Abstract
Purpose
Diagnosis and classification of primary progressive aphasia (PPA) requires confirmation of specific speech and language symptoms, highlighting the important role of speech-language pathologists in the evaluation process. The purpose of this case report is to inform speech-language pathologists regarding current practices for diagnostic assessment in PPA, describing standard approaches as well as complementary, state-of-the-art procedures that may improve diagnostic precision.
Method
We describe the diagnostic evaluation of a 49-year-old woman with complaints of progressive word-finding difficulty. She completed standard neurological, neuropsychological, and speech-language evaluations, as well as magnetic resonance and positron emission tomography imaging of her brain. In addition, a history of developmental speech, language, and learning abilities was obtained, as well as genetic testing and assessment of cerebrospinal fluid biomarkers. We discuss the evaluation results in the context of the most current research related to PPA diagnosis.
Conclusion
Detailed behavioral assessment, thorough intake of symptom history and neurodevelopmental differences, multimodal neuroimaging, and comprehensive examination of genes and biomarkers are of paramount importance for detecting and characterizing PPA, with ramifications for early behavioral and/or pharmacological intervention.
Supplemental Material
The diagnosis of primary progressive aphasia (PPA) is made when a patient has a predominant and progressive loss of communication caused by neurodegenerative disease that targets speech and language regions of the brain (Gorno-Tempini et al., 2004; Mesulam, 1982). Diagnosis of PPA requires confirmation of specific speech and language symptoms, highlighting the important contribution of speech-language pathologists (SLPs) during the evaluation process. Approaches to assessment and clinical characterization have evolved along with diagnostic terminology for PPA. Initially, PPA was characterized using a binary classification system with two predominant subtypes: nonfluent PPA (also referred to as progressive nonfluent aphasia; Grossman et al., 1996;Neary et al., 1998; Turner et al., 1996) and fluent PPA (also referred to as semantic dementia; Neary et al., 1998; Snowden et al., 1989; Warrington, 1975). As understanding of the clinical manifestations of PPA has grown, a tripartite clinical classification scheme emerged and was formalized in international consensus criteria for diagnosis (Gorno-Tempini et al., 2011). Current consensus criteria delineate clinical, imaging, neuropathological, and genetic features of each of the three variants of PPA.
Contemporary approaches to PPA diagnosis continue to evolve with the discoveries of novel imaging and genetic biomarkers and studies investigating symptom trajectories and neurodevelopmental patterns. Procedures for a multidisciplinary evaluation of typical PPA cases have been previously outlined for a general clinical audience (Marshall et al., 2018), and speech-language assessment procedures were recently summarized (Henry & Grasso, 2018). However, best practices for PPA diagnosis via a comprehensive, multidisciplinary evaluation have yet to be described for an SLP audience. This case report illustrates the application and interpretation of current assessment tools, including magnetic resonance imaging (MRI), positron emission tomography (PET) imaging, fluid biomarkers, genetic testing, neurodevelopmental history-taking, and state-of-the-art speech-language and cognitive testing. First, we outline modern diagnostic procedures that may complement standard assessments and improve diagnostic precision, with a particular focus on issues relevant to SLPs. Subsequently, we will apply these diagnostic best practices to an illustrative case of PPA and discuss the clinical decision-making process as well as recommendations for clinical management.
Establishing a PPA Diagnosis and Clinical Phenotpying
According to the international consensus criteria for PPA (Gorno-Tempini et al., 2011), clinical diagnosis is a two-step process: The first step involves determining whether an individual's pattern and trajectory of symptoms meet criteria for PPA, and the second step includes PPA variant classification. The earliest criteria for PPA diagnosis included a decline in speech and/or language that occurred in isolation from other symptoms for at least 2 years after disease onset (Mesulam, 1982, 2001; Mesulam & Weintraub, 1992). However, the “2-year rule” was ultimately thought to hinder diagnosis of PPA at early or mild stages of the disease (Mesulam et al., 2012). Currently, a clinical diagnosis of PPA requires predominant and progressive speech and/or language symptoms during initial stages of the disease, that these deficits are the primary limitation to activities of daily living, and that impairments cannot be better explained by psychiatric, behavioral, or nondegenerative central nervous system disorders.
The second diagnostic step is classification by PPA clinical variant, when possible. The international consensus criteria for PPA define three clinical variants based on core impairments and associated features (see Gorno-Tempini et al., 2011, for more details). Semantic variant PPA (svPPA) is characterized by core impairments in both single-word comprehension and confrontation naming, and at least three of four associated features: loss of object knowledge (especially for items that are less frequent or less familiar), surface dyslexia or dysgraphia, spared repetition, and spared grammar and motor speech. A diagnosis of logopenic variant PPA (lvPPA) occurs when both core impairments in repetition of sentences/phrases as well as word retrieval in spontaneous speech and confrontation naming are present, and at least three of four associated features: phonemic errors in spontaneous speech and naming, spared single-word comprehension and object knowledge, spared motor speech, and absence of frank agrammatism. Nonfluent/agrammatic variant PPA (nfvPPA) is indicated with at least one of two core deficits, either agrammatism in language production or apraxia of speech, and at least two of three associated features: impaired comprehension of syntactically complex sentences, spared single-word comprehension, and spared object knowledge.
Diagnosis by clinical variant has important implications for managing a patient's symptoms. Because each PPA variant is associated with a particular pattern of deficits and neuropathological cause, classification can inform recommendations for appropriate behavioral or pharmacological interventions. In addition, each clinical variant is associated with a unique pattern and trajectory of decline (Brambati et al., 2015; Faria et al., 2014; Hsieh et al., 2012; Rogalski et al., 2011, 2014; Van Langenhove et al., 2016), and information regarding probable patterns of symptom evolution can help the patient's family prepare for current and future management of the disease.
For each PPA variant, diagnosis can be further supported by imaging if the clinical symptoms are accompanied by evidence of particular patterns of atrophy, hypoperfusion, or glucose hypometabolism in the brain (i.e., an imaging-supported diagnosis; Gorno-Tempini et al., 2011): for svPPA, involvement of the anterior temporal lobe in the left hemisphere (greater than right); for lvPPA, left posterior perisylvian or parietal involvement; and for nfvPPA, left posterior fronto-insular involvement.
Etiology
The etiology of neurodegeneration for each of the three variants of PPA has been linked to different pathological processes. There is a strong association between lvPPA and Alzheimer's disease (AD) neuropathology (Deramecourt et al., 2010; Josephs et al., 2008; Mesulam et al., 2008; Rohrer et al., 2012; Spinelli et al., 2017). Semantic and nonfluent/agrammatic variants of PPA are considered two of three forms of frontotemporal dementia (FTD), the third being behavioral variant FTD, a nonaphasic phenotype that is characterized by progressive deterioration of behavior and/or cognition (Rascovsky et al., 2011). With respect to the two FTD PPA variants, the majority of svPPA cases have been linked to the transactive response DNA binding protein 43 kDa (TDP-43) Type C (Cairns et al., 2007; Hodges et al., 2009; Josephs et al., 2011; Mackenzie et al., 2011; Rohrer, Geser, et al., 2010), and less commonly with TDP-43 Type B and tauopathies including Pick's disease and globular glial tauopathy (Spinelli et al., 2017). The neuropathological profile of nfvPPA is the most heterogeneous and includes 4R-tauopathies such as progressive supranuclear palsy and corticobasal degeneration (Chare et al., 2014; Deramecourt et al., 2010; Mesulam et al., 2014; Santos-Santos et al., 2016). Atypical neuropathologies associated with nfvPPA include Pick's disease (a 3R-tauopathy) and TDP-43 Type A (Spinelli et al., 2017).
Genetic Basis
PPA is usually sporadic, but in rare cases, there may be an underlying genetic basis. Although genetic testing is not currently standard procedure in the assessment of PPA, emerging research in the genetics of PPA has demonstrated it may serve as a complementary tool for diagnosis. In the largest genetic screening study of PPA to date, 14 of 403 cases (or 3.5%) had gene mutations, primarily in C9ORF72 and GRN (Ramos et al., 2019). In addition, nine of the 14 genetic cases had a first or second degree relative with a clinical diagnosis of dementia, suggesting most genetic PPA cases present with a family history of dementia. Previous case reports have also reported mutations in MAPT (Munoz et al., 2007; Tacik et al., 2017; Villa et al., 2011), including a study with 13 familial cases of PPA (Pickering-Brown et al., 2008). If motor function is affected (e.g., motor speech), pathological abnormalities in the FUS gene may be indicative of amyotrophic lateral sclerosis (Vance et al., 2009). In addition, pathological abnormalities in the TARDBP gene have been linked to various FTD phenotypes, including svPPA (Floris et al., 2015; González-Sánchez et al., 2018).
Due to the association between lvPPA and AD neuropathology, the genetics of AD is also important to consider. APP, PSEN1, and PSEN2 are known to cause AD and should be tested in patients with a family history of Alzheimer's dementia (see Loy et al., 2014, for a review). For sporadic cases of AD, genetic variations of Apolipoprotein E (ApoE) should be examined. While most people have the ApoE E3/E3 genotype, people with E3/E4 and E4/E4 genotypes are 3 and 8 times more likely to develop AD, respectively (Karch & Goate, 2015; Pericak-Vance & Haines, 2002). In contrast, individuals with the ApoE E2/E2 genotype have a 66%, 87%, and 99.6% lower odds ratio of developing AD compared to those with the ApoE E2/E3, ApoE E3/E3, and ApoE E4/E4 genotypes, respectively (Reiman et al., 2020).
Interestingly, some research suggests that unclassifiable PPA cases (where PPA criteria are met but classification by clinical variant is not possible) or mixed PPA cases (where classification criteria for two or more clinical variants are met) are more likely to have a genetic basis (Rohrer, 2014). Such unclassifiable and mixed PPA cases have been reported in 10%–41% of patients across studies (Gil-Navarro et al., 2013; Harris et al., 2013; Mesulam et al., 2012; Sajjadi et al., 2012; Utianski et al., 2019).
Overview of Evaluation Procedures
Patient History
In current clinical practice, diagnosis and classification of PPA are determined by a clinician (typically a behavioral neurologist) based on the patient's history of symptoms and family history, a neurological examination, a comprehensive evaluation of speech, language, and cognition, and clinical brain imaging. A detailed history helps to establish the symptom pattern at onset and the development of clinical features over time. Obtaining the patient's history usually involves an informal interview with the patient and/or their caregiver as well as medical chart review. The history should include the first symptoms observed; a timeline of symptom progression; how current symptoms impact activities of daily living; any concurrent psychiatric, memory, visuospatial, behavioral, and nondegenerative nervous system disorders; a developmental history with an emphasis on learning differences; and patient and family medical history.
There is great value in obtaining a clinical history directly from the patient, but severity of language and cognitive symptoms should be considered. Less impaired patients may be able to provide thoughtful insight regarding the onset and progression of symptoms. A patient interview also provides an opportunity for the clinician to begin forming impressions of the patient's ability to communicate. The following strategies and considerations can be applied when speaking with individuals with suspected PPA:
Use simple, frequent, and literal words because patients may have word or object knowledge loss (as in svPPA).
Use short sentences because patients may have impaired phonological working memory (as in lvPPA).
Speak in sentences with canonical word order (e.g., subject–verb–object) and offer encouragement to use multimodal communication such as writing, typing, or pointing to a picture board because patients may have difficulty understanding complex grammatical structures and present with motor speech disorders (as in nfvPPA).
For more severely impaired patients, a comprehensive interview may not be feasible, though the interaction can be shortened and used as an opportunity to build rapport.
