Abstract
Background
To identify the real high-risk group among Japanese de novo metastatic prostate cancer patients who fit CHAARTED or LATITUDE criteria.
Methods
We retrospectively studied patients who fitted CHAARTED (292 patients) and LATITUDE (294 patients) criteria from Japanese multi-institutions. All patients received androgen deprivation therapy with bicalutamide as an initial treatment. Factors related to overall survival (OS) and progression-free survival were statistically analyzed.
Results
The median OS was 55.5 months and 60.0 months in patients who met the CHAARTED and the LATITUDE criteria, respectively. In patients who met CHAARTED criteria, lactate dehydrogenase (LDH) (hazard ratio (HR) 2.63, P < 0.0001) and C-reactive protein (CRP) (HR 1.65, P = 0.042) were independent risk factors for OS. In patients who met the LATITUDE criteria, Gleason score (GS) ≥9 (HR 1.77, P = 0.0326) and LDH (HR 2.62, P < 0.0001) were independent risk factors for OS. Modified CHAARTED criteria by adding LDH and CRP showed a significant difference in OS (HR 2.55, P < 0.0001) with a comparative median OS (31.8 months) to placebo of CHAARTED trial (32.2 months). Modified LATITUDE criteria by adding GS ≥9 and LDH showed a significant difference in OS (HR 2.66, P < 0.0001) with a comparative median OS (32.7 months) to placebo of LATITUDE trial (34.7 months).
Conclusion
Modified criteria may potentially elucidate the true “high volume” and “high risk” patients in the Japanese cohort who require early intensive therapy.
Keywords: Androgen deprivation therapy, CHAARTED study, Lactate dehydrogenase, LATITUDE study, Metastatic castration naïve prostate cancer
1. Introduction
With the widespread use of prostate-specific antigen (PSA) screening, more and more patients are being found with early prostate cancer; however, there are still many cases of de novo metastatic prostate cancer at the time of diagnosis. Unlike early-stage prostate cancer, metastatic prostate cancer, which often responds to androgen deprivation therapy (ADT) at first, will eventually lead to lethal castration-resistant prostate cancer (CRPC).1,2 Recently, although there are some treatment options for CRPC, including new antiandrogen agents, therapeutic resistance finally develops in the majority of patients, resulting in lethal disease within several years.3, 4, 5
In the current era of precision medicine, genomic or molecular markers start to play a role in working out the treatment strategy of advanced prostate cancer.6 It has been reported that androgen receptor-axis-targeted (ARAT) agents are less effective when ARv7 is expressed.7 DNA repair gene mutations and PTEN loss are linked to the favorable response for poly ADP ribose polymerase (PARP) and serine/threonine kinase Akt inhibitors, respectively.8,9 However, those genome-based treatments are not applicable in the upfront treatment of high-risk prostate cancer. Therefore, it is important how to stratify risk by clinical factors.
One of the newer treatment strategies is the upfront treatment with ARAT or docetaxel in de novo metastatic castration naïve prostate cancer (mCNPC) patients. Previous phase III LATITUDE and CHAARTED clinical trials showed significant survival advantage of upfront abiraterone acetate or docetaxel treatment with ADT compared with ADT alone among “high risk” or “high volume” de novo mCNPC patients.10,11 However, a subanalysis of the LATITUDE study among Japanese patients showed no statistical survival advantage.12,13 In the study, when comparing the placebo arm, the Japanese subgroup seems to have better overall survival (OS) rate than the overall population’s survival rate.12 This suggests that there is a wide range of prognoses among the high-risk groups selected in this study. Furthermore, the criteria for selecting upfront treatment should be carefully considered, especially among Asian patients.
In this study, we focused on “high risk” or “high volume” cases and investigated additional prognostic factors in Asian patients.
2. Materials and methods
2.1. Patients and clinical variables
We retrospectively enrolled 318 de novo mCNPC patients at Chiba University Hospital and affiliated institutions between 1999 and 2017. All patients were diagnosed with prostatic adenocarcinoma by the pathological examination of needle biopsy specimens and underwent computed tomography (CT) and 99mtechnetium-methylene-diphosphate (99mTc-MDP) bone scintigraphy. Radiologists determined the TNM stage according to International Cancer Control classifications. All patients received combined ADT and bicalutamide as initial treatment. Blood tests and computed tomography were undergone in all cases, and radiologists determined the TNM stage according to the Union for International Cancer Control classification.
