Abstract
DESCRIPTION:
The purpose of this Clinical Practice Update Expert Review is to provide clinicians with guidance on the diagnosis and management of atrophic gastritis, a common preneoplastic condition of the stomach, with a primary focus on atrophic gastritis due to chronic Helicobacter pylori infection—the most common etiology—or due to autoimmunity. To date, clinical guidance for best practices related to the diagnosis and management of atrophic gastritis remains very limited in the United States, which leads to poor recognition of this preneoplastic condition and suboptimal risk stratification. In addition, there is heterogeneity in the definitions of atrophic gastritis, autoimmune gastritis, pernicious anemia, and gastric neoplasia in the literature, which has led to confusion in clinical practice and research. Accordingly, the primary objective of this Clinical Practice Update is to provide clinicians with a framework for the diagnosis and management of atrophic gastritis. By focusing on atrophic gastritis, this Clinical Practice Update is intended to complement the 2020 American Gastroenterological Association Institute guidelines on the management of gastric intestinal metaplasia. These recent guidelines did not specifically discuss the diagnosis and management of atrophic gastritis. Providers should recognize, however, that a diagnosis of intestinal metaplasia on gastric histopathology implies the diagnosis of atrophic gastritis because intestinal metaplasia occurs in underlying atrophic mucosa, although this is often not distinctly noted on histopathologic reports. Nevertheless, atrophic gastritis represents an important stage with distinct histopathologic alterations in the multistep cascade of gastric cancer pathogenesis.
METHODS:
The Best Practice Advice statements presented herein were developed from a combination of available evidence from published literature and consensus-based expert opinion. No formal rating of the strength or quality of the evidence was carried out. These statements are meant to provide practical advice to clinicians practicing in the United States.
Keywords: Gastric Cancer, Gastric Intestinal Metaplasia, Helicobacter pylori, Screening, Surveillance, Early Cancer Detection, Endoscopy, Best Practice, Atrophic Gastritis
BEST PRACTICE ADVICE STATEMENTS
BEST PRACTICE ADVICE 1:
Atrophic gastritis is defined as the loss of gastric glands, with or without metaplasia, in the setting of chronic inflammation mainly due to Helicobacter pylori infection or autoimmunity. Regardless of the etiology, the diagnosis of atrophic gastritis should be confirmed by histopathology.
BEST PRACTICE ADVICE 2:
Providers should be aware that the presence of intestinal metaplasia on gastric histology almost invariably implies the diagnosis of atrophic gastritis. There should be a coordinated effort between gastroenterologists and pathologists to improve the consistency of documenting the extent and severity of atrophic gastritis, particularly if marked atrophy is present.
BEST PRACTICE ADVICE 3:
Providers should recognize typical endoscopic features of atrophic gastritis, which include pale appearance of gastric mucosa, increased visibility of vasculature due to thinning of the gastric mucosa, and loss of gastric folds, and, if with concomitant intestinal metaplasia, light blue crests and white opaque fields. Because these mucosal changes are often subtle, techniques to optimize evaluation of the gastric mucosa should be performed.
BEST PRACTICE ADVICE 4:
When endoscopic features of atrophic gastritis are present, providers should assess the extent endoscopically. Providers should obtain biopsies from the suspected atrophic/metaplastic areas for histopathological confirmation and risk stratification; at a minimum, biopsies from the body and antrum/incisura should be obtained and placed in separately labeled jars. Targeted biopsies should additionally be obtained from any other mucosal abnormalities.
BEST PRACTICE ADVICE 5:
In patients with histology compatible with autoimmune gastritis, providers should consider checking antiparietal cell antibodies and anti-intrinsic factor antibodies to assist with the diagnosis. Providers should also evaluate for anemia due to vitamin B-12 and iron deficiencies.
BEST PRACTICE ADVICE 6:
All individuals with atrophic gastritis should be assessed for H pylori infection. If positive, treatment of H pylori should be administered and successful eradication should be confirmed using non-serological testing modalities.
BEST PRACTICE ADVICE 7:
The optimal endoscopic surveillance interval for patients with atrophic gastritis is not well-defined and should be decided based on individual risk assessment and shared decision making. A surveillance endoscopy every 3 years should be considered in individuals with advanced atrophic gastritis, defined based on anatomic extent and histologic grade.
BEST PRACTICE ADVICE 8:
The optimal surveillance interval for individuals with autoimmune gastritis is unclear. Interval endoscopic surveillance should be considered based on individualized assessment and shared decision making.
BEST PRACTICE ADVICE 9:
Providers should recognize pernicious anemia as a late-stage manifestation of autoimmune gastritis that is characterized by vitamin B-12 deficiency and macrocytic anemia. Patients with a new diagnosis of pernicious anemia who have not had a recent endoscopy should undergo endoscopy with topographical biopsies to confirm corpus-predominant atrophic gastritis for risk stratification and to rule out prevalent gastric neoplasia, including neuroendocrine tumors.
BEST PRACTICE ADVICE 10:
Individuals with autoimmune gastritis should be screened for type 1 gastric neuroendocrine tumors with upper endoscopy. Small neuroendocrine tumors should be removed endoscopically, followed by surveillance endoscopy every 1–2 years, depending on the burden of neuroendocrine tumors.
BEST PRACTICE ADVICE 11:
Providers should evaluate for iron and vitamin B-12 deficiencies in patients with atrophic gastritis irrespective of etiology, especially if corpus-predominant. Likewise, in patients with unexplained iron or vitamin B-12 deficiency, atrophic gastritis should be considered in the differential diagnosis and appropriate diagnostic evaluation pursued.
BEST PRACTICE ADVICE 12:
In patients with autoimmune gastritis, providers should recognize that concomitant autoimmune disorders, particularly autoimmune thyroid disease, are common. Screening for autoimmune thyroid disease should be performed.
