Abstract
Objective:
To identify the prevalence and characteristics of people living with dementia (PLWD) lost to follow-up (LTFU) from a specialized dementia care clinic and to understand factors influencing patient follow-up status.
Methods:
We conducted a retrospective chart review of PLWD seen at a dementia care clinic 2012–2017 who were deceased as of 2018 (n = 746). Participants were evaluated for follow-up status at the time of death. Generalized linear regression was used to analyze demographic and diagnostic characteristics by follow-up status. Text extracted from participant medical records was analyzed using qualitative content analysis to identify reasons patients became LTFU.
Results:
Among PLWD seen at a dementia care clinic, 42% became LTFU before death, 39% of whom had chart documentation describing reasons for loss to follow-up. Increased rates of LTFU were associated with female sex (risk ratio 1.27, [95% confidence interval 1.09–1.49]; p = 0.003), educational attainment of high school or less (1.34, [1.13–1.61]; p = 0.001), and death in a long-term care facility (1.46, [1.19–1.80]; p = 0.003). Commonly documented reasons for not returning for care at the clinic included switching care to another provider (42%), logistical difficulty accessing care (26%), patient-family decision to discontinue care (24%), and functional challenges in accessing care (23%).
Conclusions:
PLWD are LTFU from specialized memory care at high rates. Attention to care coordination, patient–provider communication, and integrated use of alternative care models such as telehealth are potential strategies to improve care.
Keywords: access to care, ambulatory, care coordination, continuity of care, dementia, end-of-life, lost to follow-up, memory care, mixed methods, outpatient
1 |. INTRODUCTION
Dementia care clinics and other specialist care models have been recommended as a path to quality dementia care in outpatient settings.1–3 In the United States, these centers provide interdisciplinary care for a range of dementia syndromes and are often associated with large urban health centers serving wide catchment areas.4 Specialized care programs are associated with earlier dementia diagnosis,5 decreased nursing home placements,6 lower hospitalization rates,7 and reduced caregiver burden.8 Additionally, people living with dementia (PLWD) report improved quality of life in the 6 months after referral to dementia care clinics3,9 and improved behavioral, psychological, and depressive symptoms at 1 year.8
However, despite support for the positive benefits of specialized outpatient dementia care, visit frequency tends to decrease toward the end of life.10 While some decline in visit frequency is due to planned transition to long-term care facilities,11 it is unclear what proportion is driven by loss to follow-up (LTFU). This is especially relevant given that decreased care continuity for PLWD who are living at home is associated with increased hospitalization rates, emergency department visits, and healthcare spending.12 As a result, optimizing care continuity in a dementia care practice is a potential target area for interventions to improve care. However, there is a gap in the literature regarding the incidence of LTFU and factors associated with LTFU from outpatient dementia care.
This study aims to identify the prevalence and characteristics of people living with dementia (PLWD) LTFU from a specialized dementia care clinic in the United States and to understand factors contributing to LTFU. Understanding the breadth and drivers of this issue will provide important insights to inform future interventions to help PLWD and their caregivers’ access care as needed throughout the disease trajectory.
2 |. MATERIALS AND METHODS
2.1 |. Design
In this study, we examined patient characteristics by follow-up status using a retrospective cohort obtained through chart review data of deceased patients with a diagnosis of dementia seen at the University of California, San Francisco’s Memory and Aging Center (MAC).
Follow-up status was determined from the documented follow-up plan at the final in-person clinic visit. Participants who did not return to clinic 3 or more months after their recommended return date were classified as LTFU. By relying on the recommended follow-up plan at the last clinic visit, we were able to more accurately distinguish between planned discontinuation of care versus a deviation from clinician-recommended care.13 This study was approved by the institution’s review board. Data available on request due to privacy/ethical restrictions.
2.2 |. Study participants
To evaluate the full trajectory of dementia care at the end of life, we conducted a chart review of all patients with a diagnosed dementia syndrome seen at the MAC at least once between 2012 and 2017 and who were deceased as of year-end 2018. Deceased status was determined through documentation in the electronic medical record (EMR), clinic research records, or the California Department of Public Health and Vital Records death certificate database.14 Dementia diagnoses were determined from the final documented clinic diagnosis and were reviewed and verified by a behavioral neurologist (GN). For analytical and comparative purposes, diagnoses were grouped by common symptomatology into the following categories: (1) Alzheimer’s disease, (2) Parkinson’s disease dementia/dementia with Lewy bodies, (3) diagnoses that impair primarily motor function including corticobasal syndrome and progressive supranuclear palsy, (4) diagnoses that primarily impair behavior including frontotemporal dementia, and (5) diagnoses that primarily impair language including primary progressive aphasias.
Of the 6254 unique MAC patients seen within window, 3115 were diagnosed with a dementia syndrome and 750 were deceased as of year-end 2018. Four additional participants were excluded due to special privacy restrictions on their EMR. A total of 746 participants met inclusion criteria (Figure 1).
FIGURE 1.
