Abstract
Our previous studies demonstrated that prenatal in utero growth restriction impairs postnatal intestinal function. Thus, improving postpartal intestinal absorption capacity and growth by manipulating the maternal diet prepartum is of importance. This work was conducted to determine whether supplementation of N-carbamylglutamate (NCG) or rumen-protected L-arginine (RP-Arg) increased fetal intestinal amino acid (AA) profiles in intrauterine growth retardation (IUGR) fetuses. On d 35 of gestation, Hu ewes (n = 32) carrying twin fetuses were randomized into 4 groups (8 ewes and 16 fetuses in each group), where diets were as follows: 100% of nutrient requirements recommended by National Research Council (NRC, 2007) (CON); 50% of nutrient requirements recommended by NRC (2007) (RES); RES + RP-Arg (20 g/d), (RES + ARG); and RES + NCG (5 g/d), (RES + NCG). On d 110 of gestation, both fetal and maternal tissues were collected and weighed. Compared with RES, solute carrier family 1, member 5 (SLC1A5) was upregulated (P < 0.05) within fetal jejunum, duodenum and ileum when supplementing NCG and RP-Arg. Relative to RES, RP-Arg or NCG supplementation to RES resulted in upregulation (P < 0.05) of peptide transporter 1 protein abundance within the fetal ileum. NCG or RP-Arg supplementation to RES also upregulated phosphorylated mechanistic target of rapamycin (pmTOR)-to-mTOR ratio in the fetal ileum induced by IUGR (P < 0.05). As a result, during IUGR, supplementation of Arg or NCG affected intestinal AA profiles in the fetus in part through controlling mTOR signal transduction as well as AA and peptide transport. Future studies should be conducted to understand the role (if any) of the placenta on the improvement of growth and AA profiles independent of the fetal intestine. This would help demonstrate the relative contribution of intestinal uptake in fetal life.
Keywords: Intrauterine growth restriction, L-Arginine, Fetal sheep, N-carbamylglutamate, Intestinal amino acid
1. Introduction
Nutrient insufficiency to the conceptus alters function and growth of the placenta, leading to intrauterine growth retardation (IUGR) (Wu et al., 2006). Although from a conceptual point of view the situation may be evident, actual identification of nutritional insufficiencies may be more complex. According to most neonatologists, a newborn that is small for gestational date, likely due to IUGR, is one whose weight falls below the 10th percentile (Romo et al., 2009). The issue of IUGR is important for both human health and animal production (Dong et al., 2016). Previous studies suggested that placentas from animals with IUGR had impaired nutrient exchange ability (Mandò et al., 2011), induced lower antioxidant capacity (Zadrożna et al., 2009) and offspring from those dams suffered from an extended period of severe endoplasmic reticulum stress (Yung et al., 2012). The process of IUGR disrupted normal intestinal maturation, thereafter impairing gut physiological functions and morphological structure (Wang et al., 2015). Our previous study demonstrated that IUGR impaired postpartal intestinal function (Zhang et al., 2018a), but the recognition that this study did not measure intrauterine intestinal function and that the observed changes could be due to improved placental delivery of critical nutrients is helpful.
Great attention has been paid to how L-arginine (L-Arg) affects intestinal mucosal physiology. For example, dietary Arg supplementation helps trigger intestinal mucosal growth in IUGR suckling lambs (Zhang et al., 2019a). For pigs fed with mold-contaminated feed, supplementation of 1% Arg in the diet increased solute carrier family 7, member 1 (SLC7A1) mRNA abundance within the ileum, and solute carrier family 7, member 7 (SLC7A7) within the jejunum (Yin et al., 2014). N-carbamylglutamate (NCG) is a metabolically-stable analogue of N-acetylglutamate that is less degradable in the rumen than Arg. It can enhance endogenous Arg synthesis, increase intestinal growth and improve reproductive performance (Chacher B et al., 2012; Sun et al., 2018). Thus, NCG is considered as an Arg enhancer. Apart from increasing plasma Arg concentration, NCG supplementation improves gestational outcomes in sheep (Zhang et al., 2016a). In our previous research, dietary NCG or rumen-protected Arg (RP-Arg) supplemented to underfed ewes enhanced fetal small intestinal weight and fetal body weight (BW), but the underlying mechanisms were not completely assessed (Zhang et al., 2016a).
The role of the intestine in nutrient digestion and absorption as well as its metabolic activity in the context of animal growth are well known (Shortt et al., 2018). At the level of amino acid (AA) absorption, the abundance of specific transporters is critical for their utilization by peripheral tissues or intestinal cells (Chen et al., 2018). In the present study, the general hypothesis was that dietary NCG or Arg affects fetal intestinal AA profiles through modulating the abundance of peptide transporters and AA profiles along with mechanistic target of rapamycin (mTOR) signal transduction during IUGR in undernourished ewes. This study aimed to examine how RP-NCG and Arg affected fetal intestinal AA profiles, AA and peptide transporter protein and mRNA abundance, and the underlying impact of the mTOR signaling pathway on regulating the above events.
2. Materials and methods
2.1. Animals
All experimental procedures were approved by the Ethics Committee of Yangzhou University (SYXK2013-0057). Hu ewes used in our experiment were raised within an indoor barn in the Jiangyan Experimental Station (Taizhou, Jiangsu Province, China). Heating radiators were provided in the barn for maintaining a mean temperature of 15.3 ± 0.88 °C. Light conditions were automatically controlled to simulate the outdoor environment photoperiod. This work used a total of 48 multiparous Hu ewes with average BW of 40.1 ± 1.2 kg, and all animals were of similar age (18.5 ± 0.5 months) and body condition score (BCS; range, 0 to 5 [from emaciated to obese]; mean, 2.6 ± 0.2; Russel et al., 1969). After drenching with 0.2 mg/kg anti-endoparasite ivermectin, each animal was administered progestogen sponges at a dose of 30 mg (Pharmp) into the vagina for 12 d. Oestrous behavior was monitored at 08:00 and 16:00 from d 2 after the removal of the pessary through 3 vasectomized rams. Fresh semen was used to conduct artificial insemination into ewes at 48 h after the sponge was withdrawn (d 0 of gestation); then, for the following 35 d, ewes were kept within separate pens (1.05 m × 1.60 m). On d 35 of gestation, fetal numbers carried by all ewes were counted through ultrasonic examination using the Asonics Microimager 1,000 sector scanner manufactured by Ausonics. Thereafter, 32 ewes that carried twin fetuses were used in subsequent experiments. The diet composition (Appendix Table 1) was designed to satisfy 100% of estimated nutritional requirements of pregnant sheep according to the National Research Council (NRC, 2007). The main nutrients were as follows: crude protein (CP), metabolizable energy (ME), acid detergent fiber (ADF), natural detergent fiber (NDF), ether extract (EE), calcium (Ca), and phosphorus (P). During d 0 to 35, 100% of nutrient requirements recommended by NRC (2007) were fed to all ewes once a day at 08:00, and ewes had free access to water (Zhang et al., 2016a).