During the interview or chart review, obtaining information regarding handedness and developmental history can be useful for the diagnostic team. Information about handedness may be relevant for interpreting imaging findings that deviate from the left-lateralized pattern of involvement typically observed in PPA. Additionally, a greater proportion of non-right-handed individuals with svPPA has been documented compared to the general population (Miller et al., 2013). Notably, a greater prevalence of developmental dyslexia has been observed in individuals with lvPPA relative to other PPA variants, and lvPPA patients with developmental learning differences are reported to have a relatively younger age-of-onset and better performance on global cognitive assessments than those without a significant developmental history (Miller et al., 2013, 2019). Since dyslexia is highly heritable (Darki et al., 2012), it may be informative to inquire about possible developmental learning differences in patients' first- and second-degree relatives, especially when the patient's neurodevelopmental history is unclear.
Speech-Language and Neuropsychological Assessment
Skilled professionals in speech, language, and cognition, including SLPs and neuropsychologists, should play an integral role in the diagnostic process. Specifically, SLPs' expertise in communication disorders may be especially valuable in the detection and characterization of subtle or complex speech and language features (see Henry & Grasso, 2018, for details on comprehensive assessment of speech and language in PPA), which is critical for classifying a patient by PPA variant. For example, SLPs are trained in differentiating between apraxic speech errors and phonemic errors in speech production, and assessing whether word retrieval difficulty stems from degraded semantic, phonological, or motoric processes.
The domains examined in a comprehensive speech and language battery are listed in Table 1, along with example tasks for assessing each domain and the expected behavioral pattern for each PPA variant. Global assessments of speech and language function, such as the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007), may help with determining overall severity and identifying patterns of deficits, but should not be the sole instrument used for evaluating speech and language symptoms. Because documentation of specific features (or their absence) is critical to PPA diagnosis and classification by clinical variant, it is important for examiners to evaluate multiple linguistic domains, including semantic, phonological, and syntactic processing, as well as written language and motor speech. The typical evaluation will comprise assessments of spontaneous speech, confrontation naming, repetition, single-word and sentence comprehension, verbal and nonverbal semantic processing, reading and spelling, and also a motor speech battery consisting of tasks of varying articulatory difficulty (i.e., diadochokinesis as well as production of single syllables, multisyllabic words, phrases, and sentences).
Table 1.
Domains of speech and language, example tasks, and expected observations in a comprehensive evaluation of primary progressive aphasia (PPA).
| Domain | Example tasks | Expected observations in svPPA | Expected observations in nfvPPA | Expected observations in lvPPA |
|---|---|---|---|---|
| Connected speech | Answer open-ended questions; verbal picture description | Use of simple or vague language; semantic paraphasias, circumlocution | Slow rate of speech; speech sound errors; simple or agrammatic sentences | Frequent word-finding difficulty; circumlocution; phonemic paraphasias |
| Motor speech | Diadochokinesis (DDK) | No motoric deficit | Consonant and/or vowel distortions on DDK | Phonological sequencing errors on sequential motor rates |
| Repetition | Repeat words/sentences | Relatively preserved | Difficulty with polysyllabic words and long sentences | Difficulty with polysyllabic words and long sentences |
| Auditory comprehension | Spoken word-to-picture matching; follow commands | Difficulty on words or commands involving low frequency nouns or verbs | Relatively intact auditory comprehension; difficulty following commands with complex grammar | Word comprehension relatively intact; difficulty following longer commands |
| Reading | Regular/irregular word and pseudoword reading; passage reading | Difficulty with irregular words | Slow rate; speech sound errors; agrammatic text reading | Difficulty with pseudowords |
| Writing | Regular/irregular word and pseudoword spelling; written picture description | Difficulty with irregular words | Spelling words relatively intact; simple or agrammatic sentences | Difficulty with pseudowords |
| Naming | Generative naming; confrontation naming | Impaired; semantic paraphasias | Relatively preserved | Impaired; phonemic paraphasias and/or circumlocution |
| Semantics | Match semantically related words and/or pictures | Low accuracy on low frequency words and less common objects | Relatively preserved | Relatively preserved |
| Phonology | Phoneme deletion; phoneme blending | Relatively preserved | Impaired in some patients | Impaired |
| Syntax | Building sentences with word cards; sentence-to-picture matching | Relatively preserved, but difficulty if sentences involve low frequency words | Impaired on complex sentences | Impaired on long sentences |
Note. svPPA = semantic variant primary progressive aphasia; nfvPPA = nonfluent/agrammatic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia.
Examination of cognitive abilities is also standard practice in an evaluation of PPA to rule out other diagnoses or to identify cognitive impairments that may have emerged during the progression of the disease. A cognitive screening tool, such as the Mini-Mental State Examination (MMSE; Folstein et al., 1975), can provide a gross estimation of functional impairment. However, for PPA patients, the MMSE may overestimate the severity of cognitive impairments because the test relies heavily on language comprehension and production abilities. A standard neuropsychological evaluation should also include assessments of memory, learning, arithmetic calculation, executive function, and visuospatial function (Kramer et al., 2003). In all three PPA variants, cognitive deficits arise during advanced stages of the disease. However, recent research suggests that visuospatial impairment, executive function deficits, and dyscalculia may develop earlier in lvPPA (Ramanan et al., 2019; Rohrer, Ridgway, et al., 2010; Tippett et al., 2019; Watson et al., 2018).
Subjective instruments can also be utilized during the evaluation to aid in the interpretation of more objective measures. The Geriatric Depression Scale (Yesavage et al., 1982) is a common questionnaire used for assessing mood in older adults and can be helpful for contextualizing patient symptoms or performance. Clinicians can also use instruments, such as the Clinical Dementia Rating scale (Morris, 1993), to characterize and monitor a patient's global level of impairment across multiple cognitive and functional domains. The Clinical Dementia Rating scale uses information from semistructured interviews with the patient and a reliable informant (e.g., caregiver or spouse) to rate the domains of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care.
Brain Imaging
MRI and other types of brain imaging may provide context for behavioral findings and can rule out other neurological causes for observed deficits. MRI is a noninvasive method commonly used for visualizing cortical and subcortical brain structures. For individuals with PPA, a structural MRI scan would be expected to show left-lateralized atrophy, indicated by cortical thinning and widening of sulci in the syndrome-specific left hemisphere regions noted above.
Additional neuroimaging techniques may complement clinical evaluations and structural brain scans for a more definitive PPA diagnosis. Arterial spin labeling MRI or single-photon emission computed tomography (which is less commonly used) can be used to measure blood flow or perfusion in the brain. Hypoperfused areas are indicated by regions of decreased blood flow, whether or not atrophy is observed. Similarly, PET imaging using the radioactive tracer F-18 fluorodeoxyglucose (FDG-PET) can localize regions of hypometabolism, or reduced glucose uptake, indicative of decreased neural function.
Important and emerging clinical applications of PET imaging involve the use of radioactive tracers to detect the presence of β-amyloid plaques and neurofibrillary tau tangle aggregation. For lvPPA, AD is the most common neuropathological finding at autopsy and is characterized by the brain's accumulation of both β-amyloid plaques and tau tangles, which trigger downstream neural dysfunction and atrophy (Chare et al., 2014; Kirshner, 2012; Mesulam et al., 2008; Rohrer et al., 2012; Santos-Santos et al., 2018; Spinelli et al., 2017). Accumulation of β-amyloid plaques, which may occur decades before an individual develops clinical symptoms, can be detected with PET imaging using nuclear ligand tracers that selectively bind to β-amyloid plaques (i.e., “amyloid-PET” imaging). A “positive” amyloid-PET scan, illustrated by increased tracer uptake in the cortex relative to a reference region, such as the cerebellar cortex, is a strong indication for underlying AD neuropathology only when the individual has cognitive or language symptoms. Across the three PPA variants, high rates of amyloid positivity have been observed in lvPPA, whereas svPPA and nfvPPA are more often associated with amyloid negativity (Santos-Santos et al., 2018). Amyloid-PET images of asymptomatic individuals should be interpreted with caution because amyloid positivity has also been observed in cognitively healthy people over the age of 50 years with no clear relationship between amyloid burden and cognitive abilities (Hedden et al., 2013).
Similar to amyloid-PET, aggregation of tau tangles can also be detected with PET imaging using nuclear ligand tracers that selectively bind to tau tangles (i.e., “tau-PET” imaging). However, unlike amyloid-PET, increased tau-PET tracer uptake in left posterior perisylvian or parietal regions has been strongly linked to the cognitive symptoms observed in lvPPA (Josephs et al., 2018; Ossenkoppele et al., 2016). To a lesser extent, tau-PET tracer uptake in left posterior fronto-insular areas has also been associated with symptoms in nfvPPA (Josephs et al., 2018; Tsai et al., 2019; Utianski et al., 2018). For svPPA, the utility of tau-PET imaging remains unclear (Josephs et al., 2018; Tsai et al., 2019; Whitwell et al., 2019).
Cerebrospinal Fluid Biomarkers
Additional neuropathological evidence for AD can be acquired by inspecting cerebrospinal fluid (CSF) for the 42 amino acid form of the amyloid-β peptide (Aβ42), total tau (t-tau), and phosphorylated tau (p-tau; Blennow & Hampel, 2003; see Blennow et al., 2010, for a review). AD is indicated with a reduction of CSF Aβ42, a biomarker for Aβ metabolism, and the formation of plaques (Fagan et al., 2006; Forsberg et al., 2008; Strozyk et al., 2003; Tapiola et al., 2009). Increased levels of p-tau in CSF is specific to AD pathology (Blennow, 2004), whereas high levels of t-tau in CSF are found in AD and other neurological conditions involving neuronal damage including stroke, traumatic brain injury, and Creutzfeldt–Jakob disease (Hesse et al., 2001; Öst et al., 2006; Riemenschneider et al., 2003; Tapiola et al., 2009). Extraction of these biomarkers is conducted by lumbar puncture, an invasive procedure involving the insertion of a needle into the spinal canal to extract CSF. A comprehensive review by Blennow et al. (2015) reported that, within AD and prodromal AD, levels of Aβ42, p-tau, and t-tau are respectively half, double, and triple the amount seen in controls. A summary of typical neuropathological and imaging findings for the PPA variants is shown in Table 2.
Table 2.
Neuropathological findings associated with the three primary progressive aphasia (PPA) variants.
| Variable | svPPA | nfvPPA | lvPPA |
|---|---|---|---|
| Disease epicenter | Anterior temporal lobe (L>R) | Left posterior fronto-insular cortex | Left posterior perisylvian or parietal cortex |
| Typical neuropathology | TDP-43C | Tau (CBD/PSP) | Alzheimer's disease (AD) |
| Modality | Evidence for PPA | ||
| Structural MRI (T1) | a Predominant atrophy or cortical thinning in disease epicenter | ||
| Perfusion imaging (ASL, MRI, or SPECT) | a Predominant hypoperfusion (lower signal) in disease epicenter | ||
| FDG-PET (F-18 fluorodeoxyglucose PET) | a Predominant hypometabolism (lower signal) in disease epicenter | ||
| Tau-PET ( b 18F-Flortaucipir PET) | Predominant tau aggregation (high signal) in disease epicenter in lvPPA and (to a lesser extent) in nfvPPA; the relation between tau aggregation and symptoms is unclear for svPPA | ||
| Amyloid-PET ( b 11C-labeled Pittsburgh Compound-B PET) | Elevated levels of amyloid (high signal) distributed along cortex indicates AD, which is closely associated with lvPPA; little or no amyloid (low signal) in nfvPPAand svPPA | ||
| Cerebrospinal fluid (CSF) via lumbar puncture | Decreased levels of Aβ and increased levels of total tau and phosphorylated tau compared to healthy controls indicates probable AD pathology, which is strongly linked to lvPPA | ||
Note. svPPA = semantic variant primary progressive aphasia; nfvPPA = nonfluent/agrammatic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; CBD = corticobasal degeneration; PSP = progressive supranuclear palsy; MRI = magnetic resonance imaging; ASL = arterial spin labeling; SPECT = single-photon emission computed tomography.
