Abstract
The kidney has a remarkable regenerative capacity. In response to ischemic or toxic injury, proximal tubule cells can proliferate to rebuild damaged tubules and restore kidney function. However, severe acute kidney injury (AKI) or recurrent AKI events can lead to maladaptive repair and disease progression from AKI to chronic kidney disease (CKD). The application of single cell technologies has identified injured proximal tubule cell states weeks after AKI, distinguished by a pro-inflammatory senescent molecular signature. Epigenetic studies highlighted dynamic changes in the chromatin landscape of the kidney following AKI and described key transcription factors linked to the AKI response. The integration of multi-omic technologies opens new possibilities to improve our understanding of AKI and the driving forces behind the AKI-to-CKD transition, with the ultimate goal of designing tailored diagnostic and therapeutic strategies to improve AKI outcomes and prevent kidney disease progression.
Keywords: acute kidney injury, multi-omics, epigenomics, single-cell RNA-sequencing, ATAC sequencing
Introduction
Acute kidney injury (AKI), diagnosed by a sudden increase in serum creatinine and/or decrease of urine volume, is a highly prevalent condition associated with increased morbidity and mortality as well as significant health care costs [1–4]. For instance, current evidence suggests that at least 25% of all hospitalized COVID-19 patients develop AKI and that these patients have a significantly higher mortality risk than COVID-19 patients without AKI [5]. Patients surviving AKI are at increased risk of transitioning to chronic and end-stage kidney disease [3,4,6]. According to the 2018 US Renal Data System annual report, only 52.6% of hospitalized Veteran Affairs patients, who met the AKI diagnosis criteria, were given the diagnosis AKI, calling for more awareness for AKI in hospital routines [7]. Furthermore, early diagnosis of AKI is limited by a lack of sensitive biomarkers, and available treatment options for AKI continue to rely on hemodynamic optimization, avoidance of nephrotoxicity and renal replacement therapy.
Proximal tubule cells, the most abundant cell type in the kidney, are responsible for a large part of renal fluid and solute re-absorption and have emerged as major players in adaptive and maladaptive responses to AKI [8]. Due to their high metabolic activity and dependence on oxidative metabolism, ischemia rapidly leads to ATP-depletion, accumulation of reactive oxygen species and ultimately apoptosis or necrosis of proximal tubule cells (Fig. 1). Proximal tubule cells that survive an ischemic event have the capacity to dedifferentiate, proliferate and rebuild damaged tubules, thus playing an essential part in the adaptive repair process to restore kidney function [9–11]. However, maladaptive proximal tubule cells, characterized by a pro-inflammatory, pro-fibrotic phenotype, have also been implicated in disease progression from AKI to CKD, a complex process involving inflammation, vascular rarefication and extracellular matrix production by activated pericytes and myofibroblasts (Fig. 1) [8,12].
Figure 1. Overview of AKI and processes.
Proximal tubule cells are highly susceptible to ischemia, which can lead to cell death through apoptosis and regulated (pyroptosis, ferroptosis, necroptosis) as well as unregulated necrosis and sloughing of both dead and viable cells into the tubular lumen. Surviving proximal tubule cells (PTCs) can dedifferentiate and proliferate to restore kidney architecture and function. However, maladaptive repair can also lead to disease progression to chronic kidney disease (CKD). This process is characterized by vascular rarefication, pericyte to myofibroblast differentiation, enhanced fibrous extracellular matrix (ECM) deposition, tubular loss and chronic inflammation. Nfkb1+ failed-repair PTCs, characterized by a senescence-associated secretory phenotype (SASP), likely play an important role in the AKI-to-CKD transition.
Omics technologies examining genomic organization, gene expression and protein products, like assay for transposase-accessible chromatin using sequencing (ATAC-seq), mRNA-sequencing and mass spectrometry, respectively, have dramatically improved molecular insight into cellular responses initiated by AKI. Most recently, single cell applications of these approaches enabled the study of cellular events at unprecedentedly high resolution. These and other multi-omics approaches hold the promise of a comprehensive understanding of the regulatory mechanisms governing kidney disease, opening new avenues for biomarker and drug discovery. This manuscript reviews recent findings applying omics technology to kidney injury and repair and considers future applications of multi-omic approaches to advance understanding and treatment of kidney injury and kidney disease.
Genomics
Genomic studies aim to identify the genetic basis of a disease by hypothesis-driven targeted sequencing of candidate genes or hypothesis-free whole genome sequencing. Here, we will focus on genome-wide association studies (GWAS), which use an unbiased approach to map sequence variants across the whole genome to phenotypic features like disease traits and allow candidate gene identification for mechanistic studies (Fig. 2). Compared to other disease traits, the available number of GWAS interrogating the genetic background of AKI is scarce. This is partially due to the complex and heterogenic nature of AKI as a disease trait. AKI can be caused by a multitude of different factors notably: ischemia, sepsis, nephrotoxic drugs and obstructive nephropathy. Current evidence suggests that the underlying molecular and cellular mechanism of AKI have cause-specific components [13,14], but the consensus definition of AKI - a change in serum creatinine and urine output - does not account for different AKI causes.
Figure 2. Schematic of selected omics technologies.