2.2. Experimental procedure
In this experiment, RP-Arg was purchased from Beijing Feeding Feed Science Technology Co. (Beijing, China). NCG was provided by the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Changsha, China). On d 35 of gestation, twin-bearing Hu ewes (n = 32) were assigned to 4 groups (8 ewes and 16 fetuses in each group), where diets were as follows: 100% of nutrient requirements recommended by NRC (2007) (CON), 50% of nutrient requirements recommended by NRC (2007) (RES), RES + RP-Arg (20 g/d, RES + ARG), and RES + NCG (5 g/d, RES + NCG) groups (Zhang et al., 2016a, 2016b, 2018b). The RP-Arg contained 50% L-Arg; the NCG contained 50% NCG. Thus, the actual additional amounts of RP-Arg and NCG provided 10 g/d L-Arg and 2.5 g/d NCG, respectively, which were added into the pelleted mixed diet. The RP-Arg ruminal protection was estimated at ≥ 85%, whereas RP-Arg intestinal release was estimated at ≥ 90% and both were calculated according to a published procedure (Chacher et al., 2012). Using the spray-congealing and spray-drying processes, RP-Arg was processed from phospholipids and glycerides, as described by Eldem et al. (1991). The Arg doses were determined on the basis of prior research with pregnant sheep, given parenteral Arg (Lassala et al., 2010; Satterfield et al., 2013) and supplemented with RP-Arg (Zhang et al., 2018b). The NCG dose was determined on the basis of prior research using piglets (Zeng et al., 2012), dairy cows (Chacher et al., 2014) and pregnant sheep (Zhang et al., 2018b). The restriction of nutrients (50% of NRC recommendations) was attained through providing 1/2 of the total daily diet determined for satisfying 100% of nutrient requirements recommended by NRC (2007). In addition, we detected BW at intervals of 10 d from d 35 of gestation, and adjusted feed intake according to changes of BW (Zhang et al., 2016a).
2.3. Chemical analyses
Feed samples were analyzed for CP, dry matter (DM), Ca, P, and EE (methods 990.02, 930.15, 968.08, 965.17, and 920.39, respectively; AOAC, 1990). The ADF and NDF concentrations were quantified according to Van et al. (1991). The gross energy (GE) in dietary ingredients and feces was measured using a bomb calorimeter (C200; IKA Works Inc., Staufen, Germany). The GE in the urine sample was determined according to Deng et al. (2014).
2.4. Fetal intestinal sample collection
On d 110 of gestation, animals were rendered unconscious using a captive bolt gun (Supercash Mark 2; Acceles and Shelvoke); afterwards, they were killed by exsanguination. The fetal small intestine was immediately taken out from the abdominal cavity and was divided into the duodenum (approximately 8-cm segment after the stomach), the jejunum (about half of the small intestine below the duodenum), and the ileum (the left part of the small intestine). The weight of the fetal small intestine was recorded (Dong et al., 2016). Ten grams of phosphate buffered saline (PBS)-washed duodenal, jejunal, and ileal tissue samples were collected followed by immediate freezing in liquid nitrogen as well as preservation at −80 °C for later analyses (Yin et al., 2009).
2.5. Concentrations of nitric oxide synthase (NOS) and nitric oxide (NO) in fetal intestine
Concentrations of NOS and NO in fetal intestinal samples were measured using commercially available detection kits (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) in line with specific protocols (Liu et al., 2009). The protein content was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Results of NO content analysis were calculated as micromole per gram (μmol/g) of protein. In addition, total NOS (TNOS) and inducible NOS (iNOS) activities were measured using the NOS activity detection kit. Thereafter, TNOS was subtracted from iNOS to determine the activity of constitutive NOS (cNOS). Results of tNOS and iNOS activities were calculated as units per gram (U/g) of protein. Afterwards, the optical density was determined at 450 nm. We set inter-assay CV as <15% (Guo et al., 2017).
2.6. Analysis of AA concentrations in fetal intestine
The AA profiles were measured in duodenal, jejunal, and ileal samples by reverse-phase HPLC (HP1100; Agilent) using norleucine as an internal standard in accordance with published methods (Bidlingmeyer et al., 1984; Sun et al., 2018).
2.7. mRNA abundance
Total RNA extraction and RT-PCR analysis were carried out according to previous protocols (Zhang et al., 2019a). In brief, total RNA was extracted with Trizol followed by DNase I treatment to remove genomic DNA. Electrophoresis (A260/A280, Beckman DU-800, Beckman Coulter, Inc., Fullerton, CA, USA) was used to assess the purity and quality of RNA samples, separately. Subsequently, the ABI 7900 PCR system (ABI Biotechnology, Eldersburg, MD, USA) was employed for real-time PCR. The Primer 5.0 software was adopted to design primers for specific genes (Table 1), with β-actin being the endogenous control. The abundance of each target mRNA was expressed relative to β-actin mRNA. The 2−ΔΔCt approach was used to calculate target gene relative abundance. Target gene mRNA abundance in treatment groups was determined compared with that of the CON group (i.e. fold-difference or change; Zhang et al., 2018a).
Table 1.
Primer sequences used for real-time PCR.
| Gene | Sequence (5′–3′) | GenBank accession number |
|---|---|---|
| SLC7A1 | F: ACCTAATGTCCATCGGCACTCT | XM_012184646.2 |
| R: ATCGCTGCTGCTCACCAACT | ||
| SLC1A1 | F: TTGGTCTGCGTGCTGTCGT | XM_004004350.3 |
| R: CATGGCATCCACTGTGCTAACT | ||
| SLC7A7 | F: CAGTTGCATTATCCTGCTTCGGT | XM_004010352.3 |
| R: GCCTATCGGGCTCCTTCCAG | ||
| SLC1A5 | F: GATCGTGGAGATGGAGGAT | AF334818 |
| R: TGACTGCTTCGAGGATGATG | ||
| SLC15A1 | F: GGGCACCGCCATCTATCAC | NM_001009758.1 |
| R: CAGACACGCAAGGCTTTATCC | ||
| β-actin | F: TCTGGCACCACACCTTCTAC | NM_001009784.1 |
| R: TCTTCTCACGGTTGGCCTTG |
SLC7A1 = solute carrier family 7 (cationic amino acid transporter, y+ system), member 1; SLC1A1 = solute carrier family 1 (neuronal/epithelial high-affinity glutamate transporter, system XAG), member 1; SLC7A7 = solute carrier family 7 (amino acid transporter light chain, y+ L system), member 7; SLC1A5 = solute carrier family 1 (alanine-serine-cysteine amino acid transporter 2), member 5; SLC15A1 = solute carrier family 15 (peptide transporter 1), member 1.
