Abstract
Dormancy is an evolutionarily conserved protective mechanism widely observed in nature. A pathological example is found during cancer metastasis, where cancer cells disseminate from the primary tumor, home to secondary organs, and enter a growth-arrested state, which could last for decades. Recent studies have pointed toward the microenvironment being heavily involved in inducing, preserving, or ceasing this dormant state, with a strong focus on identifying specific molecular mechanisms and signaling pathways. Increasing evidence now suggests the existence of an interplay between intracellular as well as extracellular biochemical and mechanical cues in guiding such processes. Despite the inherent complexities associated with dormancy, proliferation, and growth of cancer cells and tumor tissues, viewing these phenomena from a physical perspective allows for a more global description, independent from many details of the systems. Building on the analogies between tissues and fluids and thermodynamic phase separation concepts, we classify a number of proposed mechanisms in terms of a thermodynamic metastability of the tumor with respect to growth. This can be governed by interaction with the microenvironment in the form of adherence (wetting) to a substrate or by mechanical confinement of the surrounding extracellular matrix. By drawing parallels with clinical and experimental data, we advance the notion that the local energy minima, or metastable states, emerging in the tissue droplet growth kinetics can be associated with a dormant state. Despite its simplicity, the provided framework captures several aspects associated with cancer dormancy and tumor growth.
Keywords: cancer dormancy, metastability, tissue growth, phase separation, extracellular matrix
Cell and tissue growth is tightly regulated by intracellular as well as extracellular mechanisms. The latter, comprising both cell–cell and cell–extracellular matrix (ECM) interactions, provides a wide variety of biochemical and biophysical signals, which critically affect growth kinetics and fate (1). In particular, the physical/mechanical role of the surrounding microenvironment is being increasingly recognized in many physiological processes such as stem cell maintenance, differentiation (2), and tissue morphogenesis and adaptation (3, 4), as well as pathological conditions like tumor progression (5, 6). Nonetheless, the focus of most studies has overwhelmingly weighed toward the role of specific genes and proteins involved in these processes and less toward providing a physical framework for a more global description. This is hardly surprising, considering the complexity of biological systems.
From a thermodynamic point of view, living entities are considered “open systems” lying in a highly unpredictable “far-from-equilibrium” state, as opposed to their nonliving counterparts (7). Nonetheless, thermodynamic phase transitions occurring in open physical systems, such as the Bénard instability or the Belousov–Zhabotinski chemical reaction, where stationary states emerge by virtue of energy and material exchange with the environment, have prompted physicists to compare certain aspects of biological systems with thermodynamic phase transitions and metastable or critical states (8–12). Such parallels range from associating healthy-to-cancerous transformations, epithelial-to-mesenchymal transitions, and tumor invasion with first-order far-from-equilibrium phase transitions (8, 13) or modeling cancer dormancy as a critical phenomenon using evolutionary game theory approaches (9).
Despite intrinsic differences across biological species, dormancy can be regarded as an evolutionarily conserved mechanism exploited by a multitude of organisms, such as insects, bacteria, worms, or plant seeds, which enables them to enter a growth-arrested state under hostile conditions (14–16). Such a mechanism must be reversible to ensure regrowth upon restoration of favorable settings. Notably, the physiological state of most mammalian adult stem cells is quiescent (or dormant) (17), reentering the cell cycle only when required. This low metabolic state prevents their exhaustion and lowers the probability of acquiring oncogenic mutations upon cell division (17).
A pathological example of dormancy, on the other hand, can be found during cancer metastasis, which involves the dissemination of detached cells from the primary tumor mass into distant organs. Here, disseminated cancer cells (DCCs) require growth-friendly conditions for metastatic progression; if the secondary organ does not foster metastatic growth, cancer cells can recapitulate the evolutionarily conserved mechanism of older organisms and switch into a dormant state (18, 19).
The consideration that almost half of all forms of cancer metastasis become clinically detectable years or even decades after primary tumor resection points toward the presence of an invisible latent stage of metastatic colonization (20). This challenges the hypothesis of an otherwise linear or exponential growth between primary tumor and metastatic site (21, 22). The question that arises then is: What is the cause for such nonlinearity in growth kinetics? A plausible answer would be that changes in the microenvironment at the dissemination site might provide the stimuli necessary for dormancy of DCCs, until either accumulation of genetic aberrations and/or microenvironment alterations trigger their awakening (20).
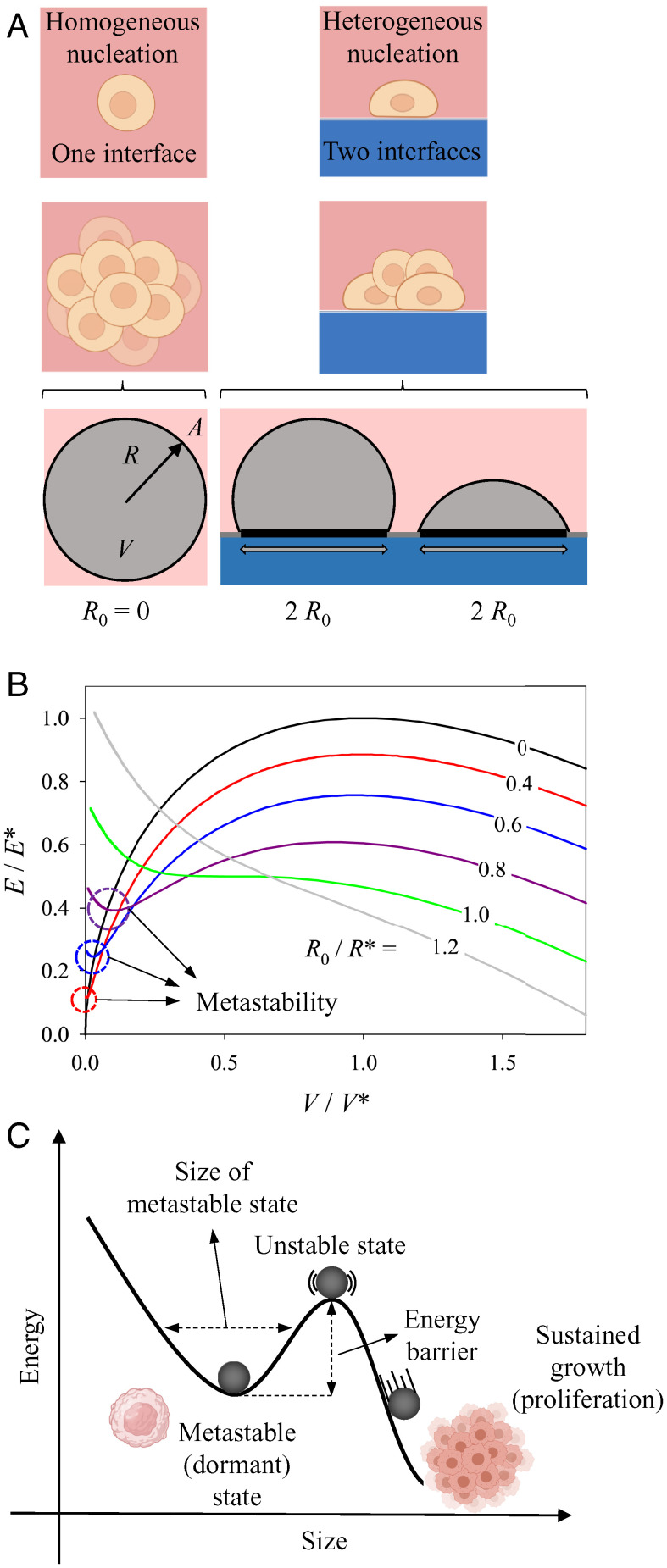