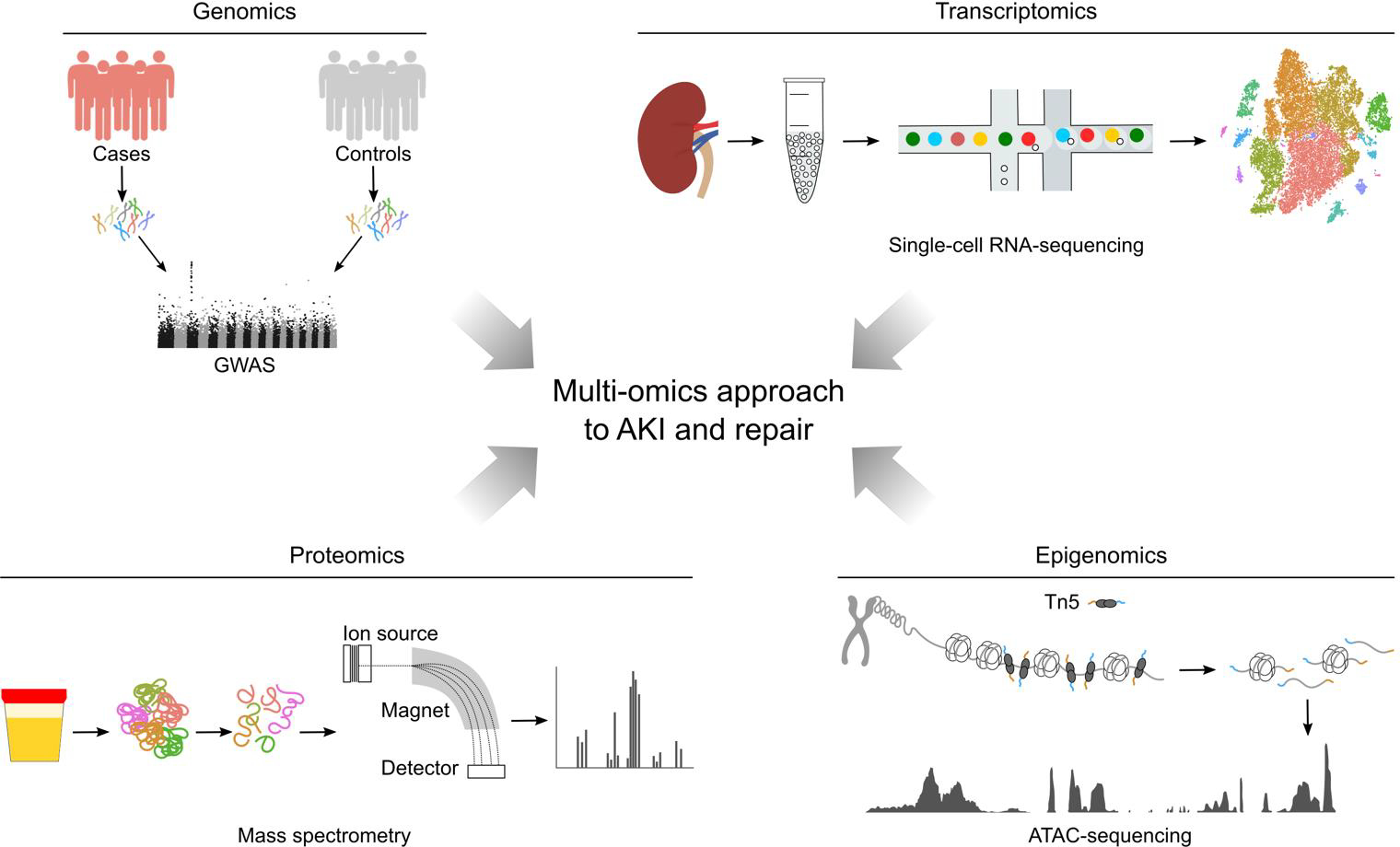
A simplified workflow for a genome-wide association study (GWAS) is illustrated (top left). To study an association between genetic variants and a specific trait, whole-genome sequencing or genotyping using single-nucleotide polymorphism (SNP) arrays is performed on a population and statistical association tests are used to identify SNPs associated with the trait of interest. For single-cell RNA-sequencing (top right), a single-cell suspension is prepared, single-cells are isolated and the mRNA molecules from the cell (or nucleus) captured on beads for subsequent conversions into cDNA. cDNA’s are amplified and sequences determined by next-generation sequencing (NGS) and sequence mapping to the genome. For epigenomic analysis (bottom right), an assay for transposase-accessible chromatin (ATAC) is followed by NGS sequencing and sequence mapping to visualize genome-wide chromatin accessibility. Hyperactive Tn5 transposase (Tn5) is used to specifically cleave sites of open chromatin and simultaneously insert sequencing adapters for NGS. Regions of increased chromatin accessibility are shown as ATAC peaks. For proteomics analysis by mass spectrometry (bottom left), proteins are extracted, digested into peptides and ionized. In the mass spectrometer, ions are accelerated, subjected to a magnetic field and their mass-to-charge ratio is measured, allowing for protein identification and quantification.
To date, two larger GWAS studies interrogated the genetic background of AKI. The first study showed an association of single nuclear polymorphisms (SNPs) in the GRM7|LMCD1-AS1 and the BBS9 locus with coronary bypass graft surgery related AKI in a discovery cohort of 873 patients and a replication cohort [15]. The second combined patients from two distinct cohorts to form a discovery population of 1429 critically ill patients and identified four AKI associated SNPs in close proximity to either the interferon regulatory factor 2 (IRF2) or the transcription factor T-box 1 (TBX1) [16]. Interestingly, IRF2 has been linked to pyroptosis and a regulatory role in the immune system [17,18] and TBX1 is part of the T-box gene family, a group of transcription factors with important roles in cell fate and cell state regulation [19]. However, the association between AKI and these loci could not be reproduced in a prospective cohort of critically ill patients, which were specifically genotyped for the identified SNPs [20]. This underlines the necessity of even larger sample sizes for future AKI GWAS and illustrates the difficulty in identifying robust genetic risk factors for heterogeneous diseases like AKI.
In addition, expression quantitative trait loci (eQTL) analyses will likely aid the translation of insights gained by GWAS to disease mechanisms. eQTL analyses aim to identify loci that explain a fraction of the gene expression variance in a tissue and can be used to link SNPs in the non-coding genome to target gene expression. For example, an integrative approach combining GWAS, eQTL and functional experiments led to the identification of genes involved in the pathogenesis of CKD [21–23].