The following variables were analyzed at diagnosis: age, PSA, Gleason score (GS), haemoglobin (Hb), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), clinical T stage, lymph node metastasis, and extent of disease (EOD) score. EOD score was used as a classification of the number of bone metastasis in five stages on a bone scan as follows: 0, none; 1, one to five bone metastases; 2, six to twenty bone metastases; 3, more than twenty bone metastases, but not “super scan,” and 4, “super scan.”14 We analyzed progression-free survival (PFS) and OS. PFS was defined as progression of disease based on investigator assessment of either an imaged-based or clinical symptomatic progression or an increase of PSA despite castrate levels of serum testosterone (<50 ng/dl). The definition of these progressions was referred to Prostate Cancer Working Group 2 (PCWG2) criteria.
2.2. Definition of risk classification
In the present study, we used high-volume criteria of the CHAARTED trial or the high-risk criteria of the LATITUDE trial. The high-volume criteria of the CHAARTED trial meet the presence of visceral metastases or four or more bone lesions with at least one beyond the vertebral bodies and pelvis.11 The high-risk criteria of the LATITUDE trial meet at least two of the three factors: Gleason score of 8 or more, at least three bone lesions, and the presence of measurable visceral metastasis.10
2.3. Compliance with ethical standards
The current study was approved by the institutional review board (approval number 3504). All patients agreed with participation in the study.
2.4. Statistical analysis
The Mann-Whitney U test and χ2 test were used for comparative analyses between two groups. Kaplan-Meier methods (log-rank test) and Cox proportional hazard models were used to analyze clinical outcomes and prognostic factors. Multivariate analysis was performed with those clinical factors showing statistical significance in univariate analyses. All statistical analyses were performed using JMP version 13.0.0 software (SAS Institute, Cary, NC). Values of P < .05 were considered statistically significant in this study.
3. Results
3.1. Patient characteristics
The characteristics of 318 patients enrolled in the present study are shown in Table 1. The median observation period was 35.1 months. The median (range), age at diagnosis, initial PSA, Hb, LDH, ALP, NLR and CRP were 73 years (46–93 years), 450.3 ng/mL (2.3–24913.3 ng/mL), 13.0 g/dl (5.5–17.0 g/dl), 208 U/l (126–3896 U/l), 408 U/l (102–21417 U/l), 2.6 (0.73–10.34) and 0.2 mg/dl (0.01–24.9 mg/dl), respectively. Two hundred and four patients (64.2%) were diagnosed with GS 9 or higher. Visceral metastases were identified in 59 patients (18.6%). As the location of distant metastases, 259 patients (81.4%) had bone metastases, 6 patients (1.9%) had liver metastases and 53 patients (16.7%) had lung metastases.
Table 1.
Characteristics of patients
| Characteristics | |
|---|---|
| No. patients | 318 |
| Median age at diagnosis (range) (years) | 73 (46–93) |
| Median initial PSA (range) (ng/ml) | 450.3 (2.3–24913.3) |
| Gleason score sum, n (%) | |
| ≤7 | 13 (4.1) |
| 8 | 75 (23.6) |
| ≥9 | 204 (64.2) |
| undiagnosed | 26 (8.2) |
| T stage, n (%) | |
| ≤2c | 76 (23.9) |
| ≥3a | 242 (76.1) |
| N stage, n (%) | |
| positive | 207 (65.1) |
| M stage, n (%) | |
| 1b/1c | 259 (81.4)/59 (18.6) |
| EOD score, n (%) | |
| 0 | 14 (4.5) |
| 1 | 59 (18.9) |
| 2 | 92 (29.4) |
| 3 | 121 (38.7) |
| 4 | 27 (8.6) |
| location of visceral metastasis, n (%) | |
| liver | 6 (1.9) |
| lung | 53 (16.7) |
| Median baseline blood examination | |
| Hb (range) (g/dl) | 13.0 (5.5–17.0) |
| LDH (range) (U/l) | 208 (126–3896) |
| ALP (range) (U/l) | 408 (102–21417) |
| NLR (range) | 2.6 (0.73–10.34) |
| CRP (range) (mg/dl) | 0.2 (0.01–24.9) |
| CHAARTED high volume, n (%)a | 294 (92.5) |
| LATITUDE high risk, n (%)b | 292 (91.8) |
| Median progression-free survival (months) | 12.7 |
| Median overall survival (months) | 59.2 |
| Progression-free survival rate (%) | 3-yr: 23.3% |
| 5-yr: 14.1% | |
| Overall survival rate (%) | 3-yr: 69.4% |
| 5-yr: 49.2% | |
ALP, alkaline phosphatase; CRP, C-reactive protein; EOD, extent of disease; Hb, haemoglobin; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PSA, prostate-specific antigen.
Defined as the presence of visceral metastases or 4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.
Defined as the presence of two of the three following factors: GS ≥8, ≥3 bone lesions, measurable visceral metastasis.
