Abstract
Purpose
The development of myopia in guinea pigs can be inhibited by attenuating scleral hypoxia by increasing choroidal blood perfusion (ChBP). In this study, we reduced ChBP through surgical and pharmacological methods to determine the effect on myopia development. We also determined whether ChBP was reduced by quinpirole, a drug that enhances form-deprivation myopia (FDM).
Methods
ChBP was reduced in the right eyes of guinea pigs via transection of the temporal ciliary arteries or daily injections of phenylephrine into the inferior peribulbar space for one week during normal ocular growth. Other guinea pigs were subjected to two weeks of monocular FDM—with facemasks, along with daily injections of quinpirole, a dopamine D2 receptor agonist, to enhance the FDM. Changes in refraction, axial length, ChBP, and choroidal thickness (ChT) were measured in both treated and fellow eyes of the treatment and control groups. Scleral hypoxia labeling with pimonidazole adducts and α-smooth muscle actin (α-SMA) protein were also measured.
Results
Surgical and pharmacological reduction of ChBP induced myopia development in the treated eyes. These treatments rendered the scleral hypoxia and increased scleral α-SMA expression. Furthermore, quinpirole injections, which increased the magnitude of myopia, augmented the FDM-associated reductions in ChBP and ChT and increased the levels of scleral hypoxia and α-SMA protein.
Conclusions
Decreased ChBP in guinea pigs leads to scleral hypoxia and scleral myofibroblast transdifferentiation with increased α-SMA expression, ultimately resulting in myopia development. In future clinical trials, ChBP reduction can serve as a potential biomarker for early detection of myopia development.
Keywords: myopia, choroidal blood perfusion, scleral hypoxia, scleral myofibroblast transdifferentiation, guinea pigs
Myopia (short-sightedness) is the most common refractive error worldwide, and the prevalence of myopia has markedly increased over the last few decades.1,2 High myopia (more negative than −6.00 diopters [D]) increases the risk of pathologic ocular complications such as glaucoma, retinal detachment, and myopic macular degeneration, all of which can result in irreversible vision loss.3,4 Although treatments such as orthokeratology,5 atropine,6 and increased time outdoors7 significantly reduce the rate of progression of myopia in clinical studies, the exact underlying molecular mechanisms of both the development and the control of myopia are still elusive.
It is well established, both in experimental models and clinical myopia, that the reduction of scleral thickness is closely associated with the increase of axial length (AL) that occurs during the development of myopia.8,9 Reduced scleral thickness is accompanied by a rapid turnover of the extracellular matrix (ECM) components,10,11 which includes declines in scleral proteoglycan and collagen syntheses and increases in collagen degradation.12,13 Our previous study, using single-cell RNA sequencing, suggested that the hypoxia-inducible factor-1α (HIF-1α) signaling pathway was activated, leading to the transdifferentiation of fibroblasts into myofibroblasts that expressed low level of type I collagen.14 These changes subsequently enabled scleral ECM remodeling that led to the development of myopia in mice and guinea pigs. The possible involvement of HIF-1α in myopia has also been reported in other animal studies with tree shrews15,16 and rabbits.17 Consequently it is reasonable to consider that hypoxia might play a key role in scleral myofibroblast transdifferentiation, scleral ECM remodeling, and myopia development.
The highly vascular choroid is sandwiched between the retina and the sclera, and choroidal vessels supply oxygen and nutrients to the underlying retinal pigment epithelium (RPE), outer retina, and the overlying sclera.18 Previous animal studies have found that the choroid thins during myopia development and thickens during recovery, in chicks,19,20 guinea pigs,21,22 and marmosets.23 Such changes in choroidal thickness (ChT) would impact choroidal blood perfusion (ChBP) and the oxygenation of the adjacent scleral tissue. Recently we reported that both ChT and ChBP were significantly decreased by both form-deprivation myopia (FDM) and lens-induced myopia (LIM) in guinea pigs.22 Similarly, significant reductions in ChT and ChBP have been observed clinically in high myopes.24,25 Furthermore, the antimyopia treatments atropine, apomorphine, and intense light all significantly inhibit the development of myopia and the associated decrease in ChBP.26 These observations are consistent with a report by Li et al.,27 who found that inhibition of circRNA-FoxO1 expression alleviated choroidal vascular dysfunction, improved choroidal vascular perfusion, and retarded the progression of myopia. These findings confirmed the strong correlation between the changes in ChBP and myopia development, but the causal relationship between ChBP and myopia still remains unclear. Our previous study found that increasing ChBP with prazosin attenuated scleral hypoxia and inhibited myopia development in guinea pigs;26 however, the impact of reduced ChBP on myopia development still needs to be explored further.
In the current study, we examined in guinea pigs the impact of reducing ChBP, by surgically (transecting the temporal ciliary arteries) and pharmacologically (with the vasoconstrictor phenylephrine), on the development of myopia and on the associated changes in scleral hypoxia and scleral myofibroblast transdifferentiation. We also included experiments with quinpirole, which enhances FDM,28 to study the impact of FDM enhancement on ChBP, scleral hypoxia, and scleral myofibroblast transdifferentiation.
Material and Methods
Animals
Three-week-old pigmented guinea pigs (Cavia porcellus, English shorthair stock, tricolor strain, n = 90) were randomly assigned to different experimental groups as described below. The animals were housed under a 12:12-hour light/dark cycle, with lights on at 8 AM and off at 8 PM, while the room temperature was maintained at 25°C. The illuminance at the cage floor was approximately 300 lux. The animals had free access to standard food, and fresh vegetables were provided twice a day. This research was approved by the Animal Care and Ethics Committee at the Wenzhou Medical University (Wenzhou, China). All procedures and care of animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental Design
The right eyes of the guinea pigs underwent experimental manipulations, whereas the left eyes served as the untreated, internal (same-individual) controls.
Experiment 1: Impact of Experimentally Decreasing ChBP on Myopia, Scleral Hypoxia, and Scleral Myofibroblast Transdifferentiation
The effect of reduced ChBP was assessed using two experimental paradigms. In Experiments 1.1 and 1.2, the ciliary arteries around the temporal side of the optic disc of the right eyes were transected, resulting in partial reduction of ChBP.22 These eyes (n = 12) were compared with the right eyes of a sham-operated group (n = 11) in which the temporal ciliary arteries were only exposed but not transected. In Experiment 1.1, refraction and AL were measured (as described below) at the baseline and one week after surgery, and ChBP and ChT were measured (as described below) at the baseline and five minutes and one week after the surgical procedure. In Experiment 1.2, the level of induced scleral hypoxia was assessed by immunodetection of injected pimonidazole hydrochloride one week after surgery (as described below). Pimonidazole becomes reductively activated specifically in hypoxic cells, where it forms immuno-detectable adducts with thiols in proteins, peptides, and amino acids.26,29 The scleral content of α-smooth muscle actin protein (α-SMA, a marker of the transdifferentiation of scleral fibroblasts into myofibroblasts) was determined by quantitative western blotting, and hematoxylin and eosin staining and electroretinogram (ERG) recordings were used to determine whether the retinal structure and function were affected, one week after surgery.