Transcriptomics
The advent of new scalable technologies to study the transcriptome of cells within the injured kidney has revolutionized our understanding of the cellular responses to AKI, uncovering new cell states and highlighting the dynamically changing transcriptional landscape of the repairing kidney. Kidney bulk mRNA-sequencing over the course of one year after AKI demonstrated temporal-specific gene expression changes related to tubular injury, proliferation, repair, fibrosis and immunity [24]. This mouse AKI biobank data showed that inflammation-associated genes were still upregulated one year after AKI, suggesting persistent inflammation as a driving factor of disease progression to CKD. Bulk mRNA-sequencing of routine kidney transplant biopsies allowed for profiling of the short- and long-term transcriptional changes in human kidney transplants triggered by the ischemia/reperfusion injury (IRI) during transplantation [25]. All kidney biopsies taken in the first hours after reperfusion showed a similar transcriptional profile characterized by activation of immediate-early response genes, many of which encode transcriptional regulatory factors. At 3- and 12-months post transplantation, however, two divergent trajectories associated with either recovery or progression to CKD could be delineated. The CKD-like trajectory showed a downregulation in the expression of genes related to mitochondrial biogenesis and an upregulation of inflammation and fibrosis associated genes, as observed in the mouse model of AKI [24,25]. Both mouse and human studies suggest a prominent role for T and B cell activity and the appearance of centers of tertiary lymphoid tissue in long-term outcomes of AKI [25,26]. Analysis of the mouse AKI biobank and human kidney transplant datasets argue for an auto-antigen driven B cell activity, promoting disease progression through sustained immune response, one year after AKI [27].
Although bulk mRNA-sequencing has proven highly informative in AKI studies, cell type specific responses are not differentiated by this technique. Genetic approaches labeling distinct cell types add greater precision and additional insight. For example, translating ribosome affinity purification (TRAP) uses an eGFP-tagging of the L10a ribosomal subunit to identify the specific mRNA subset in individual cell types undergoing active translation [28]. The first TRAP study in AKI research employed a genetic model of CRE-recombinase-mediated activation of the eGFP-L10a cassette specifically within nephron, stromal, vascular or immune cell types and showed distinct transcriptional changes linked to each cellular compartment following AKI [29]. Many genes related to renal tubular function were downregulated 24 hours after AKI. In contrast, genes involved in anti-apoptotic pathway activity, cell proliferation and cell movement were markedly upregulated. TRAP analysis of proximal tubule cells highlighted by the injury marker Kim-1 (encoded by Havcr1) showed the majority of Kim-1 positive cells successfully repaired two weeks after AKI, although 15% retained expression of Kim-1 and the injury marker Sox9 [10], highlighting a persistently injured cell state [30]. Clonal analysis revealed that expansion of Kim-1 positive clones repopulated damaged proximal tubules, supporting a model of repair through dedifferentiation of proximal tubule cells, rather than through a specific stem/progenitor cell population [30]. The transcription FoxM1 was highly upregulated in Kim-1 positive proximal tubule cells and shown to drive proximal tubule proliferation in vitro in an epidermal growth factor receptor-dependent manner [30].
High-throughput single-cell mRNA-sequencing approaches examining whole cell mRNA sequencing profiles (scRNA-seq) or nuclear localized mRNA transcripts (snRNA-seq) have dramatically enhanced AKI studies. These approaches provide a deep molecular insight into gene expression at the cell level when combined with powerful computational approaches, identifying variable cell types and cell states throughout the organ [31,32]. scRNA-seq starts from a suspension of single cells or single nuclei, which are labeled with unique molecular tags and lysed (Fig. 2). The mRNA is subsequently reverse transcribed, amplified and sequenced. A snRNA-seq study on kidneys subjected to unilateral ureteral obstruction (UUO) identified two novel proximal tubule cell clusters 14 days after UUO, one of which had a strong proliferative signature, while the other expressed genes involved in inflammation and cell movement, like Ccl2, Il34, Cxcl1 and Dock10 [33].
A similar cell population is evident in a snRNA-seq study profiling the injured kidney after IRI [34]. In the first hours post IRI, there is downregulation of normal proximal tubule gene expression and a marked transcriptional response including immediate early genes and the known injury markers Havcr1 and Krt20 [24,25,34]. By day two post IRI, a large fraction of proximal tubule cells showed either a proliferative signature or regained the transcriptional profile characteristic for differentiated proximal tubule cells. However, a small population of pro-inflammatory, pro-fibrotic cells, characterized by expression of Vcam1 in most cells, and co-expression of Ccl2 in a subset, predicted activity of the NF-κB family of transcription factors. While proliferating proximal tubule cells decreased two weeks after AKI, pro-inflammatory, pro-fibrotic proximal tubule cells increased, suggesting a linkage between failed tubular repair and the observed pro-inflammatory, pro-fibrotic cell population (Fig. 1). Interestingly, deconvolution analysis of bulk RNA-sequencing studies suggested a similar population increased as the kidney aged normally, and after transplantation.
In line with the above study [34], a genetically selective focus on injured proximal tubule cells four weeks after a milder AKI, using Krt20-T2A-CRE-ERT2 mice to label and purify injured proximal tubule cells, showed a similar injured cellular phenotype: a Vcam1+/Ccl2+ injured proximal tubule cell population which was further distinguished by a strong pro-inflammatory (e.g. Ccl2, Vcam1, Cxcl1, Il34), and pro-fibrotic (e.g. Pdgfb, Col4a1) transcriptional signature, with marked activation of NF-κB-, TNF- and AP-1 signaling [35]. These injured proximal tubule cells shared features of a secretory associated senescent phenotype (SASP) identified in other injured organs (Fig. 1) [35,36]. In contrast to previous AKI studies [12], no significant G2/M cell cycle arrest was observed in this population. Additional fate-mapping experiments of cycling cells showed the majority of Vcam1+/Ccl2+ proximal tubule cells, localized to the corticomedullary boundary, traced back to proximal tubule cells proliferating in the first days after AKI. However, cortical Vcam1+/Ccl2+ proximal tubule cells likely represented secondary sites of injury, not related to early replication-associated proximal tubule repair.