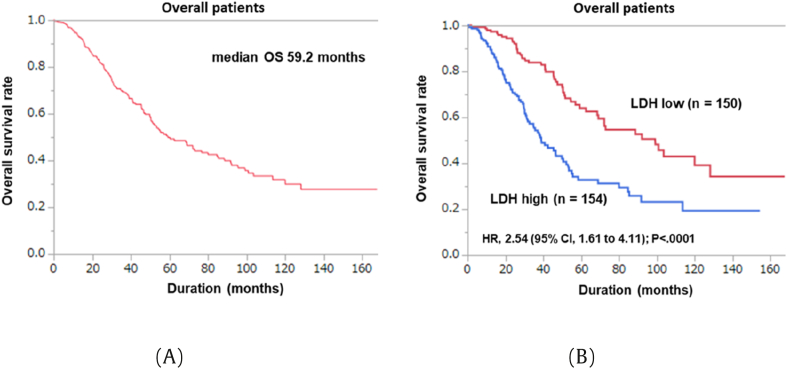
The number (percentage) of patients who fitted for the criteria of CHAARTED (high volume) or LATITUDE (high risk) was 294 (92.5%) and 292 (91.8%), respectively. Median PFS and OS were 12.7 and 59.2 months, respectively (Fig. 1A).
Fig. 1.
Kaplan-Meier analysis of the overall survival rates: (A) Overall; (B) LDH high and low groups.
3.2. Univariate and multivariate analyses of overall survival in our cohort
The clinical factors associated with OS are listed in Table 2. On univariate Cox regression analysis, age (≥73 years) (hazard ratio (HR) 1.63, P = 0.0038), high GS (≥9) (HR 1.69, P = 0.0068), T stage (≥T3b) (HR 1.68, P = 0.0121), positive lymph node (LN) metastasis (HR 2.07, P = 0.0001), low Hb (<13 g/dl) (HR 1.60, P = 0.0062), high LDH (≥208 U/l) (HR 2.43, P < 0.0001), high ALP (≥408 U/l) (HR 1.76, P = 0.0011), and high CRP (≥0.2 mg/dl) (HR 1.88, P = 0.0030) were associated with poor OS. On multivariate analysis, high LDH (≥208 U/l) (HR 2.54, P < 0.0001) was an independent factor to predict poor OS (Fig. 1B).
Table 2.
Univariable and multivariable cox proportional hazard regression models for overall survival
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (≥73) | 1.63 | 1.17–2.29 | 0.0038 | 1.39 | 0.88–2.22 | 0.1574 |
| initial PSA (≥450) | 0.98 | 0.70–1.36 | 0.8942 | |||
| GS ≥ 9 | 1.69 | 1.15–2.55 | 0.0068 | 1.62 | 0.98–2.75 | 0.0587 |
| T stage (≥T3b) | 1.68 | 1.11–2.62 | 0.0121 | 0.99 | 0.58–1.79 | 0.9800 |
| N stage (positive) | 2.07 | 1.42–3.09 | 0.0001 | 1.61 | 0.97–2.76 | 0.0643 |
| Hb (<13) | 1.60 | 1.14–2.25 | 0.0062 | 1.14 | 0.71–1.85 | 0.5915 |
| LDH (≥ 208) | 2.43 | 1.72–3.47 | < 0.0001 | 2.54 | 1.61–4.11 | < 0.0001 |
| ALP (≥408) | 1.76 | 1.25–2.49 | 0.0011 | 1.46 | 0.91–2.36 | 0.1145 |
| NLR (≥2.6) | 0.85 | 0.60–1.20 | 0.3521 | |||
| CRP (≥0.2) | 1.88 | 1.24–2.91 | 0.0030 | 1.41 | 0.87–2.32 | 0.1659 |
ALP, alkaline phosphatase; CI, confidence interval; CRP, C-reactive protein; GS, Gleason score sum; Hb, haemoglobin; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PSA, prostate-specific antigen.
