Abstract
Background:
The role of Gram-negative anaerobic periodontal pathogens in periodontal diseases has led to the loss of tooth-supporting structures. These diseases can be prevented by the inhibition of bacterial biofilm on the tooth surfaces. Many treatment modalities have been tried to prevent periodontal diseases. With the rise in resistance to synthetic antimicrobials, there is a requirement to develop natural antimicrobials for the control of periodontitis.
Aim:
The aim of the study was to evaluate and compare the efficacy of garlic (Allium sativum) and guava (Psidium guajava) extracts on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans using time-kill assay.
Materials and Methods:
Aqueous garlic extract (AGaE), ethanolic garlic extract (EGaE), aqueous guava extract (AGuE), and ethanolic guava extract (EGuE) were prepared. Time-kill assays were performed on P. gingivalis and A. actinomycetemcomitans. The aqueous and ethanolic extracts of guava and garlic were compared to assess the maximum bactericidal potency.
Results:
The comparison of time-kill assay of AGaE and AGuE on P. gingivalis showed a statistically significant difference at 2 h (t = 5.29, P < 0.01), 4 h (t = −4.867, P < 0.01), and 6 h (t = −3.647, P < 0.001). The comparison of time-kill assay of EGaE and EGuE on A. actinomycetemcomitans showed a statistically significant difference at 2 h (t = 4.54, P < 0.01) and highly significant difference at 4 h (t = 6.57, P < 0.001).
Conclusions:
The, judicious use of these phytomedicinal products could be cost-effective and also the adverse effects caused due to the long-term usage of synthetic antimicrobials can be avoided.
Keywords: Aggregatibacter actinomycetemcomitans, Allium sativum, antimicrobial, garlic, guava, Porphyromonas gingivalis, Psidium guajava, time-kill assay
Introduction
The role of Gram-negative anaerobic periodontal pathogens in periodontal diseases has been well-documented.[1] The Gram-negative anaerobes mainly Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are found to be linked with the onset and/or development of periodontitis. These organisms express a number of virulence factors which have been implicated in causing periodontal attachment loss.[2] Among these virulence factors, cysteine proteases including arg-gingipains play a major role in P. gingivalis virulence by degrading the host tissue and activating the host pro-inflammatory mediators, thus neutralizing the host immune systems.[3] Leukotoxin of A. actinomycetemcomitans is one of the major endotoxins causing periodontal disease.[4] Long-standing exposure of the periodontal tissues to these microbial toxins results in the loss of supporting structures such as periodontal ligament and alveolar bone, ultimately leading to the loss of teeth.[5]
Developing a better diagnosis and a cost-effective way of curing periodontal disease is required. These diseases can be prevented by the inhibition of bacterial biofilm on the tooth surfaces. This includes the mechanical debridement and the use of antimicrobial agents.[6]
Various researches have shown the inhibitory effect of various antimicrobial agents on the oral biofilm. Nevertheless, these antimicrobials might have some ill effects such as staining of teeth, gastrointestinal disturbance, and risk of developing antibacterial resistance. Hence, a naturally available herbal antimicrobial that interferes with the development of dental plaque is the need of the hour.[7]
Garlic (Allium sativum) is considered as an ancient medicine and known to have antimicrobial properties.[8,9] It has been observed that the resistance to allicin that is one of the active compounds in garlic is thousand times less compared to certain synthetic antimicrobials.[10] Garlic exhibits antimicrobial effect against a variety of oral microorganisms, including Gram-negative periodontal pathogens.[11]
Psidium guajava is a phytotherapic plant commonly known as guava. Guava has been demonstrated for its antimicrobial, antiparasitic, antioxidant, antigenotoxic, anticancer, and antihyperglycemic effects.[12] The leaves of guava have been reported to be used for the maintenance of oral hygiene.[13] The antibacterial activity of guava extract against cariogenic bacteria Lactobacillus acidophilus is reported to be similar to that of chlorhexidine mouthrinse.[14]
Even though guava and garlic are naturally available medicinal plants with proven antimicrobial property, the literature on its effect on periodontal pathogens and their virulence factors and enzymes are scanty. Very few studies have evaluated the efficacy of garlic and guava as phytomedicines against periodontal pathogens.[11,15,16,17] However, there is no literature found regarding comparison of the effect of garlic and guava on oral Gram-negative microbes. Thus, the present study aimed to evaluate and compare the efficacy of guava (P. guajava) and garlic (A. sativum) on P. gingivalis and A. actinomycetemcomitans using time-kill assay.