For a better description of the role of the microenvironment in modulating this process, we took advantage of the well-known concepts of stability and metastability for growing droplets of a thermodynamic phase. The idea is that a cancer cell aggregate (described as a droplet) grows by proliferation and migration of cancer cells within a normal tissue (the parent phase). When assuming that the cancer cell aggregate has a tendency to grow that is counterbalanced by an unfavorable interface between the droplet and the surrounding normal tissue, one obtains a formal analogy with thermodynamics of phase separation. The associated nucleation process can be 1) homogeneous, that is, occurring fully inside the parent phase (that is, the normal tissue) or 2) heterogeneous when the droplet grows in contact with a surface, so that the total interface between the nucleating droplet and the parent phase is reduced (see Fig. 2A). In this description, growth arrest (or dormancy) would correspond to a “metastable” state, which can be defined as a state of local (as opposed to global) minimum of energy, so that further growth requires to overcome a barrier. As a consequence, the system can reside in this local minimum, until enough energy is gained to leave this state and to progress toward a global minimum of energy (23). A crossing of the barrier may result from various perturbations, such as fluctuations in the driving force for growth or a decrease in the barrier height through a modification of the microenvironment. In this sense, metastability is the resilience of the system against small mechanobiological perturbations (24–28) that would ultimately lead to unrestricted growth (beyond the unstable state; see the sketch in Fig. 2C). In particular, we assume the growing tissue “droplet” to behave like a fluid, in that the relaxation of local stress concentrations is much faster than growth itself. Under these conditions, the Young–Laplace law leads to droplet shapes with constant curvature and kinetic metastability (see details in SI Appendix) that results from the fact that a small increase in droplet volume would lead to a negative change in growth rate (24, 29).
Fig. 2.
Spherical droplet adhering to a flat wetting surface. (A) Graphical illustration of homogeneous and heterogeneous nucleation, where the former corresponds to a nonadhering spherical droplet (left circle) with radius , surface area , and volume and the latter to a sphere adhering to a wetting surface on a circular patch of radius . (B) Energy diagram as a function of volume changes when the droplet adheres to a wetting surface. The parameters , , and are the energy, volume, and radius of a critical spherical droplet, respectively. The black line with is the curve for a nonadherent spherical droplet, while the remaining curves show what happens when the same droplet adheres to a wetting surface on a contact circular patch with a radius 40 to 120% of the critical radius, , as indicated. For detailed mathematical derivations, see equations in SI Appendix. (C) Example of an energy diagram illustrating a local energy minima or metastable state, an unstable state and sustained growth with the analogous proliferative state of cancer cells (illustrations created with BioRender.com).
In this respect, some parallels can be drawn with critical states [also known as the emergence of multistability (8)], such as their reduced sensitivity to the details of the system, while acquiring increased susceptibility to environmental perturbations (8, 9, 11). Building on the fact that, at least for spherical shapes, tissue growth models fall under the same category as phase separation models of nodular structures (24, 29, 30) (e.g., growth of a particle within a solution, or diffusion along dislocation lines or grain boundaries), here we propose that cancer dormancy shares several traits of thermodynamic metastability. The duration (or stability) of this “dormant” state will then depend on the activation energy barrier, which, in thermodynamic terms, can be described as the amount of energy that needs to be overcome in order to transition from particle nucleation to its critical size and eventual growth.
We argue that the ability of cells and tissues to grow vs. remain dormant is highly sensitive to their interaction with the microenvironment, which introduces the emergence of one or more metastable states, assumed to be proxies of dormancy. Different modes of dormancy induction have been proposed, spanning from drug-induced survival of subpopulations of cells under chemotherapy treatment, paracrine signaling from vasculature and niche cells, biochemical factors (i.e., hypoxia or nutrients), and ECM-induced dormancy (31). Here we focus on more physical aspects of the microenvironment, and based on experimental data (Fig. 1) we simplistically classify three distinct classes of interactions: 1) cells adhering to a wetting flat surface in the form of a spherical cap, 2) a spherical droplet enclosed by an elastic sheath as a mechanical interpretation of ECM-mediated confinement, and 3) a spherical droplet with size-dependent limited growth due to lack of nutrients and oxygen, leading to cell apoptosis deep inside the tissue. We then put these results in the context of recent modeling, experimental, and clinical data from both physiological and pathological examples of cell and tissue growth. Notably, the concepts proposed here are not meant to replace cellular or genetic mechanisms but to provide a complementary physical description with a more global perspective for the role of the microenvironment in mediating the described processes. In other terms, thermodynamics dictates the possible, while mutations direct the probable (32).
