Abstract
Primary cilium, first described in the 19th century in different cell types and organisms by Alexander Ecker, Albert Kolliker, Aleksandr Kowalevsky, Paul Langerhans, and Karl Zimmermann (Ecker, 1844; Kolliker, 1854; Kowalevsky, 1867; Langerhans, 1876; Zimmermann, 1898), play an essential modulatory role in diverse aspects of nervous system development and function. The primary cilium, sometimes referred to as the cell’s ‘antennae’, can receive wide ranging inputs from cellular milieu, including morphogens, growth factors, neuromodulators, and neurotransmitters. Its unique structural and functional organization bequeaths it the capacity to hyper-concentrate signaling machinery in a restricted cellular domain approximately one-thousandth the volume of cell soma. Thus enabling it to act as a signaling hub that integrates diverse developmental and homestatic information from cellular milieu to regulate the development and function of neural cells. Dysfunction of primary cilia contributes to the pathophysiology of several brain malformations, intellectual disabilities, epilepsy, and psychiatric disorders. This review focuses on the most essential contributions of primary cilia to cerebral cortical development and function, in the context of neurodevelopmental disorders and malformations. It highlights the recent progress made in identifying the mechanisms underlying primary cilia’s role in cortical progenitors, neurons and glia, in health and disease. A future challenge will be to translate these insights and advances into effective clinical treatments for ciliopathies.
1. Introduction
Primary cilia are microtubule based antennae-like sensory organelles extended by a vast majority of mammalian cells (Bloodgood, 2009; Fliegauf, Benzing, & Omran, 2007; Hilgendorf, Johnson, & Jackson, 2016; Reiter & Leroux, 2017; Sengupta, 2017). Primary cilia are anchored to the cell by mother centriole, the older of the pair of centrioles, which together with the pericentrosomal matrix, make up the centrosome complex. The primary cilia are structurally and functionally distinct from the motile cilia, that line epithelia and power fluid movement (Ringers, Olstad, & Jurisch-Yaksi, 2020). Disruption of the physiological function of primary cilia in humans lead to a broad range of multi-organ disease phenotypes, including obesity, renal, hepatic, and pancreatic cyst formation, situs abnormalities, congenital heart defects, anosmia, retinal degeneration, postaxial polydactyly, bronchiectasis, hypogonadism, infertility, hearing loss, facial anomalies, liver fibrosis, and brain malformations (Fliegauf et al., 2007; Hildebrandt, Benzing, & Katsanis, 2011; Lancaster & Gleeson, 2009; McIntyre et al., 2012; Mitchison & Valente, 2017; Park, Jang, & Lee, 2019; Reiter & Leroux, 2017; Sattar & Gleeson, 2011; Thomas, Boutaud, Reilly, & Benmerah, 2019; Valente, Rosti, Gibbs, & Gleeson, 2014). These disorders resulting from aberrant primary cilia function include Bardet-Biedl syndrome (BBS), nephronophthisis (NPHP), Senior-Loken syndrome (SNLS), Alstrom syndrome (ALMS), Meckel syndrome (MKS), Joubert syndrome (JBTS), Oral-facial-digital Type I (OFD 1), polycystic kidney diseases (PKD), Jeune asphyxiating thoracic dystrophy (JATD), Ellis van Creveld (EVC), and Leber congenital amaurosis (LCA) (see Reiter & Leroux, 2017 and Ringers et al., 2020 for comprehensive reviews of motile and non-motile ciliopathies).
Abnormalities in the formation, connectivity, and function of the central nervous system (CNS) underlie the neurological syndromes triggered by primary cilia malfunction (Caspary, Larkins, & Anderson, 2007; Doherty, 2009; Guo et al., 2015; Novarino, Akizu, & Gleeson, 2011; Parisi, 2019; Romani, Micalizzi, & Valente, 2013; Valente et al., 2014). These neurological syndromes include developmental delay, intellectual disabilities, autism spectrum disorder, mood disorders, epilepsy, abnormal respiratory rhythms, hypotonia, ataxia, oculomotor apraxia, and mirror movement synkinesis. The structural anomalies such as microcephaly, neuronal heterotopia, disrupted neuronal layer malformation, absent or reduced growth and decussation of axonal tracts (e.g., superior cerebellar peduncles (SCP), corticospinal tract (CST), corpus callosum (CC), and central pontine tracts), and cerebellar hypoplasia associated with these functional outcomes indicate the significance of physiological function of primary cilia in progenitors, neurons, and glia during human CNS development (Doherty, 2009; Guo et al., 2015; Juric-Sekhar, Adkins, Doherty, & Hevner, 2012; Parisi, 2019; Romani et al., 2013; Valente et al., 2014).
The human cerebral cortex forms as a result of coordinated unfolding of a series of inter-related developmental events (Kwan, Sestan, & Anton, 2012; Molnár et al., 2019; Fig. 1A). During early embryonic development, an undifferentiated sheet of pseudostratified neuroepithelial cells in telencephalic vesicles end their interkinetic nuclear migration, spanning the apical–basal axis, and transform into radial glial progenitors. Radial glial cells (RGCs), through their function as a source of neurogenic progenitors and neuronal migratory guides, provide a template for the formation of the cerebral cortex. RGCs in the pallium divide symmetrically and asymmetrically to expand the pool of progenitors and generate neurons, respectively. During the neurogenic period, asymmetric division of RGCs generates cortical neurons and neurogenic intermediate progenitors (IPs) or outer radial glial cells (oRGCs). Symmetric proliferation of IPs and oRGCs serve to rapidly expand the cortical neuronal population, particularly the upper layer neurons. The cell soma of radial progenitors remain apically positioned in the ventricular zone, with a long basal process extending toward the pial surface, thereby enabling the orderly generation and guidance of new neurons in the cerebral cortex. Migration of clonally related neurons along related radial glial basal processes leads to the formation of cortical radial columns (or radial units) and laminar organization of neurons. As neurogenesis nears its completion, progenitors either morphologically transform or undergo a final division to generate astrocytes. Oligodendrocytes are generated similarly from progenitors in the subpallium. In contrast, microglia, originating from early erythromyeloid progenitors (EMPs) in the extra-embryonic yolk sac, enter the CNS in association with waves of hematopoiesis, prior to the proliferation of neural progenitors (Thion, Ginhoux, & Garel, 2018). The developmental balance between different progenitor subtypes and the resultant differences in the generation and guidance of distinct classes of neurons and glia within the developing cerebral cortex enables the emergence of distinctly different cortical areas with characteristic patterns of neuronal and glial density and diversity. Once appropriately positioned, connectivity between the two main types of neurons, excitatory projection neurons with spiny dendrites and long axons and inhibitory interneurons with short axons and non-spiny dendrites, form the basic neuronal circuitry in the cerebral cortex. The distinct cellular architecture of these neuronal subtypes and their interactions with glia (i.e., astrocytes, oligodendroglia, and microglia) constrains the types of circuits formed and thus the excitatory/inhibitory (E/I) balance and the functional competence of these cortical circuits. Primary cilia play a diverse and essential modulatory role in these major developmental events underlying cerebral cortical formation and function (Fig. 1B-M).
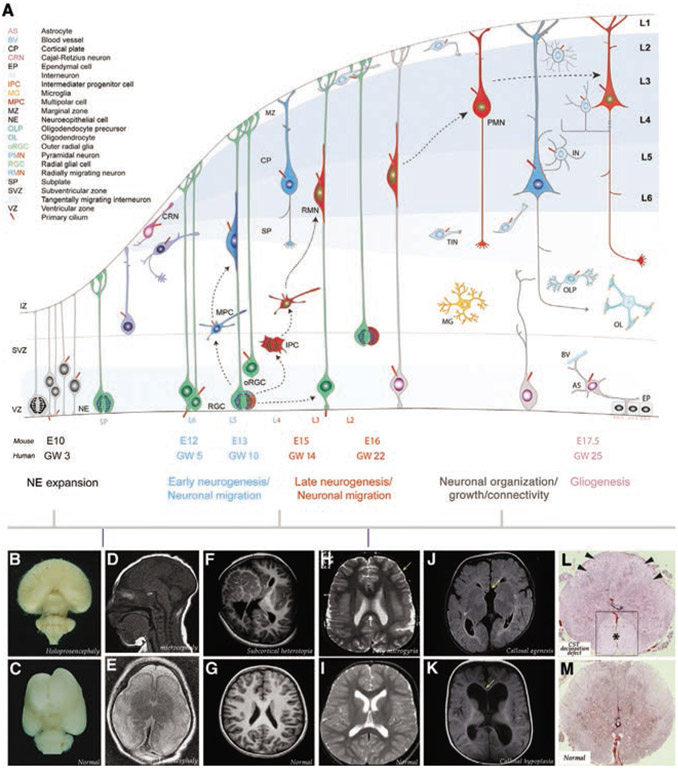
Fig. 1.
Development of the cerebral cortex and cortical malformations associated with primary ciliary dysfunction. (A) During early development, symmetrically dividing neuroepithelial cells (NPs) in the ventricular zone (VZ;blue) assume radial glial identity. Radial glia cells (RGCs) divide symmetrically to expand the RGC pool or asymmetrically to generate neurons and other progenitors (intermediate progenitor cells [IPCs] or outer radial glial cells [oRGC]). Newborn neurons assume a multipolar morphology and then migrate away from the germinal zones, guided by the basal processes of the RGCs, to reach the mantle layers. The first-born neurons settle within the preplate (PP) to form the nascent cortical plate (CP). Continued addition of subsequently generated neurons then split the PP into the marginal zone (MZ) and the subplate (SP), leading to sequential formation of deep (L6 and L5; blue) and upper layers (L4, L3 and L2; red) neurons of the cerebral cortex. Intermediate progenitor cells (IPCs) or outer radial glial cells (oRGC) derived from radial glia undergo symmetric neurogenic divisions in the SVZ to generate upper layer neurons. Concurrent to radial migration of projection neurons, tangentially migrating interneurons originating in the ganglionic eminence invade the cortical plate. At the end of neurogenesis, the radial glial scaffold is dismantled, progenitors become gliogenic, generate cortical and subependymal zone (SEZ) astrocytes (Ast), and give rise to a layer of ependymal cells (EL). Early embryonic microglial (MG) invasion into the developing cerebral wall and oligodendrocyte precursor (OLP) generation and differentiation into oligodendrocytes (OL) are not illustrated. AS, astrocyte; BV, blood vessel; CP, cortical plate; CRN, Cajal-Retzius neuron; EP, ependymal cell; IN, interneuron; IPC, intermediate progenitor cell; MG, microglia; MPC, multipolar cell; MZ, marginal zone; NPC, neuroepithelial progenitor cell; OLP, oligodendrocyte precursor; OL, oligodendrocyte; oRGC, outer radial glia; PMN, Pyramidal neuron; RGC, radial glial cell, RMN, radially migrating neuron; SP, subplate; SVZ, subventricular zone, TIN, tangentially migrating interneuron; VZ, ventricular zone. A timeline of mouse embryonic (E) day or human gestational week (GW) relevant to the initial stages of the respective developmental events are indicated. (B–M) Primary cilia dysfunction during distinct stages of cortical development contributes to specific cortical malformations. (B, C) Shh mutations, likely disrupting cilia mediated Shh signaling, in NPs lead to holoprosencephaly. (D–G) Primary cilia malfunction in RGCs or migrating neurons can promote microcephaly (D), lissencephaly (E), and subcortical heterotopia (F, G). Primary cilia dysfunction in postmigratory neurons contributes to poly microgyria (H[arrow], I), callosal agenesis (arrow, J) or hypoplasia (arrow, K), and CST decussation defects (L, M [asterisk in boxed area indicates enlarged, nondecussating anterior CST in HE stained cervical spinal cord]). Images are reproduced from Monuki and Walsh (2001) (B, C), Shamseldin et al. (2015) (D, E; RTTN mutation), Uzquiano et al. (2019) (F, G; RPGRIP1L mutation), Kheradmand Kia et al. (2012) (H, I; RTTN mutation), Putoux et al. (2011) (J, K; KIF7 mutation), and Juric-Sekhar et al. (2012) (L, M; OFD1 mutation). These mutations directly or indirectly disrupt primary cilia signaling and maintenance.
The majority of the cellular components of the developing cerebral cortex, including neuroepithelial cells, progenitors, migrating neurons, postmigratory neurons, and astrocytes, have active primary cilia (Fig. 2; Arellano, Guadiana, Breunig, Rakic, & Sarkisian, 2017; Bishop, Berbari, Lewis, & Mykytyn, 2007; Green & Mykytyn, 2014; Guemez-Gamboa, Coufal, & Gleeson, 2014; Sarkisian & Guadiana, 2015; Sengupta, 2017). Presence of primary cilia in mature oligodendrocytes and microglial cells has yet to be conclusively demonstrated. Primary cilia in the developing cortical cells are highly dynamic in terms of their ability to undergo changes in shape, length, and orientation during development (Fig. 2B; Higginbotham et al., 2012, 2013). Although the detection of primary cilia in different cortical cells with transmission or scanning electron microscopy analyses or immunolabeling with pancilia-specific antibodies (e.g., anti-ACIII or Arl13b) do not reveal their functional diversity, primary cilia in each of these cell types are highly likely to be specialized to subserve cell-type specific functions during cortical development. Supporting this possibility, expression mapping of cilia-associated genes across different cell types during cerebral cortical development reveals distinct cell-type specific expression profiles (Loo et al., 2019; Fig. 3). How this molecular specification of primary cilia in different cortical cells translates into developmental stage- and cell type-specific functions during cerebral cortical formation remains open. However, the spectrum of primary cilia related developmental brain malformations indicate the significance of this sensory organelle for the appropriate development and differentiation of cortical progenitors, neurons, and glia and the resultant orderly progression of cerebral cortical formation and organization.
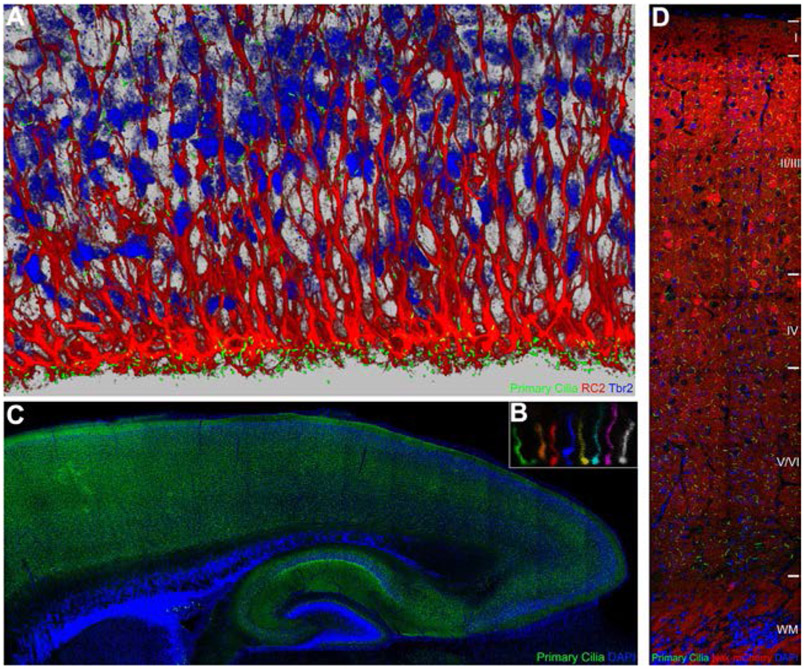
Fig. 2.
Primary cilia in the embryonic and postnatal cerebral cortex. Primary cilia (green) in the ventricular zone of the developing mouse cerebral cortex (E16) were labeled with hGFAP-Cre induced Sstr3-GFP (A). Radial glia and intermediate progenitors were co-labeled with RC2 (red) and anti-Tbr2 (blue) antibodies, respectively. (B) Live imaging of a primary cilium in the embryonic cerebral wall (E16), over 5.5 h, indicates its dynamic nature. Images at different time points were pseudocolored to illustrate the changing shape, length, and orientation. (C) Projection neuronal primary cilia in postnatal brains (P7) were labeled with Nex-Cre induced Sstr3-GFP (B). Neurons across cortical layers display primary cilia of varying shapes and orientation (C).
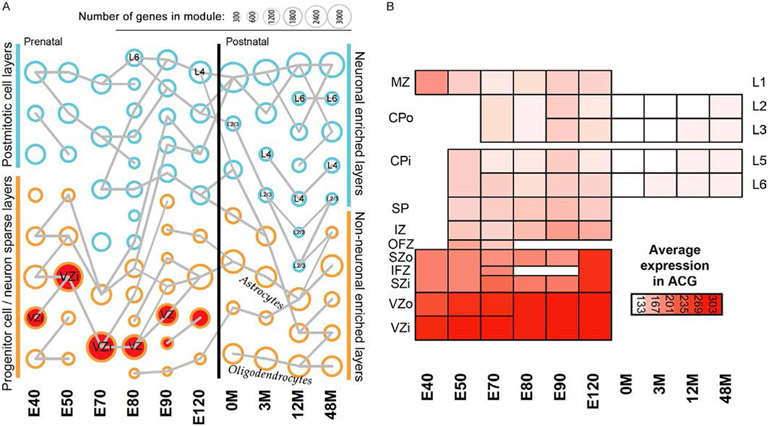
Fig. 3.
