Abstract
Background
With the advancement of the world population aging, more attention should be paid to the prognosis of elderly patients with acute coronary syndrome (ACS). Triglyceride-glucose (TyG) index is a reliable indicator of insulin resistance (IR) and is closely related to traditional risk factors of cardiovascular disease (CVD). However, the effect of TyG index on the prognosis of long-term adverse events in elderly ACS patients has not been reported. This study evaluated the prognostic power of TyG index in predicting adverse events in elderly ACS patients.
Methods
In this study, 662 ACS patients > 80 years old who were hospitalized from January 2006 to December 2012 were enrolled consecutively and the general clinical data and baseline blood biochemical indicators were collected. The follow-up time after discharge was 40–120 months (median, 63 months; interquartile range, 51‒74 months). In addition, the following formula was used to calculate the TyG index: Ln [fasting TG (mg/dL) × FBG (mg/dL)/2], and patients were divided into three groups according to the tertile of the TyG index.
Results
The mean age of the subjects was 81.87 ± 2.14 years, the proportion of females was 28.10%, and the mean TyG index was 8.76 ± 0.72. The TyG index was closely associated with the traditional risk factors of CVD. In the fully-adjusted Cox regression model, the Hazard ratio (95% CI) of all-cause mortality (in tertile 3) was 1.64 (1.06, 2.54) and major adverse cardiac event (MACE) (in tertile 3) was 1.36 (1.05, 1.95) for each SD increase in the TyG index. The subgroup analyses also confirmed the significant association of the TyG index and long-term prognosis.
Conclusion
The TyG index is an independent predictor of long-term all-cause mortality and MACE in elderly ACS patients.
Keywords: Triglyceride glucose index, Elderly patient, Acute coronary syndrome, Insulin resistance, Prognosis
Background
Coronary heart disease (CHD) is the number one killer threatening human health, and the incidence increases significantly with age [1]. With the aggravation of population aging, the proportion of elderly patients hospitalized for acute coronary syndrome (ACS) is increasing, and studies showed that more than 30% of patients are over 75 years old [2, 3]. The risk of bleeding and acute myocardial infarction post-percutaneous coronary intervention (re-MI) at 1-year follow-up is more than doubled in elderly patients [4]. Advanced aging has a profound impact on the health of the elderly. With the increase of age, changes in cardiovascular structure and function accelerate the progression of CHD, and elderly patients have more severe lesions [5]. Insulin resistance (IR) is the main pathogenesis of diabetes mellitus (DM) and is a common marker of systemic inflammatory response and metabolic disorders, which is closely related to the progression of coronary atherosclerosis [6–8]. However, IR was not a traditional risk factor of CHD [9]. Multiple studies have shown that the TyG index is associated with multiple risk factors of CHD, such as hypertension, diabetes, obesity and metabolic syndrome, and it also can predict the prognosis of patients with CHD and in-stent restenosis (ISR) [10–15]. However, among the clinical studies on the prognosis of ACS patients, there are few studies on the predictive value of the TyG index, especially with regard to elderly people (who have not been clearly reported), and the pathophysiological mechanism has not been clarified. In this study, we analyzed the TyG index after admission and the incidence of main cardiovascular adverse events within 10 years after discharge of elderly ACS patients, aiming to investigate whether the TyG index is an independent risk factor for the long-term prognosis of elderly ACS patients.
Methods
Study population
A total of 720 patients aged 80 years and above who were hospitalized for coronary angiography due to ACS symptoms in the Cardiology Department of Chinese People’s Liberation Army (PLA) General Hospital from January 2006 to December 2012 were enrolled. Of these patients, 699 provided informed consent and were included in the study. During the follow-up period, 37 were lost to follow up, and 662 were finally available for our statistical analysis. The follow-up rate was 94.7%. The main exclusion criteria were as follows: (1) patients with severe valvular heart disease, pulmonary hypertension, severe liver insufficiency, rheumatoid arthritis, malignant tumors, infectious diseases, or body mass index (BMI) ≥ 45 kg/m2; (2) patients with familial hypertriglyceridemia (triglyceride ≥ 5.65 mmol/L); and (3) patients with neuropsychiatric disorders that prevented them from cooperating with the researcher. Most importantly, our study was performed in line with the Declaration of Helsinki and was approved by Ethics Service Center of Chinese PLA General Hospital, and all patients signed the informed consent.
Intervention and management
Coronary intervention and periprocedural management were performed according to current guidelines in the Cardiac Interventional Center of PLA General Hospital to confirm the diagnosis of CHD, and all angiography results were analyzed using the same image analysis software. Loading doses of aspirin (300 mg) and clopidogrel (300 mg) were given before the intervention. The severity of coronary artery stenosis was recorded by Gensini score, and the experimental data recorders passed uniform professional training. According to the results of coronary angiography, patients were given individualized interventions, including intensive treatment with medicine, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), and long-term follow-up observation was conducted after discharge.
Data collection and definitions
We recorded general information (age, gender, BMI, heart rate, blood pressure, left ventricular ejection fraction (LVEF), and Gensini score), cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, previous myocardial infarction (MI), previous stroke, chronic kidney injury (CKD) and current smoking), fasting blood biochemical indicators at 6 am the next day after admission (total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), the estimated glomerular filtration rate (eGFR), fasting blood glucose (FBG), uric acid (UA), calculated TyG index), cardiovascular medication experience (aspirin, clopidogrel, statins, β-blockers, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB)), culprit artery (left anterior descending artery (LAD), left circumflex artery (LCX), left main coronary artery (LM), right coronary artery (RCA), and multivessel lesion), and treatment strategies (intensive treatment with medicine, PCI and CABG).
The TyG index was calculated by the following formula: Ln [fasting TG (mg/dL) × FBG (mg/dL)/2] [16]. The diagnosis of T2DM included: (1) FPG ≥ 7.0 mmol/L, and/or random blood glucose (RBG) ≥ 11.1 mmol/L, and/or 2 h plasma glucose after oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L [17]. Hyperlipidemia was defined as: ICD-10 code E78 with lipid-lowering agents or serum total cholesterol ≥ 240 mg/dL [16].