A comprehensive atlas of the injury response to unilateral-ischemia reperfusion (UIR) performing scRNA-seq at multiple time-points from day one to day 14 post UIR revealed an elevated expression in injured cells of Sox4 and Cd24a, genes identified for roles in renal development [37]. A novel cell population, “mixed identity cells”, was also reported exhibiting co-expression of proximal tubule (Slc34a1), thick ascending limb (Umod) or collecting duct (Aqp2) markers.
scRNA-seq data from mouse folic acid nephropathy and UUO models showed that reduced expression of differentiation markers like solute carriers in injured proximal tubule cells is associated with downregulation of genes involved in metabolic processes, such as fatty acid oxidation [38]. The nuclear receptor Esrra was identified as an important link between proximal tubule differentiation and metabolism, since it directly regulated the expression of metabolic and proximal tubule specific genes. This, and other scRNA-seq studies, have also highlighted a previously unappreciated diversity of immune cells in the diseased kidney [38,39].
One important limitation of all discussed transcriptomics techniques is the absence of spatial information. Novel transcriptomics platforms now make it possible to visualize mRNA expression spatially by annealing and fixing tissue sections directly onto uniquely barcoded probes, imaging the tissue section and performing reverse transcription in situ followed by probe release and sequencing [40]. The sequenced transcriptomes can subsequently be mapped back onto the imaged tissue section. This spatial transcriptomics technology allowed the specific localization of the two transcriptionally distinct cell types of the proximal tubule segment S3 [41] to cortex and outer stripe of outer medulla, respectively [42]. In different murine AKI models, deconvolution of individual spatial transcriptomic spots using scRNA-seq data revealed AKI model-specific patterns of immune cell infiltration and enabled the identification of a distinct Atf3-expressing proximal tubule cell population, colocalizing with neutrophils after IRI [43]. These studies illustrate the potential for spatial-temporal omics to predict microenvironmental controls on cellular responses following AKI.
Epigenomics
Cell-type specific programs of differential gene expression depend on epigenetic modification of chromatin (for example, histone methylation and acetylation) and DNA (for example, by cytosine methylation at CpG residues) in response to DNA-binding transcriptional regulators and associated factors. An accumulating body of evidence suggests an important regulatory role for epigenetic mechanisms in AKI and renal repair [44–46]. Given excellent in-depth reviews [44–47], we only briefly highlight selected recent epigenomics studies in AKI research.
Positively charged histone proteins provide the core for packing negatively charged DNA, through electrostatic interactions, into nucleosomes – the structural unit of chromosomes [44]. Modifications to the amino-terminal tails of histones alter the chromatin structure or recruit chromatin modifiers, thus altering gene expression. An unbiased mass spectrometry screen of 63 different histone marks in the healthy kidney revealed widespread histone modifications with a compartment-specific pattern [48]. In this bulk tissue analysis, few histone marks showed a quantitatively significant change >5% after UUO. Interestingly, complementary changes in different marks on the same amino acid were reported, suggesting a coordinated response to AKI [48].
H3K4me3-highlighted promotors were compared with H3K27ac-marked enhancers following AKI using chromatin immunoprecipitation and next-generation sequencing (ChIP-seq) [49]. Active enhancer sites displayed a more dynamic change in response to AKI. Chromatin modifications at active enhancer sites associated with altered binding of transcription factors such as Hnf4a, Gr and Stat3. Hnf4a is a key transcription factor in the specification and differentiation of proximal tubule cells [50]. Hnf4a binding to enhancer elements active in normal proximal tubule cells decreased in response to AKI [49]. This likely contributes to the AKI-induced dedifferentiation of injured proximal tubule cells. Stat3 is a key transcriptional regulator in an inflammatory network with NF-κB and activator protein-1 (AP-1) regulatory factors [51]. AP-1 pathway activation is highly conserved as an initial response to mouse AKI and human kidney transplant associated IRI [24,25]. Stat3 engagement at the Junb locus in response to AKI suggests a role for Stat3 in activation of AP-1 transcriptional components [49]. Further, inhibition of BET (bromodomain and extra-terminal) dependent enhancer activation impaired recovery post-AKI, highlighting the important role of enhancer dynamics in AKI repair [49].
ATAC-seq has become the most widely used methodology for assessing the openness of chromatin and inferring activity of regulatory and transcribed genomic regions. Rapidly, the ATAC-seq technology has moved from sensitive bulk tissue assays with only 500 cells, to single nuclear analysis, and most recently parallel analysis of chromatin state and gene expression in the same cell [41,52–54]. ATAC-seq uses hyperactive Tn5 transposase to cut at open chromatin and tag cut DNA with DNA adaptors to facilitate library construction and DNA sequencing (Fig. 2). Given only two target sites in each genome, ATAC data is sparse, and snATAC studies utilize computational approaches to group cells with similar snATAC profiles, and to integrate with independently generated sc or snRNA-seq datasets.
A recent study profiled the cellular heterogeneity in healthy adult human kidney samples with computationally integrated snATAC-seq and snRNA-seq datasets [55]. Interestingly, the study identified a subpopulation of VCAM1-expressing proximal tubule cells, that showed reduced activity of HNF4A and increased activity of NF-κB family members, partially resembling pro-inflammatory, pro-fibrotic injured proximal tubule cells identified in the mouse kidney several weeks after AKI [34,35,55]. The abundance of this cell population increased over time in aging mice and was higher in human diabetic kidneys compared to healthy controls. Thus, some cellular responses seem to be conserved between different kidney diseases and across species. However, to what extent this cell population contributes to age-related loss of kidney function and kidney disease progression requires further investigation.