Ciliopathy gene expression profile in the developing primate brain. (A) Gene expression levels of distinct populations of cells in individual cortical layers were profiled in rhesus monkey brain at 10 different pre and postnatal ages (E40, E50, E70, E80, E90, E120, 0M, 3M, 12M, and 48M; Bakken et al., 2016). Ciliopathy associated genes (confirmed and candidate) were significantly enriched among genes co-expressed in the developing cortex, primarily in the proliferative niche and new born neurons. Circle size indicates how many genes are in the module. Modules significantly enriched for risk genes associated with ciliopathies are in red (P < 0.1). Modules from adjacent ages with the most highly significant gene overlap are connected by gray lines. Though the number of ciliopathy genes overlapping with the other modules did not reach statistical significance, different ciliopathy genes are expressed in these modules (see Table 1). (B) Average expression pattern of cilia associated genes found in at least two enriched modules. Heat maps are organized by specific cortical layer and age as described in Bakken et al. (2016). MZ, marginal zone; CPo/CPi, outer/inner cortical plate; SP, subplate; IZ, intermediate zone; SZ, subventricular zone; VZo/VZi, outer/inner ventricular zone; L2-6, cortical neuronal layers 2–6.
2. Progenitors, primary cilia, and associated brain developmental malformations
The organization of the ventricular niche where radial glial cells, intermediate progenitors, and outer radial glial progenitors actively form, divide, and interact with their daughter neuron or glial cells, depends on primary cilia signaling (Guo et al., 2015; Higginbotham et al., 2013; Lepanto, Badano, & Zolessi, 2016; Valente et al., 2014). In proliferating progenitors, primary cilia are disassembled during cell division and ciliary membrane remnants may preferentially associate with the mother centriole. During neurogenesis, asymmetric inheritance of the old mother centriole by daughter radial glial progenitors facilitates the maintenance of radial progenitor identity (Wang et al., 2009). Asymmetric inheritance of the primed mother centriole and thus, rapid extension of a primary cilium by one of the daughter cells is thought to provide differential competence for that daughter cell to respond to environmental cues in the ventricular niche (Basten & Giles, 2013; Delgehyr & Spassky, 2014). In dividing radial progenitors, WDR62 enables centrosome protein CEP170’s localization to the basal body of the primary cilium, where CEP170 recruits a microtubule depolymerizing factor, KIF2A, to dismantle the cilium (Zhang et al., 2019). Disruption of this pathway and other WDR62 linked networks vital for cilia formation (e.g., WDR62-CPAP-IFT88) lead to microcephaly (Shohayeb et al., 2020; Zhang et al., 2019).
Disruption of primary cilia activity in early radial progenitors via Arl13b deletion disrupt their apical-basal polarity, leading to drastic malformation of the cerebral cortex (Higginbotham et al., 2013). Shh mutations in humans, likely disrupting cilia mediated Shh signaling, lead to holoprosencephaly where defective early patterning of neuroepithelial cells results in the failure of the forebrain to develop into two cerebral cortical hemispheres (Fig. 1B and C; Monuki & Walsh, 2001; Nanni et al., 1999). Further, primary cilia in RGCs regulate the size of the surface of their ventricular apical domains via the mTORC1 pathway. Genetic deletion of primary cilia in RGCs (via conditional deletion of Ift88 or Kif3a in RGCs) leads to misorientation of the mitotic spindle in radial glia, increased basal progenitors, dilation of brain ventricles (ventriculomegaly), and eventual hydrocephalus (Foerster et al., 2017). In contrast to RGCs, the dynamics and significance of primary cilia in intermediate progenitors and outer radial glia remains to be fully defined. In the postnatal germinal niche of the forebrain, the ventricular–subventricular zone, where there is ongoing progenitor proliferation and neurogenesis, the primary cilia is required for neurogenesis (Tong et al., 2014).
Several human ciliopathy mutations that disrupt ciliogenesis in progenitors, primarily via disrupting centrosome or centriolar functions, lead to primary microcephaly (Fig. 1D), where disrupted progenitor proliferation causes a reduction in the generation of appropriate complement of neurons and glia necessary to form a normal cerebral cortex. For example, mutations in centrosome related genes PCNT, PLK4, ASPM, WDR62, CPAP, DNYLT1, and C2CD3 disrupt appropriate patterns of ciliogenesis or cilia maintenance, post cell-division, thus disrupting the ability of progenitors to use primary cilia to sense essential environmental cues present in the CSF (e.g., SHH) or ventricular niche (e.g., IGF) necessary for progenitor differentiation (Bilguvar et al., 2010; Bober & Jackson, 2017; Boczek et al., 2018; Bond et al., 2002; Gabriel et al., 2016; Lehtinen et al., 2011; Li et al., 2011; Nicholas et al., 2010; Thauvin-Robinet et al., 2014). In addition, mutations in genes necessary for primary cilia structural maintenance, trafficking, or signaling, such as KIF2A, RPGRIP1L, Arl13b or Inpp5e, also disrupt the ventricular niche of the developing cerebral cortex and leads to microcephaly or subcortical heterotopia (Bielas et al., 2009; Broix et al., 2018; Cavallin et al., 2017; Higginbotham et al., 2013; Poirier et al., 2013; Uzquiano et al., 2019). Microcephaly in humans is often presented with hydrocephalus. Many of the genes disrupting primary cilia in progenitors can also affect motile cilia in ependymal cells lining the ventricles necessary for CSF flow. This may contribute to defective ependymal cell differentiation, ciliogenesis, and or motile ciliary beating frequency changes in these cells and thus hydrocephaly (Ringers et al., 2020).
Opposite of microcephaly, megalencephaly results from over proliferation of progenitors. Removal of CEP83 in RGCs disrupts distal appendage (DAP) assembly and thus impairs the anchoring of the centrosome to the apical membrane and primary ciliogenesis (Shao et al., 2020). CEP83 deficiency leads to megalencephaly and biallelic mutations in human CEP83 cause intellectual disability (Failler et al., 2014). Furthermore, an activating mutation of AKT in humans cause hemimegalencephaly (Poduri et al., 2012). Primary cilia activation leads to phospho AKT localization at the base of primary cilium (Christensen, Morthorst, Mogensen, & Pedersen, 2017; Suizu et al., 2016). If and how the primary cilium is affected in human cortical progenitors with AKT mutations and how it contributes to brain overgrowth in these patients remains to be fully examined.
3. Migrating neurons and primary cilium in the developing cerebral cortex
Following their birth in the ventricular zone, newborn neurons migrate several thousand cell soma size distances through a complex cellular milieu to reach their final resting position in different cortical layers and areas. Both radially migrating projection neurons and tangentially migrating interneurons in the cerebral cortex display a dynamic primary cilium. Primary cilia change in length, rotate, branch, and appear to probe their surroundings in migrating inter neurons (Higginbotham et al., 2012). Cilia in neurons localize to the leading process during migration, but move closer to the cell soma during periods of pausing when they became much more dynamic in their movement and length. Electron tomography studies of interneuronal primary cilia further indicate that they cycle between elongated, surface-exposed and shorter, intracellular phases, in coordination with the cell migration stages (Baudoin et al., 2012). The localization and orientation of the primary cilium are also altered depending on the state of migration in migrating neuroblasts of the postnatal rostral migratory stream (Matsumoto et al., 2019). In contrast, signaling defective primary cilia in neurons do not display such dynamism (Higginbotham et al., 2012). Neurons with mutant cilia paused longer during migration and were unable to respond to micro gradients of guidance cues, suggesting that the reduction in cilia dynamics may reflect an inability to efficiently sense guidance cues in the migratory environment (Higginbotham et al., 2012).
Intriguingly, primary cilia activity modulates both radially and tangentially migrating neurons in the cerebral cortex (Baudoin et al., 2012; Guo et al., 2015; Higginbotham et al., 2012; Park et al., 2018). Radial migration involves close intercellular adhesive interactions with radial glial migratory guides, whereas tangential migration occurs for the most part in a cell-substrate independent manner. After they invade the developing cerebral wall, tangentially migrating neurons turn radially, along radial glia processes, to populate the emerging cortical layers (Yokota et al., 2007). Neuronal primary cilia are hypothesized to cell autonomously bind and transduce signals from gradients of guidance molecules, leading to changes in neuronal morphology and movement (Higginbotham et al., 2012). Shh signaling via primary cilia may enable tangentially migrating interneurons to turn radially and populate appropriate cortical plate regions (Baudoin et al., 2012). However, primary cilia may differentially affect the chemotaxic and haptotaxic mechanisms necessary for radial or tangential neuronal migration. Further, it remains untested if primary cilia and the growth cones in the leading processes of migrating neurons transduce different signals related to the directed movement of neurons.
Human genetic mutations of EML1 that perturb anterograde trafficking from the Golgi apparatus to primary cilia and thus the maintenance of primary cilia, lead to subcortical heterotopia, characterized by ectopic radial progenitor localization, disrupted neuronal migration, and aberrant placement of neurons away from their predestined cortical layers (Fig. 2F and G; Uzquiano et al., 2019). RTTN mutations associated with dysmorphic primary cilium can cause lissencephaly (Fig. 2E), a cortical neuronal migration defect resulting from an inability to properly maintain neuronal migration during cortical development (Kwan et al., 2012; Shamseldin et al., 2015). MTOR mutations affecting autophagy mediated ciliogenesis disrupts radial neuronal migration and leads to focal cortical dysplasia in FMCD patients with somatic mutations in MTOR (Di Nardo & Sahin, 2018; Park et al., 2019, 2018). MTOR mutant neurons without primary cilium or severely shortened primary cilium do not undergo normal radial migration and appropriate laminar placement when compared to wild type neurons in the same brain (Park et al., 2018), further demonstrating a role for primary cilia signaling in cortical neuronal migration.
Recent studies combining real time neuronal migration assays with live imaging of second messenger pathways in the ciliary compartment are beginning to provide new insights into how directional information from guidance cues in the developing cortex may be conveyed through the primary cilium to trigger changes in neuronal migration (Stoufflet et al., 2019). In embryonic, postnatal and adult migrating neurons, ciliary Adenylate Cyclase 3 (AC3) activity generates cAMP, which in turn activates centrosomal Protein Kinase A (PKA). Live-imaging with cAMP biosensors reveals a periodic cAMP hotspot at the centrosome, which appears to be necessary for centrosome/nucleus coupling and nucleokinesis of neurons. Delocalization of PKA from the centrosome leads to migratory defects. Primary cilia and its anchoring centrosome may form a cAMP-signaling unit to dynamically regulate neuronal migration (Stoufflet et al., 2019). Considering the cilium’s proximity to the nucleus and its link to the centrosome-associated microtubule network, such primary cilia generated cAMP-signaling may promote rapid and efficient local and transcriptional signal transduction underlying nucleokinesis and directional migration of neurons.
4. Dendrites, axons, neuronal connectivity and primary cilium
As neurons arrive at their appropriate laminar locations, they extend dendrites and axons to form appropriate connections with other neurons in cortex and sub cerebral regions (Banker, 2018; Barnes & Polleux, 2009). Axonal pathway defects are a common feature in many human ciliopathies (Guo et al., 2015; Reiter & Leroux, 2017; Valente et al., 2014; Fig. 2J-M). Appropriate axonal growth and pathfinding are essential for the wiring of the developing brain (Chedotal & Richards, 2010; Engle, 2010). Joubert Syndrome Related disorders (JSRD), a subset of ciliopathies, are characterized by consistent and distinct axonal malformations (Brancati, Dallapiccola, & Valente, 2010; Engle, 2010; Parisi, 2009). An axonal tract malformation called the molar tooth sign (MTS) is a diagnostic feature of JSRD. MTS results from thickened, elongated, and horizontally misoriented superior cerebellar peduncles (SCPs) that fail to decussate in the midbrain (Brancati et al., 2010; Juric-Sekhar et al., 2012; Parisi, 2009; Sattar & Gleeson, 2011; Senocak, Oguz, Haliloglu, Topcu, & Cila, 2010). SCPs are formed by the axons of deep cerebellar nuclei (DCN). In addition to SCP malformation, absent or reduced decussation of the corticospinal tract (CST), corpus callosum (CC), and transverse pontine tracts are frequently observed in JSRD patients (Brancati et al., 2010; Engle, 2010; Hildebrandt et al., 2011; Jissendi-Tchofo et al., 2015; Juric-Sekhar et al., 2012; Poretti et al., 2007; Romani et al., 2013). Consistent with these axonal anomalies and the resultant changes in brain wiring, JSRD patients are often diagnosed with delayed development, intellectual disabilities, autism spectrum disorder (ASD), muscular hypotonia, breathing difficulties, epilepsy, and ataxia (Akizu et al., 2013, 2014; Alvarez Retuerto et al., 2008; Novarino et al., 2011; Reiter & Leroux, 2017). Further, mutations in KIF7, a ciliary kinesin, leads to callosal agenesis or hypoplasia with associated intellectual disabilities (Putoux et al., 2011; Fig. 2J-K). Callosal axon malformations are also seen in ciliopathies such as Oral-facial-digital Syndrome resulting from mutations in OFD1 or TCTN3, necessary for centriole elongation and ciliogenesis or transition zone organization and SHH signaling, respectively (Guo et al., 2015; Thauvin-Robinet et al., 2013; Thomas et al., 2012). These diverse axonal phenotypes related to primary cilia malfunctions in humans strongly indicate the significance of ciliary signaling to axonal tract growth and connectivity.
During the formation of neuronal connectivity in the brain, axons are guided by tropic and trophic gradients of chemoattractant or chemorepellent cues toward their target. Axons extend in large tracts or fascicles and follow stereotyped pathways to reach their appropriate synaptic targets in distant brain regions. Such group extension of axons toward appropriate targets is necessary for neuronal wiring in the brain (Barnes & Polleux, 2009; Chedotal & Richards, 2010; Cionni et al., 2018; Engle, 2010; Paolino, Fenlon, Suárez, & Richards, 2018). The axon-dendrite polarization of neurites and initial extension of axons appear to be unaffected in the absence of primary cilia signaling (Guo et al., 2019). However, branching, fasciculation and crossing of axons and thus their accurate final projections and connectivity are disrupted in cilia mutants (Grochowsky & Gunay-Aygun, 2019; Guo et al., 2015; Parisi, 2019; Poretti, Huisman, Scheer, & Boltshauser, 2011; Qian, Song, & Ming, 2019; Thomas et al., 2019; Ware, Gunay-Aygun, & Hildebrandt, 2011). How does primary cilia signaling selectively modulate the fasciculation, decussation, and targeting of axons?
A motile growth cone that properly responds to guidance cues, and axon-axon interactions that enable axon sorting and fasciculation are both crucial to ensure the fidelity of axonal growth and navigation during development (Nishikimi, Oishi, & Nakajima, 2013; Raper & Mason, 2010; Wang & Marquardt, 2013; Yu & Bargmann, 2001). The axonal growth cone motility and direction relies on the interplay between filopodia that sense environmental cues for pathfinding, and lamellipodia that support persistent axonal outgrowth (Mattila & Lappalainen, 2008; Mejillano et al., 2004). Growth cones of neurons with aberrant primary cilia show altered filopodia vs. lamellipodia balance (Guo et al., 2019). Further, adhesion molecules such as Pcdh17, essential to mediate axon-axon recognition (Hayashi et al., 2014; Hoshina et al., 2013), fail to localize to axon-axon contacts along cilia mutant axonal shafts (Guo et al., 2019). The combined disruption in axonal growth cone dynamics and axon-axon interactions may thus contribute to the axonal pathfinding and fasciculation defects evident in neurons with defective primary cilia (Guo et al., 2015, 2019). Some of the axonal tract defects in ciliopathies, particularly callosal malformations, may also involve abnormal guidepost cell positioning and associated inappropriate midline crossing (Laclef et al., 2015; Putoux et al., 2019).
Once they reach their appropriate targets, axons establish proper connections by extending terminal axon arbors (Courchet et al., 2013; Gibson & Ma, 2011; Kalil & Dent, 2014; Kalil, Li, & Hutchins, 2011). Upper layer cortical neurons with disrupted cilia grow a long primary axon with significantly reduced terminal arbors at targets in ipsi and contra lateral cortex, thus failing to sufficiently innervate their targets to form appropriate neuronal connections (Guo et al., 2019). Neuron intrinsic properties (e.g. neuronal activity, cAMP and Ca2+ gradients) are known to converge onto signaling nodes (e.g. PI3K/AKT/GSK3β, Rho GTPases) to shape axonal growth, branching and targeting (Kalil & Dent, 2014). But how do primary cilia convey such signals to axons?