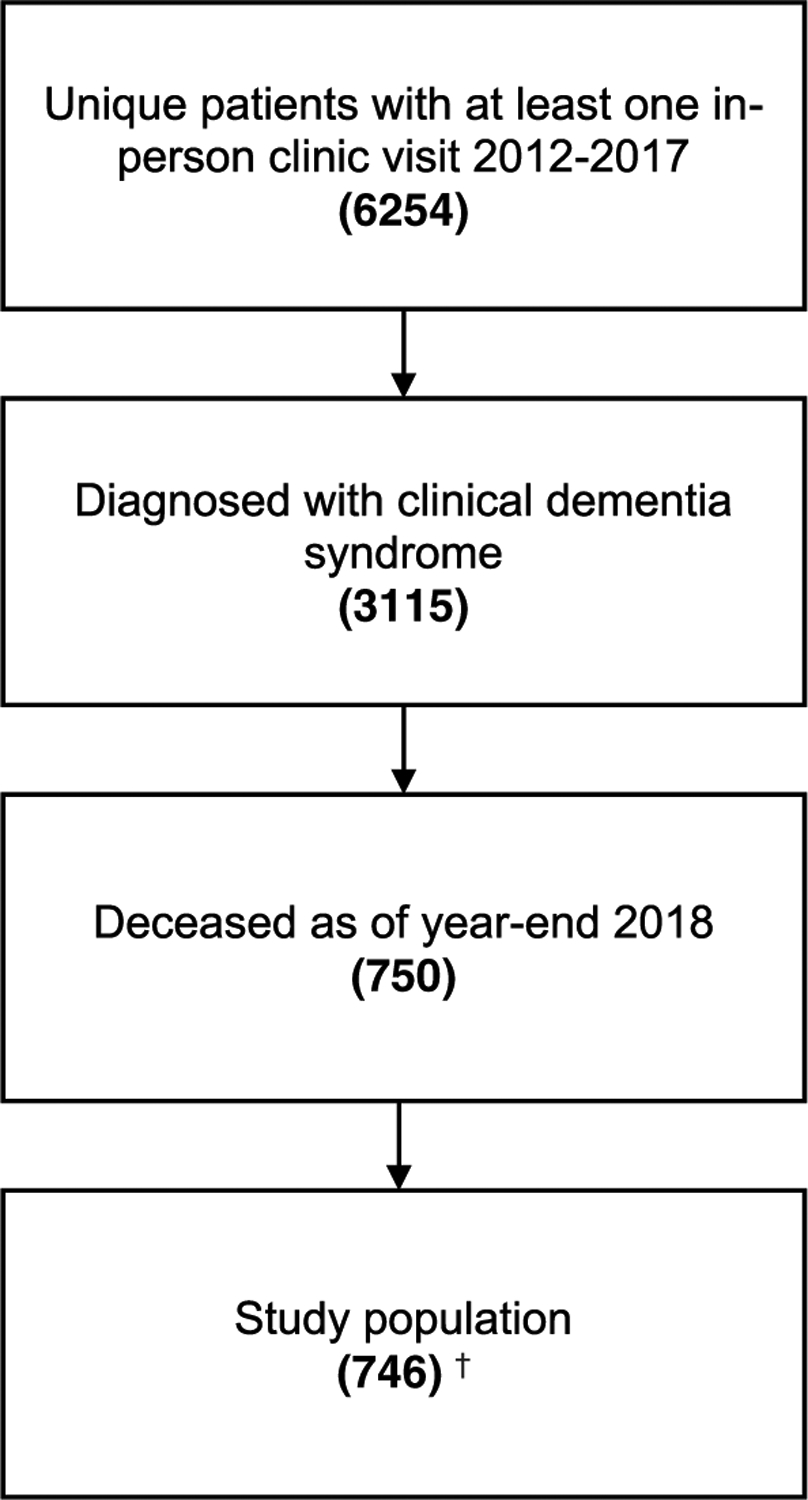
Sample selection
2.3 |. Data collection
We abstracted data on patient demographics, diagnostic information, healthcare utilization, and clinic follow-up status from patient EMRs (NB). To ensure accuracy, 5% of data were independently double coded and reviewed for discrepancies (NB, TR, BP). Appropriate values were determined through group consensus with less than 2% of data requiring correction.
For participants who met criteria for LTFU, we reviewed notes from all clinic encounters after and including the final visit note and abstracted any text related to the effort to schedule continuing dementia care.
2.4 |. Statistical analysis
We used descriptive statistics to summarize the baseline characteristics of the study population, proportion of participants LTFU, and to contextualize the quality of interactions with the MAC (number of total in-person clinic visits, as well as emails, phone encounters, home, and telehealth visits with the MAC after the final visit). We used generalized linear regression with a log link and Poisson distribution with robust variance estimator to investigate the relationship between patient characteristics and follow-up status, an established method of analyzing relative risk ratios using retrospective cohort data.15,16 Outcome variables included (1) whether participants had a planned clinic follow-up at their final MAC visit and (2) whether participants were LTFU or continued with follow-up visits until death. Predictor characteristics included clinical dementia diagnosis, age and self-reported sex, race/ethnicity, education, marital status, and primary language. Driving time to clinic was estimated using zip code data and calculated via Google Maps using a uniform arrival time.17 Associations were tested in both a bivariate and a multivariate model that adjusted for age, sex, education, and primary dementia diagnosis. A subgroup analysis comparing patients LTFU with and without documented reasons for LTFU was conducted using the same methodology. Results are presented as risk ratios (RR) and adjusted risk ratios (ARR) with corresponding 95% confidence intervals (CI). Statistical analyses were conducted using Stata v.16 (Stata Corp.).
2.5 |. Qualitative analysis
For participants LTFU, we analyzed text extracted from the patients’ medical charts using inductive and deductive qualitative content analysis to identify reasons for LTFU. Data were abstracted from chart documentation of face-to-face and telephone encounters with patient-families by clinic staff and clinicians. Excerpts were reviewed and discussed over multiple meetings to develop thematic categories operationalized as codes (NB, SG, SM). These included deductive codes based on existing scholarship (e.g., financial or insurance difficulties) and inductive codes derived from the team’s preliminary review of the data (e.g., functional challenges).18,19 We further refined and developed this framework through application and discussion until a high degree of consensus was reached (NB, SM). Coding of the full dataset was performed by one team member (NB) using Microsoft Excel (version 16.0); 25% of the data was independently double coded to ensure validity (SM) with interrater reliability over 95%. For each element of text, coders applied all relevant qualitative codes.
3 |. RESULTS
Demographic and clinical characteristics of participants are described in Table 1. Most participants self-identified as white (71%), 51% identified as female, and 66% of participants reported at least some college education. Of the 746 deceased PLWD first seen at the MAC between 2012 and 2017, 42% became LTFU before death (Figure 2). One-way driving time from a patient’s neighborhood (estimated by zip code) to the clinic was a median of 30 min, with an average of 65 min. The median driving distance one way was 24.6 miles. Individuals became LTFU a median of 0.99 years and an average of 1.28 years prior to death (25th, 75th percentiles: 0.48, 1.82 years, respectively).