Atrophic gastritis (AG) is a preneoplastic condition defined by replacement of appropriate gastric glandular structures with connective tissue (nonmetaplastic atrophy) or a different, non-native epithelium (metaplastic atrophy) on a background of chronic inflammation.1–3 AG is considered the first of a multistep precancerous cascade, with more advanced stages including gastric intestinal metaplasia (IM), dysplasia, and ultimately gastric adenocarcinoma.4 The 2 most common etiologies of AG are chronic infection with Helicobacter pylori and autoimmunity, with the former recognized as the dominant etiology. It is important for providers to recognize the broad spectrum of clinical manifestations of AG, which includes both gastric and extragastric manifestations.5
Epidemiology
The estimated prevalence of AG is up to 15% in US populations, and may be greater in specific populations with a higher baseline prevalence of H pylori or incidence of gastric adenocarcinoma, such as non-White racial/ethnic minority groups and early-generation immigrants from high-risk countries.6–12 AG is typically asymptomatic and may go undiagnosed, or with nonspecific symptoms that may occur later in the course.13 In addition, the inconsistent reporting of AG on histopathology contributes to the underdiagnosis of this condition. Based on a meta-analysis, the rate ratio of AG incidence in patients with vs without H pylori infection was 5.0 (95% confidence interval, 3.1–8.3) and AG incidence was very low (<1% annually) among H pylori–uninfected individuals, supporting the strong relationship between H pylori and AG.10 Other risk factors for nonautoimmune AG include age, tobacco use, high-salt diet, and possibly chronic bile acid reflux.14,15
Autoimmune gastritis (AIG) is significantly less common than H pylori–associated AG (HpAG). The prevalence has been estimated to approximate 0.5%–2%, although this might be an overestimation.16–18 The prevalence increases with age and the presence of other autoimmune diseases (eg, autoimmune thyroid diseases and type 1 diabetes mellitus).19 For example, it is estimated that up to one-third of patients with autoimmune thyroid disease have AIG.20 Women have a higher prevalence of AIG compared to men. In marked contrast to HpAG, racial and ethnic variation is not prominent in AIG.19 Pernicious anemia (PA)—a late-stage complication of AIG characterized by megaloblastic anemia due to vitamin B-12 deficiency19—is even rarer, with an estimated prevalence of 0.15%–1%.18,21
Natural History of Atrophic Gastritis as a Preneoplastic Condition
The estimates for the risk of progression from AG to high-grade dysplasia or gastric adenocarcinoma vary widely and reflect the heterogeneity of study populations and study design. It is estimated that the risk of progression of AG to gastric adenocarcinoma ranges from 0.1 % to 0.3 % per year (similar to the estimated rate of malignant progression from nondysplastic Barrett’s esophagus or low-risk colorectal adenomas), but may be higher, depending on AG severity, extent, concomitant IM, and other factors.22–24 As a late-stage manifestation of AIG, the diagnosis of PA identifies patients who have had corpus-predominant AG for at least several years. One meta-analysis of 27 studies demonstrated a nearly 7-fold significantly higher relative risk of gastric cancer in patients with vs without PA; although most individual studies suggest a 2- to 4-fold higher risk.25
Patients with chronic AG, particularly AIG given the corpus-predominant atrophy, are also at increased risk of type I NETs.26 These tumors develop as a consequence of the parietal cell loss, which leads to reduced gastric acid secretion and downstream persistent hypergastrinemia, enterochromaffin-like cell (ECL) hyperplasia, and, in a small percentage, ECL dysplasia and gastric NETs.27 Based on longitudinal cohort studies, the incidence rate of type I gastric NETs in patients with chronic AG is estimated as 0.4–0.7% per year,26,28 with variability across the literature.29–31
Histopathologic Features of Atrophic Gastritis
The pathogenesis of AG involves the interaction between environmental and genetic determinants to a varying extent depending on the primary trigger, namely H pylori infection vs autoimmunity. In some individuals, chronic H pylori gastritis can advance to atrophy (loss of gastric glands) with or without replacement by metaplastic epithelium. In AIG, autoantibodies against parietal cells and intrinsic factor along with T-cell–mediated destruction of the gastric oxyntic mucosa leads to the characteristic pattern of corpus-predominant atrophy with antral sparing that distinguishes AIG from HpAG. That said, overlap exists between these phenotypes, both related to pathogenesis (eg, molecular mimicry between H pylori antigens and the gastric H+/K+ ATPase has been proposed as a trigger for AIG19,32) and clinical considerations.19 Regardless of the etiology, the diagnosis of AG should be confirmed by histopathology (Figure 1A–H) (Best Practice Advice [BPA] 1).
Figure 1.
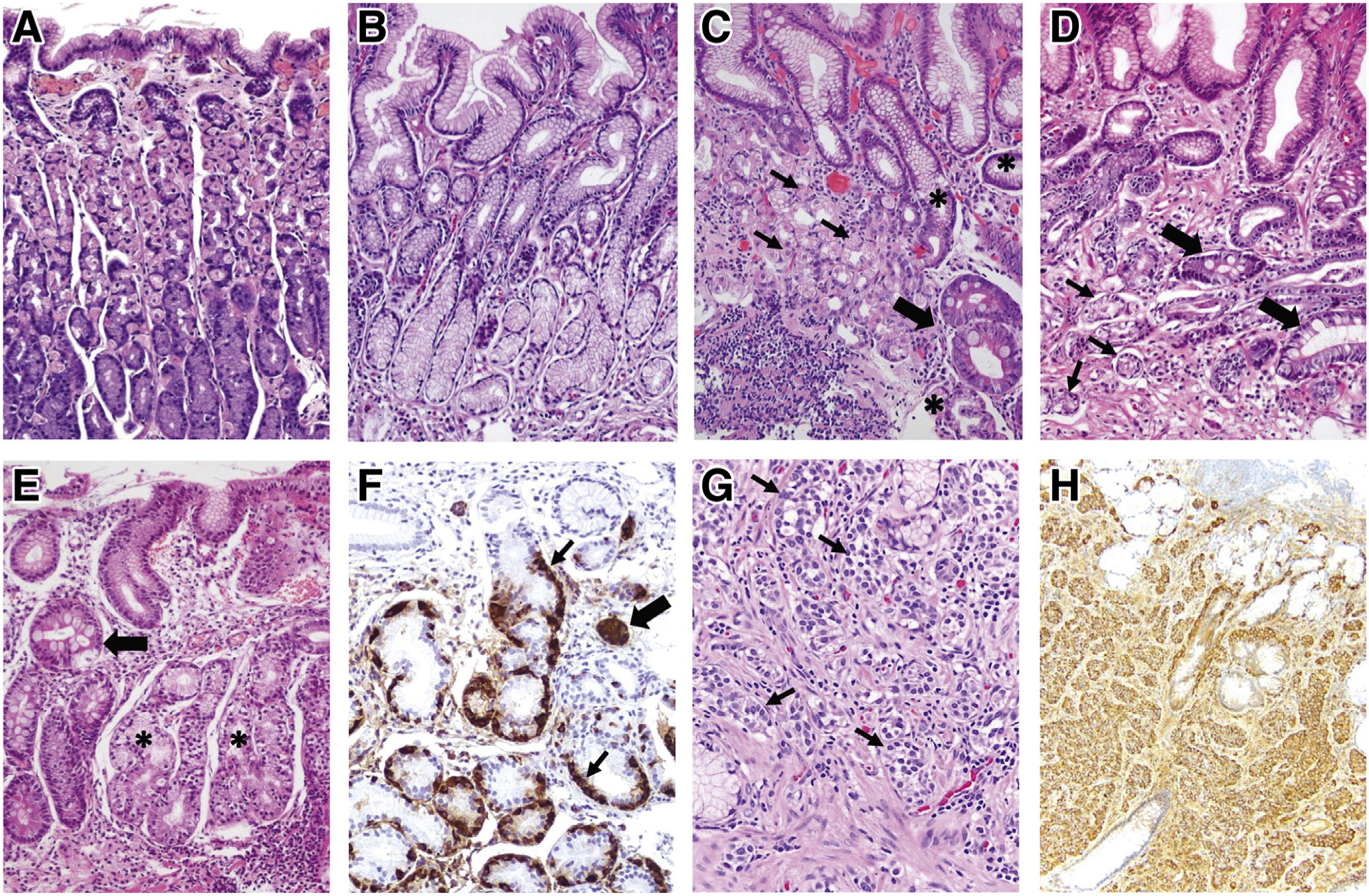
Histopathologic features of normal gastric mucosa, chronic AG, and gastric NET. (A) Normal gastric oxyntic mucosa, characterized by short foveolae (pits) and tightly packed, straight glands. The glands are primarily lined by parietal (pink) cells, which predominate in the upper two-thirds, and by chief (purple) cells at the base. (B) Normal antral mucosa, with wider foveolae and loosely packed glands, primarily lined by mucus-secreting cells. (C, D) H pylori–associated AG. (C) Corpus mucosa with chronic inflammation, moderate loss of oxyntic glands, pseudopyloric metaplasia (asterisks), and IM (thick arrow). Remaining parietal cells (thin arrows) are forming short, disorganized glands. In marked oxyntic atrophy (not shown), there may be complete absence of parietal and chief cells, making the histologic findings indistinguishable from those of antral atrophy. (D) Antral mucosa with shrunk, vanishing glands (thin arrows) and foci of IM (thick arrows) surrounded by fibromuscular tissue in the lamina propria. (E, F) Oxyntic mucosa showing fully developed autoimmune gastritis. (E) Complete absence of parietal and chief cells replaced by pseudopyloric (asterisks) and intestinal (arrow) metaplasia, in a background of chronic inflammation. In this case, glands with pseudopyloric metaplasia show ECL cell hyperplasia, highlighted in (F) with chromogranin A stain. Linear (thin arrows) and micronodular (thick arrow) ECL cell hyperplasia are observed. In earlier stages of autoimmune gastritis, the destruction of oxyntic glands by infiltrating lymphocytes (not shown) and the corpus-predominant pattern of inflammatory and atrophic changes strongly suggests this condition. (G, H) Gastric type 1 ECL cell NET. (G) The tumor is composed of well-differentiated cells with monomorphic round nuclei, arranged in small nests (arrows) infiltrating the lamina propria. The neuroendocrine differentiation is confirmed using chromogranin A stain (H).