Imaging-supported criteria for variant classification in Gorno-Tempini et al. (2011).
Alternative labels for 18F-Flortaucipir and 11C-labeled Pittsburgh Compound-B are 18F-AV-1451 and 11C-PiB, respectively.
Genetic Testing
When behavioral and imaging findings are unclear, genetic testing is an additional tool that can be used to inform differential diagnosis. Genetic testing requires the patient to provide a biological specimen such as saliva or blood. A typical genetic testing procedure involves a buccal swab, in which cell samples are collected from inside the patient's mouth along their cheek. The chromosomes, proteins, and DNA from the cells are then examined for abnormalities. Genetic testing may be especially informative when findings from behavioral testing and neuroimaging are inconclusive.
Current Study
In the following case report, we illustrate the components of a comprehensive PPA evaluation, including both standard assessments and state-of-the-art diagnostic procedures. We discuss the case of a 49-year-old female patient who presented with progressive deterioration of speech and language that significantly affected activities of daily living and eventually led to her early retirement. She and her husband provided a developmental history, a timeline of symptoms, and information regarding her current functional status. Comprehensive neurological, speech, language, and cognitive testing were conducted. In addition, neuroimaging data were acquired, which included a high-resolution structural brain image and amyloid-PET, tau-PET, and FDG-PET imaging. Finally, CSF biomarkers were obtained, genetic testing was conducted, and a family history was gathered to further inform diagnostic decision making.
Case Report
Clinical History
DR was a right-handed, 49-year-old retired pediatrician who was seen at the Memory and Aging Center at the University of California, San Francisco (UCSF) with a primary complaint of progressive word-finding difficulty. Onset of symptoms began approximately 5 years prior to the evaluation. Her initial symptom was forgetting words during conversations but remembering them later, typically after a long delay. Her husband reported that she compensated for this difficulty by using words with similar meanings or sounds, or by simplifying her language during conversations. Over the next few years, language symptoms began to significantly impact her personal and professional life and became noticeable to friends and colleagues. For example, she required assistance spelling familiar words, exhibited increasing forgetfulness for names of people and objects, and had difficulty sequencing tasks in a logical manner and following conversations. DR was first seen locally by a neurologist and neuropsychologist, at which time FTD was introduced as a potential diagnosis. However, FDG-PET results indicated reduced glucose uptake in posterior brain regions. Soon after, she was seen at a university hospital where she was given the diagnoses of AD and PPA.
Subsequent to the initial neurological evaluation, DR left her medical practice, and she and her husband both noticed a striking decline in her language over the course of approximately 1 year. She spoke even less, used simpler language, began losing track of her own speech in conversations, and experienced greater difficulty with writing. She stopped driving and became more tearful. As the disorder progressed, she described several occasions where she experienced visual hallucinations in the form of shapes, figures, or fictional characters, but was aware they were not actually there. She was prescribed 15 mg daily of donepezil and 10 mg daily of citalopram, but these medications did not yield any change to her hallucinations. Her husband reported that citalopram, however, did result in slight improvements in mood.
Academic, Family, and Social History
DR's academic performance was reported to have been generally strong, although she mentioned spelling difficulty in childhood and stated that she failed calculus in medical school. She practiced medicine for nearly 20 years and specialized in pediatrics. At the time of her UCSF evaluation, she lived with her husband and two children. DR's family history was significant for several relevant factors. In her immediate family, one of her sons was diagnosed with dyslexia, and her mother reported a history of reading difficulty. Her maternal grandmother had memory loss in her later years, but lived past 100 years of age. Probable psychiatric illness was reported in one maternal relative, and an unspecified mental illness was indicated in one paternal relative.
Clinical Assessment
Throughout DR's evaluation at UCSF, she was loquacious, speaking excessively during and between testing sessions. Her behavior was gregarious and, at times, overly friendly, but she was interpersonally warm. On several occasions, she remarked that her husband is “good to look at” and she became tearful when talking about how “the poet inside of [her is]…watching [her] self fade away.” She frequently requested repetition of task instructions and auditorily presented stimuli. In general, she required additional time to complete testing due to difficulty with the tasks and her insistence on finishing tasks as accurately as possible.
During neurological examination, DR struggled with imitating the Luria sequence (a sequence of three hand motions repeated 3 times), indicating some difficulty with motor sequence learning. On motor examination, no pronator drift was noted, but there was notable parietal/sensory drift upward in the right upper extremity, indicating potential sensorimotor dysfunction. Muscle tone was normal. Rapid finger tapping was slightly slower on the right compared to the left, and reflexes were more brisk on the right compared to the left, both consistent with asymmetric involvement of the left motor cortex. However, there was no evidence of snout, rooting, jaw-jerk, palmomental, Hoffman's, or grasp reflexes, indicating relatively normal involuntary motor responses to sensory stimuli. Hearing was intact to finger rub bilaterally.
Tables 3 and 4 show scores from neuropsychological and speech-language assessments. On the Geriatric Depression Scale (Yesavage & Sheikh, 1986), she scored 17 out of 30 points, indicating mild depression. On a global test of cognition (MMSE; Folstein et al., 1975), DR earned a score of 19 of 30 points. She lost points on items involving orientation to time and place, verbal memory, and phrase repetition. Digit span forward was 4, and backward was 2. She demonstrated poor immediate and delayed verbal recall of a list of nine words (California Verbal Learning Test; Delis et al., 1987); when presented with 27 words to test recognition, she identified all nine words but also selected 10 false positive distractors. Visual memory performance was impaired, and her approach was disorganized. She drew seven of 17 components of a complex figure 10 min after copying it; however, she was able to recognize the correct figure from a field of four choices. She was unable to understand the verbal instructions on a test of executive function, which required her to alternate between ascending numbers and sequential days of the week (Modified Trails B). DR was also unable to do the Stroop inhibition task where she needed to name the ink color for color words while inhibiting reading the word itself (e.g., the word “blue” written in the color red). DR was accurate on only two of five simple arithmetic problems. Copy of the complex figure was largely accurate, but she performed poorly on the Digit Location subtest of the Visual Object and Space Perception battery (Warrington & James, 1991). In summary, impaired performance was observed in memory and learning, executive function, and arithmetic calculation, and results were mixed on assessment of visuospatial abilities.
Table 3.
DR's scores from her comprehensive clinical assessment (Part 1 of 2).
| Domain | Test | DR | Normative data mean score (SD) |
|---|---|---|---|
| Global Measures | MMSE | 19/30 | 29 (1.6) a |
| WAB-R Aphasia Quotient | 75.3/100 | ≥ 93.8 b | |
| CDR | 1/3 | 0.0 (0.0) c | |
| Mood | GDS | 17/30 | — |
| Memory and Learning | Digit Span Forward | 4 | 7.2 (0.9) d |
| Digit Span Backward | 2 | 5.4 (1.3) d | |
| CVLT: Four consecutive trials | 2,5,4,5 or 16/36 | 28.7 (3.1) c | |
| CVLT: After 30 s | 4/9 | 7.9 (1.6) c | |
| CVLT: After 10 min | 4/9 | 7.3 (1.6) c | |
| CVLT Recognition | 9/9, 10 false positives | 8.7 (0.9) c | |
| Complex Figure Recall | 7/17 | 11.3 (3.1) d | |
| Complex Figure Recognition | Correct | — | |
| Executive Function | Modified Trails B (lines per minute) | Discontinued | 37.2 (9.8) c |
| Stroop Inhibition | Discontinued | — | |
| Math | Calculations | 2/5 | 4.5 (0.5) c |
| Visuospatial | VOSP: Number Location | 2/10 | 8.6 (1.7) e |
| Complex Figure Copy | 15/17 | 15.0 (1.4) d | |
| Motor Speech | MSE Apraxia Rating | 0/7 | — |
| MSE Dysarthria Rating | 0/7 | — | |
| Auditory Comprehension | WAB-R Yes/No Questions | 60/60 | — |
| WAB-R Sequential Commands | 18/80 | — | |
| Repetition | WAB-R Repetition | 76/100 | 99.5 (0.9) c |
| MSE Multisyllabic Word Repetition | 18/30 | — | |
| MSE Sentence Repetition | 36/62 | — | |
| Bayles: Short Meaningful | 12/60 | 59.9 (0.4) f | |
| Bayles: Short Nonmeaningul | 46/60 | 59.8 (3.2) f | |
| Bayles: Long Frequent | 90/90 | 90.0 (0.0) f | |
| Bayles: Long Meaningful | 30/90 | 89.7 (0.7) f | |
| Bayles: Long Nonmeaningful | 4/90 | 86.4 (1.4) f |
Note. For MSE Apraxia and Dysarthria Ratings: 0 indicates no impairment present; severity ratings range from 1 (minimal) to 7 (profound). Dashes indicate no normative data available. MMSE = Mini-Mental State Examination (Folstein et al., 1975); WAB-R = Western Aphasia Battery–Revised (Kertesz, 2007); CDR = Clinical Dementia Rating (Morris, 1993); GDS = Geriatric Depression Scale (Yesavage & Sheikh, 1986); CVLT = California Verbal Learning Test (Delis et al., 1987); VOSP = Visual Object and Space Perception (Warrington & James, 1991); MSE = Motor Speech Exam (Wertz, LaPointe, & Rosenbek, 1984); Bayles = Bayles Repetition Test (Bayles et al., 1996).
Normative data are derived from:
Crum et al. (1993) for individuals 45-49 years of age with college experience or higher.
Table 4.
DR's scores from her comprehensive clinical assessment (Part 2 of 2).
| Domain | Test | DR | Normative data mean score (SD) |
|---|---|---|---|
| Naming | BNT | 48/60 | 55.4 (3.6) a |
| WAB-R Object Naming | 51/60 | — | |
| WAB-R Category Fluency: Animals in 1 min | 4 | 20.6 (5.1) b | |
| Phonemic Fluency: D-words in 1 min | 4 | 11.3 (3.1) b | |
| WAB-R Sentence Completion | 8/10 | — | |
| WAB-R Responsive Naming | 10/10 | — | |
| Semantics | WAB-R Auditory Word Comprehension | 57/60 | — |
| PPVT | 14/16 | 15.5 (0.7) c | |
| PPT Pictures Version | 42/52 | 50.9 (1.1) d | |
| Affect Matching: Faces and Emotions | 13/16 | 13.5 (1.5) e | |
| Phonological Processing |
APB Phoneme Deletion in Words | 4/10 | 9.9 (0.2) f |
| APB Phoneme Blending - Words and Pseudowords | 9/20 | 17.4 (2.7) f | |
| APB Phoneme Replacement | Discontinued | 26.3/30 (2.7) f | |
| Reading | ABRS Reading: Regular Words | 18/18 | 18.0 (0.0) f |
| ABRS Reading: Irregular Words | 16/18 | 17.8 (0.4) f | |
| ABRS Reading: Pseudowords | 15/18 | 16.6 (1.0) f | |
| Grandfather Passage Reading Rate | 64.5 WPM | — | |
| Spelling | ABRS Spelling: Regular Words | 8/10 | 9.6 (0.6) f |
| ABRS Spelling: Irregular Words | 1/10 | 9.1 (1.0) f | |
| ABRS Spelling: Pseudowords | 6/10 | 9.2 (0.7) f | |
| Syntax | Auditory Sentence–Picture Matching | 29/36 | — |
| NAT | 5/12 | — |
Note. Dashes indicate no normative data available. BNT = Boston Naming Test (Kaplan et al., 2001); WAB-R = Western Aphasia Battery–Revised (Kertesz, 2007); PPVT = Peabody Picture Vocabulary Test (Dunn & Dunn, 1981); PPT = Pyramids and Palm Trees (Howard & Patterson, 1992); APB = Arizona Phonological Battery (Rapcsak et al., 2009); ABRS = Arizona Battery of Reading and Spelling (Beeson et al., 2010); Auditory Sentence-Picture Matching (Wilson et al., 2010); NAT = Northwestern Anagram Test (Thompson et al., 2011); WPM = words per minute.