TABLE 1.
Participant characteristics
| Total | Continued follow-ups | Lost to follow-up | No planned follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| N. | % | No. | % | No. | % | No. | % | |
| Agea | ||||||||
| Under 65 | 89 | 12% | 25 | 28% | 38 | 43% | 26 | 29% |
| 65–74 | 179 | 24% | 58 | 32% | 83 | 46% | 38 | 21% |
| 75–84 | 253 | 34% | 100 | 40% | 107 | 42% | 46 | 18% |
| 85+ | 225 | 30% | 86 | 38% | 86 | 38% | 53 | 24% |
| Sex | ||||||||
| Male | 365 | 49% | 155 | 42% | 143 | 39% | 67 | 18% |
| Female | 381 | 51% | 114 | 30% | 171 | 45% | 96 | 25% |
| Race/ethnicity | ||||||||
| White, non-Hispanic | 513 | 69% | 186 | 36% | 223 | 43% | 104 | 20% |
| Asian | 78 | 11% | 34 | 44% | 28 | 36% | 16 | 21% |
| Black/African-American | 28 | 4% | 11 | 39% | 11 | 39% | 6 | 21% |
| Hispanic/Latinx | 39 | 5% | 14 | 36% | 13 | 33% | 12 | 31% |
| Other | 44 | 6% | 12 | 27% | 19 | 43% | 13 | 30% |
| Unknown/not documentedb | 44 | 6% | 12 | 27% | 20 | 45% | 12 | 27% |
| Education | ||||||||
| High school or less | 167 | 22% | 42 | 25% | 83 | 50% | 42 | 25% |
| College or some college | 294 | 39% | 120 | 41% | 118 | 40% | 56 | 19% |
| Graduate or professional | 200 | 27% | 75 | 38% | 82 | 41% | 43 | 22% |
| Unknown/not documented | 85 | 11% | 32 | 38% | 31 | 36% | 22 | 26% |
| Primary dementia diagnosisc | ||||||||
| Alzheimer’s disease | 387 | 52% | 140 | 36% | 164 | 42% | 83 | 21% |
| Dementia with Lewy bodies | 126 | 17% | 54 | 43% | 51 | 40% | 21 | 17% |
| Other motor-based dementia | 76 | 10% | 30 | 39% | 27 | 36% | 19 | 25% |
| Frontotemporal dementia | 42 | 6% | 13 | 31% | 21 | 50% | 8 | 19% |
| Language-based dementia | 36 | 5% | 11 | 31% | 15 | 42% | 10 | 28% |
| Other | 79 | 11% | 21 | 27% | 36 | 46% | 22 | 28% |
| Location of death | ||||||||
| Single family residence | 210 | 28% | 84 | 40% | 74 | 35% | 52 | 25% |
| Long-term care facility | 253 | 34% | 67 | 26% | 137 | 54% | 49 | 19% |
| Hospital/acute care facility | 139 | 19% | 66 | 47% | 51 | 37% | 22 | 16% |
| Other | 7 | 1% | 4 | 57% | 1 | 14% | 2 | 29% |
| Unknown/not documented | 137 | 18% | 48 | 35% | 51 | 37% | 38 | 28% |
| Primary language | ||||||||
| English | 650 | 87% | 232 | 36% | 274 | 42% | 144 | 22% |
| Other | 93 | 13% | 36 | 39% | 39 | 42% | 18 | 19% |
| Unknown/not documented | 3 | 0% | 1 | 33% | 1 | 33% | 1 | 33% |
| Total no. MAC clinic visits | ||||||||
| One | 200 | 27% | 51 | 26% | 95 | 48% | 54 | 27% |
| Two | 110 | 15% | 32 | 29% | 57 | 52% | 21 | 19% |
| Three | 84 | 11% | 29 | 35% | 40 | 48% | 15 | 18% |
| Four | 64 | 9% | 27 | 42% | 24 | 38% | 13 | 20% |
| Five or more | 288 | 39% | 130 | 45% | 98 | 34% | 60 | 21% |
| Driving distance (min) | ||||||||
| <30 | 338 | 45% | 134 | 40% | 129 | 38% | 75 | 22% |
| 30–60 | 209 | 28% | 71 | 34% | 93 | 44% | 45 | 22% |
| 60–120 | 112 | 15% | 42 | 38% | 47 | 42% | 23 | 21% |
| 120–180 | 28 | 4% | 12 | 43% | 10 | 36% | 6 | 21% |
| 180+ | 59 | 8% | 10 | 17% | 35 | 59% | 14 | 24% |
Note: Percentages of total reflect share of columns; other percentages reflect share of row.
Age at final clinic visit to the UCSF Memory and Aging Center.
Information not documented in medical record.
Alzheimer’s dementias: Alzheimer’s disease (AD), posterior cortical atrophy; Lewy body dementias (LBD): LBD, LBD-AD, Parkinson’s disease dementia; motor-based dementias: corticobasal syndrome, progressive supranuclear palsy; language-based dementias: semantic dementia, logopenic and non-fluent primary progressive aphasia.
Determined from United States Census demographic data based on participant’s zip code.
FIGURE 2.