Materials and Methods
Preparation of guava and garlic extract
Aqueous garlic extract (AGaE), ethanolic garlic extract (EGaE), aqueous guava extract (AGuE), and ethanolic guava extract (EGuE) were prepared equivalent to the previously reported studies in the literature.[15,16]
Microbes and growth condition
Periodontal pathogens such as P. gingivalis and A. actinomycetemcomitans were utilized from the stock culture for the present study. Kanamycin blood agar was used to isolate and Oxoid anaerobic jar was used for cultivating P. gingivalis. Dentaid agar was used to isolate and candle jar technique was used to cultivate A. actinomycetemcomitans.[15,16]
Inoculum preparations
The colonies were transferred to the brain heart infusion (BHI) broth with a sterile straight wire. The turbidity of the suspensions of the bacteria was calibrated with a photometric device to 0.5 McFarland turbidity standards.
Growth kill assay
Serial dilutions of garlic/guava (test extracts) were made to estimate the time-kill assay. A set of 10 tubes were taken and numbered from 1 to 10. One milliliter of the extract to be tested was taken in the first tube. BHI broth (0.5 ml) was added to the remaining tubes numbered from 2 to 10. 0.5 ml of the first tube extract was transferred to the second tube which consisted of BHI broth and it was mixed thoroughly. 0.5 ml from the second tube was serially transferred to the third tube until the 9th tube. 0.5 ml was discarded from the 9th tube and the 10th tube, i.e., the last tube, acted as a control [Figure 1].
Figure 1.
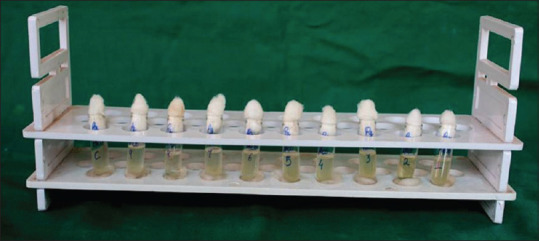
Serial dilutions performed for time-kill assay
The solutions of garlic extract were thus serially diluted and concentrations at 500, 250, 125, 62.5, 31.25, 16.6, 8.3, 4, and 2 mg/ml for EGaE and concentrations at 500, 250, 125, 62.5, 31.25, 16.6, 8.3, 4, and 2 μl/ml for AGaE were obtained. Similarly, the guava extract solutions were serial diluted and concentrations at 500, 250, 125, 62.5, 31.25, 16.6, 8.3, 4, and 2 mg/ml for EGuE and concentrations at 500, 250, 125, 62.5, 31.25, 16.6, 8.3, 4, and 2 μl/ml for AGuE were obtained. 0.1 ml of culture cells (107 cells) were then inoculated into the tube containing test extract. Then directly, it was plated and colonies were observed at 0 h. The minimal inhibitory concentration (MIC) was defined as the lowest concentration of the extract that completely inhibited the growth of the organisms.
The anaerobic jar for P. gingivalis and CO2 jar for A. actinomycetemcomitans were used for culturing. At the end of 2 h again, the first tube was plated. The same procedure was repeated after every 2 h, i.e., after 4 h, 6 h, and 24 h. Then, the plates were incubated in either CO2 jar or anaerobic jar as per the requirement. After 48 h of incubation at 37°C, the plates were taken out and the colonies were calculated.
Statistical analysis
One-way ANOVA was applied to analyze the significance of difference of colonies at various time intervals. Post hoc Bonferronis test was used to determine the pairwise significance of difference in the means of colony formed if the differences across the time intervals were statistically significant. The comparisons of the extracts were analyzed by unpaired t-test. All the analyses were carried out using SPSS version 20.0 (SPSS Version 20.0. Armonk, NY: IBM Corp.) and the statistical significance was tested at a 5% level.
Results
Minimal inhibitory concentration of garlic and guava extracts
The AGaE exhibited MIC at 16.6 μl/ml and EGaE exhibited MIC at 62.5 mg/ml on P. gingivalis. The MIC for the AGaE aqueous extract was determined at 62.5 μl/ml on A. actinomycetemcomitans, whereas A. actinomycetemcomitans was completely resistant to all the concentrations of the EGaEs [Table 1].
Table 1.