The estimated glomerular filtration rate (eGFR) was calculated by the Chinese modified Modification of Diet in Renal Disease equation [18]:
Standardized creatinine (Scr) was calculated by the calibration equation [19]:
Chronic Kidney Disease was defined as eGFR < 60 mL/min/1.73 m2.
Based on coronary angiography results, the multivessel lesion was defined as the number of culprit vessels with significantly diameter stenosis ≥ 50% was more than 2.
Endpoints and follow-up
A clinical follow-up was performed once every 12 months after discharge via family interview, telephone record or medical records in the event of outcomes, and the follow-up period lasted up to 10 years. The main outcomes of our study were the occurrence of major adverse cardiac event (MACE), including nonfatal AMI, coronary artery revascularization (PCI or CABG) and all-cause mortality (cardiac or non-cardiac mortalities).
Statistical analysis
Distributions of characteristics of the participants were illustrated according to the TyG index tertiles. The measurement data of normal distribution were expressed as mean ± standard deviation (SD), and if the variances were homogeneous, the t-test was used; if the variances were not homogeneous, the rank-sum test was used. The measurement data of non-normal distribution were represented by medians with interquartile range (IQR). The enumeration data were expressed numerically and differences between groups were assessed using the chi-square test. Analysis of variance was used to compare data between groups. In addition, the associations between the TyG index and clinical parameters were assessed by using Pearson’s correlation test. Unadjusted survival curves were generated in Kaplan–Meier plots with log-rank tests.
Univariate Cox regression analysis (Hazard ratio [HR], 95% CI) was used to identify the factors associated with all-cause mortality and MACE. P < 0.05 meant the statistical significance of the difference. We used Cox proportional hazards models to estimate the association between TyG index and all-cause mortality and MACE. Three regression models were built and potential confounders were adjusted in these models. Model 1 was the unadjusted model, model 2 was the partially adjusted model that was controlled for age and sex, and model 3 was the fully adjusted model that was controlled for variables in model 2 plus BMI, SBP, DBP, LVEF, Gensini score, hypertension, diabetes, hyperlipidemia, previous MI, previous stroke, CKD, current smoking, TC, LDL-C, HDL-C, eGFR, UA, aspirin, clopidogrel, statin, β-blocker, ACEI/ARB, LM lesion, multivessel lesion and treatment.
The TyG index was transformed into three categorical variables for the primary analysis. For the trend test, the newly categorical variable was also recoded a continuous variable and entered into the regression models. We also standardized the TyG index, then put it into the regression models to determine the relationships between the increase of TyG per SD and endpoints.
Additionally, we conducted subgroup analyses to explore whether the associations between the TyG index and all-cause mortality and MACE was modified by the following variables: sex, hypertension, diabetes, previous stroke, previous MI, hyperlipidemia, CKD, current smoking, BMI and multivessel lesion. Interactions between the TyG index and each of the above variables were tested. Findings are reported by hazard ratios (HR) and 95% confidence intervals (CI).
Two-sided P < 0.05 was considered statistically significant. All data in this study were processed using SPSS software Version 23.0 (IBM Corporation, Armonk, NY, USA), the statistical software packages R (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Results
Baseline characteristics of study participants by TyG index
A total of 662 elderly patients with ACS were enrolled in this study, with an average age of 81.87 ± 2.14 years, the female proportion was 28.10%, and the average TyG index was 8.76 ± 0.72. Patients were divided into three groups according to the tertile of TyG index. Participants with higher TyG index were more likely to have elevated BMI, HR, TC, TG, LDL-C, FBG, UA, GENSINI score, and lower height, LVEF, HDL-C, and eGFR. In the high TyG index group, the proportion of patients with female, hypertension, DM, hyperlipidemia, previous stroke, and multivessel lesion were likely to be higher (Table 1).
Table 1.
Baseline characteristics of patients stratified by tertile of TyG index
| Variable | Total | Tertile 1 | Tertile 2 | Tertile 3 | P-value |
|---|---|---|---|---|---|
| N | 662 | 221 | 221 | 220 | – |
| General conditions | |||||
| Age, years | 81.87 ± 2.14 | 81.88 ± 2.04 | 82.15 ± 2.38 | 81.58 ± 1.94 | 0.062 |
| Female, n (%) | 186 (28.10%) | 35 (15.84%) | 61 (27.60%) | 90 (40.91%) | < 0.001 |
| Height, cm | 165.32 ± 8.25 | 166.36 ± 7.50 | 165.32 ± 8.72 | 164.28 ± 8.39 | 0.025 |
| Weight, kg | 67.20 ± 10.68 | 66.52 ± 10.73 | 67.31 ± 10.26 | 67.76 ± 11.06 | 0.463 |
| BMI, kg/m2 | 24.57 ± 3.40 | 24.01 ± 3.40 | 24.62 ± 3.30 | 25.07 ± 3.43 | 0.004 |
| HR, beat/min | 74.81 ± 14.02 | 73.15 ± 13.54 | 74.13 ± 12.27 | 77.15 ± 15.79 | 0.008 |
| SBP, mmHg | 137.08 ± 21.79 | 136.85 ± 22.00 | 137.33 ± 21.04 | 137.06 ± 22.40 | 0.902 |
| DBP, mmHg | 71.44 ± 12.12 | 71.96 ± 11.83 | 71.19 ± 11.94 | 71.15 ± 12.60 | 0.731 |