AG is a slow process, in which, after years of mucosal inflammation, gastric glands gradually decrease in size and may disappear completely. The glands may be replaced with connective tissue (nonmetaplastic atrophy, usually referred to as atrophy) or with a different type of epithelium (metaplastic atrophy, ie, IM or pseudopyloric metaplasia). IM is the most frequently diagnosed histopathologic manifestation of AG.
In HpAG, atrophic changes arise initially in the incisura and the antrocorporal transitional mucosa as small foci with loss of glands and IM. Over time, these foci coalesce to form larger patches of atrophic/metaplastic mucosa along the lesser curvature and the antrum, and eventually spread to the corpus/fundus, followed by the loss of gastric acidity. In AIG, the typical histologic manifestation is corpus-predominant AG, with destruction of individual oxyntic glands by lymphocytes.33 In both conditions, there may be progressive loss of oxyntic glands, and increasingly prominent pseudopyloric and IM. ECL hyperplasia and type 1 gastric NETs can develop, indicating a state of hypergastrinemia. 34,35 If antral atrophy is seen in AIG, concomitant HpAG should be considered. If the anatomic location of the biopsies is uncertain, special stains (eg, gastrin [antrum], pepsinogen I [corpus]) may discriminate between antral mucosa and pseudopyloric metaplasia, which can be avoided if the biopsy specimens from the corpus and antrum/incisura are placed in separate specimen jars following the updated Sydney protocol (discussed below).
In clinical practice, the ability to report AG depends on presentation of antral and corpus biopsy specimens in separate jars, and correct orientation of the specimens during the paraffin-embedding process. Of note, gastric IM is readily identified irrespective of mucosal thickness of the biopsy specimen and orientation.
Severity and topographic distribution of atrophic lesions are well-established determinants of gastric cancer risk. These prognosticators are incorporated in 2 classification systems for risk assessment: Operative Link for Gastritis Assessment (OLGA) and Operative Link for Gastric Intestinal Metaplasia Assessment (OLGIM) (see Supplementary Figure 1A and B for details).36,37 Both OLGA and OLGIM are advocated by international guidelines for risk stratification of individuals diagnosed with precancerous gastric mucosal changes38,39; however, reporting of OLGA/OLGIM stage has not gained a foothold in clinical practice in the United States. Barriers to routine incorporation in practice should be identified and addressed. At the very least, providers should be aware that the presence of IM on gastric histology almost invariably implies the diagnosis of AG, and the presence of extensive atrophy and metaplasia are associated with an increased cancer risk.40 There should be a coordinated effort between gastroenterologists and pathologists to improve the consistency of documenting AG severity and extent, particularly if marked atrophy is present (BPA 2).
Diagnostic Workup
Endoscopic Evaluation for Atrophic Gastritis
The endoscopic appearance of AG may be subtle. Endoscopists should perform a high-quality examination following a systematic approach in order to maximize diagnostic yield. First, endoscopists should ensure excellent mucosal visualization, which necessitates adequate air insufflation and mucosal cleansing, and should spend sufficient time carefully examining the gastric mucosa.41,42 Defoaming and mucolytic agents, such as simethicone and 1 % N-acetylcysteine, may be considered because water irrigation alone may be insufficient for mucosal washing.43–45 The entire gastric lumen should be examined for the overall appearance of the mucosa, including the color and texture, appearance of submucosal blood vessels, and the architecture of the gastric rugae, followed by targeted examinations of focal abnormalities using high-definition white-light endoscopy (HD-WLE) or image-enhanced techniques, such as narrow-band imaging (NBI). Photographic documentation should be obtained to cover the cardia and fundus, lesser and greater curvature of corpus and antrum, incisura angularis, and pylorus (Figure 2).
Figure 2.