Minimal inhibitory concentration of garlic and guava extracts
| 500 | 250 | 125 | 62.5 | 31.25 | 16.6 | 8.3 | 4 | 2 | Control | |
|---|---|---|---|---|---|---|---|---|---|---|
| MIC of garlic on P. gingivalis | ||||||||||
| AGaE | S | S | S | S | S | R | R | R | R | R |
| EGaE | S | S | S | R | R | R | R | R | R | R |
| MIC of garlic on A. actinomycetemcomitans | ||||||||||
| AGaE | S | S | S | R | R | R | R | R | R | R |
| EGaE | R | R | R | R | R | R | R | R | R | R |
| MIC of guava on P. gingivalis | ||||||||||
| AGuE | S | S | S | S | S | S | S | R | R | R |
| EGuE | S | S | S | S | S | S | S | S | R | R |
| MIC of Guava on A. actinomycetemcomitans | ||||||||||
| AGuE | S | S | S | S | S | R | R | R | R | R |
| EGuE | S | S | S | S | S | S | S | S | S | R |
S: Susceptible; R: Resistant; MIC: Minimal inhibitory concentration; AGaE: Aqueous garlic extract; EGaE: Ethanolic garlic extract; AGuE: Aqueous guava extract; EGuE: Ethanolic guava extract; P. gingivalis: Porphyromonas gingivalis, A. actinomycetemcomitans: Aggregatibacter actinomycetemcomitans
The AGuE exhibited MIC at 4 μl/ml, whereas EGuE exhibited MIC at 2 μl/ml for P. gingivalis. A. actinomycetemcomitans showed lesser resistance to ethanolic extracts. The MIC for the AGuE was determined at 16.6 μl/ml, whereas A. actinomycetemcomitans was completely susceptible to all the concentrations of the EGuE [Table 1].
Time-kill assay of garlic extracts
The garlic extracts exhibited only bacteriostatic activity for both the organisms. Bactericidal effect on P. gingivalis was not evident over the first 2 h of incubation; however, bacteriostatic activity was noticed between 2 and 6 h. The aqueous extract showed greater bacteriostatic activity when compared to the ethanolic extract, followed by a gradual increase in the colony-forming units and bacteriostatic activity was not observed at 24 h [Table 2 and Figure 2]. The control tube showed no drop in colony count during the same period.
Table 2.
Time-kill assay for Porphyromonas gingivalis by garlic extracts
| Extracts | Time (h) |
Significance |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 24 | F | P | |
| AGaE | 130.8 | 70.6 | 111.4 | 112.0 | 500 | 843.84 | <0.001* |
| EGaE | 141.4 | 177.6 | 199.8 | 177.4 | 500 | 1031.58 | <0.001* |
| t | −0.69 | −9.20 | −8.96 | −7.04 | |||
| P | 0.50 | <0.001** | <0.001** | <0.001** | NS | ||
Post hoc Bonferronis test: AGaE 0 h versus 4 h and 6 h; 2 h versus 4 h and 6 h; 4 h versus 6 h – Nonsignificant. EGaE 0 h versus 2 h, 2 h versus 4 h and 6 h; 4 h versus 6 h – Nonsignificant. *Significant, **Highly significant; NS: Nonsignificant, AGaE: Aqueous garlic extract, EGaE: Ethanolic garlic extract
Figure 2.
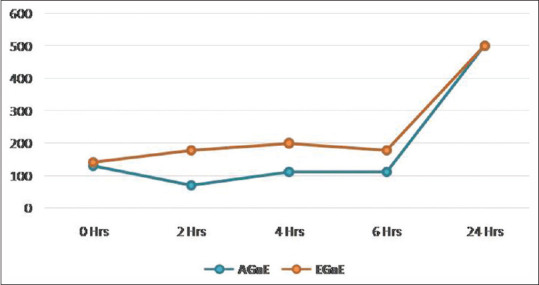
Time-kill assay for Porphyromonas gingivalis by garlic extracts
The A. actinomycetemcomitans showed resistance to the bactericidal activity of the garlic extracts. Bacteriostatic activity was noticed between 0 and 2 h incubation period for aqueous and ethanolic extracts. Later on, a gradual increase in the colonies was observed up to 24 h [Table 3 and Figure 3].
Table 3.
Time-kill assay for Aggregatibacter actinomycetemcomitans by garlic extracts
| Extracts | Time (h) |
Significance |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 24 | F | P | |
| AGaE | 73.6 | 38.4 | 47.6 | 68.4 | 500 | 419.29 | <0.001** |
| EGaE | 215.2 | 58.6 | 60.6 | 56.4 | 500 | 91.11 | <0.001** |
| t | −3.02 | −2.12 | −1.07 | 0.72 | |||
| P | <0.05* | 0.06 | 0.31 | 0.48 | NS | ||
Post hoc Bonferronis test: AGaE 0 h versus 2 h, 4 h, and 6 h; 2 h versus 4 h and 6 h; 4 h versus 6 h – Nonsignificant. EGaE 0 h versus 2 h, 4 h,6 h, and 24 h, 2 h versus 4 h and 6 h; 4 h versus 6 h – Nonsignificant. *Significant, **Highly significant. NS: Nonsignificant, AGaE: Aqueous garlic extract; EGaE: Ethanolic garlic extract
Figure 3.