2.8. Determination of protein abundance by Western blotting
Total protein from fetal ileal tissue was extracted with a commercial kit (Beyotime Biotechnology, Jiangsu, China) and homogenized according to the protocol of the manufacturer (Zhang et al., 2018c). The bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) was used to measure the protein content. The extracted protein sample (typically 30 μg) was denatured, separated in parallel by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis (Bio-Rad, Richmond, CA, USA), and then electroblotted onto polyvinylidene fluoride (PVDF) membranes (0.45 μm, Millipore, Billerica, MA, USA). Then, the membrane was incubated for 1 h with 5% (wt/vol) of bovine serum albumin to block nonspecific binding, followed by overnight incubation at 4 °C with a primary antibody (Zhang et al., 2018c). Primary antibodies displayed below were used in this study, including β-actin (dilution at 1:5,000, 60008-1, Proteintech); total Akt (sc1083, 1:1,000, Abcam); phospho-Akt (Ser473) (1:1,000, sc 6732, Abcam); total mTOR (1:500, 20657-1-AP, Proteintech); phospho-mTOR (Ser2448) (1:2,000, ab137133, Abcam); total S6 kinase 1 (S6K1) (1:1,000, sc15098, Abcam); phospho-S6K1 (Thr389) (1:1,000, sc16542, Abcam); AA transporter light chain, y+ L system, member 7 (y+LAT2) (1:500, sc35673, Santa); alanine-serine-cysteine AA transporter 2 (ASCT2) (1:500, sc-87896, Santa); cationic AA transporter, y+ system, member 1 (CAT1, 1:500, sc-66824, Santa); peptide transporter 1 (PEPT1) (1:500, sc19965, Santa); and epithelial/neuronal high-affinity glutamate transporter, system XAG, member 1 (EAAT3) (1:1,000, ab41776, Abcam). The secondary antibodies were goat anti-mouse IgG HRP (1:5,000; Antgene Biotech) and goat anti-rabbit IgG HRP (1:5,000; Antgene Biotech). After washing 3 times, the membrane was incubated with secondary antibody at room temperature for 1 h. After washing, immunoreactive bands were visualized using an ECL Western blotting detection system (Fijifilm, Tokyo, Japan). Band intensities were estimated by a densitometry measurement using Image J software (Wayne Rasband, Maryland, USA) and the target protein was normalized to β-actin. Values for phosphorylation of each protein were normalized for total protein. The ratio of phosphorylated to total protein is presented next to the blots.
2.9. Statistical analysis
Statistical comparisons among the four groups of ewes killed on d 110 of gestation were conducted using one-way analysis of variance (ANOVA). All results are presented as least square means ± SEM. In addition, fetal sex was introduced in the original model, and no significance was detected (P > 0.05); thus, it was excluded from the final model. Only maternal nutritional treatment was used as the fixed effect in the model. Fetus within treatment was the random effect. All fetal measures were subjected to least squares ANOVA using the General Linear Models procedures of SAS (SAS Inst. Inc., Cary, NC) with pairwise comparisons. Differences across treatments were determined by the Duncan's multiple range test. All analyses were performed using the statistical package SAS (version 9.1). Differences in means were considered statistically significant when a P value was ≤0.05.
3. Results
3.1. Maternal BW and DMI, fetal BW and organ weights
Relative to CON groups, the maternal BW and DMI in the RES group was lower (P < 0.05; Appendix Table 2), and maternal RP-Arg or NCG supplementation did not alter (P > 0.05) maternal BW in RES ewes. In comparison with the RES group, ewes supplemented with Arg and NCG increased fetal BW and small intestinal weight (P < 0.05; Zhang et al., 2016a).
3.2. NOS and NO concentrations in fetal intestine
The fetal duodenal, jejunal and ileal concentrations of TNOS, NO, cNOS and iNOS in RES ewes decreased (P < 0.05) relative to those in CON ewes (Fig. 1). However, NCG or Arg supplementation alleviated changes induced by RES on concentrations of TNOS, NO, cNOS and iNOS in fetal intestine (P < 0.05).
Fig. 1.
Fetal NO (A), TNOS (B), iNOS (C) and eNOS (D) concentration of intestine in CON, RES, RES + ARG and RES + NCG groups on d 110 of gestation. Results are expressed as means ± SEM (n = 16 per group). CON, ewes fed 100% of nutrient requirements recommended by NRC (2007); RES, 50% of nutrient requirements recommended by NRC (2007); RES + ARG, 50% of nutrient requirements recommended by NRC (2007) and 20 g/d RP-Arg; RES + NCG, 50% of nutrient requirements recommended by NRC (2007) and 5 g/d NCG. a, b, c Means labeled with different letters indicate statistical significance (P < 0.05). NO = nitric oxide; NOS = nitric oxide synthase; TNOS = total NOS; iNOS = inducible NOS; cNOS = constitutive NOS.
3.3. Fetal intestinal AA profiles
With the exception of alanine, histidine, glutamate and aspartate, fetal AA concentrations in duodenum, ileum and jejunum in the RES group decreased by 14.1% to 54.3%, relative to CON group (P < 0.05; Table 2, Table 3). NCG or Arg supplementation in the diet alleviated changes induced by RES on cysteine, Arg, isoleucine, leucine, citrulline, ornithine, proline and glutamine concentrations in fetal duodenum, ileum and jejunum (P < 0.05).
Table 2.
Fetal intestinal essential amino acid concentrations (nmol/g wet weight) in CON and RES ewes and in RES ewes supplemented with Arg or NCG on d 110 of gestation1.