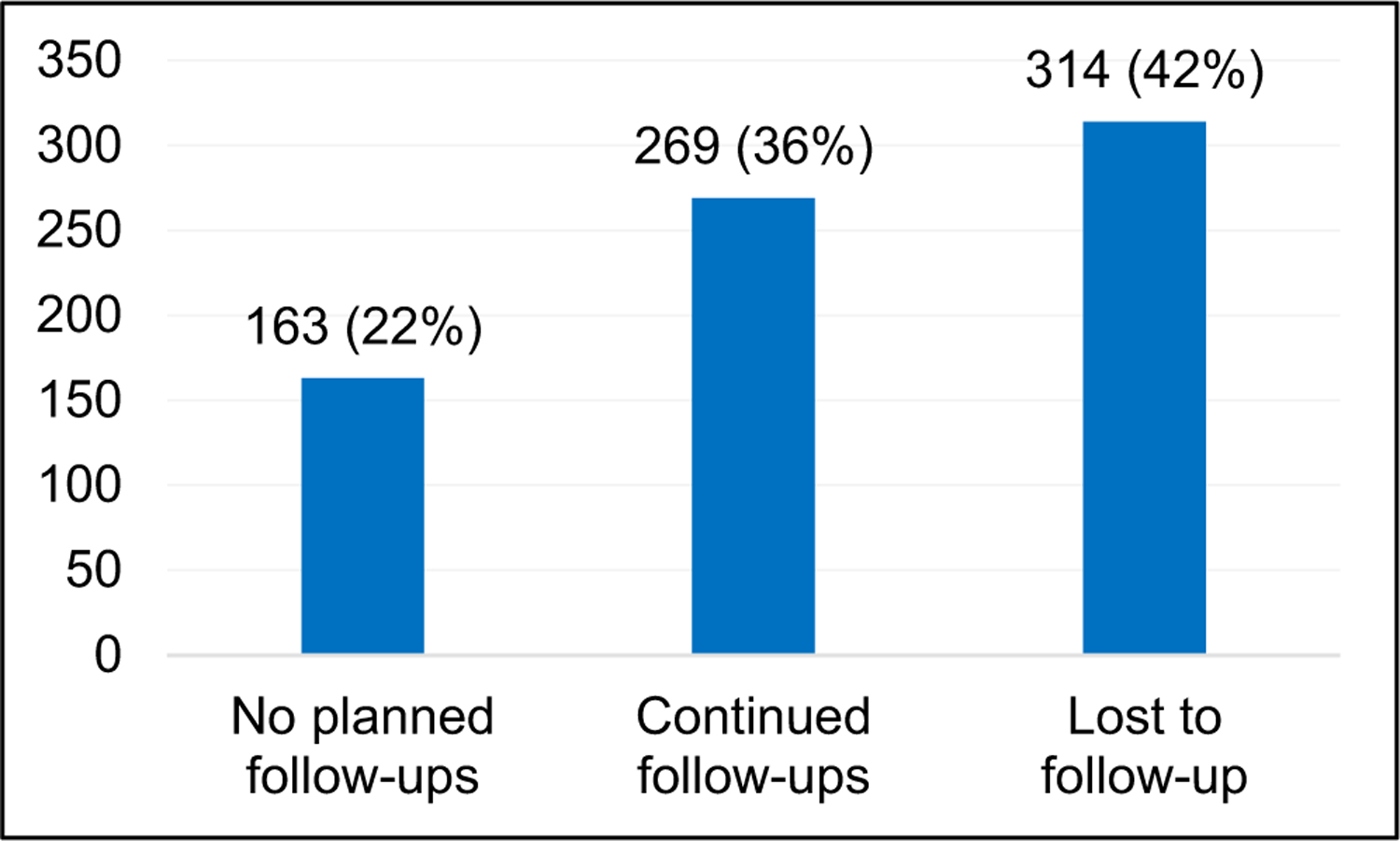
Participants by final follow-up status
Overall, PLWD had a median of three visits before death, though patients had as many as 30 visits and as few as one. A two-sample t-test showed that PLWD who were LTFU had fewer total visits on average than those who continued with follow-ups (4.4 vs. 5.8 mean visits, p = 0.0001); median visits were three and four, respectively. The range of visits was similar for those who continued follow-ups versus those LTFU, at 1–30 and 1–28 total visits, respectively.
Of patients LTFU, 232 (74%) had at least one email or phone contact with the clinic after their final-in person visit, and 62 (20%) had five or more phone or email contacts. Eleven patients characterized as LTFU from in-person visits (4%) received a telehealth visit with a MAC provider after their final in-clinic visit and five patients (2%) received their final MAC encounter as home visits.
Table 2 shows the association between participant characteristics and loss to follow-up. Women with dementia were 1.27 times more likely to become LTFU than men (44.9% vs. 39.2%, p = 0.003) and individuals without college education were 1.34 times more likely to become LTFU than people with some college or more (49.7% vs. 40.5%, p = 0.001). PLWD who died in long-term care facilities were 1.46 times more likely to be LTFU than those who died at home (54.2% vs. 35.2%, p = 0.003). Patients with a driving distance of 3 h or more were 1.69 times more likely to become LTFU than those with a commute of 30 min or less (59.3% vs. 38.2%, p < 0.001).
TABLE 2.
Relative risk of loss to follow-up by patient characteristic
| Variables | Risk ratio (95% CI), binary | Adjusted risk ratio (95% CI), multivariatea |
|---|---|---|
| Age | ||
| Under 65 | Ref. | Ref. |
| 75–84 | 0.86 (0.67 – 1.09) | 0.91 (0.70 – 1.16) |
| 65–74 | 0.98 (0.77–1.24) | 1.02 (0.79–1.32) |
| 85+ | 0.83 (0.65–1.06) | 0.84 (0.64–1.11) |
| Sex | ||
| Male | Ref. | |
| Female | 1.25*** (1.07–1.46) | 1.27*** (1.09–1.49) |
| Race/ethnicity | ||
| White, non-Hispanic | Ref. | Ref. |
| Asian | 0.83 (0.62–1.11) | 0.90 (0.67–1.21) |
| Black/African-American | 0.88 (0.59–1.32) | 0.75 (0.48–1.18) |
| Hispanic/Latinx | 0.92 (0.60–1.41) | 0.89 (0.56–1.40) |
| Education | ||
| College or some college | Ref. | Ref. |
| High school or less | 1.34*** (1.12–1.60) | 1.34*** (1.13–1.61) |
| Graduate or professional | 1.05 (0.86–1.28) | 1.08 (0.89–1.31) |
| Primary dementia diagnosis | ||
| Alzheimer’s dementia | Ref. | Ref. |
| Dementia with Lewy bodies | 0.90 (0.72–1.12) | 0.88 (0.70–1.10) |
| Other motor-based dementia | 0.88 (0.66–1.18) | 0.80 (0.57–1.12) |
| Frontotemporal dementia | 1.14 (0.86–1.52) | 1.08 (0.80–1.46) |
| Language-based dementia | 1.07 (0.76–1.51) | 1.07 (0.74–1.55) |
| Location of death | ||
| Single family residence | Ref. | Ref. |
| Long-term care facility | 1.43*** (1.18–1.74) | 1.46*** (1.19–1.80) |
| Acute care facility | 0.93 (0.71–1.21) | 0.95 (0.72–1.25) |
| Preferred language | ||
| Non-English | Ref. | Ref. |
| English | 1.10 (0.94–1.29) | 1.03 (0.80–1.32) |
| Driving distance (min) | ||
| <30 | Ref. | Ref. |
| 30–60 | 1.16 (0.96–1.39) | 1.20* (0.99–1.45) |
| 60–1.20 | 1.08 (0.85–1.36) | 1.08 (0.84–1.39) |
| 120–1.80 | 0.93 (0.58–1.49) | 0.91 (0.54–1.52) |
| 180+ | 1.59*** (1.30–1.93) | 1.69*** (1.36–2.10) |
Note: Confidence Interval (95%) in parentheses.