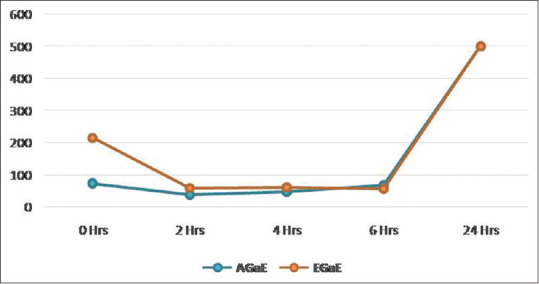
Time-kill assay for Aggregatibacter actinomycetemcomitans by garlic extracts
Time-kill assay of AGaE and EGaE was compared for both the microbes used in the study [Tables 2 and 3]. The comparisons analyzed by unpaired t-test showed statistical significance with P. gingivalis at 2 h (t = −9.205, P < 0.001), 4 h (t = −8.962, P < 0.001), and 6 h (t = −7.046, P < 0.001).
However, statistical significance was seen only at the beginning at 0 h between AGaE and EGaE on A. actinomycetemcomitans.
Time-kill assay of guava extracts
A statistically significant decrease in the colonies of P. gingivalis was seen up to 6 h in both AGuE and EGuE. Bacteriostatic activity was seen during 4–6 h in case of both the type of guava extracts where there was no statistically significant difference in the colonies count from 4 to 6 h. There was a raise in the colony-forming units with no bacteriostatic activity observed at 24 h [Table 4 and Figure 4].
Table 4.
Time-kill assay for Porphyromonas gingivalis by guava extracts
| Extracts | Time (h) |
Significance |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 24 | F | P | |
| AGuE | 142.2 | 131.4 | 102.4 | 99.6 | 500 | 71318.96 | <0.001* |
| EGuE | 126.0 | 95.8 | 74.6 | 70.0 | 500 | 28879.90 | <0.001* |
| T | 6.48 | 19.90 | 10.34 | 11.92 | |||
| P | <0.001** | <0.001** | <0.001** | <0.001** | NS | ||
Post hoc Bonferronis test: Both AGuE and EGuE for 4 h versus 6 h – NS. *Significant, NS: Nonsignificant; AGuE: Aqueous guava extract; EGuE: Ethanolic guava extract; **Highly Significant
Figure 4.
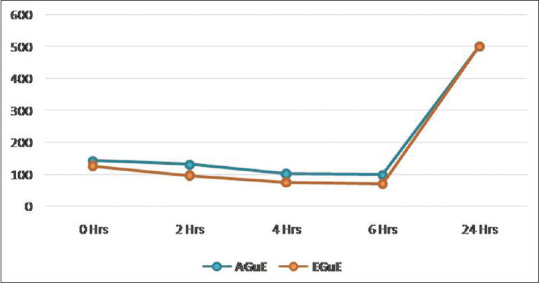
Time-kill assay for Porphyromonas gingivalis by guava extracts
The A. actinomycetemcomitans colonies count decreased statistically up to 4 h with aqueous extract and there was an increase in the colony-forming units with no bacteriostatic activity at 6 h and 24 h. However, with ethanolic extract, there was a statistically significant decrease in the colonies count up to 6 h with no significant difference from 2 h to 6 h, indicating bacteriostatic activity between 2 and 6 h [Table 5 and Figure 5]. Control cell suspensions without guava extract showed no drop in viability over the same period.
Table 5.
Time-kill assay for Aggregatibacter actinomycetemcomitans by guava extracts
| Extracts | Time (h) |
Significance* |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 24 | F | P | |
| AGuE | 132.0 | 98.2 | 72.4 | 101.6 | 500 | 85394.20 | <0.001* |
| EGuE | 128.2 | 92.6 | 86.4 | 84.4 | 500 | 23372.97 | <0.001* |
| T | 1.182 | 2.701 | −10.1 | 10.42 | |||
| P | 0.271 | <0.05* | <0.001** | <0.001** | NS | ||
Post hoc Bonferronis test: AGuE 2 h versus 6 h NS. EGuE 2 h versus 4 h, 2 h versus 6 h, 4 h versus 6 h NS. *Significant. NS: Nonsignificant; AGuE: Aqueous guava extract; EGuE: Ethanolic guava extract; **Highly Significant
Figure 5.