Cell type detection and regulatory predictions are improved by co-analyzing snRNA-seq and snATAC-seq in the same nucleus [41]. This technique was recently employed to characterize healthy human kidney tissue and AKI and CKD biopsies revealing novel cell diversity in the normal kidney, and highlighting comparable injury states in mouse and human AKI, as well as altered thick ascending limb cell states characterized by expression of Prom1 and Dcdc2 [56]. Additionally, spatial transcriptomics localized myofibroblasts and immune cells in CKD samples to sites of proximal tubule injury [56].
Proteomics and metabolomics
The study of proteins and metabolites in plasma, urine and kidney tissue using gel electrophoresis and mass spectrometry complements transcriptomic and epigenomic tools. Mass spectrometry can identify and quantify macromolecules (e.g. proteins) and cellular metabolites within a complex tissue, measuring molecularly distinct mass-charge properties of molecules when ionized and subjected to a magnetic or electric field (Fig. 2) [57]. Many proteomics studies have been directed to the identification of biomarkers for AKI (comprehensively reviewed elsewhere [57–59]). For example, urinary tissue inhibitor metalloproteinase-2 (TIMP2) and IGF-binding protein-7 (IGFBP7) levels were shown to be predictive of death and kidney failure in critically ill patients with AKI [60]. Treatment of TIMP2 and IGFBP7 co-positive patients with supportive care measures according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines for patients at high risk for AKI, reduced AKI development in the first 72 hours after cardiac surgery, but did not affect mortality and need for renal replacement therapy [61]. These results and findings from other biomarker studies are encouraging. However, the ideal biomarker or more likely biomarker panel for AKI risk assessment, targeted AKI prevention, predictive diagnosis and possibly even therapeutic guidance in routine clinical practice has yet to be identified.
Besides the drive for effective AKI biomarkers, proteomics can also be used to interrogate AKI pathophysiology [62]. A preclinical study of cisplatin-induced AKI revealed a weak correlation between transcriptome and proteome in the injured kidney [63]. The study suggested that the discrepancy between the two approaches was driven by protein changes in the extracellular compartment, for example, in the complement system [63]. Interestingly, the extent of dissociation between RNA and protein expression was strongly correlated with injury severity as indicated by serum creatinine and blood urea nitrogen levels [63]. This highlights the relevance of posttranscriptional changes in the AKI response.
Recently, near-single-cell proteomics profiling, which enables quantitative proteomic measurements from as little as 10 to 100 laser capture micro-dissected kidney cells, has been used to identify proteins specific to the glomerular and the proximal tubular compartment in the healthy kidney [64]. Some of the most enriched compartment-specific proteins correlated well with expression of the corresponding gene in the podocyte and the proximal tubule cluster of a scRNA-seq dataset, respectively. Furthermore, more than 40 protein markers can be detected simultaneously on a single tissue section by imaging mass cytometry (IMC), thus allowing for the study of the proteome in spatial context [65]. IMC of the healthy human kidney revealed unexpected cellular heterogeneity and identified a rare megalin/aquaporin-1/vimentin (Lrp2/Aqp1/Vim) positive cell type potentially reflecting an injured (Vim+) proximal tubule (Lrp2+/Aqp1+) cell state [65]. The application of these technologies to AKI research will provide critical insights into the proteomic landscape of injury and repair.
The study of the kidney metabolome (molecules smaller than 1500 Da [62]) has further advanced an understanding of AKI pathophysiology. Metabolomic profiling of injured kidney tissue using liquid chromatography-mass spectrometry led to the finding that de novo nicotinamide adenine dinucleotide (NAD+) synthesis is impaired in AKI [66]. Downregulation of the mitochondrial biogenesis regulator PGC1α in AKI resulted in local NAD+ depletion, while PGC1α overexpression increased renal NAD+ levels and reduced AKI [66]. Unbiased urine screens have also demonstrated increased levels of the NAD+ precursor quinolate in the urine of mice after IRI [67], resulting from a reduction of renal quinolate phosphoribosyltransferase (QPRT), a key enzyme in de novo NAD+ synthesis [67]. Reduced renal QPRT levels were associated with higher AKI susceptibility [67]. Oral niacinamide treatment salvaged AKI-induced renal NAD+ biosynthetic deficiency in mice and a small Phase 1 clinical trial has suggested a potential renal benefit of niacinamide treatment in patients undergoing cardiac surgery [67]. A metabolomics study of human kidney transplant biopsies pre- and post-reperfusion showed a distinctly different metabolic profile of transplants with future delayed graft function compared to transplants without delayed graft function [68]. Transplants with future delayed graft function were characterized by persistent ATP/GTP catabolism, suggesting early restoration of energy homeostasis as a therapeutic goal in AKI prevention [68].
Mass-spectrometry imaging (MSI) is a powerful technique to visualize the spatial distribution of metabolites, proteins or lipids, with cellular and subcelluar resolution [69]. MSI analysis of injured kidneys showed lipidomic changes early after injury, which could be used to differentiate between mild and severe ischemia as early as two hours post injury [70,71].
Conclusions and Perspective
Available omics technologies have propelled our understanding of AKI and repair. The integration or simultaneous acquisition of different omics datasets will further enhance insight into AKI and corroborative findings from orthogonal approaches will give additional confidence to identified mechanisms. New insights, tools and informational resources are accruing with the investment by the NIDDK in several large consortia including the Kidney Precision Medicine Project and the Rebuilding a Kidney Program. The great challenge of this era of “big data” is to infer biologically relevant mechanisms from highly complex data sets, to democratize access to these data through user-friendly viewing and data analysis platforms, and ultimately, to translate our increasing pathophysiologic insights into diagnostic and therapeutic strategies that improve patient care and AKI outcomes.
Highlights.
Integrated multi-omics enable a comprehensive understanding of complex cellular mechanisms.
GWAS can predict risk loci for acute kidney injury.
Single cell RNA-sequencing revealed pro-inflammatory failed-repair proximal tubule cells.
Acute kidney injury leads to chromatin changes in the kidney, relevant to outcome.
Spatial transcriptomics allows the study of injury and repair processes in spatial context.
