Abstract
A biomarker describes a measurable indicator of a patient's clinical condition that can be measured accurately and reproducibly. Biomarkers offer utility for diagnosis, prognosis, early disease recognition, risk stratification, appropriate treatment (theranostics), and trial enrichment for patients with sepsis or suspected sepsis. In this narrative review, we aim to answer the question, "Do biomarkers in patients with sepsis or septic shock predict mortality, multiple organ dysfunction syndrome (MODS), or organ dysfunction?" We also discuss the role of pro- and anti-inflammatory biomarkers and biomarkers associated with intestinal permeability, endothelial injury, organ dysfunction, blood–brain barrier (BBB) breakdown, brain injury, and short and long-term mortality. For sepsis, a range of biomarkers is identified, including fluid phase pattern recognition molecules (PRMs), complement system, cytokines, chemokines, damage-associated molecular patterns (DAMPs), non-coding RNAs, miRNAs, cell membrane receptors, cell proteins, metabolites, and soluble receptors. We also provide an overview of immune response biomarkers that can help identify or differentiate between systemic inflammatory response syndrome (SIRS), sepsis, septic shock, and sepsis-associated encephalopathy. However, significant work is needed to identify the optimal combinations of biomarkers that can augment diagnosis, treatment, and good patient outcomes.
Keywords: Biomarker, Systemic inflammatory response, Sepsis, Septic shock, Sepsis-associated encephalopathy
Introduction
A biomarker describes a measurable indicator of biological status in normal and pathogenic processes. It may be helpful as a theranostic for identifying suitable patients for therapeutic intervention and titrating the degree and/or duration of intervention. A biomarker should be accurate and reproducible. In the ideal scenario, the biomarker (or combination of biomarkers) should offer both high specificity and sensitivity for diagnosing a condition, but either alone may be adequate as a 'rule-in' or 'rule-out' test.
Sepsis represents a dysregulated immune response to infection that leads to organ dysfunction [1]. Host response biomarkers play a critical role in diagnosis, early recognition of organ dysfunction, risk stratification, prognostication, and patient management, including antibiotic stewardship. Biomarkers may also be helpful for trial enrichment to identify suitable patients and/or risk categorization for an intervention. A wide range of biomarkers, measured by a host of different technologies, are being investigated to discriminate a systemic inflammatory response syndrome (SIRS) rapidly, which is an excessive defensive body's response to a harmful stressor (for example, infection, trauma, surgery, acute inflammation, ischemia or reperfusion, or cancer) [2] or early identification of infection-triggered organ dysfunction (sepsis). Also, the quick sepsis related organ failure assessment (qSOFA) is intended to raise suspicion of sepsis and encourage additional action; although, qSOFA is not a substitute for SIRS [3]. These biomarkers include measurement of acute-phase proteins, cytokines, chemokines, damage-associated molecular patterns (DAMPs), endothelial cell markers, leukocyte surface markers, non-coding RNAs, miRNA, and soluble receptors, as well as metabolites and alterations in gene expression (transcriptomics). Biomarkers may help stratify septic patients into biological phenotypes, for example, hyperinflammatory versus immunosuppressive. Biomarkers can also be used to identify gut permeability, blood–brain barrier (BBB) permeability, probability of hospital readmission, and longer-term outcomes [4, 5].
The causative pathogen replicates and releases its constituents such as endo- and exotoxins, and DNA. These constituents are designated pathogen-associated molecular patterns (PAMPs) [6, 7]. PAMPs are recognized by both pattern-recognition receptors (PRRs) and non-PRRs, which are essential components of the immune system [8, 9]. PRRs include several families, including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NOD)-like receptors (NLRs), a retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), C-type lectin receptors (CLRs), and intracellular DNA-sensing molecules. Non-PRRs include receptors for advanced glycation end products (RAGE), triggering receptors expressed on myeloid cells (TREM), and G-protein-coupled receptors (GPCRs) [10]. The sensing of PAMPs by immune cell receptors triggers a cascade of signaling pathways that activate multiple transcription factors to promote the production and release of pro- and anti-inflammatory mediators such as acute-phase proteins, cytokines, chemokines, as well as antimicrobial peptides, which are needed to eliminate the invading pathogen [11].
The host immune response and pathogen virulence factors will both trigger cell injury and/or induce cell stress. These results in the release of endogenous molecules (DAMPs), exacerbating the inflammatory response. DAMPs are recognized by the same immune receptors that recognize PAMPs [12, 13]. Many DAMPs have been identified, with some currently used as inflammatory biomarkers. Examples include proteins and cellular molecules related to nucleic acids, such as heat shock proteins (HSPs), the high mobility group box 1 (HMGB-1), and members of the S100 family [12, 14, 15]. The immune response may induce vascular endothelial damage disrupting tight junctions (T.J.), increasing gut permeability, and potentially facilitating translocation of pathogens and/or their PAMPs from the gut to the bloodstream and lymphatics, thereby amplifying the systemic inflammatory response [16]. In addition, an increase of BBB permeability allows circulating immune cells to enter the brain, triggering or exacerbating glial cell activation [17]. These events could trigger an intense and excessive host response activating coagulation and fibrinolytic systems, activating or suppressing hormonal, bioenergetic, and metabolic pathways, and inducing macro- and microcirculatory changes with a net result of multiple organ dysfunction. In the past few decades, researchers have studied each inflammatory response stage during SIRS, sepsis, and septic shock, metabolites associated with inflammatory cascades, and cellular components that could be used as biomarkers. These biomarkers could help identify endothelial damage, intestinal permeability, organ failure, BBB breakdown and predict rehospitalization, short- and long-term mortality, and cognitive consequences in survivors [18].
For this narrative review, we addressed the question, "Do biomarkers in patients with sepsis or septic shock predict mortality, MODS, or organ dysfunction?" Studies were identified by searching PubMed/MEDLINE (National Library of Medicine) databases for peer-reviewed journal articles published until October 2021. The abovementioned databases were searched with the following combinations of keywords: ("inflammatory response syndrome" OR "SIRS" OR "sepsis" OR "septic shock" OR "sepsis-associated encephalopathy" OR "SAE") AND ("markers" OR "biomarkers" OR "biological markers" OR "biological measures" OR "molecular predictor"). We omitted review articles, in vitro studies, and animal studies.
The humoral innate immune response, cytokines, and chemokines
The humoral innate immune response consists of multiple components, including fluid phase pattern recognition molecules (PRMs) and the complement system. PRMs include C-reactive protein (CRP), serum amyloid P component (SAP), and pentraxin 3 (PTX-3) [19]. The rise in CRP level is primarily induced by interleukin (IL)-6 and IL-1β acting on the gene responsible for CRP transcription during the acute phase of an inflammatory process. CRP is a pentameric acute-phase reactant protein whose conformation facilitates the ability to trigger complement activation and activate platelets, monocytes, and endothelial cells [20]. Furthermore, CRP is one of the most widely used and investigated biomarkers [21]. A prospective multicenter cohort study followed 483 adult patients who survived hospitalization for sepsis for up to one year. IL-6, high-sensitivity C reactive protein (hs-CRP), soluble programmed death-ligand 1 (sPD-L1), E-selectin, and intercellular adhesion molecule 1 (ICAM-1) were evaluated at five-time points during and after hospitalization. A comparison was made between a phenotype with hyperinflammation (high levels of IL-6 and hs-CRP) and a phenotype of immunosuppression (high sPD-L1 levels). Compared with a normal phenotype, both hyperinflammation and immunosuppression phenotypes had higher 6-month hospital readmission rates and 1-year mortality rates, both all-cause and attributable to cardiovascular or cancer [22].
Pentraxin (PTX-3) is secreted by macrophages, dendritic cells, macrophages, fibroblasts, mesangial cells, and glial cells under pathogen or inflammatory stimuli [19]. Plasma PTX-3 was assessed on days 1, 2, and 7 in 958 patients with sepsis or septic shock included in the Albumin Italian Outcome Sepsis (ALBIOS) study. The researchers assessed a possible association between PTX-3 levels and clinical severity, organ dysfunction, treatment, and mortality within 90 days. PTX-3 levels were elevated at the onset of sepsis and increased with illness severity and the number of organ dysfunctions. PTX-3 levels decreased between days 1 to 7, but this was less prominent in patients with septic shock [23]. In a prospective observational analysis, PTX-3, IL-6, procalcitonin (PCT), and lactate combined showed excellent performance in predicting 28-day all-cause mortality among patients diagnosed with sepsis or septic shock and superior to the Sequential Organ Failure Assessment (SOFA) score [24].
In a prospective pilot study of markers of complement activation in sepsis, higher C4d (3.5-fold), factor Bb (6.1-fold), C3 (0.8-fold), C3a (11.6-fold), and C5a (1.8-fold) levels were seen compared with healthy volunteer controls [25]. In another study of 49 sepsis patients, 34 developed disseminated intravascular coagulation (DIC), and eight died. Patients with DIC had lower C3 levels and higher SC5b-9 levels. On stratifying by SC5b-9 quartile (ng/mL: low: < 260, moderate: 260–342, high: 343–501, highest: > 501), coagulation parameters were most deranged in the highest quartile with prolonged thrombocytopenia and higher mortality (33%) [26].
The activation of PRRs culminates in the stimulation of transcription factors resulting in the expression and secretion of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-1-β, IL-6, and interferons (IFNs). These inflammatory mediators are required for host defense against pathogens and activation of the adaptive immune response. A retrospective study evaluated a broad panel of cytokines and found IL-1β, IL-6, IL-8, MCP-1, IL-10, and plasminogen activator inhibitor 1 (PAI-1) levels were increased in the acute phase of sepsis in both critically and non-critically ill patients. In addition, levels of IL-10 (days 1, 2, and 4), IL-6 and PAI-1 (days 2 and 4), and IL-8 (day 4) increased in critically ill patients compared to non-critically ill [27]. In summary, hs-CRP, IL-6, and PAI-1 circulatory levels may have utility in stratifying a hyperinflammatory patient phenotype.
DAMPs
DAMPs are endogenous danger molecules released from damaged or stressed cells. These molecules activate the innate immune system through interaction with PRRs. DAMPs contribute to the host defense but can also promote pathological inflammatory responses. Calprotectin, a protein found in the cytosol of neutrophils and macrophages, is released under cell stress or damage. In a mixed population study, plasma calprotectin levels were higher in sepsis than in trauma patients and other medical conditions. Calprotectin levels were higher in patients who did not survive for 30 days. Plasma PCT did not differ between the groups or as a prognosticator of the outcome. Receiver operating characteristic (ROC) analysis, used as a sepsis biomarker, had a higher area under the curve (AUC) value for calprotectin (AUC: 0.79) compared to PCT (AUC: 0.49) [28].
A prospective study evaluated IL-6, HMGB-1, and neutrophil gelatinase-associated lipocalin (NGAL) in 14 septic patients and 16 patients without sepsis admitted to the ICU. In patients with sepsis, IL-6 decreased levels were associated with ICU survival; NGAL levels rose in non-survivors, while HMGB-1 levels were unchanged in both survivors and non-survivors regardless of complications [29].
Endothelial cells and BBB markers
The first step in endothelial and BBB injury is the breakdown and destruction of proteins followed by release into the bloodstream. These proteins or peptides can be evaluated as a marker of endothelial cells and BBB integrity [30]. Plasma levels of occludin (OCLN), claudin-5 (CLDN-5), zonula-occludens 1 (ZO-1), PCT, and lactate were assessed in 51 septic patients. OCLN and ZO-1 were elevated with disease severity and positively correlated with the Acute Physiology and Chronic Health Evaluation II (APACHE-II) and SOFA scores and lactate levels. The predictive value for in-hospital mortality of ZO-1 was comparable to that of lactate levels, APACHE-II, and SOFA scores but superior to OCLN and PCT [31]. In a case series of brain autopsies from adults who died from sepsis, 38% had no OCLN expression in the endothelium of cerebral microvessels. BBB damage was associated with higher maximum SOFA scores and PCT levels > 10 μg/L. BBB damage in the cerebellum was more common with CRP values > 100 mg/L [32]. Soluble fms-like tyrosine kinase 1 (sFlt-1), soluble E-selectin (sE-selectin), soluble intercellular adhesion molecule 1 (sICAM-1), soluble vascular cell adhesion molecule 1 (sVCAM-1), and PAI-1 were evaluated in another studies. All these evaluated endothelial biomarkers were associated with sepsis severity. sFlt-1 had the strongest association with the SOFA score, while sFlt-1 and PAI-1 had the highest area under the operating receiver characteristic curve for mortality [33].
Syndecan-1 is a structural component of the endothelium. Antithrombin, PAI-1, syndecan-1, VCAM-1, E-selectin, IL-1β, IL-6, IL-8, HMGB-1, and histone-H3 were increased in septic patients compared with healthy controls. Non-survivors had a higher syndecan-1 level compared with survivors. On day one, an association was seen between syndecan-1 levels and APACHE-II, SOFA, DIC scores, hemostatic markers, IL-1β, IL-8, and PAI-1. Day 1 syndecan-1 levels were also significantly higher in patients with DIC and had reliable discriminative power to predict both DIC development and subsequent mortality [34].
The serum biomarker, calcium-binding protein B (S100B), reflects BBB disruption, glial cell injury, and activation. S100B is used to evaluate brain injury severity and predict outcomes from stroke, traumatic brain injury, encephalopathy, and delirium [35]. A prospective cohort study demonstrated that day three values for predicting 180-day mortality were superior to day one (0.731 vs. 0.611) [36]. Patients with sepsis-associated encephalopathy also had elevated levels. In another observational study of 22 patients with septic shock, delirium was present in ten. The odds ratio for the risk of developing delirium with an S100B > 0.15 μg/L was 18.0. Patients with delirium had higher plasma levels of IL-6. S100B and IL-6 levels were positively correlated [37]. S100B, PAI-1, angiopoietin (Ang)-2, ZO-1, and OCLN are the main biomarkers currently used to evaluate the vascular injury and BBB permeability.
Gut permeability markers
Critically ill patients show an increase in gut permeability, which may trigger a systemic inflammatory response syndrome and multiple organ dysfunction syndromes (MODS) [38]. Plasma zonulin levels were higher in sepsis patients compared to a post-surgical control group or healthy volunteers [39]. In another study, serum levels of intestinal fatty acid-binding protein (I-FABP) were higher in patients with sepsis and higher still in those with septic shock. Serum D-lactic acid levels were also elevated with sepsis but did not differentiate severity. Neither I-FABP nor D-lactic acid could prognosticate [40].