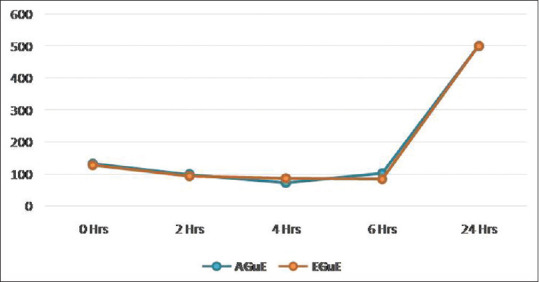
Time-kill assay for Aggregatibacter actinomycetemcomitans by guava extracts
Time-kill assay of AGuE and EGuE was compared for P. gingivalis and A. actinomycetemcomitans [Tables 4 and 5]. The comparisons analyzed by unpaired t-test showed statistical significance with P. gingivalis at 0 h (t = 6.485, P < 0.001), 2 h (t = 19.901, P < 0.001), 4 h (t = 10.346, P < 0.001), and 6 h (t = 11.926, P < 0.001) and with A. actinomycetemcomitans at 2 h (t = 2.701, P < 0.05), 4 h (t = −10.1, P < 0.001), and 6 h (t = 10.42, P < 0.001).
Comparison of aqueous extracts of guava and garlic on Porphyromonas gingivalis
Time-kill assay of AGuE and AGaE was compared for P. gingivalis. The comparisons showed statistically significant difference at 2 h (t = 5.29, P < 0.01), 4 h (t = −4.867, P < 0.01), and 6 h (t = −3.647, P < 0.001) [Table 6].
Table 6.
Comparison of aqueous extracts of guava and garlic on Porphyromonas gingivalis
| Micro-organism | Time | Extract | n | Mean±SD | Significance |
|
|---|---|---|---|---|---|---|
| t | P | |||||
| P. gingivalis | 0 h | AGuE | 5 | 142.2±2.2 | 0.88 | 0.401 |
| AGaE | 5 | 130.8±28.6 | ||||
| 2 h | AGuE | 5 | 131.4±1.9 | 5.29 | <0.01* | |
| AGaE | 5 | 70.6±25.6 | ||||
| 4 h | AGuE | 5 | 102.4±3.2 | −4.86 | <0.01* | |
| AGaE | 5 | 111.4±2.5 | ||||
| 6 h | AGaE | 5 | 99.6±2.9 | −3.64 | <0.01* | |
| AGaE | 5 | 112.0±7.0 | ||||
| 24 h | AGuE | 5 | 500.0±0.0 | 0.00 | NS | |
| AGaE | 5 | 500.0±0.0 | ||||
*Significant. NS: Nonsignificant; AGaE: Aqueous garlic extract; AGuE: Aqueous guava extract; SD: Standard deviation; P. gingivalis: Porphyromonas gingivalis
Comparison of ethanolic extracts of guava and garlic on Porphyromonas gingivalis
Time-kill assay of EGuE and EGaE was compared for P. gingivalis. The comparisons showed statistically high significant difference at 2 h (t = −32.85, P < 0.001), 4 h (t = −12.45, P < 0.001), and 6 h (t = −11.95, P < 0.001) [Table 7].
Table 7.
Comparison of ethanolic extracts of guava and garlic on Porphyromonas gingivalis
| Micro-organism | Time | Extract | n | Mean±SD | Significance |
|
|---|---|---|---|---|---|---|
| t | P | |||||
| P. gingivalis | 0 h | EGuE | 5 | 126.0±5.0 | −1.76 | 0.116 |
| EGaE | 5 | 141.4±18.8 | ||||
| 2 h | EGuE | 5 | 95.8±3.4 | −32.85 | <0.001** | |
| EGaE | 5 | 177.6±4.3 | ||||
| 4 h | EGuE | 5 | 74.6±5.0 | −12.45 | <0.001** | |
| EGaE | 5 | 199.8±21.9 | ||||
| 6 h | EGuE | 5 | 70.0±4.6 | −11.95 | <0.001** | |
| EGaE | 5 | 177.4±19.5 | ||||
| 24 h | EGuE | 5 | 500.0±0.0 | 0.00 | NS | |
| EGaE | 5 | 500.0±0.0 | ||||
**Highly significant. NS: Nonsignificant; EGuE: Ethanolic guava extract; EGaE: Ethanolic garlic extract; SD: Standard deviation; P. gingivalis: Porphyromonas gingivalis
Comparison of aqueous extracts of guava and garlic on Aggregatibacter actinomycetemcomitans
Time-kill assay of AGuE and AGaE was compared for A. actinomycetemcomitans. The comparisons showed statistically high significant difference at 2 h (t = 9.62, P < 0.01) and significant difference at 0 h (t = 3.64, P < 0.01) and 6 h (t = 4.59, P < 0.01) [Table 8].