| Item | CON2 | RES3 | RES + ARG4 | RES + NCG5 | SEM | P-value |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| Arginine | 1,892a | 1,443c | 1,601b | 1,619b | 22.1 | 0.014 |
| Cysteine | 991a | 453c | 643b | 661b | 11.9 | 0.009 |
| Histidine | 223 | 219 | 227 | 225 | 6.3 | 0.208 |
| Isoleucine | 386a | 271c | 329b | 321b | 8.7 | 0.021 |
| Leucine | 493a | 374c | 434b | 442b | 16.3 | 0.012 |
| Lysine | 798a | 567c | 669b | 678b | 13.4 | 0.014 |
| Methionine | 132a | 83c | 102b | 108b | 8.1 | 0.007 |
| Phenylalanine | 286a | 192b | 198b | 201b | 5.8 | 0.012 |
| Threonine | 419a | 267b | 272b | 276b | 8.6 | 0.007 |
| Tryptophan | 156a | 109b | 108b | 113b | 7.3 | 0.019 |
| Valine | 682a | 513b | 519b | 523b | 13.1 | 0.008 |
| Jejunum | ||||||
| Arginine | 1,677a | 1,378b | 1,623a | 1,638a | 32.3 | 0.007 |
| Cysteine | 863a | 486c | 615b | 626b | 15.6 | 0.021 |
| Histidine | 219 | 223 | 225 | 218 | 7.3 | 0.308 |
| Isoleucine | 311a | 208c | 256b | 261b | 8.1 | 0.009 |
| Leucine | 498a | 364c | 431b | 445b | 12.4 | 0.016 |
| Lysine | 783a | 536b | 544b | 551b | 15.8 | 0.024 |
| Methionine | 134a | 99b | 101b | 102b | 6.9 | 0.008 |
| Phenylalanine | 212a | 141b | 148b | 142b | 10.7 | 0.012 |
| Threonine | 348a | 253b | 267b | 259b | 9.4 | 0.009 |
| Tryptophan | 156a | 108b | 112b | 116b | 10.9 | 0.019 |
| Valine | 684a | 462b | 458b | 471b | 23.7 | 0.007 |
| Ileum | ||||||
| Arginine | 1,598a | 1,007c | 1,326b | 1,332b | 19.3 | 0.008 |
| Cysteine | 684a | 348c | 493b | 504b | 18.3 | 0.013 |
| Histidine | 215 | 218 | 217 | 212 | 9.7 | 0.142 |
| Isoleucine | 335a | 208c | 259b | 262b | 7.3 | 0.007 |
| Leucine | 498a | 366c | 419b | 421b | 11.7 | 0.009 |
| Lysine | 784a | 603b | 599b | 606b | 11.2 | 0.005 |
| Methionine | 114a | 61c | 88b | 93b | 5.8 | 0.008 |
| Phenylalanine | 232a | 178b | 183b | 181b | 9.2 | 0.016 |
| Threonine | 369a | 279b | 282b | 286b | 11.7 | 0.009 |
| Tryptophan | 148a | 94b | 98b | 92b | 6.4 | 0.009 |
| Valine | 664a | 478b | 488b | 492b | 20.8 | 0.023 |
a,b,c Means in a row with superscripts without a common letter differ (P < 0.05).
Data are means and pooled SEM (n = 16 per group).
CON, ewes fed 100% of nutrient requirements recommended by NRC (2007).
RES, ewes fed 50% of nutrient requirements recommended by NRC (2007).
RES + ARG, ewes fed 50% of nutrient requirements recommended by NRC (2007) and supplemented with 20 g/d RP-Arg.
RES + NCG, ewes fed 50% of nutrient requirements recommended by NRC (2007) and supplemented with 5 g/d NCG.
Table 3.
Fetal intestinal non-essential amino acid concentrations (nmol/g wet weight) in CON and RES ewes and in RES ewes supplemented with Arg or NCG on d 110 of gestation1.
| Item | CON2 | RES3 | RES + ARG4 | RES + NCG5 | SEM | P-value |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| Alanine | 6,012 | 5,988 | 6,057 | 6,102 | 201.3 | 0.108 |
| Aspartate | 489 | 479 | 491 | 471 | 11.7 | 0.201 |
| Glutamate | 5,892 | 5,738 | 5,809 | 5,799 | 112.1 | 0.113 |
| Glutamine | 1,986a | 1,705c | 1,837b | 1,865b | 35.8 | 0.012 |
| Citrulline | 512a | 327c | 409b | 412b | 7.3 | 0.006 |
| Ornithine | 135a | 89c | 109b | 112b | 6.3 | 0.024 |
| Proline | 4,078a | 3,009c | 3578b | 3,606b | 82.9 | 0.014 |
| Jejunum | ||||||
| Alanine | 6,372 | 6,299 | 6,302 | 6,339 | 134.7 | 0.211 |
| Aspartate | 463 | 458 | 466 | 461 | 10.6 | 0.113 |
| Glutamate | 6,124 | 6,098 | 6,079 | 6,102 | 105.4 | 0.236 |
| Glutamine | 2,011a | 1,629c | 1,817b | 1,892b | 51.1 | 0.009 |
| Citrulline | 377a | 289c | 323b | 329b | 9.4 | 0.009 |
| Ornithine | 105a | 67c | 81b | 85b | 5.2 | 0.011 |
| Proline | 3,109a | 2,257c | 2,688b | 2,701b | 101.8 | 0.007 |
| Ileum | ||||||
| Alanine | 6,289 | 6,305 | 6,267 | 6,328 | 126.8 | 0.108 |
| Aspartate | 511 | 519 | 515 | 517 | 10.9 | 0.118 |
| Glutamate | 6,598 | 6,608 | 6,569 | 6,572 | 108.3 | 0.101 |
| Glutamine | 1,703a | 1,405c | 1,599b | 1,616b | 35.9 | 0.023 |
| Citrulline | 427a | 348c | 391b | 388b | 9.4 | 0.008 |
| Ornithine | 126a | 83c | 103b | 109b | 4.98 | 0.014 |
| Proline | 3,721a | 2,801c | 3,306b | 3,312b | 98.6 | 0.006 |
a,b,c Means in a row with superscripts without a common letter differ (P < 0.05).
Data are means and pooled SEM (n = 16 per group).
CON, ewes fed 100% of nutrient requirements recommended by NRC (2007).
RES, ewes fed 50% of nutrient requirements recommended by NRC (2007).
RES + ARG, ewes fed 50% of nutrient requirements recommended by NRC (2007) and supplemented with 20 g/d RP-Arg.
RES + NCG, ewes fed 50% of nutrient requirements recommended by NRC (2007) and supplemented with 5 g/d NCG.
3.4. AA and peptide transporter mRNA abundance in fetal intestine
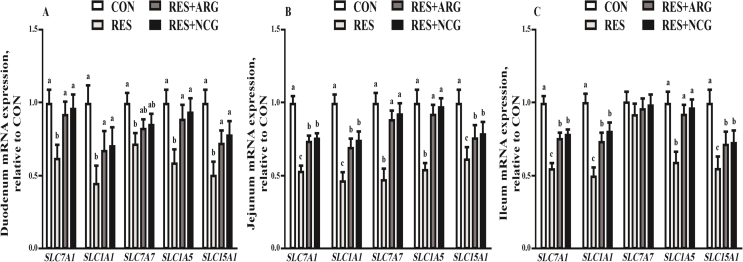
Relative to CON, fetal jejunal, ileal, and duodenal mRNA abundance of solute carrier family 1 (epithelial/neuronal high affinity glutamate transporter, system XAG), member 1 (SLC1A1), solute carrier family 1 (alanine-serine-cysteine AA transporter 2), member 5 (SLC1A5), solute carrier family 15 (peptide transporter 1), member 1 (SLC15A1) and SLC7A1 decreased by 38.3% to 53.8% among RES ewes (P < 0.05) (Fig. 2). Relative to the RES group, abundance of SLC1A1, SLC7A1, SLC15A1 and SLC1A5 (P < 0.05) in the RES + NCG and RES + ARG groups was 34.9% to 63.6% greater. NCG or Arg supplementation normalized the RES-induced changes in fetal intestinal SLC1A5 abundance (P < 0.05).