Non-coding RNAs and miRNA
A non-coding RNA (ncRNA) is an RNA molecule transcribed from DNA but not translated into proteins. A microRNA is a small non-coding RNA molecule that functions in RNA silencing and post-transcriptional gene expression regulation. ncRNAs and mRNAs are being studied as sepsis biomarkers. For example, long non-coding metastasis-associated lung adenocarcinoma transcript 1 (lnc-MALAT1) and micro RNA (miR)-125a were increased in sepsis patients compared with healthy controls and positively correlated with APACHE-II score, SOFA score, serum creatinine, CRP, TNF-α, IL-1β, IL-6, and IL-8. The lnc-MALAT1/miR-125a axis was also a predictor of increased 28-day mortality risk [41]. In another study lnc-MALAT1 expression was increased in acute respiratory distress syndrome (ARDS) patients compared to non-ARDS patients (AUROC: 0.674). Nonsurvivors compared to survivors (AUROC: 0.651) and positively correlated with APACHE-II and SOFA scores, CRP, PCT, TNF-α, IL-1β, IL-6, and IL-17 [42]. Long non-coding RNA maternally expressed gene 3 (lnc-MEG3), and the lnc-MEG3/miR-21 axis were increased, while miR-21 expression was decreased in sepsis patients compared with healthy controls. lnc-MEG3 (AUROC: 0.887) and the lnc-MEG3/miR-21 ratio (AUROC: 0.934) had high values for predicting elevated sepsis risk, while miR-21 (AUROC: 0.801) gave excellent predictive value for a reduced sepsis risk [43]. A further study showed miR-125a and miR-125b expressions were elevated in sepsis patients compared with healthy controls and were predictive of sepsis risk—miR-125a (AUROC: 0.749) and miR-125b (AUROC: 0.839). No correlation was seen between miR-125a and CRP, TNF-α, IL-6, IL-17, and IL-23 in however, miR-125b was positively associated with these cytokines. miR-125a failed to predict 28-day mortality risk (AUROC: 0.588) in sepsis patients, whereas miR-125b was superior (AUROC: 0.699) [44].
Membrane receptors, cell proteins, and metabolites
Cell surface receptors are receptors incorporated into the plasma membrane of cells and act on cell signaling by receiving or binding to extracellular molecules. After detecting such molecules, the production of metabolites occurs. In one study, the cluster of differentiation (CD)-13, CD14, CD25, CD64, and human leukocyte antigen (HLA-DR) showed acceptable sensitivity and specificity for mortality prediction (CD13 AUROC:0.824; CD64 0.843; and HLA-DR 0.804) while CD14 and CD25 did not predict mortality [45]. nCD64 expression, in a further study, nCD64, PCT, CRP, and SOFA scores were higher in septic patients, with nCD64 having the highest AUC (0.879) for differentiating a positive microbial culture. This was superior to PCT (0.868), SOFA score (0.701), CRP (0.609), and white blood cell (WBC) count. In predicting 28-day mortality, the combination of nCD64 and SOFA score had an AUROC of 0.91 versus 0.882 for the combination of PCT and SOFA [46].
A meta-analysis of 19 studies enrolling 3012 patients evaluated the value of PCT (AUROC 0.84) and presepsin (0.87 AUROC) for diagnosing sepsis. The pooled sensitivities and specificities were 0.80 and 0.75 for PCT and 0.84 and 0.73 for presepsin [47]. In one study, levels of presepsin, PCT, CRP, and WBC were higher in sepsis patients than in a SIRS group with AUROC values of 0.954 (presepsin), 0.874 (PCT), 0.859 (CRP), and 0.723 (WBC). The cut-off of presepsin for discriminating between sepsis and SIRS was 407 pg/ml, with sensitivity and specificity values of 98.6% and 82.6%, respectively [48]. In a study of septic children, TREM-1 levels were higher in septic shock patients [49].
Hormones and peptide precursors
Adrenomedullin (ADM) is synthesized in different tissues, including the adrenal cortex, kidney, lung, blood vessels, and heart. It has biological properties, including vasodilating, inotropic, diuretic, natriuretic, and bronchodilating. In one study, mid-regional pro adrenomedullin (MR-proADM) was an independent predictor of five different organ failures (respiratory, coagulation, cardiovascular, neurological, and renal), compared to lactate which predicted three (coagulation, cardiovascular and neurological), PCT two (cardiovascular and renal) and CRP (none) [50]. MR-proADM most accurately identified patients with a high likelihood of further disease progression compared to other biomarkers and clinical scores [51]. A total of 1089 individuals with either sepsis (142) or septic shock (977) were included in a randomized controlled study. The MR-proADM level within the first 24 h after sepsis diagnosis was associated with 7-day mortality (AUROC 0.72 and p < 0.001) and 90-day mortality (AUROC 0.71 and p < 0.001). Patients with declining PCT levels but persistently high MR-proADM levels on day-1 or day-4 had a substantially higher mortality risk of 19.1 (8.0–45.9) and 43.1 (10.1–184.0), respectively [52]. Adult patients hospitalized to ICU had their bioactive-ADM levels measured in this retrospective observational study. This study comprised a total of 1867 patients, 632 septic patients, and 267 septic shock patients. The median bioactive-ADM was 74 pg/mL in sepsis patients, 107 pg/mL in septic shock, and 29 pg/mL in non-septic patients. The association of elevated bioactive-ADM and mortality in sepsis patients and the ICU population resulted in O.R.s of 1.23 and 1.22, respectively [53]. In addition, the MR-proADM is potentially removal by continuous renal replacement therapy (CRRT) [54].
N-terminal (N.T.)-prohormone BNP (NT-proBNP) is a non-active prohormone produced by the heart and released in response to changes in intracardiac pressure. Higher levels of NT-proBNP at 24 h after sepsis onset were associated with lower short physical performance battery (SPPB) scores at 12 months and lower handgrip strength at 6-month and 12-month follow-up. NT-proBNP levels during the acute phase of sepsis may thus be a valuable indicator of a greater risk of long-term impairment in physical function and muscle strength in sepsis survivors [55]. In another study, NT-proBNP levels on admission were higher in-hospital non-survivors (7908 pg/mL) compared with survivors (3479 pg/mL). AUROC curves of admission and 72-h NT-proBNP levels for hospital mortality were 0.631 and 0.648, respectively [56].
PCT is produced in the thyroid's C cells and converted to calcitonin, with no PCT released into the bloodstream. During inflammatory processes, PCT is produced directly by stimulating bacterial components or induced by various inflammatory mediators such as IL-6 and TNF-α. PCT and presepsin had similar performance in predicting positive sepsis results with AUROC values of 0.75 and 0.73, respectively [57]. Another investigation gave AUROC values of 0.87 for PCT and 0.78 for presepsin in predicting bacteremia [58]. Plasma levels of presepsin and PCT were progressively higher in sepsis and septic shock than in non-septic patients. Presepsin was superior to PCT in diagnosing severe community-acquired pneumonia [59], while PCT was marginally superior in another study of septic patients admitted to intensive care [60]. On the other hand, MR-proADM had a better predictive value for septic shock. This study concluded that PCT, MR-proADM, and presepsin are complementary biomarkers that could have utility in the management of septic patients. In an intention-to-treat study comparing PCT versus no PCT guidance, there was no significant difference in 28-day mortality (25.6% PCT versus 28.2% no PCT, p = 0.34). The use of procalcitonin did not impact investment decisions as measured by the frequency of therapeutic and diagnostic interventions. [61].
Neutrophil-related biomarkers
High levels of resistin collected on day 1 of ICU admission were associated with an increased likelihood of developing new organ failure, whereas high myeloperoxidase (MPO) levels on day one were associated with an increased risk of developing incident organ failure for clotting and kidney systems [62].
Soluble receptors
Soluble trigger receptor expressed in the myeloid cell-1 (sTREM-1) is a TREM family member. This receptor offers excellent potential as a biomarker for infectious diseases as it can be measured in different biological fluids, including serum, pleural fluid, sputum, and urine [63]. However, a meta-analysis of 2418 patients enrolled in 19 studies showed serum sTREM-1 had only moderate accuracy in diagnosing patients with suspected sepsis [63]. Combining sTREM-1 with clinical variables offered more significant mortality discrimination compared to clinical variables alone [64]. In a multicenter prospective cohort study, soluble tumor necrosis factor receptor type 1 (sTNFR1) levels > 8861 pg/ml predicted 30-day mortality [65].
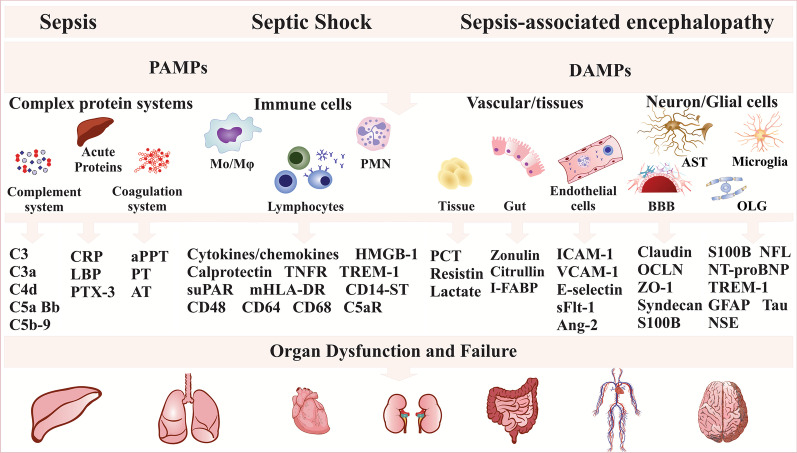
Patients with sepsis or septic shock displayed higher levels of the soluble form of the urokinase plasminogen activator receptor (suPAR), PCT, and lactate on days 1, 2, 4, and 7 of admission, with lactate and suPAR being the best risk stratifies for suspected infection [66]. Levels of suPAR and PCT levels were higher in sepsis patients than in a SIRS group with AUROC values of 0.89 and 0.82, respectively [67]. Serum sPD-L1 levels were increased in non-survivors compared with survivors with similar prognostic accuracy for 28-day mortality as APACHE-II and SOFA scores [68]. See Tables 1 and 2 for further information, as well as Fig. 1.
Table 1.
Different roles of the biomarkers in sepsis
| Biomarker | Function | References |
|---|---|---|
| Acute-phase proteins | ||
| CRP, hsCRP | Response to infection and other inflammatory stimuli | [4, 69, 70] |
| Predictive for increased 28-day mortality in patients with sepsis | ||
| Hyperinflammatory phenotype | ||
| Complement | Prognosis of disease severity | [25, 71] |
| Proteins | C5a can be predictive for DIC | |
| PTX-3 | Discrimination of sepsis and septic shock | [72, 73] |
| Diagnosis of sepsis and septic shock during the first week in the ICU | ||
| Prediction of septic shock | ||
| Cytokines and chemokines | ||
| IL-10 | Hypoinflammatory phenotype | [22, 71] |
| MCP-1 | It differentiates patients with septic shock from patients with sepsis | [73, 74] |
| Mortality prognosis at 30 days and six months | ||
| TNF-α, IL-1β, IL-6 | IL-6 all-cause mortality prognosis at 30 days and six months | [27, 74] |
| IL-1β and IL-6 acute phase of sepsis | ||
| It was increased in the hyperinflammatory phenotype | ||
| Organ dysfunction prognosis | ||
| DAMPs | ||
| Calprotectin | PCT to distinguish between patients with sepsis and patients without sepsis in the ICU | [28] |
| Predictive for 30-day mortality | ||
| HMGB-1 | Worst prognosis and higher 28-day mortality | [75, 76] |
| Endothelial cells and BBB markers | ||
| Syndecan-1 | Increase related to sepsis severity | [34] |
| Discriminative power for DIC and subsequent mortality | ||
| VLA-3 (a3β1) | Indicative of sepsis | [77, 78] |
| Discrimination of sepsis and SIRS | ||
| Ang-1 | It stabilizes the endothelium and inhibits vascular leakage by constitutively activating the Tie-2 receptor | [79] |
| Ang-2/Ang-1, Ang-1/Tie-2 ratio has a prognosis for 90-day mortality in sepsis and septic shock in the ICU higher than the PCT and SOFA score | ||
| Independent and effective predictors of SOFA score changes | ||
| Ang-2 | It can disrupt microvascular integrity by blocking the Tie-2 receptor, which results in vascular leakage | [73, 79] |
| Individuals with septic shock had higher levels of Ang-2 than those with sepsis | ||
| CLDN-5 | The absence of CLDN-5 may indicate damage to endothelial cells during sepsis | [31] |
| OCLN | Increase related to sepsis severity and positive correlation with SOFA scores | [31, 32] |
| Predictive of mortality | ||
| The absence of OCLN in the cerebral microvascular endothelium was related to more severe disease and intense inflammatory response | ||
| PAI-1 | Sepsis severity prognosis | [33, 34] |
| Predictor of mortality | ||
| An increase may indicate DIC | ||
| sICAM-1 | Sepsis severity prognosis | [33, 79] |