The primary cilium, with a volume ~1/1000 of the soma (Phua, Lin, & Inoue, 2015), is home to diverse signaling machineries, including receptor tyrosine kinases (e.g., IGFR, EGFR, MET, PDGFR-α, Trk receptors p75NTR and TrkB; (Armato, Chakravarthy, Chiarini, PrÃ, & Whitfield, 2011; Christensen, Clement, Satir, & Pedersen, 2012) and GPCRs (e.g., Smo, Frizzled receptor FZD3, Sstr3, HTR6, NPY2R, NPY5R, DRD1, DRD2, MchR1, GPR161, GPR120 (Choi et al., 2011; Christensen et al., 2012; Green & Mykytyn, 2014; Hilgendorf et al., 2016; Mukhopadhyay & Rohatgi, 2014; Nichols, Floyd, Bruinsma, Narzinski, & Baranski, 2013; Omori et al., 2015; Schou, Pedersen, & Christensen, 2015), their downstream effectors (e.g., PI3 kinase (Franco et al., 2014), Inpp5b, Inpp5e (Conduit, Dyson, & Mitchell, 2012; Jacoby et al., 2009), Ocrl (Phua et al., 2015), AKT (Zhu, Shi, Wang, & Liao, 2009), AC3, 5 and 6 (Phua et al., 2015), and second messengers (e.g., PIP3, PIP2, Ca2+ and cAMP; (DeCaen, Delling, Vien, & Clapham, 2015; Delling, DeCaen, Doerner, Febvay, & Clapham, 2013). The primary cilium thus provides a unique environment where signaling components are highly concentrated in a small cellular domain to rapidly trigger and facilitate efficient signaling cascade activation and crosstalk (Gherman, Davis, & Katsanis, 2006; Ishikawa, Thompson, Yates, & Marshall, 2012; Kohli et al., 2017; Narita, Kozuka-Hata, Nonami, et al., 2012; Sigg et al., 2017; Van Dam et al., 2019, 2013; Mick et al., 2015).
Using optogenetic or chemogenetic methods to exert precise control of cilia-specific signaling events, the cellular and physiological impact of the signaling emanating selectively from the primary cilium on developing neurons and neural circuit formation are beginning to be understood (Guo et al., 2017, 2019). Optogenetic or chemogenetic manipulation of ciliary signaling events at multiple levels, including receptor activation (e.g., GPCRs), downstream effector activation (e.g., PI3K, AKT), and second messenger production (i.e., cAMP via ACIII), reveal that exogenously induced deregulation of ciliary signaling cascades rapidly alters growth cone behavior and filopodia/lamellipodia balance, to a large extent recapitulating the aberrant axonal growth cone behavior seen in primary cilia mutant neurons. Conversely, activation of the ciliary-phosphoinositide phosphatase (Inpp5e) pathway, that antagonizes PI3K/AKT signaling, induces an opposite effect, causing axons to retract filopodia and extend lamellipodia. These results suggest that disrupted primary cilia driven PI3K/AKT signaling homeostasis may be an underlying cause of aberrant axonal growth dynamics in cilia mutant neurons and that signals emanating from primary cilia have the capacity to remotely and rapidly regulate axonal growth behavior (Guo et al., 2015, 2019).
Once triggered, PI3K/AKT signaling co-opts downstream kinase cascades and second messengers (e.g. Ca2+, cAMP, IP3, PIP3) to amplify and propagate signals for cell-wide, long-distance signaling (Civelli, 2012; Gavi, Shumay, Wang, & Malbon, 2006; Lemmon & Schlessinger, 2010). Ciliary GPCR signaling can induce axonal Ca2+ waves down to the growth cone (Guo et al., 2019). Localized activation of GPCR or PI3K/AKT signaling are known to trigger cell-wide Ca2+ waves through Ca2+ influx via Ca2+ channels on the plasma membrane, IP3-mediated Ca2+ release from ER internal stores, and Ca2+/calmodulin signaling mediated by calcium binding proteins such as CaMKII (Averaimo & Nicol, 2014; Henle et al., 2011; Schneider et al., 2008; Zheng & Poo, 2007). Various Ca2+ channels localize to primary cilia (e.g., PKD1-L1 and 2-L1; DeCaen et al., 2015; Phua et al., 2015; Pablo, DeCaen, & Clapham, 2017). CaMKIIβ, known to amplify Ca2+ signals, is localized to the base of cilia (Puram et al., 2011). Furthermore, Shh-Smo signaling, likely mediated via primary cilia, can downregulate cAMP levels and protein kinase A activity in commissural neurons to allow Sema3-mediated repulsion of commissural axons at midline crossing (Parra & Zou, 2010). Even though the exact mechanisms that enable ciliary signaling to trigger axonal Ca2+ wave and its integration with axonal PI3K/AKT signaling waves are yet to be fully elucidated, signaling effectors downstream of PI3K/AKT, such as TSC/mTOR, GSK3β, and CREB, are known to regulate cytoskeleton organization, transcriptional and translational programs involved in neuronal morphogenesis and synaptic plasticity. Particularly, human patients and genetic models with mutations in PI3K/AKT signaling pathway components (e.g. PTEN, AKT1, TSC1/2) show aberrant axonal growth and disorganization of axon tracts (Choi et al., 2008; Geoffroy et al., 2015; Huang, Chen, & Page, 2016; Kwon et al., 2006; Shin et al., 2017; Tsai & Sahin, 2011). Elevated PI3K/AKT activity and altered downstream mTOR, GSK3β and CREB activity were evident in JSRD-related mouse neuronal cilia mutant models (Guo et al., 2017, 2019; Higginbotham et al., 2012). Further, transcriptional networks modulated by JSRD genes such as ARL13B and ZNF423 converge onto neuronal developmental programs including neuronal differentiation, cytoskeleton organization, and neural survival, potentially through AKT signaling network (Guo et al., 2019). These observations raise the intriguing hypothesis that PI3K/AKT signaling may serve as a converging node to integrate ciliary receptor signaling necessary for both rapid axonal growth modulation and long-term neuronal development programs.
Primary cilia signaling is known to modulate dendritic branching and spine formation in cortical neurons, possibly via cAMP and Shh-dependent pathways (Guadiana et al., 2013; Guo et al., 2017; Harwell et al., 2012; Lesiak, Brodsky, Cohenca, Croicu, & Neumaier, 2018). Disrupting primary cilia signaling via overexpression of 5HT6 and SSTR3 in cortical neurons, induces the formation of elongated and branched primary cilia, increases levels of intraflagellar transport proteins such as Kif3a, and reduces dendritic outgrowth and branching. These deficits are rescued when ciliary signaling capacity is restored with ACIII (Guadiana et al., 2013).
Intriguingly, both dendritic spines and cilia share a common function, i.e., to sense and transduce extracellular signals, with cilia being receptive to a broader range of extracellular cues than dendritic spines, which receive input signals primarily from presynaptic neurons and surrounding astrocytes or microglia (Nechipurenko, Doroquez, & Sengupta, 2013: Thion et al., 2018). Despite this commonality, if and how they influence each other remains an open question in neuronal functional biology. Intriguingly, synaptic integration that occurs in dendritic spines appears to require a functional primary cilium. Conditional deletion of cilia in hippocampal neurons induced defective dendritic refinement and synapse formation (Kumamoto et al., 2012). Importantly, unlike major axonal pathway defects detectable in MRI of ciliopathy patient brains, neuronal dendritic disruptions are yet to be systematically probed in genotyped ciliopathy patient brain tissue or organoid models.
5. Primary cilia’s impact on neural circuit maintenance and function
In ciliopathies such JSRD, nearly two thirds of the affected individuals function in the intellectually disabled range, indicative of ongoing neural circuit malfunction (Grochowsky & Gunay-Aygun, 2019; Parisi, 2019). Psychomotor delay and epilepsy in subsets of ciliopathy patients further indicates neural circuit disruptions (Canning et al., 2018; Grochowsky & Gunay-Aygun, 2019). DISC1, a major susceptibility gene for psychiatric syndromes, regulates the formation and maintenance of primary cilia and targeting of dopamine D2 receptors to ciliary membrane (Marley & von Castro, 2012; Marley & von Zastrow, 2010). A diverse number of neuro-psychiatric risk genes affect the formation or maintenance of primary cilia (Marley & von Castro, 2012). A decrease in the percentage of cells (olfactory neuronal precursors [ONP]) with primary cilia was noticed in schizophrenia (SCZ) and bipolar disorder (BD) patients (Muñoz-Estrada, Lora-Castellanos, Meza, Alarcón Elizaldes, & Benítez-King, 2017). Missense mutations in RTTN or AH1, resulting in short and dysmorphic primary cilia, cause polymicrogyria, characterized by abnormal cortical neuronal organization and circuits (Fig. 1H and I; Dixon-Salazar et al., 2004; Kheradmand Kia et al., 2012). Lithium, a commonly used antimanic mood stabilizer, elongates primary cilia (Miyoshi, Kasahara, Miyazaki, & Asanuma, 2009; Muñoz-Estrada et al., 2017) and is known to rescue brain malformations in mouse models of JSRD ciliopathy (Ferland et al., 2004). Interneurons (INs) with Arl13b deficient primary cilia (Arl13bcKO-IN) display reduced synaptic bouton density and size (Guo et al., 2017). Patch-clamp electrophysiological recordings of interneuron-projection neuron circuits in brains with defective interneuronal primary cilia indicate a decrease in mIPSC frequency in projection neurons (Guo et al., 2017). In contrast, no changes in mIPSC amplitude, mEPSC frequency, or mEPSC amplitude were evident. The specific decrease in the inhibitory synaptic input onto projection neurons in cilia mutant mice (Arl13bcKO-IN), leads to excitatory/inhibitory (E/I) synaptic imbalance in the IN-PN circuit. Intriguingly, accumulation and expression of somatostatin receptor 3 (Sstr3), a neurotransmitter receptor, is disrupted in the primary cilia of Arl13b deficient INs. Normal ciliary signaling involves ectocytosis of activated Sstr3 from ciliary tips (Nager et al., 2017). Disrupted ciliary targeting of neurotransmitter receptors such as Sstr3 may underlie cilia driven E/I imbalance in these mice. Consistent with this possibility, induced expression of Sstr3 in cilia mutant interneurons rescues their synaptic and E/I imbalance deficits (Guo et al., 2017). These observations indicate that primary cilia signaling is a non-synaptic signaling mechanism through which environmental signals can shape and refine interneuronal networks and this mechanism might be compromised in ciliopathy patients with neuro-behavioral or epilepsy phenotypes.
6. Glial diversity (astrocytes, oligodendrocytes, microglia), function, and primary cilia in the developing cerebral cortex
Primary cilia functions and signaling in developing glial cells (astrocytes, oligodendendrocytes, and microglia) of the central nervous system remain for the most part uncharacterized. The vast majority of astrocytes in the postnatal cerebral cortex express an individual primary cilium (Kasahara, Miyoshi, Murakami, Miyazaki, & Asanuma, 2014). Primary cilia signaling is necessary for the neurogenic function of astrocyte like precursors in mature hippocampus (Breunig et al., 2008; Han et al., 2009). Altered functional states such as seizure activity shortens astroglial cilia length (Sterpka et al., 2020). In contrast, primary cilia are either absent or severely malformed in aberrantly proliferating, invasive astrocytes in astrocytomas or gliomas (Basten & Giles, 2013; Han et al., 2009; Han & Alvarez-Buylla, 2010; Moser, Fritzler, & Rattner, 2009; Sarkisian & Semple-Rowland, 2019; Seeger-Nukpezah & Golemis, 2012). Primary cilia are present in oligodendrocyte precursor cells and ciliary Shh signaling is critical for the generation of myelinating oligodendrocytes from these precursors (Bhattarai, 2015; Falcon-Urrutia, Carrasco, Lois, Palma, & Roth, 2015; Sanchez & Armstrong, 2018). Although loss of primary cilia in differentiated oligodendrocytes was noticed in vitro (Falcon-Urrutia et al., 2015), the presence of primary cilia in subsets of myelinating mature oligodendrocytes in vivo remains possible (Sanchez & Armstrong, 2018). Immunolabeling with common primary ciliary markers such as ACIII did not detect primary cilia in microglial cells. However, the lack of primary cilia in these cells remains to be fully validated with the use of other established ciliary markers and electron microscopy analysis. Further, comprehensive characterization of the presence and function of primary cilia in different glial cells of the cerebral cortex, spanning the entire spectrum of cortical development, is necessary to fully evaluate the functional impact of glial primary cilia in cerebral cortex.
7. Future directions: Primary cilia’s role in brain development and neurodevelopmental disorders
The primary cilium is structured and positioned to be able to generate a fast and robust response to a wide range of extracellular signals present in the cellular milieu of the developing brain. The diffusion barrier at the base of the cilium allows a cell to maximize the local concentration of a plethora of signaling receptors and their downstream effectors within cilia. The design of the primary cilium enables high levels of second messengers to be sustained more easily within the narrow geometry of the cilium than in other regions of the cell where diffusion through a larger space could lead to a rapid diminishment of the signal. The primary cilium’s proximity to the nucleus and its link to the centrosome-associated microtubule network may promote rapid and efficient intracellular signal transduction. Understanding in real time how signaling emanating from primary cilia is conveyed to far flung domains of a neuron, including, dendritic, spines, axonal growth cones, AIS, and nucleus (or similarly, between primary cilium and distinct cell domains in non-neural cell types of the CNS) will be essential to fully evaluate the cell biological significance of this sensory organelle in nervous system development and disease (Fig. 4). Further, understanding the crosstalk that occurs between intracellular signaling cascades that originate in distinct cellular compartments (e.g., primary cilium, growth cone, dendritic spine, glial end feet etc.) of neural cells during development will help define the primary cilia-specific pathways vital for normal CNS development.
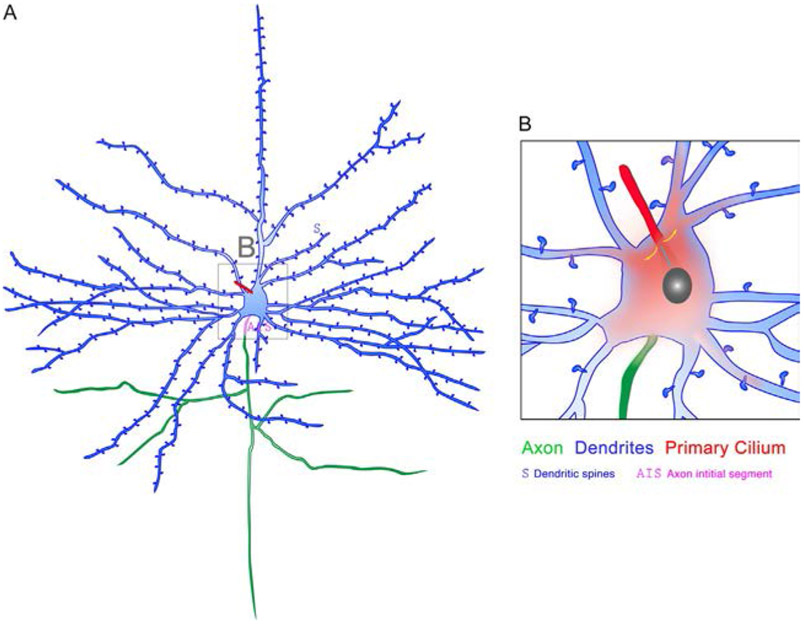
Fig. 4.
Understanding the functional importance of primary cilia in neurons. Interrogating the selective effect of signaling emanating from primary cilia on axons and dendrites and defining how signaling spreads from the primary cilium to distal domains of neurons (red gradient) and to the nucleus (cyan arrow) to influence neuronal activity and behavior are challenges yet to be met. A similar dearth of knowledge exists for the importance of primary cilia signaling during glial cell development and behavior.
Considering that the vast majority of ciliopathies have their origins in primary cilia malfunction during embryonic development (Fig. 3), better modeling of cilia dysfunction and rescue in relevant human models, such as iPSC derived brain organoids, or exploration of CRISPR/Cas9-based targeted editing of ciliary gene defects in utero during development will help open up new therapeutic strategies to address ciliopathies at their origin.
Centrosomes play an essential role in ciliogenesis and cilia maintenance. Often human mutations affecting primarily the centrosomes lead to secondary structural and functional changes in primary cilia, causing a spectrum of outcomes not entirely attributable to primary cilia signaling. Further development of unbiased screening assays for the discovery and interrogation of mutations that exclusively affect the signaling function of neuronal or glial primary cilium and its underpinning cilia-specific machinery will shed new light on the functional importance of primary cilia in the nervous system.
Neuronal or glial primary cilia may produce a “ciliary signaling signature” that elicits cilia-specific impact on neuronal or glial events such as calcium waves, second messenger cascades, cytoskeletal rearrangements, adhesion, and transcriptional regulation necessary for proper neuronal or glial generation, growth, differentiation, and interactions. In this regard, examining if primary cilia in distinct cell types are as molecularly specified as the cell types they subserve, will be highly informative. Future efforts to decipher the precise molecular mediators of communication between neuronal or glial cilia and their cellular environment in the context of brain development will enable us to decisively delineate how primary cilia convey environmental signals to modulate brain formation and organization. Since ciliary proteins, such as Arl13b, can also exert cilia-independent functions in neural cells (Ferent et al., 2019), a vigilant approach that excludes non-ciliary functions of cilia localized proteins should be part of this effort.