| LVEF, % | 55.65 ± 9.91 | 57.06 ± 9.42 | 55.08 ± 10.12 | 54.82 ± 10.06 | 0.034 |
| Gensini score | 53.65 ± 42.65 | 40.79 ± 34.51 | 59.78 ± 41.27 | 60.41 ± 48.29 | < 0.001 |
| Risk factors, n (%) | |||||
| Hypertension | 511 (77.19%) | 158 (71.49%) | 173 (78.28%) | 180 (81.82%) | 0.032 |
| Diabetes | 231 (34.89%) | 46 (20.81%) | 68 (30.77%) | 117 (53.18%) | < 0.001 |
| Hyperlipidemia | 151 (22.81%) | 36 (16.29%) | 54(24.43%) | 61 (27.73%) | 0.011 |
| Previous MI | 120 (18.13%) | 34 (15.38%) | 44 (19.91%) | 42 (19.09%) | 0.421 |
| Previous stroke | 138 (20.85%) | 43 (19.46%) | 46 (20.81%) | 49 (22.27%) | 0.042 |
| CKD | 78 (11.78%) | 19 (8.60%) | 29 (13.12%) | 30 (13.64%) | 0.195 |
| Current smoking | 164 (24.77%) | 67 (30.32%) | 47 (21.27%) | 50 (22.73%) | 0.061 |
| Baseline blood features | |||||
| TC, mmol/L | 4.11 ± 0.97 | 3.87 ± 0.89 | 4.10 ± 0.91 | 4.36 ± 1.04 | < 0.001 |
| TG, mmol/L | 1.39 ± 0.72 | 0.84 ± 0.22 | 1.28 ± 0.33 | 2.04 ± 0.82 | < 0.001 |
| LDL-C, mmol/L | 2.36 ± 0.84 | 2.19 ± 0.75 | 2.38 ± 0.82 | 2.52 ± 0.91 | < 0.001 |
| HDL-C, mmol/L | 1.13 ± 0.36 | 1.23 ± 0.40 | 1.13 ± 0.36 | 1.01 ± 0.28 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 70.85 ± 23.11 | 73.69 ± 17.27 | 69.93 ± 19.77 | 68.93 ± 30.11 | 0.003 |
| FBG, mmol/L | 7.05 ± 4.13 | 5.32 ± 1.17 | 6.42 ± 1.75 | 9.40 ± 6.17 | < 0.001 |
| UA, umol/L | 351.59 ± 149.66 | 335.69 ± 200.17 | 356.29 ± 103.22 | 362.85 ± 127.46 | 0.001 |
| TyG index | 8.76 ± 0.72 | 8.08 ± 0.61 | 8.72 ± 0.15 | 9.47 ± 0.42 | < 0.001 |
| Cardiovascular medications, n (%) | |||||
| Aspirin | 642 (96.98%) | 210 (95.02%) | 217 (98.19%) | 215 (97.73%) | 0.110 |
| Clopidogrel | 632 (95.47%) | 208 (94.12%) | 216 (97.74%) | 208 (94.55%) | 0.136 |
| Statin | 615 (92.90%) | 206 (93.21%) | 203 (91.86%) | 206 (93.64%) | 0.748 |
| β-blocker | 418 (63.14%) | 122 (55.20%) | 147 (66.52%) | 149 (67.73%) | 0.011 |
| ACEI/ARB | 364 (54.98%) | 107 (48.42%) | 126 (57.01%) | 124 (59.55%) | 0.040 |
| Angiography, n (%) | |||||
| LAD lesion | 560 (84.59%) | 184 (83.26%) | 192 (86.88%) | 184 (83.64%) | 0.511 |
| LCX lesion | 383 (57.85%) | 129 (58.37%) | 128 (57.92%) | 126 (57.27%) | 0.601 |
| RCA lesion | 429 (64.80%) | 144 (65.16%) | 137 (61.99%) | 148 (67.27%) | 0.251 |
| LM lesion | 109 (16.47%) | 26 (11.76%) | 39 (17.65%) | 44 (20.00%) | 0.056 |
| Multivessel lesion | 466 (70.39%) | 138 (62.44%) | 164 (74.21%) | 164 (74.55%) | 0.007 |
| Treatment, n (%) | 0.081 | ||||
| Intensive medication | 241 (36.40%) | 94 (42.53%) | 71 (32.13%) | 76 (34.55%) | |
| PCI | 405 (61.18%) | 125 (56.56%) | 142 (64.25%) | 138 (62.73%) | |
| CABG | 16 (2.42%) | 2 (0.90%) | 8 (3.62%) | 6 (2.73%) | |
Data are shown as mean ± standard deviation (SD) or number (n) of patients. △P < 0.05, △△P < 0.01, Tertile 1 vs. Tertile 2; #P < 0.05, ##P < 0.01, Tertile 1 vs. Tertile 3; *P < 0.05, **P < 0.01, Tertile 2 vs. Tertile 3. P values in bold are < 0.05
BMI, body mass index; HR heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; CKD, chronic kidney disease; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein-C; HDL-C, high-density lipoprotein-C; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; UA, uric acid; TyG, triglyceride glucose; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; LM, left main coronary artery; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting
Correlations between TyG index and clinical variables
In order to identify the association between the TyG index and risk factors, Spearman’s rank or Pearson’s correlation analysis was performed. As shown in Table 2, the TyG index was positively correlated with BMI, Gensini score, TC, TG, LDL-C, FBG and UA, and negatively correlated with DBP, LVEF, HDL-C. Except for TC and FBG, the TyG index had the highest positive correlation with Gensini score and the highest negative correlation with HDL-C.
Table 2.
Correlations between TyG index and traditional cardiovascular risk factors
| Variable | Correlation coefficient | P value |
|---|---|---|
| Age | − 0.0672 | 0.0841 |
| BMI | 0.0920 | 0.0179 |
| SBP | − 0.0491 | 0.2068 |
| DBP | − 0.1049 | 0.0069 |
| LVEF | − 0.0778 | 0.0453 |
| Gensini score | 0.1963 | < 0.001 |
| TC | 0.1742 | < 0.001 |
| TG | 0.7982 | < 0.001 |
| LDL-C | 0.1151 | 0.0030 |
| HDL-C | − 0.2370 | < 0.001 |
| eGFR | − 0.0599 | 0.1234 |
| FBG | 0.5524 | < 0.001 |
| UA | 0.0804 | 0.0386 |
P values in bold are < 0.05
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein-C; HDL-C, high-density lipoprotein-C; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; UA, uric acid
Univariate regression analysis of long-term prognostic correlation among three groups
The follow-up time of this study was 40–120 months (median, 63 months; interquartile range, 51‒74 months), and the main outcomes were all-cause mortality and MACE. All-cause mortality and MACE of the three groups were analyzed by univariate Cox regression. As presented in Table 3, that age, LVEF, Gensini score, DM, previous stroke, CKD, TC, TG, LDL-C, eGFR, FBG, TyG index, aspirin, LM lesion, and multivessel lesion were found to be independent risk factors for all-cause mortality in ACS patients. Meanwhile, SBP, LVEF, Gensini score, DM, previous stroke, CKD, TC, TG, eGFR, FBG, TyG index, aspirin, LM lesion, and multivessel lesions were found to be independent risk factors for MACE in ACS patients.
