang A., Rais-Bahrami S., Rastinehad A. Magnetic resonance imaging guided focal laser therapy of prostate cancer: follow up results from a single center phase I trial. J Urol. 2018;199:e339–e340. [Google Scholar]
- 31.Taneja S.S., Press B., Huang R., Deng F.M., Rosenkrantz A. Interim follow up of a phase II trial of MRI-ultrasound fusion biopsy guided prostate cancer (PCA) focal therapy by bipolar radiofrequency ablation. J Urol. 2018;199:e376. [Google Scholar]
- 32.Orczyk C., et al. Prostate radiofrequency ablation focal treatment (PRORAFT): results of a prospective development study for localised prostate cancer. J Urol. 2018;199:e376. doi: 10.1097/JU.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 33.Rubinsky B. Irreversible electroporation in medicine. Technol Canc Res Treat. 2007;6:255–259. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 34.King M.T., et al. Long-term outcomes of magnetic resonance image-guided partial prostate brachytherapy for favorable-risk prostate cancer. J Clin Oncol. 2018;36:138. [Google Scholar]
- 35.Cosset J.M., et al. Focal brachytherapy for selected low-risk prostate cancers: a pilot study. Brachytherapy. 2013;12:331–337. doi: 10.1016/j.brachy.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Saito K., et al. Focal therapy for prostate cancer using I125seed implantation: hemiablative brachytherapy for patients selected using extended biopsy and MRI. Eur Urol. 2013;12:e578. [Google Scholar]
- 37.Azzouzi A.R., et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017;18:181–191. doi: 10.1016/S1470-2045(16)30661-1. [DOI] [PubMed] [Google Scholar]
- 38.Gill I.S., et al. Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: extended followup and analyses of effectiveness. J Urol. 2018;200:786–793. doi: 10.1016/j.juro.2018.05.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray K.S., et al. Pilot study to assess safety and clinical outcomes of irreversible electroporation for partial gland ablation in men with prostate cancer. J Urol. 2016;196:883–890. doi: 10.1016/j.juro.2016.02.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Bos W., et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018;121:716–724. doi: 10.1111/bju.13983. [DOI] [PubMed] [Google Scholar]
- 41.Valerio M., et al. Nanoknife electroporation ablation trial: a prospective development study investigating focal irreversible electroporation for localized prostate cancer. J Urol. 2017;197:647–654. doi: 10.1016/j.juro.2016.09.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.