Significance
Tumor-infiltrating macrophages are involved in tumor development, progression, and metastasis. Much recent attention has focused on the modification of macrophage functions in tumors as a basis for cancer treatments. SIRPα and SIRPβ1 are present in macrophages and function as a regulator for phagocytosis. We found that monotherapy with an anti-SIRPα/SIRPβ1 antibody (Ab) suppresses tumor formation by bladder and mammary cancer cells in mice. This effect is likely mediated by promoting the cytotoxic and phagocytic activity of macrophages against tumor cells. Notably, the antitumor effect of the Ab is largely dependent on its binding to SIRPβ1 on macrophages. Our findings reveal mechanisms of antitumor action of the anti-SIRPα/SIRPβ1 Ab and targeting of SIRPβ1 as a potential strategy for cancer immunotherapy.
Keywords: antibody, cancer, macrophage, immunotherapy, SIRPα
Abstract
The interaction of signal regulatory protein α (SIRPα) on macrophages with CD47 on cancer cells is thought to prevent antibody (Ab)-dependent cellular phagocytosis (ADCP) of the latter cells by the former. Blockade of the CD47-SIRPα interaction by Abs to CD47 or to SIRPα, in combination with tumor-targeting Abs such as rituximab, thus inhibits tumor formation by promoting macrophage-mediated ADCP of cancer cells. Here we show that monotherapy with a monoclonal Ab (mAb) to SIRPα that also recognizes SIRPβ1 inhibited tumor formation by bladder and mammary cancer cells in mice, with this inhibitory effect being largely dependent on macrophages. The mAb to SIRPα promoted polarization of tumor-infiltrating macrophages toward an antitumorigenic phenotype, resulting in the killing and phagocytosis of cancer cells by the macrophages. Ablation of SIRPα in mice did not prevent the inhibitory effect of the anti-SIRPα mAb on tumor formation or its promotion of the cancer cell–killing activity of macrophages, however. Moreover, knockdown of SIRPβ1 in macrophages attenuated the stimulatory effect of the anti-SIRPα mAb on the killing of cancer cells, whereas an mAb specific for SIRPβ1 mimicked the effect of the anti-SIRPα mAb. Our results thus suggest that monotherapy with Abs to SIRPα/SIRPβ1 induces antitumorigenic macrophages and thereby inhibits tumor growth and that SIRPβ1 is a potential target for cancer immunotherapy.
Macrophages are innate immune cells that show phenotypic heterogeneity and functional diversity; and they play key roles in development, tissue homeostasis and repair, and in cancer, as well as in defense against pathogens (1–3). In the tumor microenvironment (TME), macrophages are exposed to a variety of stimuli, including cell–cell contact, hypoxia, as well as soluble and insoluble factors such as cytokines, chemokines, metabolites, and extracellular matrix components (2, 4). These environmental cues promote the acquisition by macrophages of protumorigenic phenotypes that facilitate tumor development, progression, and metastasis as well as suppress antitumor immune responses (2, 4). A high density of macrophages within tumor tissue is associated with poor prognosis in patients with various types of cancer, including that of the bladder or breast (5–7). Depletion of macrophages in the TME or the reprogramming of these cells to acquire antitumorigenic phenotypes has been shown to ameliorate the immunosuppressive condition and result in a reduction in tumor burden in both preclinical and clinical studies (2, 4, 8, 9). Macrophages within the TME have therefore attracted much attention as a potential therapeutic target for cancer immunotherapy.
Signal regulatory protein α (SIRPα) is a transmembrane protein that possesses one NH2-terminal immunoglobulin (Ig)-V–like and two Ig-C domains in its extracellular region, as well as immunoreceptor tyrosine-based inhibition motifs in its cytoplasmic region (10, 11). The extracellular region of SIRPα interacts with that of CD47, another member of the Ig superfamily of proteins, with this interaction constituting a means of cell–cell communication. The expression of SIRPα in hematopoietic cells is restricted to the myeloid compartment—including macrophages, neutrophils, and dendritic cells (DCs)—whereas CD47 is expressed in most normal cell types as well as cancer cells (12, 13). The interaction of SIRPα on macrophages with CD47 on antibody (Ab)-opsonized viable cells such as blood cells or cancer cells prevents phagocytosis of the latter cells by the former (13–15), with this negative regulation of macrophages being thought to be mediated by SHP1, a protein tyrosine phosphatase that binds to the cytoplasmic region of SIRPα (14). Indeed, blockade of the CD47–SIRPα interaction by Abs to either SIRPα or CD47, in combination with a tumor-targeting Ab such as rituximab (anti-CD20), was found to enhance the Ab-dependent cellular phagocytosis (ADCP) activity of macrophages for cancer cells that do not express SIRPα, resulting in marked suppression of tumor formation in mice (15–19). Targeting of SIRPα in combination with a tumor-targeting Ab therefore provides a potential approach to cancer immunotherapy dependent on enhancement of the ADCP activity of macrophages for cancer cells. In contrast, the effect of Abs to SIRPα in the absence of a tumor-targeting Ab on the phagocytosis by macrophages of, as well as on tumor formation by, cancer cells that do not express SIRPα was minimal or limited.
We have now further examined the antitumor efficacy of a monoclonal Ab (mAb) to mouse SIRPα (MY-1) (20) in immunocompetent mice transplanted subcutaneously with several types of murine cancer cells that do not express SIRPα. This Ab prevents the binding of mouse CD47 to SIRPα and cross-reacts with mouse SIRPβ1 (15). We found that monotherapy with MY-1 efficiently attenuated the growth of tumors formed by bladder or mammary cancer cells. In addition, MY-1 markedly promoted the induction of antitumorigenic macrophages able to target these cancer cells. Furthermore, our results suggest that SIRPβ1 on macrophages likely participated in the antitumorigenic effect of MY-1.
Results
MY-1 Attenuates the Growth of Tumors Formed by Murine Bladder or Mammary Cancer Cells in Mice.