Fig. 2.
The duodenal (A), jejunal (B) and ileal (C) mRNA expressions of fetal amino acid and peptide transporters in CON, RES, RES + ARG and RES + NCG groups on d 110 of gestation. Results are expressed as means ± SEM (n = 16 per group). CON, animals raised with 100% of nutrient requirements recommended by NRC (2007); RES, 50% of nutrient requirements recommended by NRC (2007); RES + ARG, 50% of nutrient requirements recommended by NRC (2007) and 20 g/d RP-Arg; RES + NCG, 50% of nutrient requirements recommended by NRC (2007) and 5 g/d NCG. a, b, c Means labeled with different letters indicate statistical significance (P < 0.05). SLC7A1 = solute carrier family 7 (cationic amino acid transporter, y+ system), member 1; SLC1A1 = solute carrier family 1 (neuronal/epithelial high-affinity glutamate transporter, system XAG), member 1; SLC7A7 = solute carrier family 7 (amino acid transporter light chain, y+ L system), member 7; SLC1A5 = solute carrier family 1 (alanine-serine-cysteine amino acid transporter 2), member 5; SLC15A1 = solute carrier family 15 (peptide transporter 1), member 1.
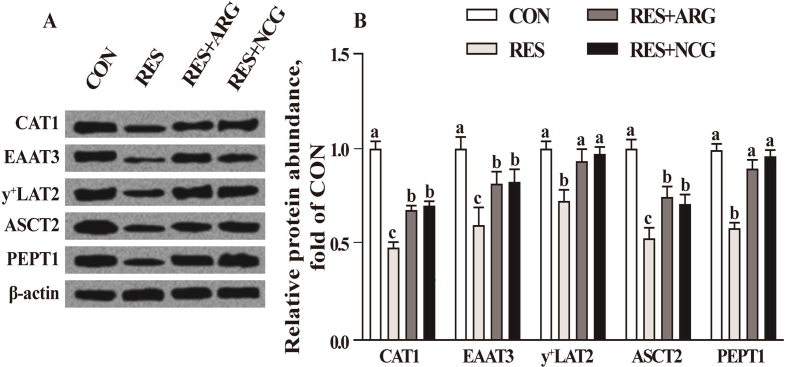
3.5. Fetal ileal AA and peptide transporter protein abundance
Relative to CON ewes, the ileal levels of EAAT3, CAT1, PEPT1, ASCT2, and y+LAT2 proteins decreased by 35.2% to 51.7% (P < 0.05) among RES fetuses (Fig. 3). Relative to RES, Arg and NCG supplementation in the diet resulted in 41.5% to 61.2% greater (P < 0.05) EAAT3, CAT1, PEPT1, ASCT2, and y+LAT2 protein abundance in fetal ileum.
Fig. 3.
The ileal levels of peptide transporters and amino acids of fetuses in CON, RES, RES + ARG and RES + NCG groups on d 110 of gestation. SLC1A1, SLC7A1, SLC1A5, SLC15A1 and SLC7A7 were responsible for encoding EAAT3, CAT1, ASCT2, PEPT1 and y+LAT2, separately. (A) Typical charts showing the Western blotting analysis. (B) Densitometric data were presented in the manner of relative levels and normalized based on β-actin. Results are expressed as means ± SEM (n = 16 per group). CON, animals raised with 100% of nutrient requirements recommended by NRC (2007); RES, 50% of nutrient requirements recommended by NRC (2007); RES + ARG, 50% of nutrient requirements recommended by NRC (2007) and 20 g/d RP-Arg. RES + NCG, 50% of nutrient requirements recommended by NRC (2007) and 5 g/d NCG. a, b, c Means labeled with different letters indicate statistical significance (P < 0.05). CAT1 = cationic amino acid transporter, y+ system, member 1; EAAT3 = neuronal/epithelial high-affinity glutamate transporter, system XAG, member 1; y+ LAT2 = amino acid transporter light chain, y+ L system, member 7; ASCT2 = alanine-serine-cysteine amino acid transporter 2; PEPT1 = peptide transporter 1.
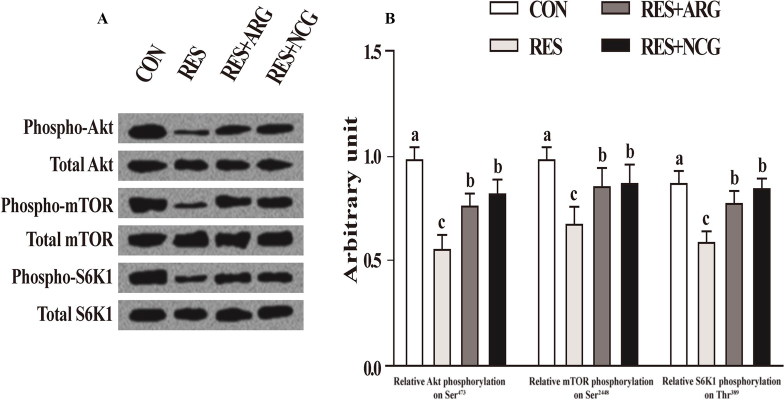
3.6. Fetal ileal phosphorylation levels of S6K1, mTOR and Akt
Relative to CON, pAKt-to-Akt, pS6K1-to-S6K1 and pmTOR-to-mTOR ratios in fetal ileum decreased by 38.7%, 31.2%, and 24.6% in RES group (P < 0.05), respectively (Fig. 4). NCG or Arg supplementation alleviated changes induced by RES on the pAKt-to-Akt, pmTOR-to-mTOR, and pS6K1-to-S6K1 ratios in fetal ileum (P < 0.05).
Fig. 4.
The mTOR signaling pathways of fetal ileum in CON, RES, RES + ARG and RES + NCG groups on d 110 of gestation. (A) Typical charts showing Western blotting analysis. (B) Relative Akt phosphorylation on Ser473, with the values of phosphorylated Akt being normalized for total Akt; Relative mTOR phosphorylation on Ser2448, with the values of phosphorylated mTOR being normalized for total mTOR; Relative S6K1 phosphorylation on Thr389, with the values of phosphorylated S6K1 being normalized for total S6K1. Results are expressed as means ± SEM (n = 16 per group). CON, animals raised with 100% of nutrient requirements recommended by NRC (2007); RES, 50% of nutrient requirements recommended by NRC (2007); RES + ARG, 50% of nutrient requirements recommended by NRC (2007) and 20 g/d RP-Arg; RES + NCG, 50% of nutrient requirements recommended by NRC (2007) and 5 g/d NCG. a, b, c Means labeled with different letters indicate statistical significance (P < 0.05). Akt = α serine–threonine kinase; mTOR = mammalian target of rapamycin; S6K1 = S6 kinase 1.