In Experiments 1.3 and 1.4, in the experimental group the α1-adrenoceptor agonist phenylephrine (n = 16), a vasoconstrictor, was used to decrease ChBP, whereas the control group received normal saline solution (NS) vehicle (n = 16), by daily injections for one week into the inferior peribulbar space of the right eyes. Details regarding dose, vehicle, and route of administration are provided below. Refraction and AL measurements were obtained at baseline and after phenylephrine or normal saline treatments. In Experiment 1.3, the effects of phenylephrine injection on refraction, AL, ChBP, and ChT were assessed. In Experiment 1.4, the phenylephrine-induced presence of hypoxia-labeled pimonidazole adducts in the sclera and the expression of scleral α-SMA were also measured after treatment.
Experiment 2: Impact of Quinpirole on Decreases in ChBP, Scleral Hypoxia, and Scleral Myofibroblast Transdifferentiation
In Experiments 2.1 and 2.2, the right eyes of all animals were covered with a facemask for two weeks to induce FDM.30 The form-deprived eyes (n = 17) were treated by peribulbar injection of quinpirole, a dopamine D2 receptor agonist,28 to augment the FDM, while the masked right eyes of the FDM control animals (n = 18) were injected with vitamin C vehicle, for the entire two weeks (as described below). Refraction and AL were measured at baseline and after two weeks of FDM, in the fellow unmasked eyes and in the FDM eyes that had received either quinpirole or vitamin C injections. In Experiment 2.1, the effects of quinpirole on the development of FDM, refraction, AL, ChT, and ChBP were evaluated. In Experiment 2.2, the effects of quinpirole injections on hypoxia-labeled scleral pimonidazole adducts and expression of scleral α-SMA were measured after treatment.
Pharmaceutical Preparation and Administration
Phenylephrine (Abcam, Cambridge, MA, USA) was dissolved in normal saline solution, and quinpirole (Tocris, Glasgow, UK) was dissolved in Milli-Q water containing 0.1% ascorbic acid (Sigma-Aldrich Corp., St. Louis, MO, USA) as an antioxidant. After applying one drop of topical anesthetic (0.5% proparacaine hydrochloride; Alcon, Puurs, Belgium), the drugs (100 µL solution containing 20 µg phenylephrine or 100 ng quinpirole) were injected once daily (9:00 AM) into the inferior peribulbar space of the right eyes; animals in vehicle control groups were injected with the respective vehicle (100 µL). All injections were administered in dim red light. The left (fellow) eyes remained untreated throughout the experiment.
Optical Coherence Tomography Angiography (OCTA)
OCTA is a novel method for visualizing the retinal and choroidal vascular layers in a depth-resolved and noninvasive fashion.31 The animals were subjected to inhalation anesthesia (3% isoflurane, 1 L/min) during these measurements. ChT and ChBP were measured using the Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) as described previously.22 Three different formats of B-scans (a structural OCT image, an OCTA image, and the merged overlay of these two) were exported for further ChT and ChBP analyses. The images were analyzed using custom-designed software (MATLAB R2017a; MathWorks, Natick, MA, USA) to obtain both ChT and ChBP simultaneously.22
Biometric Measurements
Refractive measurements were carried out without cycloplegia in a dark room using a custom-built eccentric infrared photorefractor, as described previously.32 The mean of three readings was recorded as the final refractive error. AL (from the anterior corneal surface to the nerve fiber layer of the retina) was measured with A-scan ultrasonography (11 MHz, AVISO Echograph Class I-Type Bat; Quantel Medical, Clermont-Ferrand, France),32 after anesthetizing the cornea with a drop of 0.5% proparacaine hydrochloride (Alcon). Ten traces were captured and averaged for each eye as the final AL value.
Immunolabeling of Scleral Hypoxia Signals
After their respective treatment periods, the animals received a peribulbar injection of pimonidazole hydrochloride (Hypoxyprobe, 600 µg, HP3-100 Kit; HPI, Burlington, MA, USA). Then, 45 minutes after injection, the animals were terminally anesthetized with an overdose of sodium pentobarbital and euthanized via cervical dislocation. The eyes were immediately enucleated, and the anterior segment was removed. The posterior eyecups were first fixed by immersion in 0.1 M phosphate-buffered 4% paraformaldehyde at room temperature for 30 minutes and then dehydrated in 30% sucrose for 24 hours. Afterward they were embedded in a mounting medium (Neg-50; Thermo Fisher Scientific, Waltham, MA, USA) frozen in liquid nitrogen, and stored at −80°C until sectioning. Sections 12 µm thick were cut at −20°C using a cryostat microtome (Leica CM1860 UV; Leica Microsystems, Solms, Germany), and sections were mounted and air-dried on coated glass slides (Maixin Biotechnologies, Fujian, China). Nonspecific labeling was blocked by incubating slides for two hours at room temperature with 10% normal donkey serum, followed by incubation overnight at 4°C with anti-pimonidazole antibody (1:100; PAb2627AP, HPI). After rinsing the sections with phosphate-buffered saline solution, they were incubated for two hours at room temperature with secondary antibody (FITC conjugated donkey anti-rabbit IgG, 1:400; Invitrogen, Waltham, MA, USA). Sections incubated in buffer alone, without primary antibody, were used as negative controls.
Images of stained sections were acquired using a confocal microscope (LSM880 META; Carl Zeiss Meditec, Göttingen, Germany) under magnification ×20. The intensities of the hypoxia signals, indicated by pimonidazole staining, were analyzed with Zeiss ZEN 2.3 software (Carl Zeiss Meditec) as described previously.26
Western Blot Analysis
After one- or two-week treatment periods, the animals were terminally anesthetized with an overdose of sodium pentobarbital and euthanized via cervical dislocation. The eyes were immediately enucleated and the whole sclera (posterior to the corneal limbus, and excluding the optic nerve, retina, and choroid) was isolated. Scleral protein was extracted as described earlier.33 Briefly, scleral samples were disrupted and homogenized in lysis buffer (RIPA Lysis Buffer; Beyotime Biotechnology, Shanghai, China) containing protease inhibitors (PMSF, Beyotime Biotechnology, Shanghai, China; cOmplete Mini, Roche, Basel, Switzerland). A ball mill (MM400; Retsch, Düsseldorf, Germany) and an ultrasonic crusher (JY92-2D; Ningbo Scientz Biotechnology, Ningbo, China) were used for homogenization. After centrifuging the samples at 14,000g for 10 minutes at 4°C, the supernatant was collected, and protein concentrations were estimated (BCA, Beyotime Biotechnology).
The amounts of α-SMA protein were determined by Western blotting. Primary antibodies against α-SMA (1:1500, ab5694; Abcam) and α-tubulin (1:1000, ab7291; Abcam) were used. Densitometric analyses of protein bands were carried out using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and the signal intensities were normalized to the signal intensity of α-tubulin. A series of protein dilutions was tested under these conditions to confirm that band densities in the blot images were in the quasi-linear range between the threshold and saturation limits.