3.3. Modified CHAARTED criteria
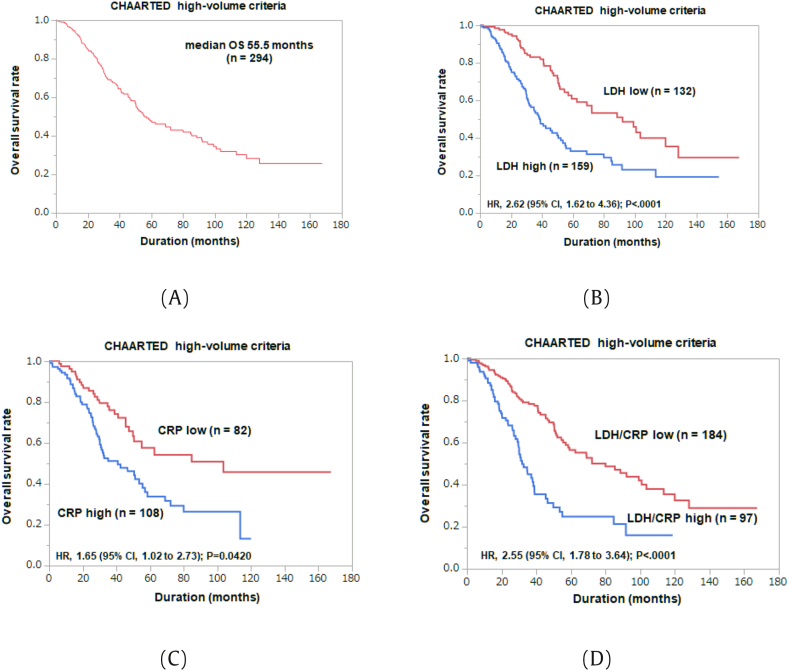
Next, we identified and evaluated 294 patients who met “high volume” criteria in CHAARTED study (Table 3). Median OS of this extracted cohort was 55.5 months (Fig. 2A). On univariate Cox regression analysis, age (≥73 years) (HR 1.79, P = 0.0009), high GS (≥9) (HR 1.66, P = 0.0127), T stage (≥T3b) (HR 1.75, P = 0.0137), positive LN metastasis (HR 2.00, P = 0.0004), high LDH (≥205 U/l) (HR 2.31, P < 0.0001), high ALP (≥352 U/l) (HR 1.61, P = 0.0118), and high CRP (≥0.19 mg/dl) (HR 2.06, P = 0.0013) were associated with poor OS. On multivariate analysis, high LDH (≥205 U/l) (HR 2.63, P < 0.0001) and high CRP (≥0.19 mg/dl) (HR 1.65, P = 0.0420) were independent predictors of poor OS (Fig. 2B and C).
Table 3.
Univariable and multivariable cox proportional hazard regression models for overall survival in CHAARTED high-volume group
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (≥73) | 1.79 | 1.27–2.54 | 0.0009 | 1.55 | 0.98–2.51 | 0.0636 |
| initial PSA (≥485) | 0.92 | 0.65–1.29 | 0.6248 | - | - | - |
| GS ≥ 9 | 1.66 | 1.11–2.54 | 0.0127 | 1.47 | 0.87–2.59 | 0.1499 |
| T stage (≥T3b) | 1.75 | 1.12–2.90 | 0.0137 | 1.14 | 0.63–2.21 | 0.6755 |
| N stage (positive) | 2.00 | 1.35–3.04 | 0.0004 | 1.66 | 0.99–2.90 | 0.0555 |
| Hb (<13.3) | 1.4 | 0.99–2.01 | 0.0575 | - | - | - |
| LDH (≥ 205) | 2.31 | 1.61–3.35 | < 0.0001 | 2.63 | 1.62–4.36 | < 0.0001 |
| ALP (≥352) | 1.61 | 1.11–2.39 | 0.0118 | 1.52 | 0.93–2.54 | 0.1003 |
| NLR (≥2.6) | 0.77 | 0.54–1.11 | 0.1641 | - | - | - |
| CRP (≥ 0.19) | 2.06 | 1.32–3.29 | 0.0013 | 1.65 | 1.02–2.73 | 0.0420 |
ALP, alkaline phosphatase; CI, confidence interval; CRP, C-reactive protein; GS, Gleason score sum; Hb, haemoglobin; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PSA, prostate-specific antigen.
Fig. 2.
Kaplan-Meier analysis of the overall survival rates for patients fitted for CHAARTED high-volume criteria: (A) Overall; (B) LDH high and low groups; (C) CRP high and low groups; (D) LDH/CRP high and low groups.
We added LDH ≥205 U/l and CRP ≥0.19 mg/dl as risk factors to CHAARTED criteria and re-evaluated the prognosis of the modified high-risk group. Median OS in modified high-risk group (n = 97) was 31.8 months, compared with 80 months in low-risk group (n = 184) (HR 2.55, P < 0.0001) (Fig. 2D).