Normative data are derived from:
Tombaugh & Hubley (1997) for individuals 45-54 years of age.
The University of Arizona Aphasia Research Project; 34 healthy adults, mean age = 62.9 (11.4), range: 34–85; mean education = 15.8 years (2.8), range: 12–22 years, male:female 13:21.
A normal cohort from the UCSF Memory and Aging Center; 39 healthy adults, mean age = 68.3 (5.6), range: 53-82; mean education = 17.6 years (1.6), range: 14-20 years, male:female 12:27.
With regard to speech-language assessment, DR showed impairments across several tasks, including spontaneous speech, auditory verbal comprehension, repetition, and naming. On a general speech-language measure (WAB-R; Kertesz, 2007), her aphasia quotient was 75.3/100. DR spoke at a relatively normal rate, and her speech was well articulated, with no evidence of motoric impairment. Spontaneous speech was tangential, and prominent anomia was observed, with frequent pauses, mazes, revisions, phonemic paraphasias, and circumlocutions. For example, when asked to describe herself as a student, she said:
I am a…I'm…I don't have any siblings. Um I've always been…um…uh an A…student. Um…I've always been…um a science person from you know…. My my parents didn't know what to do with that because they…were both more…they're both teachers. And you know…they had not…you know sometimes in the…later stages when you um…uh…you know like…when it's like high school or something like that you can…have different things but…m-my dad…um…was in the…uh um you know in the…the higher up…kind of stuff.
No apraxia of speech or dysarthria was observed during a motor speech examination. Auditory comprehension was unimpaired for yes/no questions, but comprehension difficulty was noted on multistep commands. Repetition of simple words and common phrases and sentences was relatively accurate, but she struggled with repeating multisyllabic words as well as uncommon phrases and sentences. Confrontation naming performance was impaired on the Boston Naming Test (Kaplan et al., 2001), with errors primarily consisting of semantic and phonological paraphasias and circumlocutions. Phonemic cues were beneficial for six out of 12 object names. Generative naming of animals (semantic fluency) and words beginning with the letter “d” (phonemic fluency) were also poor, as she was only able to produce four of each within 1 min. However, lexical retrieval was less impaired during sentence completion and in response to wh-questions on the WAB-R. Her performance across these tasks indicated underlying severe verbal short-term memory and lexical retrieval deficits.
With regard to semantic processing, DR made only a few errors on auditory word-to-picture matching tasks (WAB-R Auditory Word Comprehension and a shortened version of the Peabody Picture Vocabulary Test; Dunn & Dunn, 1981) as well as a written emotion-to-face matching task. She demonstrated some impairment on a test of semantic associates (Pyramids and Palm Trees; Howard & Patterson, 1992), which involves selecting one of two pictures (e.g., palm tree and pine tree) that is more closely related to a target picture (e.g., pyramid).
Phonological manipulation tasks presented the most striking difficulty for DR across all language assessments. These tasks included deleting or replacing a phoneme from a word/pseudoword to create a new word/pseudoword and blending three sequential phonemes into a word/pseudoword (Arizona Phonological Battery; Henry et al., 2016; Rapcsak et al., 2009). She produced correct responses for less than half of items on the phoneme deletion (words) and blending (words and pseudowords) tasks. Phoneme deletion in pseudowords and phoneme replacement in words and pseudowords were discontinued due to profound difficulty with these tasks. DR was impaired on both reading and spelling tasks (Arizona Reading and Spelling Battery; Beeson et al., 2010). She exhibited the greatest difficulty on spelling irregular words (e.g., overregularization errors such as bole for “bowl”) and pseudowords (e.g., phonologically implausible errors such as dib for “dusp”). Passage reading was slow due to frequent pauses and numerous attempts at self-correcting phonemic paralexias. Performance on syntax comprehension (auditory sentence-to-picture matching; Wilson et al., 2010) was relatively better than syntax production (shortened version of the Northwestern Anagram Test; Thompson et al., 2011), but both scores indicated some impairment. For syntax comprehension, complex or long sentences were particularly difficult for her. It should be noted that the syntax production task required her to order word cards to create a sentence that matched a picture. Given the executive demands of this task, DR's poor performance is consistent with her difficulty with executive function tasks from the neuropsychological assessment.
Imaging and CSF Results
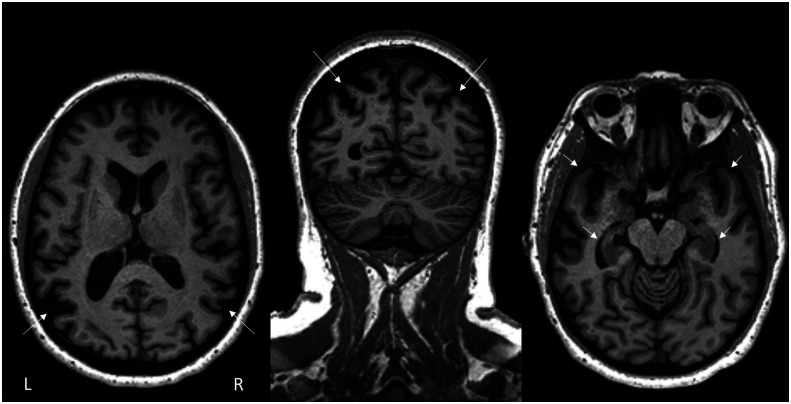
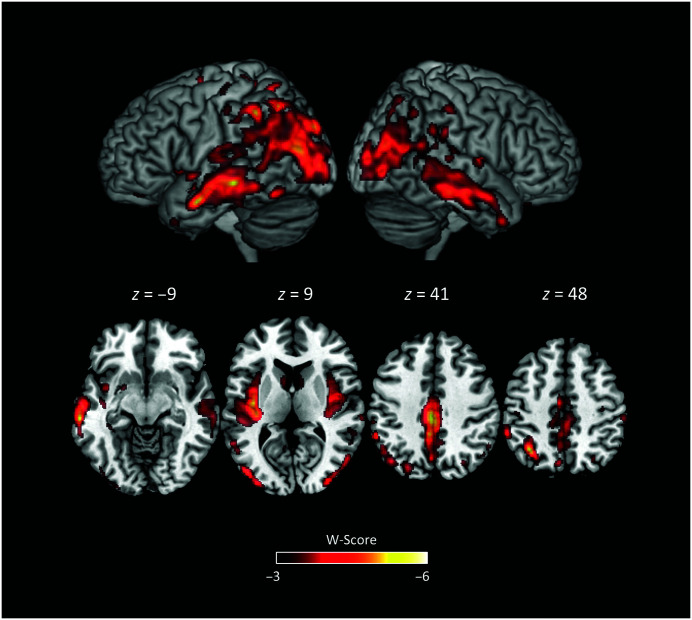
The neurologist's review of the structural MRI scan noted the presence of bilateral temporoparietal atrophy dorsally and posteriorly, with greater involvement of the left hemisphere than the right. Mild atrophy was also noted in bilateral hippocampi and anterior temporal lobes. Figure 1 shows the structural MRI scan with representative axial and coronal slices. In addition to the clinical review of the MRI scan, results from a single-subject voxel-based morphometry (VBM) analysis 1 compared DR's brain to those of a control group of N = 534 neurologically healthy adults (age range: 44–99 years; M ± SD = 68.7 ± 9.1; 302 women; see Supplemental Material S1 for more details). Figure 2 shows statistically significant differences between DR's brain and a control group using a standardized W-score (similar to a z score, but regresses out effects of age, gender, total intracranial volume, and scanner type; Ossenkoppele et al., 2015). VBM results demonstrated the greatest reduction in gray matter relative to controls in (left hemisphere more than right) inferior and superior parietal lobes, extending inferiorly and anteriorly to temporal cortices and posteriorly to occipital cortices. Significant atrophy was also noted in the insula (left greater than right), as well as medial regions including middle and posterior cingulate and precuneus.
Figure 1.
Arrows highlight the neurologist's impressions of DR's structural MRI scan. Greater atrophy was noted in the left hemisphere compared to the right. The left and middle images show parietal atrophy on axial and coronal views, respectively. The right image highlights anterior temporal lobe (top arrows) and hippocampal atrophy (bottom arrows).
Figure 2.
Single-subject voxel-based morphometry analysis of DR compared to a group of neurologically healthy adults. More negative W-score indicates greater difference from the group of healthy adults after controlling for age, gender, total intracranial volume, and scanner type.
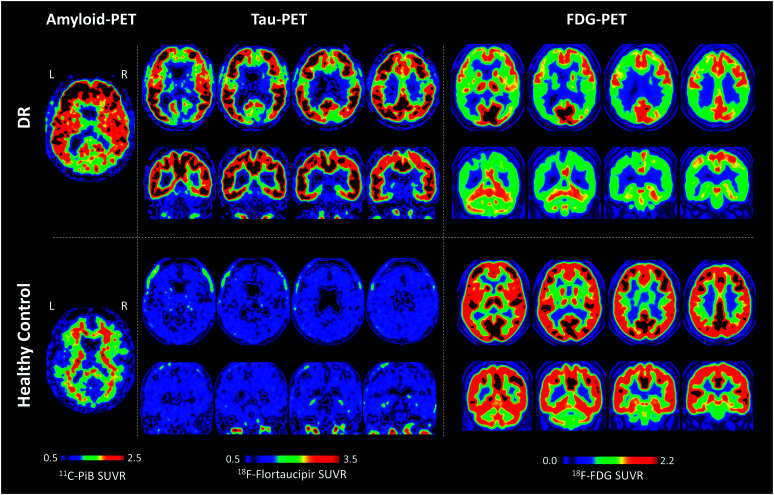
Amyloid-PET imaging was positive, as indicated by high standardized uptake value ratios (SUVRs) across the cortex (see Figure 3 left panel with results from a 64-year-old cognitively normal woman for comparison). The SUVR measure indicates the tracer's binding to β-amyloid plaques in the cortex relative to the radiotracer's binding in the cerebellum, typically devoid of β-amyloid plaques until very late stages in AD. In a cognitively healthy individual, one would expect limbic and heteromodal cortices (regions affected in AD) to show lack of the tracer's binding (i.e., present a clear contrast between gray and white matter; Dickerson et al., 2009; La Joie et al., 2012; Landau et al., 2011). However, Figure 3 (top row, left panel) demonstrates that DR's amyloid-PET scan was diffusely positive in the cortical gray matter, with SUVR greater than 2.5 in some frontal regions, and a general loss of gray–white matter contrast.
Figure 3.
Amyloid-, Tau- and FDG-PET results from DR (top row), amyloid- and tau-PET results from a 64-year-old cognitively normal female control participant (bottom row, left and middle panels) and FDG-PET from an age-matched (49-year-old) female control participant (bottom, right panel). PET = positron emission tomography; FDG = F-18 fluorodeoxyglucose; SUVR = standardized uptake value ratio.
Tau-PET results showed very elevated binding in fronto-temporo-parietal regions bilaterally and also in the posterior cingulate and precuneus. The SUVR indicates the tracer's binding to neurofibrillary tau tangle aggregates in the cortex relative to uptake in the inferior cerebellum. Again, higher-than-normal SUVR values were observed in the tau-PET scan (see Figure 3 , middle panel with results from the same 64-year-old cognitively normal woman for comparison).