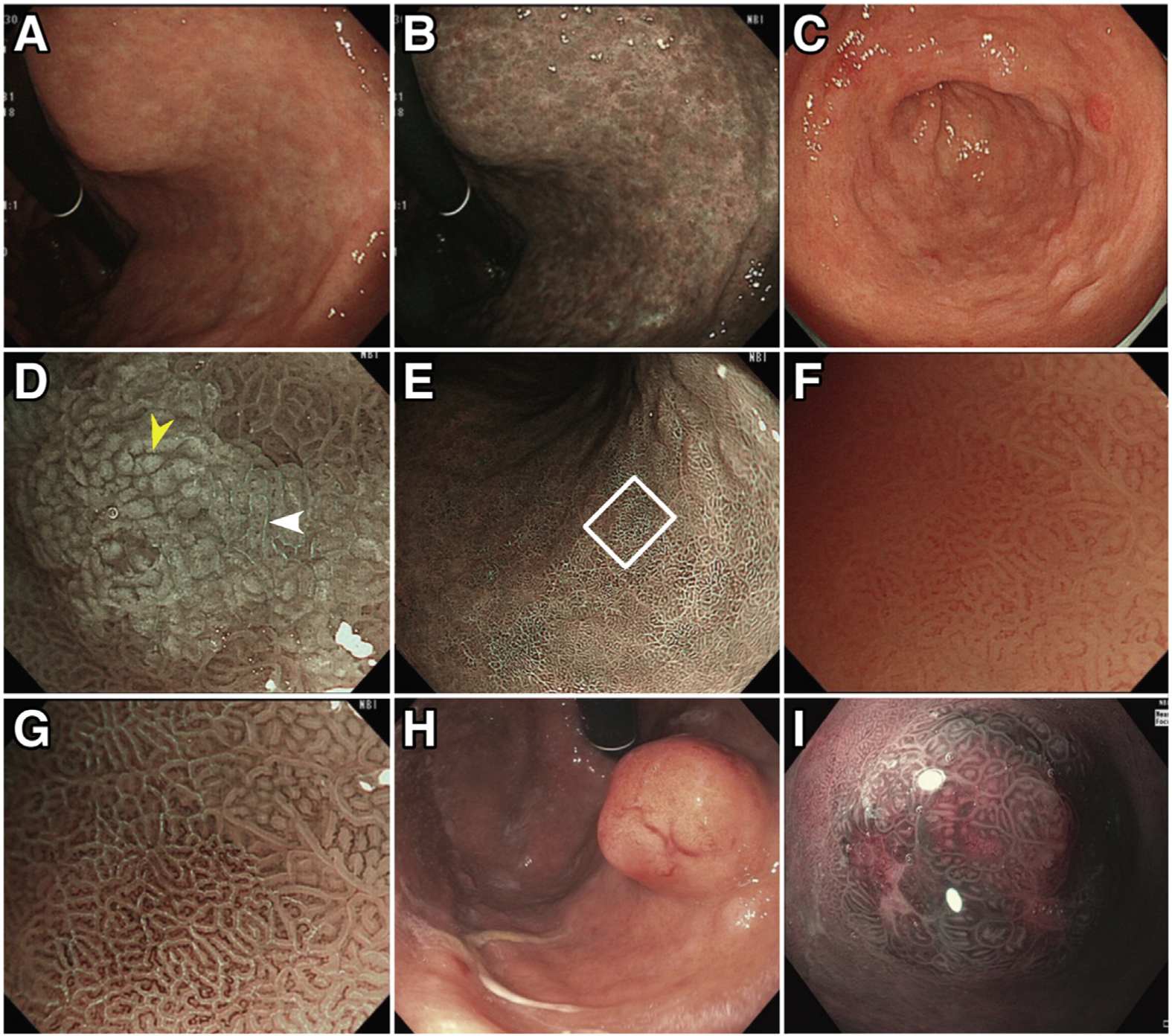
Typical endoscopic appearance of chronic AG, IM, and gastric NET. Characteristic endoscopic features of chronic AG include pale appearance of mucosa, loss of gastric rugal folds, and prominence of submucosal blood vessels due to thinning of the atrophied gastric epithelium, as shown in (A) HD-WLE and (B) NBI. Changes representing IM are frequently found in chronic AG (C–G). On HD-WLE, the areas with IM typically appear mildly nodular (C). On magnifying NBI (D), characteristic signs of IM include the LBC (white arrowhead) and white opaque field (WOF, yellow arrowhead) (or white opaque substance [WOS]). The LBC sign refers to the fine, blue-white lines on the crests of the epithelial surface, which correspond to histologic finding of the brush border (microvilli). The WOF (or WOS) is caused by light scattering at microscopic lipid droplets that accumulate in the mucosa of IM. Both LBC and WOF/WOS signs are best visualized using magnifying NBI. IM in the flat gastric mucosa is shown in (E) (nonmagnifying NBI image). (F, G) Magnified views of IM (white square [E]) with and without NBI, respectively, with abundant LBCs visible in (G). Note that areas of IM with LBC coexist with non-IM mucosa (right upper corner of [G]). (H) and (I) demonstrate endoscopic appearance of gastric NETs on HD-WLE (H) and near-focus NBI (I).
Providers should recognize that HpAG and AIG have different patterns of gastric mucosa involvement. In the former, the atrophy typically initiates in the gastric antrum and expands proximally, and may involve the entire stomach in severe cases. In 1969, Kimura and Takemoto proposed a classification system for AG based on the extent of the atrophic border.46 In this system, gastric atrophy is categorized as closed (C) or open (O) type, each with a grade based on the extent of atrophic border (Supplementary Figure 2). Multiple reports have consistently demonstrated that severe or extensive atrophy (02–03 types) has significantly higher cumulative risk of gastric cancer compared with mild atrophy (C1–C2 types)47–49; these findings are concordant with those based on OLGA/OLGIM systems described above. In AIG, because of the destruction of parietal cells by autoantibodies, the areas of atrophy primarily involve the gastric corpus and fundus with characteristic sparing of the antrum. In the early phase of the AIG, the mucosal changes are usually subtle except for nonspecific erythema, and the diagnosis of AIG can be missed without taking biopsies.17 With progressive loss of parietal cells, the mucosa of the entire gastric body appears atrophic.
Compared to conventional WLE, HD-WLE offers significantly improved sensitivity for identifying premalignant mucosal changes (Figure 2).50–52 Atrophic mucosa typically has a pale appearance, with increased visibility of submucosal blood vessels due to thinning of the gastric mucosa and loss of gastric folds (BPA 3). Frequently, a border of atrophic mucosa can be identified (Figure 2). In a Swedish study, the sensitivity and specificity of absence of rugal folds for moderate to severe AG in the gastric corpus were 67 % and 85 %, respectively.53 A combination of magnifying endoscopy and chromoendoscopy or image-enhanced techniques (eg, NBI) provides more detailed evaluation of gastric mucosa and microvascular architecture. Although magnifying endoscopy is not routinely available in the United States, the near-focus function of the newer-generation HD endoscopes, which are available in the Unites States, does provide better differentiation of mucosal abnormalities compared to the older-generation HD-WLE54 (Figure 2).
Because IM is an indicator of AG, providers should similarly be able to recognize endoscopic features of IM (BPA 3). Compared to AG, IM can be more reliably identified using HD-WLE, with the sensitivity further improved with image-enhancing technology, such as NBI.52,55,56 Relevant to US practice, prospective multicenter study using HD-WLE with NBI showed a sensitivity and specificity of 87% and 97% for the diagnosis of IM and 92% and 99% for the diagnosis of dysplasia, even without using magnifying endoscopy.57 On HD-WLE, the areas with IM typically appear mildly nodular with ridged or tubulovillous mucosal patterns.52,57 The “light blue crest” (LBC) sign, defined as fine, blue-white lines on the crests of the epithelial surface (Figure 2G), is characteristic for IM, with sensitivity and specificity approximately 90%, and positive and negative likelihood ratio 8.98 and 0.12, respectively, based on one meta-analysis.58,59 The so-called white opaque fields (or “white opaque substance”) (Figure 2D), which is the result of microscopic lipid droplets that accumulate in the mucosa of gastric tumors and IM, is also a useful marker for IM, with high specificity (100%; 95% confidence interval, 85%–100%) and limited sensitivity (50%; 95% confidence interval, 40%–50%) in 1 study.60
Biopsy Protocol
In the United States, the diagnosis of AG requires histopathologic confirmation. Because of the higher risk in patients with extensive AG vs AG limited to the antrum, providers should follow the updated Sydney protocol for obtaining topographical biopsies.3 This protocol has close to 100% sensitivity in identifying H pylori colonization as well.61 The protocol requires 5 gastric biopsies, which should be placed in separately labeled jars3: 2 from the antrum along the lesser and greater curvature, within 2–3 cm of the pylorus; 2 from the gastric corpus (including 1 from the lesser curvature at 4 cm proximal to the incisura angularis and the other from the middle portion of the greater curvature of the gastric body at 8 cm from the cardia), and 1 from the incisura angularis. Because AG/IM frequently involves the incisura angularis, providers should not skip this site when obtaining biopsies.62–64 If the cost incurred by separating each of these sites is a concern, at a minimum the biopsies should be placed in 2 separate specimen jars labeled antrum/incisura and body. Targeted biopsies should be obtained from any visible mucosal abnormalities and placed in appropriately labeled specimen jars (BPA 4).