| Prognosis of 90-day mortality in patients with sepsis and septic shock in the ICU | ||
| S100B | It is associated with delirium in septic shock | [36, 37, 80] |
| Prognosis of severe organ dysfunction | ||
| Shortest survival in 180 days | ||
| Diagnosis of sepsis-associated encephalopathy | ||
| E-selectin | Sepsis severity prognosis | [33] |
| Predicts mortality | ||
| Increase related to SOFA and APACHE-II | ||
| sFlt-1 | Prognosis of sepsis severity and SOFA score, | [33] |
| The prognosis for morbidity and mortality | ||
| sVCAM-1 | Prognosis of sepsis severity and 28-day mortality | [33, 79, 81] |
| Prognosis of 90-day mortality in patients with severe sepsis and septic shock in the ICU | ||
| Risk of septic shock | ||
| ZO-1 | Prognosis of sepsis severity and correlation with APACHE-II and SOFA scores | [31, 32] |
| Predictor of mortality | ||
| Diagnostic capability for MODS | ||
| Gut permeability markers | ||
| Citrulline | The decrease may indicate early acute bowel dysfunction | [82, 83] |
| I-FABP | Risk of septic shock | [40] |
| Indicates early intestinal damage in patients with sepsis and septic shock | ||
| Zonulin | Indicates intestinal permeability during sepsis and SIRS | [39] |
| D-lactic acid | Indicates early intestinal damage in patients with sepsis and septic shock | [40] |
| Non-coding RNAs | ||
| Lnc-MALAT1 | The distinction between septic and non-sepsis patients | [41, 42] |
| Positive correlation with APACHE-II | ||
| Sepsis severity prognosis | ||
| High risk of ARDS | ||
| Predictive for high mortality | ||
| The increase can distinguish ARDS from non-ARDS | ||
| lnc-MEG3 | The increase is predictive of sepsis | [43] |
| 28-day mortality risk | ||
| miRNA | ||
| miR-125a, miR-125b | Prognosis of more significant disease severity | [44, 84, 85] |
| Distinguishes patients with sepsis from patients without sepsis | ||
| miR-125b: increased risk of mortality in patients with sepsis | ||
| miR-125a: risk of sepsis and increased mortality | ||
| Membrane receptors, cell proteins, and metabolites | ||
| CD64 | Prognosis of disease severity | [46] |
| 28-day mortality predictor | ||
| Early diagnosis of infection | ||
| CD68 | Prognosis of disease severity | [86] |
| Microglial activation | ||
| NFL | Indicates risk and severity of sepsis-associated encephalopathy | [87] |
| NFH | Indicates risk and severity of sepsis-associated encephalopathy | [87] |
| NSE | Diagnosis of sepsis-associated encephalopathy | [80, 88] |
| 30-day mortality risk | ||
| Risk of delirium | ||
| Neuronal injury marker in sepsis | ||
| Presepsin | Initial diagnosis and sepsis risk stratification | [48] |
| Potential marker to distinguish Gram ( +) and Gram (-) bacterial infection | ||
| TREM-1 | Sepsis indicator | [89–91] |
| An early distinction between sepsis and SIRS | ||
| Predictive of septic shock | ||
| Peptide precursor of the hormone and hormone | ||
| MR-proADM | Discrimination of survivors and non-survivors | [92] |
| Organ dysfunction marker | ||
| PCT | Diagnosis of sepsis | [66, 90] |
| Predicts Bacterial Infection | ||
| NT-proBNP | In the acute phase of sepsis it indicates a risk of long-term impairment of physical function and muscle strength | [55] |
| Predict mortality risk | [52] | |
| Neutrophil, cells, and related biomarkers | ||
| Lactate | Predictive of mortality | [93] |
| Risk stratification of patients with suspected infection | ||
| MPO | Increase in patients with DIC | [94, 95] |
| Indicates organ dysfunction | ||
| Mortality predictor at 28 and 90 days | ||
| Risk of septic shock | ||
| NET generation | ||
| Resistin | Sepsis indicator | [96, 97] |
| Risk of septic shock | ||
| 28-day mortality predictor | ||
| Soluble receptors | ||
| sPD-L1 | Prognosis of disease severity | [4, 68] |
| 28-day mortality predictor | ||
| Indicates immunosuppression | ||
| suPAR | Predictive mortality at 7 and 30 days | [66] |
| Risk of patients with suspected infection | ||
| sTNFR-1 | Prognosis of 28-day mortality | [81, 98] |
| Risk of septic shock | ||
| Risk of delirium | ||
| Lipoproteins | ||
| LDL-C | Protective effect against sepsis | [99] |
| The decrease can cause a risk of sepsis and admission to the ICU | ||
| HDL | Low levels: mortality prognosis and adverse clinical outcomes | [100, 101] |
| Predictive for MODS | ||
| T-chol | The decrease has a worse prognosis in sepsis | [102] |
Ang-1 angiopoietin-1, Ang-2 angiopoietin-2, APACHE-II acute physiology and chronic health evaluation II, ARDS acute respiratory distress syndrome, CD cluster of differentiation, CLDN-5 claudin-5, CRP C reactive protein, DAMPs damage-associated molecular patterns, DIC disseminated intravascular coagulation, HDL high-density lipoprotein, HMGB1 high mobility group box 1, hsCRP high-sensitivity C reactive protein, ICU intensive care unit, I-FABP intestinal fatty acid binding protein, IL interleukin, LDL low-density lipoprotein, lnc-MALAT1 long non-coding metastasis-associated lung adenocarcinoma transcript 1, lnc-MEG3 long non-coding RNA maternally expressed gene 3, MCP-1 monocyte chemoattractant protein-1, miR-125a micro RNA-125a, miR-125b micro RNA-125b, MODS multiple organ dysfunction syndrome, MPO myeloperoxidase, MR-proADM mid-regional pro adrenomedullin, NFL neurofilament light, NFH neurofilament heavy, NSE neuron specific enolase, NT-proBNP N-terminal pro-brain natriuretic peptide, OCLN occludin, OR odds ratio, PAI-1 plasminogen activator inhibitor 1, PCT procalcitonin, PTX-3 pentraxin-3), S100B calcium-binding protein B, sFlt-1 soluble fms-like tyrosine kinase 1, sICAM-1 soluble intercellular adhesion molecule 1, SIRS systemic inflammatory response syndrome, SOFA sequential organ failure assessment, sPD-L1 soluble programmed death ligand 1, sTNFR1 soluble tumor necrosis factor receptor type 1, suPAR soluble form of the urokinase plasminogen activator receptor, sVCAM-1 soluble vascular cell adhesion molecule 1, T-chol total cholesterol, TNF-α tumour necrosis factor alpha, TREM-1 triggering receptor expressed on myeloid cells-1, VLA-3/a3β1 integrin alpha 3 beta 1, ZO-1 zonula-occluden 1
Table 2.
Biomarkers for sepsis, septic shock, and sepsis-associated encephalopathy
| Biomarker | Sample | Demographic | Specificity (%) | Sensitivity (%) | Cut-off | R2 | AUC | Clinical relevance | References |
|---|---|---|---|---|---|---|---|---|---|
| Acute-phase proteins | |||||||||
| CRP, hsCRP | Plasma and serum | Sepsis = 483 | – | – | 15–20 mg/dl | – | – | ↑ hsCRP hyperinflammatory phenotype | [22] |
| Mean age mean = 60.5 | – | – | – | – | – | ↑ hsCRP day 1 to 2, 95.8% | |||
| ♂ 54.9% | – | – | – | – | – | ↑ hsCRP, 23 patients (25.8%) at 3, 26 patients (30.2%) at 6, and 23 patients (25.6%) at 12 months | |||
| Plasma | Sepsis = 43 | – | – | – | – | 0.51, 0.56, and 0.48 | CRP, day 1, 3, and 8 to predict 30-day mortality p = 0.836, p = 0.059, and p = 0.819, respectively | [74] | |
| Septic shock n = 93 | |||||||||
| Age = 26 to 88 | |||||||||
| Plasma | Sepsis = 17 | 61.54% | 52.17% | 9 mg/dl | – | 0.684 | ↑ hsCRP sepsis versus control group, p = 0.008 | [103] | |
| Control = 19 | |||||||||
| Age = 52.18 | |||||||||
| ♂ 63% | |||||||||
| Serum | Sepsis = 38 | 100% | 88.40% | 8.02 mg/l | – | 0.98 | ↑ CRP in septic patients compared to control group, p = 0.001 | [104] | |
| Septic shock = 31 Control = 40 | |||||||||
| Age = 37 to 95 | |||||||||
| ♂ 57.89% | |||||||||
| Serum | Sepsis = 27 | 75.00% | 78.00% | 7.4 mg/dl | – | 0.825 | ↑ hsCRP sepsis versus control group, p = 0.001 | [105] | |
| Septic shock = 23 | – | – | – | – | 0.751 | ↑ hsCRP septic shock versus sepsis, p = 0.002 | |||
| Control = 20 | – | – | – | 0.686 | – | ↑ hs-CRP level versus SOFA, p < 0.001 | |||
| Age = 85 | |||||||||
| ♂57.89% | |||||||||
| Blood | Sepsis = 33 | 90.70% | 98.60% | 407 pg/ml | - | 0.859 | ↑ CRP in sepsis patients compared in SIRS group, p < 0.05 | [48] | |
| Severe sepsis = 24 | |||||||||
| Septic shock = 15 | |||||||||
| SIRS = 23 | |||||||||
| Normal = 20 | |||||||||
| Mean age = 62.1 | |||||||||
| Serum | Sepsis = 119 | - | - | - | - | - | ↑ CRP and SOFA score in the sepsis group compared to the control group, p < 0.05 | [46] | |
| Septic shock = 32 | ↔ Septic shock group when compared with sepsis group, p = 0.086 | ||||||||
| Control = 20 | – | – | – | – | – | ↔ Diagnosing positive infection culture in patients with sepsis, p = 0.071 | |||
| – | – | – | – | 0.609 | |||||
| Serum | Severe sepsis = 34 | – | – | – | – | – | ↔ CRP did not differentiate septic shock and severe sepsis | [89] | |
| Septic shock = 53 | |||||||||
| Age = 2 mo to 16 years | |||||||||
| Complement | Plasma | Sepsis = 20 | – | – | – | – | – | ↑ Sepsis (C4d 3.5-fold; Factor Bb 6.1-fold; C3 0.8-fold; C3a 11.6-fold; and C5a 1.8-fold) versus control | [25] |
| Proteins | Control = 10 | ↑ C5a ↓ SOFA | |||||||
| Age = 85 | – | – | – | 0.18 | – | ||||
| ♂57.89% | – | ||||||||
| PTX-3 | Plasma | Sepsis = 73 | – | – | – | 0.36 | – | ↑ PTX-3 versus APACHE-II, and SOFA, p = 0.0001 | [72] |
| Control = 77 | – | – | 31.4 ng/ml | – | – | Sepsis versus SIRS, p > 0.05 | |||
| Septic Shock = 140 | – | – | – | – | – | Sepsis versus septic shock, p = 0.0001 | |||
| Age = 26 to 88 | – | – | – | – | – | ↑Sepsis/septic shock versus control, p ≤ 0.001 | |||
| ♂57.89% | – | – | – | – | 0.82 and 0.73 | Sepsis and septic shock discrimination on day 1 | |||
| Plasma | Sepsis = 17 | – | – | – | – | – | ↑ PTX-3 sepsis, septic shock, and post-surgery infection versus control group, p < 0.05 | [73] | |
| Septic shock = 26 | ↑ PTX-3 sepsis shock versus sepsis, p < 0.0001 | ||||||||
| Post-surg. Inf. = 33 | – | – | – | – | 0.798 | ||||
| Control = 25 | |||||||||
| Cytokines and chemokines | |||||||||
| IL-10 | Plasma | Sepsis = 208 Control = 210 | – | – | – | – | − 0.161 | ↑ miR-126 correlated negatively with IL-10, p = 0.020 | [106] |
| Plasma | Sepsis = 309 | – | – | – | – | − 0.166 | ↑ lncRNA ITSN1-2 correlated negatively with IL-10, p = 0.003 | [107] | |
| Mean age = 57,3 ± 9,7 | |||||||||
| Control = 330 | |||||||||
| Mean age = 55,8 ± 9,7 | |||||||||
| MCP-1 | Plasma | Sepsis = 43 | – | – | – | – | 0.64, 0.51, and 0.51 | MCP-1, day 1, 3, and 8 to predict 30-day mortality, p = 0.004, p = 0.948, and p = 0.948, respectively | [74] |
| Septic shock n = 93 | |||||||||
| Age = 26 to 88 | |||||||||
| Plasma | Sepsis = 17 | – | – | – | – | – | ↑ MCP-1 sepsis, septic shock and post-surgery infection versus control group, p < 0.05 | [73] | |
| Septic shock = 26 | ↑ MCP-1 sepsis shock versus sepsis, p = 0.0059 | ||||||||
| Post-surg. Inf. = 33 | – | – | – | – | 0.71 | ||||
| Control = 25 | |||||||||
| TNF-α, IL-1β, IL-6 | Serum | Sepsis = 288 | – | – | – | – | – | ↑ Sepsis TNF-α, IL-1β, IL-6, and IL-8 compared to the control group, p < 0.001 | [70] |
| Mean age = 58.2 ± 11.2 | ↑ TNF-α, IL-1β, IL-6, and IL-8 were negatively correlated with surviving sepsis patients, p < 0 .001 | ||||||||
| Control = 290 | – | – | – | – | − 0.270, | ||||
| Mean age = 56.8 ± 12.1 | − 0.310, | ||||||||
| − 0.254, and | |||||||||
| − 0.256 | |||||||||
| Plasma | Sepsis = 483 | – | – | 102 pg/dl | – | – | ↑ IL-6, 72 patients (74.2%) at 3 months, 62 (70.5%) at 6 months, and 59 (66.3%) at 12 months | [22] | |
| Age mean = 60.5 | |||||||||
| ♂ 54.9% | |||||||||
| Serum | Sepsis = 43 | – | – | – | – | 0.69, 0.70, and 0.68 | ↑ IL-6, day 1, 3, and 8 to predict 30-day mortality, p = 0.0001, p = 0.0001, and p = 0.012, respectively | [74] | |
| Septic shock n = 93 | |||||||||
| Age = 26 to 88 | |||||||||
| Serum | Sepsis = 39 | – | – | 12,704—111,372 | – | – | ↑ IL-6 septic patients with DIC, p = 0.01 | [34] | |
| Control = 15 | pg/ml | ||||||||
| Age ≥ 18 years | |||||||||
| DAMPs | |||||||||
| Calprotectin | Plasma | Sepsis = 77 | 56% | 81% | 1.3 mg/l | – | – | ↑ Calprotectin, sepsis versus trauma patients, p < 0.001 | [28] |
| Trauma = 32 | – | – | – | – | – | ↑ Calprotectin at admission was ↑ in non-survivors than in survivors at day 30, p < 0.01 | |||
| HMGB-1 | Serum | Sepsis = 247 | – | – | 3.6 ng/ml | – | – | ↑ HMGB-1 sepsis versus control, p < 0.001 | [75] |