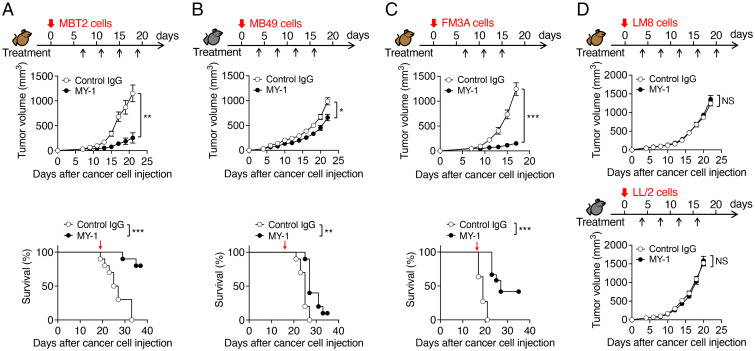
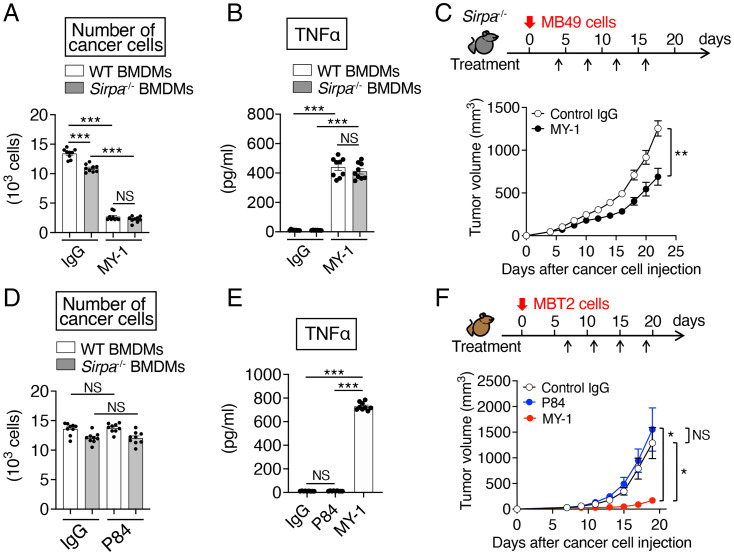
MY-1 recognizes and binds to the NH2-terminal Ig-V–like domain of mouse SIRPα and thereby efficiently blocks the transinteraction of CD47 with SIRPα (15). We first examined the antitumor effect of monotherapy with MY-1 on several types of murine cancer cells in immunocompetent mice. We initiated treatment with MY-1 or control IgG at 4 or 7 d after subcutaneous injection of cancer cells and then monitored tumor growth. Treatment of mice with MY-1 markedly inhibited the growth of tumors formed by the murine bladder cancer cell lines MBT2 or MB49 in syngeneic C3H/HeN (C3H) or C57BL/6J (B6) mice, respectively, compared with control IgG (Fig. 1 A and B), although the effect of MY-1 on the latter cell line was smaller than that on the former. Moreover, treatment with MY-1 prolonged the survival of mice injected with these bladder cancer cell lines (Fig. 1 A and B). Similarly, treatment with MY-1 markedly inhibited the growth of tumors formed by FM3A murine mammary cancer cells in syngeneic C3H mice and prolonged the survival of these animals (Fig. 1C). In contrast, MY-1 had no effect on tumor burden in C3H or B6 mice injected subcutaneously with syngeneic LM8 osteosarcoma cells or LL/2 lung cancer cells, respectively (Fig. 1D). These results suggested that monotherapy with the SIRPα mAb efficiently suppressed the growth of tumors formed by certain cancer cell types, including bladder and mammary cancer cells.
Fig. 1.
MY-1 attenuates the growth of tumors formed by murine bladder or mammary cancer cells in mice. (A–C) Tumor volume (Upper) and Kaplan–Meier survival curves (Lower) for immunocompetent mice injected subcutaneously with MBT2 (A), MB49 (B), or FM3A (C) cells and treated intraperitoneally with MY-1 or control IgG (each at a dose of 200 μg) according to the indicated schedule. Data for tumor volume are means ± SEM [n = 10 (A), 18 (B), or 11 or 12 (control IgG or MY-1, respectively) (C) mice per group examined in two (A and C) or three (B) separate experiments]. Survival data are for 10 (A and B) or 11 or 12 (control IgG or MY-1, respectively) (C) mice per group examined in two separate experiments. Red arrows (Lower) indicate the last dose of treatment. (D) Tumor volume for immunocompetent mice injected subcutaneously with LM8 (Upper) or LL/2 (Lower) cells and treated intraperitoneally with MY-1 or control IgG (each at 200 μg) according to the indicated schedule. Data are means ± SEM (n = 10 mice per group examined in two separate experiments). *P < 0.05, **P < 0.01, ***P < 0.001, NS by two-way repeated-measures ANOVA with the Greenhouse–Geisser correction and Šídák’s multiple-comparison test (tumor volume) or the log-rank test (survival). NS, not significant (in all figures where found).
We next tested which types of immune cells participated in inhibition of tumor growth by MY-1 in the MBT2 model. Five days after cancer cell injection, we treated mice with an mAb (clone AFS98) to the mouse colony-stimulating factor 1 receptor (CSF1R) (21) to deplete macrophages in tumors, and the animals were further treated at 7 d and every 4 d thereafter with the same mAb in combination with either MY-1 or control IgG. Flow cytometry revealed that tumor-infiltrating macrophages (CD45+CD11b+F4/80+Ly6Clow cells) were largely depleted at 4 d after the first injection of anti-CSF1R (SI Appendix, Figs. S1A and S2A). Such macrophage depletion was associated with almost complete prevention of the suppressive effect of MY-1 on tumor growth (SI Appendix, Fig. S1A). Treatment with an mAb to mouse CD8α (clone YTS169.4) (22) effectively depleted CD8+ T cells in the spleen (SI Appendix, Figs. S1B and S2B), whereas such depletion only partially suppressed the antitumor effect of MY-1 on MBT2 cells (SI Appendix, Fig. S1B). By contrast, although natural killer (NK) cells were efficiently depleted with polyclonal Abs (pAbs) to asialoganglioside GM1 (23) (SI Appendix, Figs. S1C and S2C), such depletion had no effect on the inhibition of tumor growth by MY-1 (SI Appendix, Fig. S1C). These results thus suggested that macrophages are largely responsible for the elimination of murine bladder cancer cells by MY-1 in vivo.
MY-1 Promotes the Killing of Murine Bladder Cancer Cells by Macrophages.
Given that we found that macrophages are important for the antitumor effect of MY-1 on murine bladder cancer cells, we next examined how MY-1 might inhibit tumor growth by regulating macrophage function in the TME. We previously showed that monotherapy with MY-1 markedly suppressed the growth of tumors formed by the murine renal cancer cell line RENCA (15), which expresses SIRPα at a high level (SI Appendix, Fig. S3A). This inhibitory effect of MY-1 was thought to be attributable both to ADCP by macrophages of MY-1–opsonized tumor cells as well as to simultaneous blockade by MY-1 of CD47–SIRPα signaling that negatively regulates such ADCP activity (15). However, all the murine cancer cell lines (MBT2, MB49, FM3A, LM8, and LL/2) tested for the effect of MY-1 monotherapy in the present study (Fig. 1) showed minimal expression of SIRPα but substantial CD47 expression (SI Appendix, Fig. S3A). Moreover, coculture of mouse bone marrow–derived macrophages (BMDMs) with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled (SIRPα-positive) RENCA cells in the presence of MY-1 for 4 h resulted in marked phagocytosis of the latter cells by the former, consistent with our previous data (15) (SI Appendix, Fig. S3B), whereas MY-1 had no substantial effect on the phagocytosis of CFSE-labeled (SIRPα-negative) MBT2 or MB49 cells (SI Appendix, Figs. S3B and S4A). The inhibitory effect of MY-1 monotherapy on the growth of tumors formed by MBT2 or MB49 cells was thus not likely due to promotion of ADCP of MY-1–opsonized cancer cells by macrophages.