Serologic Diagnosis
Serum pepsinogens (PGs) reflect both the functional and morphologic status of the gastric mucosa and are useful markers of extensive atrophy.65 Chief and mucous neck cells in the gastric corpus and fundic glands secrete both PG I and PG II, while PG II (but not PG I) is also produced by pyloric glands and Brunner’s glands. At least in regions with high gastric cancer incidence (with most studies from East Asia), PG I levels (<70 μg/L) and low PG I:II ratio (<3.0) demonstrate a high sensitivity and specificity for severe corpus atrophy.66 However, PG testing is not available for routine clinical use in the United States.
In patients with histology compatible with AIG, providers should check parietal cell antibodies (PCAs) and intrinsic factor antibodies (IFA) to assist with the diagnosis (BPA 5). PCA is the most sensitive serum biomarker for AIG, but false positives are not uncommon, as PCA can be elevated in H pylori infection and other autoimmune diseases.19,67 IFA has low sensitivity (<30% in many studies) but high specificity, and is more often positive later in the disease course.32,68 Autoantibody positivity might also predate clinical presentation of AIG, particularly in individuals with other autoimmune disorders.19,69
Management
Test and Treat for Helicobactor pylori
The vast majority of patients with AG have evidence of current or prior infection of H pylori.70,71 Irrespective of etiology, patients with AG should be tested for H pylori and, if positive, H pylori should be eradicated. Subsequent non-serologic H pylori testing should be performed to confirm successful eradication (BPA 6). Normal gastric mucosa may be restored over time in some patients with AG after successful H pylori eradication,72 although most patients may have passed a “point-of-no-return” in which the gastric mucosal damage cannot be reversed despite H pylori eradication. 73 For these patients, their risk remains elevated, particularly among those with extensive or moderate to severe atrophy (eg, OLGA/OLGIM III/IV), providing the clinical rationale for endoscopic surveillance even after successful H pylori eradication, a practice supported by international guidelines (Supplementary Table 1). Nevertheless, despite persistent signs of AG, H pylori eradication does still appear to reduce the risk of gastric cancer.74
Endoscopic Surveillance of Atrophic Gastritis
Overall, only a small minority of patients with AG will have neoplastic complications. There is a lack of prospective, randomized controlled trials to support the benefits of performing routine surveillance endoscopy for patients with AG, namely reduced gastric cancer–related morbidity and mortality. However, multiple observational studies consistently demonstrate a strong association between severe AG (based on histology, anatomic distribution, or OLGA/OLGIM III/IV stages) and increased risk of gastric adenocarcinoma; this provides the justification for endoscopic surveillance for these patients to increase the likelihood of detection of gastric cancer at an early stage when resection with curative intent is possible.37,75–79 In addition to risk stratification for gastric neoplasia, endoscopic surveillance in patients with AG should also consider comorbidities, as well as patient values and priorities.80,81 Providers should consider performing endoscopic surveillance every 3 years in patients with advanced AG. However, it should be recognized that optimal surveillance intervals remain to be determined, and shorter or longer intervals may be appropriate depending on individual risk assessment (BPA 7). An algorithm for clinical management of AG is shown in Figure 3.
Figure 3.
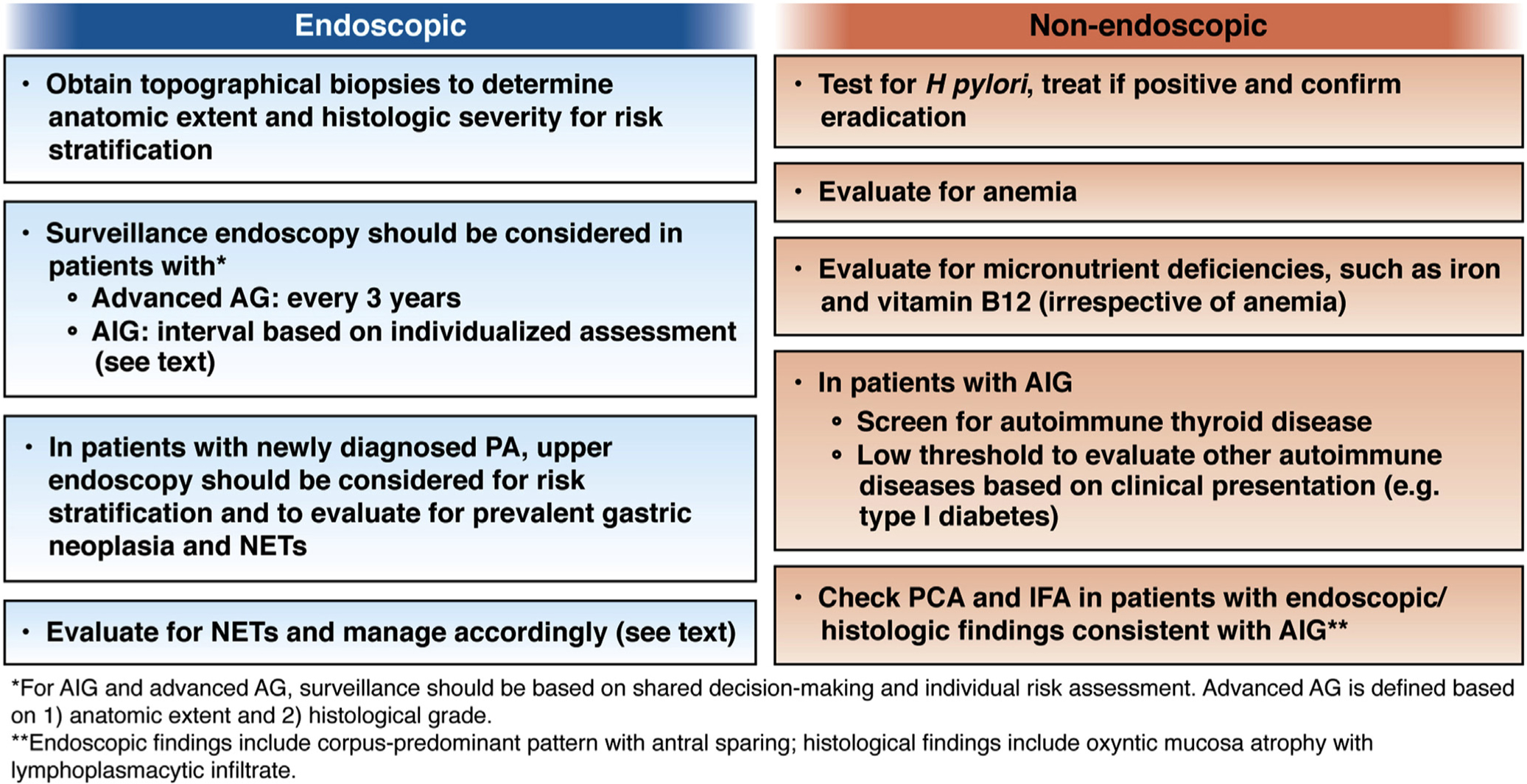
Algorithm for clinical management of AG.