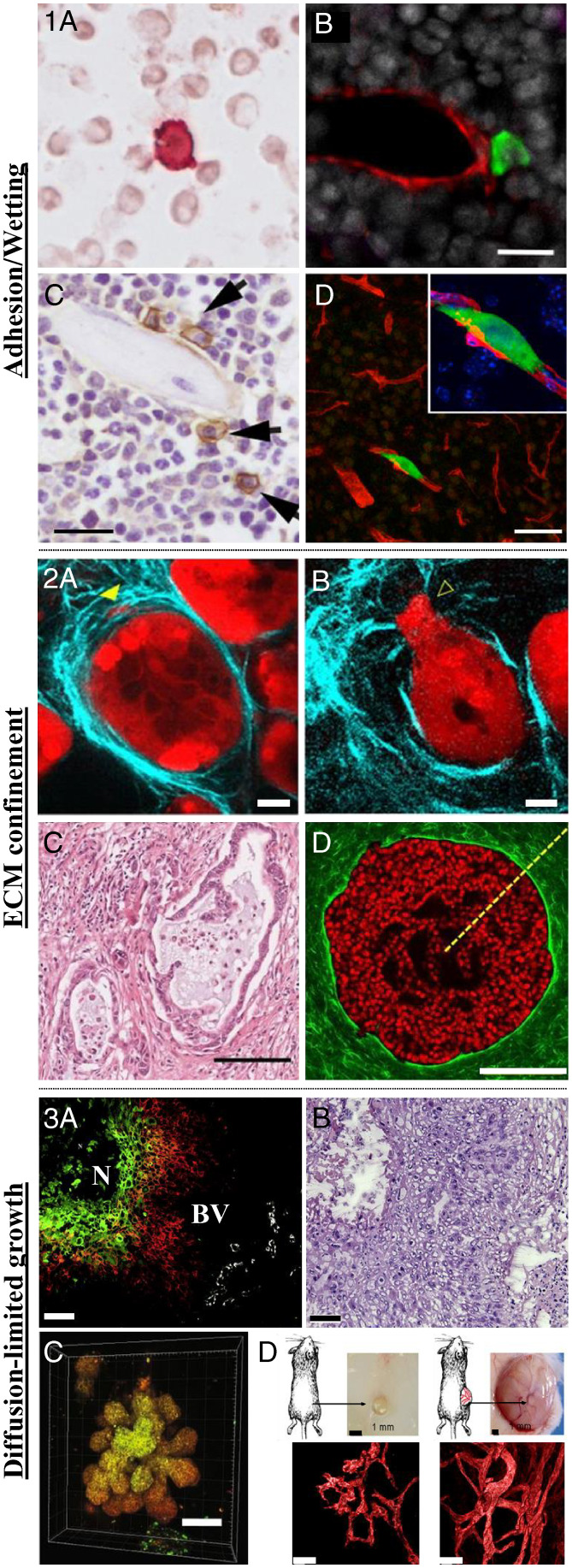
Fig. 1.
Microenvironment-mediated cancer dormancy. (1) Adhesion/wetting. (1A) Disseminated breast cancer cell (red) in the proximity of neighboring cells in the bone marrow of patients with ductal carcinoma in situ (DCIS), the earliest and most noninvasive form of breast cancer (400-fold magnification). Adapted from ref. 33. (1B) Disseminated breast cancer cell (green) adhering to the microvasculature (red) of the bone marrow in a mouse model. (Scale bar, 20 µm.) Adapted from ref. 34. (1C) Disseminated MM cells (brown) adhere to the endosteal surface of trabecular bone in a mouse model. (Scale bar, 20 µm.) Adapted from ref. 35. (1D) Disseminated breast cancer cell (green) adhere to the microvasculature (red) of the brain in a mouse model. (Scale bar, 50 µm.) Adapted from ref. 36. (2) Mechanical confinement. (2A and 2B) Pancreatic ductal carcinoma (red) surrounded by collagen ECM (cyan) before (2A) and after (2B) invasion of the surrounding murine tissue. (Scale bar, 20 µm.) Adapted from ref. 37. (2C) Histopathological staining of collagen (pink) shows a dense sheath of collagen surrounding the tumor core in a patient diagnosed with pancreatic cancer. (Scale bar, 100 µm.) Adapted from ref. 38. (2D) Noninvasive mammary epithelial spheroid (red) embedded in a 3D collagen hydrogel. (Scale bar, 200 µm.) Adapted from ref. 39. (3) Diffusion of oxygen and nutrients. (3A) Fluorescent image of a human biopsy of squamous cell carcinoma of the larynx shows that the outer cells (red) which are close to the blood vessels (BV, white) to be less hypoxic than the cells at the core of the tumor (green cells) close to the black necrotic (N) region. (3B) Hematoxylin and eosin histological staining of the same region. (Scale bar, 50 µm.) Adapted from ref. 40. (3C) Fate mapping of hypoxic cells in 3D spheroid cultures reveal higher hypoxia levels at the core of the spheroids (yellow region) as opposed to the outer region (orange). (Scale bar, 100 µm.) Adapted from ref. 41. (3D) Human tumor cells injected subcutaneously in mice reveal slower tumor size with impaired and less developed vasculature for dormant, nonangiogenic tumors (Left) as opposed to fast-growing angiogenic tumors (Right). (Scale bar of vasculature images, 40 µm.) Adapted from ref. 42.
Role of Adhesion
The majority of cells and tissues in the body are not just suspended in a void but require either cell–cell or cell–ECM anchorage in order to grow and proliferate. Meanwhile, adhesion has been shown to be directly involved in many steps of the metastatic cascade, including dissemination, homing, and dormancy. It has been previously reported that DCCs can adhere either close to the vasculature (i.e., perivascular niche) (34, 36) (Fig. 1, 1B and 1D) or in the proximity of bones (i.e., endosteal niche) (35) of the target organs (Fig. 1, 1C). To better understand the role of adhesion in mediating dormancy and tissue growth, we start by observing the growth kinetics of an unconfined spherical droplet, well-known from classical nucleation theory (Fig. 2 B and C). This simple model shows that a size-dependent parameter, named the critical radius (), exists, which, if exceeded, will result in the sustained growth of the droplet; otherwise, it will shrink and disappear (24) (Fig. 2B). More specifically, droplets with a volume smaller than will shrink to zero while those larger than this size will see an unlimited growth. This means that in a classical nucleation process, as in the case of a tumor suspended in a void, there is no metastable droplet of finite size; instead, it either disappears or grows. This aspect is also visible in earlier theories of cancer growth (see figure 5 of ref. 43), where the tumor either grows or shrinks, depending on whether its size is above or below a critical dimension. Indeed, the critical size corresponds to a maximum of the energy curve (Fig. 2B) and, thus, to an unstable state (Fig. 2C). In Fig. 2, we restrict ourselves to a qualitative stability analysis, because the growth kinetics as well as actual values of the critical size will be different depending on the details of any specific tissue (type of cells, ECM composition, etc.).
We then model adhesion by including a wetting substrate to which the previously unsupported tissue nodule can now adhere (Fig. 2A). From a modeling point of view, this wetting surface provides a boundary condition, which limits the growth of an otherwise unconfined spherical droplet, also known as surface-directed phase separation (44–47). We and others have previously shown that this simple physical constraint can lead to the emergence of extremely complex structural shapes (24, 29, 48, 49).
Based on this, we hypothesize that the tissue adheres to a surface (that is “liked” by the tissue in the droplet) without costs in energy and in contrast to the interface with the foreign microenvironment. Fig. 2A shows two configurations for a droplet taking the shape of a spherical cap adhering to a circular patch of radius on the flat surface. Outside this patch, the adherence is not favorable (in physical terms, the droplet would not wet the foreign microenvironment outside the circular patch).