| – | – | – | – | 0.51 and 0.53 | HMGB-1, day 0 and 3, survivor = non-survivor | ||||
| – | – | – | – | – | HMGB-1 does not have predictive value for organ failure and outcome | ||||
| Endothelial cells and BBB markers | |||||||||
| Syndecan-1 | Serum | Sepsis = 39 | – | – | – | – | – | ↑ Syndecan-1 in sepsis versus control, p < 0.0001 | [34] |
| Control = 15 | – | – | – | – | – | ↑ Syndecan-1 non-survivors on days 1, 2, and 4 | |||
| Age | – | – | – | – | 0.54 and 0.59 | ↑ Syndecan-1 versus DIC on day 1 and 2, p = 0.0004 and p = 0.0002, subsequently | |||
| Age ≥ 18 years | – | – | 189–1301 ng/ml | – | – | ↑ Syndecan-1 in septic patient with DIC, p < 0.01 | |||
| VLA-3 (a3β1) | Neutrophil | SIRS = 9 | – | – | – | – | – | ↑ α3β1 (VLA-3, CD49c/CD29) on neutrophils of septic patients, p < 0.05 | [77] |
| Sepsis = 15 | |||||||||
| Control = 7 | |||||||||
| Sepsis = 6 | – | – | – | – | – | ↑ β1 (CD29), on neutrophils of sepsis patients, p < 0.05 | [78] | ||
| Control = 5 | |||||||||
| Ang-1 | Serum | Severe sepsis = 48 | – | – | – | – | – | ↑ Ang-1 severe sepsis compared with shock septic, p < 0.01 | [79] |
| Septic shock = 54 | ↓ Ang-1/Tie-2 in non-survivors, p < 0.001 | ||||||||
| Age ≥ 18 years | – | – | 3.81–16.1 ng/ml | – | – | ||||
| Plasma | SIRS = 943 | – | – | – | – | – | ↑ Ang-1 was associated with a reduced risk of shock. OR: 0.77 | [81] | |
| Sepsis = 330 | ↑ Ang-1 was higher in survivor versus non-survivor, p < 0.001 | ||||||||
| Shock = 216 | – | – | 5719 pg/ml | – | – | ||||
| Pneumonia = 169 | |||||||||
| Others = 152 | |||||||||
| Age = 55.1 ± 16.1 | |||||||||
| ♂ 63.9% | |||||||||
| Ang-2 | Serum | Severe sepsis = 48 | – | – | – | – | – | ↑ Ang-2 severe sepsis compared with shock septic, p < 0.02 | [79] |
| Septic shock = 54 | ↓ Ang-2/Ang-1 in non-survivors, p < 0.001 | ||||||||
| Age ≥ 18 years | – | – | – | – | – | ||||
| Plasma | SIRS = 943 | – | – | – | – | – | ↑ Ang-2 was associated with an increased risk of shock, OR: 1.63 | [81] | |
| Sepsis = 330 | – | – | 42.063 pg/ml | – | – | ↑ Ang-2 non-survivor, p < 0.001 | |||
| Shock = 216 | |||||||||
| Pneumonia = 169 | |||||||||
| Others = 152 | |||||||||
| Age = 55.1 ± 16.1 | |||||||||
| ♂ 63.9% | |||||||||
| CLDN-5 | Serum | Sepsis = 11 | – | – | – | – | – | ↑ CLDN-5 was not associated with MODS and the non-MODS group | [31] |
| Severe sepsis = 18 | – | – | – | – | 0.157 and 0.087 | ↑ CLDN-5 was not correlated with SOFA or APACHE score, p = 0.270, p = 0.542 | |||
| Septic shock = 22 | – | – | – | – | – | Did not predict mortality | |||
| Serum | Sepsis = 11 | – | – | – | – | – | CLDN-5 was absent from the endothelium | [32] | |
| Severe sepsis = 18 | |||||||||
| Septic shock = 22 | |||||||||
| OCLN | Serum | Sepsis = 11 | – | – | – | – | – | ↑ OCLN in severe sepsis and septic shock than in sepsis, p < 0.05 | [31] |
| Severe sepsis = 18 | – | – | – | – | – | ↑ OCLN in non-survivors compared with survivors, p < 0.01 | |||
| Septic shock = 22 | – | – | – | – | 0.337 | ↑ OCLN positively correlated with SOFA, p < 0.016 | |||
| – | – | – | – | 0.224 | ↔ OCLN levels were not correlated with the APACHE-II, p < 0.085 | ||||
| Brain tissue autopsies | Sepsis = 47 | – | – | – | – | – | ↓ OCLN, cerebellar endothelium damage, ↑ CRP ≥ 100 mg/l | [32] | |
| – | – | – | – | – | 38% of patients (18/47) had no expression of OCLN in the BMVECs | ||||
| – | – | – | – | – | 34% of patients (16/47) had MOFs | ||||
| – | – | – | – | – | 74.5% of patients (35/47) had septic shock | ||||
| – | – | – | – | – | The deceased with BBB damage had SOFA scores six versus 14, p = 0.04 | ||||
| PAI-1 | Plasma | Sepsis = 63 | – | – | – | – | 0.85 | ↑ PAI-1 to predict mortality, p < 0.05 | [33] |
| Severe sepsis = 61 | – | – | – | 0.45 | – | ↑ PAI-1 correlated with the SOFA score at 24 h, p < 0.0001 | |||
| Septic shock = 42 | – | – | – | 0.58 | – | ↑ PAI-1 correlation with APACHE-II score, p < 0.0001 | |||
| Age = 60 ± 17 | – | – | – | – | – | ↑ Severe sepsis ↑ sFlt-1, p < 0.0001 | |||
| Serum | Sepsis = 39 | – | – | 15.5–49.9 | – | – | ↑ PAI-1 in patients with DIC, p = 0.016 | [34] | |
| Control = 15 | ng/ml | ||||||||
| Age ≥ 18 years | |||||||||
| sICAM-1 | Plasma | Sepsis = 63 | – | – | – | – | – | ↑ Severe sepsis ↑ sICAM-1, p < 0.001 | [33] |
| Severe sepsis = 61 | – | – | – | 0.15 | – | ↑ sICAM-1 correlated with SOFA score at 24 h, p < 0.03 | |||
| Septic shock = 42 | – | ↑ sICAM-1 correlate with APACHE-II score, p < 0.05 | |||||||
| Age = 60 ± 17 | – | – | – | 0.17 | – | ||||
| Serum | Severe sepsis = 48 | – | – | 1.297–1787 ng/ml | - | – | ↑ sICAM-1 in non-survivors, p < 0.001 | [79] | |
| Septic shock = 54 | – | – | – | – | – | ↑ sICAM-1, predictor of 90 day-mortality, p < 0.0001 | |||
| Age ≥ 18 years | – | – | – | – | – | ↑ sICAM-1, septic shock compared to severe sepsis p < 0.01 | |||
| S100B | Serum | Septic shock = 22 | – | – | > 0.15 μg/l | – | – | ↑ Delirium was present in 10/22 of the patients (45.5%) | [37] |
| – | – | – | – | – | OR: 18.0, for risk of developing delirium S-100β > 0.15 μg/l | ||||
| – | – | – | – | 0.489 | ↑ S100 β correlate positively with and IL-6 p = 0.021 | ||||
| Serum | Sepsis = 104 | 80.0% and | 66.1 and | 0.226 and | – | – | ↑ S100B cut-of value for day 1 and 3 | [36] | |
| Sepsis-associated encephalopathy = 59 | 84.44% | 69.49% | 0.144 μg/l | – | – | ↑ S100B in sepsis-associated encephalopathy day 1 to day 3 compared with non- sepsis-associated encephalopathy, p < 0.001 | |||
| non- sepsis-associated encephalopathy = 45 | – | – | – | – | 0.728 and 0.819 | ↑ S100B on days 1 and 3 to predict sepsis-associated encephalopathy | |||
| – | – | – | – | 0.61 | ↑ S100B on day 1 to predict 180 day-mortality | ||||
| 84.44% | 69.49% | 0.529 μg/l | – | 0.731 | ↑ S100B on day 3 to predict 180 day-mortality | ||||
| 93.33% | 50.00% | 0.266 μg/l | |||||||
| Serum | Sepsis = 21 | – | – | – | – | 0.082, 0.082, | ↑ S100B did not correlate with GCS, EEG pattern, or SOFA scores | [108] | |
| Age = 68.7 | and 0.124 | ||||||||
| E-selectin | Plasma | Sepsis = 63 | – | – | – | – | 0.77 | ↑ Predict mortality | [33] |
| Severe sepsis = 61 | – | – | – | – | – | ↑ Severe sepsis ↑ sE-selectin, p < 0.001 | |||
| Septic shock = 42 | – | – | – | 0.27 | – | ↑ sE-selectin correlated with SOFA score at 24 h, p < 0.0001 | |||
| Age = 60 ± 17 | – | – | – | 0.31 | – | ↑ sE-selectin correlated with APACHE-II score, p < 0.0001 | |||
| sFlt-1 | Plasma | Sepsis = 63 | – | – | – | - | 0.85 | ↑ sFlt-1 to predict mortality, p < 0.05 | [33] |
| Severe sepsis = 61 | – | – | – | 0.36 | – | ↑ sFlt-1 associated with organ dysfunction | |||
| Septic shock = 42 | – | – | – | 0.63 | – | ↑ sFlt-1 correlation with ↑ IL-6, p < 0.0001 | |||
| Age = 60 ± 17 | – | – | – | 0.6 | – | ↑ sFlt-1 correlated with SOFA score at 24 h, p < 0.0001 | |||
| – | – | – | 0.64 | – | ↑ sFlt-1 correlated with APACHE-II score, p < 0.0001 | ||||
| sVCAM-1 | Plasma | Sepsis = 63 | – | – | – | – | 0.78 | ↑ Predict mortality | [33] |
| Severe sepsis = 61 | – | – | – | – | – | ↑ Severe sepsis ↑ sVCAM-1, p < 0.002 | |||
| Septic shock = 42 | – | – | – | 0.45 | – | ↑ sE-selectin correlated with SOFA at 24 h, p < 0.0001 | |||
| Age = 60 ± 17 | – | – | – | 0.38 | – | ↑ sVCAM-1 correlated with APACHE-II s, p < 0.0001 | |||
| Serum | Severe sepsis = 48 | – | – | 369–467 µg/l | – | – | ↑ sVCAM in non-survivors, p < 0.001 | [79] | |
| Septic shock = 54 | – | – | – | – | – | ↑ sVCAM, predictor of 90 day-mortality, p < 0.0001 | |||
| Age ≥ 18 years | – | – | – | – | – | ↑ sVCAM, septic shock compared to severe sepsis, p < 0.01 | |||
| Plasma | SIRS = 943 | – | – | – | – | – | ↑ s-VCAM was associated with an increased risk of shock. OR: 1.63 | [81] | |
| Sepsis = 330 | ↑ sVCAM-1 non-survivor, p < 0.001 | ||||||||
| Shock = 216 | – | – | 819 pg/ml | – | – | ||||
| Pneumonia = 169 | |||||||||
| Others = 152 | |||||||||
| Age = 55.1 ± 16.1 | |||||||||
| ♂ 63.9% | |||||||||
| ZO-1 | Serum | Sepsis = 11 | – | – | – | – | – | ↑ ZO-1 in severe sepsis and septic shock compared to sepsis, p < 0.05 | [31] |
| Severe sepsis = 18 | – | – | – | – | – | ↑ ZO-1 in non-survivors compared with survivors, p < 0.01 | |||
| Septic shock = 22 | – | – | – | – | – | ↑ ZO-1 in MODs group | |||
| – | – | – | – | 0.502 and 0.380 | ↑ ZO-1 was positively correlated with SOFA and APACHE-II scores, p < 0.001 and p < 0.006 | ||||
| Brain tissue autopsies | Sepsis = 47 | – | – | – | – | – | ZO-1 is absent from the endothelial cells in the cerebrum and endothelium | [32] | |
| Gut permeability markers | |||||||||
| Citrulline | Plasma | Septic shock = 16 | – | – | – | – | – | Citrulline was positively correlated with plasma arginine (r2 = 0.85) and glutamine (r2 = 0.90) concentrations in both groups, and significantly inversely correlated with CRP (r2 = 0.10) | [109] |
| (Survivors = 8 | ↓ Citrulline in patients with digestive bacterial translocation | ||||||||
| Age = 60 ± 16.5 | |||||||||
| Non-survivor = 8 | |||||||||
| Age = 62.9 ± 18.5 | – | – | – | – | – | ||||
| Plasma | Sepsis/ARDS = 44 | – | – | – | – | – | ↓ Citrulline in all patients | [83] | |
| Sepsis/NO ARDS = 91 | – | – | 6 and 10.1 uM | – | – | ↓ ARDS compared to the no ARDS group, p = 0.002 | |||
| Age = 55 ± 16 | – | – | – | – | – | Citrulline levels were associated with ARDS | |||
| I-FABP | Serum | Sepsis = 30 | – | – | 27.46 and 36.95 μg/l | – | – | ↑ I-FABP sepsis and septic shock group, p < 0.01 | [40] |
| Septic shock = 30 | – | ↑ I-FABP no difference between survivors and non-survivors | |||||||
| Control = 20 | – | – | – | – | |||||
| Zonulin | Plasma | Sepsis = 25 | – | – | 6.61 ng/ml | – | – | ↑ Zonulin sepsis compared to post-surgical and control groups, p = 0.008 | [39] |
| Post-surgical = 18 | – | – | – | – | – | No difference between survivors and non-survivors, p = 0.305 | |||
| Control = 20 | – | – | – | – | 0.01, − 0.46, − 0.19, and 0.10 | ↑ Zonulin, no correlation with CRP, APACHE-II, SAPSII, SOFA, p = 0.997, p = 0.077, p = 0.491, and p = 0.671, subsequently | |||
| D-lactic acid | Serum | Sepsis = 30 | – | – | 15.32 and 27.95 mg/l | – | – | ↑ D-lactic acid sepsis and septic shock groups, p < 0.01 | [40] |
| Septic shock = 30 | – | – | – | – | ↑ D-lactic acid is no different between survivors and non-survivors | ||||
| Control = 20 | |||||||||
| Non-coding RNAs | |||||||||
| Lnc-MALAT1 | Plasma | Sepsis = 196 | – | – | – | – | 0.866 | ↑ Lnc‐MALAT1/miR‐125a axis in sepsis patients | [41] |
| Age = 58.2 ± 11.2 | – | – | – | – | – | ↑ Lnc‐MALAT1 relative expression in sepsis patients | |||
| Control = 196 | – | – | – | – | – | Lnc-MALAT1/miRNA-125a axis discriminates sepsis patients from healthy controls and exhibits a positive association with general disease severity, organ injury, inflammation level, and mortality in sepsis patients | |||
| Age = 57.1 ± 12.1 | |||||||||
| Plasma | Sepsis = 152 | 68.50% | 65.90% | – | – | 0,674 (ARDS | ↑ lnc-MALAT1 correlates with raised ARDS risk, disease severity, and increased mortality in septic patients | [42] | |
| Age = 59.7 ± 11.2 | 38.30% | 88.60% | – | – | 0.651 | High mortality in sepsis patients | |||
| – | – | – | – | – | Lnc-MALAT1 expression was positively correlated with inflammatory factor levels (CRP, PCT, TNF-α, IL-1β, IL-6, and IL-17) in septic patients | ||||
| Plasma | Sepsis = 120 | – | – | – | – | 0.91 | ↑ lnc-MALAT1 in septic patients, distinguishing patients with sepsis from control | [110] | |
| Control = 60 | – | – | – | – | 0.836 | Septic shock patients compared to patients without septic shock | |||
| – | – | – | – | 0.886 | Non-survivors compared to surviving patients | ||||
| – | – | – | – | – | ↑ Lnc-MALAT1 expression was an independent risk factor for sepsis, septic shock, and poor prognosis | ||||
| lnc-MEG3 | Plasma | Sepsis = 219 | – | – | – | – | 0,887 | ↑ lnc‐MEG3 expression predicting elevated sepsis risk | [43] |
| Control = 219 | – | – | – | – | 0.934 | lnc-MEG3/miR-21 axis predicting elevated sepsis risk | |||
| Age = 56.5 ± 10.3 | – | – | – | – | 0.801 | miR-21 was predicting reduced sepsis risk | |||