Table 3.
Results of univariate Cox regression analysis
| Variable | All-cause mortality | MACE | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.08 (1.02, 1.15) | 0.0117 | 1.03 (0.98, 1.09) | 0.2401 |
| Male | 1.05 (0.77, 1.43) | 0.7774 | 1.15 (0.88, 1.51) | 0.3181 |
| BMI | 0.96 (0.92, 1.00) | 0.0839 | 0.98 (0.95, 1.02) | 0.3472 |
| SBP | 0.99 (0.99, 1.00) | 0.0581 | 0.99 (0.99, 1.00) | 0.0105 |
| DBP | 0.99 (0.98, 1.01) | 0.3366 | 1.00 (0.99, 1.01) | 0.5432 |
| LVEF | 0.96 (0.95, 0.98) | < 0.0001 | 0.97 (0.96, 0.99) | < 0.0001 |
| Gensini score | 1.01 (1.00, 1.01) | < 0.0001 | 1.01 (1.00, 1.01) | < 0.0001 |
| Hypertension | 1.07 (0.76, 1.49) | 0.7124 | 0.93 (0.71, 1.24) | 0.6376 |
| Diabetes | 1.46 (1.10, 1.93) | 0.0084 | 1.41 (1.11, 1.79) | 0.0050 |
| Hyperlipidemia | 0.81 (0.57, 1.15) | 0.2304 | 0.94 (0.71, 1.26) | 0.6878 |
| Previous MI | 1.05 (0.75, 1.49) | 0.7642 | 1.14 (0.85, 1.52) | 0.3852 |
| Previous stroke | 1.50 (1.09, 2.04) | 0.0116 | 1.54 (1.18, 2.01) | 0.0014 |
| CKD | 2.30 (1.62, 3.25) | < 0.0001 | 1.81 (1.31, 2.48) | 0.0003 |
| Current smoking | 1.08 (0.78, 1.48) | 0.648 | 1.10 (0.84, 1.44) | 0.4967 |
| TC | 1.26 (1.09, 1.45) | 0.0015 | 1.14 (1.01, 1.29) | 0.0295 |
| TG | 1.22 (1.03, 1.44) | 0.0190 | 1.27 (1.09, 1.48) | 0.0018 |
| LDL-C | 1.25 (1.06, 1.48) | 0.0074 | 1.14 (0.99, 1.31) | 0.0738 |
| HDL-C | 0.68 (0.44, 1.04) | 0.0783 | 0.70 (0.49, 1.01) | 0.0594 |
| eGFR | 0.98 (0.97, 0.98) | < 0.0001 | 0.99 (0.98, 0.99) | < 0.0001 |
| FBG | 1.05 (1.03, 1.06) | < 0.0001 | 1.04 (1.02, 1.06) | < 0.0001 |
| UA | 1.00 (1.00, 1.00) | 0.0035 | 1.00 (1.00, 1.00) | 0.0160 |
| TyG index | 1.75 (1.42, 2.16) | < 0.0001 | 1.60 (1.32, 1.92) | < 0.0001 |
| Aspirin | 0.38 (0.21, 0.70) | 0.0019 | 0.43 (0.25, 0.75) | 0.0031 |
| Clopidogrel | 0.60 (0.34, 1.05) | 0.0711 | 0.63 (0.39, 1.03) | 0.0653 |
| Statin | 0.98 (0.59, 1.64) | 0.9511 | 1.40 (0.86, 2.30) | 0.1785 |
| β-blocker | 1.06 (0.80, 1.42) | 0.6721 | 1.23 (0.96, 1.59) | 0.1021 |
| ACEI/ARB | 1.11 (0.84, 1.48) | 0.4501 | 1.13 (0.89, 1.43) | 0.3199 |
| LM lesion | 1.53 (1.09, 2.14) | 0.0141 | 1.78 (1.34, 2.35) | < 0.0001 |
| Multivessel lesion | 1.44 (1.04, 2.00) | 0.0299 | 1.62 (1.22, 2.15) | 0.0009 |
P values in bold are < 0.05
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; CKD, chronic kidney disease; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein-C; HDL-C, high-density lipoprotein-C; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; UA, uric acid; TyG, triglyceride glucose; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LM, left main coronary artery
Association between TyG index and long-term prognosis
During the follow-up period, a total of 277 MACEs occurred and 201 of these were attributed to all-cause mortality. As shown in Table 4, for each SD increase in the TyG index, the HR (95% CI) of all-cause mortality was 1.28 (1.06, 1.56) in the fully adjusted regression model. The fully adjusted HRs (95%CIs) for all-cause mortality for subjects in tertile 2 and tertile 3 were 1.18 (0.79, 1.79) and 1.64 (1.06, 2.54), respectively. The increased risk of all-cause mortality from tertile 1 to tertile 3 was statistically significant (P for trend = 0.0204).
Table 4.
Multivariable Cox regression analyses for the association between TyG index and all-cause mortality
| TyG index | Events (No.), n (%) | HR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Per SD increase | 201 (30.36) | 1.50 (1.29, 1.73)*** | 1.56 (1.34, 1.81)*** | 1.28 (1.06, 1.56)* |
| Tertile 1 | 44 (19.91) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Tertile 2 | 64 (28.96) | 1.42 (0.96, 2.08) | 1.45 (0.98, 2.12) | 1.18 (0.79, 1.79) |
| Tertile 3 | 93 (42.27) | 2.32 (1.62, 3.33)*** | 2.54 (1.76, 3.67)*** | 1.64 (1.06, 2.54)* |
| P for trend | < 0.001 | < 0.001 | 0.0204 | |
Model 1: unadjusted
Model 2: adjusted for age and sex
Model 3: adjusted for age, gender, BMI, SBP, DBP, LVEF, Gensini score, hypertension, diabetes, hyperlipidemia, previous MI, previous stroke, CKD, current smoking, TC, LDL-C, HDL-C, eGFR, UA, aspirin, clopidogrel, statin, β-blocker, ACEI/ARB, LM lesion, multivessel lesion and treatment
*P < 0.05
**P < 0.01
***P < 0.001
Similarly, the significant association between the TyG index and MACE was also found in the fully adjusted regression model (Table 5). For each SD increase in the TyG index, the HR (95% CI) of MACE rate was 1.21 (1.02, 1.43) in the fully adjusted regression model. The fully adjusted HR (95%CIs) for MACE in tertile 3 was 1.36 (1.05, 1.95). The increased risk of MACE from tertile 1 to tertile 3 was statistically significant (P for trend = 0.0214).