Additional risk factors that should be considered for informing surveillance intervals include the quality of baseline endoscopy, family history of gastric cancer, immigration history from geographic regions with high incidence of gastric cancer, persistent H pylori infection, smoking history and dietary factors, among others.80,82 This risk stratification-based approach is overall consistent with current guidelines from different professional societies on surveillance for chronic AG and GIM (Supplementary Table 1).
The optimal surveillance strategy for individuals with AIG is unclear (BPA 8). The current European Society for Gastrointestinal Endoscopy guidelines advocate performing surveillance endoscopy at 3–5 years in patients with AIG.38 In patients with PA, observational studies suggest that the risk of gastric adenocarcinoma might be highest within the first year of diagnosis. Reflective of this, the American Society of Gastrointestinal Endoscopy advocates that an upper endoscopy be performed within 6 months of the diagnosis of PA.83 The development of upper gastrointestinal symptoms in patients with PA should also prompt diagnostic endoscopy (BPA 9).
Management of Gastric Neuroendocrine Tumors
Gastric NETs associated with AG represent approximately 80%–90% of all gastric NETs and are overwhelmingly categorized as type 1.84 Small NETs are typically asymptomatic and often diagnosed incidentally. These usually appear as small to tiny nodules <10 mm and are most often found at the gastric corpus or fundus; they are typically well-differentiated with an indolent course.85–88 Endoscopically, they present as polypoid lesions in slight yellow or red color on HD-WLE (Figure 2H–I). The prognosis is determined by the size, depth of invasion, and mitotic activity of the tumor. The rate of metastasis is <10% in gastric NETs ≤2 cm but approaches 20 % in NETs >2 cm.89 Small gastric NETs <1 cm are generally amenable to endoscopic resection. The optimal interval for endoscopic surveillance has not been well defined. Providers should resect all small NETs <1 cm endoscopically, and should consider surveillance endoscopy every 1–2 years, depending on the burden of NETs (BPA 10). For gastric NETs >1–2 cm, providers should consider endoscopic ultrasound to assess depth of tumor invasion and presence of local metastasis to help guide further management.90 Surgical resection is appropriate for NETs >2 cm, with invasion past the submucosa, or with evidence of lymph node metastasis.89,90
Management of Coexisting Conditions Associated With Atrophic Gastritis
Patients with corpus-predominant AG, irrespective of etiology, are at an increased risk of developing iron and vitamin B-12 deficiency due to reduced gastric acid secretion and intrinsic factor. Iron deficiency is common, with some series reporting this in up to 50% of patients with corpus-predominant AG, and often presents much earlier than the manifestation of B-12 deficiency.19 Providers should therefore evaluate for iron and vitamin B-12 deficiency in patients with AG, especially if corpus-predominant; likewise, in patients with unexplained iron or vitamin B-12 deficiency, AG should be considered in the differential diagnosis and appropriate diagnostic evaluation pursued (BPA 11).
Providers should recognize that there is an established association between AIG and other autoimmune diseases, especially autoimmune thyroid disease, perhaps related to shared genetic susceptibility loci.91–93 Screening for autoimmune thyroid disease should be considered in patients diagnosed with AIG. Providers should also have a low threshold to evaluate for other associated autoimmune diseases, including type 1 diabetes mellitus and Addison’s disease, if the clinical picture is consistent94 (BPA 12).
Conclusions
Providers should recognize AG as an important, albeit frequently underdiagnosed, condition with both gastric and extragastric manifestations. Patients with severe AG should be considered for endoscopic surveillance for the purpose of early gastric cancer detection and may require additional management considerations, including attention to micronutrient deficiencies, particularly iron and vitamin B-12 deficiencies. Coordinated efforts between gastroenterologists and pathologists are needed to improve the diagnosis and characterization of AG. In addition, collaborative efforts are also needed, particularly in the form of comparative clinical trials in US populations, in order to refine risk stratification algorithms and optimize surveillance strategies for patients with AG.
Supplementary Material
Acknowledgments
This Expert Review was commissioned and approved by the American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee and the AGA Governing Board to provide timely guidance on a topic of high clinical importance to the AGA membership, and underwent internal peer review by the Clinical Practice Update Committee and external peer review through standard procedures of Gastroenterology.
The authors would like to sincerely thank Dr Noriya Uedo (Department of Gastrointestinal Oncology, Osaka International Cancer Institute, Japan), Dr Arnoldo Riquelme (Pontificia Universidad Católica de Chile, Chile), Dr Alberto Espino (Pontificia Universidad Católica de Chile, Chile), and Dr Kay Washington (Vanderbilt University Medical Center, Nashville, TN) for providing endoscopic and histologic images used in this manuscript, as well as for their kind correspondence.
Funding
Shailja C. Shah is supported by an American Gastroenterological Association Research Scholar Award (2019) and a Veterans Affairs Career Development Award ICX002027A01. Dan Li is supported by a Delivery Science Grant and the Physician Researcher Program of the Kaiser Permanente Northern California Division of Research. M. Blanca Piazuelo is supported by US National Institutes of Health grants P01CA028842, R01CA190612, P01CA116087, R21AI142042, R01DK058587, 5P30 DK058404, and Department of Defense grant W81XWH-18–1-0301.
Abbreviations used in this paper:
- AG
atrophic gastritis
- AIG
autoimmune gastritis
- BPA
Best Practice Advice
- ECL
enterochromaffin-like
- ESGE
European Society of Gastrointestinal Endoscopy
- HD-WLE
high-definition white-light endoscopy
- HpAG
Helicobacter pylori–associated atrophic gastritis
- IFA
intrinsic factor antibody
- IM
intestinal metaplasia
- LBC
light blue crest
- NBI
narrow-band imaging
- OLGA
Operative Link for Gastritis Assessment
- OLGIM
Operative Link for Gastric Intestinal Metaplasia Assessment
- PA
pernicious anemia
- PCA
parietal cell antibody
- PG
pepsinogen
Footnotes
Conflicts of interest
This author disclosed the following: Shailja C. Shah serves as a consultant for Phathom Pharmaceuticals. The remaining authors disclose no conflicts.
Supplementary Material
Note: The first 25 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.06.078.