First, it is observed that with adhesion to a surface, a minimum value of the energy appears at small volumes (red 40%, blue 60%, and purple 80%) (Fig. 2B). This minimum indicates the existence of a metastable droplet whose size is directly related to the radius of the adhesion patch, or, in other words, the amount of surface cells adhere to. In the context of cancer dormancy, it has been reported in multiple in vitro studies that adhesion of breast or prostate cancer cells to ECM-derived proteins such as fibronectin or collagen results in the up-regulation of transmembrane cell–ECM receptor integrins, which confer tumor cells with enhanced survival abilities in the form of a growth-arrested dormant phenotype (50–54). Likewise, it has been reported that at the site of primary breast tumors there is a great abundance of collagenous proteins, which bind to Discoidin Domain Receptors 1 and 2 (DDR1 and DDR2) transmembrane receptor kinases, which foster adhesion and endow tumor cells with stemness properties via STAT3 signaling (55, 56). Knocking out DDR1 in mouse models resulted in increased tumor malignancy and metastatic potential (57).
On the metastatic site, Montagner et al. (58) recently revealed that dormant disseminated breast cancer cells in the lung of mice secrete fibronectin fibrils, which leads to the activation of an integrin-mediated prosurvival mechanism. Seemingly, Carlson et al. (34) showed that disseminated tumor cells residing in the perivascular niche of the bone marrow are protected against chemotherapy by adhering to the vascular endothelium (Fig. 1, 1B). Surprisingly, deleting the integrin-mediated adhesion between the tumor cells and their niche resulted in enhanced chemosensitization and prevention of bone metastasis, irrespective of cell cycle and proliferation state of the cells (34). Using intravital imaging, Lawson et al. (35) observed multiple myeloma (MM) cells switching to a dormant state after adhering to the endosteal surface of trabecular bones (Fig. 1, 1C). Gene expression analysis revealed that cell adhesion molecules such as Vcam1 and Axl were highly up-regulated in dormant MMs, rendering them resistant to chemotherapy (35). Highlighting the importance of engaging with adhesion molecules, and not just their expression, recent work has shown that breast cancer cells that directly engage with specific “integrin subtype-specific peptidomimetics” immobilized on a surface via nanolithography display significant increase in survival upon exposure to chemotherapeutics (59).
Analogous behavior to dormant cancer cells was observed for adult quiescent hematopoietic stem cells, which, when presented with substrates coated with bone marrow-inspired ECM ligands or adherence junctions, showed reduced cycling and maintenance of long-term multipotency (60–62).
Nonetheless, an important observation from the growth kinetics of the adhering droplet is that, even though the size of the metastable state increases with adhesion, at the same time its stability, or, in other words, the activation barrier for growth (as measured by the energy difference between the minimum and the maximum of the curve), decreases (red 40%, blue 60%, purple 80%) (Fig. 2B). Concurrently, with increasing the size of the patch of radius the maximum value of the energy decreases and, when the radius of the adhesion patch is equal to or exceeds the critical radius of a spherical droplet, there is no more stability (green 100% and grey 120% curves) (Fig. 2B). This indicates that, above a certain threshold, increasing adhesion will result in the droplet leaving its dormant state, which is, apparently, in contrast to its previously described role in inducing and preserving dormancy (34, 50–57).
An example illustrating this controversy comes from a recent study featuring the effects of inflammation on dormant breast and prostate DCCs in the lungs of mice (63). Before inducing inflammation, DCCs resided in a dormant state with expressed but not engaged integrin receptors (low degree of adhesion). After tobacco smoke or bacterial lipopolysaccharide exposure, activated neutrophils were recruited to the lungs, where they formed neutrophil extracellular traps, which released ECM-degrading enzymes, resulting in the cleavage of ECM proteins, in particular laminin. These cleaved proteins then engaged with DCCs integrin receptors (high degree of adhesion), triggering their awakening and subsequent metastatic growth. Inhibiting this interaction by means of blocking antibodies prevented such outcome (63). Several other studies have similarly pointed to integrin or other adhesion molecules as essential mediators in the downstream signaling leading to the awakening of dormant DCCs (64–69).
Efforts in deciphering this seemingly paradoxical effect of adhesion have led to recognizing a graded rather than a binary role for adhesion on dormancy vs. proliferation of cancer cells (70–72). Within these lines, it had been previously reported that dormant DCCs express lower levels of integrin compared to their activated form (64, 73). Despite its simplicity, our model manages to capture this graded effect of adhesion: 1) Low adhesion (proportional to the size of the adhesion patch) leads to the emergence of a metastable (dormant) state, 2) increasing the adhesion patch lowers the energy required to exit this state, 3) until the size of the adhesion patch reaches the critical size of the droplet, resulting in the loss of metastability. Nevertheless, the concept of adhesion-mediated cancer dormancy is still in its infancy and far more experimental models are needed to validate the proposed thesis.
Importantly, the single size-dependent parameter, which controls both the size and the stability of the metastable droplet, is the radius of the adhesive patch, or in other words, the contact area to which cells/tissues adhere. The central role of a size-dependent parameter has been seemingly observed for tissues growing on substrates with different geometries (29). For a tissue growing on top of a hollow cylinder, for instance, a maximum height exists which the tissue will tend to reach, and above which tissue growth will stop. This resembles the case of fracture gaps present in osteotomy (74), where bone at each free end of the fracture grows to bridge the gap. Based on this model, it can be predicted that if the size of the segmental bone defect is bigger than a critical value, complete bone filling and healing will not take place (75).