Evaluation of the FDG-PET images primarily revealed hypometabolic areas in temporal, parietal, and frontal regions that were greater in the left than the right hemisphere (see Figure 3 , right panel with results from an age-matched, 49-year-old, cognitively normal woman for comparison). FDG-PET SUVR values indicate brain glucose utilization as compared to glucose uptake in the pons. The brain was more hypometabolic dorsally and posteriorly in comparison to ventral and anterior areas. Details regarding methodology for MRI and PET imaging and analyses can be found in Supplemental Material S1.
CSF biomarkers indicated that the level of Aβ42 peptide in the CSF was approximately half of that seen in controls. While the level of p-tau was nearly 3 times greater than the control level, the level of t-tau was almost 6 times greater. These results were consistent with probable AD neuropathology.
Genetic Testing Results
DR was tested for common pathogenic variants of gene mutations known to cause dementia. She was negative for pathogenic variants associated with AD including APP, PSEN1, and PSEN2, as well as negative for those associated with FTD including C9ORF72, GRN, and MAPT. Results showed she was ApoE 3/3, a finding that is not associated with dementia. Lastly, findings were also negative for TARDBP and FUS, both of which are associated with FTD and amyotrophic lateral sclerosis.
Discussion
This case report illustrates a comprehensive and multidisciplinary evaluation conducted with a 49-year-old woman with complaints of progressive word-finding difficulty. Taken together, the assessments revealed a complex, early-onset, nongenetic case of probable AD with an initial lvPPA syndrome. The following discussion will elaborate on the interpretation of findings from DR's comprehensive evaluation and examine how they relate to her PPA diagnosis.
The first step in the diagnostic process was determining whether DR met diagnostic criteria for PPA. Her most prominent clinical feature and primary complaint were her difficulties with speech and language, specifically, pervasive word-finding difficulty. At symptom onset, she and her caregiver reported behaviors consistent with word-finding difficulty in conversation including semantic and phonemic paraphasias and circumlocution. Importantly, aphasia was the most prominent symptom in her clinical presentation at onset, and her speech and language symptoms worsened to the point where she struggled in socializing and completing everyday work responsibilities. The clinical team determined that this patient satisfied the diagnostic inclusion criteria for PPA.
Ruling out exclusionary factors for PPA required careful consideration. This is often the case when a patient is assessed several years post-onset and the diagnostic team must tease apart symptoms that were likely predominant at disease onset from symptoms that arose during the evolution of the disease. DR's medical history and clinical presentation did not indicate a nondegenerative nervous system or medical disorder, nor a prominent initial behavioral disturbance, and psychiatric status was assessed to be within normal limits. To establish that the patient's speech-language symptoms were isolated enough to meet PPA criteria, the diagnostic team needed to discern whether the patient had prominent initial episodic memory, visual memory, and visuo-perceptual impairments, especially given her poor performance on tests of verbal memory and learning, executive function, and visuospatial abilities. In individuals with PPA and probable or definite AD pathology, episodic and visual memory impairments typically emerge with progression of the disease (Rohrer et al., 2012). Findings from recent studies also suggest that visuospatial and executive deficits are part of the symptom complex of lvPPA rather than a symptom of disease progression (Ramanan et al., 2019; Tippett et al., 2019; Watson et al., 2018). Similarly, dyscalculia is frequently observed in lvPPA and typically present in other parietal lobe syndromes (Rohrer, Ridgway, et al., 2010). Taking this into account with DR's overall history and pattern of symptoms, the diagnostic team ultimately concluded that her nonlinguistic cognitive impairments were not exclusionary and agreed that she satisfied criteria for PPA.
With regard to classification by clinical variant, the diagnostic team was confident that DR did not meet core criteria for nfvPPA. This diagnosis requires the presence of either agrammatic language production or effortful/halting speech with inconsistent speech sound errors and distortions (apraxia of speech). Evaluation of her spontaneous speech revealed intelligible, grammatical speech disrupted by frequent word-finding difficulty. In addition, the motor speech exam was unremarkable.
We considered the remaining two PPA variants, each of which presents with prominent anomia but with a different underlying cause of impairment. In DR's case, the underlying deficit appeared to be more phonological than semantic. Lexical retrieval errors and phonological paraphasias in spontaneous speech and confrontation naming were pervasive and prominent, and her repetition impairment followed a classic pattern typically seen in lvPPA. It was relatively easier for her to repeat long, overlearned phrases on the Bayles Repetition Test (Bayles et al., 1996; That must have…costed…cost…ugh…it must have been a pretty penny for “That must have cost a pretty penny”) as compared to nonmeaningful phrases that were either short (Cracked emanel enam emmanual I can't get that word for “Cracked enamel surface”) or long (Something about um… for “Loud ambassadors freeze stable waves”). Generally, repetition is relatively preserved in svPPA. DR also demonstrated difficulty carrying out sequential commands, a very reduced digit span, and impaired performance on the comprehension of long, complex sentences and phonological manipulation tasks. This pattern is consistent with phonological working memory impairment.
On the Arizona Battery of Reading and Spelling (Beeson et al., 2010), DR demonstrated a pattern of overregularization errors (reading /pɪnt/ for pint) and phonologically implausible errors (spelling “temenant” for tenement). However, her developmental and family history raised the possibility of an undiagnosed developmental reading disability, making it difficult to fully interpret the nature of these errors. Developmental learning disorders such as dyslexia are more prevalent in lvPPA compared to other variants (Miller et al., 2013, 2019). Although DR also presented with some evidence of semantic deficits, these impairments were much less pronounced. She made a few errors on the WAB-R Auditory Word Recognition subtest and the shortened version of the Peabody Picture Vocabulary Test, and several errors on the pictures version of the Pyramids and Palm Trees Test. However, it is possible that impaired executive functioning affected her ability to complete these tasks, which required selecting a target from an array of distractors. Participants with executive function deficits may fail to inhibit distractors, or may have difficulty orienting to the task for nonsemantic reasons. The latter was observed on the Pyramids and Palm Trees Test, as DR required frequent verbal reminders to select which of the two pictures on the bottom “goes best” with the one on top.
The neuroimaging and CSF results provided additional helpful information to inform DR's diagnosis. Positive amyloid-PET and tau-PET scans, reduced levels of Aβ42, and elevated levels of total tau and phosphorylated tau indicated probable underlying AD pathology, which is most associated with lvPPA. A common pattern of results across the VBM, FDG-PET, and tau-PET images suggests neurodegeneration in bilateral posterior temporoparietal cortices typically affected by the disease, with more pronounced involvement of the left parietal cortex compared to the right in the VBM and FDG-PET results. The VBM results also showed that the left inferior occipital gyrus was affected, which may be linked to DR's visual hallucinations (Holroyd et al., 2000).
Results from DR's genetic testing were unremarkable, but did rule out a clear genetic contribution to her disease. Her clinical and neuroimaging results strongly indicated early-onset AD (younger than age 65 years), and her genetic testing results demonstrated that her syndrome was not caused by dominantly inherited genes associated with the disease (e.g., Chartier-Harlin et al., 1991; van Duijn et al., 1994). She had the typical ApoE E3/E3 genotype and was negative for any other dementia-related genetic mutations including TARDBP, C9ORF72, GRN, MAPT, FUS, APP, PSEN1, and PSEN2. Together with findings from the clinical evaluation, the diagnostic team determined that DR's current presentation of symptoms was consistent with an initial lvPPA syndrome and early-onset AD pathology with predominant language symptoms, followed by the emergence of executive and memory difficulties.
Following DR's diagnosis, we provided the patient and family with several recommendations to facilitate and promote communication, which continued to be her most prominent area of difficulty. We encouraged DR's caregiver to monitor her hearing ability, since degraded auditory input will exacerbate auditory comprehension difficulties. We advised that communication partners speak to DR in short sentences no longer than four to five words, as this was her maximum forward digit span. We suggested that she continue participating in her local AD support group and dementia advocacy support group as these social activities can enrich patients' daily lives with community and purpose, and may alleviate some depressive symptoms. We also recommended that she increase her dosage of citalopram to 20 mg daily to target mood and daytime fatigue. Finally, she and her husband decided to enroll in our restitutive speech-language treatment research program aimed at improving object naming in svPPA and lvPPA (see Henry et al., 2019, for details on lexical retrieval treatment). Briefly, the 10-week treatment included twice weekly sessions with an SLP augmented by daily homework, which, together, provided guided, structured, and functional lexical retrieval practice. The benefit of treatment was demonstrated in improved confrontation naming accuracy for trained and untrained words at posttreatment. She improved from 25% to 90% accuracy for trained object names and modestly improved from 33% to 50% accuracy for untrained objects. Subjectively, she endorsed decreased word-finding difficulties and reduced frustration on a posttreatment survey.
Conclusion
In summary, this report outlined a comprehensive PPA evaluation using an illustrative case of a patient with suspected PPA. We have highlighted the critical diagnostic procedures and complex points of discussion that contributed to DR's diagnosis. Advances in clinical research and diagnostic procedures are of paramount importance for detecting PPA as early as possible and for identifying the likely pathological basis, particularly in cases that are behaviorally complex or mixed in their speech-language presentation. Neural and genetic biomarkers provide objective measures that can significantly contribute to clinical diagnosis, affording opportunities for beneficial treatments. As this case highlights, evidence-based treatment of PPA can have positive effects in mild-to-moderate cases (e.g., Beeson et al., 2011; Henry et al., 2019, 2018; Jokel et al., 2016), and this underscores the importance of early and accurate diagnosis and timely speech-language intervention.
Supplementary Material
Acknowledgments
This work was supported by P01AG019724 and P30AG062422 from the National Institute on Aging awarded to Bruce L. Miller, R01DC016291 from the National Institute on Deafness and Other Communication Disorders awarded to Maya L. Henry, and R01NS05091 from the National Institute of Neurological Disorders and Stroke and K24DC015544 from the National Institute on Deafness and Other Communication Disorders awarded to Maria Luisa Gorno-Tempini. Voxel-based morphometry analyses were performed using the Brainsight system, developed at University of California, San Francisco by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov and powered by the voxel-based lesion-symptom mapping script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the University of California, San Francisco Chancellor's Fund for Precision Medicine.
Funding Statement
This work was supported by P01AG019724 and P30AG062422 from the National Institute on Aging awarded to Bruce L. Miller, R01DC016291 from the National Institute on Deafness and Other Communication Disorders awarded to Maya L. Henry, and R01NS05091 from the National Institute of Neurological Disorders and Stroke and K24DC015544 from the National Institute on Deafness and Other Communication Disorders awarded to Maria Luisa Gorno-Tempini. Voxel-based morphometry analyses were performed using the Brainsight system, developed at University of California, San Francisco by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov and powered by the voxel-based lesion-symptom mapping script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the University of California, San Francisco Chancellor's Fund for Precision Medicine.
Footnote
Quantitative analysis of a structural MRI is not standard practice in routine clinical settings but is sometimes employed in academic medical settings to supplement the neurologist or radiologist's impression with a data-driven analysis.