| – | – | – | – | 0.704 | lnc‐MEG3 predicting 28‐day mortality risk | ||||
| – | – | – | – | 0.669 | lnc‐MEG3/miR‐21 axis predicting 28‐day mortality risk | ||||
| – | – | – | – | – | ↑ lnc-MEG3/miR-21 axis, while ↓ miR-21 expression was decreased in sepsis patients | ||||
| - | lnc-MEG3 expression and lnc‐MEG3/miR‐21 axis positively correlated, whereas miR‐21 expression negatively correlated with APACHE-II, SOFA, and inflammatory molecules in sepsis patients | ||||||||
| ↑ lnc‐MEG3 relative expression and lnc‐MEG3/miR‐21 axis in deaths than that in survivor | |||||||||
| miRNA | |||||||||
| miR-125a, miR-125b | Plasma | Sepsis = 120 | – | – | – | – | 0.557 | ↔ miR‐125a expression between groups of patients and not differentiate sepsis patients from controls | [84] |
| Control = 120 | – | – | – | – | 0.658 | ↑ miR-125b in sepsis patients and can distinguish sepsis patients from control healths | |||
| 59.1 ± 12.1 | – | – | – | – | – | Positive correlation between miR‐125a and miR‐125b in sepsis patients and controls | |||
| – | – | – | – | – | miR-125a was not correlated with APACHE-II or SOFA score, while miR-125b was positively associated with both scores | ||||
| – | – | – | – | – | ↓ miR-125b in survivors compared with non‐survivors | ||||
| – | – | – | – | – | ↑ miR-125b, but not miR‐125a, is correlated with ↑ disease severity, inflammation, and ↑ mortality in sepsis patients | ||||
| Plasma | Sepsis = 126 | – | – | – | – | 0.817 | ↓ miR-125a good predictive values for sepsis risk | [85] | |
| Control = 125 | – | – | – | – | 0.843 | ↑ lnc‐ANRIL/miR‐125a axis for sepsis risk | |||
| Age = 56.6 ± 13 | – | – | – | – | 0.745 | ↓ miR‐125a expression in deaths than those in survivors | |||
| – | – | – | – | 0.785 | ↑ lnc‐ANRIL/miR‐125a differentiating deaths from survivors | ||||
| – | – | – | – | – | lnc-ANRIL/miR-125a axis positively correlated, and miR-125a was negatively associated with disease severity and inflammation in sepsis patients | ||||
| Plasma | Sepsis = 150 | – | – | – | – | 0.749 and 0.839 | ↑ miR-125a and miR-125b distinguish sepsis patients from controls | [111] | |
| Age = 56.9 ± 10.3 | – | – | – | – | 0.588 | miR-125a to predict 28-day mortality risk | |||
| Control = 150 | – | – | – | – | 0.699 | miR-125b had a potential value in predicting elevated 28-day mortality risk | |||
| Age = 55.1 ± 11.4 | – | – | – | – | – | miR-125a failed to predict the 28-day mortality risk in sepsis patients | |||
| – | – | – | – | – | 1. The predictive value of miR‐125b for sepsis risk | ||||
| – | – | – | – | ||||||
| miR‐125a and miR‐125b relative expressions were positively associated with disease severity in sepsis patients | |||||||||
| Plasma | Sepsis = 196 | – | – | – | – | – | ↑ lnc‐MALAT1/miR‐125a axis in sepsis patients, p < 0.001 | [41] | |
| Age = 58.2 ± 11.2 | – | – | – | – | 0.931 | lnc-MALAT1/miRNA-125a axis discriminates sepsis patients from control | |||
| Control = 196 | – | – | – | – | 0.866 | lnc-MALAT1 discriminates sepsis patients from control | |||
| Age = 57.1 ± 12.1 | |||||||||
| Membrane receptors, cell proteins, and metabolites | |||||||||
| CD64 | Blood | Sepsis = 119 | – | – | – | – | – | ↑ nCD64 and SOFA score in the sepsis compared to control p < 0.05 | [46] |
| Septic shock = 32 | – | – | 4.1, 9, and 2.2 MFI | – | – | ↑ Sepsis and septic shock compared to control p < 0.001 | |||
| Control = 20 | – | – | – | – | 0.879 | nCD64 in bacterial infection | |||
| – | – | – | – | 0.888 | ↑ AUC of nCD64 combined with SOFA than that of any other parameter alone or in combination | ||||
| – | – | – | – | 0.85 | CD64 for predicting death | ||||
| – | – | – | – | 0.916 | Combination of nCD64 and SOFA score | ||||
| – | – | 4.1 versus 8.9 MFI | – | – | ↑ nCD64 survivors versus non-survivors p < 0.001 | ||||
| Blood | Sepsis = 20 | 0.82, 0.67 and 0.67 | 0.67, 0.76, and 0.76 | < 90.40, < 3.01, and < 0.825 | – | 0.843, 0.824, and 0.804 | ↑ CD64, ↓CD13, and ↓HLA-DR predict mortality in septic patients | [45] | |
| Age = 54.35 ± 17.97 | |||||||||
| Control = 20 | |||||||||
| Age = 51.55 ± 13.37 | |||||||||
| CD68 | Brain | Septic shock = 16 | – | – | – | – | – | ↑CD68 in the hippocampus (1.5 fold), putamen (2.2 fold), and cerebellum (2.5 fold) in patients with sepsis than control patients | [86] |
| Age = 8.9–71.7 | |||||||||
| Control = 15 | |||||||||
| Age = 65.2–87.4 | |||||||||
| NFL | CSF and plasma | Sepsis = 20 | – | – | 1723.4, 1905.2 | – | – | Day 1 – sepsis versus control p > 0.05 | [87] |
| Age = 66.7 ± 14.0 | – | – | 2753.1, 2208.0 | – | – | Day 3 – sepsis versus control p > 0.05 | |||
| Control = 5 | – | – | 5309.6, 3701.3 pg/ml | – | – | Day 7 – sepsis versus control p > 0.05 | |||
| Age = 61.2 ± 24.7 | – | – | – | – | – | ↑ NFL in patient septic compared to control from day 1 p = 0.0063 | |||
| – | – | – | – | – | ↑ NFL patients with sepsis-associated encephalopathy p = 0.011 | ||||
| – | – | – | – | – | ↑ NFL correlated with the severity of sepsis-associated encephalopathy p = 0.022 | ||||
| – | – | – | – | ↑ NFL at CSF in non-survivors compared to survivors p = 0.012 | |||||
| NFH | CSF and plasma | Sepsis = 20 | – | – | 17.6, 100.3 | - | - | Day 1 – sepsis versus control p > 0.05 | [87] |
| Age = 66.7 ± 14 | – | – | 18.9, 163.1 | – | – | Day 3 – sepsis versus control p > 0.05 | |||
| Control = 5 | – | – | 164.3, 519.9 | – | – | Day 7 – sepsis versus control p = 0.016 | |||
| Age = 61.2 ± 24.7 | – | – | ng/ml | – | – | ↑ NFH from day 1 in septic patients p = 0.043 | |||
| NSE | Serum | Sepsis/ sepsis-associated encephalopathy = 48 | – | – | 24.87 and 15.49 ng/ml | – | – | ↑ NSE in sepsis-associated encephalopathy group versus no- sepsis-associated encephalopathy group p = 0.003 | [80] |
| Age = 56 ± 16 Sepsis/non- sepsis-associated encephalopathy = 64 | 24.15 ng/ml | Diagnostic of sepsis-associated encephalopathy | |||||||
| Age = 52 ± 17 | 82.80% | 54.20% | – | – | 0.664 | ↔ NSE, sepsis-survivors versus sepsis-non-survivors p = 0.108 | |||
| – | – | – | – | ||||||
| Plasma | Sepsis = 124 | – | – | > 12.5 ug/l | – | – | 23.3%, increased risk of 30-day mortality, p = 0.006, and a 29.3% increased risk of delirium p = 0.005 | [88] | |
| Mean age = 52–71 | – | – | – | – | – | ↑ NSE is associated with mortality p = 0.003, and delirium in critically ill septic patients p < 0.001 | |||
| CSF and plasma | Sepsis/ sepsis-associated encephalopathy = 12 | – | – | Eight versus 3.8 ng/ml | – | – | ↑ CSF NSE in sepsis group compared to controls p < 0.05 | [112] | |
| Control = 21 | ↔ Plasma NSE sepsis group versus control group | ||||||||
| Mean age = 67.8 ± 1 2.1 | – | – | – | – | – | ||||
| Presepsin | Blood | Sepsis = 33 | 90.70% | 98.60% | 407 pg/ml | – | 0.954 | ↑ Presepsin in sepsis patients compared to SIRS group p < 0.05 | [48] |
| Severe sepsis = 24 | ↑ Presepsin and APACHE-II score in severe sepsis group than sepsis group p < 0.05 | ||||||||
| Septic shock = 15 | – | – | – | – | – | ↑ Presepsin and APACHE-II score in septic shock group compared to severe sepsis group p < 0.05 | |||
| SIRS = 23 | |||||||||
| Normal = 20 | – | – | – | – | – | ||||
| Mean age = 62.1 | |||||||||
| TREM-1 | Serum | Severe sepsis = 34 | – | – | 129 pg/ml versus 105 pg/ml | – | – | ↑ TREM-1 levels in septic shock compared to severe sepsis | [89] |
| Septic shock = 53 | – | – | – | – | – | ↔ TREM-1 did not differentiate between septic shock and severe sepsis | |||
| Age = 2 mo to 16 years | 56% | 60% | 116.47 pg/ml | – | 0.62 | Predict septic shock | |||
| 52% | 71% | 116.47 pg/ml | – | 0.63 | Predict mortality | ||||
| – | – | – | – | – | ↔ TREM-1 non-survivors versus survivors | ||||
| Serum | SIRS = 38 | 73.30% | 71.10% | ≥ 133 pg/ml | – | – | sTREM-1 cut-off for sepsis | [113] | |
| Sepsis = 52 | – | – | – | – | – | ↑ sTREM-1 in sepsis group p = 0.001 | |||
| Age = 20 to 92 | – | – | – | – | – | ↑ sTREM-1 in the patients with positive blood culture p = 0.002 | |||
| Plasma and leukocytes | Septic shock = 60 Postoperative = 30 | 100% | 98.30% | 30.0 pg/ml | – | – | ↑ sTREM-1 plasma in septic shock compared to control and postoperative groups p < 0.05 | [91] | |
| Control = 30 | – | – | – | – | – | ↑ sTREM-1 compared with postoperative group p < 0.05 | |||
| – | – | – | – | 0.955 | ↑ TREM-1 expression on human monocytes of a septic shock compared to control and postoperative groups p < 0.05 | ||||
| Peptide precursor of the hormone and hormone | |||||||||
| MR-proADM | Plasma | Sepsis/bacterial isolate = 39 | 78% | 74.20% | ≥ 1.5 | – | 0.82 | ↑ MR-proADM sepsis versus control p < 0.0001 | [92] |
| Sepsis w/bacterial isolate = 23 | 80% | 89.36% | ≥ 1.70 | – | 0.92 | ↑ MR-proADM septic shock versus control p < 0.0001 | |||
| Septic shock = 47 | 77.40% | 59.60% | > 3.00 | – | 0.7 | ↑ MR-proADM septic shock versus sepsis p < 0.0001 | |||
| Control = 50 | – | – | 4.37 versus 2.34 nmol/l | – | – | ↑ MR-proADM, non-survivor versus survivor p < 0.0001 | |||
| Bio-ADM | Sepsis = 632 | – | – | – | – | Median sepsis patients = 74 pg/mL; septic shock = 107 pg/mL, and 29 pg/mL in non-septic patients | [53] | ||
| Septic shock = 267 | – | – | – | Mortality in sepsis patients OR of 1.23 | |||||
| Non-septic = 1235 | – | – | – | – | ↑ Dialysis: OR 1.97 in sepsis patients | ||||
| – | – | 70 pg/mL | ↑ bio-ADM ↑ Use of vasopressors, OR 1.33 | ||||||
| – | – | 108 pg/mL | – | Survivors and non-survivors in sepsis | |||||
| – | – | – | Youden’s index derived threshold of performed better | ||||||
| – | – | – | ↑ bio-ADM non-survivors | ||||||
| – | |||||||||
| – | |||||||||
| – | |||||||||
| PCT | Serum | Sepsis = 59 | – | – | – | – | – | ↑ PCT p < 0.0005 | [66] |
| Severe sepsis/septic shock = 71 | – | – | 0.67 versus 3.81 | – | – | Survivor versus non-survivor at seven days | |||
| Mean age = 80 | – | – | 0.48 versus 1.82 ng/mL | – | – | Survivor versus non-survivor at 30 days | |||
| Serum | SIRS = 38 | 65.79% | 67.33% | 1.57 ng/ml | – | – | PCT cut-off for sepsis | [113] | |
| Sepsis = 52 | – | – | – | – | – | ↑ PCT in sepsis group, p = 0.01 | |||
| Age = 20 to 92 | |||||||||
| Serum | Sepsis = 79 | – | – | – | – | – | ↑ PCT concentrations in patients with sepsis and infection | [114] | |
| Age = newborn to 12 | ↓ PCT concentrations with antibiotic treatment | ||||||||
| Control = 21 | – | – | – | – | |||||
| Age = newborn to 10 | |||||||||
| Blood | Sepsis = 119 | – | – | 17.1, 1.8, and 0.04 ng/ml | – | – | ↑ PCT septic shock and sepsis compared to the control group p < 0.001 | [46] | |
| Septic shock = 32 | – | – | 1.8 and 9.2 ng/ml | – | – | ↑ PCT levels in survivors versus non-survivors p > 0.001 | |||
| Control = 20 | |||||||||
| Blood | Sepsis = 33 | 90.70% | 98.60% | 407 pg/ml | – | 0.874 | ↑ PCT sepsis patients compared to SIRS group p < 0.05 | [48] | |
| Severe sepsis = 24 | – | – | – | – | – | ↑ PCT and APACHEII score in severe sepsis group compared to sepsis group p < 0.05 | |||
| Septic shock = 15 | |||||||||
| SIRS = 23 | |||||||||
| Normal = 20 | |||||||||
| Mean age = 62.1 | |||||||||
| Plasma | Sepsis and shock septic = 1089 | ↔ There was no statistic difference in the primary outcome regarding PCT-guidance 27.9% versus no PCT-guidance 22.9% to predict mortality p = 0.18 | [61] | ||||||
| PCT-guidance n = 279 | ↔ PCT-guidance versus no PCT-guidance there was no statistic difference in 28-day mortality, 25.6% versus 28.2% p = 0.34 | ||||||||
| No PCT-guidance n = 267 | |||||||||
| Serum | Severe sepsis = 34 | – | – | 129 pg/ml versus 105 pg/ml | – | – | ↔ PCT did not differentiate septic shock from severe sepsis | [89] | |
| Septic shock = 53 | |||||||||
| Age = 2 mo to 16 years | |||||||||
| NT-proBNP | Serum | Sepsis = 60 | – | – | 1.209 ng/l | – | – | ↑ NT-proBNP level at 24 h after sepsis diagnosis | [55] |
| Severe sepsis = 89 | – | – | – | – | – | ↑ NT-proBNP levels at 24 h after sepsis onset were associated with ↓ SPPB scores at 12 months p < 0.05, and ↓ handgrip strength at six and 12-month follow-up p < 0.001 | |||
| Septic shock = 47 | |||||||||
| Age = 59.1 ± 15.1 | |||||||||