Role of Mechanical Confinement
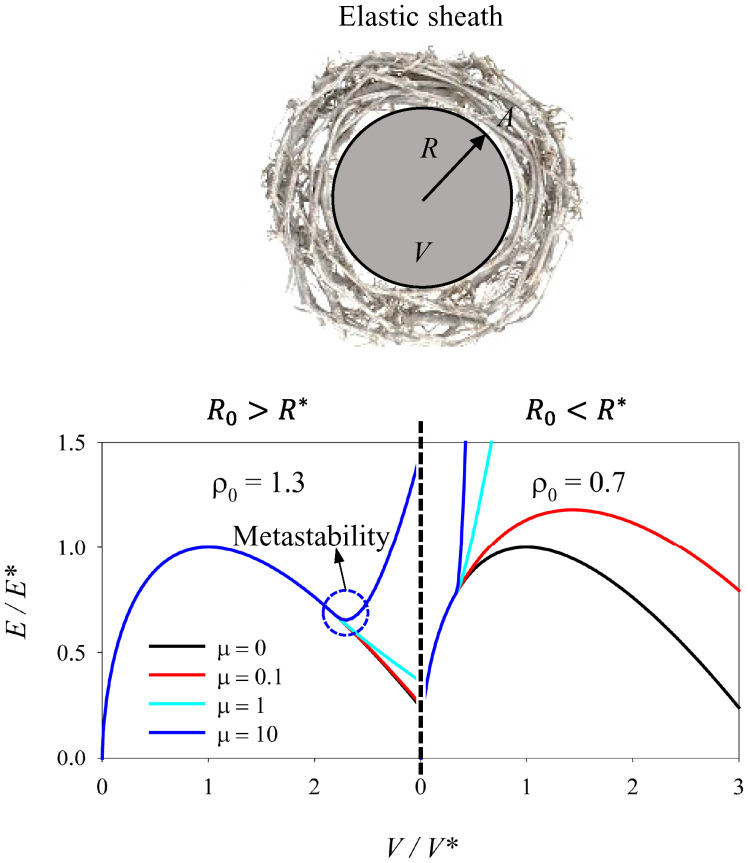
Growing tumors within different organs have been shown to be surrounded by a plethora of cells and ECM which restrict their growth in such confined microenvironment, while being subjected to mechanical compression (76, 77). Premalignant and less-invasive tumors in both humans and mouse models have been widely reported of being surrounded by dense ECM fibers (37, 38) (Fig. 1 2A–2D). Seemingly, single cancer cells have been observed to reside within the dense collagen stroma enclosing breast tumors (78). Here we seek to understand how mechanical confinement provided by an elastic sheath, meant to resemble a fibrous capsule with predominantly elastic properties, restrains the growth of a spherical droplet starting from a volume V0 (corresponding to a sphere of radius ) (Fig. 3).
Fig. 3.
Spherical droplet mechanically confined by an elastic sheath. Graphical illustration and energy diagram of a spherical droplet mechanically confined by an elastic sheath meant to resemble an ECM with predominantly elastic properties, as a function of volume changes. shows the relation between the unstretched radius of the sheath () and the critical radius of the droplet (). characterizes the stiffness of the elastic sheath in relation to the critical energy of the droplet () and . For detailed mathematical derivations, see equations in SI Appendix.
Given that the elastic energy of the sheath increases with the volume of the droplet, if is lower than 1 (), or in other words if the initial radius of the sphere is lower than its critical radius, there is no minimum in the total energy. Instead, for and for a sufficiently stiff sheath (μ), a minimum of the energy appears for a size larger than (how much larger will depend on the stiffness of the elastic sheath). There are, however, important differences from the previous case, where the droplet growth was controlled only by adhesion to a substrate: 1) Now the metastable state (corresponding to the minimum of the energy) is at a relatively large size of the droplet (necessarily larger than its critical size , given that the metastable state emerges at ), 2) the energy barrier (the difference between the maximum and the minimum energy) to exit the dormant state toward further growth is predicted to be very large or even infinite. In this sense, such a state would represent a stable state and not a metastable one, unless the elastic sheath is damaged or degraded in some way. A change from elastic to viscous properties of the ECM or proteolytic degradation could reflect such variations (55).
Indeed, in benign tumors or early-stage carcinoma in situ, growth is contained by a basement membrane which needs to be breached either by proteolytic degradation through matrix metalloproteinases secreted by tumor and stromal cells or through force-mediated remodeling and rupture for successful tissue invasion and tumor progression (55, 79). Intuitively, recent in vitro experiments have shown that highly proliferative cancer cells encapsulated in nondegradable stiff synthetic hydrogels display several phenotypic features of dormant cancer cells (80). This resemblance is reduced if cells were allowed to grow in degradable hydrogels (80). In a less-intuitive in vitro study, it was observed that even within nondegradable hydrogels with matching elastic moduli, breast cancer cell cycle progression can be modulated as a function of the stress relaxation (viscosity) properties of the surrounding material (81). Specifically, encapsulation within hydrogels with slow stress relaxation led to a higher number of growth-arrested cells compared to the faster stress-relaxing hydrogels (81). As for the case of adhesion, mechanical confinement has been shown to play a role in stem cell maintenance as well. Hematopoietic stem and progenitor cells within spatially constrained microcavities or embedded in hydrogels of a higher degree of cross-linking maintained a quiescent and undifferentiated state as opposed to less-confining microenvironments (82, 83).
Theoretical models of tumor growth within a surrounding tissue have similarly highlighted the importance of the ECM’s physical properties in driving tumor progression. Basan et al. (43), for instance, have shown that if the surface tension of the ECM basement membrane surrounding the tumor increases faster than the radius of the tumor, cancer expansion will eventually stop, resembling a dormant state. Building on the previously described (84) vertex model of solid–liquid transition of a two-dimensional (2D) confluent monolayer, Merkel and Manning (85) generalized a three-dimensional (3D) tissue-based vertex model introducing the ECM as a spring network with interfacial line tension () surrounding a tissue. Interestingly, above a certain critical tension (), a cavitational instability occurs, resulting in a compression-induced fluidization of the tissue. Below this critical tension (), tissue boundaries become unstable due to cavities and empty spaces hindering cell–ECM coupling (86). In the compact regime, the tissue is not affected by the network tension and its state is a function of cell shape as defined by 2D vertex models (84). On the other hand, when is low, the tissue becomes sensitive to the network and its phase will depend on both shape and cell–cell alignment (86). Based on these results, the authors postulated that prior to invasion cell alignment might lead to tumor solidification, which, upon breaching through the basement membrane, will switch to a fluid behavior (relative to the external ECM), leading to tumor migration (86). Importantly, this and previous work suggests that in solid-to-liquid transitions the origin of rigidity remains purely geometric and therefore robust among different biological systems for both 2D and 3D configurations (84, 85).
These examples underline the importance of the ECM’s confining role, which, when coupled with specific mechanical properties, could grant (or prevent) tumor growth and progression by means of protease-dependent and/or independent mechanisms.
Role of Diffusion of Oxygen and Nutrients
The high proliferation rate of tumor cells requires extensive nutrient and oxygen supply. Nonetheless, this is impaired by the highly dense tumor mass, whose growth is mediated by a turnover/balance between apoptotic and proliferating cells (87), until hypoxia-mediated signaling triggers ECM remodeling and subsequent vascularization promoting tumor progression (40–42, 88) (Fig. 1, 3A–3D). Several studies have associated tumor dormancy with impaired vasculature in both primary and secondary sites (42, 89), which can be interrupted by angiogenic bursts within the tumor microenvironment (90).