Acknowledgements
We apologize to all researchers whose work could not be discussed due to space limitations. We thank Dr. Pietro E. Cippà for critical reading of the manuscript. L.M.S. Gerhardt was supported by the German Research Foundation (DFG) with a postdoctoral scholarship (GE 3179/1-1). Work in A.P. McMahon’s laboratory is supported by grants from the NIDDK (DK126024, 54364, 126925) and Chan Zuckerberg Initiative (CZIF2019-002430).
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
LMSG: none.
APM is a scientific advisor on kidney-related approaches to human disease for Novartis, eGenesis, Iviva and Trestle Biotherapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. JASN 2005, 16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, et al. : International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. The Lancet 2015, 385:2616–2643. [DOI] [PubMed] [Google Scholar]
- 3.Hsu RK, Hsu C-Y: The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin Nephrol 2016, 36:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. New England Journal of Medicine 2014, 371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, Cantaluppi V: Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol 2021, doi:10.1038/s41581-021-00452-0. • This review provides a detailed overview over the current literature related to COVID-19-associated acute kidney injury.
- 6. Zuk A, Bonventre JV: Recent Advances on Acute Kidney Injury and its Consequences and Impact on Chronic Kidney Disease. Curr Opin Nephrol Hypertens 2019, 28:397–405.30925515 • This excellent review summarizes the pathophysiologic mechanisms underlying the transition from acute to chronic kidney injury and highlights the role of mitochondrial dysfunction, inflammation, senescence and cell death in this process.
- 7.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, et al. : US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2019, 73:A7–A8. US Renal Data System 2018 Annual Data Report available from: https://www.usrds.org/media/2282/2018_volume_1_ckd_in_the_us.pdf [Accessed 11 April 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015, 11:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 2014, 111:1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP: Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Reports 2015, 12:1325–1338. [DOI] [PubMed] [Google Scholar]
- 11.Kang HM, Huang S, Reidy K, Han SH, Chinga F, Susztak K: Sox9 positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Rep 2016, 14:861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 2010, 16:535–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Shen TH, Werth M, Forster C, Deng R, Bruck E, et al. : Unique Transcriptional Programs Identify Subtypes of AKI. JASN 2017, 28:1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar D, Gharib SA, Zager RA, Johnson A, Denisenko O, Bomsztyk K: Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int 2015, 88:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stafford-Smith M, Li Y-J, Mathew JP, Li Y-W, Ji Y, Phillips-Bute B, Milano CA, Newman MF, Kraus WE, Kertai MD, et al. : Genome-wide association study of acute kidney injury after coronary bypass graft surgery identifies susceptibility loci. Kidney Int 2015, 88:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Lu Q, Cheng Y, Belcher JM, Siew ED, Leaf DE, Body SC, Fox AA, Waikar SS, Collard CD, et al. : A Genome-Wide Association Study to Identify Single-Nucleotide Polymorphisms for Acute Kidney Injury. Am J Respir Crit Care Med 2017, 195:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayagaki N, Lee BL, Stowe IB, Kornfeld OS, O’Rourke K, Mirrashidi KM, Haley B, Watanabe C, Roose-Girma M, Modrusan Z, et al. : IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci Signal 2019, 12. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kündig TM, Amakawa R, Kishihara K, Wakeham A: Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 1993, 75:83–97. [PubMed] [Google Scholar]
- 19.Papaioannou VE: The T-box gene family: emerging roles in development, stem cells and cancer. Development 2014, 141:3819–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renken IJE, Vilander LM, Kaunisto MA, Vaara ST, Snieder H, Keus F, van der Horst ICC, Pettilä V: No Association between Genetic Loci near IRF2 and TBX1 and Acute Kidney Injury in the Critically Ill. Am J Respir Crit Care Med 2019, 201:109–111. [DOI] [PubMed] [Google Scholar]
- 21. Gu X, Yang H, Sheng X, Ko Y-A, Qiu C, Park J, Huang S, Kember R, Judy RL, Park J, et al. : Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci Transl Med 2021, 13. • This study showed that beta-mannosidase (MANBA) expression was lower in the kidneys of patients with risk loci for chronic kidney disease. In mouse models of kidney fibrosis, knock out of Manba led to increased fibrosis, which was associated with alterations in endocytosis and autophagy pathways.
- 22.Qiu C, Huang S, Park J, Park Y, Ko Y-A, Seasock MJ, Bryer JS, Xu X-X, Song W-C, Palmer M, et al. : Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat Med 2018, 24:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan KM, Susztak K: Unravelling the complex genetics of common kidney diseases: from variants to mechanisms. Nature Reviews Nephrology 2020, 16:628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, et al. : Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cippà PE, Sun B, Liu J, Chen L, Naesens M, McMahon AP: Transcriptional trajectories of human kidney injury progression. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y, Boor P, Fukuma S, Klinkhammer BM, Haga H, Ogawa O, Floege J, Yanagita M: Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney International 2020, 98:448–463. [DOI] [PubMed] [Google Scholar]
- 27. Cippà PE, Liu J, Sun B, Kumar S, Naesens M, McMahon AP: A late B lymphocyte action in dysfunctional tissue repair following kidney injury and transplantation. Nat Commun 2019, 10.30602777 • The report shows dysfunctional repair after AKI is linked to local immunomodulation. Evidence from transcriptomics of human transplant biopsies and of mouse kidneys 18 months after IRI suggested that late autoantigen driven B cell activity drives ongoing inflammation and fibrosis in the AKI-to-CKD transition.
- 28.Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N: Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat Protoc 2014, 9:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, et al. : Cell-specific translational profiling in acute kidney injury. J Clin Invest 2014, 124:1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang-Panesso M, Kadyrov FF, Lalli M, Wu H, Ikeda S, Kefaloyianni E, Abdelmageed MM, Herrlich A, Kobayashi A, Humphreys BD: FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J Clin Invest 2019, 129:5501–5517.31710314 • This study used lineage-tracing of Kim-1 positive injured proximal tubule cells to demonstrate that Kim-1 positive clones expand during the repair process. Translating ribosome affinity purification of Kim-1 positive cells showed transcriptional signatures of both maladaptive and adaptive repair two weeks after AKI.