Multivariate model included age, sex, education, and primary dementia diagnosis.
p < 0.01,
p < 0.05,
p < 0.1.
Of the 314 participants identified as LTFU, 39% (n = 123) had documentation indicating why they did not return to clinic. A comparative subgroup analysis showed that those who live 30–60 min driving distance from the MAC and were more likely to have a documented reason for loss to follow-up than those living less than 30 min away who were LTFU (1.52, p = 0.025). Similarly, people with some college education were more likely to have a reason documented than those with less education (1.61, p = 0.018). There were no significant differences between patients LTFU who did and did not give an explanation with respect to age, race, sex, language, primary dementia diagnosis, or location of death.
We identified multiple themes in the chart notes on follow-up care planning. A description of all identified themes and example quotes are provided in Table 3. Percentages describing results of content analysis are given relative to total participants LTFU who had a documented reason for loss to follow-up (n = 123).
TABLE 3.
Reasons lost to follow-up
| Reasonsa | Definitions & examples | Quotes |
|---|---|---|
| Transferred care to another provider/facility | Note indicates the patient plans to continue follow-ups with outside care team after final MAC visit This can include other neurologist, PCP, or care at a facility if indicated as replacement for care provided by MAC. | “Patient’s wife called to cancel his appt for [date]. Per [wife] he will be moving and continuing his care at [long-term care facility].” -Provider note |
| Patient on hospice | Patient/caregiver canceled follow-up MAC appointment and cited being on or transitioning to hospice in the context of discontinuing MAC care. | “Received a follow-up call from [Caregiver, wife] regarding annual follow with [MAC Provider]. Wife states that patient has been immobile and in hospice for the last 6 months, and will not be returning for further work up.” -Provider note |
| Logistical challenges (distance to clinic, difficult transfer from facility) | Patient is not continuing care with MAC because it is difficult or inconvenient for some reason that is not related to the patient’s function/condition, for example, distance to clinic, location, or having moved away. | “Patient’s husband called to cancel appt for tomorrow. He said it’s very hard to get to [clinic] from where they live and will not reschedule at [this] time.” -Provider note |
| Financial or insurance difficulties | Patient/caregiver not following up with MAC due to factors related to finances or insurance matters. For example, insurance change, cost of visit, financial hardship. | “Spoke to [Caregiver] this morning regarding scheduling a follow-up for [Patient]. Unfortunately the patient has switched over to [insurance provider] for health coverage and will not be able to return to [clinic], since [their insurance] only covers in network providers.” -Provider note |
| Functional challenges (mobility, behavior) | Patient/caregiver not following up with MAC due to difficulty having to do with the patient’s behavior, mobility, or function(s). | “[Wife] would like to cancel the follow-up appointment scheduled for [date]… [She] will not be able to bring him to clinic because she is no longer able to transfer him from a wheelchair.” -Provider note |
| Patient/family decision-assessed follow-up to be not necessary, desirable or worth it | Patient/caregiver indicates a judgment or preference that informs their decision to not continue at the MAC. | “I feel like we have learned all we are going to learn from someone of your expertise, [MAC Provider]. Basically, with each visit, I learn that the disease is running its normal course of events. No news.” -Caregiver email |
Multivariate model included age, sex, education, and primary dementia diagnosis.
3.1 |. Transfer of care to another provider, facility, or hospice
The most frequently documented theme regarding discontinuation of care at the MAC was transfer of care to another provider or facility (42%). This was often stated as a desire to continue care with a local physician:
[Caregiver] was very impressed and happy with our service here but will follow-up with local physician. – Provider note from caregiver calling in response to scheduling reminder
Another common reason patients left care was following a transition to a residential care facility. Fourteen (11%) specifically referenced transition to hospice in the context of discontinuing care with the MAC.
While many patient-families did not provide clarification as to why they were transferring care, several (39%) went onto describe increasing logistical and/or functional challenges as additional contributing factors.