References
- 1.Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther 2002; 16:1249–1259. [DOI] [PubMed] [Google Scholar]
- 2.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20:1161–1181. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet 1975;2:58–60. [DOI] [PubMed] [Google Scholar]
- 5.Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis 2019;51:1621–1632. [DOI] [PubMed] [Google Scholar]
- 6.Namekata T, Miki K, Kimmey M, et al. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol 2000;151:820–830. [DOI] [PubMed] [Google Scholar]
- 7.Song H, Held M, Sandin S, et al. Increase in the prevalence of atrophic gastritis among adults age 35 to 44 years old in northern Sweden between 1990 and 2009. Clin Gastroenterol Hepatol 2015;13:1592–1600.e1. [DOI] [PubMed] [Google Scholar]
- 8.Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev 2006;15:1083–1094. [DOI] [PubMed] [Google Scholar]
- 9.Choi CE, Sonnenberg A, Turner K, et al. High prevalence of gastric preneoplastic lesions in east asians and hispanics in the USA. Dig Dis Sci 2015;60:2070–2076. [DOI] [PubMed] [Google Scholar]
- 10.Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol 2010;25:439–448. [DOI] [PubMed] [Google Scholar]
- 11.Altayar O, Davitkov P, Shah SC, et al. AGA technical review on gastric intestinal metaplasia-epidemiology and risk factors. Gastroenterology 2020;158:732–744.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen T, Ramsey D, Graham D, et al. The prevalence of Helicobacter pylori remains high in African American and Hispanic veterans. Helicobacter 2015;20:305–315. [DOI] [PubMed] [Google Scholar]
- 13.Annibale B, Esposito G, Lahner E. A current clinical overview of atrophic gastritis. Expert Rev Gastroenterol Hepatol 2020;14:93–102. [DOI] [PubMed] [Google Scholar]
- 14.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010;105:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JH, Kim YS, Heo NJ, et al. High salt intake is associated with atrophic gastritis with intestinal metaplasia. Cancer Epidemiol Biomarkers Prev 2017;26:1133–1138. [DOI] [PubMed] [Google Scholar]
- 16.Notsu T, Adachi K, Mishiro T, et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern Med 2019;58:1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JY, Cornish TC, Lam-Himlin D, et al. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol 2010;34:1591–1598. [DOI] [PubMed] [Google Scholar]
- 18.Kuipers EJ. Pernicious anemia, atrophic gastritis, and the risk of cancer. Clin Gastroenterol Hepatol 2015;13:2290–2292. [DOI] [PubMed] [Google Scholar]
- 19.Lenti MV, Rugge M, Lahner E, et al. Autoimmune gastritis. Nat Rev Dis Primers 2020;6:56. [DOI] [PubMed] [Google Scholar]
- 20.Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med 1999;159:1726–1730. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223–243. [DOI] [PubMed] [Google Scholar]
- 22.Vannella L, Lahner E, Osborn J, et al. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment Pharmacol Ther 2010; 31:1042–1050. [DOI] [PubMed] [Google Scholar]
- 23.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, et al. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol 2004; 57:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuwenburg SAV, Mommersteeg MC, Eikenboom EL, et al. Factors associated with the progression of gastric intestinal metaplasia: a multicenter, prospective cohort study. Endosc Int Open 2021;9:E297–E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375–382. [DOI] [PubMed] [Google Scholar]
- 26.Vannella L, Sbrozzi-Vanni A, Lahner E, et al. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther 2011; 33:1361–1369. [DOI] [PubMed] [Google Scholar]
- 27.Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 2012;95:74–87. [DOI] [PubMed] [Google Scholar]
- 28.Lahner E, Esposito G, Pilozzi E, et al. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol 2015;50:856–865. [DOI] [PubMed] [Google Scholar]
- 29.Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol 2004;99:23–32. [DOI] [PubMed] [Google Scholar]
- 30.Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev 2014:CD010623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol 2010;105:2563–2569. [DOI] [PubMed] [Google Scholar]
- 32.Kulnigg-Dabsch S Autoimmune gastritis. Wien Med Wochenschr 2016;166:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittman ME, Voltaggio L, Bhaijee F, et al. Autoimmune metaplastic atrophic gastritis: recognizing precursor lesions for appropriate patient evaluation. Am J Surg Pathol 2015;39:1611–1620. [DOI] [PubMed] [Google Scholar]
- 34.Hall SN, Appelman HD. Autoimmune gastritis. Arch Pathol Lab Med 2019;143:1327–1331. [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Iwafuchi M, Ueki J-I, et al. Gastric carcinoid tumors without autoimmune gastritis in Japan: a relationship with Helicobacter pylori infection. Dig Dis Sci 2002;47:579–585. [DOI] [PubMed] [Google Scholar]
- 36.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007;56:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–1158. [DOI] [PubMed] [Google Scholar]
- 38.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 39.Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019;68:1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gawron AJ, Shah SC, Altayar O, et al. AGA technical review on gastric intestinal metaplasia-natural history and clinical outcomes. Gastroenterology 2020; 158:705–731.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JM, Huo SM, Lee HH, et al. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology 2017;153:460–469.e1. [DOI] [PubMed] [Google Scholar]
- 42.Teh JL, Tan JR, Lau LJF, et al. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol 2015;13:480–487.e2. [DOI] [PubMed] [Google Scholar]
- 43.Burke E, Harkins P, Moriarty F, Ahmed I. Does premedication with mucolytic agents improve mucosal visualization during oesophagogastroduodenoscopy: a systematic review and meta-analysis. Surg Res Pract 2021;2021:1570121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monrroy H, Vargas JI, Glasinovic E, et al. Use of N-acetylcysteine plus simethicone to improve mucosal visibility during upper GI endoscopy: a double-blind, randomized controlled trial. Gastrointest Endosc 2018;87:986–993. [DOI] [PubMed] [Google Scholar]
- 45.Neale JR, James S, Callaghan J, Patel P. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: a randomized, controlled, endoscopist-blinded study. Eur J Gastroenterol Hepatol 2013;25:778–783. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K, Takemoto T. An endoscopic recognition ofthe atrophic border and its significance in chronic gastritis. Endoscopy 1969;3:87–97. [Google Scholar]
- 47.Masuyama H, Yoshitake N, Sasai T, et al. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion 2015; 91:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shichijo S, Hirata Y, Niikura R, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc 2016;84:618–624. [DOI] [PubMed] [Google Scholar]
- 49.Toyoshima O, Yamaji Y, Yoshida S, et al. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc 2017;31:2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ang TL, Pittayanon R, Lau JYW, et al. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol 2015; 27:1473–1478. [DOI] [PubMed] [Google Scholar]
- 51.Panteris V, Nikolopoulou S, Lountou A, et al. Diagnostic capabilities of high-definition white light endoscopy for the diagnosis of gastric intestinal metaplasia and correlation with histologic and clinical data. Eur J Gastroenterol Hepatol 2014;26:594–601. [DOI] [PubMed] [Google Scholar]