4. Discussion
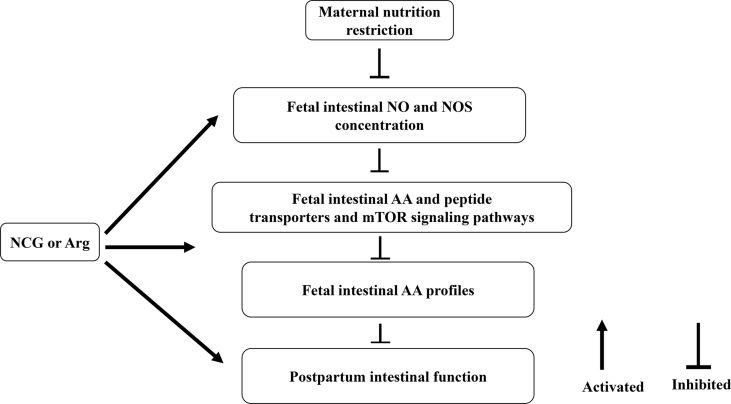
Pregnancy in ewes is unequally divided into 3 periods: early gestation (d 0 to 45 gestation), mid-gestation (d 46 to 115 of gestation) and late-gestation (d 116 of gestation to birth; Pillai et al., 2017). Maternal nutrition from early to mid-gestation in ewes is one of the main factors affecting fetal growth and organ development (Ford et al., 2007; Sun et al., 2018). It has been reported that the growth rate of fetal lambs on d 100 to 120 of gestation is higher compared with d 121 to 147 (Lassala et al., 2011). These data demonstrated that maternal under-nutrition during early-to-mid-gestation in ewes is more important to fetuses than in late-gestation (Sun et al., 2018). According to our previous study, NCG or Arg supplementation from d 35 to 110 of pregnancy ameliorated IUGR experienced by the fetuses carried by the ewes, and enhanced fetal BW and small intestinal development and growth (Zhang et al., 2016a). In the present study, the results showed that dietary NCG or Arg increased IUGR fetal intestinal AA profiles. This effect was probably related to changes in peptide transporters/AA profiles in fetal intestine and/or the protein signal transduction in the mTOR and insulin-associated pathways (Fig. 5). These observed changes may be due to nutritional and endocrine improvements related to overall better fetal nutrition and AAs transferred across the placenta in ewes supplemented with these long lasting and enhancing products related to Arg metabolism. Future studies should be conducted on understanding the role of the placenta on the improvement of growth and AA profiles independent of the fetal intestine.
Fig. 5.
Possible mechanism of Arg or NCG on the regulation of the fetal intestinal AA profiles during IUGR in undernourished ewes. NCG = N-carbamylglutamate; Arg = L-arginine; NO = nitric oxide; NOS = nitric oxide synthase; AA = amino acid; mTOR = mammalian target of rapamycin. IUGR = intrauterine growth retardation.
Nitric oxide is produced by NO synthase using Arg in almost every cell type. Increasing evidence demonstrates that NO can regulate metabolism of AA, fatty acids and glucose in mammals (Jobgen et al., 2006). The small intestine exerts a vital role in catabolizing numerous dietary non-essential and essential AA (such as the branched-chain AAs, phenylalanine, lysine and methionine; Wu, 1998). Arg and NO play indispensable roles in maintaining the integrity of intestinal mucosa through mechanisms dependent on focal adhesion kinases, which can be reflected by enterocyte growth (Yin et al., 2014). Degradation of Arg to NO via iNOS and cNOS is carefully controlled at the whole body, tissue and cell levels. Thus, Arg supplementation in the diet may serve as an essential part to maintain normal biological functions that involve NO signaling (Wu et al., 2009). This idea is supported by our results on Arg, NOS and NO levels in fetal intestine in RES ewes treated with NCG or Arg.
AA play important roles in nutrition, immune response and growth performance (Fontana et al., 2016). Each AA has its own unique and tissue-specific metabolic function. Typically, reduced glutamine and proline concentrations in intestine of IUGR fetuses suggests that their degradation might be increased, which may possibly restrict AA availability to peripheral tissues. The above AA (glutamine and proline) exert important roles in regulating the synthesis of tissue proteins (Yin et al., 2014). Glutamine and its metabolite glutamate have a beneficial role in stimulating fetal intestinal development and neonatal growth (Zhu et al., 2018; Duan et al., 2014). Furthermore, most citrulline formed in enterocytes of suckling piglets is converted to Arg locally (Wu et al., 2009). Additionally, the decreased Arg concentration in fetal intestine, as well as the reduced proline, ornithine, and citrulline concentrations (all participated in synthesis of Arg) of RES ewes was similar to previous findings in IUGR pigs (Wang et al., 2012). Similarly, the elevated citrulline, Arg, proline and ornithine concentrations in fetal small intestine of RES ewes supplemented with Arg and NCG agreed with prior findings from piglets (Yao et al., 2011), suckling lambs (Zhang et al., 2019b) and humans (Nagasaka et al., 2006). Mechanistically, the reduced level of citrulline, the precursor of Arg, indicated the presence of intestinal dysfunction in animals as well as humans (Crenn et al., 2008). Thus, the increased concentrations of citrulline and glutamine in fetal intestinal mucosa in RES ewes treated with Arg or NCG further indicated improved intestinal functions in fetuses.
AA absorption is largely dependent on specific transport proteins located within the enterocyte membrane (Wu, 2013). The basic, acidic and neutral systems are primarily responsible for intestinal AA absorption, allowing for transport of neutral AA, glutamate, aspartate as well as cationic AA + cysteine (Broer, 2008). The SLC1A5, SLC7A7, SLC7A1, SLC1A1, and SLC15A1 have been identified as the major intestinal transporters for neutral, basic, and acidic AA and peptides, respectively (Broer, 2008). To test whether the alterations of AA profiles in fetal intestine were related to the absorption process, the mRNA and protein abundance of AA transporters, including CAT1, EAAT3, y+LAT2, ASCT2, and PEPT1 (encoded by SLC7A1, SLC1A1, SLC7A7, SLC1A5, and SLC15A1, respectively), were examined. In this study, most AA transporters in fetal intestine were up-regulated due to NCG and Arg supplementation, indicating that these might be a possible mechanism to alleviate the adverse impacts on intestinal absorption in offspring induced by IUGR. The abundance of AA transporters increased in the fetal intestines when NCG and Arg were fed to RES animals, which agreed with changes in AA profiles in fetal intestine. This suggested that the enhanced AA profiles in response to Arg and NCG might partly occur through controlling nutrient transporter expression in the intestine.