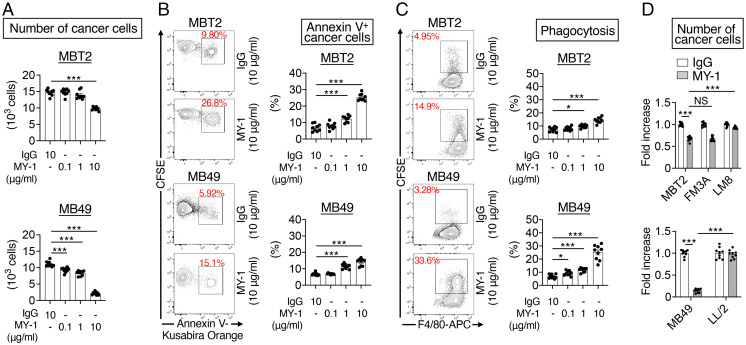
We next examined whether MY-1 stimulated the killing of MBT2 and MB49 cells by macrophages in vitro. CFSE-labeled MBT2 or MB49 cells were cocultured with BMDMs from C3H or B6 mice, respectively, and treated with either MY-1 or control IgG for 16 h. Treatment with MY-1 resulted in a marked reduction in the number of MBT2 or MB49 cells (CFSE+F4/80– cells) in a concentration-dependent manner (Fig. 2A and SI Appendix, Fig. S4B). MY-1 treatment also increased the proportion of annexin V+CFSE+F4/80– (apoptotic or dead) cancer cells among all CFSE+F4/80– cancer cells (Fig. 2B and SI Appendix, Fig. S4B) as well as that of CFSE+F4/80+ BMDMs (BMDMs that had likely phagocytosed CFSE-labeled cancer cells) among all F4/80+ BMDMs (Fig. 2C and SI Appendix, Fig. S4A). By contrast, MBT2 or MB49 cells exposed to MY-1 in the absence of BMDMs showed no loss of viability (SI Appendix, Fig. S5). These results thus suggested that MY-1 stimulates the killing activity of macrophages for MBT2 and MB49 cells, leading to inhibition of the growth of tumors formed by these bladder cancer cells in vivo. Moreover, the F(ab')2 fragment of MY-1 promoted the killing of MBT2 cells by C3H BMDMs to an extent similar to that observed with intact MY-1 (SI Appendix, Fig. S6), suggesting that this effect of MY-1 is independent of the interaction between the Fc region of the Ab and the Fcγ receptor on macrophages. MY-1 also reduced the number of murine mammary cancer FM3A cells during coculture with BMDMs, whereas it had no such effect on murine osteosarcoma LM8 or lung cancer LL/2 cells (Fig. 2D), consistent with the effects of MY-1 monotherapy on the growth of tumors formed by these cancer cells in vivo (Fig. 1 C and D).
Fig. 2.
MY-1 promotes the killing of murine bladder cancer cells by macrophages. (A–C) CFSE-labeled MBT2 or MB49 cells were incubated for 16 h with BMDMs from C3H or B6 mice, respectively, as well as with MY-1 or control IgG at the indicated concentrations. The cells were then harvested for flow cytometric determination of the number of cancer cells (CFSE+F4/80–) (A), the percentage of apoptotic or dead cancer cells (annexin V+CFSE+F4/80) among all cancer cells (CFSE+F4/80–) (B), and the percentage of CFSE+F4/80+ BMDMs (BMDMs that had phagocytosed CFSE-labeled cancer cells) among all F4/80+ BMDMs (C). Representative plots are shown for B and C. Quantitative data are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group). (D) CFSE-labeled MBT2, FM3A, or LM8 cells (Upper) or CFSE-labeled MB49 or LL/2 cells (Lower) were incubated for 16 h with BMDMs from C3H mice (Upper) or B6 mice (Lower) as well as with MY-1 or control IgG (each at 10 μg/mL). The number of cancer cells was then determined as in A. Data are expressed as fold increase relative to the corresponding value for cells treated with control IgG and are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group). *P < 0.05, ***P < 0.001, NS (Welch and Brown–Forsythe ANOVA with Dunnett’s T3 multiple-comparison test).
MY-1 Promotes Polarization of Mouse Macrophages toward an M1-Like Phenotype.
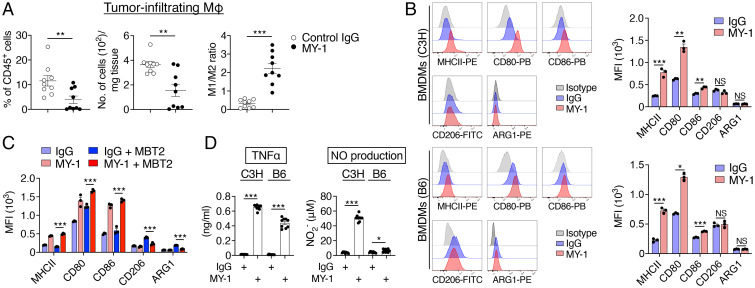
Tumor-infiltrating macrophages are thought to be derived from bone marrow monocytes or tissue-resident macrophages and to be polarized toward the protumorigenic M2-like phenotype rather than the antitumorigenic M1-like phenotype (2, 24, 25). We found that treatment of mice bearing MBT2 tumors with MY-1 resulted in a reduction in the frequency and total number of tumor-infiltrating macrophages (CD45+CD11b+Ly6ClowF4/80+ cells) as well as an increase in the M1 (F4/80+MHCIIhigh)/M2 (F4/80+CD206+) macrophage ratio apparent 21 d after cancer cell injection (Fig. 3A and SI Appendix, Fig. S2A). Indeed, culture of either C3H or B6 BMDMs with MY-1 for 48 h resulted in a marked increase in the expression of M1 macrophage markers, including MHCII, CD80, and CD86 (24, 25), compared with that apparent after treatment with control IgG (Fig. 3B). Such culture of BMDMs with MY-1 had no effect on the expression of the M2 macrophage markers CD206 or arginase 1 (ARG1) (24, 25) (Fig. 3B). Moreover, exposure of cocultures of C3H BMDMs and MBT2 cells to MY-1 for 24 h also resulted in a marked increase in the expression of MHCII, CD80, and CD86 on BMDMs (Fig. 3C). Coculture with MBT2 cells increased the expression of CD206 and ARG1 in BMDMs compared with that apparent for BMDMs cultured alone, and this up-regulation of CD206 and ARG1 was markedly attenuated by MY-1 treatment (Fig. 3C). Exposure of either C3H or B6 BMDMs alone to MY-1 also increased the production of tumor necrosis factor α (TNFα) and nitric oxide (NO) (Fig. 3D), both of which are produced by proinflammatory M1 macrophages (24, 25). Moreover, the F(ab')2 fragment of MY-1 promoted the expression of M1 macrophage makers MHCII, CD80, and CD86 and the production of TNFα in C3H BMDMs (SI Appendix, Fig. S6 D and E). These results thus suggested that MY-1 promotes the polarization of macrophages in the TME toward an M1-like phenotype.
Fig. 3.
MY-1 promotes the polarization of mouse macrophages toward an M1-like phenotype. (A) C3H mice were injected subcutaneously with MBT2 cells and treated with control IgG or MY-1 (each at 200 μg) every 4 d beginning 7 d after cell injection. At 21 d after cancer cell injection, immune infiltrates of tumors were analyzed by flow cytometry for the frequency of macrophages (CD45+CD11b+Ly6ClowF4/80+ cells) among all viable CD45+ cells (Left), the absolute number of macrophages (Middle), and the ratio of M1-like macrophages (CD45+CD11b+Ly6ClowF4/80+MHCIIhigh cells) to M2-like macrophages (CD45+CD11b+Ly6ClowF4/80+CD206+ cells) (Right). MΦ, macrophages. (B) BMDMs from C3H (Upper) or B6 (Lower) mice were treated with control IgG or MY-1 (each at 10 μg/mL) for 48 h and then subjected to flow cytometric analysis of the expression of MHCII, CD80, CD86, CD206, and ARG1. Representative overlaid flow cytometry histograms (Left) and quantitative data for median fluorescence intensity (MFI) (Right) from three separate experiments are shown. (C) MFI for MHCII, CD80, CD86, CD206, and ARG1 expression in C3H BMDMs cultured with or without MBT2 cells and in the presence of control IgG or MY-1 (each at 10 μg/mL) for 24 h. (D) C3H or B6 BMDMs were treated with control IgG or MY-1 (each at 10 μg/mL) for 48 h, after which culture supernatants were collected and assayed for TNFα and NO. All quantitative data are means ± SEM for n = 9 mice per group examined in three separate experiments (A), for three separate experiments (n = 3 for each group) (B and C), or for three separate experiments, each performed in triplicate (n = 9 per group) (D). *P < 0.05, **P < 0.01, ***P < 0.001, NS (two-tailed Welch’s t test).