Table 5.
Multivariable Cox regression analyses for the association between TyG index and MACE
| TyG index | Events (No.), n (%) | HR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Per SD increase | 277 (41.84) | 1.40 (1.22, 1.60)*** | 1.47 (1.28, 1.69)*** | 1.21 (1.02, 1.43)* |
| Tertile 1 | 70 (31.67) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Tertile 2 | 92 (41.63) | 1.31 (0.96, 1.79) | 1.34 (0.98, 1.83) | 1.09 (0.78, 1.52) |
| Tertile 3 | 115 (52.27) | 1.86 (1.38, 2.50)*** | 2.02 (1.49, 2.74)*** | 1.36 (1.05, 1.95)* |
| P for trend | < 0.001 | < 0.001 | 0.0214 | |
Model 1: unadjusted
Model 2: adjusted for age and sex
Model 3: adjusted for age, gender, BMI, SBP, DBP, LVEF, Gensini score, hypertension, diabetes, hyperlipidemia, previous MI, previous stroke, CKD, current smoking, TC, LDL-C, HDL-C, eGFR, UA, aspirin, clopidogrel, statin, β-blocker, ACEI/ARB, LM lesion, multivessel lesion and treatment
*P < 0.05
**P < 0.01
***P < 0.001
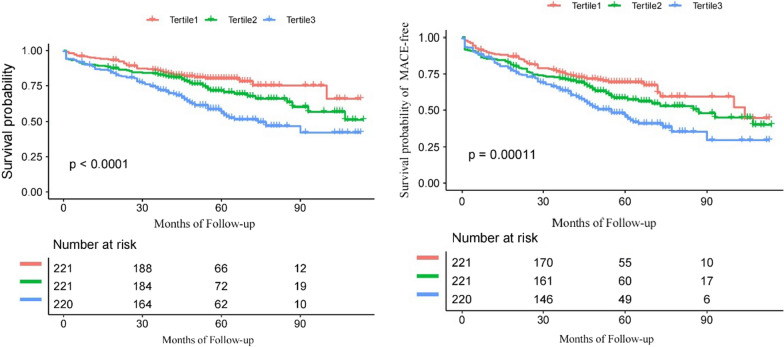
Kaplan Meier curves of the long-term survival and survival of MACE-free in elderly ACS patients for the TyG index tertiles are presented in Fig. 1. The survival probability and the survival probability of MACE-free in the tertile 1 group was significantly higher than that in the tertiel 3 group (P < 0.001). The cumulative incidence of all-cause death and MACE increased with higher tertiles of the TyG index.
Fig. 1.
Kaplan–Meier survival curves of survival probability (A) and survival probability of MACE-free (B) for the elderly ACS patients
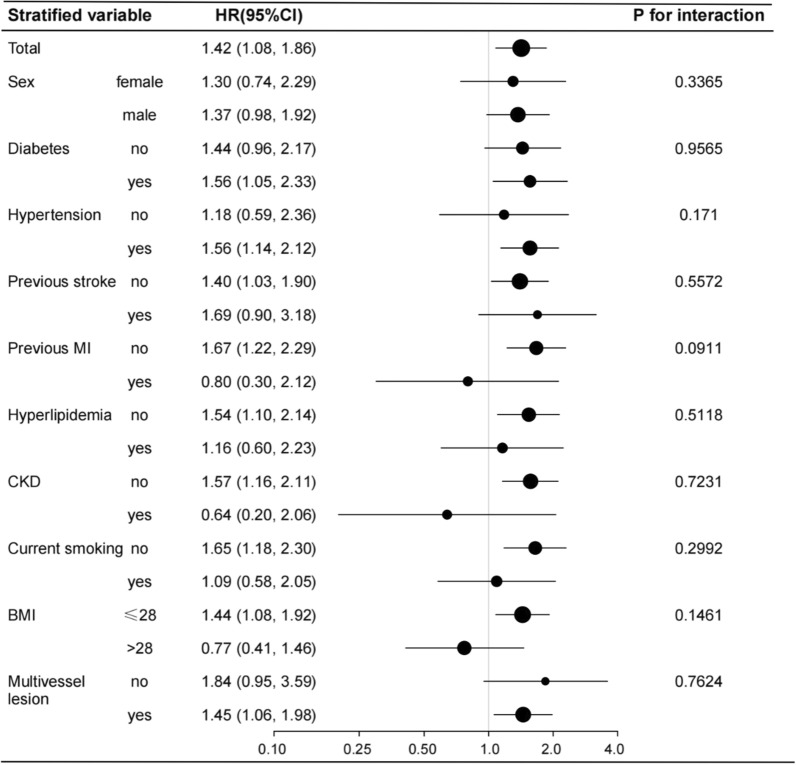
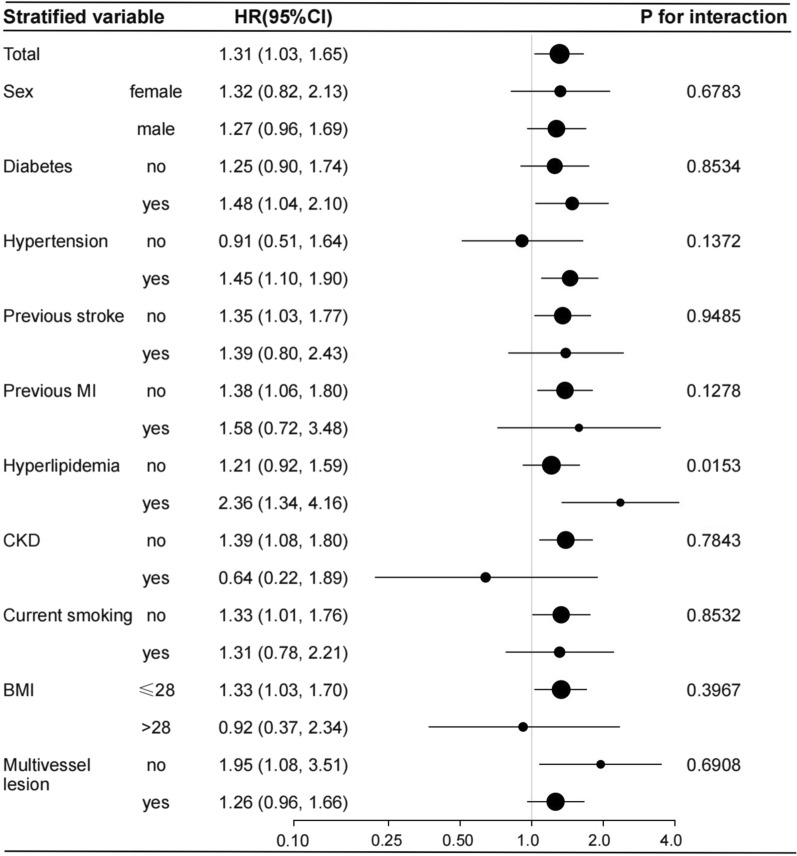
Subgroup analyses for association between TyG index and long-term prognosis
Several stratified analyses were performed to further examine the relationship between the TyG index and prognosis. As shown in Fig. 2, the significantly association between TyG index and all-cause mortality in the subgroups of diabetes [HR (95% CI), 1.56 (1.05, 2.33)], those with hypertension [HR (95% CI), 1.14 (1.05, 2.12)], those without previous stoke [HR (95% CI), 1.40 (1.03, 1.90)], those without previous MI [HR (95% CI), 1.67 (1.22, 2.29)], those without hyperlipidemia [HR (95% CI), 1.54 (1.10, 2.14)], those without CKD [HR (95% CI), 1.57 (1.16, 2.11)], those no smoking [HR (95% CI), 1.65 (1.18, 2.30)], those with BMI ≤ 28 kg/m2 [HR (95% CI), 1.44 (1.08, 1.92)], those with multivessel disease [HR (95% CI), 1.45 (1.06, 1.98)], were consistent with the total [HR (95% CI), 1.42 (1.08, 1.86)]. Likewise, the significantly association between TyG index and MACE in the subgroups of diabetes, those with hypertension, those without previous stoke, those without previous MI, those with hyperlipidemia, those without CKD, those no smoking, those with BMI ≤ 28 kg/m2, those without multivessel disease, were consistent with the total. And Hyperlipidemia-stratified analyses showed a significant trend of TyG index (P for interaction = 0.0153) for MACE among the hyperlipidemia group (Fig. 3).
Fig. 2.
Forest plot investigating the association between the TyG index and all-cause mortality in different subgroups. Adjusted for age, gender, BMI, SBP, DBP, LVEF, Gensini score, hypertension, diabetes, hyperlipidemia, previous MI, previous stroke, CKD, current smoking, TC, LDL-C, HDL-C, eGFR, UA, aspirin, clopidogrel, statin, β-blocker, ACEI/ARB, LM lesion, multivessel lesion except for the stratified variable