Environmental inputs such as growth factors and nutrients can regulate metabolism and growth of eukaryotic cells via mTOR (Saxton and Sabatini, 2017). Mammalian target of rapamycin, the threonine/serine protein kinase identified within the phosphoinositide 3-kinase-related kinase (PIKK) family, constitutes the catalytic subunits in 2 protein complexes, which are referred to as mTOR complex 1 (mTORC1) and 2 (mTORC2). mTOR signaling can coordinate the metabolism of AA and the responses to the availability of AA (Javedan et al., 2016). In previous studies, Arg was suggested to enhance intestinal cell migration as well as the synthesis of intestinal proteins in vitro, partly through triggering activation of mTOR as well as S6K1 (Wu et al., 2009). The fact that fetuses with IUGR decreased mTOR phosphorylation levels in the ileum relative to those in normal fetuses, underscored the key role of this pathway within intestinal mucosa in ruminants. Phosphorylation of mTOR increased its function. Consequently, the greater concentrations of AA intermediates of Arg metabolism and synthesis (such as proline, ornithine, and citrulline) together with the increased p-mTOR and p-Akt levels in ileal mucosa of Arg and NCG-treated animals, underscored the significance of the above signal transduction pathways to the mitigation of the negative effects of IUGR.
5. Conclusion
Overall, NCG or Arg supplementation to underfed ewes can help mitigate certain negative effects of IUGR on fetal intestinal AA profiles. The benefits of Arg and NCG on intestinal AA profiles occur in part through controlling mTOR signal transduction as well as AA and peptide transport in fetuses experiencing IUGR. Supplementation with critical AA to the mother may improve intestinal absorptive capacity, allowing better postpartal growth, although this was not shown in the present study. This is an important finding overall, but the current data ignores the likely role of the key nutrient delivery to the fetus by the placenta rather than absorption of nutrients across the fetal intestine. Thus, further studies need to be conducted to explore the role of the placenta in these pregnancies on improving growth and AAs independent of the fetal intestine. Such studies will help demonstrate the relative contribution of intestinal uptake in fetal life.
Author contributions
Hao Zhang and Mengzhi Wang designed the research; Xiaoyun Liu, Yi Zheng and Ying Zhang conducted the research; Hao Zhang analyzed the data and wrote the paper; Juan J. Loor, Hongrong Wang and Mengzhi Wang revised the manuscript; Hao Zhang and Mengzhi Wang had primary responsibility for the final content. All authors read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The research was supported by the fund for the National Natural Science Foundation of China (31902180), the Science and Technology Innovation Project of Yangzhou University (2019CXJ152), the Top Talents Award Plan of Yangzhou University (2019), the funds from State Key Laboratory of Sheep Genetic Improvement and Healthy Production (2021ZD07) and the Cyanine Project of Yangzhou University (2020).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
The appendix to this article can be found online at https://doi.org/10.1016/j.aninu.2021.12.001.
Appendix. Supplementary data
The following is the supplementary data to this article:
References
- AOAC . Association of Official Analytical Chemists; Washington, D.C: 1990. Official methods of analysis. [Google Scholar]
- Bidlingmeyer B.A., Cohen S.A., Tarvin T.L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984;336:93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Chacher B., Wang D.M., Liu H.Y., Liu J.X. Degradation of l-arginine and N-carbamoyl glutamate and their effect on rumen fermentation in vitro. Ital J Anim Sci. 2012;11:374–377. [Google Scholar]
- Chacher B., Zhu W., Ye J.A., Wang D.M., Liu J.X. Effect of dietary N-carbamoylglutamate on milk production and nitrogen utilization in high-yielding dairy cows. J Dairy Sci. 2014;97:2338–2345. doi: 10.3168/jds.2013-7330. [DOI] [PubMed] [Google Scholar]
- Chen C., Yin Y., Tu Q., Yang H. Glucose and amino acid in enterocyte: absorption, metabolism and maturation. Front Biosci. 2018;23:1721–1739. doi: 10.2741/4669. [DOI] [PubMed] [Google Scholar]
- Crenn P., Messing B., Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Dong L., Zhong X., He J., Zhang L., Bai K., Xu W., et al. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr. 2016;35:399–407. doi: 10.1016/j.clnu.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Deng K.D., Jiang C.G., Tu Y., Zhang N.F., Liu J., Ma T., et al. Energy requirements of Dorper crossbred Ewe lambs. J Anim Sci. 2014;92:2161–2169. doi: 10.2527/jas.2013-7314. [DOI] [PubMed] [Google Scholar]
- Duan J., Yin J., Wu M., Liao P., Deng D., Liu G., et al. Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino acid transporters in young pigs. PLoS One. 2014;9:e112357. doi: 10.1371/journal.pone.0112357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldem T., Speiser P., Hincal A. Optimization of spray-dried and congealed lipid micropellets and characterization of their surface morphology by scanning electron microscopy. Pharm Res (N Y) 1991;8:47–54. doi: 10.1023/a:1015874121860. [DOI] [PubMed] [Google Scholar]
- Fontana L., Cummings N.E., Apelo S.I.A., Neuman J.C., Kasza I., Schmidt B.A., et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.P., Hess B.W., Schwope M.M., Nijland M.J., Gilbert J.S., Vonnahme K.A., et al. Maternal undernutrition during early to mid-gestation in the Ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85:1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- Guo Y.X., Nie H.T., Sun L.W., Zhang G.M., Deng K.P., Fan Y.X., et al. Effects of diet and arginine treatment during the luteal phase on ovarian NO/PGC-1α signaling in ewes. Theriogenology. 2017;96:76–84. doi: 10.1016/j.theriogenology.2017.03.028. [DOI] [PubMed] [Google Scholar]
- Javedan G., Shidfar F., Davoodi S.H., Ajami M., Gorjipour F., Sureda A., et al. Conjugated linoleic acid rat pretreatment reduces renal damage in ischemia/reperfusion injury: unraveling antiapoptotic mechanisms and regulation of phosphorylated mammalian target of rapamycin. Mol Nutr Food Res. 2016;60:2665–2677. doi: 10.1002/mnfr.201600112. [DOI] [PubMed] [Google Scholar]
- Jobgen W.S., Fried S.K., Fu W.J., Meininger C.J., Wu G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lassala A., Bazer F.W., Cudd T.A., Datta S., Keisler D.H., Satterfield M.C., et al. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr. 2010;140:1242–1248. doi: 10.3945/jn.110.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassala A., Bazer F.W., Cudd T.A., Datta S., Keisler D.H., Satterfield M.C., et al. Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J Nutr. 2011;141:849–855. doi: 10.3945/jn.111.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Han J., Huang J., Wang X., Wang F., Wang J. Dietary l-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian-Australas J Anim Sci. 2009;22:1667–1675. [Google Scholar]