The neural circuit malfunctions associated with ciliopathies highlight the importance of investigating the possible participation of primary ciliary signaling in experience-driven refinement of cortical neuronal connectivity during critical periods or during learning and memory (Baraban et al., 2009; Chattopadhyaya et al., 2004; Southwell, Froemke, Alvarez-Buylla, Stryker, & Gandhi, 2010). Intriguingly, IGF1, a ligand for lgf1 R receptors in cilia (Higginbotham et al., 2013; Lehtinen et al., 2011), has been identified as a sensory experience regulated gene necessary to sculpt the synaptic connections of vasoactive intestinal peptide INs (Mardinly et al., 2016). In neurodegenerative disorders such as Huntington disease (HD), pathogenic polyQ expansion of huntingtin (HTT) protein causes centrosomal accumulation of PCM1 and abnormally long primary cilia in striatal cells, contributing to dysregulated circuit homeostasis in this disease (Keryer et al., 2011). It is thus conceivable that ciliary signaling is continuously required in mature neurons for the maintenance and modulation of neuronal network homeostasis. Moreover, the source and the molecular identity of the various environmental cues, including neurotransmitters or neuromodulators, that can initiate or modulate primary cilia signaling in mature neurons, and whether such signaling enables neurons to function cooperatively (Karnani et al., 2016) to modulate the dynamics of a circuit underlying specific functions or behavior are yet to be fully characterized. In this regard, the role of primary cilia signaling via ciliary melanocortin-4 receptors (MC4R) in hypothalamic neurons to regulate food intake and body weight provides an informative example (Siljee et al., 2018). Future efforts aimed at decisively resolving the above outlined issues will further our understanding of the fundamentally vital modulatory role primary cilia play in cerebral cortical formation, function, and disorders.
Table 1.
Ciliopathy and cilia-associated genes in gene expression modules of different cell types in the developing primate brain (related to Fig. 3).
| a | |||
|---|---|---|---|
| Cortical gene expression modules | Total number of genes in module |
% Ciliopathy genes in module |
List of ciliopathy (confirmed and candidate) genes in module |
| BAKKEN2016_WGCNA_E40_M00 | 2484 | 2.254428341 | ADCY6,AIPL1,AKAP9,ARF4,ARL2BP,ARL6,B9D1,BBS4,BBS7,CCNO, CEP104,CEP19,CP110,DCDC2,DNAH5,DNAI1,DNAL4,DNALI1,EFCAB7, HNF1B,HYDIN,INVS,IQCE,KIF24,LCA5,LRAT,LRRC49,LRRC6,MLF1, MST1,NEK1,NIN,NPHP3,NPHP4,OFD1,PACRG,PIH1D3,PKD1, POMGNT1,PTCH1,PTK7,PTPDC1,RAB3IP,RFX3,ROPN1L,RPE65, RSPH1,SPAG1,SPAG6,SSX2IP,TEKT1,TMEM107,TMEM17,TPRA1,TUBE1, TUBGCP6 |
| BAKKEN2016_WGCNA_E40_M01 | 1621 | 1.480567551 | CCDC28B,CEP170,CRB3,CTTN,DCTN1,KIF17,KIF3A,KIF3B,KIF3C, KIF5C,KIFAP3,MARK4,MCHR1,MXI1,NME7,OCRL,PRKACB,RAB11A, SDCCAG8,7-SEP,SNX10,TTBK2,USP9X,WDR60 |
| BAKKEN2016_WGCNA_E40_M02 | 803 | 2.615193026 | CCDC151,CETN2,GALNT11,GNAS,GSK3A,IFT140,IFT52,INPP5E, KATNB1,KIF2A,MAPRE1,ODF2,PDE6D,PEBP1,PPP2R1A,PRKAR2B, RAB11B,RAB11FIP3,TUBB,TUBB2B |
| BAKKEN2016_WGCNA_E40_M03 | 1271 | 1.41620771 | ANO2,ARMC4,CNGB1,CYS1,DNAH6,GLI1,MAK,MORN3,PKHD1, PRKACA,RAB17,RDH12,RSG1,RSPH4A,SUFU,TTC21A,TTC25, ZMYND10 |
| BAKKEN2016_WGCNA_E40_M04 | 596 | 1.006711409 | KIF27,KIF7,MDM1,RSPH3,STK38L,TAPT1 |
| BAKKEN2016_WGCNA_E40_M05 | 1107 | 1.445347787 | C2CD3,CCDC22,CEP250,CEP89,CNTROB,GPR161,HTT,IFT122,MCRS1, MKKS,NUBP1,NUBP2,PAFAH1B1,RABL5,SSNA1,TCTN3,TULP3 |
| BAKKEN2016_WGCNA_E40_M06 | 1863 | 1.986044015 | AURKA,B9D2,BBS2,BBS9,CEP192,CEP72,CEP76,CEP97,CLUAP1,DNAL1, EVC2,FAM161A,FGFR1OP,GNAI2,GNAI3,HEATR2,IFT88,INPP5B, KIAA0586,MKS1,NEK9,ORC1,PLK1,POC1A,RAB23,RAB28,RILPL2, RTTN,SAV1,2- SEP,SMO,SYNE2, TCTN1,TMEM138,TMEM231,TTC17,WRAP73 |
| BAKKEN2016_WGCNA_E40_M07 | 1603 | 4.304429195 | AHI1,ALMS1,ANKS6,ARL13B,ASAP1,ATXN10,BBS10,BBS5,BUB1B, CC2D2A,CCDC14,CENPF,CENPJ,CEP120,CEP128,CEP135,CEP152, CEP290,CEP350,CEP63,CEP78,CETN3,CRB1,CROCC,CSPP1,DZIP1, EFHC1,FAM58A,FOXJ1,GLI3,HSPB11,IFT172,IFT57,IFT74,IFT80,IQCB1, IQUB,KCNJ13,KIF14,LZTFL1,MNS1,NEK2,PARD3,PCM1,PHF17,PIK3R4, PKD2,PLK4,POC1B,RFX2,RPGRIP1L,SASS6,SPA17,SPAG16,SPATA7, SPICE1,STIL,STK3,STK39,TEX9,TMEM216,TMEM67,TRIM32,TRIP11, TTC30A,TTC30B,TTC8,WDR19,WDR35 |
| BAKKEN2016_WGCNA_E40_M08 | 1093 | 0.640439158 | MAL,MERTK,RP2,TBX3,TUBA4A,WNK1,ZIC2 |
| BAKKEN2016_WGCNA_E50_M00 | 3475 | 1.928057554 | AHI1,AIPL1,ANKS6,ANO2,ARF4,ARL2BP,ARL6,ARMC4,BBS10,BBS7, CCNO,CEP290,CEP72,CEP97,CETN2,CNGB1,CP110,CSPP1,CYS1, DCDC2,DNAH5,DNAH6,GPR161,HNF1B,HYDIN,IFT140,INPP5E,INVS, IQCE,KCNJ13,KIF17,KIF27,LCA5,LRAT,LRRC6,MAK,MCHR1,MKKS, MLF1,MST1,NEK1,NPHP3,PKD1,PKHD1,PRKACA,PTPDC1,RAB17, RFX3,RPE65,RSG1,RSPH1,RSPH3,RSPH4A,SPAG6,SPATA7,SSX2IP, SUFU,TCTN3,TEKT1,TMEM67,TRIM32,TTC17,TTC21A,TTC30A, TUBE1,WDR35,ZMYND10 |
| BAKKEN2016_WGCNA_E50_M01 | 1295 | 1.467181467 | CEP19,EFCAB7,GALNT11,KIF2A,LRRC49,MORN3,NIN,NME7,PACRG, PRKACB,PRKAR2B,RAB3IP,RDH12,ROPN1L,SNX10,TAPT1,TMEM17, TTBK2,USP9X |
| BAKKEN2016_WGCNA_E50_M02 | 1304 | 1.303680982 | AKAP9,ATXN10,CCDC28B,CEP104,CEP170,CRB3,CTTN,HSPB11,KIF3A, KIF3B,KIF3C,KIF5C,KIFAP3,MXI1,OCRL,RAB11A,SDCCAG8 |
| BAKKEN2016_WGCNA_E50_M03 | 799 | 1.627033792 | CCDC151,DCTN1,GSK3A,KATNB1,MAPRE1,MARK4,PDE6D,PEBP1, POMGNT1,PPP2R1A,RAB11B,TUBB,TUBB2B |
| BAKKEN2016_WGCNA_E50_M04 | 518 | 1.158301158 | DNAL4,GNAS,IFT52,NUBP2,TUBGCP6,WRAP73 |
| BAKKEN2016_WGCNA_E50_M05 | 1079 | 1.668211307 | B9D2,C2CD3,CCDC22,CEP250,CEP89,CNTROB,FAM58A,GNAI2,HTT, IFT122,INPP5B,KIF7,MCRS1,NUBP1,PAFAH1B1,RAB11FIP3,RABL5, SSNA1,TUBA4A |
| BAKKEN2016_WGCNA_E50_M06 | 2916 | 3.429355281 | ADCY6,ALMS1,ARL13B,ASAP1,AURKA,B9D1,BBS2,BBS4,BBS5,BBS9, BUB1B,CC2D2A,CCDC14,CENPF,CENPJ,CEP120,CEP128,CEP135, CEP152,CEP192,CEP350,CEP63,CEP76,CEP78,CETN3,CLUAP1,CRB1, CROCC,DNAI1,DNAL1,DNALI1,DZIP1,EFHC1,EVC2,FAM161A, FGFR1OP,FOXJ1,GLI1,GLI3,GNAI3,HEATR2,IFT172,IFT57,IFT74,IFT80, IFT88,IQCB1,iQuB,KIAA0586,KIF14,KIF24,LZTFL1,MDM1,MKS1,MNS1, NEK2,NEK9,NPHP4,ODF2,ORC1,PARD3,PCM1,PHF17,PIH1D3,PIK3R4, PKD2,PLK1,PLK4,POC1A,POC1B,PTCH1,PTK7,RAB23,RAB28,RFX2, RILPL2,RP2,RPGRIP1L,RTTN,SASS6,SAV1,2-SEP, SMO,SPA17,SPAG1,SPAG16,SPICE1,STIL,STK3,STK38L,STK39,SYNE2, TCTN1,TEX9,TMEM107,TMEM138,TMEM216,TMEM231,TPRA1, TRIP11,TTC30B,TTC8,TULP3,WDR19,ZIC2 |
| BAKKEN2016_WGCNA_E50_M07 | 578 | 0.346020761 | MAL,TBX3 |
| BAKKEN2016_WGCNA_E50_M08 | 477 | 1.257861635 | MERTK,OFD1,7-SEP,TTC25,WDR60,WNK1 |
| BAKKEN2016_WGCNA_E70_M00 | 3085 | 1.458670989 | ADCY6,ANKS6,ARL2BP,ARMC4,ASAP1,CEP72,CEP78,CEP89,CLUAP1, CP110,CRB3,DCDC2,DNAH5,DNAH6,EFHC1,HNF1B,HYDIN,INPP5E, INVS,KIF27,LRAT,MAL,MCHR1,MKKS,MLF1,MORN3,MST1,NEK1, NPHP3,PKD1,POMGNT1,PTK7,PTPDC1,ROPN1L,RPE65,SAV1,SPA17, SPAG1,TBX3,TRIM32,TTC17,TTC21A,TTC30B,TUBGCP6,WNK1 |
| BAKKEN2016_WGCNA_E70_M01 | 1240 | 1.048387097 | CEP19,GALNT11,IQCE,KIF3A,LRRC49,MAK,NIN,NME7,PRKACB, RAB3IP,RDH12,SNX10,TMEM17 |
| BAKKEN2016_WGCNA_E70_M02 | 1405 | 0.996441281 | CCDC28B,DCTN1,GSK3A,KIF2A,KIF3B,KIF3C,KIF5C,KIFAP3,OCRL, PEBP1,PPP2R1A,PRKAR2B,RAB11A,SDCCAG8 |
| BAKKEN2016_WGCNA_E70_M03 | 1164 | 2.319587629 | AKAP9,ARF4,ARL6,ATXN10,BBS10,BBS7,CEP104,CEP120,CEP170, CEP350,CSPP1,EFCAB7,FAM58A,HSPB11,IFT57,IFT80,MXI1,OFD1,RFX3, SPATA7,SSX2IP,TAPT1,TTBK2,TUBE1,USP9X,WDR35,WDR60 |
| BAKKEN2016_WGCNA_E70_M04 | 1116 | 1.702508961 | AIPL1,ANO2,CCNO,CEP290,CEP97,CNGB1,CYS1,KIF17,LRRC6,MARK4, PKHD1,PRKACA,RAB17,RSG1,RSPH4A,SPAG6,SUFU,TTC25,ZMYND10 |
| BAKKEN2016_WGCNA_E70_M05 | 1315 | 2.053231939 | B9D2,C2CD3,CCDC22,CEP250,CETN2,CNTROB,CTTN,DNAL4,GNAS, GPR161,HTT,IFT122,IFT140,IFT52,KATNB1,KIF7,MAPRE1,MCRS1, NPHP4,NUBP1,NUBP2,PDE6D,RAB11B,RSPH3,TUBB,TUBB2B,WRAP73 |
| BAKKEN2016_WGCNA_E70_M06 | 3116 | 3.498074454 | AHI1,ALMS1,ARL13B,AURKA,B9D1,BBS2,BBS4,BBS5,BBS9,BUB1B, CC2D2A,CCDC14,CCDC151,CENPF,CENPJ,CEP128,CEP135,CEP152, CEP192,CEP63,CEP76,CETN3,CRB1,CROCC,DNAI1,DNAL1,DNALI1, DZIP1,EVC2,FAM161A,FGFR1OP,FOXJ1,GLI1,GLI3,GNAI2,GNAI3, HEATR2,IFT172,IFT74,IFT88,INPP5B,IQCB1,IQUB,KCNJ13,KIAA0586, KIF14,KIF24,LCA5,LZTFL1,MDM1,MERTK,MKS1,MNS1,NEK2,NEK9, ODF2,ORC1,PACRG,PAFAH1B1,PARD3,PCM1,PHF17,PIH1D3,PIK3R4, PKD2,PLK1,PLK4,POC1A,POC1B,PTCH1,RAB11FIP3,RAB23,RAB28, RABL5,RFX2,RILPL2,RP2,RPGRIP1L,RSPH1,RTTN,SASS6,2-SEP,7-SEP, SMO,SPAG16,SPICE1,SSNA1,STIL,STK3, STK38L,STK39,SYNE2,TCTN1,TCTN3,TEKT1,TEX9,TMEM107, TMEM138,TMEM216,TMEM231,TMEM67,TPRA1,TRIP11,TTC30A,TTC8, TUBA4A,TULP3,WDR19,ZIC2 |
| BAKKEN2016_WGCNA_E80_M00 | 3065 | 1.924959217 | ADCY6,AIPL1,ANKS6,ANO2,ARL2BP,ARMC4,ASAP1,BBS9,CCDC151, CCNO,CEP135,CEP290,CEP350,CEP72,CEP78,CEP97,CNGB1,CP110, CRB3,CYS1,DNAH6,DNAL1,EFHC1,GLI1,HNF1B,HYDIN,INVS,KIF27, LRAT,LRRC6,MAL,MCHR1,MORN3,MST1,NIN,NPHP4,ODF2,PKD1, PKHD1,PRKACA,PTPDC1,RAB17,RFX3,RPE65,RSG1,RSPH3,RSPH4A, SAV1,SPAG1,SPAG6,SUFU,TCTN1,TCTN3,TMEM67,TPRA1,TTBK2, TTC21A,TTC25,WDR60 |
| BAKKEN2016_WGCNA_E80_M01 | 936 | 0.854700855 | KIFAP3,NME7,RAB3IP,RDH12,ROPN1L,SNX10,TAPT1,TMEM17 |
| BAKKEN2016_WGCNA_E80_M02 | 434 | 1.152073733 | AHI1,KIF17,MAK,OCRL,POMGNT1 |
| BAKKEN2016_WGCNA_E80_M03 | 691 | 2.604920405 | AKAP9,ARL6,ATXN10,CEP104,CEP170,CEP19,GALNT11,IFT57,KIF2A, KIF3A,LRRC49,MKKS,PRKACB,PRKAR2B,SPA17,SSX2IP,TRIM32, USP9X |