Importance of TNFα for MY-1–Induced Killing of Murine Bladder Cancer Cells by Macrophages.
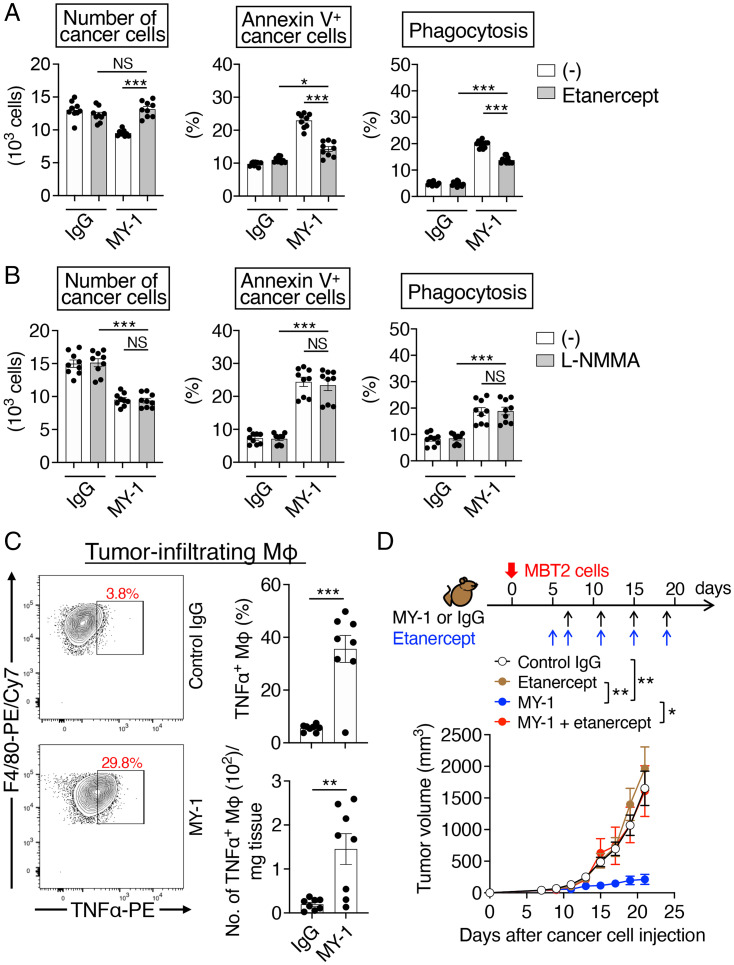
TNFα and NO, both of which are produced by activated macrophages, have the potential to mediate killing of tumor cells (26, 27). With the use of an inhibitor of TNFα, etanercept (a recombinant Fc fusion protein of the p75 human TNF receptor) (28), as well as an inhibitor of NO synthase, NG-methyl-L-arginine acetate salt (L-NMMA) (29), we next tested whether these cytotoxic factors indeed participate in the MY-1–induced killing of MBT2 cells by BMDMs. Exposure of cocultures of MBT2 cells and C3H BMDMs to etanercept for 16 h attenuated the reduction in cancer cell number as well as the increase both in the proportion of annexin V+ cancer cells and in the extent of phagocytosis of cancer cells by BMDMs induced by MY-1 (Fig. 4A). Etanercept also suppressed the MY-1–induced killing of MB49 cells by BMDMs, albeit to a lesser extent compared with its effect on that of MBT2 cells (SI Appendix, Fig. S7A). In contrast, L-NMMA did not affect the MY-1–induced killing of MBT2 or MB49 cells by BMDMs in vitro (Fig. 4B and SI Appendix, Fig. S7B).
Fig. 4.
Importance of TNFα for the MY-1–induced killing of murine bladder cancer cells by macrophages. (A and B) CFSE-labeled MBT2 cells were incubated for 16 h with C3H BMDMs in the presence of control IgG or MY-1 (each at 10 μg/mL) as well as of vehicle (–), etanercept at 10 μg/mL (A), or L-NMMA at 300 μΜ (B). The number of cancer cells (Left), the percentage of annexin V+ cancer cells (Middle), and the percentage of BMDMs that had phagocytosed CFSE-labeled cancer cells (Right) were then determined as in Fig. 2 A–C. (C) C3H mice injected subcutaneously with MBT2 cells were treated with control IgG or MY-1 as in Fig. 1A. At 21 d after cancer cell injection, immune infiltrates of tumors were analyzed by flow cytometry for the frequency of TNFα+ macrophages among all viable macrophages (Upper Right) and the absolute number of TNFα+ macrophages (Lower Right). Representative plots are also shown (Left). (D) Tumor volume for C3H mice injected subcutaneously with MBT2 cells and treated with the indicated agents according to the indicated schedule. All quantitative data are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group) (A and B); for n = 8 mice per group examined in three experiments (C); or for n = 9 (control IgG, etanercept, or MY-1 + etanercept) or n = 10 (MY-1) mice per group examined in two experiments (D). *P < 0.05, **P < 0.01, ***P < 0.001, NS by Welch and Brown–Forsythe ANOVA with Dunnett’s T3 multiple-comparison test (A and B), by the two-tailed Welch’s t test (C), or by two-way repeated-measures ANOVA with the Greenhouse–Geisser correction and Tukey’s multiple-comparison test (D).
Treatment of MBT2 tumor–bearing mice with MY-1 increased the proportion of TNFα-positive macrophages in the tumors (Fig. 4C and SI Appendix, Fig. S2A), and neutralization of TNFα by additional treatment with etanercept prevented the inhibitory effect of MY-1 on tumor growth (Fig. 4D). Moreover, we found that MBT2 cells, as well as MB49 and FM3A cells, were more susceptible to TNFα-induced apoptosis or cell death compared with LM8 or LL/2 cells (SI Appendix, Fig. S7C). These results suggested that TNFα is a key factor in the MY-1–dependent antitumor effect of macrophages on murine bladder and mammary cancer cells both in vitro and in vivo.
Importance of Mouse SIRPβ1 for MY-1–Induced Killing of Murine Bladder Cancer Cells by Macrophages.
We next examined whether SIRPα on mouse macrophages is indeed important for the inhibitory effect of MY-1 on the growth of tumors formed by murine bladder cancer cells in syngeneic mice. The MY-1–induced reduction in the number of MB49 cells cultured with BMDMs from SIRPα-deficient (Sirpa−/−) mice on the B6 background (30) was similar to that apparent for MB49 cells cultured with BMDMs from wild-type (WT) mice (Fig. 5A). Ablation of SIRPα also did not affect the MY-1–induced production of TNFα by BMDMs (Fig. 5B). Deletion of Sirpa in B6 mice did not appear to greatly influence either the growth of tumors formed by MB49 cells or the inhibitory effect of MY-1 on MB49 tumor growth (Fig. 5C and SI Appendix, Fig. S8). These results suggested that the antitumor effect of MY-1 on murine MB49 bladder cancer cells is largely independent of SIRPα on macrophages. We also found that P84, another mAb to mouse SIRPα, did not induce the killing of MB49 cells by either WT or Sirpa−/− BMDMs (Fig. 5D). In addition, P84 did not promote the production of TNFα or affect the expression of MHCII, CD80, CD86, CD206, or ARG1 by WT BMDMs (Fig. 5E and SI Appendix, Fig. S9). Treatment with P84 also did not inhibit the growth of tumors formed by MBT2 cells in C3H mice (Fig. 5F).