ERG
Scotopic and photopic flash ERGs, evoked by stimuli delivered to a custom-built Ganzfeld dome, were recorded with corneal and reference electrodes connected to a computer-based system (Q450SC UV; Roland Consult, Wiesbaden, Germany) as previously described.34 After overnight dark adaptation, the animals were anesthetized during measurements by inhalation with 3% isoflurane, 1 L/min, and the pupils were dilated with tropicamide (Santen Pharmaceutical Co. Ltd., Osaka, Japan). Flashes at five levels of scotopic stimulus intensity (−3.699, −2.201, −0.699, 0.301, and 0.799 log cd ⋅ s/m2) were used for eliciting the dark-adapted (rod-dominated) ERG. After 10 minutes of light adaptation with a rod-saturating background (1.398 log cd ⋅ s/m2), flashes at three levels of photopic intensity (−0.201, 0.301, and 0.799 log cd ⋅ s/m2) were superimposed on this white background for light-adapted (cone-dominated) ERG recording. The operational approaches were similar to those described previously.35
Statistical Analysis
All data were expressed as mean ± standard deviation. All data groups were tested for normality of distribution and equality of variance to verify that parametric statistics could be used. To compensate for inter-individual differences between growth rates and responses to treatment in different animals, we used interocular differences to indicate the effect on the treated eye. To validate this approach, we confirmed that the measured values in untreated eyes were not significantly affected by different treatments of the treated eye (Supplementary Table S1). Repeated measures analysis of variance (ANOVA) was used to compare interocular differences (for treated eye minus untreated fellow eye) of refraction and AL in different groups, with groups as factors and times as the repeated measures. ERG parameters were estimated by repeated measures ANOVA, with groups as factors and eyes and stimulus intensities as the repeated measures. For immunofluorescence experiments, independent t-tests were used to compare the data in pairs of different treatment groups. For protein expression experiments, the data did not meet the equality of variance criterion; therefore the Mann-Whitney U test was used to compare the differences between values in the various treatment groups, and the Wilcoxon signed rank test was used to assess the differences between values for treated and fellow eyes. In Experiment 1.1, comparisons of ChT and ChBP between groups before and at five minutes and one week after transection of temporal ciliary arteries were performed by repeated measures ANOVA, with groups as factors and times as the repeated measures. In Experiment 1.3 and in Experiment 2.1, repeated measures ANOVA was also used in the intergroup comparison of ChT and ChBP, with groups as factors and eyes as the repeated measures. Multiple testing analyses were carried out using Bonferroni post-hoc corrections. All statistical analyses were performed using SPSS software (SPSS version 25.0, Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
Results
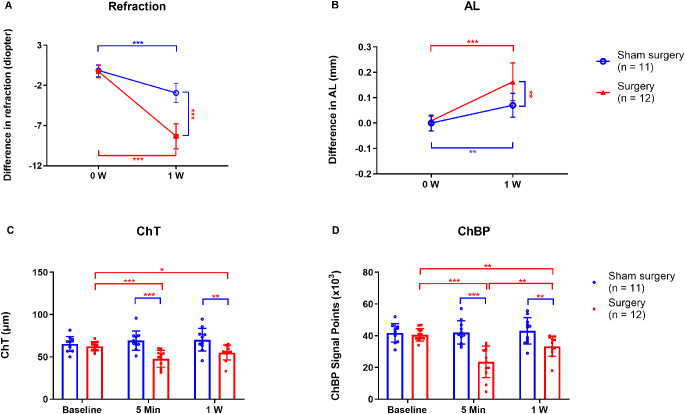
Experiment 1.1 Transection of Temporal Ciliary Arteries Induced Myopia Progression
As determined by repeated measures ANOVA, the main effects on refraction and AL were significant in both treatment groups (transected temporal ciliary arteries and sham-operated) and times (baseline and one week after surgery) (Table 1A). Additionally, the interaction effects of groups and times on both refraction and AL were also significant (Table 1A). One week after surgery, the sham-operated eyes developed a small but significant degree of myopia compared to the fellow eyes (interocular difference: −2.93 ± 1.20 D, P < 0.001, Fig. 1A). However, after transection of the temporal ciliary arteries, the degree of myopia was much greater (interocular difference: −8.33 ± 1.57 D, P < 0.001, Fig. 1A), and the interocular difference in refraction was significantly greater in the artery-transected animals compared to the sham-operated animals (P < 0.001, Fig. 1A). In parallel with the refractive changes, the interocular difference in AL of eyes with transected temporal ciliary arteries (0.16 ± 0.07 mm) was significantly greater than that in the sham-operated eyes (0.07 ± 0.05 mm; P = 0.002; Fig. 1B). The increases in interocular differences were due to myopic shifts in the artery-transected eyes, and not to hyperopic shifts in the fellow control eyes (see Supplementary Table S1).
Table 1A.
Main and Interaction Effects of Transection of Temporal Ciliary Arteries on Refraction and AL as Determined by Repeated Measures ANOVA
| Refraction | AL | |||
|---|---|---|---|---|
| Source | F1,21 | P | F1,21 | P |
| Group | 54.065 | <0.001 | 12.098 | 0.002 |
| Time1 | 366.749 | <0.001 | 68.571 | <0.001 |
| Time1 * Group | 86.299 | <0.001 | 9.547 | 0.006 |
Figure 1.
Refraction, AL, ChT, and ChBP in eyes with transected temporal ciliary arteries and sham-operated eyes. Comparisons of the interocular differences in (A) refraction and (B) AL, at the beginning (0 W) and end (1 W) of the treatment period. Comparisons of (C) ChT and (D) ChBP in the arterial-transection and sham-operated groups, at baseline (0 W), 5 min, and 1 W after surgery. W, week. *P < 0.05, **P < 0.01, and ***P < 0.001, repeated-measures ANOVA with Bonferroni correction.
As determined by repeated measures ANOVA, the main effects on ChT and ChBP were statistically significant in both treatment groups (the arterial-transection and sham-operated) and at all times (baseline, 5 min, and one week after surgery) (Table 1B). Additionally, the interaction effects of groups and times on both ChT and ChBP were also significant (Table 1B). The baseline ChT in the operated eye was 62.65 ± 5.39 µm, and 5 minutes after transection of the temporal ciliary artery it decreased to 47.80 ± 9.83 µm (P < 0.001, Fig. 1C); one week after surgery, ChT tended to be greater than at 5 minutes, but the difference was not statistically significant (P = 0.13, Fig. 1C). Similarly, ChBP was significantly smaller five minutes after transection of the temporal ciliary arteries (23.61 ± 9.91 × 103), than at baseline (40.60 ± 3.88 × 103; P < 0.001; Fig. 1D), but it was significantly greater one week after surgery (33.41 ± 6.34 × 103) than after 5 minutes (23.61 ± 9.91 × 103; P = 0.002; Fig. 1D). In contrast, in the sham-operated group there were no significant differences in ChT and ChBP at either time after treatment (Figs. 1C, 1D).
Table 1B.
Main and Interaction Effects of Transection of Temporal Ciliary Arteries on ChT and ChBP as Determined by Repeated Measures ANOVA
| ChT | ChBP | |||
|---|---|---|---|---|
| Source | F2,42 | P | F2,42 | P |
| Group | 15.694 | 0.001 | 16.866 | 0.001 |
| Time2 | 3.802 | 0.030 | 14.632 | <0.001 |
| Time2 * Group | 10.388 | <0.001 | 15.192 | <0.001 |
Group, transected temporal ciliary arteries and sham-operated; Time1, baseline, and after one week of transected temporal ciliary arteries and sham surgery; Time2, baseline, five minutes, and after one week of transected temporal ciliary arteries and sham surgery; AL: axial length.