3.4. Modified LATITUDE criteria
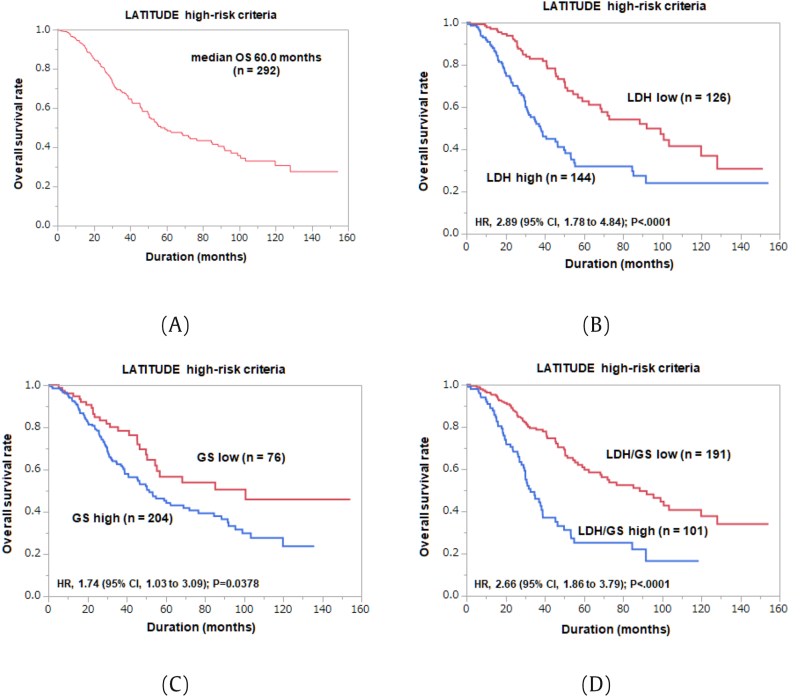
Similarly, we identified and evaluated 292 patients who met “high risk” criteria in LATITUDE study (Table 4). Median OS of this extracted cohort was 60.0 months (Fig. 3A). On univariate Cox regression analysis, age (≥73 years) (HR 1.57, P = 0.0116), high GS (≥9) (HR 1.70, P = 0.0103), positive LN metastasis (HR 1.91, P = 0.0010), low Hb (<13.0 g/dl) (HR 1.56, P = 0.0132), high LDH (≥208 U/l) (HR 2.37, P < 0.0001), high ALP (≥405 U/l) (HR 1.78, P = 0.0014), and high CRP (≥0.20 mg/dl) (HR 1.84, P = 0.0065) were associated with poor OS. On multivariate analysis, high GS (≥9) (HR 1.77, P = 0.0326) and high LDH (≥208) (HR 2.62, P < 0.0001) were independent predictors of poor OS (Fig. 3B and C).
Table 4.
Univariable and multivariable cox proportional hazard regression models for overall survival in the LATITUDE high-risk group
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (≥73) | 1.57 | 1.11–2.23 | 0.0116 | 1.37 | 0.86–2.21 | 0.1849 |
| initial PSA (≥447) | 0.88 | 0.62–1.24 | 0.4506 | - | - | - |
| GS ≥9 | 1.70 | 1.13–2.66 | 0.0103 | 1.77 | 1.05–3.16 | 0.0326 |
| T stage (≥T3b) | 1.46 | 0.97–2.29 | 0.0729 | - | - | - |
| N stage (positive) | 1.91 | 1.29–2.91 | 0.0010 | 1.61 | 0.97–2.79 | 0.0655 |
| Hb (<13.0) | 1.56 | 1.10–2.23 | 0.0132 | 1.10 | 0.66–1.81 | 0.7224 |
| LDH (≥ 208) | 2.37 | 1.65–3.43 | < 0.0001 | 2.62 | 1.62–4.31 | < 0.0001 |
| ALP (≥405) | 1.78 | 1.25–2.57 | 0.0014 | 1.41 | 0.88–2.31 | 0.1578 |
| NLR (≥2.6) | 0.84 | 0.59–1.22 | 0.3615 | - | - | - |
| CRP (≥0.20) | 1.84 | 1.18–2.89 | 0.0065 | 1.41 | 0.84–2.36 | 0.1909 |
ALP, alkaline phosphatase; CI, confidence interval; CRP, C-reactive protein; GS, Gleason score sum; Hb, haemoglobin; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PSA, prostate-specific antigen.
Fig. 3.
Kaplan-Meier analysis of the overall survival rates for patients fitted for LATITUDE high-risk criteria: (A) Overall; (B) LDH high and low groups; (C) GS high and low groups; (D) LDH/GS high and low groups.
We added high GS ≥9 and LDH ≥208 U/l as risk factors to LATITUDE criteria and re-evaluated the prognosis of the modified high-risk group. Median OS in the modified high-risk group (n = 101) was 32.7 months, compared with 88.6 months in the low-risk group (n = 191) (HR 2.66, P < 0.0001) (Fig. 3D).