| BAKKEN2016_WGCNA_E80_M04 | 534 | 0.561797753 | KIF3B,KIF3C,KIF5C |
| BAKKEN2016_WGCNA_E80_M05 | 364 | 0.549450549 | CCDC28B,PEBP1 |
| BAKKEN2016_WGCNA_E80_M06 | 834 | 2.757793765 | ARF4,BBS10,BBS5,BBS7,CEP120,CEP76,CETN2,CSPP1,EFCAB7,FAM161A, HSPB11,IFT80,MDM1,MLF1,PHF17,PIK3R4,RAB11A,RAB23,RAB28, RPGRIP1L,SPATA7,TTC30A,TUBE1 |
| BAKKEN2016_WGCNA_E80_M07 | 373 | 0.536193029 | IQCE,TUBGCP6 |
| BAKKEN2016_WGCNA_E80_M08 | 660 | 2.272727273 | C2CD3,CEP250,CTTN,DCTN1,DNAL4,GSK3A,HTT,IFT122,IFT140, INPP5E,KATNB1,MARK4,NUBP2,PPP2R1A,RAB11B |
| BAKKEN2016_WGCNA_E80_M09 | 1096 | 1.551094891 | B9D2,CCDC22,CEP89,CNTROB,FAM58A,GNAS,GPR161,IFT52,MAPRE1, MCRS1,NUBP1,PDE6D,SSNA1,TTC17,TUBB,TUBB2B,WRAP73 |
| BAKKEN2016_WGCNA_E80_M10 | 835 | 2.395209581 | ARL13B,CCDC14,CEP192,CETN3,GNAI3,HEATR2,IFT88,IQCB1,LCA5, NEK1,POC1B,RP2,RTTN,SASS6,7-SEP,STK38L, STK39,TRIP11,TTC8,WDR35 |
| BAKKEN2016_WGCNA_E80_M11 | 371 | 0.808625337 | MERTK,MXI1,RSPH1 |
| BAKKEN2016_WGCNA_E80_M12 | 1952 | 3.790983607 | ALMS1,AURKA,B9D1,BBS2,BBS4,BUB1B,CC2D2A,CENPF,CENPJ, CEP128,CEP152,CEP63,CLUAP1,CRB1,CROCC,DCDC2,DNAH5,DNAI1, DNALI1,DZIP1,EVC2,FGFR1OP,FOXJ1,GLI3,GNAI2,IFT172,IFT74, INPP5B,IQUB,KCNJ13,KIAA0586,KIF14,KIF24,KIF7,LZTFL1,MKS1,MNS1, NEK2,NEK9,NPHP3,ORC1,PACRG,PAFAH1B1,PARD3,PCM1,PIH1D3, PKD2,PLK1,PLK4,POC1A,PTCH1,PTK7,RAB11FIP3,RABL5,RFX2, RILPL2,2-SEP,SMO,SPAG16,SPICE1,STIL,STK3,SYNE2,TEKT1,TEX9, TMEM107,TMEM138,TMEM216,TMEM231,TTC30B,TUBA4A,TULP3, WDR19,ZIC2,ZMYND10 |
| BAKKEN2016_WGCNA_E80_M13 | 296 | 1.351351351 | OFD1,SDCCAG8,TBX3,WNK1 |
| BAKKEN2016_WGCNA_E90_M00 | 3082 | 1.26541207 | ADCY6,AIPL1,ANKS6,ANO2,ARL2BP,CEP192,CEP250,CEP89,CEP97, CNGB1,CP110,CRB3,CYS1,FAM161A,HNF1B,INPP5E,INVS,IQCE,KIF7, LRAT,MCHR1,MST1,NIN,NPHP4,OFD1,PKD1,PKHD1,PRKACA,RAB17, RFX3,RPE65,RSG1,SAV1,SDCCAG8,SUFU,TRIP11,TUBA4A,TUBGCP6 |
| BAKKEN2016_WGCNA_E90_M01 | 1285 | 1.322957198 | AKAP9,CEP170,CEP19,CEP72,GALNT11,KIF2A,KIF3A,KIFAP3,LRRC49, NME7,PRKACB,PRKAR2B,RAB3IP,SNX10,SSX2IP,TAPT1,TMEM17, USP9X |
| BAKKEN2016_WGCNA_E90_M02 | 941 | 0.743889479 | KIF17,KIF3B,KIF3C,KIF5C,MKKS,OCRL,RDH12 |
| BAKKEN2016_WGCNA_E90_M03 | 852 | 1.76056338 | C2CD3,CCDC28B,DCTN1,GPR161,GSK3A,HTT,KATNB1,MARK4, PDE6D,PEBP1,PPP2R1A,RAB11A,TTC17,TUBB,TUBB2B |
| BAKKEN2016_WGCNA_E90_M04 | 923 | 1.19176598 | CNTROB,CTTN,DNAL4,GNAS,HSPB11,IFT52,MCRS1,NUBP1,NUBP2, ODF2,RAB11B |
| BAKKEN2016_WGCNA_E90_M05 | 1043 | 2.396931927 | ARF4,ARL6,ASAP1,ATXN10,BBS10,BBS5,BBS7,CEP104,CEP120,CEP76, CSPP1,EFCAB7,FAM58A,IFT57,IFT80,MDM1,MLF1,PIK3R4,RAB23, RAB28,SPATA7,STK39,TRIM32,TUBE1,WRAP73 |
| BAKKEN2016_WGCNA_E90_M06 | 587 | 1.022146508 | CEP290,CEP350,MXI1,PHF17,TTBK2,WDR60 |
| BAKKEN2016_WGCNA_E90_M07 | 336 | 0.595238095 | MAL,TBX3 |
| BAKKEN2016_WGCNA_E90_M08 | 363 | 0.275482094 | MERTK |
| BAKKEN2016_WGCNA_E90_M09 | 712 | 2.106741573 | B9D2,CCDC22,CETN2,EVC2,GNAI2,IFT140,KIAA0586,MAPRE1, PAFAH1B1,POMGNT1,RAB11FIP3,RABL5,SSNA1,TCTN1,TMEM138 |
| BAKKEN2016_WGCNA_E90_M10 | 1615 | 3.343653251 | ARL13B,AURKA,BBS2,BUB1B,CC2D2A,CCDC14,CENPF,CENPJ,CEP152, CEP63,CETN3,CRB1,DZIP1,GLI1,GLI3,GNAI3,IFT74,IFT88,INPP5B, IQCB1,KIF14,LCA5,MKS1,MNS1,NEK1,NEK2,NEK9,NPHP3,ORC1, PARD3,PKD2,PLK1,PLK4,POC1A,POC1B,PTCH1,PTK7,PTPDC1,RILPL2, RP2,RTTN,SASS6,2-SEP,7-SEP,SMO,SPICE1,STIL,STK3,STK38L,SYNE2, TEX9,TMEM107,TMEM216,TULP3,ZIC2 |
| BAKKEN2016_WGCNA_E90_M11 | 454 | 12.99559471 | AHI1,ALMS1,ARMC4,B9D1,BBS4,BBS9,CCDC151,CCNO,CEP128,CEP135, CEP78,CLUAP1,CROCC,DCDC2,DNAH5,DNAH6,DNAI1,DNAL1, DNALI1,EFHC1,FGFR1OP,FOXJ1,HEATR2,HYDIN,IFT122,IFT172,IQUB, KCNJ13,KIF24,KIF27,LRRC6,LZTFL1,MAK,MORN3,PACRG,PCM1, PIH1D3,RFX2,ROPN1L,RPGRIP1L,RSPH1,RSPH3,RSPH4A,SPA17, SPAG1,SPAG16,SPAG6,TCTN3,TEKT1,TMEM231,TMEM67,TPRA1, TTC21A,TTC25,TTC30A,TTC30B,TTC8,WDR19,WDR35,ZMYND10 |
| BAKKEN2016_WGCNA_E90_M12 | 248 | 0.403225806 | WNK1 |
| BAKKEN2016_WGCNA_E120_M00 | 3095 | 1.357027464 | ADCY6,AHI1,AIPL1,ANO2,AURKA,C2CD3,CCDC14,CCDC22,CENPJ, CEP104,CEP135,CEP192,CEP350,CEP89,CEP97,CNGB1,CRB3,CTTN, CYS1,FAM161A,FAM58A,GPR161,IFT57,IFT80,KIF17,LRAT,MAPRE1, MCHR1,NIN,ODF2,PAFAH1B1,PKD1,POMGNT1,PRKACA,RAB17, RAB23,RPE65,SASS6,SSNA1,SUFU,SYNE2,TMEM17,TUBGCP6 |
| BAKKEN2016_WGCNA_E120_M01 | 1673 | 1.434548715 | ARF4,ASAP1,CEP19,CEP72,CEP76,GALNT11,INVS,KIF2A,KIF3A,KIFAP3, LRRC49,MKKS,NME7,PKHD1,PRKACB,PRKAR2B,RAB3IP,RDH12, SNX10,SSX2IP,STK39,TAPT1,TRIM32,USP9X |
| BAKKEN2016_WGCNA_E120_M02 | 1182 | 0.846023689 | CCDC28B,IQCE,KIF3B,KIF3C,KIF5C,OCRL,PDE6D,PEBP1,TUBB, TUBB2B |
| BAKKEN2016_WGCNA_E120_M03 | 921 | 1.520086862 | CEP250,CNTROB,DCTN1,GNAS,GSK3A,HTT,INPP5E,MARK4,MCRS1, NUBP1,NUBP2,PPP2R1A,RAB11B,WRAP73 |
| BAKKEN2016_WGCNA_E120_M04 | 1167 | 1.199657241 | AKAP9,ARL6,ATXN10,BBS7,CEP120,CEP170,EFCAB7,PHF17,PIK3R4, RAB11A,RAB28,TTBK2,TTC17,TUBE1 |
| BAKKEN2016_WGCNA_E120_M05 | 594 | 1.346801347 | DZIP1,KIF14,MAL,ORC1,PLK1,7-SEP,TBX3,TUBA4A |
| BAKKEN2016_WGCNA_E120_M06 | 2158 | 1.343836886 | BUB1B,CENPF,CEP63,GLI1,GLI3,GNAI2,GNAI3,KIF7,MERTK,MXI1, NEK9,NPHP3,PACRG,PARD3,PKD2,POC1A,POC1B,PTCH1,PTK7, RAB11FIP3,RILPL2,RP2,2-SEP,SMO, STK3,STK38L,TMEM216,TPRA1,ZIC2 |
| BAKKEN2016_WGCNA_E120_M07 | 1253 | 8.858739026 | ALMS1,ANKS6,ARL13B,ARL2BP,ARMC4,B9D1,B9D2,BBS10,BBS2,BBS4, BBS5,BBS9,CC2D2A,CCDC151,CCNO,CEP128,CEP152,CEP290,CEP78, CETN2,CETN3,CLUAP1,CP110,CRB1,CROCC,CSPP1,DCDC2,DNAH5, DNAH6,DNAI1,DNAL1,DNAL4,DNALI1,EFHC1,EVC2,FGFR1OP,FOXJ1, HEATR2,HNF1B,HSPB11,HYDIN,IFT122,IFT140,IFT172,IFT52,IFT74, IFT88,INPP5B,IQCB1,IQUB,KATNB1,KCNJ13,KIAA0586,KIF24,KIF27, LCA5,LRRC6,LZTFL1,MAK,MDM1,MKS1,MLF1,MNS1,MORN3,MST1, NEK1,NEK2,NPHP4,OFD1,PCM1,PIH1D3,PLK4,PTPDC1,RABL5,RFX2, RFX3,ROPN1L,RPGRIP1L,RSG1,RSPH1,RSPH3,RSPH4A,RTTN,SAV1, SDCCAG8,SPA17,SPAG1,SPAG16,SPAG6,SPATA7,SPICE1,STIL,TCTN1, TCTN3,TEKT1,TEX9,TMEM107,TMEM138,TMEM231,TMEM67,TRIP11, TTC21A,TTC25,TTC30A,TTC30B,TTC8,TULP3,WDR19,WDR35, WDR60,ZMYND10 |
| BAKKEN2016_WGCNA_E120_M08 | 398 | 0.251256281 | WNK1 |
| BAKKEN2016_WGCNA_0M_M00 | 2960 | 1.959459459 | ADCY6,AIPL1,ALMS1,ANKS6,ANO2,ASAP1,B9D1,BBS4,CCDC151,CCNO, CENPF,CENPJ,CEP128,CEP135,CEP250,CEP350,CEP97,CNGB1,CP110, CTTN,DNAH6,FGFR1OP,HSPB11,IFT172,INPP5E,IQUB,KCNJ13,KIF24, LRAT,LRRC6,MAK,MCHR1,MNS1,MORN3,ORC1,PAFAH1B1,PIH1D3, PKD1,PLK4,POC1A,PRKACA,RAB11A,RAB11B,RAB3IP,RILPL2,RSPH4A, SAV1,SDCCAG8,SPAG1,SPAG6,SUFU,TMEM107,TMEM138,TMEM67, TTC17,USP9X,WDR60,ZMYND10 |
| BAKKEN2016_WGCNA_0M_M01 | 3173 | 2.111566341 | AHI1,ATXN10,AURKA,B9D2,C2CD3,CCDC22,CCDC28B,CEP19,CEP72, CEP76,DCTN1,DNAH5,DNAL4,FAM58A,GALNT11,GPR161,GSK3A,HTT, IFT122,IFT57,INVS,KATNB1,KIF17,KIF2A,KIF3A,KIF3B,KIF3C,KIF5C, KIFAP3,LRRC49,MARK4,MCRS1,MKKS,NEK2,NME7,NPHP4,NUBP1, NUBP2,OCRL,PDE6D,PEBP1,PHF17,PKHD1,PPP2R1A,PRKAR2B,RAB17, RABL5,RDH12,ROPN1L,RSPH1,RSPH3,SNX10,SPA17,SSX2IP,STIL, SYNE2,TCTN1,TCTN3,TEKT1,TMEM17,TRIM32,TTBK2,TUBA4A, TUBB,TUBB2B,TUBGCP6,WRAP73 |
| BAKKEN2016_WGCNA_0M_M02 | 714 | 1.120448179 | BBS9,CC2D2A,CYS1,DCDC2,DNAL1,GNAS,HNF1B,IQCE |
| BAKKEN2016_WGCNA_0M_M03 | 1079 | 1.853568119 | AKAP9,ARF4,ARL6,BBS5,CEP192,CEP78,CETN2,CLUAP1,EFCAB7, EFHC1,FAM161A,IFT52,IFT80,LZTFL1,MLF1,PIK3R4,RAB28,RFX3, RPGRIP1L,SPATA7 |
| BAKKEN2016_WGCNA_0M_M04 | 783 | 2.426564496 | ARL2BP,BBS10,BBS7,BUB1B,CEP120,CEP170,CEP63,CSPP1,LCA5,MDM1, NIN,PRKACB,7-SEP,SPICE1,TAPT1,TTC30A,TTC8,TUBE1,WDR19 |
| BAKKEN2016_WGCNA_0M_M05 | 1853 | 1.726929304 | BBS2,CNTROB,CRB3,CROCC,DNAI1,EVC2,GLI3,GNAI2,GNAI3,IFT140, KIAA0586,MST1,NEK1,NEK9,NPHP3,PARD3,PKD2,POC1B,PTCH1,PTK7, RAB11FIP3,RFX2,RPE65,2-SEP,SMO,SPAG16,STK3,STK38L,TMEM216, TTC21A,TULP3,ZIC2 |
| BAKKEN2016_WGCNA_0M_M06 | 526 | 1.330798479 | DZIP1,GLI1,KIF7,MERTK,MXI1,ODF2,TBX3 |
| BAKKEN2016_WGCNA_0M_M07 | 1353 | 3.17812269 | ARL13B,ARMC4,CCDC14,CEP104,CEP152,CEP290,CEP89,CETN3,CRB1, DNALI1,FOXJ1,HEATR2,HYDIN,IFT74,IFT88,INPP5B,IQCB1,KIF14, KIF27,MAL,MAPRE1,MKS1,OFD1,PACRG,PCM1,PLK1,POMGNT1, PTPDC1,RAB23,RP2,RSG1,RTTN,SASS6,SSNA1,STK39,TEX9,TMEM231, TPRA1,TRIP11,TTC25,TTC30B,WDR35,WNK1 |
| BAKKEN2016_WGCNA_3M_M00 | 2279 | 2.237823607 | ADCY6,AIPL1,ALMS1,ANKS6,ANO2,ARMC4,B9D1,BBS4,CENPJ,CEP128, CEP135,CEP350,CEP78,CEP97,CLUAP1,CNGB1,CROCC,CSPP1,EFHC1, FOXJ1,GLI1,HNF1B,HYDIN,IFT74,INPP5B,KCNJ13,KIF14,KIF24,KIF27, KIF7,LRAT,LRRC6,MAK,MORN3,MST1,NIN,ORC1,PIH1D3,PKHD1, PLK1,PRKACA,PTK7,RAB11A,RAB11B,RAB3IP,SAV1,SDCCAG8,SUFU, TEX9,TTC21A,WDR19 |
| BAKKEN2016_WGCNA_3M_M01 | 2318 | 1.98446937 | AHI1,ARL6,ASAP1,ATXN10,C2CD3,CCNO,CYS1,DNAH6,DNAL1, DNAL4,GNAS,GSK3A,HTT,IFT57,INPP5E,INVS,IQCE,KATNB1,KIF3A, KIF3B,KIF3C,KIF5C,KIFAP3,LRRC49,MARK4,NME7,NPHP4,OCRL, PEBP1,POC1A,RAB17,RSPH1,RSPH3,SNX10,SSX2IP,STIL,TCTN3,TEKT1, TMEM17,TMEM67,TRIM32,TTBK2,TTC17,TUBA4A,TUBB,USP9X |
| BAKKEN2016_WGCNA_3M_M02 | 1448 | 1.726519337 | B9D2,BBS9,CCDC151,CCDC22,CEP76,CETN2,DCTN1,FAM58A, FGFR1OP,GALNT11,IFT172,IFT80,LZTFL1,MKKS,NUBP1,NUBP2,PDE6D, ROPN1L,SPA17,SPAG1,SPAG6,SPATA7,TUBGCP6,WDR60,WRAP73 |
| BAKKEN2016_WGCNA_3M_M03 | 584 | 2.739726027 | AURKA,BBS7,BUB1B,CCDC28B,CENPF,CTTN,GPR161,IFT122,MCRS1, PHF17,PKD1,PPP2R1A,RDH12,SYNE2,TTC25 |
| BAKKEN2016_WGCNA_3M_M04 | 678 | 2.212389381 | CC2D2A,CEP72,DCDC2,DNAH5,HSPB11,KIF17,MCHR1,MLF1,NEK2, PRKAR2B,PTCH1,RFX3,RSPH4A,TUBB2B,ZMYND10 |