Hematoxylin and eosin staining revealed no significant changes in retinal structure between the surgery and sham-operated groups at one week (Supplementary Figs. S1A–D). Similarly, a-wave amplitudes were not significantly different between these groups, under either scotopic or photopic conditions (Supplementary Figs. S2A, S2B). The b-wave amplitudes in the eyes with transected ciliary arteries were lower than those in the corresponding fellow eyes at stimulus intensities of 0.301 log cd⋅ s/m2 and 0.799 log cd ⋅ s/m2, under both scotopic and photopic conditions (Supplementary Figs. S2C, S2D). However, b-wave amplitudes in the arterial-transection and sham-operated eyes were not significantly different, under either scotopic or photopic conditions (Supplementary Figs. S2C, S2D).
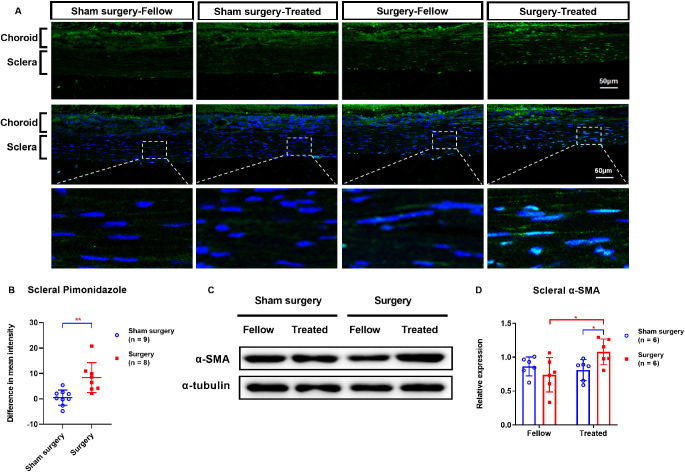
Experiment 1.2 Transection of Temporal Ciliary Arteries Induced Scleral Hypoxia and Induced Scleral Myofibroblast Transdifferentiation
One week after transection of the temporal ciliary arteries, the intensities of hypoxia signals were greater in the operated eyes than in the fellow eyes (Fig. 2A). Furthermore, the interocular difference in scleral hypoxia signals was much greater in the treated groups (8.36 ± 5.88) than in the sham-operated groups (0.52 ± 2.99; P = 0.003; Fig. 2B).
Figure 2.
Comparisons of pimonidazole labeling (A–B) and Western blot analysis of α-SMA expression (C–D) in scleras of eyes with transected temporal ciliary arteries or sham operations, one week after surgery. (A) First row, hypoxia signals (green). Second row, hypoxia signals (green) plus DAPI nuclear staining (blue). Third row, higher magnification details of the boxed scleral areas in the second row. Fellow, eyes contralateral to those with either transected temporal ciliary arteries or sham operations; Treated, eyes subjected to either transection of the temporal ciliary arteries or sham surgery. *P < 0.05, **P < 0.01, and ***P < 0.001; independent t-test, Mann-Whitney U test, or Wilcoxon signed rank test, as appropriate.
On the other hand, at one week after the surgery, the scleral α-SMA protein level was significantly greater in the eyes with transected temporal ciliary arteries (1.08 ± 0.19) than in the corresponding fellow eyes (0.74 ± 0.25; P = 0.046; Figs. 2C, 2D). Furthermore, scleral α-SMA protein levels were higher in surgery eyes (1.08 ± 0.19) than in sham-operated eyes (0.81 ± 0.15; P = 0.037; Figs. 2C, 2D). There were no statistically significant post-surgical changes in scleral α-SMA protein levels in the sham-operated eyes or the corresponding fellow eyes (Fig. 2C, 2D).
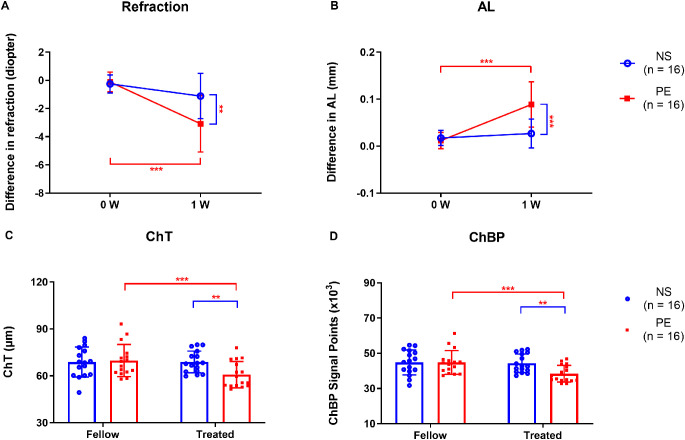
Experiment 1.3 Phenylephrine Reduced ChT and ChBP and Promoted the Development of Myopia
As determined by repeated measures ANOVA, the main effects on refraction and AL were significant in both treatment groups (phenylephrine and normal saline groups) and times (baseline and one week of injections), as were the interaction effects of groups and times on both refraction and AL (Table 2A). For animals in a normal visual environment, the interocular difference in refraction was greater after one week of daily phenylephrine injections (−3.08 ± 2.00 D) than after normal saline vehicle injections (−1.11 ± 1.61 D; P = 0.005; Fig. 3A). Similarly, the interocular difference in AL was greater in the phenylephrine-treated group (0.09 ± 0.05 mm) than in the normal saline solution–treated group (0.03 ± 0.03 mm; P < 0.001; Fig. 3B). The increases in interocular difference were due to myopic shifts in the phenylephrine-treated eyes, and not due to hyperopic shifts in the fellow control eyes (Supplementary Table S1).
Table 2A.
Main and Interaction Effects of Phenylephrine on Refraction and AL as Determined by Repeated Measures ANOVA
| Refraction | AL | |||
|---|---|---|---|---|
| Source | F1,30 | P | F1,30 | P |
| Group | 5.631 | 0.024 | 10.804 | 0.003 |
| Time | 40.033 | <0.001 | 40.727 | <0.001 |
| Time * Group | 13.066 | 0.001 | 20.944 | <0.001 |
Figure 3.
Refraction, AL, ChT, and ChBP in the normal saline (NS) and phenylephrine (PE) groups of animals in a normal visual environment. Comparison of the interocular differences in (A) refraction and (B) AL in the NS group and PE group at the beginning (0 W) and end (1 W) of the treatment period. Comparison of (C) ChT and (D) ChBP after 1 W. W, week; Fellow, eyes contralateral to those given peribulbar injections of either normal saline or phenylephrine; Treated, eyes given injections of either normal saline or phenylephrine; NS, normal saline; PE, phenylephrine. *P < 0.05, **P < 0.01, and ***P < 0.001, repeated-measures ANOVA with Bonferroni correction.