Table 8.
Comparison of aqueous extracts of guava and garlic on Aggregatibacter actinomycetemcomitans
| Micro-organism | Time | Extract | n | Mean±SD | Significance |
|
|---|---|---|---|---|---|---|
| t | P | |||||
| A. actinomycetem comitans | 0 h | AGuE | 5 | 132.0±2.4 | 3.64 | <0.01* |
| AGaE | 5 | 73.6±35.7 | ||||
| 2 h | AGuE | 5 | 98.2±2.8 | 9.62 | <0.001** | |
| AGaE | 5 | 38.4±13.5 | ||||
| 4 h | AGuE | 5 | 72.4±2.0 | 2.14 | 0.097 | |
| AGaE | 5 | 47.6±25.7 | ||||
| 6 h | AGuE | 5 | 101.6±2.6 | 4.59 | <0.01* | |
| AGaE | 5 | 68.4±15.9 | ||||
| 24 h | AGuE | 5 | 500.0±0.0 | 0.00 | NS | |
| AGaE | 5 | 500.0±0.0 | ||||
*Significant, **Highly significant. NS: Nonsignificant; SD: Standard deviation; A. actinomycetemcomitans: Aggregatibacter actinomycetemcomitans
Comparison of ethanolic extracts of guava and garlic on Aggregatibacter actinomycetemcomitans
Time-kill assay of EGuE and EGaE was compared for A. actinomycetemcomitans. The comparisons showed statistically significant difference at 2 h (t = 4.54, P < 0.01) and highly significant difference at 4 h (t = 6.57, P < 0.001) [Table 9].
Table 9.
Comparison of ethanolic extracts of guava and garlic on Aggregatibacter actinomycetemcomitans
| Micro-organism | Time | Extract | n | Mean±SD | Significance |
|
|---|---|---|---|---|---|---|
| t | P | |||||
| A. actinomycetem comitans | 0 h | EGuE | 5 | 128.2±6.7 | −1.97 | 0.084 |
| EGaE | 5 | 215.2±98.2 | ||||
| 2 h | EGuE | 5 | 92.6±3.6 | 4.54 | <0.01* | |
| EGaE | 5 | 58.6±16.3 | ||||
| 4 h | EGuE | 5 | 86.4±2.3 | 6.57 | <0.001** | |
| EGaE | 5 | 60.6±8.4 | ||||
| 6 h | EGuE | 5 | 84.4±2.6 | 1.87 | 0.098 | |
| EGaE | 5 | 56.4±33.3 | ||||
| 24 h | EGuE | 5 | 500.0±0.0 | 0.00 | NS | |
| EGaE | 5 | 500.0±0.0 | ||||
*Significant, **Highly significant. NS: Nonsignificant; EGuE: Ethanolic guava extract; EGaE: Ethanolic garlic extract; A. actinomycetemcomitans: Aggregatibacter actinomycetemcomitans
Discussion
Periodontal disease is known as an immune modulatory disease which results in the destruction of periodontal tissue, loss of attachment, and alveolar bone resorption. P. gingivalis and A. actinomycetemcomitans are known to be associated with periodontitis.[18] P. gingivalis is one of the most important bacteria causing periodontal disease, and it can colonize in the subgingival area, which may begin the process of periodontal disease, thereby activating other Gram-negative bacteria species to colonize and further infect periodontal tissue. Inhibition or elimination of the subgingival periodontopathogens like P. gingivalis and A. actinomycetemcomitans is the key for the successful treatment of periodontitis.[18,19,20]
In the present study, the inhibitory effect of aqueous and ethanolic extracts of garlic and guava on P. gingivalis and A. actinomycetemcomitans was evaluated using the time-kill assay. The efficacy of aqueous extracts of guava was compared with that of garlic; similarly, the ethanolic extracts of guava were compared with that of garlic.
Time-kill assay of AGaE and EGaE was compared for both the microorganisms revealed statistical significance for P. gingivalis at 2 h, 4 h, and 6 h. However, statistical significance was seen only at the beginning at 0 h between aqueous and EGaEs on A. actinomycetemcomitans.