3.2 |. Logistical challenges
Difficulty with logistical coordination of visits at the MAC was a common reason that participants became LTFU (26%). This usually came up in the context of driving distance. Other instances included difficulty coordinating an appointment time and travel with caregiver work schedules and caregiver difficulty in getting the patient to the clinic:
Per [Caregiver] it is very difficult for the patient to come to the city… they decided they do their best to care for the patient and will not need to schedule a follow-up visit here at this time. – Provider note from caregiver calling in response to scheduling reminder
Additionally, six participants (5%) cited insurance issues as a specific reason for discontinuing care:
Daughter called and said due to insurance issues, patient will be seen at [other network] going forward. – Provider note from caregiver calling to cancel visit
3.3 |. Functional challenges
When difficulty making clinic visits was specifically associated with the patient’s deteriorating health or functional status, or when patients and families cited advanced illness as a reason to discontinue visits, we classified the reason for loss to follow-up as a functional rather than logistical challenge (23%):
[Caregiver] called and stated that family does not wish to proceed with any further care for the patient because patient is too ill to travel. – Provider note from caregiver calling to cancel visit
Patient is hard to transport and is too advanced according to caregiver for any further care. Does not wish to reschedule. – Provider note from caregiver calling to cancel visit
3.4 |. Patient or family preference to discontinue follow-up
Twenty-four percent of patients and families indicated that the discontinuation of care was based on family preferences and priorities rather than something hindering their continued care (e.g., functional decline or logistics). For some, this decision was in the context of increasing illness burden:
Since [my father’s] first visit with you his overall health condition has deteriorated precipitously… we are trying to reduce the amount of times of seeing another doctor that is for longer term therapy, rather than shorter one. I would like to cease his care with you. – Caregiver email to cancel visit
For others, the decision to discontinue care was tied to the idea that continued follow-up would have minimal additional value:
[Daughter] feels that her father does not need to follow up w/ MAC [as] there’s no cure for her dad. – Provider note from caregiver calling to cancel visit
4 |. DISCUSSION
Literature on patient and caregiver follow-up with formal dementia care services is limited. Existing research focuses on access to and utilization of various healthcare services but does not address continuation of specialty care.20–22 This study directly explores factors associated with loss to follow-up from an outpatient dementia care center.
Results highlight the importance of this phenomenon in later-stage dementia care: over 40% of patients at the clinic were ultimately LTFU.
Our data show that patients who were LTFU had slightly fewer clinic visits overall. However, the average number of visits was greater than four, with some patients having as many as 28 visits before becoming LTFU. Therefore, high rates of LTFU are not necessarily driven by patients with one or two encounters—LTFU also occurs among patients who have established care over several visits. Similarly, a long visit history does not necessarily convey protection from becoming LTFU.
Women with dementia were also more likely to be LTFU than men. As approximately two-thirds of informal dementia caregivers are women,23 it is possible that women who are care-receivers are less likely to have caregivers well positioned to support their continued care in a dementia clinic. This is supported by a study of individuals with Parkinson disease, which found that informal care-giving resources are lower for woman than men.24
Individuals without college education were more likely to be LTFU than those with some college education. There is extensive documentation supporting a strong positive association between education and income.25,26 Individuals with less financial resources may also have less access to paid caregiver support, and informal caregivers may have reduced access to transportation or less flexibility to take time off from work to accommodate memory care visits, all of which could contribute to a greater risk of loss to follow-up. Although few individuals in this study directly cited financial issues as a reason for discontinuing care, this trend suggests access to fewer resources and the resulting burden on patient-families is a relevant factor influencing loss to follow-up.
Patients with less education were also less likely to provide a reason for becoming LTFU. Possible explanations include reduced comfort communicating care challenges with the healthcare team or higher intrinsic rates of reasons that were sensitive or difficult to disclose, such as financial challenges.
Patients who died in long-term care facilities were more likely to be LTFU than those who died at home. This is likely driven by decreased utilization of specialty care upon transition to long-term care facilities.27
Approximately a quarter of patient-families cited functional challenges as a reason for discontinuing follow-ups; a quarter cited logistical issues such as transportation. Most patients likely traveled by car as the median travel distance was over 20 miles each way. Interestingly, although patients with a driving distance to clinic of three or more hours were more likely to become LTFU than those with a commute of less than 30 min, there were no significant differences observed in LTFU rates for patients with driving times in between these ranges. suggesting that specialty dementia care is relatively unaffected by all but the longest travel times. This likely reflects differences in access to local dementia specialty care and poses a particular challenge for patients in rural areas without local specialty care options.
Despite stated difficulties coming to clinic, few patients received follow-up via telehealth or home visits. Many patients had informal follow-ups by phone or email, however, with 20% receiving five or more contacts. This raises an important question regarding the role other models of care delivery can play in mitigating factors that drive high loss to follow-up, particularly for patients with advanced illness who remain in the community. Studies show that more than 60% of people with moderately severe dementia live at home28 and most PLWD and their caregivers express a desire to remain at home given appropriate support.29,30 Home-based medical care (HBMC) often serve a disproportionately high number of patients with a diagnosis of dementia.31 Consistent access to HBMC would alleviate transportation and functional challenges. The transition to virtual visits has the potential to overcome transportation issues, limited geographic options, travel time, and other similar challenges posed by in-person care delivery.27,28 While telemedicine might be an avenue for maintaining continuity of care for PLWD who live at home, barriers related to Internet connectivity or video-capable devices suggest that telemedicine may not be sufficient for all patients.32–34 Individuals with low socioeconomic status or low educational attainment are particularly vulnerable in this context, as they are both more likely to be LTFU from specialty dementia care and known to face greater challenges with receiving care via telehealth.35
Research has shown that caregivers’ perceived lack of need is an important driver of the non-use of services.36 Additionally, caregiver satisfaction with outpatient memory care has been shown to decline over time.37 Our results indicate these factors also contribute to loss to follow-up: a frequently cited reason for loss to follow-up was a patient or caregiver decision to discontinue care due to perception that additional follow-up was unnecessary or not worth pursuing. This highlights the difficulty in communicating the value of continued memory care visits to patient-families and the importance of communication between the patient-family and their provider. Additionally, as many patients ultimately transfer their care locally or to a residential facility, greater alignment between providers and patient/families earlier in the patient’s illness trajectory may allow the clinic to better prepare and later support the patient-family through their care transition.
All patients who were classified as LTFU in this study were recommended to return to clinic for follow-up at the time of their final visit. Although circumstances change as patient function declines over time, the non-functional factors endorsed by participants as reasons LTFU suggest that illness progression alone is not responsible for the high rate of LTFU seen at this outpatient dementia care center. These findings suggest an opportunity to improve care via better upstream patient–provider communication around patient-families’ ability and willingness to continue care in an outpatient in-person setting. This also highlights an opportunity to provide structured guidance via telehealth by a member of the care team. An example model is such as Care Ecosystem, which is a telephone and Internet-based supportive care intervention that has been shown to improve PLWD quality of life and caregiver burden, depression and self-efficacy.38
This study has several limitations to consider. The use of retrospective EMR data resulted in missing observations for several key variables where data was not available. MAC patients are more likely to self-identify as white, are more educated, and live in wealthier areas than the general population. Thus, the factors cited and the distribution in the sample may not match those of patient groups in dissimilar settings. Given the relative privilege of the study population, however, it is striking how often patient-families reported functional–logistical challenges as reasons for discontinuing care. This highlights the incredible challenge these issues present to patient-families, even for those who are relatively well resourced.