As determined by repeated measures ANOVA, the main effects on ChT and ChBP were significant in fellow and treated eyes (Table 2B). The interaction effects of groups and eyes on both ChT and ChBP were also significant (Table 2B). On the other hand, there were no significant differences in ChT and ChBP between normal saline solution–treated eyes and the corresponding untreated fellow eyes (Figs. 3C, 3D). However, the ChT of phenylephrine-treated eyes (60.82 ± 8.40 µm) was less than that in untreated fellow eyes (69.82 ± 10.29 µm; P < 0.001; Fig. 3C), and ChBP was also lower in the phenylephrine group (38.37 ± 4.76 × 103) than in contralateral fellow eyes (44.81 ± 6.78 × 103; P < 0.001; Fig. 3D). Furthermore, ChT was less in phenylephrine-treated eyes (60.82 ± 8.40 µm) than in normal saline solution–treated eyes (68.94 ± 6.93 µm; P = 0.006; Fig. 3C); and ChBP was significantly lower in phenylephrine-treated eyes (38.37 ± 4.76 × 103) than in normal saline solution–treated eyes (44.30 ± 5.24 × 103; P = 0.002; Fig. 3D).
Table 2B.
Main and Interaction Effects of Phenylephrine on ChT and ChBP as Determined by Repeated Measures ANOVA
| ChT | ChBP | |||
|---|---|---|---|---|
| Source | F1,30 | P | F1,30 | P |
| Group | 1.520 | 0.227 | 2.232 | 0.146 |
| Eye | 13.148 | 0.001 | 19.999 | <0.001 |
| Eye * Group | 13.690 | 0.001 | 14.692 | 0.001 |
Group, phenylephrine and normal saline groups; Time, baseline and after one week of injections with either phenylephrine or normal saline; Eye, fellow and treated eyes; AL, axial length.
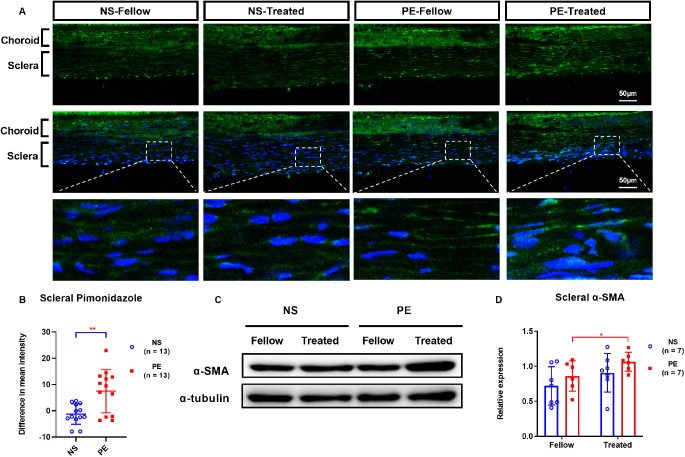
Experiment 1.4 Phenylephrine-Induced Scleral Hypoxia and Scleral Myofibroblast Transdifferentiation
After one week of daily peribulbar phenylephrine injections, the intensity of hypoxia signals in the sclera, as indicated by pimonidazole labeling, was significantly greater in the phenylephrine-treated eyes than in their fellow eyes (Fig. 4A). Furthermore, the mean difference in scleral hypoxia signals between the treated and fellow eyes was much larger in phenylephrine-treated eyes (7.49 ± 8.28) than in normal saline control eyes (−1.25 ± 3.86; P = 0.002; Fig. 4B).
Figure 4.
Comparisons of scleral labeling for hypoxia with pimonidazole (A–B) and of the Western blot analysis of scleral α-SMA protein content (C–D), in normal saline (NS) and phenylephrine (PE) groups one week after treatment. (A) First row, hypoxia signals (green). Second row, hypoxia signals (green) plus DAPI nuclear staining (blue). Third row, higher magnification details of the boxed scleral areas in the second row. Fellow: eyes contralateral to those injected with either normal saline or phenylephrine; Treated: eyes injected with either normal saline or phenylephrine; NS, normal saline; PE, phenylephrine. *P < 0.05, **P < 0.01, and ***P < 0.001, independent t-tests, Mann-Whitney U test, or Wilcoxon signed rank test, as appropriate.
On the other hand, there was no significant difference between scleral α-SMA levels in the normal saline solution control eyes and their fellow eyes (Figs. 4C, 4D). However, the scleral α-SMA level was significantly greater in phenylephrine-treated eyes (1.06 ± 0.14) than in untreated fellow eyes (0.86 ± 0.22; P = 0.046; Fig. 4D).
Experiment 2.1. Quinpirole Promoted FDM and Augmented the Reductions of ChT and ChBP Induced by FDM
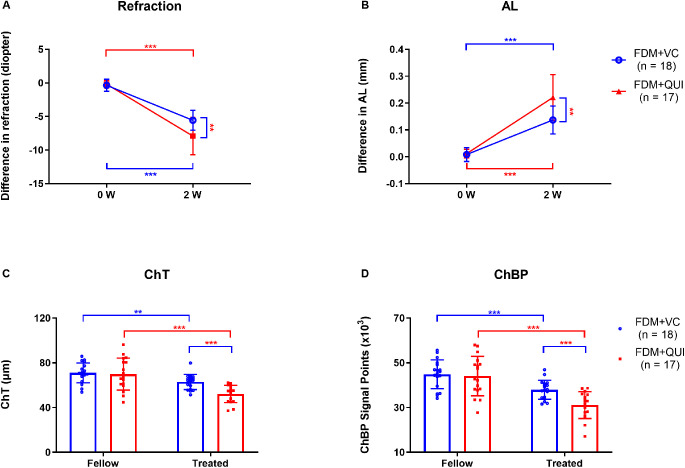
As determined by repeated measures ANOVA, the main effects on refraction and AL were statistically significant in both treatment groups (FDM+QUI and FDM+VC) and times (baseline and two weeks of daily FDM treatment with quinpirole or vitamin C vehicle alone). Similarly, the interaction effects of groups and times on refraction and AL were also significant (Table 3A). Form deprivation produced −5.56 ± 1.48 D myopic refraction in the vitamin C–injected eyes after two weeks of treatment. The interocular difference in refraction between the quinpirole-injected FDM eyes and the untreated fellow eyes (−7.91 ± 3.28 D) was significantly more negative (myopic) than the difference between the vitamin C–injected FDM and their fellow eyes (−5.56 ± 1.48 D; P = 0.003; Fig. 5A). In parallel, the interocular difference in AL between the quinpirole-injected FDM and the fellow eyes (0.22 ± 0.08 mm) was greater than that between the vitamin C–injected FDM and fellow eyes (0.14 ± 0.05 mm; P = 0.001; Fig. 5B). The increases in interocular difference were due to myopic shifts in the quinpirole-injected FDM eyes, and not due to hyperopic shifts in the fellow control eyes (Supplementary Table S1).
Table 3A.
Main and Interaction Effects of FDM+QUI on Refraction and AL as Determined by Repeated Measures ANOVA
| Refraction | AL | |||
|---|---|---|---|---|
| Source | F1,33 | P | F1,33 | P |
| Group | 8.545 | 0.006 | 12.765 | 0.001 |
| Time | 243.707 | <0.001 | 183.573 | <0.001 |
| Time * Group | 9.289 | 0.005 | 10.400 | 0.003 |
Figure 5.