Fig. 3.
Forest plot investigating the association between the TyG index and MACE in different subgroups. Adjusted for age, gender, BMI, SBP, DBP, LVEF, Gensini score, hypertension, diabetes, hyperlipidemia, previous MI, previous stroke, CKD, current smoking, TC, LDL-C, HDL-C, eGFR, UA, aspirin, clopidogrel, statin, β-blocker, ACEI/ARB, LM lesion, multivessel lesion except for the stratified variable
Discussion
In this study, 662 elderly ACS patients were performed coronary angiography and further treatments, clinical information were collected and followed-up ten years. To the best of our knowledge, this study was the first one to explore the relationship between TyG index and the all-cause mortality and MACE in elderly ACS patients, with a focus on long-term outcomes. The main findings of our study were as follows: (1) As a reliable indicator for IR, TyG index has a significant correlation with traditional risk factors of CHD, which corresponded to previous studies [20–22]. Furthermore, we also found that except for TG and FBG, the TyG index had the highest positive correlation with the degree of coronary artery stenosis and the highest negative correlation with HDL-C; (2) TyG index had a significant effect on all-cause mortality and MACE rate. In univariate regression analysis, the all-cause mortality and MACE rate of patients increased significantly with the increase of TyG index; (3) in the fully adjusted model, the TyG index, as a continuous or categorical variable, was independently correlated with the increase of all-cause mortality and MACE rate; (4) there was a graded, positive association between TyG index and all-cause mortality and MACE; and (5) taking the TyG index into consideration was likely to have significant clinical value in early risk stratification of elderly patients with ACS.
Traditional risk factors of CHD include age, hypertension, DM, hyperlipidemia, smoking, etc. [23, 24]. Among them, DM, hyperlipidemia, and other major risk factors have been widely concerned by many scholars, but elderly ACS patients have not received enough attention. With the aging population rapidly growing worldwide, the situation of CHD in the elderly is becoming increasingly serious, and the harm and burden brought by this disease to the country, society, and family become more and more prominent. Most of the previous studies on TyG index collected middle-aged people, paid little attention to the elderly [25–27]. In addition, most of these studies have not performed stratified analyses to further validate and discuss the association between TyG index and long-term outcomes. As we all know, with the acceleration of the global aging process, the number of the elderly has increased dramatically, which has become the main goal of today's medical care. Considering the potential prognostic significance of TyG index, it is important to figure out the ability of TyG index to evaluate elderly patients with ACS. In this study, we selected elderly patients with ACS as objects, which has a good application prospect.