References
- Bayles, K. A. , Tomoeda, C. K. , & Rein, J. A. (1996). Phrase repetition in Alzheimer's disease: Effect of meaning and length. Brain and Language, 54(2), 246–261. https://doi.org/10.1006/brln.1996.0074 [DOI] [PubMed] [Google Scholar]
- Beeson, P. M. , Rising, K. , Kim, E. S. , & Rapcsak, S. Z. (2010). A treatment sequence for phonological alexia/agraphia. Journal of Speech, Language, and Hearing Research, 53(2), 450–468. https://doi.org/10.1044/1092-4388(2009/08-0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, P. M. , King, R. M. , Bonakdarpour, B. , Henry, M. L. , Cho, H. , & Rapcsak, S. Z. (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 724–736. https://doi.org/10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney, R. J. , Henry, M. L. , Babiak, M. , Pressman, P. S. , Santos-Santos, M. A. , Narvid, J. , Mandelli, M. L. , Strain, P. J. , Miller, B. L. , Rankin, K. P. , Rosen, H. J. , & Gorno-Tempini, M. L. (2016). Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex, 82, 147–163. https://doi.org/10.1016/j.cortex.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow, K. (2004). Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRX, 1(2), 213–225. https://doi.org/10.1602/neurorx.1.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow, K. , Dubois, B. , Fagan, A. M. , Lewczuk, P. , de Leon, M. J. , & Hampel, H. (2015). Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimer's & Dementia, 11(1), 58–69. https://doi.org/10.1016/j.jalz.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow, K. , & Hampel, H. (2003). CSF markers for incipient Alzheimer's disease. The Lancet Neurology, 2(10), 605–613. https://doi.org/10.1016/S1474-4422(03)00530-1 [DOI] [PubMed] [Google Scholar]
- Blennow, K. , Hampel, H. , Weiner, M. , & Zetterberg, H. (2010). Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Reviews Neurology, 6(3), 131–144. https://doi.org/10.1038/nrneurol.2010.4 [DOI] [PubMed] [Google Scholar]
- Brambati, S. M. , Amici, S. , Racine, C. A. , Neuhaus, J. , Miller, Z. , Ogar, J. , . Dronkers, N. , Miller, B. L. , Rosen, H. , & Gorno-Tempini, M. L. (2015). Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser-based morphometry study. NeuroImage: Clinical, 8, 345–355. https://doi.org/10.1016/j.nicl.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns, N. J. , Bigio, E. H. , Mackenzie, I. R. , Neumann, M. , Lee, V. M.-Y. , Hatanpaa, K. J. , White, C. L., III. , Schneider, J. A. , Grinberg, L. T. , Halliday, G. , Duyckaerts, C. , Lowe, J. S. , Holm, I. E. , Tolnay, M. , Okamoto, K. , Yokoo, H. , Murayama, S. , Woulfe, J. , Munoz, D. G. , … Mann, D. M. A. (2007). Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathologica, 114(1), 5–22. https://doi.org/10.1007/s00401-007-0237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chare, L. , Hodges, J. R. , Leyton, C. E. , McGinley, C. , Tan, R. H. , Kril, J. J. , & Halliday, G. M. (2014). New criteria for frontotemporal dementia syndromes: Clinical and pathological diagnostic implications. Journal of Neurology, Neurosurgery, & Psychiatry, 85(8), 865–870. https://doi.org/10.1136/jnnp-2013-306948 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin, M.-C. , Crawford, F. , Houlden, H. , Warren, A. , Hughes, D. , Fidani, L. , Goate, A. , Rossor, M. , Roques, P. , Hardy, J. , & Mullan, M. (1991). Early-onset Alzheimer's disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature, 353(6347), 844–846. https://doi.org/10.1038/353844a0 [DOI] [PubMed] [Google Scholar]
- Crum, R. M. , Anthony, J. C. , Bassett, S. S. , & Folstein, M. F. (1993). Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA, 269(18), 2386–2391. https://doi.org/10.1001/jama.1993.03500180078038 [PubMed] [Google Scholar]
- Darki, F. , Peyrard-Janvid, M. , Matsson, H. , Kere, J. , & Klingberg, T. (2012). Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biological Psychiatry, 72(8), 671–676. https://doi.org/10.1016/j.biopsych.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Delis, D. C. , Kramer, J. H. , Kaplan, E. , & Ober, B. A. (1987). California Verbal Learning Test–Adult Version: Manual. The Psychological Corporation. [Google Scholar]
- Deramecourt, V. , Lebert, F. , Debachy, B. , Mackowiak-Cordoliani, M. A. , Bombois, S. , Kerdraon, O. , Buée, L. , Maurage, C.-A. , & Pasquier, F. (2010). Prediction of pathology in primary progressive language and speech disorders. Neurology, 74(1), 42–49. [DOI] [PubMed] [Google Scholar]
- Dickerson, B. C. , Bakkour, A. , Salat, D. H. , Feczko, E. , Pacheco, J. , Greve, D. N. , Grodstein, F. , Wright, C. I. , Blacker, D. , Diana Rosas, H. , Sperling, R. A. , Atri, A. , Growdon, J. H. , Hyman, B. T. , Morris, J. C. , Fischl, B. , & Buckner, R. L. (2009). The cortical signature of Alzheimer's disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex, 19(3), 497–510. https://doi.org/10.1093/cercor/bhn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, L. M. , & Dunn, L. M. (1981). Peabody Picture Vocabulary Test–Revised. AGS. [Google Scholar]
- Fagan, A. M. , Mintun, M. A. , Mach, R. H. , Lee, S.-Y. , Dence, C. S. , Shah, A. R. , LaRossa, G. N. , Spinner, M. L. , Klunk, W. E. , Mathis, C. A. , DeKosky, S. T. , Morris, J. C. , & Holtzman, D. M. (2006). Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Annals of Neurology, 59(3), 512–519. https://doi.org/10.1002/ana.20730 [DOI] [PubMed] [Google Scholar]
- Faria, A. V. , Sebastian, R. , Newhart, M. , Mori, S. , & Hillis, A. E. (2014). Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: Potential clinical significance. Aphasiology, 28(8–9), 948–963. https://doi.org/10.1080/02687038.2014.911241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris, G. , Borghero, G. , Cannas, A. , Di Stefano, F. , Murru, M. R. , Corongiu, D. , Cuccu, S. , Tranquilli, S. , Cherchi, M. V. , Serra, A. , Loi, G. , Marrosu, M. G. , Chiò, A. , & Marrosu, F. (2015). Clinical phenotypes and radiological findings in frontotemporal dementia related to TARDBP mutations. Journal of Neurology, 262(2), 375–384. https://doi.org/10.1007/s00415-014-7575-5 [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Forsberg, A. , Engler, H. , Almkvist, O. , Blomquist, G. , Hagman, G. , Wall, A. , Ringheim, A. , Långström, B. , & Nordberg, A. (2008). PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiology of Aging, 29(10), 1456–1465. https://doi.org/10.1016/j.neurobiolaging.2007.03.029 [DOI] [PubMed] [Google Scholar]
- Gil-Navarro, S. , Lladó, A. , Rami, L. , Castellví, M. , Bosch, B. , Bargalló, N. , Lomeña, F. , Reñé, R. , Montagut, N. , Antonell, A. , Molinuevo, J. L. , & Sánchez-Valle, R. (2013). Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dementia and Geriatric Cognitive Disorders, 35(1–2), 106–117. https://doi.org/10.1159/000346289 [DOI] [PubMed] [Google Scholar]
- González-Sánchez, M. , Puertas-Martín, V. , Esteban-Pérez, J. , García-Redondo, A. , Borrego-Hernández, D. , Méndez-Guerrero, A. , Llamas-Velasco, S. , Herrero-San Martin, A. , Cordero-Vázquez, P. , Herrero-Manso, M. C. , Pérez-Martínez, D. A. , & Villarejo-Galende, A. (2018). TARDBP mutation associated with semantic variant primary progressive aphasia, case report and review of the literature. Neurocase, 24(5–6), 301–305. https://doi.org/10.1080/13554794.2019.1581225 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Dronkers, N. F. , Rankin, K. P. , Ogar, J. M. , Phengrasamy, L. , Rosen, H. J. , Johnson, J. K. , Weiner, M. W. , & Miller, B. L. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. https://doi.org/10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, M. , Mickanin, J. , Onishi, K. , Hughes, E. , D'Esposito, M. , Ding, X.-S. , Alavi, A. , & Reivich, M. (1996). Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer's disease. Journal of Cognitive Neuroscience, 8(2), 135–154. https://doi.org/10.1162/jocn.1996.8.2.135 [DOI] [PubMed] [Google Scholar]
- Harris, J. M. , Gall, C. , Thompson, J. C. , Richardson, A. M. , Neary, D. , du Plessis, D. , Pal, P. , Mann, D. M. A. , Snowden, J. S. , & Jones, M. (2013). Classification and pathology of primary progressive aphasia. Neurology, 81(21), 1832–1839. https://doi.org/10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Hedden, T. , Oh, H. , Younger, A. P. , & Patel, T. A. (2013). Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology, 80(14), 1341–1348. https://doi.org/10.1212/WNL.0b013e31828ab35d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. L. , & Grasso, S. M. (2018). Assessment of individuals with primary progressive aphasia. Seminars in Speech and Language, 39(3), 231–241. https://doi.org/10.1055/s-0038-1660782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. L. , Hubbard, H. I. , Grasso, S. M. , Dial, H. R. , Beeson, P. M. , Miller, B. L. , & Gorno-Tempini, M. L. (2019). Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. Journal of Speech, Language, and Hearing Research, 62(8), 2723–2749. https://doi.org/10.1044/2018_JSLHR-L-18-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. L. , Hubbard, H. I. , Grasso, S. M. , Mandelli, M. L. , Wilson, S. M. , Sathishkumar, M. T. , Fridriksson, J. , Daigle, W. , Boxer, A. L. , Miller, B. L. , & Gorno-Tempini, M. L. (2018). Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain, 141(6), 1799–1814. https://doi.org/10.1093/brain/awy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. L. , Rising, K. , DeMarco, A. T. , Miller, B. L. , Gorno-Tempini, M. L. , & Beeson, P. M. (2013). Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain and Language, 127(2), 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. L. , Wilson, S. M. , Babiak, M. C. , Mandelli, M. L. , Beeson, P. M. , Miller, Z. A. , & Gorno-Tempini, M. L. (2016). Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience, 28(2), 210–222. https://doi.org/10.1162/jocn_a_00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse, C. , Rosengren, L. , Andreasen, N. , Davidsson, P. , Vanderstichele, H. , Vanmechelen, E. , & Blennow, K. (2001). Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neuroscience Letters, 297(3), 187–190. https://doi.org/10.1016/S0304-3940(00)01697-9 [DOI] [PubMed] [Google Scholar]
- Hodges, J. R. , Mitchell, J. , Dawson, K. , Spillantini, M. G. , Xuereb, J. H. , McMonagle, P. , Nesytor, P. J. , & Patterson, K. (2009). Semantic dementia: Demography, familial factors and survival in a consecutive series of 100 cases. Brain, 133(1), 300–306. https://doi.org/10.1093/brain/awp248 [DOI] [PubMed] [Google Scholar]
- Holroyd, S. , Shepherd, M. L. , & Downs, J. H., III. (2000). Occipital atrophy is associated with visual hallucinations in Alzheimer's disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(1), 25–28. https://doi.org/10.1176/jnp.12.1.25 [DOI] [PubMed] [Google Scholar]