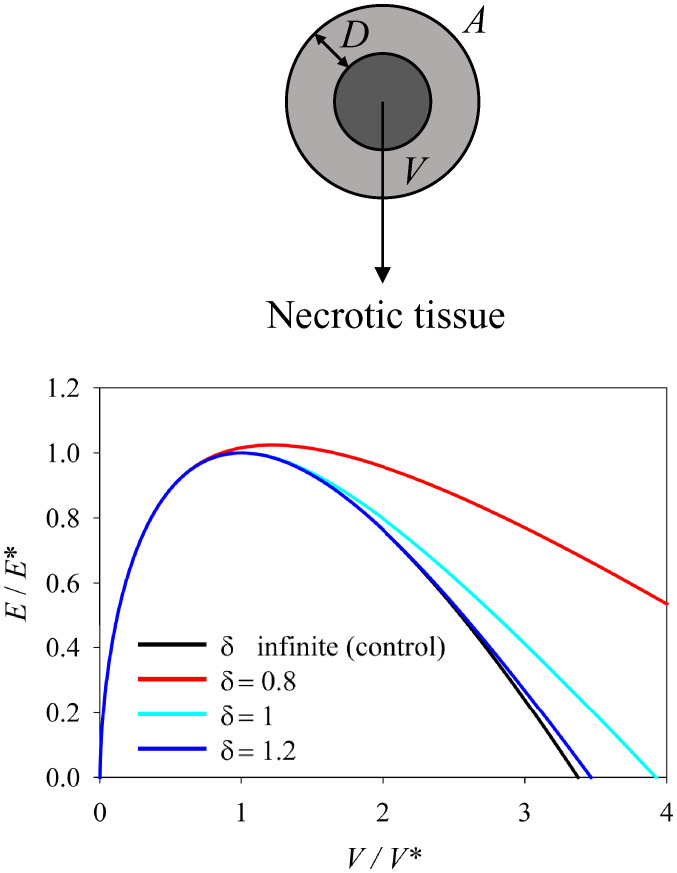
To model such behavior with a very simplistic approach, here we consider a tissue that cannot survive below a distance D from the surface, as it gets necrotic due to limited diffusion of oxygen and nutrients Fig. 4. Hence, the volume is reduced to the outer shell with thickness D, as soon as the radius of the droplet R > D.
Fig. 4.
Spherical droplet with size-dependent growth. Graphical illustration and energy diagram of a spherical droplet with size-dependent growth as a function of volume changes. The assumed tissue cannot survive below a distance D from the surface due to necrosis associated with limited diffusion of oxygen and nutrients. is the ratio of the thickness of the viable part (D) and the critical radius of the droplet (). For detailed mathematical derivations, see equations in SI Appendix.
When looking in more detail at this model, it appears that this function has no minimum outside ρ = 0 for any value of D, meaning that no metastable state is predicted which could be interpreted in terms of dormancy. In line with our physical framework, and in accordance with clinical data, Monte Carlo simulations have predicted that cancer dormancy as a function of balanced apoptosis/proliferation alone has low survival probabilities, suggesting that accounting for an alternative growth-arrested or dormant state is indispensable to explain the strikingly high recurrence of late-stage metastatic relapse (91).
Discussion and Concluding Remarks
The nonlinear progression of tumor growth at the primary compared to the secondary site has led cancer biologists and clinicians to emphasize more than ever the role of the tumor microenvironment in modulating the growth of DCCs in this invisible phase of metastasis (20). Inspired by nucleation theory and concepts from thermodynamic phase separation, here we propose that the emergence of metastable states in the growth kinetics of surface-directed or enclosed droplets can be assumed as proxies of cancer dormancy. Importantly, and in line with experimental and clinical data, we show that the only scenarios where a metastable state is predicted is when physical aspects of the microenvironment are included in the model: adhesion and mechanical-mediated confinement. It is worth noting that the concepts of phase separation and nucleation are not new to biology: At the subcellular level, a fast-growing body of evidence is pointing toward liquid–liquid phase separation underlying the organization of membrane-free biomolecular condensates (92), known to participate in a wide variety of intracellular processes such as DNA damage response (93), protein translocation (94, 95), and signal transduction (96). Importantly, nucleation was shown to govern growth initiation and size control of these condensates (97). These, in turn, interact with existing substrates which energetically favor their coacervation (agglomeration), as shown for chromatin during spindle formation (98), ribosomal RNA for the nucleolus (99), or centrioles for centrosomes (100, 101).
To achieve an optimal level of functionality, biological systems require adaptability and robustness during growth and development or upon environmental perturbations. Being near critical and metastable states during phase transitions allows the system to reach a balance between stability from the ordered and versatility from the disordered state, respectively (10, 102). Slow-driven processes in particular, such as evolution (103), morphogenesis (11), or dormancy (9), have proven to fit analogies of criticality, metastability, and phase transition processes. Their long time scales and environmental interaction-dominated nature leads to large fluctuations at transition points, which results in high energy gains upon even small perturbations (11, 104). Studies on brain neural networks have shown that the number of metastable states is highest at the critical point, where the role of the ordered state is to optimize information storage, while the disordered state maximizes transmission efficiency (105). Extensive empirical evidence from gene expression patterns (106), morphogenesis (11), cell growth (107), and dormancy (9), alongside recent computational and analytical models from information theory (102), substantiate this hypothesis (108).
With this in mind, here we propose that cancer dormancy can be regarded as an example where an equilibrium between stability and adaptability is achieved: DCCs reside in a growth-arrested state (e.g., mediated by adhesion or confinement), acquiring several survival traits against an otherwise hostile environment (robust ordered state). Concurrently, this metabolically less-committing noncycling state allows DCCs to activate specific molecular pathways which act by influencing their sensitivity to changes in the microenvironment, or by actively fostering growth-favoring/suppressing conditions (adaptability state) (109). In this article, we have not considered adaptability, as the aim was to advance the idea of viewing dormancy from a metastability theory perspective. Future work could address this point by introducing a chemical potential, where spheroid growth rate is not constant but rather a variable which depends on the presence/absence of growth factors (29), or alterations of surface tension due to changes in cytoskeletal contractility of the cells within the tissue (110, 111).
Notably, the physical framework of microenvironment-mediated dormancy proposed in this article does not oppose or replace genetic/molecular signaling or other subcellular mechanisms involved in the process. Instead, it proposes a multiscale hand-to-hand relationship, where the microenvironment feeds information to cells/tissues, and vice versa.
Supplementary Material
Acknowledgments
S.B. and A.C. acknowledge funding by the Deutsche Forschungsgemeinschaft Emmy Noether grant CI 203/2-1.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111046118/-/DCSupplemental.