- 31.Potter SS: Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol 2018, 14:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang B, Lee JH, Bang D: Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 2018, 50:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu H, Kirita Y, Donnelly EL, Humphreys BD: Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. JASN 2019, 30:23–32.30510133 • This report showed that snRNA-seq captured the diversity of renal cell populations better than scRNA-seq with equivalent gene detection sensitivity. snRNA-seq of kidneys after UUO identified two novel proximal tubule clusters, one characterized by a proliferative signature, the other by expression of inflammation and cell movement related genes.
- 34. Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A 2020, 117:15874–15883.32571916 • This study performed snRNA-seq at different timepoints after IRI and identified a pro-inflammatory, pro-fibrotic proximal tubule cell population that increased in abundance until two weeks post IRI and was still present at six weeks after IRI suggesting failed tubular repair.
- 35.Gerhardt LMS, Liu J, Koppitch K, Cippà PE, McMahon AP: Single-nuclear transcriptomics reveals diversity of proximal tubule cell states in a dynamic response to acute kidney injury. Proc Natl Acad Sci U S A 2021, 118:e2026684118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J: The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010, 5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudman-Melnick V, Adam M, Potter A, Chokshi SM, Ma Q, Drake KA, Schuh MP, Kofron JM, Devarajan P, Potter SS: Single-Cell Profiling of AKI in a Murine Model Reveals Novel Transcriptional Signatures, Profibrotic Phenotype, and Epithelial-to-Stromal Crosstalk. J Am Soc Nephrol 2020, 31:2793–2814.33115917 • This report identified “mixed identity” cells co-expressing markers of proximal tubule cells and thick ascending limb or collecting duct cells after AKI, suggesting unexpected plasticity of injured tubule cells.
- 38. Dhillon P, Park J, Hurtado del Pozo C, Li L, Doke T, Huang S, Zhao J, Kang HM, Shrestra R, Balzer MS, et al. : The Nuclear Receptor ESRRA Protects from Kidney Disease by Coupling Metabolism and Differentiation. Cell Metabolism 2021, 33:379–394.e8.33301705 • This study demonstrated that differentiation of proximal tubule cells is associated with expression of metabolism related genes. The nuclear receptor Esrra was identified as a link between cell differentiation and metabolism.
- 39. Conway BR, O’Sullivan ED, Cairns C, O’Sullivan J, Simpson DJ, Salzano A, Connor K, Ding P, Humphries D, Stewart K, et al. : Kidney Single-Cell Atlas Reveals Myeloid Heterogeneity in Progression and Regression of Kidney Disease. J Am Soc Nephrol 2020, 31:2833–2854.32978267 • This study revealed a surprising heterogeneity of myeloid cells in the kidney after AKI, which changed dynamically over the course of repair.
- 40.Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, et al. : Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353:78–82. [DOI] [PubMed] [Google Scholar]
- 41.Cao J, Cusanovich DA, Ramani V, Aghamirzaie D, Pliner HA, Hill AJ, Daza RM, McFaline-Figueroa JL, Packer JS, Christiansen L, et al. : Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 2018, 361:1380–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Janosevic D, Myslinski J, McCarthy TW, Zollman A, Syed F, Xuei X, Gao H, Liu Y-L, Collins KS, Cheng Y-H, et al. : The orchestrated cellular and molecular responses of the kidney to endotoxin define a precise sepsis timeline. Elife 2021, 10. • This report provided a detailed profiling of the transcriptomic changes to endotoxemia in kidney cell types over time. Integration of scRNA-seq and spatial transcriptomics data showed a distinct localization of the two different proximal tubule S3 segment subtypes to the cortex and the outer stripe of the outer medulla, respectively.
- 43. Melo Ferreira R, Sabo AR, Winfree S, Collins KS, Janosevic D, Gulbronson CJ, Cheng Y-H, Casbon L, Barwinska D, Ferkowicz MJ, et al. : Integration of spatial and single-cell transcriptomics localizes epithelial cell-immune cross-talk in kidney injury. JCI Insight 2021, 6:147703.34003797 • This study showed different spatial patterns of immune cell infiltration in the injured kidney after IRI and cecal ligation puncture. A combination of spatial transcriptomics and scRNA-seq identified a role of Atf3-expressing proximal tubule cells in neutrophil chemotaxis after IRI.
- 44. Guo C, Dong G, Liang X, Dong Z: Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nature Reviews Nephrology 2019, 15:220–239.30651611 • An excellent review summarizing current knowledge on the epigenetic regulation of AKI and repair, also highlighting epigenetic-modifying drugs as potential therapeutic options for AKI.
- 45.Fontecha-Barriuso M, Martin-Sanchez D, Ruiz-Andres O, Poveda J, Sanchez-Niño MD, Valiño-Rivas L, Ruiz-Ortega M, Ortiz A, Sanz AB: Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrology Dialysis Transplantation 2018, 33:1875–1886. [DOI] [PubMed] [Google Scholar]
- 46.Hyndman KA: Histone deacetylases in kidney physiology and acute kidney injury. Semin Nephrol 2020, 40:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang J, Zhuang S: Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin Sci (Lond) 2019, 133:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hewitson TD, Holt SG, Samuel CS, Wigg B, Smith ER: Profiling histone modifications in the normal mouse kidney and after unilateral ureteric obstruction. American Journal of Physiology-Renal Physiology 2019, 317:F606–F615.31268352 • This study showed compartment-specific differences in histone mark distribution in the normal kidney and histone modifications in response to UUO.
- 49. Wilflingseder J, Willi M, Lee HK, Olauson H, Jankowski J, Ichimura T, Erben R, Valerius MT, Hennighausen L, Bonventre JV: Enhancer and super-enhancer dynamics in repair after ischemic acute kidney injury. Nat Commun 2020, 11.31896763 • This study revealed that acute kidney injury leads to dynamic changes in the enhancer landscape of the kidney, relevant to AKI outcome.