| Plasma | Sepsis = 142 | 4 (2.6–8.8) versus 8.2 nmol/L (5.2–12.6) | - | ↑ NT-proBNP levels in non-survivors compared with survivors p < 0.01. ↔ CRP did not change in survivors and non-survivors | [52] | ||||
| Septic shock = 947 | - | ↑ NT-proBNP prediction of 28-day mortality in total population, sepsis group, and shock septic group, respectively | |||||||
| 0.73, 0.73, and 0.72 | |||||||||
| Neutrophil, cells, and related biomarkers | |||||||||
| Lactate | Plasma | Sepsis = 59 | – | – | – | – | ↑ Lactate p < 0.0005 | [66] | |
| Severe sepsis/septic shock = 71 | – | – | 1.7 versus 3.4 | – | Survivor versus non-survivor at seven days | ||||
| Mean age = 80 | – | – | 1.6 versus 2.2 | – | Survivor versus non-survivor at 30 days | ||||
| – | – | mmol/l | 0.79 and 0.77 | Predictors of mortality at 7 and 30 days p = 0.001 | |||||
| Serum | Non- sepsis-associated encephalopathy = 2513 Sepsis-associated encephalopathy = 2474 | – | – | – | – | – | ↑ Lactate predicted 30-day mortality of patients with sepsis-associated encephalopathy, OR: 1.19 p < 0.0005 | [93] | |
| Blood | Sepsis = 33 | 90.70% | 98.60% | 407 pg/ml | – | 0.859 and 0.723 | ↑ Lactate and APACHE-II score in severe sepsis group compared to sepsis group p < 0.05 | [48] | |
| Severe sepsis = 24 | – | – | – | – | – | ↑ APACHE-II score and lactate in septic shock group when compared with severe sepsis group p < 0.05 | |||
| Septic shock = 15 | |||||||||
| SIRS = 23 | |||||||||
| Normal = 20 | |||||||||
| Mean age = 62.1 | |||||||||
| Serum | Severe sepsis = 34 | – | – | – | – | – | ↔ Lactate did not differentiate septic shock from severe sepsis | [89] | |
| Septic shock = 53 | |||||||||
| Age = 2 mo to 16 years | |||||||||
| MPO | Plasma | Sepsis = 957 | – | – | 128.1 ng/ml | – | – | ↑ MPO day 1 and progressively decreased until day 7 | [94] |
| – | – | – | – | – | ↑ MPO increase on days on days 1, 2, and 7 in 90-day non-survivors p < 0.003, p = 0.03, and p = 0.001 | ||||
| Septic shock = 55 | – | – | – | – | – | ↑ MPO-DNA and cf-DNA in patients with septic shock on day 1 p < 0.01 | [95] | ||
| Control = 13 | – | – | – | – | – | ↑ MPO-DNA on days 3 and 7 of sepsis was associated with 28-day mortality p < 0.01 | |||
| Mean age = 68 | – | – | – | 0.303 and 0.434 | – | ↑ MPO-DNA on day 3 and 7 positive correlation with SOFA score p = 0.04 and p = 0.03, subsequently | |||
| ♂ = 71% | |||||||||
| Resistin | Plasma | Sepsis = 957 | – | – | 192.9 ng/ml | – | – | ↑ Resistin on day one and progressively decreased until day 7 | [94] |
| Mean age = 70 | – | – | – | – | – | ↑ Resistin increase on days 1, 2, and 7 in 90-day non-survivors p < 0.001 | |||
| ♂ = 60% | |||||||||
| Serum | Sepsis = 50 | 72%, 80%, and 100% | 82%, 95%, and 100% | 5.2, 6.1, and 7,5 ng/ml | – | – | ↑ Resistin levels on day 1, 4, and 7 | [115] | |
| Patient without sepsis = 22 | – | – | – | – | 0.864, 0.987, and 0.987 | ↑ Resistin levels on days 1, 4, and 7 were associated with sepsis | |||
| Control = 25 | |||||||||
| Age ≤ 12 | |||||||||
| Serum | Sepsis = 60 | – | – | 36.45 | – | – | ↑ Resistin in sepsis/septic shock groups p = 0.001 | [96] | |
| Septic shock = 42 | – | – | 48.13 versus 31.58 | – | – | ↑ Resistin levels in non-survivors versus Survivors on day 1 and 7 p < 0.001 and p < 0.001 | |||
| Control = 102 | – | – | 46.20 versus 25.22 | – | – | ↑ Resistin septic shock versus sepsis on day 1 and 3 p < 0.001 and p < 0.001 | |||
| 40.8 versus 33.4 | |||||||||
| 37.1 versus 27.4 | |||||||||
| µg/l | |||||||||
| Soluble receptors | |||||||||
| sPD-L1 | Serum | Sepsis = 483 | – | – | 0.16 ng/ml | – | – | ↑ sPD-L1 immunosuppression phenotype, ↑ risk of hospital readmission and mortality, OR = 8.26 | [22] |
| Mean age = 60.5 | ↑ sPD-L1, 45 (46.4%) at 3 months, 40 (44.9%) at 6 months, and 44 (49.4%) at 12 months | ||||||||
| ♂ 54.9% | – | – | – | – | – | ↑ sPD-L1 to predict 28-day mortality ≅ APACHE-II and SOFA scores | |||
| – | – | – | – | – | |||||
| Serum | Sepsis = 91 | – | – | 2.09 ng/ml | – | – | ↑ sPD-L1 and sPD-1 in septic patients p = 0.0001 | [68] | |
| Control = 29 | – | – | – | – | – | ↑ sPD-L1 increased in non-survivors p < 0.05 | |||
| – | – | – | – | 0.71 | ↑ sPD-L1 level to predict 28-day mortality | ||||
| suPAR | Serum | Sepsis = 59 | – | – | – | – | – | ↑ suPAR, p < 0.0005 | [66] |
| Severe sepsis/septic shock = 71 | – | – | 6.9 versus 9.8 | – | – | Survivor versus non-survivor at seven days | |||
| Mean age = 80 | – | – | 6.4 versus 9.3 | – | – | Survivor versus non-survivor at 30 days | |||
| – | – | ng/ml | 0.72 and 0.77 | Predictors of mortality at 7 and 30 days p = 0.006 | |||||
| – | – | – | – | – | ↓ suPAR from day 1 to day seven sepsis and severe sepsis/septic shock p < 0.0005 | ||||
| Serum | Sepsis = 60 | – | – | 13 | – | – | ↑ suPAR in sepsis and septic shock | [96] | |
| Septic shock = 42 | – | – | 10.5 versus 14.1 | – | – | ↑ suPAR in septic shock compared with sepsis on day one but not on day 7 p < 0.04 and p = 0.68, subsequently | |||
| Control = 102 | 11.3 versus 12.9 μg/l | ||||||||
| sTNFR-1 | Plasma | SIRS = 943 | – | – | 7719 versus 18,197 | – | – | ↑ sTNFR-1 in non-survivor versus survivor, p < 0.001 | [81] |
| Sepsis = 330 | – | – | pg/ml | – | – | ↑ sTNFR-1 sepsis compared to SIRS p < 0.001 | |||
| Shock = 216 | |||||||||
| Pneumonia = 169 | |||||||||
| Others = 152 | |||||||||
| Age = 55.1 ± 16.1 | |||||||||
| ♂ 63.9% | |||||||||
| Plasma | No delirium = 47 | – | – | 3.843 and 10.250 pg/ml | – | – | ↑ sTNFR1 and sTNFR2 delirium cutoff p = 0.005 | [98] | |
| Delirium = 31 | – | – | – | – | – | ↑ sTNFR1 and sTNFR2 in delirium group compared with non-delirium p = 0.005, and p = 0.003, subsequently | |||
| OR: 18 to sTNFR1, p = 0.004 and OR: 51 to | |||||||||
| STNFR2, p = 0.006 | |||||||||
| – | – | – | – | – | |||||
| Lipoproteins | |||||||||
| LDL | Serum | Sepsis = 594 | – | – | – | – | – | Risk of sepsis, OR, 0.86, p = 0.001and admission to the ICU, OR, 0.85; p = 0.008; but not hospital mortality, OR, | [99] |
| ↓Quartile greater risk of sepsis; OR, 1.48; and admission to the ICU, OR, 1.45, versus highest quartile | |||||||||
| ↔ When comorbidities were considered | |||||||||
| HDL | Serum | Sepsis = 63 | – | – | – | – | – | ↓ HDL in non-survivors on days 1 to 4 | [100] |
| Mean age = 72 | – | – | – | – | 0.84 | Predicts mortality within 30 days | |||
| 80% | 92% | 20 mg/dl | – | – | 83% accuracy to predict 30-day overall mortality | ||||
| – | – | – | – | – | HDL < 20 mg/dl increases attributable mortality, risk of prolonged ICU stay, and hospital-acquired infection rate | ||||
| Plasma | Suspected sepsis = 200 | 0.690 | 0.716 | 30.9 mg/dl | – | 0,749 | MODS predictor | [101] | |
| 0.699 | 0.857 | 25,1 mg/dl | – | 0,818 | Mortality in 28 days | ||||
| – | – | < 25.1 mg/dl | – | – | ↑ Mortality, p < 0.0001 in 28 days and p = 0.0007 in 90 days | ||||
| – | – | – | – | – | 74% of patients with HDL < 25.1 mg/dl required ICU compared to 35% above cutoff; development of severe acute renal dysfunction was 47% versus 21%, respectively; multiple organ dysfunction was 60% versus 25%; and mechanical ventilation was 53% versus 21% | ||||
| – | – | – | – | – | ↓ HD, the 28-day mortality is more than ten-fold higher (17.6% versus 1.5%) and a mean of 6.2 fewer days without mechanical ventilation and vasopressor support | ||||
| T-chol | Serum | Sepsis = 136 | – | – | – | – | – | ↓ T-chol associated with risk of death in septic patients p < 0.05 | [102] |
Ang-1 angiopoietin-1, Ang-2 angiopoietin-2, APACHE-II acute physiology and chronic health evaluation II, ARDS acute respiratory distress syndrome, AUC area under the curve, BBB blood–brain barrier, BMVEC brain microvascular endothelial cells, CD cluster of differentiation, CLDN-5 claudin-5, CRP C reactive protein, CSF cerebrospinal fluid, DAMPs damage-associated molecular patterns, DIC disseminated intravascular coagulation, EEG electroencephalography, GCS Glasgow coma scale, HDL high-density lipoprotein, HLA-DR human leukocyte antigen, HMGB1 high mobility group box 1, hsCRP high-sensitivity C reactive protein, I-FABP intestinal fatty acid binding protein, IL interleukin, LDL low-density lipoprotein, lnc-ANRIL long non-coding antisense non-coding RNA in the INK4 locus, lnc-MALAT1 long non-coding metastasis-associated lung adenocarcinoma transcript 1, lnc-MEG3 long non-coding RNA maternally expressed gene 3, lncRNA long non-coding RNA, MCP-1 monocyte chemoattractant protein-1, miR-125a micro RNA-125a, miR-125b micro RNA-125b, MODS multiple organ dysfunction syndrome, MOF multiple organ failure, MPO myeloperoxidase, MR-proADM mid-regional pro adrenomedullin, NFL neurofilament light, NfH neurofilament heavy, NSE neuron specific enolase, NT-proBNP N-terminal pro-brain natriuretic peptide, OCLN occludin, OR odds ratio, PAI-1 plasminogen activator inhibitor 1, PCT procalcitonin, PTX-3 pentraxin-3, RNA ribonucleic acid, S100B calcium-binding protein B, sE-Selectin soluble E-selectin, sFlt-1 soluble fms-like tyrosine kinase 1, sICAM-1 soluble intercellular adhesion molecule 1, SIRS systemic inflammatory response syndrome, SOFA sequential organ failure assessment, sPD-1 soluble programmed death protein 1, sPD-L1 soluble programmed death ligand 1, SPPB short physical performance battery, sTNFR1 soluble tumor necrosis factor receptor type 1, sTNFR2 soluble tumor necrosis factor receptor type 2, sTREM-1 soluble triggering receptor expressed on myeloid cells 1, suPAR soluble form of the urokinase plasminogen activator receptor, sVCAM-1 soluble vascular cell adhesion molecule 1, T-chol total cholesterol, TNF-α tumour necrosis factor alpha, TREM-1 triggering receptor expressed on myeloid cells-1, VLA-3/a3β1 integrin alpha 3 beta 1, ZO-1 zonula-occluden 1). ↑ increase, ↓ decrease, ↔ no difference
Fig. 1.
Sepsis, septic shock, and sepsis-associated encephalopathy biomarkers. The infection triggers a cascade of signaling pathways that activate several transcription factors and promote proinflammatory mediators such as acute-phase proteins, cytokines, chemokines, and antimicrobial peptides necessary to eliminate the invading pathogens. The unbalanced host immune response triggers vascular endothelial damage, increasing gut and BBB permeability, culminating in organ dysfunction. Ang-2 (angiopoietin-2), APP (acute phase proteins), aPPT (activated partial thromboplastin), AST (astrocytes), AT (antithrombin), BBB (blood–brain barrier), C5aR (complement component 5a receptor), CD (cluster of differentiation), CD14-ST (soluble subtype of CD14), CRP (C reactive protein), DAMPs (damage-associated molecular patterns), GFAP (glial fibrillary acidic protein), HMGB-1 (high mobility group box 1), ICAM-1 (intercellular adhesion molecule 1), I-FABP (intestinal fatty acid binding protein), LBP (lipopolysaccharide binding protein), mHLA-DR (monocytic human leukocyte antigen DR), Mo (macrophage), NFL (neurofilament light), NSE (neuron specific enolase), NT-proBNP (N-terminal pro-brain natriuretic peptide), OCLN (occludin), OLG (oligodendrocyte), PAMPs (pathogen-associated molecular patterns), PCT (procalcitonin), PMNL (polymorphonuclear leukocytes), PT (prothrombin), PTX-3 (pentraxin-3), S100B (calcium-binding protein B), sFlt-1 (soluble fms-like tyrosine kinase 1), suPAR (soluble form of the urokinase plasminogen activator receptor), TNFR (tumor necrosis factor receptor type), TREM-1 (triggering receptor expressed on myeloid cells 1), VCAM-1 (vascular cell adhesion molecule 1), ZO-1 (zonula-occluden 1)
Conclusion
Despite significant advances in treating septic patients, this disease continues to be associated with high mortality rates and high long-term cognitive dysfunction. Extensive research in the area is being performed to validate biomarkers, facilitate sepsis diagnosis, and allow an early intervention that, although primarily supportive, can reduce the risk of death. Sepsis sometimes shows a hyperinflammatory response pattern and may be followed by an immunosuppressive phase, during which multiple organ dysfunction is present. A biomarker or a panel of biomarkers could be a new avenue to predict, identify, or provide new approaches to treat sepsis.