- Mandò C., Tabano S., Colapietro P., Pileri P., Colleoni F., Avagliano L., et al. Transferrin receptor gene and protein expression and localization in human IUGR and normal term placentas. Placenta. 2011;32:44–50. doi: 10.1016/j.placenta.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Nagasaka H., Yorifuji T., Murayama K., Murakami T., Kanazawa M., Takatani T., et al. Effects of arginine treatment on nutrition, growth and urea cycle function in seven Japanese boys with late-onset ornithine transcarbamylase deficiency. Eur J Pediatr. 2006;165:618–624. doi: 10.1007/s00431-006-0143-y. [DOI] [PubMed] [Google Scholar]
- NRC . Natl. Acad. Press; Washington, DC: 2007. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. [Google Scholar]
- Pillai S., Jones A., Hoffman M., Mcfadden K., Reed S., Zinn S., et al. Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under- or over-nutrition during gestation. Transl Anim Sci. 2017;1:16–25. doi: 10.2527/tas2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo A., Carceller R., Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(Suppl 3):332–336. [PubMed] [Google Scholar]
- Russel A.J.F., Doney J.M., Gunn R.G. Subjective assessment of body fat in live sheep. J Agric Sci. 1969;72:451–454. [Google Scholar]
- Satterfield M.C., Dunlap K.A., Keisler D.H., Bazer F.W., Wu G. Arginine nutrition and fetal brown adipose tissue development in nutrient restricted sheep. Amino Acids. 2013;45:489–499. doi: 10.1007/s00726-011-1168-8. [DOI] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;68:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortt C., Hasselwander O., Meynier A., Nauta A., Fernández E.N., Putz P., et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur J Nutr. 2018;57:25–49. doi: 10.1007/s00394-017-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Zhang H., Wang Z., Fan Y., Guo Y., Wang F. Dietary rumen-protected arginine and N-carbamylglutamate supplementation enhances fetal growth in underfed ewes. Reprod Fertil Dev. 2018;30:1116–1127. doi: 10.1071/RD17164. [DOI] [PubMed] [Google Scholar]
- Van Soest, P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang W., Degroote J., Van Ginneken C., Van Poucke M., Vergauwen H., Dam T.M.T., et al. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. Faseb J. 2015;30:863–873. doi: 10.1096/fj.15-274779. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Zhou G., Liao Z., Ahmad H., Liu W., et al. Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intrauterine growth retarded piglets. Br J Nutr. 2012;108:1371–1381. doi: 10.1017/S0007114511006763. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Kim S.W., Li P., Rhoads J.M., et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Wallace J.M., Spencer T.E. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in nutrition and health. Amino Acids. 2013;45:407–411. doi: 10.1007/s00726-013-1500-6. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128(8):1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Yao K., Guan S., Li T., Huang R., Wu G., Ruan Z., et al. Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr. 2011;105:703–709. doi: 10.1017/S000711451000365X. [DOI] [PubMed] [Google Scholar]
- Yin F.G., Liu Y.L., Yin Y.L., Kong X.F., Huang R.L., Li T.J., et al. Dietary supplementation with astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids. 2009;37:263–270. doi: 10.1007/s00726-008-0142-6. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Duan J., Wu L., Chen S., Li T., et al. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin challenged pigs. Amino Acids. 2014;46:883–892. doi: 10.1007/s00726-013-1643-5. [DOI] [PubMed] [Google Scholar]
- Yung H., Hemberger M., Watson E.D., Senner C.E., Jones C.P., Kaufman R.J., et al. Endoplasmic reticulum stress disrupts placental morphogenesis: implications for human intrauterine growth restriction. Pathology. 2012;228:554–564. doi: 10.1002/path.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadrożna M., Gawlik M., Nowak B., Marcinek A., Mrowiec H., Walas S., et al. Antioxidants activities and concentration of selenium, zinc and copper in preterm and IUGR human placentas. J Trace Elem Med Biol. 2009;23:144–148. doi: 10.1016/j.jtemb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Zeng X., Huang Z., Mao X., Wang J., Wu G., Qiao S. N-Carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Sun L.W., Wang Z.Y., Wang M.T., Zhang G.M., Guo R.H., et al. Dietary N-carbamylglutamate and rumen-protected L-arginine supplementation ameliorate fetal growth restriction in undernourished ewes. J Anim Sci. 2016;94:2072–2085. doi: 10.2527/jas.2015-9587. [DOI] [PubMed] [Google Scholar]
- Zhang H., Sun L., Wang Z., Deng M., Nie H., Zhang G., et al. N-carbamylglutamate and L-arginine improved maternal and placental development in underfed ewes. Reproduction. 2016;151:623–635. doi: 10.1530/REP-16-0067. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhao F., Peng A., Dong L., Wang M., Yu L., et al. Effects of dietary l-arginine and N-carbamylglutamate supplementation on intestinal integrity, immune function, and oxidative status in intrauterine-growth-retarded suckling lambs. J Agric Food Chem. 2018;66:4145–4154. doi: 10.1021/acs.jafc.8b00726. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhao F., Nie H., Ma T., Wang Z., Wang F., et al. Dietary N-carbamylglutamate and rumen-protected l-arginine supplementation during intrauterine growth restriction in undernourished ewes improve fetal thymus development and immune function. Reprod Fertil Dev. 2018;30:1522–1531. doi: 10.1071/RD18047. [DOI] [PubMed] [Google Scholar]
- Zhang G.M., Zhang T.T., Jin Y.H., Liu J.L., Guo Y.X., Fan Y.X., et al. Effect of caloric restriction and subsequent re-alimentation on oxidative stress in the liver of Hu sheep ram lambs. Anim Feed Sci Technol. 2018;237:68–77. [Google Scholar]
- Zhang H., Peng A., Guo S., Wang M., Loor J.J., Wang H. Dietary N-carbamylglutamate and l-arginine supplementation improves intestinal energy status in intrauterine-growth-retarded suckling lambs. Food Funct. 2019;10:1903–1914. doi: 10.1039/c8fo01618f. [DOI] [PubMed] [Google Scholar]
- Zhang H., Peng A., Yu Y., Guo S., Wang M., Coleman D.N., et al. N-carbamylglutamate and l-arginine promote intestinal absorption of amino acids by regulating the mTOR signaling pathway and amino acid and peptide transporters in suckling lambs with intrauterine growth restriction. J Nutr. 2019;149:923–932. doi: 10.1093/jn/nxz016. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li T., Huang S., Wang W., Dai Z., Feng C., et al. Maternal L-glutamine supplementation during late gestation alleviates intrauterine growth restriction-induced intestinal dysfunction in piglets. Amino Acids. 2018;50:1289–1299. doi: 10.1007/s00726-018-2608-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.