- Howard, D. , & Patterson, K. (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Hsieh, S. , Hodges, J. R. , Leyton, C. E. , & Mioshi, E. (2012). Longitudinal changes in primary progressive aphasias: Differences in cognitive and dementia staging measures. Dementia and Geriatric Cognitive Disorders, 34(2), 135–141. https://doi.org/10.1159/000342347 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , Kielar, A. , Anderson, N. D. , Black, S. E. , Rochon, E. , Graham, S. , Freedman, M. , & Tang-Wai, D. F. (2016). Behavioural and neuroimaging changes after naming therapy for semantic variant primary progressive aphasia. Neuropsychologia, 89, 191–216. https://doi.org/10.1016/j.neuropsychologia.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Hodges, J. R. , Snowden, J. S. , Mackenzie, I. R. , Neumann, M. , Mann, D. M. , & Dickson, D. W. (2011). Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathologica, 122(2), 137–153. https://doi.org/10.1007/s00401-011-0839-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Martin, P. R. , Botha, H. , Schwarz, C. G. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Graff-Radford, J. , Weigand, S. D. , Senjem, M. L. , Utianski, R. L. , Drubach, D. A. , Boeve, B. F. , Jones, D. T. , Knopman, D. S. , Petersen, R. C. , Jack, C. R., Jr. , Lowe, V. J. , & Whitwell, J. L. (2018). [18F]AV-1451 tau-PET and primary progressive aphasia. Annals of Neurology, 83(3), 599–611. https://doi.org/10.1002/ana.25183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Whitwell, J. L. , Duffy, J. R. , Vanvoorst, W. A. , Strand, E. A. , Hu, W. T. , Boeve, B. F. , Graff-Radford, N. R. , Parisi, J. E. , Knopman, D. S. , Dickson, D. W. , Jack, C. R. , & Petersen, R. C. (2008). Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology, 70(1), 25–34. https://doi.org/10.1212/01.wnl.0000287073.12737.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, E. , Goodglass, H. , & Weintraub, S. (2001). Boston Naming Test. Pro-Ed. [Google Scholar]
- Karch, C. M. , & Goate, A. M. (2015). Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biological Psychiatry, 77(1), 43–51. https://doi.org/10.1016/j.biopsych.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised. The Psychological Corporation. [Google Scholar]
- Kirshner, H. S. (2012). Primary progressive aphasia and Alzheimer's disease: Brief history, recent evidence. Current Neurology and Neuroscience Reports, 12(6), 709–714. https://doi.org/10.1007/s11910-012-0307-2 [DOI] [PubMed] [Google Scholar]
- Kramer, J. H. , Jurik, J. , Sharon, S. , Rankin, K. P. , Rosen, H. J. , Johnson, J. K. , & Miller, B. L. (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology, 16(4), 211–218. https://doi.org/10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- La Joie, R. , Perrotin, A. , Barré, L. , Hommet, C. , Mézenge, F. , Ibazizene, M. , Camus, V. , Abbas, A. , Landeau, B. , Guilloteau, D. , de La Sayette, V. , Eustache, F. , Desgranges, B. , & Chételat, G. (2012). Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (Aβ) load in Alzheimer's disease dementia. The Journal of Neuroscience, 32(46), 16265–16273. https://doi.org/10.1523/JNEUROSCI.2170-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau, S. M. , Harvey, D. , Madison, C. M. , Koeppe, R. A. , Reiman, E. M. , Foster, N. L. , Weiner, M. W. , Jagust, W. J. , & the Alzheimer's Disease Neuroimaging Initiative. (2011). Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiology of Aging, 32(7), 1207–1218. https://doi.org/10.1016/j.neurobiolaging.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy, C. T. , Schofield, P. R. , Turner, A. M. , & Kwok, J. B. (2014). Genetics of dementia. The Lancet, 383(9919), 828–840. https://doi.org/10.1016/S0140-6736(13)60630-3 [DOI] [PubMed] [Google Scholar]
- Lukic, S. , Mandelli, M. L. , Welch, A. , Jordan, K. , Shwe, W. , Neuhaus, J. , Miller, Z. , Hubbard, H. I. , Henry, M. , Miller, B. L. , Dronkers, N. F. , & Gorno-Tempini, M. L. (2019). Neurocognitive basis of repetition deficits in primary progressive aphasia. Brain and Language, 194, 35–45. https://doi.org/10.1016/j.bandl.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie, I. R. , Neumann, M. , Baborie, A. , Sampathu, D. M. , Du Plessis, D. , Jaros, E. , Perry, R. H. , Trojanowski, J. Q. , Mann, D. M. A. , & Lee, V. M. (2011). A harmonized classification system for FTLD-TDP pathology. Acta Neuropathologica, 122(1), 111–113. https://doi.org/10.1007/s00401-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, C. R. , Hardy, C. J. , Volkmer, A. , Russell, L. L. , Bond, R. L. , Fletcher, P. D. , Clark, C. N. , Mummery, C. J. , Schott, J. M. , Rossor, M. N. , Fox, N. C. , Crutch, S. J. , Rohrer, J. D. , & Warren, J. D. (2018). Primary progressive aphasia: A clinical approach. Journal of Neurology, 265(6), 1474–1490. https://doi.org/10.1007/s00415-018-8762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M.-M. (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. https://doi.org/10.1002/ana.410110607 [DOI] [PubMed] [Google Scholar]
- Mesulam, M.-M. (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. https://doi.org/10.1002/ana.91 [PubMed] [Google Scholar]
- Mesulam, M.-M. , & Weintraub, S. (1992). Spectrum of primary progressive aphasia. Bailliere's Clinical Neurology, 1(3), 583–609. [PubMed] [Google Scholar]
- Mesulam, M.-M. , Weintraub, S. , Rogalski, E. J. , Wieneke, C. , Geula, C. , & Bigio, E. H. (2014). Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain, 137(4), 1176–1192. https://doi.org/10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M.-M. , Wicklund, A. , Johnson, N. , Rogalski, E. , Léger, G. C. , Rademaker, A. , Weintraub, S. , & Bigio, E. H. (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology, 63(6), 709–719. https://doi.org/10.1002/ana.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M.-M. , Wieneke, C. , Thompson, C. , Rogalski, E. , & Weintraub, S. (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(5), 1537–1553. https://doi.org/10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Z. A. , Mandelli, M. L. , Rankin, K. P. , Henry, M. L. , Babiak, M. C. , Frazier, D. T. , Lobach, I. V. , Bettcher, B. M. , Wu, T. Q. , Rabinovici, G. D. , Graff-Radford, N. R. , Miller, B. L. , & Gorno-Tempini, M. L. (2013). Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain, 136(11), 3461–3473. https://doi.org/10.1093/brain/awt242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Z. A. , Spina, S. , Pakvasa, M. , Rosenberg, L. , Watson, C. , Mandelli, M. L. , Paredes, M. F. , La Joie, R. , Rabinovici, G. D. , Rosen, H. J. , Grinberg, L. T. , Huang, E. J. , Miller, B. L. , Seeley, W. W. , & Gorno-Tempini, M. L. (2019). Cortical developmental abnormalities in logopenic variant primary progressive aphasia with dyslexia. Brain Communications, 1(1), fcz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. C. (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412–2413. https://doi.org/10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Munoz, D. G. , Ros, R. , Fatas, M. , Bermejo, F. , & de Yebenes, J. G. (2007). Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. American Journal of Alzheimer's Disease & Other Dementias, 22(4), 294–299. https://doi.org/10.1177/1533317507302320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary, D. , Snowden, J. S. , Gustafson, L. , Passant, U. , Stuss, D. , Black, S. , Freedman, M. , Kertesz, A. , Robert, P. H. , Albert, M. , Boone, K. , Miller, B. L. , Cummings, J. , & Benson, B. F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. https://doi.org/10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Ossenkoppele, R. , Pijnenburg, Y. A. L. , Perry, D. C. , Cohn-Sheehy, B. I. , Scheltens, N. M. , Vogel, J. W. , Kramer, J. H. , van der Vlies, A. E. , La Joie, R. , Rosen, H. J. , van der Flier, W. M. , Grinberg, L. T. , Rozemuller, A. J. , Huang, E. J. , van Berckel, B. N. M. , Miller, B. L. , Barkhof, F. , Jagust, W. J. , Scheltens, P. , … Rabinovici, G. D. (2015). The behavioural/dysexecutive variant of Alzheimer's disease: Clinical, neuroimaging and pathological features. Brain, 138(9), 2732–2749. https://doi.org/10.1093/brain/awv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele, R. , Schonhaut, D. R. , Schöll, M. , Lockhart, S. N. , Ayakta, N. , Baker, S. L. , O'Neil, J. P. , Janabi, M. , Lazaris, A. , Cantwell, A. , Vogel, J. , Santos, M. , Miller, Z. A. , Bettcher, B. M. , Vossel, K. A. , Kramer, J. H. , Gorno-Tempini, M. L. , Miller, B. L. , Jagust, W. J. , & Rabinovici, G. D. (2016). Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain, 139(5), 1551–1567. https://doi.org/10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öst, M. , Nylen, K. , Csajbok, L. , Öhrfelt, A. O. , Tullberg, M. , Wikkelsö, C. , Nellgård, P. , Rosengren, L. , Blennow, K. , & Nellgård, B. (2006). Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology, 67(9), 1600–1604. https://doi.org/10.1212/01.wnl.0000242732.06714.0f [DOI] [PubMed] [Google Scholar]
- Pericak-Vance, M. A. , & Haines, J. L. (2002). Alzheimer's disease. In King R. A. & Rotter J. I. (Eds.), The genetic basis of common diseases, 2nd edition (pp. 818–830). Oxford University Press. [Google Scholar]
- Pickering-Brown, S. M. , Rollinson, S. , Du Plessis, D. , Morrison, K. E. , Varma, A. , Richardson, A. M. , Neary, D. , Snowden, J. S. , & Mann, D. M. (2008). Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: Comparison with patients with MAPT and no known mutations. Brain, 131(3), 721–731. https://doi.org/10.1093/brain/awm331 [DOI] [PubMed] [Google Scholar]
- Quental, N. B. M. , Brucki, S. M. D. , & Bueno, O. F. A. (2013). Visuospatial function in early Alzheimer’s disease—The use of the Visual Object and Space Perception (VOSP) battery. PLOS ONE, 8(7), e68398. https://doi.org/10.1371/journal.pone.0068398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan, S. , Roquet, D. , Goldberg, Z.-L. , Hodges, J. R. , Piguet, O. , Irish, M. , & Lambon Ralph, M. A. (2019). Establishing two principal dimensions of cognitive variation in Logopenic Progressive Aphasia. bioRxiv, 767269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, E. M. , Dokuru, D. R. , Van Berlo, V. , Wojta, K. , Wang, Q. , Huang, A. Y. , Miller, Z. A. , Karydas, A. M. , Bigio, E. H. , Rogalski, E. , Weintraub, S. , Rader, B. , Miller, B. L. , Gorno-Tempini, M. L. , Mesulam, M.-M. , & Coppola, G. (2019). Genetic screen in a large series of patients with primary progressive aphasia. Alzheimer's & Dementia, 15(4), 553–560. https://doi.org/10.1016/j.jalz.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak, S. Z. , Beeson, P. M. , Henry, M. L. , Leyden, A. , Kim, E. , Rising, K. , Andersen, S. , & Cho, H. (2009). Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex, 45(5), 575–591. https://doi.org/10.1016/j.cortex.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky, K. , Hodges, J. R. , Knopman, D. , Mendez, M. F. , Kramer, J. H. , Neuhaus, J. , van Swieten, J. C. , Seelaar, H. , Dopper, E. G. P. , Onyike, C. U. , Hillis, A. E. , Josephs, K. A. , Boeve, B. F. , Kertesx, A. , Seeley, W. W. , Rankin, K. P. , Johnson, J. K. , Gorno-Tempini, M.-L. , … Rosen, H. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. https://doi.org/10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman, E. M. , Arboleda-Velasquez, J. F. , Quiroz, Y. T. , Huentelman, M. J. , Beach, T. G. , Casseli, R. J. , Chen, Y. , Su, Y. , Myers, A. J. , Hardy, J. , Vonsattel, J. P. , Younkin, S. G. , Bennett, D. A. , De Jager, P. L. , Larson, E. B. , Crane, P. K. , Dirk Keene, C. , Ilyas Kamboh, M. , … . Alzheimer's Disease Genetics Consortium. (2020). Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nature Communications, 11(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider, M. , Wagenpfeil, S. , Vanderstichele, H. , Otto, M. , Wiltfang, J. , Kretzschmar, H. , Vanmechelen, E. , Förstl, H. , & Kurz, A. (2003). Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Molecular Psychiatry, 8(3), 343–347. https://doi.org/10.1038/sj.mp.4001220 [DOI] [PubMed] [Google Scholar]