Fig. 5.
Role of SIRPα in the macrophage-dependent antitumor effect of MY-1 on murine bladder cancer cells. (A and D) CFSE-labeled MB49 cells were cocultured for 16 h with BMDMs from WT or Sirpa−/− mice in the presence of control IgG, MY-1 (A), or P84 (D), each at 10 μg/mL, after which the number of cancer cells was determined as in Fig. 2A. (B) WT or Sirpa−/− BMDMs were treated with control IgG or MY-1 (each at 10 μg/mL) for 48 h, after which culture supernatants were collected and assayed for TNFα. (C) Tumor volume for Sirpa−/− mice injected subcutaneously with MB49 cells and treated intraperitoneally with either control IgG or MY-1 (each at 200 μg) according to the indicated schedule. (E) TNFα production by C3H BMDMs treated with control IgG, MY-1, or P84 (each at 10 μg/mL) for 48 h was determined as in B. (F) Tumor volume for C3H mice injected subcutaneously with MBT2 cells and treated intraperitoneally with control IgG, MY-1, or P84 (each at 200 μg) according to the indicated schedule. All quantitative data are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group) (A, B, D, and E), or for n = 10 mice (C) or n = 9 (control IgG or P84) or 10 (MY-1) mice (F) per group examined in two separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001, NS by Welch and Brown–Forsythe ANOVA with Dunnett’s T3 multiple-comparison test (A, B, D, and E) or by two-way repeated-measures ANOVA with the Greenhouse–Geisser correction and Šídák's multiple-comparison test (C and F).
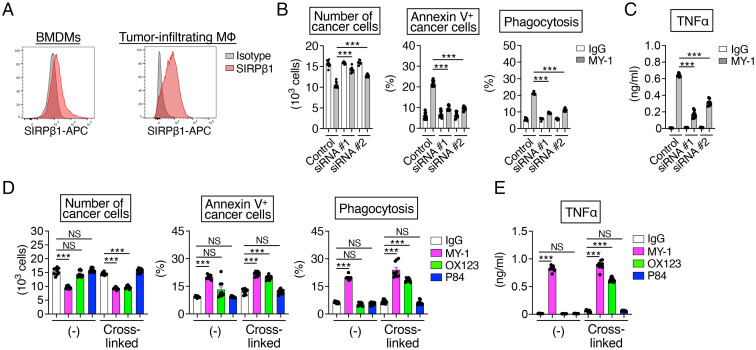
Given that MY-1 also reacts with the extracellular region of mouse SIRPβ1 (15), another member of the SIRP family, whereas P84 does not, we next examined whether mouse SIRPβ1 is important for the antitumor effect of MY-1 monotherapy on murine bladder cancer cells. Mouse SIRPβ1, like human SIRPβ1, is a transmembrane protein that possesses an NH2-terminal Ig-V–like domain and two Ig-C domains in the extracellular region as well as a short cytoplasmic tail (31, 32) and which is expressed in monocytes and macrophages (31, 33). Indeed, with the use of an mAb specific for mouse SIRPβ1 (OX123, rat IgG2a), which does not cross-react with mouse SIRPα, we detected the expression of SIRPβ1 on C3H BMDMs as well as on tumor-infiltrating macrophages isolated from MBT2 tumor–bearing mice (Fig. 6A). Mouse SIRPβ1 is thought to exist as three paralogs—SIRPβ1A, SIRPβ1B, and SIRPβ1C—that are encoded by three different genes (Sirpb1a, Sirpb1b, and Sirpb1c, respectively). The Ig-V–like domains of SIRPβ1A, SIRPβ1B, and SIRPβ1C share a high level of sequence similarity with that of mouse SIRPα (80%, 77%, and 76% identity, respectively) (SI Appendix, Fig. S10A). We therefore examined the effects of small interfering RNA (siRNA)-mediated knockdown of SIRPβ1 in mouse BMDMs on the MY-1–induced killing of cancer cells and TNFα production. Transfection of C3H BMDMs with siRNAs (#1 or #2) that target Sirpb1a, Sirpb1b, and Sirpb1c mRNAs resulted in a marked decrease in the amount of SIRPβ1 protein in these cells, whereas it did not affect that of SIRPα protein (SI Appendix, Fig. S10B). Depletion of SIRPβ1 in C3H BMDMs prevented the MY-1–induced reduction in cancer cell number as well as the increase both in the proportion of annexin V+ cancer cells and in the extent of phagocytosis of cancer cells by the BMDMs in cocultures with MBT2 cells (Fig. 6B). Moreover, knockdown of SIRPβ1 markedly attenuated the MY-1–induced production of TNFα by BMDMs (Fig. 6C). Consistently, deletion of DAP12 (DNAX activating protein of 12 kDa), a downstream molecule of SIRPβ1 (31), in BMDMs exposed to MY-1 or the F(ab')2 fragment of MY-1 also attenuated the cytotoxic and phagocytic activity against MB49 cells and TNFα production (SI Appendix, Fig. S11), suggesting that SIRPβ1–DAP12 signaling participates in the MY-1–dependent killing of murine bladder cancer cells by macrophages.
Fig. 6.
Importance of mouse SIRPβ1 for the MY-1–induced killing activity of macrophages for murine bladder cancer cells. (A) Representative flow cytometric histograms for mouse SIRPβ1 expression on BMDMs from C3H mice (Left) and on tumor-infiltrating macrophages isolated from MBT2 tumor–bearing C3H mice (Right). Data are representative of three separate experiments. (B) C3H BMDMs transfected with control or mouse SIRPβ1 (#1 or #2) siRNAs were incubated for 16 h with CFSE-labeled MBT2 cells in the presence of control IgG or MY-1 (each at 10 μg/mL). The cells were then harvested, and the number of cancer cells (Left), the percentage of annexin V+ cancer cells (Middle), and the percentage of BMDMs that had phagocytosed cancer cells (Right) were determined as in Fig. 2 A–C. (C) TNFα production by C3H BMDMs that had been transfected with the indicated siRNAs and treated with control IgG or MY-1 (each at 10 μg/mL) for 24 h. (D) CFSE-labeled MBT2 cells were cultured for 16 h with C3H BMDMs in the presence of control IgG, MY-1, OX123, or P84 (each at 10 μg/mL) or of the same agents that had been preincubated with the F(ab')2 fragment of goat pAbs to the Fc region of rat IgG so as to induce cross-linking. The number of cancer cells (Left), the percentage of annexin V+ cancer cells (Middle), and the percentage of BMDMs that had phagocytosed cancer cells (Right) were determined as in Fig. 2 A–C. (E) C3H BMDMs were treated with the indicated agents for 48 h, after which culture supernatants were harvested and assayed for TNFα. All quantitative data are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group) (B–E). ***P < 0.001, NS (Welch and Brown–Forsythe ANOVA with Dunnett’s T3 multiple-comparison test).