UCSF’s status as prominent research institution may also mediate potential discrepancies in follow-up status for rarer or more costly dementia syndromes like frontotemporal dementia (FTD).39 However, UCSF offered some key advantages as a study site: it allowed for inclusion of a broad range of rare diagnoses that would have been difficult to include in other settings, and allowed for inclusion of patients residing in both urban and rural areas given UCSF draws patients from a large area of California and its neighboring states.
This study addresses a key gap in the literature and is an important first step to identifying reasons for loss to follow-up. However, additional research is needed. Only 39% of patients LTFU had documented factors influencing their discontinuation of care. Inconsistency in documentation or clinic follow-up regarding why patients discontinued care may not accurately reflect the breadth and complexities of all factors associated with loss to follow-up. Patients who lived close to the center (within 30 min) and those who had lower education were less likely to have a documented reason for loss to follow-up, as well, suggesting this work may not capture the full range of factors impacting loss to follow-up within these populations. Similarly, while we found that many of these issues overlap, the chart notes may not have captured the full breadth of factors at play for each individual patient. Loss to follow-up related to patient or family perceptions that it was not worth pursuing additional follow-up may be meaningfully undercounted if these individuals felt a desire to avoid upsetting clinic staff with negative feedback. Finally, while most documentation on reasons for discontinued care cited conversations with caregivers, notes based on discussions with PLWD may not reflect full or accurate reasoning due to cognitive impairment. A follow-up study that uses qualitative interviews to directly explore patients and families’ reasons for loss to follow-up would help build on and further elaborate the themes identified in this effort.
The high rates of loss to follow-up from specialty memory care and factors influencing loss to follow-up identified in this work underscore the importance of early, strong, and sustained patient–provider communication that is attentive to patient and family goals of care. Exploring and integrating alternative models of dementia care delivery offers an opportunity to reduce loss to follow-up, ease care transitions, and improve care. Although this work draws insights from the specific context of American specialty dementia care, it has broad implications on the ways in which we deliver care to patients. Providers, administrators, and health policy-makers need to think critically about how to adapt our care models to adjust to the evolving needs of patients over the natural course of their illness, particularly those with progressive or incurable conditions.
Key points.
Among people living with dementia seen at a specialized memory care center, 42% became lost to follow-up (LTFU) before death.
LTFU rates were higher among women and patients with lower educational attainment.
Commonly documented reasons for not returning for care at the clinic included switching care to another provider (42%), logistical difficulty accessing care (26%), patient-family decision to discontinue care (24%), and functional challenges in accessing care (23%).
Attention to care coordination, patient–provider communication, and integrated use of alternative care models such as telehealth or home-based medical care are potential strategies to improve care.
ACKNOWLEDGMENTS
Research reported in this publication was conducted with support from the Global Brain Health Institute. All analyses, interpretations, and conclusions reached through this project are the sole responsibility of the authors. The authors thank Kanan Patel for her assistance in data acquisition.
Funding information
Global Brain Health Institute
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Morgan DG, Crossley M, Kirk A, et al. Improving access to dementia care: development and evaluation of a rural and remote memory clinic. Aging Ment Heal. 2009;13:17–30. Published online. doi: 10.1080/13607860802154432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley McCarten J, Anderson P, Kuskowski MA, McPherson SE, Borson S, Dysken MW. Finding dementia in primary care: the results of a clinical demonstration project. J Am Geriatr Soc. 2012;60: 210–217. Published online. doi: 10.1111/j.1532-5415.2011.03841.x [DOI] [PubMed] [Google Scholar]
- 3.Park MH, Smith SC, Chrysanthaki T, et al. Change in Health-related Quality of Life after referral to memory assessment services. Alzheimer Dis Assoc Disord. 2017;31:192–199. Published online. doi: 10.1097/WAD.0000000000000190 [DOI] [PubMed] [Google Scholar]
- 4.Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199–206. doi: 10.1136/pgmj.2005.040592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2):1–8. doi: 10.1136/bmjopen-2016-011146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennings LA, Laffan AM, Schlissel AC, et al. Health care utilization and cost outcomes of a comprehensive dementia care program for Medicare beneficiaries. JAMA Intern Med. 2019;179(2):161–166. doi: 10.1001/jamainternmed.2018.5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Heal. 2011;15(1):13–22. doi: 10.1080/13607863.2010.496445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuben DB, Tan ZS, Romero T, Wenger NS, Keeler E, Jennings LA. Patient and caregiver benefit from a comprehensive dementia care program: 1-year results from the UCLA Alzheimer’s and Dementia Care Program. J Am Geriatr Soc. 2019;67(11):2267–2273. doi: 10.1111/jgs.16085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S, Willis R, Matthews D, Contell F, Chan J, Murray J. Improving the quality of care for mild to moderate dementia: an evaluation of the Croydon Memory Service Model. Int J Geriatr Psychiatry. 2007;22(8):782–788. doi: 10.1002/gps.1741 [DOI] [PubMed] [Google Scholar]
- 10.McCormick WC, Hardy J, Kukull WA, et al. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49:1156–1160. Published online. doi: 10.1046/j.1532-5415.2001.49231.x [DOI] [PubMed] [Google Scholar]
- 11.Aaltonen M, Rissanen P, Forma L, Raitanen J, Jylhä M. The impact of dementia on care transitions during the last two years of life. Age Ageing. 2012. Published online. doi: 10.1093/ageing/afr133 [DOI] [PubMed] [Google Scholar]
- 12.Amjad H, Carmichael D, Austin AM, Chang CH, Bynum JPW. Continuity of care and health care utilization in older adults with dementia in fee-for-service Medicare. JAMA Intern Med. 2016;176: 1371. Published online. doi: 10.1001/jamainternmed.2016.3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd BE, Blevins M, Vaz LME, et al. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol. 2013;178:819–828. Published online. doi: 10.1093/aje/kwt030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.California Department of Public Health and Vital Records. Death Certificate Dataset, Concatenated; 2012–2017.