3.5. Correlation between high/low LDH and other clinical factors
Table 5 shows the clinical characteristics associated with LDH value, and two groups (LDH low group vs. LDH high group) were compared. The LDH high group showed significantly higher age, alkaline phosphatase, and CRP (P = 0.0173, P = 0.0005, and P = 0.0007, respectively) and lower haemoglobin (Hb) (P < 0.0001). In addition, the LDH high group showed significantly higher rates of EOD score ≥3 (P < 0.0001). High CRP was related to high EOD score, high ALP, and low Hb among patients who met CHAARTED high-volume criteria (Table S1). GS ≥9 was related to high EOD score and positive LN metastasis among patients who met the LATITUDE high-risk criteria (Table S2).
Table 5.
Comparison of clinical factors between LDH low and LDH high groups.
| LDH low | LDH high | P value | |
|---|---|---|---|
| Age (years) | 72.0 (71.3) | 73.5 (73.6) | 0.0173∗ |
| Initial PSA (ng/mL) | 391 (1347.6) | 484.3 (1569.0) | 0.4788 |
| EOD score, n | <0.0001∗ | ||
| 0 | 4 | 8 | |
| 1 | 36 | 22 | |
| 2 | 52 | 36 | |
| 3 | 53 | 63 | |
| 4 | 3 | 24 | |
| Gleason score, n | 0.8681 | ||
| ≤7 | 7 | 6 | |
| 8 | 38 | 35 | |
| ≥9 | 95 | 99 | |
| T stage, n | 0.8589 | ||
| ≤2c | 9 | 10 | |
| ≥3a | 141 | 144 | |
| N stage (positive) (%) | 65.33 (98/150) | 63.6 (98/154) | 0.7572 |
| Hb (g/dl) | 13.4 (13.4) | 12.4 (12.0) | <0.0001∗ |
| ALP (U/l) | 355.0 (655.0) | 667.5 (1379.6) | 0.0005∗ |
| NLR (ng/mL) | 2.6 (3.0) | 2.6 (3.1) | 0.4648 |
| CRP (mg/dl) | 0.1 (0.8) | 0.6 (2.4) | 0.0007∗ |
| PFS (months) | 16.6 (25.7) | 9.8 (16.3) | 0.0008∗ |
| OS (months) | 44.3 (52.4) | 29.6 (37.5) | <0.0001∗ |
ALP, alkaline phosphatase; CRP, C-reactive protein; EOD, extent of disease; Hb, haemoglobin; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PSA, prostate-specific antigen.
4. Discussion
Since Asian metastatic prostate cancer patients are known to have a favorable prognosis compared to those of Caucasians, we tried to identify the factors that will elucidate the “true high risk” patients in Asia. In the present study, we evaluated the patients who met the high-volume or high-risk criteria used in the CHAARTED and LATITUDE trials among the Japanese cohort in a multi-institutional setting. We showed that LDH was the independent prognostic factor common in these patients. Furthermore, CRP and GS ≥9 were the independent prognostic factors for the patients who met CHAARTED and LATITUDE criteria, respectively. Based on the modified CHAARTED and LATITUDE criteria, we elucidate the high-risk patients who had an equivalent prognosis of the “global high risk” patients. Based on the modified criteria we may establish the treatment strategy of de novo metastatic prostate cancer patients.
In recent years, there has been a focus on the upfront treatment of mCNPC. Large clinical trials have consistently reported the benefit of upfront treatment at the time of castration naïve prostate cancer. In metastatic prostate cancer, the addition of six courses of docetaxel to initial ADT treatment was reported to affect OS significantly.11,15 In particular, the CHAARTED study showed a 17-month OS benefit only in the high-volume group, and abiraterone also showed an improvement in OS when used in combination with initial ADT.10,16 The LATITUDE trial defined a high-risk group, and patients with two or more of the three risk factors (visceral metastases, three or more bone metastases, and Gleason score 8 or higher) were studied and met the endpoints, including OS. Furthermore, the ARCHES, ENZAMET, and TITAN studies showed, regardless of risk criteria, significantly improved radiographic PFS or OS with enzalutamide or apalutamide combined with initial ADT.17, 18, 19
On the other hand, in the Japanese subgroup analysis of the LATITUDE study, no statistical significance was observed by upfront treatment with abiraterone among high-risk patients.12,13 By adopting the high-volume criteria in the CHAARTED study, the median OS was 55.5 months in our cohort compared to 34.4 months in the placebo group of CHAARTED global trial.16 By adopting the high-risk criteria in the LATITUDE study, the median OS was 60.0 months in our cohort compared to 36.5 months in the placebo group of the LATITUDE global trial.20 Since all the cohort received bicalutamide and LH-RH agonist/antagonist in our study, like a placebo in the clinical trial, the prognostic difference of over 20 months might represent the insufficiency of the high risk or high volume risk classification among Asians.