| BAKKEN2016_WGCNA_3M_M05 | 1227 | 1.711491443 | AKAP9,ARF4,ARL2BP,BBS10,BBS5,CEP120,CEP19,CEP192,EFCAB7, FAM161A,IFT52,KIF2A,MDM1,PAFAH1B1,PIK3R4,PLK4,RAB28,RABL5, RPGRIP1L,TTC8,WDR35 |
| BAKKEN2016_WGCNA_3M_M06 | 1489 | 1.813297515 | BBS2,CEP250,CRB1,DNAI1,DNALI1,EVC2,GLI3,GNAI2,GNAI3,KIAA0586, MXI1,NEK1,NEK9,NPHP3,PACRG,PARD3,PCM1,RAB11FIP3,RFX2, RSG1,2-SEP,SMO,STK38L,TMEM107,TMEM216,TULP3,ZIC2 |
| BAKKEN2016_WGCNA_3M_M07 | 604 | 1.655629139 | CNTROB,DZIP1,IFT140,IQUB,MERTK,MKS1,ODF2,RILPL2,TBX3, TPRA1 |
| BAKKEN2016_WGCNA_3M_M08 | 1814 | 2.425578831 | ARL13B,CCDC14,CEP104,CEP152,CEP170,CEP290,CEP63,CEP89,CETN3, CP110,CRB3,HEATR2,IFT88,IQCB1,LCA5,MAL,MAPRE1,MNS1,OFD1, PKD2,POC1B,POMGNT1,PRKACB,PTPDC1,RAB23,RP2,RPE65,RTTN, SASS6,7-SEP,SPAG16,SPICE1,SSNA1,STK3,STK39,TAPT1,TCTN1, TMEM138,TMEM231,TRIP11,TTC30A,TTC30B,TUBE1,WNK1 |
| BAKKEN2016_WGCNA_12M_M00 | 2310 | 1.904761905 | AIPL1,ANO2,ARMC4,AURKA,BBS4,BBS9,BUB1B,CENPJ,CEP128,CEP192, CEP350,CEP72,CEP78,CEP97,CLUAP1,CNGB1,DNAH6,GLI1,HNF1B, IFT140,IFT172,KIF14,KIF24,LRRC6,MAK,MKS1,MORN3,NEK1,NIN, ORC1,PIH1D3,PKD1,PKHD1,PLK1,POC1A,PRKACA,RAB11B,RAB3IP, SAV1,SDCCAG8,SSNA1,TCTN1,TMEM138,ZMYND10 |
| BAKKEN2016_WGCNA_12M_M01 | 2025 | 1.827160494 | AHI1,ANKS6,ASAP1,B9D2,C2CD3,CCDC151,CCDC22,CCNO,CYS1, DCTN1,DNAL4,FAM58A,GALNT11,GNAS,KATNB1,KIF3B,KIF3C,KIF5C, KIF7,KIFAP3,MCRS1,NPHP4,OCRL,PEBP1,PPP2R1A,RAB17,RSPH1, SNX10,SSX2IP,STIL,TCTN3,TRIM32,TTBK2,TTC17,TUBA4A,TUBGCP6, WRAP73 |
| BAKKEN2016_WGCNA_12M_M02 | 588 | 2.040816327 | CETN2,DNAH5,DNAL1,FGFR1OP,KIF17,MCHR1,PDE6D,PLK4,RABL5, ROPN1L,TEKT1,TUBB |
| BAKKEN2016_WGCNA_12M_M03 | 918 | 2.178649237 | AKAP9,ARL6,CEP19,CEP76,EFCAB7,IFT57,INVS,KIF3A,LRRC49,LZTFL1, MKKS,PRKAR2B,RPGRIP1L,RSPH3,SPA17,SPAG6,SPATA7,TMEM17, USP9X,WDR60 |
| BAKKEN2016_WGCNA_12M_M04 | 615 | 1.300813008 | ADCY6,CCDC28B,DZIP1,GSK3A,HTT,INPP5E,IQCE,MARK4 |
| BAKKEN2016_WGCNA_12M_M05 | 671 | 2.682563338 | BBS7,CENPF,CTTN,GPR161,IFT122,INPP5B,MDM1,NME7,NUBP1, NUBP2,PHF17,RDH12,RSPH4A,SPAG1,SYNE2,TMEM67,TTC21A,TTC25 |
| BAKKEN2016_WGCNA_12M_M06 | 289 | 0.692041522 | MERTK,TBX3 |
| BAKKEN2016_WGCNA_12M_M07 | 431 | 1.856148492 | ATXN10,B9D1,CC2D2A,DCDC2,HSPB11,KCNJ13,NEK2,PTCH1 |
| BAKKEN2016_WGCNA_12M_M08 | 1773 | 1.861252115 | ALMS1,BBS2,CNTROB,CRB1,CROCC,DNAI1,DNALI1,EVC2,FOXJ1, GLI3,GNAI2,HYDIN,KIAA0586,NPHP3,ODF2,PACRG,PARD3, RAB11FIP3,RFX2,RILPL2,RSG1,2-SEP,SMO,SUFU,TMEM107,TMEM216, TMEM231,TPRA1,TUBB2B,TULP3,ZIC2 |
| BAKKEN2016_WGCNA_12M_M09 | 1157 | 2.679343129 | ARF4,ARL2BP,BBS10,BBS5,CEP120,CSPP1,EFHC1,FAM161A,IFT52,IFT74, IFT80,IQUB,KIF2A,LCA5,LRAT,MLF1,MNS1,PAFAH1B1,PIK3R4,RAB11A, RAB28,RFX3,RP2,SPICE1,TAPT1,TEX9,TTC30A,TTC8,TUBE1,WDR19, WDR35 |
| BAKKEN2016_WGCNA_12M_M10 | 1664 | 2.584134615 | ARL13B,CCDC14,CEP104,CEP135,CEP152,CEP170,CEP250,CEP290, CEP63,CEP89,CETN3,CP110,CRB3,GNAI3,HEATR2,IFT88,IQCB1,KIF27, MAL,MAPRE1,MST1,MXI1,NEK9,OFD1,PCM1,PKD2,POC1B,POMGNT1, PRKACB,PTK7,PTPDC1,RAB23,RPE65,RTTN,SASS6,7-SEP,SPAG16, STK3,STK38L,STK39,TRIP11,TTC30B,WNK1 |
| BAKKEN2016_WGCNA_48M_M00 | 2109 | 2.038880986 | ANO2,ARL2BP,BBS4,BBS7,BBS9,CENPJ,CEP135,CEP192,CEP97,CNGB1, CSPP1,EFCAB7,EFHC1,FGFR1OP,HNF1B,IQUB,KIAA0586,KIF14,KIF24, KIF27,LRAT,MNS1,NEK1,NME7,ORC1,PDE6D,PHF17,PIH1D3,PKHD1, PLK1,PLK4,RAB11A,RAB11B,RAB3IP,RPGRIP1L,SAV1,SDCCAG8,SPAG1, SUFU,TEX9,TMEM138,TMEM67,WDR19 |
| BAKKEN2016_WGCNA_48M_M01 | 2217 | 2.390617952 | AHI1,AIPL1,AKAP9,ARL6,ASAP1,C2CD3,CCNO,CEP19,CEP76,CYS1, DNAL1,DNAL4,FAM58A,GALNT11,GNAS,GSK3A,HTT,IFT57,INPP5E, INVS,IQCE,KATNB1,KIF3A,KIF3B,KIF3C,KIF5C,KIF7,KIFAP3,LRRC49, LZTFL1,MARK4,MORN3,OCRL,PEBP1,PRKACA,RAB17,ROPN1L, RSPH1,RSPH3,SNX10,SPA17,SPAG6,SPATA7,SSX2IP,STIL,TCTN3, TMEM17,TRIM32,TTBK2,TTC17,TTC25,TUBA4A,USP9X |
| BAKKEN2016_WGCNA_48M_M02 | 570 | 2.280701754 | CEP78,CETN2,CLUAP1,DCDC2,DNAH5,DZIP1,IFT52,KIF17,LRRC6, MCHR1,PRKAR2B,RABL5,TEKT1 |
| BAKKEN2016_WGCNA_48M_M03 | 1382 | 1.374819103 | ADCY6,ANKS6,B9D2,CCDC151,CCDC22,CCDC28B,DCTN1,DNAH6, IFT140,IFT172,INPP5B,MCRS1,NPHP4,PKD1,POC1A,TTC21A,TUBB, TUBGCP6,WRAP73 |
| BAKKEN2016_WGCNA_48M_M04 | 744 | 1.747311828 | AURKA,CENPF,CTTN,GPR161,IFT122,MDM1,MKKS,NUBP1, NUBP2,PPP2R1A,RDH12,SYNE2,WDR60 |
| BAKKEN2016_WGCNA_48M_M05 | 433 | 1.385681293 | B9D1,BUB1B,CC2D2A,GLI1,NEK2,ZMYND10 |
| BAKKEN2016_WGCNA_48M_M06 | 426 | 2.112676056 | ARMC4,BBS2,CROCC,HSPB11,KCNJ13,MERTK,ODF2,PTCH1,TBX3 |
| BAKKEN2016_WGCNA_48M_M07 | 1630 | 2.024539877 | ALMS1,CEP250,CRB1,DNAI1,DNALI1,EVC2,FOXJ1,GLI3,GNAI2,GNAI3, MAPRE1,MXI1,NIN,NPHP3,PACRG,PARD3,PTK7,RAB11FIP3,RAB23, RFX2,RILPL2,RSG1,2-SEP,SMO,TCTN1,TMEM107,TMEM216,TMEM231, TRIP11,TTC30B,TUBB2B,TULP3,ZIC2 |
| BAKKEN2016_WGCNA_48M_M08 | 719 | 1.529902643 | ARF4,ATXN10,BBS5,IFT80,MLF1,PIK3R4,RAB28,RFX3,RSPH4A,TUBE1, WDR35 |
| BAKKEN2016_WGCNA_48M_M09 | 812 | 3.448275862 | ARL13B,BBS10,CCDC14,CEP120,CEP170,CEP290,CEP350,CEP63,CETN3, CP110,FAM161A,HYDIN,IFT74,IFT88,KIF2A,LCA5,MAK,PCM1,POC1B, RP2,RPE65,SASS6,7-SEP,SPICE1,STK38L,TAPT1,TTC30A,TTC8 |
| BAKKEN2016_WGCNA_48M_M10 | 1399 | 1.858470336 | CEP104,CEP128,CEP152,CEP72,CEP89,CNTROB,CRB3,HEATR2,IQCB1, MAL,MKS1,MST1,NEK9,OFD1,PAFAH1B1,PKD2,POMGNT1,PRKACB, PTPDC1,RTTN,SPAG16,SSNA1,STK3,STK39,TPRA1,WNK1 |
|
Modules with significant enrichment of ciliopathy genes are highlighted (adj P values are below) BAKKEN2016_WGCNA_E40_M07 4.852e-7; BAKKEN2016_WGCNA_E50_M06 7.030e-9; BAKKEN2016_WGCNA_E70_M06 3.500e-7; BAKKEN2016_WGCNA_E80_M12 2.399e-6; BAKKEN2016_WGCNA_E90_M11 3.492e-29; BAKKEN2016_WGCNA_E120_M07 1.004e-41 | |||
| b | |||
| Ciliopathy and cilia associated genes | |||
| ADCY3, ADCY6, AHI1, AIPL1, AK7, AKAP9, ALMS1, ANKRD26, ANKS3, ANKS6, ANO2, ARF4, ARL13B, ARL2, ARL2BP, ARL3, ARL6, ARMC4, ASAP1, ATXN10, AURKA, B9D1, B9D2, BBIP1, BBS1, BBS10, BBS12, BBS2, BBS4, BBS5, BBS7, BBS9, BUB1B, C10ORF107, C11ORF49, C21ORF2, C21ORF59, C2CD3, C2ORF71, C5orf42, C8orf37, CATIP, CBY1, CC2D2A, CCDC103, CCDC112, CCDC114, CCDC13, CCDC14, CCDC151, CCDC18, CCDC22, CCDC28B, CCDC37, CCDC39, CCDC40, CCDC63, CCDC65, CCDC66, CCDC88A, CCNO, CDK5RAP2, CDKL5, CENPF, CENPJ, CEP104, CEP120, CEP128, CEP131, CEP135, CEP152, CEP162, CEP164, CEP170, CEP19, CEP192, CEP250, CEP290, CEP350, CEP41, CEP63, CEP72, CEP76, CEP78, CEP83, CEP89, CEP97, CETN1, CETN2, CETN3, CFAP126, CFAP161, CFAP20, CFAP36, CFAP410, CFAP52, CFAP53, CFAP54, CFAP57, CFAP58, CFAP61, CLUAP1, CNGA1, CNGA2, CNGA4, CNGB1, CNTROB, CP110, CPLANE2, CRB1, CRB3, CROCC, CRX, CSNK1D, CSPP1, CTTN, CYS1, DCDC2, DCTN1, DDX59, DISC1, DNAAF1, DNAAF2, DNAAF3, DNAAF4, DNAAF5, DNAH1, DNAH10, DNAH11, DNAH2, DNAH5, DNAH6, DNAH9, DNAI1, DNAI2, DNAJB13, DNAL1, DNAL4, DNALI1, DPCD, DRC1, DRC3, DVL1, DYNC2H1, DYNC2LI1, DYNLT1, DYX1C1, DZIP1, EFCAB7, EFHC1, EFHC2, EVC, EVC2, EXOC4, FAM161A, FAM58A, FBF1, FGFR1OP, FIGNL1, FLCN, FOPNL, FOXJ1, FTO, FUZ, GALNT11, GAS8, GDI2, GLI1, GLI2, GLI3, GLIS2, GNAI2, GNAI3, GNAS, GPBAR1, GPR161, GSK3A, GUCY2D, HEATR2, HNF1B, HSPB11, HTR6, HTT, HYDIN, HYLS1, ICK, IFT122, IFT139, IFT140, IFT172, IFT20, IFT27, IFT43, IFT46, IFT52, IFT57, IFT74, IFT80, IFT81, IFT88, IMPDH1, INPP5B, INPP5E, INTU, INVS, IQCB1, IQCE, IQCG, IQUB, KATNB1, KCNJ13, KIAA0556, KIAA0586, KIAA0753, KIAA1217, KIF11, KIF14, KIF17, KIF19, KIF24, KIF27, KIF2A, KIF3A, KIF3B, KIF3C, KIF5C, KIF7, KIFAP3, KIZ, LCA5, LRAT, LRRC49, LRRC6, LUZP1, LZTFL1, MAK, MAL, MAPRE1, MARK4, MCHR1, MCIDAS, MCRS1, MDM1, MERTK, MKKS, MKS1, MLF1, MNS1, MOK, MORN3, MST1, MXI1, MYB, NEK1, NEK2, NEK4, NEK8, NEK9, NIN, NINL, NME5, NME7, NME8, NMNAT1, NOTO, NPHP1, NPHP2, NPHP3, NPHP4, NPHP5, NPHP7, NPHP9, NUBP1, NUBP2, OCRL, ODF2, OFD1, ORC1, OSR1, PACRG, PAFAH1B1, PARD3, PCARE, PCM1, PCNT, PDE6D, PEBP1, PHF17, PIBF1, PIFO, PIH1D3, PIK3R4, PKD1, PKD2, PKD1L1, PKD2L1, PKHD1, PLK1, PLK4, POC1A, POC1B, POC5, POMGNT1, PPP1R32, PPP2CA, PPP2R1A, PRKACA, PRKACB, PRKAR1A, PRKAR2A, PRKAR2B, PROSER3, PTCH1, PTK7, PTPDC1, RAB11A, RAB11B, RAB11FIP3, RAB17, RAB23, RAB28, RAB29, RAB3IP, RAB8A, RABL5, RD3, RDH12, RFX2, RFX3, RILPL1, RILPL2, ROPN1, ROPN1L, RP1, RP1L1, RP2, RPE65, RPGR, RPGRIP1, RPGRIP1L, RSG1, RSPH1, RSPH14, RSPH3, RSPH4A, RSPH9, RTTN, SASS6, SAV1, SAXO1, SCLT1, SDCCAG8, SEPT2, SEPT7, SLC47A2, SLC4A1, SMO, SNAP25, SNX10, SPA17, SPAG1, SPAG16, SPAG17, SPAG6, SPATA7, SPEF1, SPEF2, SPICE1, SSNA1, SSTR3, SSX2IP, STIL, STK3, STK36, STK38L, STK39, STOML3, STX3, SUFU, SYNE2, TAPT1, TBC1D31, TBC1D32, TBC1D7, TBX3, TCTEX1D2, TCTN1, TCTN2, TCTN3, TEKT1, TEKT2, TEKT3, TEKT4, TEKT5, TEX9, TMEM107, TMEM138, TMEM17, TMEM216, TMEM231, TMEM237, TMEM67, TMEM80, TOPORS, TPRA1, TRAF3IP1, TRIM32, TRIP11, TTBK2, TTC17, TTC21A, TTC21B, TTC25, TTC26, TTC29, TTC30A, TTC30B, TTC8, TTLL3, TTLL5, TTLL6, TTLL9, TUB, TUBA1A, TUBA1C, TUBA4A, TUBB, TUBB2A, TUBB2B, TUBB3, TUBE1, TUBGCP6, TULP1, TULP3, ULK4, UNC119, USP33, USP9X, VHL, WDPCP, WDR19, WDR34, WDR35, WDR60, WDR62, WDR78, WNK1, WNK4, WRAP73, XPNPEP3, ZIC2, ZMYND10, ZNF423. | |||
Names of the modules from different embryonic and postnatal ages, total number of genes in each module, % of cilia-related genes in the module, and the ciliary genes in each of the modules are listed in table (a). For a listing of all the genes in different modules, see (Bakken et al., 2016). All the cilia related genes used in this analysis are listed in table (b).