- 52.Pimentel-Nunes P, Libânio D, Lage J, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016; 48:723–730. [DOI] [PubMed] [Google Scholar]
- 53.Redéen S, Petersson F, Jönsson KA, et al. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy 2003;35:946–950. [DOI] [PubMed] [Google Scholar]
- 54.Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy 2007;39:202–207. [DOI] [PubMed] [Google Scholar]
- 55.Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, et al. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc 2017;86:857–865. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Carrasco M, Esposito G, Libânio D, et al. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: a systematic review and meta-analysis. Endoscopy 2020;52:1048–1065. [DOI] [PubMed] [Google Scholar]
- 57.Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012;44:236–246. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Huang W, Du J, et al. Diagnostic yield of the light blue crest sign in gastric intestinal metaplasia: a meta-analysis. PLoS One 2014;9:e92874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy 2006;38:819–824. [DOI] [PubMed] [Google Scholar]
- 60.Kanemitsu T, Yao K, Nagahama T, et al. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: the white opaque substance marker. Endoscopy 2017;49:529–535. [DOI] [PubMed] [Google Scholar]
- 61.El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol 1999;30:72–77. [DOI] [PubMed] [Google Scholar]
- 62.Varbanova M, Wex T, Jechorek D, et al. Impact of the angulus biopsy for the detection of gastric preneoplastic conditions and gastric cancer risk assessment. J Clin Pathol 2016;69:19–25. [DOI] [PubMed] [Google Scholar]
- 63.Isajevs S, Liepniece-Karele I, Janciauskas D, et al. The effect of incisura angularis biopsy sampling on the assessment of gastritis stage. Eur J Gastroenterol Hepatol 2014;26:510–513. [DOI] [PubMed] [Google Scholar]
- 64.Kim Y-I, Kook M-C, Cho S-J, et al. Effect of biopsy site on detection of gastric cancer high-risk groups by OLGA and OLGIM stages. Helicobacter 2017;22(6): e12442. [DOI] [PubMed] [Google Scholar]
- 65.Samloff IM, Varis K, Ihamaki T, et al. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology 1982; 83:204–209. [PubMed] [Google Scholar]
- 66.Lorente S, Doiz O, Trinidad Serrano M, et al. Helicobacter pylori stimulates pepsinogen secretion from isolated human peptic cells. Gut 2002;50:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Annibale B, Marignani M, Azzoni C, et al. Atrophic body gastritis: distinct features associated with Helicobacter pylori infection. Helicobacter 1997;2:57–64. [DOI] [PubMed] [Google Scholar]
- 68.Lahner E, Norman GL, Severi C, et al. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol 2009;104:2071–2079. [DOI] [PubMed] [Google Scholar]
- 69.Tozzoli R, Kodermaz G, Perosa AR, et al. Autoantibodies to parietal cells as predictors of atrophic body gastritis: a five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun Rev 2010; 10:80–83. [DOI] [PubMed] [Google Scholar]
- 70.Annibale B, Negrini R, Caruana P, et al. Two-thirds of atrophic body gastritis patients have evidence of Helicobacter pylori infection. Helicobacter 2001;6:225–233. [DOI] [PubMed] [Google Scholar]
- 71.Annibale B, Lahner E, Santucci A, et al. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter 2007; 12:23–30. [DOI] [PubMed] [Google Scholar]
- 72.Vannella L, Lahner E, Bordi C, et al. Reversal of atrophic body gastritis after H. pylori eradication at long-term follow-up. Dig Liver Dis 2011;43. 295–259. [DOI] [PubMed] [Google Scholar]
- 73.Piazuelo MB, Bravo LE, Mera RM, et al. The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology 2021;160:1106–1117.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato M, Hayashi Y, Nishida T, et al. Helicobacter pylori eradication prevents secondary gastric cancer in patients with mild-to-moderate atrophic gastritis [published online ahead of print January 5, 2021]. J Gastroenterol Hepatol 10.1111/jgh.15396 [DOI] [PubMed] [Google Scholar]
- 75.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018;21:579–587. [DOI] [PubMed] [Google Scholar]
- 76.Rugge M, de Boni M, Pennelli G, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinicopathological follow-up study. Aliment Pharmacol Ther 2010;31:1104–1111. [DOI] [PubMed] [Google Scholar]
- 77.Leung WK, Ho HJ, Lin J-T, et al. Prior gastroscopy and mortality in patients with gastric cancer: a matched retrospective cohort study. Gastrointest Endosc 2018; 87:119–127.e3. [DOI] [PubMed] [Google Scholar]
- 78.Gong EJ, Ahn JY, Jung H-Y, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol 2014;29:301–309. [DOI] [PubMed] [Google Scholar]
- 79.Jun JK, Choi KS, Lee H-Y, et al. Effectiveness of the korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology 2017; 152:1319–1328.e7. [DOI] [PubMed] [Google Scholar]
- 80.Gupta S, Li D, El Serag HB, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esposito G, Dilaghi E, Cazzato M, et al. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig Liver Dis 2021;53:467–473. [DOI] [PubMed] [Google Scholar]
- 82.Nam JH, Choi IJ, Cho S-J, et al. Association of the interval between endoscopies with gastric cancer stage at diagnosis in a region of high prevalence. Cancer 2012;118:4953–4960. [DOI] [PubMed] [Google Scholar]
- 83.ASGE Standards of Practice Committee, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 84.La Rosa S, Rindi G, Solcia E, et al. Gastric neuroendocrine neoplasms, WHO Classification of Tumours. 5th edn. IARC Press, 2019:104–109. [Google Scholar]
- 85.Gladdy RA, Strong VE, Coit D, et al. Defining surgical indications for type I gastric carcinoid tumor. Ann Surg Oncol 2009;16:3154–3160. [DOI] [PubMed] [Google Scholar]
- 86.Uygun A, Kadayifci A, Polat Z, et al. Long-term results of endoscopic resection for type I gastric neuroendocrine tumors. J Surg Oncol 2014;109:71–74. [DOI] [PubMed] [Google Scholar]
- 87.Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology 2012;95:207–213. [DOI] [PubMed] [Google Scholar]
- 88.Borch K, Ahrén B, Ahlman H, et al. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg 2005;242:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saund MS, Al Natour RH, Sharma AM, et al. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol 2011;18:2826–2832. [DOI] [PubMed] [Google Scholar]
- 90.Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw 2018; 16:693–702. [DOI] [PubMed] [Google Scholar]
- 91.Varis K, Ihamäki T, Härkönen M, et al. Gastric morphology, function, and immunology in first-degree relatives of probands with pernicious anemia and controls. Scand J Gastroenterol 1979;14:129–139. [DOI] [PubMed] [Google Scholar]
- 92.Silveira PA, Wilson WE, Esteban LM, et al. Identification of the Gasa3 and Gasa4 autoimmune gastritis susceptibility genes using congenic mice and partitioned, segregative and interaction analyses. Immunogenetics 2001;53:741–750. [DOI] [PubMed] [Google Scholar]
- 93.Magris R, De Re V, Maiero S, et al. Low pepsinogen I/II ratio and high gastrin-17 levels typify chronic atrophic autoimmune gastritis patients with gastric neuroendocrine tumors. Clin Transl Gastroenterol 2020;11: e00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minalyan A, Benhammou JN, Artashesyan A, et al. Autoimmune atrophic gastritis: current perspectives. Clin Exp Gastroenterol 2017;10:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