In order to discuss the reason for the prognostic difference between global and Japanese prostate cancer patients, the ethnic difference may need to be considered. Previous reports have shown that the Japanese are more sensitive to hormone therapy than other ethnic groups.21 Previous genomic analysis of Asian prostate cancer showed that genomic alterations were clearly different from Western cohorts, specifically more FOXA1 mutations, and conversely, less ETS fusion and PTEN loss.22,23 The difference in the altered genomic signature may be responsible for the difference in the response rate of the ADT. Since there are no large prospective studies specific to Asians, it will be ideal for conducting the clinical trial assessing the clinical utility of the upfront treatment in mCNPC.
The biology of prostate cancer is being studied worldwide, with potential clinical applications. For example, several studies showed that ARv7 targeted the enhancers of different genes from AR full length and that the presence of ARv7 expression was associated with therapeutic resistance of new ARAT agents.7,24 Recently, PARP inhibitor was approved by the FDA for mCRPC patients with HRR mutations, which are present in 20–30% of all patients.9 However, the use of genomic markers as a criterion for treatment strategy in clinical practice has been limited.
Regarding the clinical factors, the number of visceral and bone metastases has been reported to be prognostic.25,26 The serum marker, such as testosterone (TST) nadir, as well as PSA as a biochemical marker, were also prognostic.27 However, no consensus has been obtained regarding the risk classification. The high-volume and high-risk criteria of the CHAARTED and LATITUDE trials include the number of bone metastases. A previous study reported that less than 10 bone metastases in Japanese were suitable for prognosis.28 The presence of distant lymph nodes was also reported to be a prognostic factor in Japanese oligometastatic prostate cancer patients.29 Higher serum TST values have been reported to be more responsive to novel ARAT, whereas lower TST values to be more responsive to docetaxel.30,31
The present study showed that, by adding LDH to the conventional high-risk criteria, we could select the subgroup of our cohort with a similar prognosis to the high-risk group of both studies. It has recently been reported that LDH >250 U/I, EOD score 2–4/liver metastasis, and primary GS (5 or ≤4) are independent predictors of prognosis in Japanese mCNPC.32 LDH and age were reported to be prognostic factors in sequencing abiraterone and enzalutamide in Japanese CRPC.33 Unlike other solid tumors, the relationship between high LDH levels and the prognosis of prostate cancer has been controversial. However, meta-analysis has shown that high LDH correlates significantly with poor OS and PFS in metastatic prostate cancer, highlighting the importance of LDH in mCNPC.34
This study has several limitations. First, it is a retrospective analysis of a relatively small number of patients. Second, all patients in our cohort received 80 mg of bicalutamide treatment with initial ADT, which differs from the treatment of Europeans and Americans. Third, the evaluation of the number of bone metastases depended on the judgment of radiologists. The risk classification may be slightly different from the clinical trials conducted.
In conclusion, based on the modified CHAARTED and LATITUDE criteria, the equivalent OS was observed between global and Japanese patients. Those Japanese patients may fit for upfront docetaxel or abiraterone treatment.
Conflicts of interest
None declared.
Acknowledgment
We thank all participants in this study. The present work was supported by a grant from the “Grant-in-Aid for Scientific Research” Grant Numbers JP20K09555, JP20K18133.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2021.06.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Damodaran S., Kyriakopoulos C.E., Jarrard D.F. Newly diagnosed metastatic prostate cancer: has the paradigm changed? Urol Clin North Am. 2017;44(4):611–621. doi: 10.1016/j.ucl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James N.D., Spears M.R., Clarke N.W., Dearnaley D.P., De Bono J.S., Gale J., et al. Survival with newly diagnosed metastatic prostate cancer in the "docetaxel era": data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67(6):1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 3.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L., et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Yekeduz E., Utkan G., Kanesvaran R., Urun Y. Expanding armamentarium in advanced prostate cancer management: are all novel antiandrogens the same? Prostate Int. 2021;9(1):1–5. doi: 10.1016/j.prnil.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J.S., Morgan T.M., Hong S.K. Clinical implications of genomic evaluations for prostate cancer risk stratification, screening, and treatment: a narrative review. Prostate Int. 2020;8(3):99–106. doi: 10.1016/j.prnil.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C., et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono J.S., De Giorgi U., Rodrigues D.N., Massard C., Bracarda S., Font A., et al. Randomized phase ii study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. 2019;25(3):928–936. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]