We next examined the effect of the OX123 mAb to mouse SIRPβ1 on macrophage-mediated antitumor actions. We found that exposure of cocultures of MBT2 cells and C3H BMDMs to OX123 that had been preincubated with the F(ab')2 fragment of goat pAbs to the Fc region of rat IgG resulted in a marked reduction in the number of cancer cells as well as an increase both in the proportion of annexin V+ cancer cells and in the extent of cancer cell phagocytosis by the macrophages, whereas OX123 alone had no such effects (Fig. 6D). Moreover, the cross-linked anti-mouse SIRPβ1 mAb greatly increased the production of TNFα by BMDMs (Fig. 6E). These OX123-mediated effects were similar in extent to those of MY-1 (Fig. 6 D and E). By contrast, P84, either alone or after preincubation with the F(ab')2 fragment of anti-rat IgG Fc, did not mimic these effects of OX123 and MY-1 (Fig. 6 D and E). Together, these results implicated SIRPβ1 on mouse macrophages in the antitumor effect of the anti-mouse SIRPα/SIRPβ1 mAb MY-1 on murine bladder cancer cells.
Promotion of Macrophage-Mediated Killing of Human Bladder Cancer Cells by an mAb to Human SIRPα/SIRPβ1.
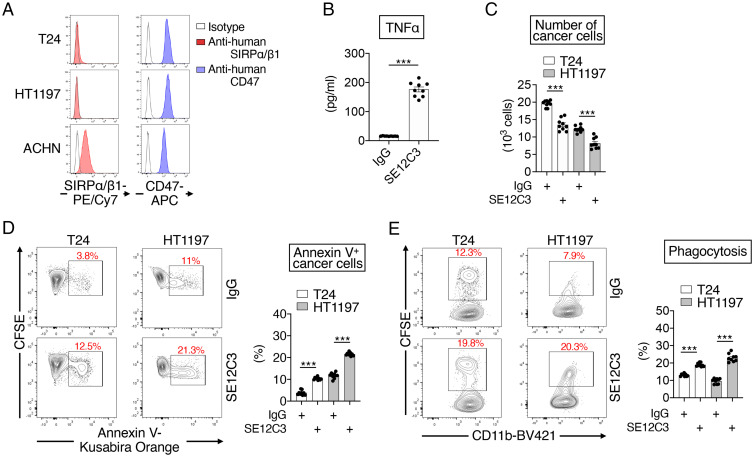
Finally, we examined whether Abs to human SIRPα that also react with human SIRPβ1 might also promote the production of TNFα by interferon γ (IFNγ)-activated human macrophages as well as the killing by these phagocytes of T24 and HT1197 human bladder cancer cells, which express SIRPα/SIRPβ1 on the cell surface only at low levels and CD47 at a high level (Fig. 7A). The SE12C3 mAb (mouse IgG1) to human SIRPα, which binds to the Ig-V–like domain of human SIRPα and cross-reacts with human SIRPβ1 (33), markedly increased TNFα production by IFNγ-activated human macrophages (Fig. 7B). In addition, exposure of cocultures of CFSE-labeled T24 or HT1197 cells and IFNγ-activated human macrophages to SE12C3 resulted in a decrease in the number of cancer (CFSE+CD11b–) cells (Fig. 7C) as well as an increase both in the percentage of annexin V+ cancer cells (annexin V+CFSE+CD11b–) among all cancer cells (CFSE+CD11b–) (Fig. 7D and SI Appendix, Fig. S4C) and in the frequency of CFSE+CD11b+ macrophages (macrophages that have phagocytosed cancer cells) among all CD11b+ macrophages (Fig. 7E and SI Appendix, Fig. S4D). The anti-human SIRPα/SIRPβ1 mAb was thus found to promote the macrophage-mediated killing of human bladder cancer cells, and may therefore inhibit the growth of tumors formed by such cells in vivo.
Fig. 7.
Promotion of macrophage-mediated killing of human bladder cancer cells by an mAb to human SIRPα/SIRPβ1. (A) Representative flow cytometric histograms for the expression of SIRPα or SIRPβ1 and CD47 on the cell surface of T24 and HT1197 human bladder cancer cells as well as on that of ACHN human renal cancer cells, which are positive for SIRPα expression (15). Data are representative of three separate experiments. (B) IFNγ-stimulated macrophages derived from human cord blood mononuclear cells were treated for 48 h with either control IgG or the SE12C3 mAb to human SIRPα/SIRPβ1 (each at 10 μg/mL), after which culture supernatants were harvested and assayed for TNFα. (C–E) CFSE-labeled T24 or HT1197 cells were cultured for 16 h with IFNγ-stimulated human macrophages in the presence of control IgG or SE12C3 (each at 10 μg/mL), after which the cells were collected for flow cytometric determination of the number of cancer cells (CFSE+CD11b–) (C), the proportion of annexin V+ cancer cells (annexin V+CFSE+CD11b–, apoptotic or dead) among all cancer cells (CFSE+CD11b–) (D), and the percentage of CFSE+CD11b+ macrophages (macrophages that had phagocytosed CFSE-labeled cancer cells) among all CD11b+ macrophages (E). Representative plots are shown (Left in D and E). All quantitative data are means ± SEM for three separate experiments, each performed in triplicate (n = 9 for each group) (B–E). ***P < 0.001 (two-tailed Welch’s t test).
Discussion
We have here revealed a therapeutic potential of Abs to SIRPα that also react with SIRPβ1 for bladder and mammary cancers that do not express SIRPα. In the absence of tumor-targeting Abs, Abs to SIRPα that block the interaction of CD47 (on cancer cells) with SIRPα (on macrophages) have been thought to have a minimal or limited effect on the phagocytosis by macrophages of, as well as on tumor formation by, these cancer cells (15, 17, 18, 34). However, we have now shown that monotherapy with MY-1, an mAb to SIRPα that also reacts with SIRPβ1, markedly suppressed the growth of tumors formed by MBT2 and MB49 murine bladder cancer cells or by FM3A mammary cancer cells in immunocompetent syngeneic mice, as well as prolonged the survival of these animals. This antitumor action of MY-1 was found to be largely dependent on macrophages but independent of macrophage-mediated ADCP of these cancer cells because these cancer cells do not express SIRPα, and MY-1 did not promote their phagocytosis by macrophages in short-term culture. By contrast, MY-1 promoted the killing activity of mouse macrophages directed toward these murine bladder and mammary cancer cells. In addition, MY-1 promoted the polarization of mouse macrophages toward an antitumorigenic phenotype in tumor-bearing mice as well as in an in vitro coculture system with cancer cells. In particular, MY-1 markedly increased the production of TNFα by macrophages in vitro as well as by tumor-infiltrating macrophages in vivo. Indeed, an inhibitor of TNFα suppressed the MY-1–induced killing of bladder cancer cells by macrophages in vitro as well as the inhibitory effect of MY-1 on the growth of tumors formed by bladder cancer cells in mice. We further showed that an mAb to human SIRPα that also reacts with human SIRPβ1 promoted the killing of human bladder cancer cells as well as the production of TNFα by human macrophages. Monotherapy with anti-SIRPα Abs such as MY-1 may therefore provide a promising type of immunotherapy for human cancers that is dependent on the induction of antitumorigenic macrophages.