Thus, garlic extract elicited its antimicrobial activity in a time-dependent manner exhibiting distinct time-kill assay, suggesting differences in the growth inhibitory response in tested isolates to it. Similar responses were observed by Yin et al.[21] and Iwalokun et al.[22] However, the exceptionality of time-kill assay on Gram-negative pathogens in the present study may be due to the structural variability between these two microorganisms.
The A. actinomycetemcomitans colonies count decreased statistically up to 4 h with aqueous extract and there was a raise in the colony-forming units with no bacteriostatic activity at 6 h and 24 h. However, with ethanolic extract, there was statistically significant decrease in the colonies count up to 6 h with no significant difference from 2 h to 6 h, indicating bacteriostatic activity between 2 and 6 h. Time-kill assay of AGuE and EGuE was compared for both the microorganisms. The comparisons revealed statistically significant results for P. gingivalis at 0 h, 2 h, 4 h, and 6 h, whereas A. actinomycetemcomitans showed statistically significant difference at 2 h, 4 h, and 6 h.
These observations interpreted that the guava extract showed its antimicrobial activity in a time-dependent fashion producing distinct time-kill assay, suggestive of differences in the growth inhibitory response of the tested microorganisms to guava extracts.
The guava extracts showed a better time-kill profile on A. actinomycetemcomitans compared to garlic extracts. Similar findings were reported by Kwamin et al.[4] stating that extracts of guava leaves and twigs contain components that efficiently neutralize the leukotoxicity of A. actinomycetemcomitans. Toma and Genet[23] showed that this leukotoxin-neutralizing activity of guava extract is stable and persist for at least 24 h; probably, this would have been one of the reasons for guava extract showing better time-kill profile on A. actinomycetemcomitans than garlic. However, in the present study, the time-kill potency of A. actinomycetemcomitans was between 2 and 6 h.
In the present study, the AGaE was more potent than the ethanolic extract, similar to observations of Roy et al.,[24] Jaber et al.,[25] and El-Mahmood and Amey[26] but in contrast with that of Debnath.[27] One of the probable explanations for this could be the evaporation of volatile components of EGaE when it was heated at 800°C.
The efficacy of ethanolic guava leaf extract was found to be better than aqueous guava leaf extract. Ethanolic extract contains tannins as well as flavonoids, whereas aqueous extract contains tannins but not flavonoids. This difference in composition of ethanolic and aqueous extract can be attributed to the difference in the solubility of various components of guava leaves in water and organic solvents.[28]
Thus, AGaE showed significant antimicrobial activity against P. gingivalis and EGuE showed maximum bactericidal activity on A. actinomycetemcomitans. However, the combination of both garlic and guava extract has to be tried out in future. Further studies and clinical trials need to be undertaken to explore the efficacy of guava and garlic in humans. Mouthwashes can be prepared and the effects can be compared with the synthetic mouthwashes available in the market. Combination of guava and garlic extract should be tried and evaluated. Garlic and guava extracts could be used as mouthwashes, local drug delivery agents in the form of gel; chip and threads could treat and manage both localized and generalized periodontitis. Hence, using these phytomedicinal extracts as an adjunct with surgical and nonsurgical therapy might result in the elimination of periodontal pathogens.
Limitations of the study
This present study is an in-vitro microbiological study; there still lies a void in research with respect to the clinical trials of these phytomedicinal agents in periodontal diseases. A second limitation is regarding the therapeutic usefulness of these phytomedicinal extracts that the constituents of garlic/guava might form a complex with blood proteins and its efficacy in the presence of bleeding at a periodontal site was not evaluated. Future targeted long-term clinical trials of these phytomedicinal remedies are required.