Acknowledgements
This work was supported by the Faillace Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth), USA (T.B.); the Graduate Program in Health Sciences, University of Southern Santa Catarina (UNESC) (T.B., JSG, and FDP), Brazil; the Alzheimer's Association and U.S. National Institute of Health/National Institute on Aging (NIH/NIA (T.B.).
Authors' contributions
Conceptualization: TB, MS, and FD-P; Writing—original draft: TB and JSG. Writing (review and editing): TB, JSG, MS, and FD-P. All authors read and approved the final manuscript.
Funding
The Alzheimer’s Association Grant number AARGDNTF-19-619645 and U.S. National Institute of Health/National Institute on Aging (NIH/NIA Grant (1RF1AG072491-01) (T.B.); MCTIC/CNPq/FNDCT/MS/SCTIE/Decit Nº 401263/2020-7 (FD-P)).
Availability of data and material
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatiana Barichello, Email: Tatiana.Barichello@uth.tmc.edu.
Felipe Dal-Pizzol, Email: piz@unesc.net.
References
- 1.Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34(2):77–84. doi: 10.1097/ACO.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty RK, Burns B. Systemic inflammatory response syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC.; 2021. [PubMed]
- 3.Vincent JL, Martin GS, Levy MM. qSOFA does not replace SIRS in the definition of sepsis. Crit Care (Lond, Engl) 2016;20(1):210. doi: 10.1186/s13054-016-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barichello T, Generoso JS, Dominguini D, Córneo E, Giridharan VV, Sahrapour TA, Simões LR, Rosa MID, Petronilho F, Ritter C, et al. Postmortem evidence of brain inflammatory markers and injury in septic patients: a systematic review. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005307. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39(3):229–246. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 7.Heckenberg SG, Brouwer MC, van de Beek D. Bacterial meningitis. Handb Clin Neurol. 2014;121:1361–1375. doi: 10.1016/B978-0-7020-4088-7.00093-6. [DOI] [PubMed] [Google Scholar]
- 8.Sellner J, Täuber MG, Leib SL. Pathogenesis and pathophysiology of bacterial CNS infections. In: Karen LR, Allan RT, editors. Handbook of clinical neurology. Amsterdam: Elsevier; 2010. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 9.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science (New York, NY) 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5(1):36–44. doi: 10.4161/viru.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu B, Wang C, Wang M, Li W, Chen F, Tracey KJ, Wang H. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev Clin Immunol. 2014;10(6):713–727. doi: 10.1586/1744666X.2014.909730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka H, Kono H, Patel Z, Rock KL. Evaluation of the contribution of multiple DAMPs and DAMP receptors in cell death-induced sterile inflammatory responses. PLoS ONE. 2014;9(8):e104741. doi: 10.1371/journal.pone.0104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2020;10(44462020005021202):1516–4446. doi: 10.1590/1516-4446-2020-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3(1):61–69. doi: 10.1016/S2213-2600(14)70246-2. [DOI] [PubMed] [Google Scholar]
- 18.Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, Petronilho F, Quevedo J, Dal-Pizzol F. Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol. 2019;56(1):186–251. doi: 10.1007/s12035-018-1048-2. [DOI] [PubMed] [Google Scholar]
- 19.Erreni M, Manfredi AA, Garlanda C, Mantovani A, Rovere-Querini P. The long pentraxin PTX3: a prototypical sensor of tissue injury and a regulator of homeostasis. Immunol Rev. 2017;280(1):112–125. doi: 10.1111/imr.12570. [DOI] [PubMed] [Google Scholar]
- 20.McFadyen JD, Zeller J, Potempa LA, Pietersz GA, Eisenhardt SU, Peter K. C-Reactive protein and its structural isoforms: an evolutionary conserved marker and central player in inflammatory diseases and beyond. Subcell Biochem. 2020;94:499–520. doi: 10.1007/978-3-030-41769-7_20. [DOI] [PubMed] [Google Scholar]
- 21.Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care (Lond, Engl) 2020;24(1):287. doi: 10.1186/s13054-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caironi P, Masson S, Mauri T, Bottazzi B, Leone R, Magnoli M, Barlera S, Mamprin F, Fedele A, Mantovani A, et al. Pentraxin 3 in patients with severe sepsis or shock: the ALBIOS trial. Eur J Clin Invest. 2017;47(1):73–83. doi: 10.1111/eci.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song J, Moon S, Park DW, Cho HJ, Kim JY, Park J, Cha JH. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock: a prospective observational study according to the Sepsis-3 definitions. Medicine. 2020;99(22):e20495. doi: 10.1097/MD.0000000000020495. [DOI] [PubMed] [Google Scholar]
- 25.Younger JG, Bracho DO, Chung-Esaki HM, Lee M, Rana GK, Sen A, Jones AE. Complement activation in emergency department patients with severe sepsis. Acad Emerg Med Off J Soc Acad Emerg Med. 2010;17(4):353–359. doi: 10.1111/j.1553-2712.2010.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe T, Kubo K, Izumoto S, Shimazu S, Goan A, Tanaka T, Koroki T, Saito K, Kawana R, Ochiai H. Complement activation in human sepsis is related to sepsis-induced disseminated intravascular coagulation. Shock (Augusta, Ga) 2020;54(2):198–204. doi: 10.1097/SHK.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto H, Ogura H, Shimizu K, Ikeda M, Hirose T, Matsuura H, Kang S, Takahashi K, Tanaka T, Shimazu T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci Rep. 2018;8(1):13995. doi: 10.1038/s41598-018-32275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson A, Tydén J, Johansson J, Lipcsey M, Bergquist M, Kultima K, Mandic-Havelka A. Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scand J Clin Lab Invest. 2020;80(2):156–161. doi: 10.1080/00365513.2019.1703216. [DOI] [PubMed] [Google Scholar]
- 29.Matsuura R, Komaru Y, Miyamoto Y, Yoshida T, Yoshimoto K, Hamasaki Y, Nangaku M, Doi K. Different biomarker kinetics in critically ill patients with high lactate levels. Diagnostics (Basel, Switzerland) 2020;10(7):454. doi: 10.3390/diagnostics10070454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barichello T, Generoso JS, Collodel A, Petronilho F, Dal-Pizzol F. The blood-brain barrier dysfunction in sepsis. Tissue Barriers. 2021;9(1):1840912. doi: 10.1080/21688370.2020.1840912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao GJ, Li D, Zhao Q, Lian J, Hu TT, Hong GL, Yao YM, Lu ZQ. Prognostic value of plasma tight-junction proteins for sepsis in emergency department: an observational study. Shock. 2016;45(3):326–332. doi: 10.1097/SHK.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 32.Erikson K, Tuominen H, Vakkala M, Liisanantti JH, Karttunen T, Syrjälä H, Ala-Kokko TI. Brain tight junction protein expression in sepsis in an autopsy series. Crit Care. 2020;24(1):385. doi: 10.1186/s13054-020-03101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, Shapiro NI. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39(5):427–432. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda M, Matsumoto H, Ogura H, Hirose T, Shimizu K, Yamamoto K, Maruyama I, Shimazu T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care. 2018;43:48–53. doi: 10.1016/j.jcrc.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 35.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6(2):121–138. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu L, Feng Q, Ai ML, Deng SY, Liu ZY, Huang L, Ai YH, Zhang L. The dynamic change of serum S100B levels from day 1 to day 3 is more associated with sepsis-associated encephalopathy. Sci Rep. 2020;10(1):7718. doi: 10.1038/s41598-020-64200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erikson K, Ala-Kokko TI, Koskenkari J, Liisanantti JH, Kamakura R, Herzig KH, Syrjälä H. Elevated serum S-100β in patients with septic shock is associated with delirium. Acta Anaesthesiol Scand. 2019;63(1):69–73. doi: 10.1111/aas.13228. [DOI] [PubMed] [Google Scholar]
- 38.Assimakopoulos SF, Triantos C, Thomopoulos K, Fligou F, Maroulis I, Marangos M, Gogos CA. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection. 2018;46(6):751–760. doi: 10.1007/s15010-018-1178-5. [DOI] [PubMed] [Google Scholar]
- 39.Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, Krenn CG, Roth GA. Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb) 2013;23(1):107–111. doi: 10.11613/BM.2013.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Liu D, Wang Y, Yan J, Yang X. Clinical significance on serum intestinal fatty acid binding protein and D-lactic acid levels in early intestinal injury of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(5):545–550. doi: 10.3760/cma.j.issn.2095-4352.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Geng F, Yu L. Long non-coding RNA MALAT1/microRNA 125a axis presents excellent value in discriminating sepsis patients and exhibits positive association with general disease severity, organ injury, inflammation level, and mortality in sepsis patients. J Clin Lab Anal. 2020;34(6):e23222. doi: 10.1002/jcla.23222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, Zhao M. High expression of long non-coding RNA MALAT1 correlates with raised acute respiratory distress syndrome risk, disease severity, and increased mortality in sepstic patients. Int J Clin Exp Pathol. 2019;12(5):1877–1887. [PMC free article] [PubMed] [Google Scholar]
- 43.Na L, Ding H, Xing E, Gao J, Liu B, Wang H, Yu J, Yu C. Lnc-MEG3 acts as a potential biomarker for predicting increased disease risk, systemic inflammation, disease severity, and poor prognosis of sepsis via interacting with miR-21. J Clin Lab Anal. 2020;34(4):e23123. doi: 10.1002/jcla.23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma miR-125a and miR-125b in sepsis: correlation with disease risk, inflammation, severity, and prognosis. J Clin Lab Anal. 2020;34(2):e23036. doi: 10.1002/jcla.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahmoodpoor A, Movassaghpour A, Talebi M, Shadvar K, Soleimanpour H. Value of flow cytometry (HLA-DR, CD14, CD25, CD13, CD64) in prediction of prognosis in critically ill septic patients admitted to ICU: a pilot study. J Clin Anesth. 2020;61:109646. doi: 10.1016/j.jclinane.2019.109646. [DOI] [PubMed] [Google Scholar]
- 46.Yin WP, Li JB, Zheng XF, An L, Shao H, Li CS. Effect of neutrophil CD64 for diagnosing sepsis in emergency department. World J Emerg Med. 2020;11(2):79–86. doi: 10.5847/wjem.j.1920-8642.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: a systematic review and meta-analysis. J Intensive Care. 2019;7:22. doi: 10.1186/s40560-019-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu B, Zhang Y, Li C, Liu C, Yao Y, Su M, Shou S. The utility of presepsin in diagnosis and risk stratification for the emergency patients with sepsis. Am J Emerg Med. 2018;36(8):1341–1345. doi: 10.1016/j.ajem.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 49.Şen S, Kamit F, İşgüder R, Yazıcı P, Bal Z, Devrim İ, Bayram SN, Karapınar B, Anıl AB, Vardar F. Surface TREM-1 as a Prognostic Biomarker in Pediatric Sepsis. Indian J Pediatr. 2020;88:134–140. doi: 10.1007/s12098-020-03355-3. [DOI] [PubMed] [Google Scholar]
- 50.Andrés C, Andaluz-Ojeda D, Cicuendez R, Nogales L, Martín S, Martin-Fernandez M, Almansa R, Calvo D, Esteban-Velasco MC, Vaquero-Roncero LM, et al. MR- proADM to detect specific types of organ failure in infection. Eur J Clin Invest. 2020;50(6):19. doi: 10.1111/eci.13246. [DOI] [PubMed] [Google Scholar]
- 51.Schuetz P, Hausfater P, Amin D, Amin A, Haubitz S, Faessler L, Kutz A, Conca A, Reutlinger B, Canavaggio P, et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care. 2015;19(377):015–1098. doi: 10.1186/s13054-015-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elke G, Bloos F, Wilson DC, Brunkhorst FM, Briegel J, Reinhart K, Loeffler M, Kluge S, Nierhaus A, Jaschinski U, et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis—a secondary analysis of a large randomised controlled trial. Crit Care (Lond, Engl) 2018;22(1):79. doi: 10.1186/s13054-018-2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundberg OHM, Lengquist M, Spångfors M, Annborn M, Bergmann D, Schulte J, Levin H, Melander O, Frigyesi A, Friberg H. Circulating bioactive adrenomedullin as a marker of sepsis, septic shock and critical illness. Crit Care (Lond, Engl) 2020;24(1):636. doi: 10.1186/s13054-020-03351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honore PM, Redant S, De Bels D. Reliability of biomarkers of sepsis during extracorporeal therapies: the clinician needs to know what is eliminated and what is not. Crit Care (Lond, Engl) 2020;24(1):553. doi: 10.1186/s13054-020-03277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Custodero C, Wu Q, Ghita GL, Anton SD, Brakenridge SC, Brumback BA, Efron PA, Gardner AK, Leeuwenburgh C, Moldawer LL, et al. Prognostic value of NT-proBNP levels in the acute phase of sepsis on lower long-term physical function and muscle strength in sepsis survivors. Crit Care. 2019;23(1):230. doi: 10.1186/s13054-019-2505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettilä V. Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35(5):1277–1283. doi: 10.1097/01.CCM.0000261893.72811.0F. [DOI] [PubMed] [Google Scholar]
- 57.Mihajlovic D, Brkic S, Uvelin A, Draskovic B, Vrsajkov V. Use of presepsin and procalcitonin for prediction of SeptiFast results in critically ill patients. J Crit Care. 2017;40:197–201. doi: 10.1016/j.jcrc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Leli C, Ferranti M, Marrano U, Al Dhahab ZS, Bozza S, Cenci E, Mencacci A. Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. J Med Microbiol. 2016;65(8):713–719. doi: 10.1099/jmm.0.000278. [DOI] [PubMed] [Google Scholar]
- 59.Klouche K, Cristol JP, Devin J, Gilles V, Kuster N, Larcher R, Amigues L, Corne P, Jonquet O, Dupuy AM. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care. 2016;6(1):59. doi: 10.1186/s13613-016-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enguix-Armada A, Escobar-Conesa R, García-De La Torre A, De La Torre-Prados MV. Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin Chem Lab Med. 2016;54(1):163–168. doi: 10.1515/cclm-2015-0243. [DOI] [PubMed] [Google Scholar]
- 61.Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, Moerer O, Weyland A, Marx G, Gründling M, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–1276. doi: 10.1001/jamainternmed.2016.2514. [DOI] [PubMed] [Google Scholar]
- 62.Bonaventura A, Carbone F, Vecchié A, Meessen J, Ferraris S, Beck E, Keim R, Minetti S, Elia E, Ferrara D, et al. The role of resistin and myeloperoxidase in severe sepsis and septic shock: results from the ALBIOS trial. Eur J Clin Investig. 2020;50:e1333. doi: 10.1111/eci.13333. [DOI] [PubMed] [Google Scholar]