We found that ablation of SIRPα in mice or macrophages did not prevent the inhibitory effect of MY-1 on the growth of tumors formed by murine bladder cancer cells or its stimulatory effect on the killing activity of macrophages for these cancer cells, suggesting that such effects of MY-1 are largely independent of SIRPα. Nevertheless, the blocking by MY-1, as an Ab to SIRPα, of the interaction between CD47 (on cancer cells) with SIRPα (on macrophages) might also contribute to its anticancer action in WT mice. We showed that SIRPβ1 most likely participates in the effects of MY-1 on tumor growth and the macrophage-mediated killing of cancer cells. SIRPβ1 is a transmembrane protein, the extracellular region of which—in particular, the NH2-terminal Ig-V–like domain—shows a high level of sequence similarity to that of SIRPα (31, 32). In addition, SIRPβ1 interacts with the adaptor protein DAP12 through its transmembrane region (31). Although the physiological role of SIRPβ1 as well as the identity of endogenous ligands for its extracellular region remain unknown, ligation of SIRPβ1 by specific Abs was previously shown to promote the phagocytic activity of macrophages toward Ab-opsonized red blood cells (RBCs) (31). Our present results now indicate that the ligation of SIRPβ1 by MY-1 promoted the killing activity of macrophages toward murine bladder cancer cells as well as the polarization of macrophages toward an M1-like phenotype and their production of TNFα. Such effects of MY-1 on macrophages were thus abolished by siRNA-mediated knockdown of SIRPβ1. Moreover, Dap12−/− macrophages showed a defect in MY-1–dependent phagocytic and cytotoxic activity against murine bladder cancer cells. Given that MY-1 reacts efficiently with the extracellular domain of SIRPβ1, the effects of monotherapy with MY-1 are likely dependent on its binding to SIRPβ1 on mouse macrophages. Moreover, our study implicates SIRPβ1 as a promising target for cancer immunotherapy. Further in vivo studies by the use of an mAb specific to SIRPβ1 will be required to validate the potential of SIRPβ1 as a therapeutic target for cancers.
Like macrophages, neutrophils express SIRPβ1 and DAP12 (35–37), as well as SIRPα, and they are thought to have tumor-suppressing or tumor-promoting properties (38). Neutrophils have been shown to exert direct antitumor activity by NO release and trogoptosis (39, 40). We confirmed that the expression of SIRPβ1 was detectable on the cell surface of neutrophils from C3H mice (SI Appendix, Fig. S12A). Exposure of cocultures of MBT2 cells with neutrophils to MY-1, the F(ab')2 fragment of MY-1, or OX123, resulted in the marked killing of MBT2 cells (SI Appendix, Fig. S12B). MY-1 likely promotes the killing activity of neutrophils toward murine bladder cancer cells by SIRPβ1 ligation. It is thus possible that neutrophils, as well as macrophages, contribute to the antitumor effect of MY-1 against murine bladder cancer cells in vivo.
The detailed molecular mechanism by which ligation of SIRPβ1 with either MY-1 or specific Abs promotes the killing of cancer cells and TNFα production by macrophages remains unclear. We showed that DAP12 likely participates in the MY-1–dependent killing of murine bladder cancer cells and production of TNFα by BMDMs. Ligation of SIRPβ1 induces tyrosine phosphorylation of DAP12 and its binding to the tyrosine kinase Syk (spleen tyrosine kinase) in mouse macrophages, resulting in activation of MAPKs (mitogen-activated protein kinases) (31). TREM1 (triggering receptor expressed on myeloid cells 1) is an Ig superfamily protein whose structure is similar to that of SIRPβ1. It consists of an extracellular domain, a transmembrane region, and a short cytoplasmic tail and also associates with DAP12 through its transmembrane region (36, 41). Indeed, cross-linking of TREM1 by specific Abs was shown to induce TNFα secretion and MAPK activation in human monocytes (36). It is thus likely that ligation of SIRPβ1 by specific Abs promotes the killing of cancer cells and the production of TNFα by macrophages through activation of a DAP12-Syk-MAPK signaling pathway.
We showed that monotherapy with MY-1 attenuated the growth of tumors formed by MB49 and MBT2 bladder cancer cells as well as FM3A mammary cancer cells, but not that of those formed by LM8 osteosarcoma or LL/2 lung cancer cells. The reason for these differences in the efficacy of MY-1 among these cancer models is unclear, but it might be related to susceptibility to TNFα-induced cytotoxicity. Indeed, neutralization of TNFα with etanercept attenuated the MY-1–dependent killing of bladder cancer cells by macrophages in vitro as well as the inhibitory effect of MY-1 on tumor growth in the MBT2 model. Furthermore, MBT2, MB49, and FM3A cells manifested a higher susceptibility to TNFα-induced cytotoxicity than did LM8 and LL/2 cells in vitro. In addition, the differences in the efficacy of MY-1 among several cancer cell lines might be related to the different expression level of phagocytic ligands on cancer cells. Phagocytosis by macrophages of cancer cells is thought to be promoted by the interaction of phagocytic ligands on cancer cells with their specific phagocytic receptors on macrophages, such as calreticulin/LRP1 (low-density lipoprotein receptor-related protein 1) and SLAMF7 (signaling lymphocytic activation molecule family member 7)/SLAMF7 interactions, and such phagocytosis is likely involved in the induction of effective antitumor immunity (42). Thus, MBT2, MB49, and FM3A cells, but not LL/2 or FM8 cells, might express phagocytic ligands specific for their phagocytic receptors on macrophages, and such ligand–receptor interaction promotes cancer cell phagocytosis in cooperation with ligation by MY-1 of SIRPβ1 on macrophages. In addition, exposure of macrophages to MY-1 might promote the expression of phagocytic receptors for their specific ligands expressed on MBT2, MB49, and FM3A cells, resulting in the efficient phagocytosis of these cancer cells.
We found that depletion of CD8+ T cells in MBT2 tumor–bearing mice resulted in partial attenuation of the inhibitory effect of MY-1 monotherapy on tumor growth. MY-1 treatment also did not inhibit the growth of tumors formed by MBT2 or MB49 cells in BALB/c nude mice (SI Appendix, Fig. S13). These findings suggest that CD8+ T cells also play a role in the MY-1–induced elimination of cancer cells in vivo. We previously showed that CD8+ T cells are important for suppression by MY-1 of tumor formation by RENCA cells (15). The therapeutic efficacy of CD47 blockade has also been shown to be dependent on adaptive immune responses in immunocompetent mouse tumor models (43–45). An engineered form of MY-1 in which the Fc domain was replaced with that of mouse IgG1 was recently shown to augment the activation of ovalbumin (OVA)-specific mouse CD8+ T cells (OT-I cells) by SIRPα-expressing mouse DCs both in vitro and in vivo (35). Inhibition of the CD47–SIRPα interaction by MY-1 is thus likely important for promotion of the cross-priming of CD8+ T cell responses by DCs or macrophages, resulting in enhancement of T cell–mediated tumor destruction. Moreover, ligation by MY-1 of SIRPβ1 on macrophages or DCs might promote macrophage-dependent stimulation of tumor antigen–specific cytotoxic T cells by enhancing phagocytosis of cancer cells. Indeed, DCs were shown to express SIRPβ1 on the cell surface, and ligation of SIRPβ1 on DCs by specific mAbs was found to regulate their phagocytic activity (46). In addition, MY-1 might also promote TNFα secretion by macrophages, thereby modulating CD8+ T cell functions against cancer cells. It was demonstrated that tumor necrosis factor receptor 2 (TNFR2), a receptor of TNFα, on CD8+ T cells participated in IL-2 production through T cell receptor (TCR)- and CD28-mediated stimulation and it promoted cell survival during the early phase of T cell activation (47). Moreover, signals mediated by TNFα and its receptors TNFR1 (on antigen-presenting cells) and TNFR2 (on CD8+ T cells) were shown to be required for effective priming, proliferation, and recruitment of tumor-specific CD8+ T cells (48).