Refraction, AL, ChT, and ChBP in the FDM+VC and FDM+QUI groups. Comparison of the interocular differences in (A) refraction and (B) AL at the beginning (0 W) and end (2 W) of the treatment period. Comparison of (C) ChT and (D) ChBP at the end of 2 weeks of treatment. W, weeks; Fellow, eyes contralateral to the FDM eyes and not subjected to either vitamin C or quinpirole injections; Treated, FDM eyes subjected to either vitamin C or quinpirole injections; VC, vitamin C; QUI, quinpirole. *P < 0.05, **P < 0.01, and ***P < 0.001, repeated-measures ANOVA with Bonferroni correction.
As determined by repeated measures ANOVA, the main effects on ChT were significant in both treatment groups (FDM+QUI and FDM+VC groups) and eyes (fellow and treated eyes), while the main effects on ChBP were significant in fellow and treated eyes (Table 3B). The interaction effects of groups and eyes on ChT and ChBP were also significant (Table 3B). The ChT of the FDM + vitamin C–treated eyes (62.95 ± 6.75 µm) was less than that of the fellow controls (71.06 ± 8.82 µm; P = 0.002; Fig. 5C). Similarly, ChBP in the FDM + vitamin C–treated eyes (37.97 ± 4.27 × 103) was lower than that in the fellow eyes (44.87 ± 6.47 × 103; P < 0.001; Fig. 5D). ChT in FDM + quinpirole–treated eyes (52.20 ± 7.74 µm) was less than that in the FDM+ vitamin C–treated eyes (62.95 ± 6.75 µm; P < 0.001; Fig. 5C); similarly, ChBP in the FDM + quinpirole–treated eyes (31.14 ± 6.00 × 103) was lower than that in FDM+vitamin C–treated eyes (37.97 ± 4.27 × 103; P < 0.001; Fig. 5D).
Table 3B.
Main and Interaction Effects of FDM+QUI on ChT and ChBP as Determined by Repeated Measures ANOVA
| ChT | ChBP | |||
|---|---|---|---|---|
| Source | F1,33 | P | F1,33 | P |
| Group | 4.429 | 0.043 | 3.719 | 0.062 |
| Eye | 57.422 | <0.001 | 94.445 | <0.001 |
| Eye * Group | 7.896 | 0.008 | 8.798 | 0.006 |
Group, FDM+QUI and FDM+VC groups; Time, baseline and after two weeks of FDM with simultaneous injections of quinpirole or vitamin C; Eye, fellow and treated eyes; AL, axial length; QUI, quinpirole; VC, vitamin C.
Experiment 2.2. Quinpirole Promoted the Scleral Hypoxia Induced by FDM and Enhanced Scleral Myofibroblast Transdifferentiation
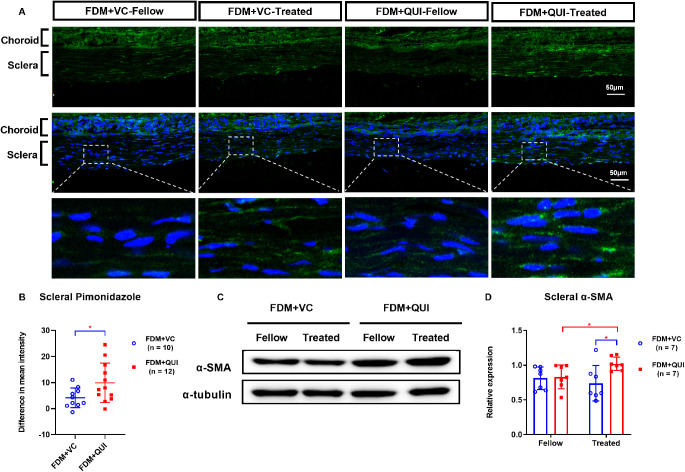
The intensity of the scleral hypoxia signals was greater in FDM + vitamin C–treated eyes than in the corresponding fellow eyes (P = 0.007, Fig 6A). This increase in hypoxia signals was enhanced further by quinpirole injection. In FDM eyes, quinpirole induced a large increase in the interocular difference of scleral hypoxia signals expression. In the FDM + quinpirole–treated eyes, the interocular difference in scleral pimonidazole intensity (9.89 ± 7.58) was greater than that in FDM + vitamin C–treated eyes (4.16 ± 3.78; P = 0.042; Fig. 6B).
Figure 6.
Comparisons of labeling with pimonidazole in scleras (A–B) and Western blot analysis of scleral α-SMA expression (C–D) in FDM+VC and FDM+QUI groups, at the end of two weeks’ treatment. First row, hypoxia signals (green). Second row, hypoxia signals (green) plus DAPI nuclear staining (blue). Third row, higher magnification of the boxed scleral areas in the second row. Fellow, eyes contralateral to the FDM eyes and not subjected to either vitamin C or quinpirole; Treated, FDM-eyes subjected to either vitamin C or quinpirole; VC, vitamin C; QUI, quinpirole. *P < 0.05, **P < 0.01, and ***P < 0.001, independent t-test, Mann-Whitney U test, or Wilcoxon signed rank test, as appropriate.
After two weeks of treatment, there were no significant differences between the interocular differences in scleral α-SMA protein levels in FDM + vitamin C–treated eyes and those in their fellow eyes (Fig. 6C). In contrast, the α-SMA level was higher in the FDM + quinpirole–treated eyes (1.02 ± 0.10) than in their fellow eyes (0.83 ± 0.17; P = 0.028; Figs. 6C-D). Furthermore, the α-SMA level was higher in FDM + quinpirole–treated eyes (1.02 ± 0.10) than in FDM + vitamin C–treated FDM eyes (0.74 ± 0.26; P = 0.025; Figs. 6C-D).
Discussion
Scleral Hypoxia Due to Decreased ChBP Induces Transdifferentiation of Fibroblasts into Myofibroblasts, Enabling ECM Remodeling and the Onset of Myopia
Choroidal structure and vasculature are critical factors that are closely linked with changes in scleral oxygenation and biochemistry, culminating in the changes in eye size and refractive state associated with emmetropization. It is well known that ChT and ChBP are reduced during myopia development.22,25,36,37 In this study, we found a strong correlation between actively decreasing the ChBP via surgical and pharmacological methods and the induction of myopia development. Additionally, we found that reduction of ChBP was correlated with a decrease of ChT, suggesting a potential role of ChBP in altering ChT. Furthermore, quinpirole injections, which increased the magnitude of FDM, also augmented the reductions in ChBP and ChT associated with the development of FDM. Overall, these findings agree with the hypothesis that the development of myopia in guinea pigs is mediated by the reduction of ChBP. The possibility remains, however, that both of these could be caused by other factors. For instance, the effects of arterial transection and adrenergic agonist treatment on ChBP and myopia development could be mediated independently, either by direct responses to the treatments themselves or via actions on other tissues such as the RPE and non-vascular elements of the choroid.