- 50. Marable SS, Chung E, Park J-S: Hnf4a Is Required for the Development of Cdh6-Expressing Progenitors into Proximal Tubules in the Mouse Kidney. JASN 2020, 31:2543–2558.32764140 • This report demonstrated that Hnf4a drives the differentiation of proximal tubule cells by enhancing expression of genes associated with proximal tubule functions like transmembrane transport and metabolism.
- 51.Ji Z, He L, Regev A, Struhl K: Inflammatory regulatory network mediated by the joint action of NF-kB, STAT3, and AP-1 factors is involved in many human cancers. PNAS 2019, 116:9453–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ: Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 2013, 10:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ: Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 2015, 523:486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agbleke AA, Amitai A, Buenrostro JD, Chakrabarti A, Chu L, Hansen AS, Koenig KM, Labade AS, Liu S, Nozaki T, et al. : Advances in Chromatin and Chromosome Research: Perspectives from Multiple Fields. Molecular Cell 2020, 79:881–901.32768408 • This review provides an excellent overview over available technologies to interrogate chromatin changes and chromosome biology.
- 55. Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, Humphreys BD: Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nature Communications 2021, 12:2190. • This study integrated snRNA-seq and snATAC-seq data of adult human kidneys, showed that this multi-omic approach can refine detection of different cell states and identified a rare VCAM1+ proximal tubule population with activated NF-κB signaling.
- 56.Lake BB, Menon R, Winfree S, Hu Q, Ferreira RM, Kalhor K, Barwinska D, Otto EA, Ferkowicz M, Diep D, et al. : An atlas of healthy and injured cell states and niches in the human kidney. bioRxiv 2021, doi: 10.1101/2021.07.28.454201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marx D, Metzger J, Pejchinovski M, Gil RB, Frantzi M, Latosinska A, Belczacka I, Heinzmann SS, Husi H, Zoidakis J, et al. : Proteomics and Metabolomics for AKI Diagnosis. Semin Nephrol 2018, 38:63–87. [DOI] [PubMed] [Google Scholar]
- 58.Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D’Agati VD, Tatonetti NP, Barasch J, Devarajan P: Molecular nephrology: types of acute tubular injury. Nature Reviews Nephrology 2019, 15:599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luft FC: Biomarkers and predicting acute kidney injury. Acta Physiologica 2021, 231:e13479. [DOI] [PubMed] [Google Scholar]
- 60.Koyner JL, Shaw AD, Chawla LS, Hoste EAJ, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA, Sapphire Investigators: Tissue Inhibitor Metalloproteinase-2 (TIMP-2)·IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 2015, 26:1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017, 43:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinschen MM, Saez-Rodriguez J: The tissue proteome in the multi-omic landscape of kidney disease. Nat Rev Nephrol 2021, 17:205–219. [DOI] [PubMed] [Google Scholar]
- 63.Späth MR, Bartram MP, Palacio-Escat N, Hoyer KJR, Debes C, Demir F, Schroeter CB, Mandel AM, Grundmann F, Ciarimboli G, et al. : The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney International 2019, 95:333–349. [DOI] [PubMed] [Google Scholar]
- 64. Sigdel TK, Piehowski PD, Roy S, Liberto J, Hansen JR, Swensen AC, Zhao R, Zhu Y, Rashmi P, Schroeder A, et al. : Near-Single-Cell Proteomics Profiling of the Proximal Tubular and Glomerulus of the Normal Human Kidney. Front Med (Lausanne) 2020, 7:499.33072769 • This report describes the application of near-single-cell proteomics to the human kidney and identifies proximal tubule and glomerulus specific proteins.
- 65. Singh N, Avigan ZM, Kliegel JA, Shuch BM, Montgomery RR, Moeckel GW, Cantley LG: Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight 2019, 4. • This report shows the imaging mass cytometry on kidney tissue, simultaneously analyzing more than 20 protein markers in spatial context.
- 66.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, et al. : PGC1α-dependent NAD biosynthesis links oxidative metabolism to renal protection. Nature 2016, 531:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, et al. : De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 2018, 24:1351–1359.30127395 • This study identified reduced quinolate phosphoribosyltransferase (QPRT) levels as a cause of reduced de novo NAD+ synthesis in AKI. Reduced QPRT levels were linked to increased AKI susceptibility. Oral niacinamide treatment increased circulating NAD+ and was associated with reduced AKI in cardiac-surgery patients.
- 68.Lindeman JH, Wijermars LG, Kostidis S, Mayboroda OA, Harms AC, Hankemeier T, Bierau J, Sai Sankar Gupta KB, Giera M, Reinders ME, et al. : Results of an explorative clinical evaluation suggest immediate and persistent post-reperfusion metabolic paralysis drives kidney ischemia reperfusion injury. Kidney International 2020, 98:1476–1488. [DOI] [PubMed] [Google Scholar]
- 69.Neumann EK, Migas LG, Allen JL, Caprioli RM, Van de Plas R, Spraggins JM: Spatial Metabolomics of the Human Kidney using MALDI Trapped Ion Mobility Imaging Mass Spectrometry. Anal Chem 2020, 92:13084–13091. [DOI] [PubMed] [Google Scholar]
- 70.Rao S, Walters KB, Wilson L, Chen B, Bolisetty S, Graves D, Barnes S, Agarwal A, Kabarowski JH: Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. Am J Physiol Renal Physiol 2016, 310:F1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Smaalen TC, Ellis SR, Mascini NE, Siegel TP, Cillero-Pastor B, Hillen LM, van Heurn LWE, Peutz-Kootstra CJ, Heeren RMA: Rapid Identification of Ischemic Injury in Renal Tissue by Mass-Spectrometry Imaging. Anal Chem 2019, 91:3575–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]