- Rogalski, E. , Cobia, D. , Harrison, T. M. , Wieneke, C. , Weintraub, S. , & Mesulam, M.-M. (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. https://doi.org/10.1212/WNL.0b013e31821ccd3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, E. , Cobia, D. , Martersteck, A. , Rademaker, A. , Wieneke, C. , Weintraub, S. , & Mesulam, M. M. (2014). Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology, 83(13), 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. (2014). The genetics of primary progressive aphasia. Aphasiology, 28(8–9), 941–947. https://doi.org/10.1080/02687038.2014.911242 [Google Scholar]
- Rohrer, J. D. , Geser, F. , Zhou, J. , Gennatas, E. D. , Sidhu, M. , Trojanowski, J. Q. , DeArmond, S. J. , Miller, B. L. , & Seeley, W. W. (2010). TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology, 75(24), 2204–2211. https://doi.org/10.1212/WNL.0b013e318202038c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. , Ridgway, G. R. , Crutch, S. J. , Hailstone, J. , Goll, J. C. , Clarkson, M. J. , Mead, S. , Beck, J. , Mummery, C. , Ourselin, S. , Warrington, E. K. , Rossor, M. N. , & Warren, J. D. (2010). Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage, 49(1), 984–993. https://doi.org/10.1016/j.neuroimage.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. , Rossor, M. N. , & Warren, J. D. (2012). Alzheimer's pathology in primary progressive aphasia. Neurobiology of Aging, 33(4), 744–752. https://doi.org/10.1016/j.neurobiolaging.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi, S. A. , Patterson, K. , Arnold, R. J. , Watson, P. C. , & Nestor, P. J. (2012). Primary progressive aphasia: A tale of two syndromes and the rest. Neurology, 78(21), 1670–1677. https://doi.org/10.1212/WNL.0b013e3182574f79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos, M. A. , Mandelli, M. L. , Binney, R. J. , Ogar, J. , Wilson, S. M. , Henry, M. L. , Hubbard, H. I. , Meese, M. , Attygalle, S. , Rosenberg, L. , Pakvasa, M. , Trojanowski, J. Q. , Grinberg, L. T. , Rosen, H. , Boxer, A. L. , Miller, B. L. , Seeley, W. W. , & Gorno-Tempini, M. L. (2016). Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurology, 73(6), 733–742. https://doi.org/10.1001/jamaneurol.2016.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos, M. A. , Rabinovici, G. D. , Iaccarino, L. , Ayakta, N. , Tammewar, G. , Lobach, I. , Henry, M. L. , Hubbard, I. , Mandelli, M. L. , Spinelli, E. , Miller, Z. A. , Pressman, P. S. , O'Neil, J. P. , Ghosh, P. , Lazaris, A. , Meyer, M. , Watson, C. , Yoon, S. J. , Rosen, H. J. , … Gorno-Tempini, M. L. (2018). Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurology, 75(3), 342–352. https://doi.org/10.1001/jamaneurol.2017.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden, J. S. , Goulding, P. J. , & Neary, D. (1989). Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology, 2(3), 167–182. [Google Scholar]
- Spinelli, E. G. , Mandelli, M. L. , Miller, Z. A. , Santos-Santos, M. A. , Wilson, S. M. , Agosta, F. , Grinberg, L. T. , Huang, E. J. , Trojanowski, J. Q. , Meyer, M. , Henry, M. L. , Comi, G. , Rabinovici, G. , Rosen, H. J. , Filippi, M. , Miller, B. L. , Seeley, W. W. , & Gorno-Tempini, M. L. (2017). Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology, 81(3), 430–443. https://doi.org/10.1002/ana.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk, D. , Blennow, K. , White, L. R. , & Launer, L. J. (2003). CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology, 60(4), 652–656. https://doi.org/10.1212/01.WNL.0000046581.81650.D0 [DOI] [PubMed] [Google Scholar]
- Tacik, P. , Sanchez-Contreras, M. , DeTure, M. , Murray, M. E. , Rademakers, R. , Ross, O. A. , Wszolek, Z. K. , Parisi, J. E. , Knopman, D. S. , Petersen, R. C. , & Dickson, D. W. (2017). Clinicopathologic heterogeneity in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) due to microtubule-associated protein tau (MAPT) p.P301L mutation, including a patient with globular glial tauopathy. Neuropathology and Applied Neurobiology, 43(3), 200–214. https://doi.org/10.1111/nan.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola, T. , Alafuzoff, I. , Herukka, S.-K. , Parkkinen, L. , Hartikainen, P. , Soininen, H. , & Pirttilä, T. (2009). Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of Neurology, 66(3), 382–389. https://doi.org/10.1001/archneurol.2008.596 [DOI] [PubMed] [Google Scholar]
- Tombaugh, T. N. , & Hubley, A. M. (1997). The 60-item Boston Naming Test: Norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology, 19(6), 922–932. https://doi.org/10.1080/01688639708403773 [DOI] [PubMed] [Google Scholar]
- Thompson, C. K. , Weintraub, S. , & Mesulam, M.-M. (2011). Northwestern Anagram Test. Northwestern University. [Google Scholar]
- Tippett, D. C. , Breining, B. , Goldberg, E. , Meier, E. , Sheppard, S. M. , Sherry, E. , Stockbridge, M. , Suarez, A. , Wright, A. E. , & Hillis, A. E. (2019). Visuomotor figure construction and visual figure delayed recall and recognition in primary progressive aphasia. Aphasiology, 1–15. https://doi.org/10.1080/02687038.2019.1670330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, R. M. , Bejanin, A. , Lesman-Segev, O. , LaJoie, R. , Visani, A. , Bourakova, V. , O'Neil, J. P. , Janabi, M. , Baker, S. , Lee, S. E. , Perry, D. C. , Bajorek, L. , Karydas, A. , Spina, S. , Grinberg, L. T. , Seeley, W. W. , Ramos, E. M. , Coppola, G. , Gorno-Tempini, M. L. , … Rabinovici, G. D. (2019). 18 F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimer's Research & Therapy, 11(1), 13. https://doi.org/10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, R. S. , Kenyon, L. C. , Trojanowski, J. Q. , Gonatas, N. , & Grossman, M. (1996). Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Annals of Neurology, 39(2), 166–173. [DOI] [PubMed] [Google Scholar]
- Utianski, R. L. , Botha, H. , Martin, P. R. , Schwarz, C. G. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Butts, A. M. , Lowe, V. J. , Jack, C. R., Jr. , Senjem, M. L. , Spychalla, A. J. , Whitwell, J. L. , & Josephs, K. A. (2019). Clinical and neuroimaging characteristics of clinically unclassifiable primary progressive aphasia. Brain and Language, 197, 104676. https://doi.org/10.1016/j.bandl.2019.104676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Whitwell, J. L. , Schwarz, C. G. , Senjem, M. L. , Tosakulwong, N. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Petersen, R. C. , Jack, C. R., Jr. , Lowe, V. J. , & Josephs, K. A. (2018). Tau-PET imaging with [18F] AV-1451 in primary progressive apraxia of speech. Cortex, 99, 358–374. https://doi.org/10.1016/j.cortex.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn, C. M. , de Knijff, P. , Cruts, M. , Wehnert, A. , Havekes, L. M. , Hofman, A. , & Van Broeckhoven, C. (1994). Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer's disease. Nature Genetics, 7(1), 74–78. https://doi.org/10.1038/ng0594-74 [DOI] [PubMed] [Google Scholar]
- Van Langenhove, T. , Leyton, C. E. , Piguet, O. , & Hodges, J. R. (2016). Comparing longitudinal behavior changes in the primary progressive aphasias. Journal of Alzheimer's Disease, 53(3), 1033–1042. https://doi.org/10.3233/JAD-160010 [DOI] [PubMed] [Google Scholar]
- Vance, C. , Rogelj, B. , Hortobágyi, T. , De Vos, K. J. , Nishimura, A. L. , Sreedharan, J. , Hu, X. , Smith, B. , Ruddy, D. , Wright, P. , Ganesalingam, J. , Williams, K. L. , Tripathi, V. , Al-Saraj, S. , Al-Chalabi, A. , Leigh, P. N. , Blair, I. P. , Nicholson, G. , de Belleroche, J. , … Shaw, C. E. (2009). Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science, 323(5918), 1208–1211. https://doi.org/10.1126/science.1165942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, C. , Ghezzi, L. , Pietroboni, A. M. , Fenoglio, C. , Cortini, F. , Serpente, M. , Cantoni, C. , Ridolfi, E. , Marcone, A. , Benussi, L. , Ghidoni, R. , Jacini, F. , Arighi, A. , Fumagalli, G. G. , Mandelli, A. , Binetti, G. , Cappa, S. , Bresolin, N. , Scarpini, E. , & Galimberti, D. (2011). A novel MAPT mutation associated with the clinical phenotype of progressive nonfluent aphasia. Journal of Alzheimer's Disease, 26(1), 19–26. https://doi.org/10.3233/JAD-2011-102124 [DOI] [PubMed] [Google Scholar]
- Watson, C. L. , Possin, K. , Allen, I. E. , Hubbard, H. I. , Meyer, M. , Welch, A. E. , Rabinovici, G. D. , Rosen, H. , Rankin, K. P. , Miller, Z. , Santos-Santos, M. A. , Kramer, J. H. , Miller, B. L. , & Gorno-Tempini, M. L. (2018). Visuospatial functioning in the primary progressive aphasias. Journal of the International Neuropsychological Society, 24(3), 259–268. https://doi.org/10.1017/S1355617717000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington, E. K. (1975). The selective impairment of semantic memory. The Quarterly Journal of Experimental Psychology, 27(4), 635–657. [DOI] [PubMed] [Google Scholar]
- Warrington, E. K. , & James, M. (1991). The Visual Object and Space Battery Perception. Thames Valley Company. [Google Scholar]
- Wertz, R. T. , LaPointe, L. L. , & Rosenbek, J. C. (1984). Apraxia of speech in adults: The disorder and its management. Grune & Stratton. [Google Scholar]
- Whitwell, J. L. , Martin, P. R. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Schwarz, C. G. , Weigand, S. D. , Sintini, I. , Senjem, M. L. , Ertekin-Taner, N. , Botha, H. , Utianski, R. L. , Graff-Radford, J. , Jones, D. T. , Boeve, B. F. , Knopman, D. S. , Petersen, R. C. , Jack, C. R. , Lowe, V. J. , & Josephs, K. A. (2019). The influence of β-amyloid on [18F]AV-1451 in semantic variant of primary progressive aphasia. Neurology, 92(7), e710–e722. https://doi.org/10.1212/WNL.0000000000006913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. M. , Dronkers, N. F. , Ogar, J. M. , Jang, J. , Growdon, M. E. , Agosta, F. , Henry, M. L. , Miller, B. L. , & Gorno-Tempini, M. L. (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience, 30(50), 16845–16854. https://doi.org/10.1523/JNEUROSCI.2547-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. , & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. https://doi.org/10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Yesavage, J. A. , & Sheikh, J. I. (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist, 5(1–2), 165–173. https://doi.org/10.1300/J018v05n01_09 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.