IR is a pathological state, which is characterized by the disorder of glucose uptake and utilization, and in the form of the decrease of insulin sensitivity. It can lead to abnormal fluctuations in blood glucose and blood lipids, manifested by increased circulating blood glucose and TC and decreased HDL-C [28]. Based on this theoretical background, TyG index is calculated according to TG and FBG, and is considered to be a reliable, convenient and inexpensive index to evaluate IR [22, 29]. The euglycemic-hyperinsulinemic clamp test is the gold standard for evaluating IR. As reported that, regardless of obesity and glucose tolerance, TyG index was negatively correlated with IR. In addition, compared with the homeostasis model assessment insulin resistance (HOMA-IR) index, TyG index showed better evaluation efficiency [30, 31]. As studies show that, TyG index is not only excellent in evaluating IR, but also closely related to coronary calcification, future cardiovascular events (including death, stroke, MI, and post-discharge revascularization), and various metabolic abnormalities, etc. and it is an independent risk factor for ACS and DES-ISR [32–35]. In this study, we found that patients with high TyG index were more likely to suffer from hypertensive, DM, stroke, hyperlipidemia and multivessel disease, which is consistent with previously reported studies [36–38]. As shown in Table 2, the TyG index had significantly positive correlation with Gensini score, and it was the first time to report the relationship between the TyG index and the degree of coronary artery stenosis in elderly ACS patients. Previous studies showed that TyG index was associated with the progression of coronary artery calcification [34]. The evaluation of noninvasive imaging such as coronary computed tomography angioplasty is limited, so the TyG index could provide a good complementary effect for coronary evaluation in this population.
With the further study of TyG index, it was found that TyG index was not only related to the traditional risk factors of CHD. Zhang et al. and Zhu et al. pointed out that if TyG index was added to the baseline risk model, the accuracy of MACE prediction in ACS and DES-ISR patients could be significantly improved [10, 39]. However, according to our 10-year follow-up study, the TyG index still has a reliable prognostic value for elderly ACS patients, especially in all-cause mortality. In addition, through subgroup analysis, we found that the association between TyG index and prognosis was stable, and this association persisted in most subgroups, including patients with DM, hypertension, stroke. It should be noted that there was a less significant interaction between TyG index and hyperlipidemia in the association between TyG index and MACE. Although the underlying mechanism is still unclear, it is necessary to consider hyperlipidemia in the prevention and management of elderly ACS patients.
Our study shows that the TyG index is a reliable indicator to evaluate the long-term prognosis of elderly patients with ACS. Although the exact mechanism is not clear, we speculate that it might be related to the fact that TyG index is a reliable index to evaluate IR. First of all, IR usually leads to the intensification of oxidative stress and inflammatory response, disturbance of glucose and lipid metabolism, activation of the renin–angiotensin–aldosterone system (RAAS), and ultimately cell damage, hypertrophy, and fibrosis occur [40, 41]. Secondly, IR might lead to excessive proliferation of vascular endothelial cells through a variety of signaling mechanisms and accelerate the progression of CHD [34]. Thirdly, as a reliable indicator to evaluate IR, TyG index is closely associated with coronary artery calcification, ISR, and various heart disease risk factors [10, 22, 34].
Strengths and limitations
Firstly, this study is the first time to select elderly ACS patients as research subjects, and we have performed a 10-year follow-up. The results of our study have stable and reliable guiding significance. Secondly, we performed the fully adjusted analysis for as many confounders related to this research as possible and sensitivity analysis for the number, presence, and absence of cardiovascular risk factors. Thirdly, the convenience, repeatability and low cost of TyG index measurement and calculation make it have important potential to predict the prognosis of elderly ACS patients. However, there are some limitations to our study. First of all, this is a single-center cohort study with relatively small sample size, and the existing selection bias may affect the results. Further studies with large samples and multi-centers are needed to verify our results. Second, only one baseline assessment of TyG index and other risk factors was performed at admission, without taking into account fluctuations in these indicators during follow-up. Third, although we adjusted for other relevant confounders including BMI and blood lipids, we did not record energy intake and nutritional habits that might affect TG levels. Finally, all subjects in this study were selected from China, which may limit the universality of our research results on a global scale.
Conclusion
The results of this study suggest TyG index, as a new surrogate marker for evaluating IR in clinical practice, is significantly associated with the degree of coronary artery stenosis, and is an independent predictor of long-term all-cause mortality and MACE in elderly ACS patients.
Acknowledgements
We thank the patient advisers for the information they provided.
Abbreviations
- CHD
Coronary heart disease
- ACS
Acute coronary syndrome
- IR
Insulin resistance
- DM
Diabetes mellitus
- TyG index
Triglyceride-glucose index
- TG
Triglyceride
- FBG
Fasting blood glucose
- PLA
People’s Liberation Army
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- CKD
Chronic kidney injury
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein-cholesterol
- HDL-C
High-density lipoprotein-cholesterol
- eGFR
The estimated glomerular filtration rate
- UA
Uric acid
- ACEI
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- LM
Left main coronary artery
- RCA
Right coronary artery
- Scr
Standardized creatinine
- MACE
Major adverse cardiac event
- AMI
Acute myocardial infarction
- IQR
Interquartile range
- HR
Hazard ratio
- HOMA-IR
Homeostasis model assessment insulin resistance
Authors’ contributions
YJ and YKS made substantial contributions to data analysis, data interpretation, and manuscript writing; JS, XLH, YL and JHW were responsible for data collection; BL, DFQ and ZJS contributed to interpretation of coronary angiography; YDC, QX, and ZHF performed manuscript revision; MZS and ZHF were responsible for the study design. All authors have full access to all the data in the study and take responsibility for the integrity and security of the data. All authors read and approved the final manuscript.
Funding
No.
Availability of data and materials
The datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the Ethics Service Center of the Chinese PLA General Hospital in China. Written informed consent was obtained from all participants.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Jiao and Yongkang Su contribute equally to the paper
Contributor Information
Mingzhi Shen, Email: shenmz301@163.com.