Data Availability
The data presented here have been deposited in Edmond, Open Access Data Repository of the Max Planck Society (https://dx.doi.org/10.17617/3.8j), or provided in the SI Appendix and within this paper.
References
- 1.Bissell M. J., Hall H. G., Parry G., How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Vining K. H., Mooney D. J., Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin K., Nelson C. M., Mechanics of development. Dev. Cell 56, 240–250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammoto T., Ingber D. E., Mechanical control of tissue and organ development. Development 137, 1407–1420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nia H. T., Munn L. L., Jain R. K., Physical traits of cancer. Science 370, eaaz0868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul C. D., et al. , Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 9, 187–206.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haken H., Synergetics (Springer, Berlin, 1978). [Google Scholar]
- 8.Davies P. C. W., Demetrius L., Tuszynski J. A., Cancer as a dynamical phase transition. Theor. Biol. Med. Model. 8, 30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu A., et al. , Cancer dormancy and criticality from a game theory perspective. Cancer Converg. 2, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petridou N. I., Heisenberg C. P., Tissue rheology in embryonic organization. EMBO J. 38, e102497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krotov D., Dubuis J. O., Gregor T., Bialek W., Morphogenesis at criticality. Proc. Natl. Acad. Sci. U.S.A. 111, 3683–3688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranft J., et al. , Fluidization of tissues by cell division and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 107, 20863–20868 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang W., et al. , A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation. iScience 24, 103252 (2021). [DOI] [PMC free article] [PubMed]
- 14.Jones S. E., Lennon J. T., Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. U.S.A. 107, 5881–5886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koornneef M., Bentsink L., Hilhorst H., Seed dormancy and germination. Curr. Opin. Plant Biol. 5, 33–36 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Kim S. K., Global analysis of dauer gene expression in Caenorhabditis elegans. Development 130, 1621–1634 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Li L., Clevers H., Coexistence of quiescent and active. Science 327, 542–545 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguirre-Ghiso J. A., Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pienta K. J., Hammarlund E. U., Brown J. S., Amend S. R., Axelrod R. M., Cancer recurrence and lethality are enabled by enhanced survival and reversible cell cycle arrest of polyaneuploid cells. Proc. Natl. Acad. Sci. U.S.A. 118, e2020838118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein C. A., Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 20, 681–694 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Uhr J. W., Pantel K., Controversies in clinical cancer dormancy. Proc. Natl. Acad. Sci. U.S.A. 108, 12396–12400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein C. A., Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Machlin S. E., An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science (Elsevier, 2007). [Google Scholar]
- 24.Dunlop J. W. C., Zickler G. A., Weinkamer R., Fischer F. D., Fratzl P., The emergence of complexity from a simple model for tissue growth. J. Stat. Phys. 180, 459–473 (2020). [Google Scholar]
- 25.Cyron C. J., Humphrey J. D., Vascular homeostasis and the concept of mechanobiological stability. Int. J. Eng. Sci. 85, 203–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyron C. J., Aydin R. C., Mechanobiological free energy: A variational approach to tensional homeostasis in tissue equivalents. ZAMM 97, 1011–1019 (2017). [Google Scholar]
- 27.Cyron C. J., Humphrey J. D., Growth and remodeling of load-bearing biological soft tissues. Meccanica 52, 645–664 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cyron C. J., Wilson J. S., Humphrey J. D., Mechanobiological stability: A new paradigm to understand the enlargement of aneurysms? J. R. Soc. Interface 11, 20140680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer F. D., Zickler G. A., Dunlop J. W. C., Fratzl P., Tissue growth controlled by geometric boundary conditions: A simple model recapitulating aspects of callus formation and bone healing. J. R. Soc. Interface 12, 20150108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vengrenovitch R. D., On the Ostwald ripening theory. Acta Metall. 30, 1079–1086 (1982). [Google Scholar]
- 31.Pradhan S., Sperduto J. L., Farino C. J., Slater J. H., Engineered in vitro models of tumor dormancy and reactivation. J. Biol. Eng. 12, 37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Crea R., Eds., Tumor Dormancy and Recurrence (Springer International Publishing, 2017). [Google Scholar]
- 33.Sänger N., et al. , Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Carlson P., et al. , Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson M. A., et al. , Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 8983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malladi S., et al. , Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray A., et al. , Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. bioRxiv [Preprint] (2021). https://www.biorxiv.org/content/10.1101/2021.02.19.431984v1 (Accessed 1 March 2021). [DOI] [PMC free article] [PubMed]
- 38.Keikhosravi A., et al. , Non-disruptive collagen characterization in clinical histopathology using cross-modality image synthesis. Commun. Biol. 3, 414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferruzzi J., et al. , Compressive remodeling alters fluid transport properties of collagen networks – Implications for tumor growth. Sci. Rep. 9, 17151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogsteen I. J., et al. , Hypoxia in larynx carcinomas assessed by pimonidazole binding and the value of CA-IX and vascularity as surrogate markers of hypoxia. Eur. J. Cancer 45, 2906–2914 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Godet I., et al. , Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 10, 4862 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almog N., Molecular mechanisms underlying tumor dormancy. Cancer Lett. 294, 139–146 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Basan M., Risler T., Joanny J. F., Sastre-Garau X., Prost J., Homeostatic competition drives tumor growth and metastasis nucleation. HFSP J. 3, 265–272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastea S., Puri S., Lebowitz J. L., Surface-directed spinodal decomposition in binary fluid mixtures. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 63, 041513 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Binder K., Puri S., Das S. K., Horbach J., Phase separation in confined geometries. J. Stat. Phys. 138, 51–84 (2010). [Google Scholar]
- 46.Puri S., Surface-directed spinodal decomposition. J. Phys. Condens. Matter 17, R101–R142 (2005). [Google Scholar]
- 47.Puri S., Binder K., Surface-directed phase separation with off-critical composition: Analytical and numerical results. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 66, 061602 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Lenz P., Fenzl W., Lipowsky R., Wetting of ring-shaped surface domains. Europhys. Lett. 53, 618–624 (2001). [Google Scholar]