Conclusions
Garlic and guava extract displayed a significant antimicrobial effect on P. gingivalis and A. actinomycetemcomitans. Garlic was found to be most effective against P. gingivalis, whereas guava showed the highest efficacy on A. actinomycetemcomitans. Time-kill assay results revealed probable use of garlic and guava as a suitable adjuvant to synthetic antimicrobials. Thus, judicious use of these naturally occurring phytomedicinal products could be cost-effective and also the adverse effects caused due to the long-term usage of synthetic antimicrobials can be avoided.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: Virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–21. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 2.Holt SC, Bramanti TE. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 3.Kuramitsu HK, Yoneda M, Madden T. Proteases and collagenases of Porphyromonas gingivalis. Adv Dent Res. 1995;9:37–40. doi: 10.1177/08959374950090010701. [DOI] [PubMed] [Google Scholar]
- 4.Kwamin F, Gref R, Haubek D, Johansson A. Interactions of extracts from selected chewing stick sources with Aggregatibacter actinomycetemcomitans. BMC Res Notes. 2012;5:203. doi: 10.1186/1756-0500-5-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol 2000. 2012;58:37–68. doi: 10.1111/j.1600-0757.2011.00415.x. [DOI] [PubMed] [Google Scholar]
- 6.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Prakash S. To detect the minimum inhibitory concentration and time-kill curve of shiitake mushroom on periodontal pathogens: An in vitro study. J Indian Soc Periodontol. 2019;23:216–9. doi: 10.4103/jisp.jisp_249_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 9.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–9. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 10.Gupta KC, Viswanthan R. Combined action of streptomycin and chloramphenicol with plant antibiotics against tubercle bacilli. I. Streptomycin and chloramphenical with cepharanthine. II. Streptomycin and allicin. Antibiot Chemother. 1995;5:24–7. [PubMed] [Google Scholar]
- 11.Bakri IM, Douglas CW. Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol. 2005;50:645–51. doi: 10.1016/j.archoralbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Gupta GK, Chahal J, Arora D. Psidium guajava Linn: Current research and future prospects. J Pharm Res. 2011;4:42–6. [Google Scholar]
- 13.Jebashree HS, Kingsley SJ, Sathish ES, Devapriya D. Antimicrobial activity of few medicinal plants against clinically isolated human cariogenic pathogens – An in vitro study. ISRN Dent. 2011;2011:541421. doi: 10.5402/2011/541421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain D, Dasar P, Nagarajappa S, Kumar S, Airen B, Warhekar S. In vitro activity of ethanolic and water extract of guava leaves at various concentrations against Lactobacillus acidophilus. J Indian Assoc Public Health Dent. 2014;12:232–6. [Google Scholar]
- 15.Shetty S, Thomas B, Shetty V, Bhandary R, Shetty RM. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. Ayu. 2013;34:445–51. doi: 10.4103/0974-8520.127732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shetty YS, Shankarapillai R, Vivekanandan G, Shetty RM, Reddy CS, Reddy H, et al. Evaluation of the efficacy of guava extract as an antimicrobial agent on periodontal pathogens. J Contemp Dent Pract. 2018;19:690–7. [PubMed] [Google Scholar]
- 17.Shetty S, Shetty RM, Rahman B, Vannala V, Desai V, Shetty SR. Efficacy of Psidium guajava and Allium sativum extracts as antimicrobial agents against periodontal pathogens. J Pharm Bioall Sci. 2020;12:S589–94. doi: 10.4103/jpbs.JPBS_206_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzo PJ, Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003;32:24–35. doi: 10.1046/j.0906-6713.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- 19.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–25. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 21.Yin MC, Chang HC, Tsao SM. Inhibitory effects of aqueous garlic extract, garlic oil and four diallyl sulphides against four enteric pathogens. J Food Drug Anal. 2002;10:120–6. [Google Scholar]
- 22.Iwalokun BA, Ogunledun A, Ogbolu DO, Bamiro SB, Jimi-Omojola J. In vitro antimicrobial properties of aqueous garlic extract against multi drug resistant bacteria and candida species from Nigeria. J Med Food. 2004;7:327–33. doi: 10.1089/jmf.2004.7.327. [DOI] [PubMed] [Google Scholar]
- 23.Toma C, Genet K. The Interaction between Guava-extract and Aggregatibacter actinomycetemcomitans Leukotoxin [Internet] [Dissertation] 2014. [Last accessed on 2020 Oct 22]. Available from: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-102414 .
- 24.Roy J, Shakaya DM, Callery PS, Thomas JG. Chemical constituents and antimicrobial activity of a traditional herbal medicine containing garlic and black cumen. Afr J Trad. 2006;3:1–7. [Google Scholar]
- 25.Jaber MA, Al-Mossawi A. Susceptibility of some multiple resistant bacteria to garlic extract. Afr J Biotechnol. 2007;6:771–6. [Google Scholar]
- 26.El-Mahmood AM, Amey JM. In vitro antibacterial activity of Parkia biglobosa (Jacq) root bark extract against some microorganisms associated with urinary infections. Afr J Biotechnol. 2007;6:1272–5. [Google Scholar]
- 27.Debnath M. Clonal propagation and antimicrobial activity of an endemic medicinal plant Steria rebaudiana. J Med Plants Res. 2005;2:48–58. [Google Scholar]
- 28.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]