- 9.Hussain M., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y., et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney C.J., Chen Y.H., Carducci M., Liu G., Jarrard D.F., Eisenberger M., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukasawa S., Suzuki H., Kawaguchi K., Noguchi H., Enjo K., Tran N., et al. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: a subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, Phase 3 study. Jpn J Clin Oncol. 2018;48(11):1012–1021. doi: 10.1093/jjco/hyy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H., Shin T., Fukasawa S., Hashine K., Kitani S., Ohtake N., et al. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: final subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, phase 3 study. Jpn J Clin Oncol. 2020;50(7):810–820. doi: 10.1093/jjco/hyaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soloway M.S., Hardeman S.W., Hickey D., Raymond J., Todd B., Soloway S., et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61(1):195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyriakopoulos C.E., Chen Y.H., Carducci M.A., Liu G., Jarrard D.F., Hahn N.M., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P., Holzbeierlein J., Villers A., Azad A., et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis I.D., Martin A.J., Stockler M.R., Begbie S., Chi K.N., Chowdhury S., et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 19.Chi K.N., Agarwal N., Bjartell A., Chung B.H., Pereira de Santana Gomes A.J., Given R., et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 20.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y., et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 21.Fukagai T., Namiki T.S., Carlile R.G., Yoshida H., Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97(6):1190–1193. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Xu C., Lee H.J., Ren S., Zi X., Zhang Z., et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature. 2020;580(7801):93–99. doi: 10.1038/s41586-020-2135-x. [DOI] [PubMed] [Google Scholar]
- 23.Conti D.V., Darst B.F., Moss L.C., Saunders E.J., Sheng X., Chou A., et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021 doi: 10.1038/s41588-020-00748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiura M., Sato H., Okabe A., Fukuyo M., Mano Y., Shinohara K.I., et al. Identification of AR-V7 downstream genes commonly targeted by AR/AR-V7 and specifically targeted by AR-V7 in castration resistant prostate cancer. Transl Oncol. 2020;14(1):100915. doi: 10.1016/j.tranon.2020.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandaglia G., Karakiewicz P.I., Briganti A., Passoni N.M., Schiffmann J., Trudeau V., et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68(2):325–334. doi: 10.1016/j.eururo.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Halabi S., Kelly W.K., Ma H., Zhou H., Solomon N.C., Fizazi K., et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamada S., Sakamoto S., Ando K., Muroi A., Fuse M., Kawamura K., et al. Nadir testosterone after long-term followup predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol. 2015;194(5):1264–1270. doi: 10.1016/j.juro.2015.03.120. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y., Sakamoto S., Rii J., Yamamoto S., Kamada S., Imamura Y., et al. How many bone metastases may be defined as high-volume metastatic prostate cancer in Asians: a retrospective multicenter cohort study. Prostate. 2020;80(5):432–440. doi: 10.1002/pros.23958. [DOI] [PubMed] [Google Scholar]
- 29.Rii J., Sakamoto S., Yamada Y., Takeshita N., Yamamoto S., Sazuka T., et al. Prognostic factors influencing overall survival in de novo oligometastatic prostate cancer patients. Prostate. 2020;80(11):850–858. doi: 10.1002/pros.24016. [DOI] [PubMed] [Google Scholar]
- 30.Ando K., Sakamoto S., Takeshita N., Fujimoto A., Maimaiti M., Saito S., et al. Higher serum testosterone levels predict poor prognosis in castration-resistant prostate cancer patients treated with docetaxel. Prostate. 2020;80(3):247–255. doi: 10.1002/pros.23938. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto S., Maimaiti M., Xu M., Kamada S., Yamada Y., Kitoh H., et al. Higher serum testosterone levels associated with favorable prognosis in enzalutamide- and abiraterone-treated castration-resistant prostate cancer. J Clin Med. 2019;8(4) doi: 10.3390/jcm8040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akamatsu S., Kubota M., Uozumi R., Narita S., Takahashi M., Mitsuzuka K., et al. Development and validation of a novel prognostic model for predicting overall survival in treatment-naive castration-sensitive metastatic prostate cancer. Eur Urol Oncol. 2019;2(3):320–328. doi: 10.1016/j.euo.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Mori K., Kimura T., Onuma H., Kimura S., Yamamoto T., Sasaki H., et al. Lactate dehydrogenase predicts combined progression-free survival after sequential therapy with abiraterone and enzalutamide for patients with castration-resistant prostate cancer. Prostate. 2017;77(10):1144–1150. doi: 10.1002/pros.23373. [DOI] [PubMed] [Google Scholar]
- 34.Li F., Xiang H., Pang Z., Chen Z., Dai J., Chen S., et al. Association between lactate dehydrogenase levels and oncologic outcomes in metastatic prostate cancer: a meta-analysis. Cancer Med. 2020 doi: 10.1002/cam4.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.