- 63.Cao C, Gu J, Zhang J. Soluble triggering receptor expressed on myeloid cell-1 (sTREM-1): a potential biomarker for the diagnosis of infectious diseases. Front Med. 2017;11(2):169–177. doi: 10.1007/s11684-017-0505-z. [DOI] [PubMed] [Google Scholar]
- 64.Wright SW, Lovelace-Macon L, Hantrakun V, Rudd KE, Teparrukkul P, Kosamo S, Liles WC, Limmathurotsakul D, West TE. sTREM-1 predicts mortality in hospitalized patients with infection in a tropical, middle-income country. BMC Med. 2020;18(1):159. doi: 10.1186/s12916-020-01627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson BJ, Calfee CS, Liu KD, Reilly JP, Kangelaris KN, Shashaty MGS, Lazaar AL, Bayliffe AI, Gallop RJ, Miano TA, et al. Plasma sTNFR1 and IL8 for prognostic enrichment in sepsis trials: a prospective cohort study. Crit Care (Lond, Engl) 2019;23(1):400. doi: 10.1186/s13054-019-2684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casagranda I, Vendramin C, Callegari T, Vidali M, Calabresi A, Ferrandu G, Cervellin G, Cavazza M, Lippi G, Zanotti I, et al. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern Emerg Med. 2015;10(6):725–730. doi: 10.1007/s11739-015-1268-7. [DOI] [PubMed] [Google Scholar]
- 67.Sharma A, Ray S, Mamidipalli R, Kakar A, Chugh P, Jain R, Ghalaut MS, Choudhury S. A comparative study of the diagnostic and prognostic utility of soluble urokinase-type plasminogen activator receptor and procalcitonin in patients with sepsis and systemic inflammation response syndrome. Indian J Crit Care Med Peer Review Off Publ Indian Soc Crit Care Med. 2020;24(4):245–251. doi: 10.5005/jp-journals-10071-23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu M, Zhang X, Chen H, Wang G, Zhang J, Dong P, Liu Y, An S, Wang L. Serum sPD-L1, upregulated in sepsis, may reflect disease severity and clinical outcomes in septic patients. Scand J Immunol. 2017;85(1):66–72. doi: 10.1111/sji.12509. [DOI] [PubMed] [Google Scholar]
- 69.Yeh CF, Wu CC, Liu SH, Chen KF. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann Intensive Care. 2019;9(1):5. doi: 10.1186/s13613-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Feng Q, Zhou S, Chen H. Downregulation of serum survivin correlates with increased inflammation, enhanced disease severity and worse prognosis in sepsis patients. Medicine. 2020;99(28):0000000000020272. doi: 10.1097/MD.0000000000020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoppensteadt D, Tsuruta K, Hirman J, Kaul I, Osawa Y, Fareed J. Dysregulation of inflammatory and hemostatic markers in sepsis and suspected disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2015;21(2):120–127. doi: 10.1177/1076029613509476. [DOI] [PubMed] [Google Scholar]
- 72.Hamed S, Behnes M, Pauly D, Lepiorz D, Barre M, Becher T, Lang S, Akin I, Borggrefe M, Bertsch T, et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. 2017;17(1):554. doi: 10.1186/s12879-017-2606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian R, Wang X, Pan T, Li R, Wang J, Liu Z, Chen E, Mao E, Tan R, Chen Y, et al. Plasma PTX3, MCP1 and Ang2 are early biomarkers to evaluate the severity of sepsis and septic shock. Scand J Immunol. 2019;90(6):7. doi: 10.1111/sji.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barre M, Behnes M, Hamed S, Pauly D, Lepiorz D, Lang S, Akin I, Borggrefe M, Bertsch T, Hoffmann U. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J Crit Care. 2018;43:21–28. doi: 10.1016/j.jcrc.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Karlsson S, Pettilä V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34(6):1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- 76.Karakike E, Adami ME, Lada M, Gkavogianni T, Koutelidakis IM, Bauer M, Giamarellos-Bourboulis EJ, Tsangaris I. Late peaks of hmgb1 and sepsis outcome: evidence for synergy with chronic inflammatory disorders. Shock. 2019;52(3):334–339. doi: 10.1097/SHK.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 77.Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent. Blood. 2014;124(24):3515–3523. doi: 10.1182/blood-2014-01-552943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarangi PP, Hyun YM, Lerman YV, Pietropaoli AP, Kim M. Role of β1 integrin in tissue homing of neutrophils during sepsis. Shock (Augusta, Ga) 2012;38(3):281–287. doi: 10.1097/SHK.0b013e31826136f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang Y, Li C, Shao R, Yu H, Zhang Q. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: a prospective observational study. Thromb Res. 2018;171:149–154. doi: 10.1016/j.thromres.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 80.Yao B, Zhang LN, Ai YH, Liu ZY, Huang L. Serum S100β is a better biomarker than neuron-specific enolase for sepsis-associated encephalopathy and determining its prognosis: a prospective and observational study. Neurochem Res. 2014;39(7):1263–1269. doi: 10.1007/s11064-014-1308-0. [DOI] [PubMed] [Google Scholar]
- 81.Mikacenic C, Hahn WO, Price BL, Harju-Baker S, Katz R, Kain KC, Himmelfarb J, Liles WC, Wurfel MM. Biomarkers of endothelial activation are associated with poor outcome in critical illness. PLoS ONE. 2015;10(10):e0141251. doi: 10.1371/journal.pone.0141251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crenn P, Neveux N, Chevret S, Jaffray P, Cynober L, Melchior JC, Annane D. Plasma L-citrulline concentrations and its relationship with inflammation at the onset of septic shock: a pilot study. J Crit Care. 2014;29(2):315.e311–316. doi: 10.1016/j.jcrc.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Ware LB, Magarik JA, Wickersham N, Cunningham G, Rice TW, Christman BW, Wheeler AP, Bernard GR, Summar ML. Low plasma citrulline levels are associated with acute respiratory distress syndrome in patients with severe sepsis. Crit Care. 2013;17(1):1–8. doi: 10.1186/cc11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu X. MiR-125b but not miR-125a is upregulated and exhibits a trend to correlate with enhanced disease severity, inflammation, and increased mortality in sepsis patients. J Clin Lab Anal. 2020;34(3):e23094. doi: 10.1002/jcla.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gui F, Peng H, Liu Y. Elevated circulating lnc-ANRIL/miR-125a axis level predicts higher risk, more severe disease condition, and worse prognosis of sepsis. J Clin Lab Anal. 2019;33(6):e22917. doi: 10.1002/jcla.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Westhoff D, Engelen-Lee JY, Hoogland ICM, Aronica EMA, van Westerloo DJ, van de Beek D, van Gool WA. Systemic infection and microglia activation: a prospective postmortem study in sepsis patients. Immun Ageing. 2019;16(18):019–0158. doi: 10.1186/s12979-019-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ehler J, Petzold A, Wittstock M, Kolbaske S, Gloger M, Henschel J, Heslegrave A, Zetterberg H, Lunn MP, Rommer PS, et al. The prognostic value of neurofilament levels in patients with sepsis-associated encephalopathy—a prospective, pilot observational study. PLoS ONE. 2019;14(1):e0211184. doi: 10.1371/journal.pone.0211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson BJ, Reilly JP, Shashaty MGS, Palakshappa JA, Wysoczanski A, Dunn TG, Kazi A, Tommasini A, Mikkelsen ME, Schweickert WD, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Şen S, Kamit F, İşgüder R, Yazıcı P, Bal Z, Devrim İ, Bayram SN, Karapınar B, Anıl AB, Vardar F. Surface TREM-1 as a Prognostic Biomarker in Pediatric Sepsis. Indian J Pediatr. 2020;23(10):020–03355. doi: 10.1007/s12098-020-03355-3. [DOI] [PubMed] [Google Scholar]
- 90.Aksaray S, Alagoz P, Inan A, Cevan S, Ozgultekin A. Diagnostic value of sTREM-1 and procalcitonin levels in the early diagnosis of sepsis. North Clin Istanb. 2016;3(3):175–182. doi: 10.14744/nci.2016.26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brenner T, Uhle F, Fleming T, Wieland M, Schmoch T, Schmitt F, Schmidt K, Zivkovic AR, Bruckner T, Weigand MA, et al. Soluble TREM-1 as a diagnostic and prognostic biomarker in patients with septic shock: an observational clinical study. Biomark Biochem Ind Expos Response Susceptibility Chem. 2017;22(1):63–69. doi: 10.1080/1354750X.2016.1204005. [DOI] [PubMed] [Google Scholar]
- 92.Spoto S, Cella E, de Cesaris M, Locorriere L, Mazzaroppi S, Nobile E, Lanotte AM, Pedicino L, Fogolari M, Costantino S, et al. Procalcitonin and MR-proadrenomedullin combination with SOFA and qSOFA Scores for Sepsis diagnosis and prognosis: a diagnostic algorithm. Shock (Augusta, Ga) 2018;50(1):44–52. doi: 10.1097/SHK.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 93.Yang Y, Liang S, Geng J, Wang Q, Wang P, Cao Y, Li R, Gao G, Li L. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care. 2020;8(45):020–00459. doi: 10.1186/s40560-020-00459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonaventura A, Carbone F, Vecchié A, Meessen J, Ferraris S, Beck E, Keim R, Minetti S, Elia E, Ferrara D, et al. The role of resistin and myeloperoxidase in severe sepsis and septic shock: Results from the ALBIOS trial. Eur J Clin Invest. 2020;50(10):13. doi: 10.1111/eci.13333. [DOI] [PubMed] [Google Scholar]
- 95.Maruchi Y, Tsuda M, Mori H, Takenaka N, Gocho T, Huq MA, Takeyama N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care. 2018;22(1):018–2109. doi: 10.1186/s13054-018-2109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karampela I, Christodoulatos GS, Kandri E, Antonakos G, Vogiatzakis E, Dimopoulos G, Armaganidis A, Dalamaga M. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: a prospective observational study. Cytokine. 2019;119:62–70. doi: 10.1016/j.cyto.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Saboktakin L, Bilan N, Ghalehgolab Behbahan A, Poorebrahim S. Relationship between resistin levels and sepsis among children under 12 years of age: a case control study. Front Pediatr. 2019;7:355. doi: 10.3389/fped.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ritter C, Tomasi CD, Dal-Pizzol F, Pinto BB, Dyson A, de Miranda AS, Comim CM, Soares M, Teixeira AL, Quevedo J, et al. Inflammation biomarkers and delirium in critically ill patients. Crit Care. 2014;18(3):1–6. doi: 10.1186/cc13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng Q, Wei WQ, Chaugai S, Leon BGC, Mosley JD, Leon DAC, Jiang L, Ihegword A, Shaffer CM, Linton MF, et al. Association between low-density lipoprotein cholesterol levels and risk for sepsis among patients admitted to the hospital with infection. JAMA Netw Open. 2019;2(1):e187223. doi: 10.1001/jamanetworkopen.2018.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chien JY, Jerng JS, Yu CJ, Yang PC. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med. 2005;33(8):1688–1693. doi: 10.1097/01.ccm.0000171183.79525.6b. [DOI] [PubMed] [Google Scholar]
- 101.Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care. 2017;38:289–294. doi: 10.1016/j.jcrc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Takegawa R, Kabata D, Shimizu K, Hisano S, Ogura H, Shintani A, Shimazu T. Serum albumin as a risk factor for death in patients with prolonged sepsis: an observational study. J Crit Care. 2019;51:139–144. doi: 10.1016/j.jcrc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Sui YD, Xin WN, Feng LL. Comparison of the clinical application values of PCT, hs-CRP and SAA detection in the early diagnosis of sepsis. Pak J Med Sci. 2020;36(7):1683–1687. doi: 10.12669/pjms.36.7.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou X, Liu C, Lian H, Xu Z, Ma L, Zang X, Sun J, Jia K, Cui L. The value of neutrophil gelatinase-associated lipocalin and citrullinated alpha enolase peptide-1 antibody in diagnosis, classification, and prognosis for patients with sepsis. Medicine. 2020;99(34):0000000000021893. doi: 10.1097/MD.0000000000021893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H, Wang X, Zhang Q, Xia Y, Liu D. Comparison of procalcitonin and high-sensitivity C-reactive protein for the diagnosis of sepsis and septic shock in the oldest old patients. BMC Geriatr. 2017;17(1):017–0566. doi: 10.1186/s12877-017-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin R, Hu H, Li L, Chen G, Luo L, Rao P. The potential of microRNA-126 in predicting disease risk, mortality of sepsis, and its correlation with inflammation and sepsis severity. J Clin Lab Anal. 2020;34(9):2. doi: 10.1002/jcla.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeng Q, Wu J, Yang S. Circulating lncRNA ITSN1-2 is upregulated, and its high expression correlates with increased disease severity, elevated inflammation, and poor survival in sepsis patients. J Clin Lab Anal. 2019;33(4):25. doi: 10.1002/jcla.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Piazza O, Russo E, Cotena S, Esposito G, Tufano R. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99(4):518–521. doi: 10.1093/bja/aem201. [DOI] [PubMed] [Google Scholar]
- 109.Crenn P, Neveux N, Chevret S, Jaffray P, Cynober L, Melchior JC, Annane D. Plasma L-citrulline concentrations and its relationship with inflammation at the onset of septic shock: a pilot study. J Crit Care. 2014;29(2):26. doi: 10.1016/j.jcrc.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 110.Chen J, He Y, Zhou L, Deng Y, Si L. Long non-coding RNA MALAT1 serves as an independent predictive biomarker for the diagnosis, severity and prognosis of patients with sepsis. Mol Med Rep. 2020;21(3):1365–1373. doi: 10.3892/mmr.2020.10923. [DOI] [PubMed] [Google Scholar]
- 111.Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma miR-125a and miR-125b in sepsis: correlation with disease risk, inflammation, severity, and prognosis. J Clin Lab Anal. 2020;34(2):23036. doi: 10.1002/jcla.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ehler J, Saller T, Wittstock M, Rommer PS, Chappell D, Zwissler B, Grossmann A, Richter G, Reuter DA, Nöldge-Schomburg G, et al. Diagnostic value of NT-proCNP compared to NSE and S100B in cerebrospinal fluid and plasma of patients with sepsis-associated encephalopathy. Neurosci Lett. 2019;692:167–173. doi: 10.1016/j.neulet.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 113.Aksaray S, Alagoz P, Inan A, Cevan S, Ozgultekin A. Diagnostic value of sTREM-1 and procalcitonin levels in the early diagnosis of sepsis. North Clin Istanb. 2017;3(3):175–182. doi: 10.14744/nci.2016.26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet (Lond, Engl) 1993;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saboktakin L, Bilan N, Ghalehgolab Behbahan A, Poorebrahim S. Relationship between resistin levels and sepsis among children under 12 years of age: a case control study. Front Pediatr. 2019;7:355. doi: 10.3389/fped.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.