Table (b) compiled from Guo, J., Higginbotham, H., Li, J., Nichols, J., Hirt, J., Ghukasyan, V., & Anton, E. S. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nature Communications, 6(7857). doi:10.1038/ncomms8857; Reiter, J. F., & Leroux, M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nature Reviews. Molecular Cell Biology, 18(9), 533–547. doi:10.1038/nrm.2017.60.
Acknowledgments
This research was supported by NIH grants MH060929 and NS to EA and the confocal imaging core of an NINDS institutional center core grant (5P30NS045892).
Footnotes
Declaration of competing financial interests
The authors declare no competing financial interests.
References
- Akizu N, Cantagrel V, Schroth J, Cai N, Vaux K, McCloskey D, et al. (2013). AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell, 154(3), 505–517. 10.1016/j.cell.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizu N, Silhavy JL, Rosti RO, Scott E, Fenstermaker AG, Schroth J, et al. (2014). Mutations in CSPP1 lead to classical Joubert syndrome. American Journal of Human Genetics, 94(1), 80–86. 10.1016/j.ajhg.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, et al. (2008). Association of common variants in the Joubert syndrome gene (AHI1) with autism. Human Molecular Genetics, 17(24), 3887–3896. 10.1093/hmg/ddn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, & Sarkisian MR (2017). Development and distribution of neuronal cilia in mouse neocortex. The Journal of Comparative Neurology, 520, 848–873. 10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armato U, Chakravarthy B, Chiarini A, Prà ID, & Whitfield JF (2011). A paradigm-changing surprise from dentate gyrus granule cells—cilium-localized p75NTR may drive their progenitor cell proliferation. Journal of Alzheimer’s Disease & Parkinsonism, 1(2), 1–3. 10.4172/2161-0460.1000e104. e104. [DOI] [Google Scholar]
- Averaimo S, & Nicol X (2014). Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits. Frontiers in Cellular Neuroscience, 8, 376. 10.3389/fncel.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, et al. (2016). A comprehensive transcriptional map of primate brain development. Nature, 535(7612), 367–375. 10.1038/nature18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G (2018). The development of neuronal polarity: A retrospective view. International Journal of Neuroscience, 38, 1867–1873. United States: 2018 the authors 0270-6474/18/381867-07$15.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG,Estrada RC,Jones DL, Sebe JY, Alfaro-Cervello C, et al. (2009). Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America, 106, 15472–15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, & Polleux F (2009). Establishment of axon-dendrite polarity in developing neurons. Annual Review of Neuroscience, 32, 347–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten SG, & Giles RH (2013). Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia, 2(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin J-P, Viou L, Launay P-S, Luccardini C, Espeso Gil S, Kiyasova V, et al. (2012). Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron, 76(6), 1108–1122. 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Bhattarai SR (2015). Characteristics of primary cilia and centrosomes in neuronal and glial lineages of the adult brain. Denton, Texas: University of North Texas. [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, et al. (2009). Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nature Genetics, 41(9), 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilguvar K, Ozturk AK, Louvi A, Kwan KY, Choi M, Tatli B, et al. (2010). Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature, 467(7312), 207–210. 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, & Mykytyn K (2007). Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. The Journal of Comparative Neurology, 505, 562–571. 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA (2009). From central to rudimentary to primary: The history of an underappreciated organelle whose time has come. The primary cilium. Methods in Cell Biology, 94, 3–52. 10.1016/S0091-679X(08)94001-2. [DOI] [PubMed] [Google Scholar]
- Bober MB, & Jackson AP (2017). Microcephalic osteodysplastic primordial dwarfism, type II: a clinical review. Current Osteoporosis Reports, 15(2), 61–69. 10.1007/s11914-017-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek NJ, Hopp K, Benoit L, Kraft D, Cousin MA, Blackburn PR, et al. (2018). Characterization of three ciliopathy pedigrees expands the phenotype associated with biallelic C2CD3 variants. European Journal of Human Genetics, 26, 1797–1809. 10.18926/AMO/53020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, et al. (2002). ASPM is a major determinant of cerebral cortical size. Nature Genetics, 32, 316–320. United States. [DOI] [PubMed] [Google Scholar]
- Brancati F, Dallapiccola B, & Valente E (2010). Joubert syndrome and related disorders. Orphanet Journal of Rare Diseases, 5(1), 20. 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, et al. (2008). Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13127–13132. 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broix L, Asselin L, Silva CG, Ivanova EL, Tilly P, Gilet JG, et al. (2018). Ciliogenesis and cell cycle alterations contribute to KIF2A-related malformations of cortical development. Human Molecular Genetics, 27, 224–238. England: The Author 2017. Published by Oxford University Press; For Permissions, please journals.permissions@oup.com. [DOI] [PubMed] [Google Scholar]
- Canning P, Park K, Goncalves J, Li C, Howard CJ, Sharpe TD, et al. (2018). CDKL family kinases have evolved distinct structural features and ciliary function. Cell Reports, 22(4), 885–894. 10.1016/j.celrep.2017.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, & Anderson KV (2007). The graded response to sonic hedgehog depends on cilia architecture. Developmental Cell, 12, 767–778. United States. [DOI] [PubMed] [Google Scholar]
- Cavallin M, Bijlsma EK, El Morjani A, Moutton S, Peeters EA, Maillard C, et al. (2017). Recurrent KIF2A mutations are responsible for classic lissencephaly. Neurogenetics, 18, 73–79. United States. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, et al. (2004). Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. Journal of Neuroscience, 24, 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A, & Richards LJ (2010). Wiring the brain: The biology of neuronal guidance. Cold Spring Harbor Perspectives in Biology, 2(6), a001917. 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, et al. (2008). Tuberous sclerosis complex proteins control axon formation. Genes & Development, 22(18), 2485–2495. 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-H, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, et al. (2011). Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proceedings of the National Academy of Sciences of the United States of America, 108(26), 10679–10684. 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Clement CA, Satir P, & Pedersen LB (2012). Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. The Journal of Pathology, 226(2), 172–184. 10.1002/path.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Morthorst SK, Mogensen JB, & Pedersen LB (2017). Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Cold Spring Harbor Perspectives in Biology. 10.1101/cshperspect.a028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cionni JM, Wong HH-W, Bressan D, Kodama L, Harris WA, & Holt CE (2018). Axon-axon interactions regulate topographic optic tract sorting via CYFIP2-dependent WAVE complex function. Neuron, 97, 1078–1093. 10.1016/j.neuron.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O (2012). Orphan GPCRs and neuromodulation. Neuron, 76(1), 12–21. 10.1016/j.neuron.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conduit SE, Dyson JM, & Mitchell CA (2012). Inositol polyphosphate 5-phosphatases; new players in the regulation of cilia and ciliopathies. FEBS Letters, 586(18), 2846–2857. 10.1016/j.febslet.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Courchet J, Lewis TL Jr., Lee S, Courchet V, Liou D-Y, Aizawa S, et al. (2013). Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell, 153(7), 1510–1525. 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, & Clapham DE (2015). Direct recording and molecular identification of the calcium channel of primary cilia. Nature, 504(7479), 315–318. 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N, & Spassky N (2014). Interplay between primary cilia and cell cycle. Medecine Sciences, 30, 976–979. France: 2014 medecine/sciences - Inserm. [DOI] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, & Clapham DE (2013). Primary cilia are specialized calcium signalling organelles. Nature, 504, 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo A, & Sahin M (2018). mTOR’ing across the cortex by chopping the cilia. Neuron, 99(1), 3–5. 10.1016/j.neuron.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, et al. (2004). Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. American Journal of Human Genetics, 75(6), 979–987. 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D (2009). Joubert Syndrome: Insight into brain development, cilium biology, and complex disease. Seminars in Pediatric Neurology, 16(3), 144–154. 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A (1844). Flimmerbewegung im Gehörorgan von Petromyzon marinus. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin, 1, 520–521. [Google Scholar]
- Engle EC (2010). Human genetic disorders of axon guidance. Cold Spring Harbor Perspectives in Biology, 2(3), a001784. 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failler M, Gee H, Krug P,Joo K, Halbritter J, Belkacem L, et al. (2014).Mutations of CEP83 cause infantile nephronophthisis and intellectual disability. American Journal of Human Genetics, 94(6), 905–915. 10.1016/j.ajhg.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon-Urrutia P, Carrasco CM, Lois P, Palma V, & Roth AD (2015). Shh signaling through the primary cilium modulates rat oligodendrocyte differentiation. PLoS One, 10(7). 10.1371/journal.pone.0133567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent J, Constable S, Gigante ED, Yam PT, Mariani LE, Legué E, et al. (2019). The ciliary protein Arl13b functions outside of the primary cilium in Shh-mediated axon guidance. Cell Reports, 29(11), 3356–3366.e3. 10.1016/j.celrep.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland R, Eyaid W, Collura R, Tully L, Hill R, Al-Nouri D, et al. (2004). Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nature Genetics, 36(9), 1008–1013. 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, & Omran H (2007). When cilia go bad: Cilia defects and ciliopathies. Nature Reviews Molecular Cell Biology, 8, 880–893. England. [DOI] [PubMed] [Google Scholar]
- Foerster P, Daclin M, Asm S, Faucourt M, Boletta A, Genovesio A, et al. (2017). mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis. Development, 144(2), 201–210. 10.1242/dev.138271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, et al. (2014). PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Developmental Cell, 28(6), 647–658. 10.1016/j.devcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, et al. (2016). CPAP promotes timely cilium disassembly to maintain neural progenitor pool. The EMBO Journal, 35(8), 803–819. 10.15252/embj.201593679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavi S, Shumay E, Wang H.-y., & Malbon CC (2006). G-protein-coupled receptors and tyrosine kinases: Crossroads in cell signaling and regulation. Trends in Endocrinology & Metabolism, 17(2), 48–54. 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A, et al. (2015). Effects of PTEN and nogo codeletion on corticospinal axon sprouting and regeneration in mice. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 35(16), 6413–6428. 10.1523/jneurosci.4013-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherman A, Davis EE, & Katsanis N (2006). The ciliary proteome database: An integrated community resource for the genetic and functional dissection of cilia. Nature Genetics, 38(9), 961–962. 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- Gibson DA, & Ma L (2011). Developmental regulation of axon branching in the vertebrate nervous system. Development, 138(2), 183–195. 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, & Mykytyn K (2014). neuronal primary cilia: an under appreciated signaling and sensory organelle in the brain. Neuropsychopharmacology, 39, 244–245. 10.1038/npp.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowsky A, & Gunay-Aygun M (2019). Clinical characteristics of individual organ system disease in non-motile ciliopathies. Translational Science of Rare Diseases, 4(1-2), 1–23. 10.3233/TRD-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, et al. (2013). Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. The Journal of Neuroscience, 33(6), 2626–2638. 10.1523/jneurosci.2906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Coufal NG, & Gleeson JG (2014). Primary cilia in the developing and mature brain. Neuron, 82(3), 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Higginbotham H, Li J, Nichols J, Hirt J, Ghukasyan V, et al. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nature Communications, 6(7857). 10.1038/ncomms8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Otis JM, Higginbotham H, Monckton C, Cheng J, Asokan A, et al. (2017). Primary cilia signaling shapes the development of interneuronal connectivity. Developmental Cell, 42(3), 286–300. e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Otis J, Suciu S, Catalano C, Xing L, Constable S, et al. (2019). Primary cilia signaling promotes axonal tract development and is disrupted in Joubert syndrome-related disorders models. Developmental Cell, 51, 759–774. 10.1016/j.devel.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, & Alvarez-Buylla A (2010). Role of primary cilia in brain development and cancer. Current Opinion in Neurobiology, 20(1), 58–67. 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, & Alvarez-Buylla A (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nature Medicine, 15(9), 1062–1065. 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, et al. (2012). Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron, 73(6), 1116–1126. 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Inoue Y, Kiyonari H, Abe T, Misaki K, Moriguchi H, et al. (2014). Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts. Developmental Cell, 30(6), 673–687. 10.1016/j.devcel.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Henle SJ, Wang G, Liang E, Wu M, Poo M.m., & Henley JR (2011). Asymmetric PI(3,4,5)P3 and Akt signaling mediates chemotaxis of axonal growth cones. Journal of Neuroscience, 31(19), 7016–7027. 10.1523/jneurosci.0216-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, et al. (2012). Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Developmental Cell, 23(5), 925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Guo J, Yokota Y, Umberger N, Su C, Li J, et al. (2013). Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nature Neuroscience, 16(8), 1000–1007. 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, & Katsanis N (2011). Ciliopathies. The New England Journal of Medicine, 364(16), 1533–1543. 10.1056/nejmra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf KI, Johnson CT, & Jackson PK (2016). The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Current Opinion in Cell Biology, 39, 84–92. 10.1016/j.ceb.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina N, Tanimura A, Yamasaki M, Inoue T, Fukabori R, Kuroda T, et al. (2013). Protocadherin 17 regulates presynaptic assembly in topographic corticobasal ganglia circuits. Neuron, 78(5), 839–854. 10.1016/j.neuron.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen Y, & Page DT (2016). Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nature Communications, 7. 10.1038/ncomms13421, 13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Thompson J, Yates JR 3rd, & Marshall WF (2012). Proteomic analysis of mammalian primary cilia. Current Biology, 22(5), 414–419. 10.1016/j.cub.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, et al. (2009). INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature Genetics, 41(9), 1027–1031. 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Jissendi-Tchofo P, Severino M, Nguema-Edzang B, Toure C, Soto Ares G, & Barkovich AJ (2015). Update on neuroimaging phenotypes of mid-hindbrain malformations. Neuroradiology, 57(2), 113–138. 10.1007/s00234-014-1431-2. [DOI] [PubMed] [Google Scholar]
- Juric-Sekhar G, Adkins J, Doherty D, & Hevner RF (2012). Joubert syndrome: Brain and spinal cord malformations in genotyped cases and implications for neurodevelopmental functions of primary cilia. Acta Neuropathologica, 123(5), 695–709. 10.1007/s00401-012-0951-2. [DOI] [PubMed] [Google Scholar]
- Kalil K, & Dent EW (2014). Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nature Reviews. Neuroscience, 15(1), 7–18. 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Li L, & Hutchins BI (2011). Signaling mechanisms in cortical axon growth, guidance and branching. Frontiers in Neuroanatomy, 5, 62. 10.3389/fnana.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Jackson J, Ayzenshtat I, Tucciarone J, Manoocheri K, Snider WG, et al. (2016). Cooperative subnetworks of molecularly similar interneurons in mouse neocortex. Neuron, 90(1), 86–100. 10.1016/j.neuron.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Miyoshi K, Murakami S, Miyazaki I, & Asanuma M (2014). Visualization of astrocytic primary cilia in the mouse brain by immunofluorescent analysis using the cilia marker Arl13b. Acta Medica Okayama, 68(6), 317–322. 10.18926/AMO/53020. [DOI] [PubMed] [Google Scholar]
- Keryer G, Pineda JR, Liot G, Kim J, Dietrich P, Benstaali C, et al. (2011). Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. The Journal of Clinical Investigation, 121(11), 4372–4382. 10.1172/JCI57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand Kia S, Verbeek E, Engelen E, Schot R, Poot RA, de Coo IF, et al. (2012). RTTN mutations link primary cilia function to organization of the human cerebral cortex. American Journal of Human Genetics, 91(3), 533–540. 10.1016/j.ajhg.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Höhne M,Jüngst C, Bertsch S, Ebert LK, Schauss AC, et al. (2017). The ciliary membrane-associated proteome reveals actin-binding proteins as key components of cilia. EMBO Reports, 18(9), 1521–1535. 10.15252/embr.201643846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolliker A (1854). Manual of human microscopical anatomy (translation by George Busk of Mikroskopische Anatomie). Philadelphia: Lippincott: Grambo and Company. [Google Scholar]