Zhenhong Fu, Email: fuzhenh@126.com.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao X, Zhang J, Guo J, Wang J, Pan Y, Zhao X, et al. Comparison of safety and efficacy between clopidogrel and ticagrelor in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. Front Pharmacol. 2021;12:743259. doi: 10.3389/fphar.2021.743259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Blas S, Cordero A, Diez-Villanueva P, Martinez-Avial M, Ayesta A, Ariza-Sole A, et al. Acute coronary syndrome in the older patient. J Clin Med. 2021;10(18):4132–4147. doi: 10.3390/jcm10184132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco M, Careggio A, Biole CA, Quadri G, Quiros A, Raposeiras-Roubin S, et al. Ticagrelor or clopidogrel after an acute coronary syndrome in the elderly: a propensity score matching analysis from 16,653 patients treated with PCI included in two large multinational registries. Cardiovasc Drugs Ther. 2021;35(6):1171–1182. doi: 10.1007/s10557-021-07213-y. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 7.Imai Y, Dobrian AD, Weaver JR, Butcher MJ, Cole BK, Galkina EV, et al. Interaction between cytokines and inflammatory cells in islet dysfunction, insulin resistance and vascular disease. Diabetes Obes Metab. 2013;15(Suppl 3):117–129. doi: 10.1111/dom.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell DS, O'Keefe JH. Lowering the triglyceride/high-density lipoprotein cholesterol and its association with the beneficial impact of pioglitazone on coronary atherosclerosis in the PERISCOPE study is likely due to lowering insulin resistance. J Am Coll Cardiol. 2011;58(7):778. doi: 10.1016/j.jacc.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 9.Menotti A, Puddu PE, Kromhout D, Kafatos A, Tolonen H. Coronary heart disease mortality trends during 50 years as explained by risk factor changes: the European cohorts of the Seven Countries Study. Eur J Prev Cardiol. 2020;27(9):988–998. doi: 10.1177/2047487318821250. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137–148. doi: 10.1186/s12933-021-01332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a meta-analysis. Front Cardiovasc Med. 2021;8:644035. doi: 10.3389/fcvm.2021.644035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si Y, Fan W, Shan W, Zhang Y, Liu J, Han C, et al. Association between triglyceride glucose index and coronary artery disease with type 2 diabetes mellitus in middle-aged and elderly people. Medicine. 2021;100(9):e25025. doi: 10.1097/MD.0000000000025025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: a cross-sectional study of Chinese adults. J Clin Hypertens. 2020;22(6):1025–1032. doi: 10.1111/jch.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz J, Bjorge T, Nagel G, Manjer J, Engeland A, Haggstrom C, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol. 2020;49(1):193–204. doi: 10.1093/ije/dyz053. [DOI] [PubMed] [Google Scholar]
- 15.Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:8. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361–368. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahuja V, Aronen P, Pramodkumar TA, Looker H, Chetrit A, Bloigu AH, et al. Accuracy of 1-hour plasma glucose during the oral glucose tolerance test in diagnosis of type 2 diabetes in adults: a meta-analysis. Diabetes Care. 2021;44(4):1062–1069. doi: 10.2337/dc20-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Qin Y, Zheng K, Gong M, Wu J, Shou W, et al. Improved glomerular filtration rate estimation using new equations combined with standardized cystatin C and creatinine in Chinese adult chronic kidney disease patients. Clin Biochem. 2014;47(13–14):1220–1226. doi: 10.1016/j.clinbiochem.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Su Y, Wang S, Sun J, Zhang Y, Ma S, Li M, et al. Triglyceride glucose index associated with arterial stiffness in chinese community-dwelling elderly. Front Cardiovasc Med. 2021;8:737899. doi: 10.3389/fcvm.2021.737899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34–41. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537. doi: 10.1155/2019/6891537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batty GD, Kivimaki M, Bell S. Comparison of risk factors for coronary heart disease morbidity versus mortality. Eur J Prev Cardiol. 2020;27(19):2232–2234. doi: 10.1177/2047487319882512. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, Navar AM, Wojdyla D, Sanchez RJ, Khan I, Elassal J, et al. Quantifying importance of major risk factors for coronary heart disease. Circulation. 2019;139(13):1603–1611. doi: 10.1161/CIRCULATIONAHA.117.031855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong S, Lee JH. The verification of the reliability of a triglyceride-glucose index and its availability as an advanced tool. Metabolomics. 2021;17(11):97–104. doi: 10.1007/s11306-021-01837-9. [DOI] [PubMed] [Google Scholar]
- 26.Tomiyama H, Shiina K. State of the art review: brachial-ankle PWV. J Atheroscler Thromb. 2020;27(7):621–636. doi: 10.5551/jat.RV17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. 2020;19(1):116–128. doi: 10.1186/s12933-020-01091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W, Tian M, Zhou Y. The relationship between insulin resistance, adiponectin and C-reactive protein and vascular endothelial injury in diabetic patients with coronary heart disease. Exp Ther Med. 2018;16(3):2022–2026. doi: 10.3892/etm.2018.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrero-Romero F, Villalobos-Molina R, Jimenez-Flores JR, Simental-Mendia LE, Mendez-Cruz R, Murguia-Romero M, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–465. doi: 10.1111/pedi.12303. [DOI] [PubMed] [Google Scholar]
- 31.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Wang Y, Chen R, Li J, Zhou J, Liu C, et al. Triglyceride glucose index combined with plaque characteristics as a novel biomarker for cardiovascular outcomes after percutaneous coronary intervention in ST-elevated myocardial infarction patients: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20(1):131–148. doi: 10.1186/s12933-021-01321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Ma W, Huang S, Lin X, Yu M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis. 2021;31(11):3184–3192. doi: 10.1016/j.numecd.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 35.Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam KW, Kwon HM, Lee YS. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci Rep. 2021;11(1):15335. doi: 10.1038/s41598-021-94631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong J, Yang H, Zhang Y, Hu Q. Triglyceride-glucose index is a predictive index of hyperuricemia events in elderly patients with hypertension: a cross-sectional study. Clin Exp Hypertens. 2021:1–6. [DOI] [PubMed]
- 38.da Silva A, Caldas APS, Rocha D, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14(6):584–593. doi: 10.1016/j.pcd.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. Predictive effect of triglycerideglucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43–55. doi: 10.1186/s12933-021-01236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambie M, Bonomini M, Davies SJ, Accili D, Arduini A, Zammit V. Insulin resistance in cardiovascular disease, uremia, and peritoneal dialysis. Trends Endocrinol Metab. 2021;32(9):721–730. doi: 10.1016/j.tem.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.