In summary, we have shown that monotherapy with Abs that recognize both SIRPα and SIRPβ1 is effective in mouse models of bladder or mammary cancer. Further study is required to elucidate the clinical potential of such treatment for these two cancer types as well as for other types of malignancy in humans.
Materials and Methods
Antibodies, reagents, animals, and detailed methods for cell culture, cancer cell engraftment and treatment, depletion of macrophages, CD8+ T cells, or NK cells in vivo, cell preparation, flow cytometry, isolation of mouse macrophages and neutrophils and human macrophages, assays for TNFα and NO production, cytotoxicity, phagocytosis, and cell viability, RNA interference, and statistical analysis can be found in SI Appendix, Materials and Methods. All animal experiments were approved by the animal care and experimentation committees of Kobe University (permit No. P190604-R2). The experiments with human umbilical cord blood were approved by the ethics committees of Kobe University Graduate School of Medicine and the Hyogo Cord Blood Bank (No. 1820) and were performed in accordance with the tenets of the Declaration of Helsinki. Human cord blood was obtained from healthy volunteers who provided informed consent (The Hyogo Cord Blood Bank, Hyogo, Japan).
Supplementary Material
Acknowledgments
We thank C. F. Lagenaur for the P84 rat mAb to mouse SIRPα, H. J. Bühring for the SE12C3 mouse mAb to human SIRPα, and T. Suganami for bone-marrow cells from Dap12−/− mice. This work was supported in part by Grants-in-Aid for Scientific Research (A) (18H04032 and 21H04807 to T.M.) and (C) (19K07385 to Y.M.) from the Japan Society for the Promotion of Science and by the Project for Cancer Research and Therapeutic Evolution of the Japan Agency for Medical Research and Development (21cm0106308h0006 to T.M.). This work was also supported in part by the Suzuken Memorial Foundation (T.M.) and the Ichiro Kanahara Foundation (T.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109923118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Wynn T. A., Chawla A., Pollard J. W., Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P., Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon S., Plüddemann A., The mononuclear phagocytic system. Generation of diversity. Front. Immunol. 10, 1893 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNardo D. G., Ruffell B., Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruffell B., Coussens L. M., Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider A. K., Chevalier M. F., Derré L., The multifaceted immune regulation of bladder cancer. Nat. Rev. Urol. 16, 613–630 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Jenkins S., Wesolowski R., Gatti-Mays M. E., Improving breast cancer responses to immunotherapy—A search for the Achilles heel of the tumor microenvironment. Curr. Oncol. Rep. 23, 55 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Beatty G. L., et al. , CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ries C. H., et al. , Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Matozaki T., Murata Y., Okazawa H., Ohnishi H., Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 19, 72–80 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Barclay A. N., Van den Berg T. K., The interaction between signal regulatory protein alpha (SIRPα) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 32, 25–50 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Oldenborg P. A., CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013, 614619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata Y., Kotani T., Ohnishi H., Matozaki T., The CD47-SIRPα signalling system: Its physiological roles and therapeutic application. J. Biochem. 155, 335–344 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Okazawa H., et al. , Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 174, 2004–2011 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Yanagita T., et al. , Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2, e89140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao M. P., et al. , Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ring N. G., et al. , Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. U.S.A. 114, E10578–E10585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata Y., et al. , Anti-human SIRPα antibody is a new tool for cancer immunotherapy. Cancer Sci. 109, 1300–1308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matlung H. L., Szilagyi K., Barclay N. A., van den Berg T. K., The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 276, 145–164 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Verjan Garcia N., et al. , SIRPα/CD172a regulates eosinophil homeostasis. J. Immunol. 187, 2268–2277 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Sudo T., et al. , Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene 11, 2469–2476 (1995). [PubMed] [Google Scholar]
- 22.Cobbold S. P., Martin G., Qin S., Waldmann H., Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature 323, 164–166 (1986). [DOI] [PubMed] [Google Scholar]
- 23.Kasai M., et al. , In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature 291, 334–335 (1981). [DOI] [PubMed] [Google Scholar]
- 24.Cheng H., Wang Z., Fu L., Xu T., Macrophage polarization in the development and progression of ovarian cancers: An overview. Front. Oncol. 9, 421 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan S., Wan G., Tumor-associated macrophages in immunotherapy. FEBS J. 288, 6174–6186 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Keller R., Keist R., Wechsler A., Leist T. P., van der Meide P. H., Mechanisms of macrophage-mediated tumor cell killing: A comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int. J. Cancer 46, 682–686 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Wang D. J., Ratnam N. M., Byrd J. C., Guttridge D. C., NF-κB functions in tumor initiation by suppressing the surveillance of both innate and adaptive immune cells. Cell Rep. 9, 90–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohler K. M., et al. , Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J. Immunol. 151, 1548–1561 (1993). [PubMed] [Google Scholar]
- 29.Hibbs J. B. Jr., Taintor R. R., Vavrin Z., Macrophage cytotoxicity: Role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476 (1987). [DOI] [PubMed] [Google Scholar]
- 30.Washio K., et al. , Dendritic cell SIRPα regulates homeostasis of dendritic cells in lymphoid organs. Genes Cells 20, 451–463 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Hayashi A., et al. , Positive regulation of phagocytosis by SIRPbeta and its signaling mechanism in macrophages. J. Biol. Chem. 279, 29450–29460 (2004). [DOI] [PubMed] [Google Scholar]
- 32.van Beek E. M., Cochrane F., Barclay A. N., van den Berg T. K., Signal regulatory proteins in the immune system. J. Immunol. 175, 7781–7787 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Seiffert M., et al. , Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38− hematopoietic cells. Blood 97, 2741–2749 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Liu J., et al. , Targeting macrophage checkpoint inhibitor SIRPα for anticancer therapy. JCI Insight 5, e134728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauttier V., et al. , Selective SIRPα blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J. Clin. Invest. 130, 6109–6123 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchon A., Dietrich J., Colonna M., Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Lucas M., et al. , Massive inflammatory syndrome and lymphocytic immunodeficiency in KARAP/DAP12-transgenic mice. Eur. J. Immunol. 32, 2653–2663 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Hedrick C. C., Malanchi I., Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol., 10.1038/s41577-021-00571-6 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Matlung H. L., et al. , Neutrophils kill antibody-opsonized cancer cells by Trogoptosis. Cell Rep. 23, 3946–3959.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Finisguerra V., et al. , MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klesney-Tait J., Turnbull I. R., Colonna M., The TREM receptor family and signal integration. Nat. Immunol. 7, 1266–1273 (2006). [DOI] [PubMed] [Google Scholar]
- 42.M. Feng et al., Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng D., et al. , Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. U.S.A. 110, 11103–11108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto-Pantoja D. R., et al. , CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 74, 6771–6783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., et al. , CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 21, 1209–1215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lahoud M. H., et al. , Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J. Immunol. 177, 372–382 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Kim E. Y., Teh H. S., Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: A functional link to CD28. J. Immunol. 173, 4500–4509 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Calzascia T., et al. , TNF-α is critical for antitumor but not antiviral T cell immunity in mice. J. Clin. Invest. 117, 3833–3845 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.