- Kowalevsky A (1867). Entwickelungsgeschichte des Amphioxus lanceolatus. Memoires de l’Academie Imperiale des Sciences de St-Petersbourg (ser VII), 11, 1–17. [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K-I, Levine J, et al. (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature Neuroscience, 15(3), 399–405. 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K, Sestan N, & Anton E (2012). Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development, 139(9), 1535–1546. 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. (2006). Pten regulates neuronal arborization and social interaction in mice. Neuron, 50(3), 377–388. 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclef C, Anselme I, Besse L, Catala M, Palmyre A, Baas D,et al. (2015). The role of primary cilia in corpus callosum formation is mediated by production of the Gli3 repressor. Human Molecular Genetics, 24(17), 4997–5014. 10.1093/hmg/ddv221. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, & Gleeson JG (2009). The primary cilium as a cellular signaling center: Lessons from disease. Current Opinion in Genetics & Development, 19, 220–229. 10.1016/J.GDE.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerhans P (1876). Zur Anatomie des Amphioxus. Archiv für Mikroskopische Anatomie, 12, 290–348. [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron, 69(5), 893–905. 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, & Schlessinger J (2010). Cell signaling by receptor tyrosine kinases. Cell, 141(7), 1117–1134. 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepanto P, Badano JL, & Zolessi FR (2016). Neuron’s little helper: The role of primary cilia in neurogenesis. Neurogenesis, 3(1). 10.1080/23262133.2016.1253363, e1253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak AJ, Brodsky M, Cohenca N, Croicu AG, & Neumaier JF (2018). Restoration of physiological expression of 5-HT6 receptor into the primary cilia of null mutant neurons lengthens both primary cilia and dendrites. Molecular Pharmacology, 94(1), 731–742. 10.1124/mol.117.111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, et al. (2011). Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nature Cell Biology, 13(4), 402–411. 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo L, Simon JM, Xing L, McCoy ES, Niehaus JK, Guo J, et al. (2019). Single-cell transcriptomic analysis of mouse neocortical development. Nature Communications, 10(1), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinly AR, Spiegel I, Patrizi A, Centofante E, Bazinet JE, Tzeng CP, et al. (2016). Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature, 531(7594), 371–375. 10.1038/nature17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, & von Castro M (2012). A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PLoS One, 7. 10.1371/journal.pone.0046647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, & von Zastrow M (2010). DISC1 regulates primary cilia that display specific dopamine receptors. PLoS One, 5(5). 10.1371/journal.pone.0010902, e10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Sawada M, García-González D, Herranz-Pérez V, Ogino T, Bang Nguyen H, et al. (2019). Dynamic changes in ultrastructure of the primary cilium in migrating neuroblasts in the postnatal brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 39(50), 9967–9988. 10.1523/JNEUROSCI.1503-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, & Lappalainen P (2008). Filopodia: molecular architecture and cellular functions. Nature Reviews. Molecular Cell Biology, 9(6), 446–454. 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- McIntyre JC, Davis EE, Joiner A, Williams CL, Tsai IC, Jenkins PM, et al. (2012). Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nature Medicine, 18, 1423–1428. 10.1038/nm.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, & Borisy GG (2004). Lamellipodial versus filopodial mode of the actin nanomachinery: Pivotal role of the filament barbed end. Cell, 118(3), 363–373. 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, et al. (2015). Proteomics of primary cilia by proximity labeling. Developmental Cell, 35(4), 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison H, & Valente E (2017). Motile and non-motile cilia in human pathology: From function to phenotypes. Journal of Pathology, 241, 294–309. 10.1002/path.4843. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, & Asanuma M (2009). Lithium treatment elongates primary cilia in the mouse brain in in cultured cells. Biochemical and Biophysical Research Communications, 388, 757–762. 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Clowry GJ, Šestan N, Alzu’bi A, Bakken T, Hevner RF, et al. (2019). New insights into the development of the human cerebral cortex. Journal of Anatomy, 235, 432–451. 10.1111/joa.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki E, & Walsh C (2001). Mechanisms of cerebral cortical patterning in mice and humans. Nature Neuroscience, 4, 1199–1206. 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- Moser JJ, Fritzler MJ, & Rattner JB (2009). Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer, 9(448). 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, & Rohatgi R (2014). G-protein-coupled receptors, Hedgehog signaling and primary cilia. Seminars in Cell and Developmental Biology, 33, 63–72. 10.1016/j.semcdb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Estrada J, Lora-Castellanos A, Meza I, Alarcón Elizaldes S, & Benítez-King G (2017). Primary cilia formation is diminished in schizophrenia and bipolar disorder: A possible marker for these psychiatric diseases. Schizophrenia Research, 195, 412–420. 10.1016/j.schres.2017.08.055. [DOI] [PubMed] [Google Scholar]
- Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, et al. (2017). An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell, 168, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, et al. (1999). The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Human Molecular Genetics, 8, 2479–2488. England. [DOI] [PubMed] [Google Scholar]
- Narita K, Kozuka-Hata H, Nonami Y, et al. (2012). Proteomic analysis of multiple primary cilia reveals a novel mode of ciliary development in mammals. Biology Open, 1(8), 815–825. 10.1242/bio.20121081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechipurenko IV, Doroquez DB, & Sengupta P (2013). Primary cilia and dendritic spines: Different but similar signaling compartments. Molecules and Cells, 36(4), 288–303. 10.1007/s10059-013-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, et al. (2010). WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nature Genetics, 42(11), 1010–1014. 10.1038/ng.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AS, Floyd DH,Bruinsma SP, Narzinski K, & Baranski TJ (2013). Frizzled receptors signal through G proteins. Cellular Signalling, 25(6), 1468–1475. 10.1016/j.cellsig.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M, Oishi K, & Nakajima K (2013). Axon guidance mechanisms for establishment of callosal connections. Neural Plasticity, 2013(2), 1–7. 10.1155/2013/149060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novarino G, Akizu N, & Gleeson JG (2011). Modeling human disease in humans: The ciliopathies. Cell, 147(1), 70–79. 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Chaya T, Yoshida S, Irie S,Tsujii T, & Furukawa T (2015). Identification of G protein-coupled receptors (GPCRs) in primary cilia and their possible involvement in body weight control. PLoS One, 10(6). 10.1371/journal.pone.0128422. e0128422–0128417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo JL, DeCaen PG, & Clapham DE (2017). Progress in ciliary ion channel physiology. The Journal of General Physiology, 149, 37–47. 10.1085/jgp.20161169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino A, Fenlon LR, Suárez R, & Richards LJ (2018). Transcriptional control of long-range cortical projections. Current Opinion in Neurobiology, 53, 57–65. 10.1016/j.conb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Parisi MA (2009). Clinical and molecular features of Joubert syndrome and related disorders. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 151C(4), 326–340. 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA (2019). The molecular genetics of Joubert syndrome and related ciliopathies: The challenges of genetic and phenotypic heterogeneity. Translational Science of Rare Diseases, 4, 25–49. 10.3233/TRD-190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Jang HJ, & Lee JH (2019). Roles of primary cilia in the developing brain. Frontiers of Cellular Neuroscience, 13(218). 10.3389/fncel.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lim J, Ramakrishina S, Kim S, Kim W, Lee J, et al. (2018). Brain somatic mutations in MTOR disrupt neuronal ciliogenesis, Leading to focal cortical dyslamination. Neuron, 99(1), 83–97. 10.1016/j.neuron.2018.05.039. [DOI] [PubMed] [Google Scholar]
- Parra LM, & Zou Y (2010). Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nature Neuroscience, 13, 29–35. United States. [DOI] [PubMed] [Google Scholar]
- Phua SC, Lin Y-C, & Inoue T (2015). An intelligent nano-antenna: Primary cilium harnesses TRP channels to decode polymodal stimuli. Cell Calcium, 58(4), 415–422. 10.1016/j.ceca.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Evrony GD, Cai X, Elhosary PC, Beroukhim R, Lehtinen MK, et al. (2012). Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron, 74(1), 41–48. 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, et al. (2013). Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nature Genetics, 45(6), 639–647. 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E, Loenneker T, Valente EM, Brancati F, Il’yasov K, et al. (2007). Diffusion tensor imaging in Joubert syndrome. American Journal of Neuroradiology, 28(10), 1929–1933. 10.3174/ajnr.A0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Huisman TA, Scheer I, & Boltshauser E (2011). Joubert syndrome and related disorders: Spectrum of neuroimagingfindings in 75 patients. American Journal of Neuroradiology, 32(8), 1459–1463. 10.3174/ajnr.A2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Kim AH, Ikeuchi Y, Wilson-Grady JT, Merdes A, Gygi SP, et al. (2011). A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nature Neuroscience, 14(8), 973–983. 10.1038/nn.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoux A, Baas D, Paschaki M, Morlé L, Maire C, Attié-Bitach T, et al. (2019). Altered GLI3 and FGF8 signaling underlies acrocallosal syndrome phenotypes in Kif7 depleted mice. Human Molecular Genetics, 28(6), 877–887. 10.1093/hmg/ddy392. [DOI] [PubMed] [Google Scholar]
- Putoux A, Thomas S, Coene KL, Davis EE, Alanay Y, Ogur G, et al. (2011). KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nature Genetics, 43(6), 601–606. 10.1038/ng.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Song H, & Ming GL (2019). Brain organoids: Advances, applications and challenges. Development, 146(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, & Mason C (2010). Cellular strategies of axonal pathfinding. Cold Spring Harbor Perspectives in Biology, 2(9), a001933. 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, & Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nature Reviews. Molecular Cell Biology, 18(9), 533–547. 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringers C, Olstad EW, & Jurisch-Yaksi N (2020). The role of motile cilia in the development and physiology of the nervous system. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 375(1792), 20190156. 10.1098/rstb.2019.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Micalizzi A, & Valente EM (2013). Joubert syndrome: Congenital cerebellar ataxia with the molar tooth. The Lancet Neurology, 12(9), 894–905. 10.1016/S1474-4422(13)70136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MA, & Armstrong RC (2018). Postnatal sonic hedgehog (Shh) responsive cells give rise to oligodendrocyte lineage cells during myelination and in adulthood contribute to remyelination. Experimental Neurology, 299, 122–136. United States: Published by Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, & Guadiana SM (2015). Influences of primary cilia on cortical morphogenesis and neuronal subtype maturation. Neuroscience, 21, 136–151. 10.1177/1073858414531074. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, & Semple-Rowland SL (2019). Emerging roles of primary cilia in glioma. Frontiers in Cellular Neuroscience, 13(55). 10.3389/fncel.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S, & Gleeson JG (2011). The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Developmental Medicine and Child Neurology, 53, 793–798. 10.1111/j.1469-8749.2011.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JW, Gao Z, Li S, Farooqi M, Tang TS, Bezprozvanny I, et al. (2008). Small-molecule activation of neuronal cell fate. Nature Chemical Biology, 4(7), 408–410. 10.1038/nchembio.95. [DOI] [PubMed] [Google Scholar]
- Schou KB, Pedersen LB, & Christensen ST (2015). Ins and outs of GPCR signaling in primary cilia. EMBO Reports, 16(9), 1099–1113. 10.15252/embr.201540530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger-Nukpezah T, & Golemis EA (2012). The extracellular matrix and ciliary signaling. Current Opinion in Cell Biology, 24(5), 652–661. 10.1016/j.ceb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P (2017). Cilia and sensory signaling: The journey from “animalcules” to human disease. PLoS Biology, 15, e2002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senocak EU, Oguz KK, Haliloglu G, Topcu M, & Cila A (2010). Structural abnormalities of the brain other than molar tooth sign in Joubert syndrome-related disorders. Diagnostic and Interventional Radiology, 16(1), 3–6. 10.4261/1305-3825.DIR.2673-09.1. [DOI] [PubMed] [Google Scholar]
- Shamseldin H, Alazami AM, Manning M, Hashem A, Caluseiu O, Tabarki B, et al. (2015). RTTN mutations cause primary microcephaly and primordial dwarfism in humans. American Journal of Human Genetics, 97(6), 862–868. 10.1016/j.ajhg.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Yang J, He M, Yu XY, Lee CH, Yang Z, et al. (2020). Centrosome anchoring regulates progenitor properties and cortical formation. Nature, 580(7801), 106–112. 10.1038/s41586-020-2139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Kim M, Jung H-J, Cha HL, Suh-Kim H, Ahn S, et al. (2017). Characterization of developmental defects in the forebrain resulting from hyperactivated mTOR signaling by integrative analysis of transcriptomic and proteomic data. Scientific Reports, 7(1), 2826. 10.1038/s41598-017-02842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohayeb B, Ho U, Yeap YY, Parton RG, Millard SS, Xu Z, et al. (2020). The association of microcephaly protein WDR62 with CPAP/IFT88 is required for cilia formation and neocortical development. Human Molecular Genetics, 29, 248–263. England: The Author(s) 2019. Published by Oxford University Press; For Permissions, please journals.permissions@oup.com. [DOI] [PubMed] [Google Scholar]
- Sigg MA, Menchen T, Lee C, Johnson J, Jungnickel MK, Choksi SP, et al. (2017). Evolutionary proteomics uncovers ancient associations of cilia with signaling pathways. Developmental Cell, 43(6), 744–762.e11. 10.1016/j.devcel.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, et al. (2018). Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nature Genetics, 50, 180–185. 10.1038/s41588-017-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, & Gandhi SP (2010). Cortical plasticity induced by inhibitory neuron transplantation. Science, 327(5969), 1145–1148. 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpka A, Yang J, Strobel M, Zhou Y, Pauplis C,& Chen X (2020). Diverged morphology changes of astrocytic and neuronal primary cilia under reactive insults. Molecular Brain, 13, 28. 10.1186/s13041-020-00571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoufflet J, Chaulet M, Doulazmi M, Fouquet C, Dubacq C, Métin C, et al. (2019). Primary cilium-dependent cAMP/PKA signaling at the centrosome regulates neuronal migration. BioRxiv. 10.1101/765925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suizu F, Hirata N, Kimura K, Edamura T, Tanaka T, Ishigaki S, et al. (2016). Phosphorylation-dependent Akt-inversin interaction at the basal body of primary cilia. The EMBO Journal, 35(12), 1346–1363. 10.15252/embj.201593003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Lee JS, Lopez E, Herranz-Perez V, Shida T, Franco B, et al. (2014). The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nature Genetics, 46(8), 905–911. 10.1038/ng.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Thomas S, Sinico M, Aral B, Burglen L, Gigot N, et al. (2013). OFD1 mutations in males: phenotypic spectrum and ciliary basal body docking impairment. Clinical Genetics, 84(1), 86–90. 10.1111/cge.12013. [DOI] [PubMed] [Google Scholar]
- Thion M, Ginhoux F, & Garel S (2018). Microglia and early brain development: An intimate journey. Science, 362(6411), 185–189. 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- Thomas S, Boutaud L, Reilly ML, & Benmerah A (2019). Cilia in hereditary cerebral anomalies. Biology of the Cell, 111, 217–231. 10.1111/boc.201900012. [DOI] [PubMed] [Google Scholar]
- Thomas S, Legendre M, Saunier S, Bessieres B, Alby C, Bonniere M, et al. (2012). TCTN3 mutations cause Mohr-Majewski syndrome. American Journal of Human Genetics, 91(2), 372–378. 10.1016/j.ajhg.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Han Y-G, Shah JK, Obernier K, Guinto CD, & Alvarez-Buylla A (2014). Primary cilia are required in a unique subpopulation of neural progenitors. Proceedings of the National Academy of Sciences of the United States of America, 111, 12438–12443. 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P, & Sahin M (2011). Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Current Opinion in Neurology, 24(2), 106–113. 10.1097/WCO.0b013e32834451c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzquiano A, Cifuentes-Diaz C, Jabali A, Romero DM, Houllier A, Dingli F, et al. (2019). Mutations in the heterotopia gene Eml1/EML1 severely disrupt the formation of primary cilia. Cell Reports, 28, 1596–1611. 10.1016/j.celrep.2019.06.096. [DOI] [PubMed] [Google Scholar]
- Valente EM, Rosti RO, Gibbs E, & Gleeson JG (2014). Primary cilia in neurodevelopmental disorders. Nature Reviews. Neurology, 10(1), 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam T, Kennedy J, van der Lee R, de Vrieze E, Wunderlich KA, Rix S, et al. (2019). CiliaCarta: An integrated and validated compendium of ciliary genes. PLoS One, 14(5), e0216705. 10.1371/journal.pone.0216705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam TJ, Wheway G, Slaats GG, SYSCILIA Study Group, Huynen MA, & Giles RH (2013). The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia, 2(1), 7. 10.1186/2046-2530-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, & Marquardt T (2013). What axons tell each other: Axon-axon signaling in nerve and circuit assembly. Current Opinion in Neurobiology, 23(6), 974–982. 10.1016/j.conb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, & Shi SH (2009). Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature, 461(7266), 947–955. 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware S, Gunay-Aygun M, & Hildebrandt F (2011). Spectrum of clinical diseases caused by disorders of primary cilia. Proceedings of the American Thoracic Society, 8, 444–450. 10.1513/pats.201103-025SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Ghashghaei H, Han C, Watson H, Campbell K, & Anton E (2007). Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS One, 2(8), 794. 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TW, & Bargmann CI (2001). Dynamic regulation of axon guidance. Nature Neuroscience, 4(Supp), 1169–1176. 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yang SL, Yang M, Herrlinger S, Shao Q, Collar JL, et al. (2019). Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors. Nature Communications, 10(1), 2612. 10.1038/s41467-019-10497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, & Poo MM (2007). Calcium signaling in neuronal motility. Annual Review of Cell and Developmental Biology, 23, 375–404. 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zhu D, Shi S, Wang H, & Liao K (2009). Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. Journal of Cell Science, 122(15), 2760–2768. 10.1242/jcs.046276. [DOI] [PubMed] [Google Scholar]
- Zimmermann KW (1898). Beitrage zur Kenntniss einiger Drusen und Epithelien. Arch. Mikrosk. Anat, 52, 552–70610. [Google Scholar]