Transection of the temporal ciliary arteries and peribulbar injections of the vasoconstrictor phenylephrine caused reduction of ChBP, induced scleral hypoxia, and increased the level of α-SMA in the sclera. Also, quinpirole injections during myopia induction further augmented these changes. Yuan et al.38 found that scleral levels of α-SMA mRNA and protein were increased in guinea pigs eyes after four weeks of FDM. These findings support an association of scleral myofibroblast transdifferentiation with the development of myopia in guinea pigs. Interestingly, we did not find that scleral α-SMA levels were increased after 2 weeks of treatment with FDM + vitamin C. This may be due to the differences in duration of form deprivation. Also, this suggests that ascorbic acid itself might modulate myopia development in the guinea pig, independently of the drug for which it served as vehicle.39
The role of hypoxia in the cause of various systemic diseases has been well documented. For example, reduced oxygen levels inhibit transdifferentiation of fibroblasts to myofibroblasts and cause a downregulation of α-SMA in wound healing sites.40 In contrast, hypoxia induces transdifferentiation of fibroblasts to myofibroblasts, with increased synthesis of α-SMA and collagen, in congenital heart disease,41 pulmonary artery adventitia,42 and tubulointerstitial fibrosis.43 However, it was also reported that hypoxia increases α-SMA expression and reduces collagen levels in cultured human scleral fibroblasts.14 The contradictory roles of hypoxia in cellular differentiation and ECM production in different tissues demand further identification of the downstream signaling factors causing hypoxia, especially in the sclera, which is a critical tissue for myopia development and a potential target for myopia control.
Choroidal blood flow is a major factor that impacts the oxygenation of the adjacent scleral tissue.18 In the present study, we found that decreased ChBP was associated with scleral hypoxia, as evidenced by the increased intensity of scleral labeling with pimonidazole, a well-established quantitative indicator of hypoxia. These changes were accompanied by increased scleral levels of α-SMA protein. Interestingly, the significant reductions in ChBP, five minutes after transection of the temporal ciliary arteries, disappeared after one week, although scleral hypoxia was still evident at that time. This suggests that even though the choroidal vascular changes are transient, as indicated by the recovery from reduced ChBP shortly after the surgery, the effect on the adjacent tissue is longer lasting. The transient reduction of ChBP might affect the scleral biochemistry by impeding ECM production, rendering the sclera thinner and more distensible, which could eventually result in excessive eye enlargement. This hypothesis is consistent with the observed onset of the myopic shift and the increased AL, which happened one week after the transection of the ciliary arteries.
The impacts of hypoxia on scleral biochemistry and myopia development were consistent with results of our earlier study, which implicated hypoxia as a key modulator of scleral ECM composition and structure during myopia development.14 More specifically, scleral HIF-1α expression, a marker of tissue hypoxia, was upregulated during both FDM and LIM and declined during recovery from myopia. Importantly, treatments that activated the HIF-1α signaling pathway also led to the transdifferentiation of fibroblasts into myofibroblasts. This change was accompanied by reduced synthesis of type I collagen, which contributed to scleral ECM remodeling and subsequent scleral thinning and myopia induction.14 Taken together, these results suggest that decreased ChBP might cause scleral hypoxia; this, in turn, could result in transdifferentiation of scleral myofibroblasts, with an increase in scleral α-SMA expression that enables scleral ECM remodeling, and ultimately, the onset and progression of myopia in guinea pigs.
The Mechanisms of Decreased Choroidal Blood Perfusion During Myopia Development
Ocular growth relies on active visual signals in response to sharp images on the retina; the absence of sufficient exposure to detailed images results in refractive errors.44 Studies in chick models have shown that myopic defocus (images of distant objects focused in front of the retinal photoreceptor cells), induced with a positive lens, results in retarded axial elongation and a hyperopic shift in refraction. On the other hand, hyperopic defocus (images of distant objects focused behind the retina), induced with a negative lens, results in an increase in axial elongation and a myopic shift in refraction. Simultaneously, myopic defocus leads to choroidal thickening, while hyperopic defocus leads to choroidal thinning; furthermore, both the rates of ChT and ocular elongation return to normal after removal of the imposed defocus.19,20 Interestingly, in line with the observations in animal models, studies in young human myopes showed that bidirectional changes in ChT occur in response to short-term imposed hyperopic and myopic defocus.45 Additionally, previous clinical studies found that an accommodative stimulus of 6 D hyperopic defocus produced an acute myopic shift and reductions in ChT and ChBP.46,47 These results suggest that the thickness of the choroid is modulated in response to the location of the image plane with respect to the retina (i.e., that ChT is modulated bidirectionally by the sign of defocus). Furthermore, the time course of these changes in ChT are fast, compared to those of the associated changes in refractive error;48,49 this indicates that choroidal thickness can potentially serve as an early biomarker of refractive change and ocular growth.
In addition to the choroidal thickening and thinning that move the retina towards the image focal plane in response to defocus, the choroid has the capacity to regulate the delivery of bioactive molecules that can be released from the retina, retinal pigment epithelium, and choroid to the sclera.18,50,51 Therefore it is plausible that thicker choroids might impede the transfer of growth-modulatory molecules to the sclera, thereby decreasing the synthesis of scleral matrix and changing its biomechanical properties, and thus slowing the rate of ocular elongation. In contrast, thinner choroids may facilitate the access of molecules to the sclera, accelerate scleral ECM remodeling, and eventually promote myopia development.
Because the choroid is positioned between the retina and the sclera, it can be presumed that this vascular tissue relays defocus signals that could be critical in ocular growth to the adjacent ocular structures. Based on this assumption, the findings of our study suggest that the myopiagenic stimulus from the retina passes through the choroid, resulting in reductions in choroidal thickness and blood flow. In the present study, we obtained experimental support for this hypothesis based on surgical and pharmacological interventions that attenuated ChBP and induced choroidal thinning and scleral hypoxia, followed by excessive axial elongation and myopia progression. Thus our findings support a critical role of ChBP in the development and the progression of myopia in guinea pigs.
Supplementary Material
Acknowledgments
The authors thank Nethrajeith Srinivasalu (The University of Newcastle, NSW), Yue Liu (Center for Eye Disease & Development, School of Optometry, University of California, Berkeley, CA, USA), and William K. Stell (Cumming School of Medicine, University of Calgary) for critical comments and editorial support in manuscript preparation.
Supported by The National Natural Science Foundation of China (82025009, 81970833, 82171094, 81830027, U20A20364), National Key Research and Development Program of China (2019YFC1710204), Key Research and Development Program of Zhejiang Province (2021C03053), and CAMS Innovation Fund for Medical Sciences (2019-I2M-5-048).