- 49.Gau H., Liquid morphologies on structured surfaces: From microchannels to microchips. Science 283, 46–49 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Ojalill M., et al. , Integrin α2β1 decelerates proliferation, but promotes survival and invasion of prostate cancer cells. Oncotarget 9, 32435–32447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrios J., Wieder R., Dual FGF-2 and intergrin α5β1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy. Cancer Microenviron. 2, 33–47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korah R., Boots M., Wieder R., Integrin α5β1 promotes survival of growth-arrested breast cancer cells: An in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 64, 4514–4522 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Barney L. E., et al. , Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci. Adv. 6, eaaz4157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henriet P., Zhong Z.-D., Brooks P. C., Weinberg K. I., DeClerck Y. A., Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc. Natl. Acad. Sci. U.S.A. 97, 10026–10031 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winkler J., Abisoye-Ogunniyan A., Metcalf K. J., Werb Z., Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao H., et al. , Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takai K., et al. , Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes Dev. 32, 244–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montagner M., et al. , Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat. Cell Biol. 22, 289–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young J. L., et al. , Integrin subtypes and nanoscale ligand presentation influence drug sensitivity in cancer cells. Nano Lett. 20, 1183–1191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kräter M., et al. , Bone marrow niche-mimetics modulate HSPC function via integrin signaling. Sci. Rep. 7, 2549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roch A., et al. , Single-cell analyses identify bioengineered niches for enhanced maintenance of hematopoietic stem cells. Nat. Commun. 8, 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi J. S., Harley B. A. C., Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 3, e1600455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albrengues J., et al. , Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barkan D., et al. , Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barkan D., et al. , Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aguirre Ghiso J. A., Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 21, 2513–2524 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Shibue T., Brooks M. W., Weinberg R. A., An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24, 481–498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibue T., Weinberg R. A., Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A. 106, 10290–10295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Er E. E., et al. , Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park S.-Y., Nam J.-S., The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 52, 569–581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barkan D., Green J. E., Chambers A. F., Extracellular matrix: A gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 46, 1181–1188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White D. E., Rayment J. H., Muller W. J., Addressing the role of cell adhesion in tumor cell dormancy. Cell Cycle 5, 1756–1759 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Aguirre-Ghiso J. A., Estrada Y., Liu D., Ossowski L., ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003). [PubMed] [Google Scholar]
- 74.Epari D. R., Schell H., Bail H. J., Duda G. N., Instability prolongs the chondral phase during bone healing in sheep. Bone 38, 864–870 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Reichert J. C., et al. , The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30, 2149–2163 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Helmlinger G., Netti P. A., Lichtenbeld H. C., Melder R. J., Jain R. K., Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15, 778–783 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Nia H. T., et al. , Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 1, 0004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raviraj V., et al. , Dormant but migratory tumour cells in desmoplastic stroma of invasive ductal carcinomas. Clin. Exp. Metastasis 29, 273–292 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Petersen O. W., Rønnov-Jessen L., Howlett A. R., Bissell M. J., Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 89, 9064–9068 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pradhan S., Slater J. H., Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials 215, 119177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nam S., et al. , Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27Kip1 signaling axis. Sci. Adv. 5, eaaw6171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller E., et al. , Distinguishing autocrine and paracrine signals in hematopoietic stem cell culture using a biofunctional microcavity platform. Sci. Rep. 6, 31951 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gvaramia D., et al. , Combined influence of biophysical and biochemical cues on maintenance and proliferation of hematopoietic stem cells. Biomaterials 138, 108–117 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Bi D., Lopez J. H., Schwarz J. M., Manning M. L., A density-independent rigidity transition in biological tissues. Nat. Phys. 11, 1074–1079 (2015). [Google Scholar]
- 85.Merkel M., Manning M. L., A geometrically controlled rigidity transition in a model for confluent 3D tissues. New J. Phys. 20, 022002 (2018). [Google Scholar]
- 86.Parker A., Marchetti M. C., Manning M. L., Schwarz J. M., How does the extracellular matrix affect the rigidity of an embedded spheroid? arXiv [Preprint] (2020). https://arxiv.org/abs/2006.16203 (Accessed 1 March 2021).
- 87.Holmgren L., O’Reilly M. S., Folkman J., Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1, 149–153 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Hanahan D., Weinberg R. A., The hallmarks of cancer. Cell 100, 57–70 (2000). [DOI] [PubMed] [Google Scholar]
- 89.O’Reilly M. S., Holmgren L., Chen C., Folkman J., Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat. Med. 2, 689–692 (1996). [DOI] [PubMed] [Google Scholar]
- 90.Indraccolo S., et al. , Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc. Natl. Acad. Sci. U.S.A. 103, 4216–4221 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor D. P., Wells J. Z., Savol A., Chennubhotla C., Wells A., Modeling boundary conditions for balanced proliferation in metastatic latency. Clin. Cancer Res. 19, 1063–1070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banani S. F., Lee H. O., Hyman A. A., Rosen M. K., Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel A., et al. , A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Lu Y., et al. , Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai D., et al. , Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 21, 1578–1589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su X., et al. , Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hyman A. A., Weber C. A., Jülicher F., Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Heald R., et al. , Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Grob A., Colleran C., McStay B., Construction of synthetic nucleoli in human cells reveals how a major functional nuclear domain is formed and propagated through cell division. Genes Dev. 28, 220–230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gönczy P., Towards a molecular architecture of centriole assembly. Nat. Rev. Mol. Cell Biol. 13, 425–435 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Zwicker D., Decker M., Jaensch S., Hyman A. A., Jülicher F., Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles. Proc. Natl. Acad. Sci. U.S.A. 111, E2636–E2645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hidalgo J., et al. , Information-based fitness and the emergence of criticality in living systems. Proc. Natl. Acad. Sci. U.S.A. 111, 10095–10100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raup D. M., The role of extinction in evolution. Proc. Natl. Acad. Sci. U.S.A. 91, 6758–6763 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Complexity S., et al. , Critical Phenomena in Natural Sciences (Springer-Verlag, 2006). [Google Scholar]
- 105.Haldeman C., Beggs J. M., Critical branching captures activity in living neural networks and maximizes the number of metastable States. Phys. Rev. Lett. 94, 058101 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Nykter M., et al. , Gene expression dynamics in the macrophage exhibit criticality. Proc. Natl. Acad. Sci. U.S.A. 105, 1897–1900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furusawa C., Kaneko K., Adaptation to optimal cell growth through self-organized criticality. Phys. Rev. Lett. 108, 208103 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Mora T., Bialek W., Are biological systems poised at criticality? J. Stat. Phys. 144, 268–302 (2011). [Google Scholar]
- 109.Werner-Klein M., et al. , Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. Nat. Commun. 11, 4977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ehrig S., et al. , Surface tension determines tissue shape and growth kinetics. Sci. Adv. 5, eaav9394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kollmannsberger P., Bidan C. M., Dunlop J. W. C., Fratzl P., Vogel V., Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 4, eaao4881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented here have been deposited in Edmond, Open Access Data Repository of the Max Planck Society (https://dx.doi.org/10.17617/3.8j), or provided in the SI Appendix and within this paper.