- 15.Suárez E, Pérez CM, Rivera R, Martínez MN. Applications of Regression Models in Epidemiology; 2017. doi: 10.1002/9781119212515 [DOI]
- 16.Richardson TS, Robins JM, Wang L. On modeling and estimation for the relative risk and risk difference. J Am Stat Assoc. 2017;112: 1121–1130. Published online. doi: 10.1080/01621459.2016.1192546 [DOI] [Google Scholar]
- 17.Google. Google Maps Driving Directions for Arrival at 10 am on June 1, 2020]. Accessed on June 8 2020. https://www.google.com/maps/dir
- 18.Kullgren JT, McLaughlin CG, Mitra N, Armstrong K. Nonfinancial barriers and access to care for U.S. adults. Health Serv Res. 2012. Published online. doi: 10.1111/j.1475-6773.2011.01308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients LTFU in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–411. Published online. doi: 10.1097/QAI.0b013e3181b843f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephan A, Bieber A, Hopper L, et al. Barriers and facilitators to the access to and use of formal dementia care: findings of a focus group study with people with dementia, informal carers and health and social care professionals in eight European countries. BMC Geriatr. 2018;18. Published online. doi: 10.1186/s12877-018-0816-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peel E, Harding R. “It’s a huge maze, the system, it’s a terrible maze”: dementia carers’ constructions of navigating health and social care services. Dementia. 2014. Published online. doi: 10.1177/1471301213480514 [DOI] [PubMed] [Google Scholar]
- 22.Weber SR, Pirraglia PA, Kunik ME. Use of services by community-dwelling patients with dementia: a systematic review. Am J Alzheimers Dis Other Demen. 2011;26:195–204. Published online. doi: 10.1177/1533317510392564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020. Published online 2020. doi: 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- 24.Dahodwala N, Shah K, He Y, et al. Sex disparities in access to caregiving in Parkinson disease. Neurology. 2018. Published online. doi: 10.1212/WNL.0000000000004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valletta R Higher education, wages, and polarization. Fed Reserv Bank San Fr Econ Lett. 2015. Published online. http://www.frbsf.org/economic-research/publications/economic-letter/2015/january/wages-education-college-labor-earnings-income/ [Google Scholar]
- 26.Tough H, Brinkhof MWG, Siegrist J, Fekete C, Fekete C. Social inequalities in the burden of care: a dyadic analysis in the caregiving partners of persons with a physical disability. Int J Equity Health. 2019;19. Published online. doi: 10.1186/s12939-019-1112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atramont A, Bourdel-Marchasson I, Bonnet-Zamponi D, Tangre I, Fagot-Campagna A, Tuppin P. Impact of nursing home admission on health care use and disease status elderly dependent people one year before and one year after skilled nursing home admission based on 2012–2013 SNIIRAM data. BMC Health Serv Res. 2017;17. Published online. doi: 10.1186/s12913-017-2620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison KL, Ritchie CS, Patel K, et al. Care settings and clinical characteristics of older adults with moderately severe dementia. J Am Geriatr Soc. 2019;69(9):1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodman C, Baillie J, Sivell S. The preferences and perspectives of family caregivers towards place of care for their relatives at the end-of-life. A systematic review and thematic synthesis of the qualitative evidence. BMJ Support Palliat Care. 2016. Published online. doi: 10.1136/bmjspcare-2014-000794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12. Published online. doi: 10.1186/1472-684X-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reckrey JM, Yang M, Kinosian B, et al. Receipt of home-based medical care among older beneficiaries enrolled in fee-for-service Medicare. Health Aff. 2020;39(8):1289–1296. doi: 10.1377/hlthaff.2019.01537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossen A, Kim H, Steinhoff A, Strieker M, Williams K. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol TeleHealth. 2015:49. Published online. doi: 10.2147/shtt.s59500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolanowski A, Fortinsky RH, Calkins M, et al. Advancing research on care needs and supportive approaches for persons with dementia: recommendations and rationale. J Am Med Dir Assoc. 2018;19: 1047–1053. Published online. doi: 10.1016/j.jamda.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69:1–2411. Published online. doi: 10.1111/jgs.17163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesser A, Burke A, Reyes J, Rohrberg T. Navigating the digital divide: literacy in underserved populations in the United States. Informatics Heal Soc Care. 2015;41(1):1–19. doi: 10.3109/17538157.2014.948171 [DOI] [PubMed] [Google Scholar]
- 36.Brodaty H, Thomson C, Thompson C, Fine M. Why caregivers of people with dementia and memory loss don’t use services. Int J Geriatr Psychiatry. 2005;20:537–546. Published online. doi: 10.1002/gps.1322 [DOI] [PubMed] [Google Scholar]
- 37.Park MH, Smith SC, Hendriks AAJ, Black N. Caregiver burden and quality of life 2 years after attendance at a memory clinic. Int J Geriatr Psychiatry. 2019;34:647–656. Published online. doi: 10.1002/gps.5060 [DOI] [PubMed] [Google Scholar]
- 38.Possin KL, Merrilees JJ, Dulaney S, et al. Effect of collaborative dementia care via telephone and Internet on quality of life, caregiver well-being, and health care use: The Care Ecosystem RandomizedClinical Trial. JAMA Intern Med. 2019;179:1658. Published online. doi: 10.1001/jamainternmed.2019.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galvin JE, Howard DH, Denny SS, Dickinson S, Tatton N. The social and economic burden of frontotemporal degeneration. Neurology. 2017;89:2049–2056. Published online. doi: 10.1212/WNL.0000000000004614 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