Disclosure: X. Zhou, None; S. Zhang, None; F. Yang, None; Y. Yang, None; Q. Huang, None; C. Huang, None; J. Qu, None; X. Zhou, None
References
- 1. Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 2. Holden BA, Fricke TR, Wilson DA, et al.. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 3. Verhoeven VJ, Wong KT, Buitendijk GH, Hofman A, Vingerling JR, Klaver CC.. Visual consequences of refractive errors in the general population. Ophthalmology. 2015; 122: 101–109. [DOI] [PubMed] [Google Scholar]
- 4. Iwase A, Araie M, Tomidokoro A, et al.. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006; 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 5. Cho P, Cheung SW.. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012; 53: 7077–7085. [DOI] [PubMed] [Google Scholar]
- 6. Yam JC, Jiang Y, Tang SM, et al.. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019; 126: 113–124. [DOI] [PubMed] [Google Scholar]
- 7. Wu PC, Chen CT, Chang LC, et al.. Increased Time Outdoors Is Followed by Reversal of the Long-Term Trend to Reduced Visual Acuity in Taiwan Primary School Students. Ophthalmology. 2020; 127: 1462–1469. [DOI] [PubMed] [Google Scholar]
- 8. McBrien NA, Cornell LM, Gentle A.. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001; 42: 2179–2187. [PubMed] [Google Scholar]
- 9. Shen L, You QS, Xu X, et al.. Scleral Thickness in Chinese Eyes. Invest Ophthalmol Vis Sci. 2015; 56: 2720–2727. [DOI] [PubMed] [Google Scholar]
- 10. Rada JA, Shelton S, Norton TT.. The sclera and myopia. Exp Eye Res. 2006; 82: 185–200. [DOI] [PubMed] [Google Scholar]
- 11. McBrien NA, Lawlor P, Gentle A.. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000; 41: 3713–3719. [PubMed] [Google Scholar]
- 12. Rada JA, Nickla DL, Troilo D.. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000; 41: 2050–2058. [PubMed] [Google Scholar]
- 13. Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA.. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003; 278: 16587–16594. [DOI] [PubMed] [Google Scholar]
- 14. Wu H, Chen W, Zhao F, et al.. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci USA. 2018; 115: E7091–E7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo L, Frost MR, He L, Siegwart JT Jr., Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013; 54: 6806–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo L, Frost MR, Siegwart JT Jr., Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014; 20: 1643–1659. [PMC free article] [PubMed] [Google Scholar]
- 17. Tong L, Cui D, Zeng J.. Topical bendazol inhibits experimental myopia progression and decreases the ocular accumulation of HIF-1alpha protein in young rabbits. Ophthalmic Physiol Opt. 2020; 40: 567–576. [DOI] [PubMed] [Google Scholar]
- 18. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallman J, Wildsoet C, Xu A, et al.. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995; 35: 37–50. [DOI] [PubMed] [Google Scholar]
- 20. Wildsoet C, Wallman J.. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35: 1175–1194. [DOI] [PubMed] [Google Scholar]
- 21. Howlett MH, McFadden SA.. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 22. Zhang S, Zhang G, Zhou X, et al.. Changes in Choroidal Thickness and Choroidal Blood Perfusion in Guinea Pig Myopia. Invest Ophthalmol Vis Sci. 2019; 60: 3074–3083. [DOI] [PubMed] [Google Scholar]
- 23. Hung LF, Wallman J, Smith EL 3rd. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000; 41: 1259–1269. [PubMed] [Google Scholar]
- 24. Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF.. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012; 32: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 25. Yang YS, Koh JW.. Choroidal Blood Flow Change in Eyes with High Myopia. Korean J Ophthalmol. 2015; 29: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X, Zhang S, Zhang G, et al.. Increased Choroidal Blood Perfusion Can Inhibit Form Deprivation Myopia in Guinea Pigs. Invest Ophthalmol Vis Sci. 2020; 61: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li D, Liu C, Sun YN, et al.. Targeting choroidal vascular dysfunction via inhibition of circRNA-FoxO1 for prevention and management of myopic pathology. Mol Ther. 2021; 29: 2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang S, Yang J, Reinach PS, et al.. Dopamine Receptor Subtypes Mediate Opposing Effects on Form Deprivation Myopia in Pigmented Guinea Pigs. Invest Ophthalmol Vis Sci. 2018; 59: 4441–4448. [DOI] [PubMed] [Google Scholar]
- 29. Aguilera KY, Brekken RA.. Hypoxia Studies with Pimonidazole in vivo. Bio Protoc. 2014; 4: e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu F, Zhou X, Zhao H, et al.. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: A non-invasive and effective model. Exp Eye Res. 2006; 82: 628–636. [DOI] [PubMed] [Google Scholar]
- 31. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G.. Optical coherence tomography angiography. Prog Retin Eye Res. 2018; 64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang L, Schaeffel F, Zhou X, et al.. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus). Invest Ophthalmol Vis Sci. 2009; 50: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 33. Pan M, Guan Z, Reinach PS, et al.. PPARgamma modulates refractive development and form deprivation myopia in Guinea pigs. Exp Eye Res. 2021; 202: 108332. [DOI] [PubMed] [Google Scholar]
- 34. Yan T, Xiong W, Huang F, et al.. Daily Injection But Not Continuous Infusion of Apomorphine Inhibits Form-Deprivation Myopia in Mice. Invest Ophthalmol Vis Sci. 2015; 56: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 35. Pan M, Jiao S, Reinach PS, et al.. Opposing Effects of PPARalpha Agonism and Antagonism on Refractive Development and Form Deprivation Myopia in Guinea Pigs. Invest Ophthalmol Vis Sci. 2018; 59: 5803–5815. [DOI] [PubMed] [Google Scholar]
- 36. Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, et al.. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Invest Ophthalmol Vis Sci. 2017; 58: 2063–2069. [DOI] [PubMed] [Google Scholar]
- 37. Fitzgerald ME, Wildsoet CF, Reiner A.. Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp Eye Res. 2002; 74: 561–570. [DOI] [PubMed] [Google Scholar]
- 38. Yuan Y, Li M, To CH, et al.. The Role of the RhoA/ROCK Signaling Pathway in Mechanical Strain-Induced Scleral Myofibroblast Differentiation. Invest Ophthalmol Vis Sci. 2018; 59: 3619–3629. [DOI] [PubMed] [Google Scholar]
- 39. Ward AH, Siegwart JT Jr., Frost MR, Norton TT. The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews. Exp Eye Res. 2016; 145: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B.. Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol. 2010; 130: 2818–2827. [DOI] [PubMed] [Google Scholar]
- 41. Zhu Y, Feng Z, Jian Z, Xiao Y.. Long noncoding RNA TUG1 promotes cardiac fibroblast transformation to myofibroblasts via miR29c in chronic hypoxia. Mol Med Rep. 2018; 18: 3451–3460. [DOI] [PubMed] [Google Scholar]
- 42. Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M.. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004; 286: C416–C425. [DOI] [PubMed] [Google Scholar]
- 43. Manotham K, Tanaka T, Matsumoto M, et al.. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004; 65: 871–880. [DOI] [PubMed] [Google Scholar]
- 44. Wallman J, Winawer J.. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 45. Moderiano D, Do M, Hobbs S, et al.. Influence of the time of day on axial length and choroidal thickness changes to hyperopic and myopic defocus in human eyes. Exp Eye Res. 2019; 182: 125–136. [DOI] [PubMed] [Google Scholar]
- 46. Zhao F, Zhang D, Zhou Q, et al.. Scleral HIF-1alpha is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine. 2020; 57: 102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woodman-Pieterse EC, Read SA, Collins MJ, Alonso-Caneiro D. Regional Changes in Choroidal Thickness Associated With Accommodation. Invest Ophthalmol Vis Sci. 2015; 56: 6414–6422. [DOI] [PubMed] [Google Scholar]
- 48. Kee CS, Marzani D, Wallman J.. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001; 42: 575–583. [PubMed] [Google Scholar]
- 49. Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007; 84: 951–959. [DOI] [PubMed] [Google Scholar]
- 50. Summers JA. The choroid as a sclera growth regulator. Exp Eye Res. 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Wildsoet CF.. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog Mol Biol Transl Sci. 2015; 134: 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.