Abstract
OBJECTIVE
To determine whether β-cell hyperresponsiveness and insulin resistance in youth versus adults in the Restoring Insulin Secretion (RISE) Study are related to increased glucagon release.
RESEARCH DESIGN AND METHODS
In 66 youth and 350 adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes (drug naive), we performed hyperglycemic clamps and oral glucose tolerance tests (OGTTs). From clamps we quantified insulin sensitivity (M/I), plasma fasting glucagon and C-peptide, steady-state glucagon and C-peptide at glucose of 11.1 mmol/L, and arginine-stimulated glucagon (acute glucagon response [AGR]) and C-peptide (ACPRmax) responses at glucose >25 mmol/L.
RESULTS
Mean ± SD fasting glucagon (7.63 ± 3.47 vs. 8.55 ± 4.47 pmol/L; P = 0.063) and steady-state glucagon (2.24 ± 1.46 vs. 2.49 ± 1.96 pmol/L, P = 0.234) were not different in youth and adults, respectively, while AGR was lower in youth (14.1 ± 5.2 vs. 16.8 ± 8.8 pmol/L, P = 0.001). Significant age-group differences in insulin sensitivity, fasting C-peptide, steady-state C-peptide, and ACPRmax were not related to glucagon. Fasting glucose and glucagon were positively correlated in adults (r = 0.133, P = 0.012) and negatively correlated in youth (r = −0.143, P = 0.251). In both age-groups, higher fasting glucagon was associated with higher fasting C-peptide (youth r = 0.209, P = 0.091; adults r = 0.335, P < 0.001) and lower insulin sensitivity (youth r = −0.228, P = 0.066; adults r = −0.324, P < 0.001). With comparable fasting glucagon, youth had greater C-peptide and lower insulin sensitivity. OGTT suppression of glucagon was greater in youth.
CONCLUSIONS
Youth with IGT or recently diagnosed type 2 diabetes (drug naive) have hyperresponsive β-cells and lower insulin sensitivity, but their glucagon concentrations are not increased compared with those in adults. Thus, α-cell dysfunction does not appear to explain the difference in β-cell function and insulin sensitivity in youth versus adults.
Introduction
The incidence of type 2 diabetes in youth is increasing in the U.S. and other parts of the world (1,2). Based on a comparison of the rates of glycemic failure observed with similar interventions in pediatric (Treatment Options for Type 2 Diabetes in Adolescents and Youth [TODAY]) and adult (A Diabetes Outcome Progression Trial [ADOPT]) studies (3,4), it has been suggested that the course of the disease in youth is more aggressive than that typically observed in adults due to a greater rate of loss of β-cell function (5,6).
The Restoring Insulin Secretion (RISE) Study has made important contributions to our understanding of the differences in rates of β-cell failure and responses to interventions in youth and adults with dysglycemia. In RISE, approaches were examined to preserve or improve β-cell function in youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes through its three studies: Pediatric Medication, Adult Medication, and Adult Surgery. The RISE studies used the same assessment methods and a central laboratory, allowing for a direct comparison of determinants of glucose metabolism in youth and adults (7). Baseline assessments in the two age-groups revealed that youth were more insulin resistant and for their degree of insulin sensitivity had hyperresponsive β-cells, such that at any matched degree of insulin sensitivity, youth were releasing greater amounts of C-peptide and insulin than adults. This increase in β-cell secretory work was evident when examined with use of both intravenous and oral glucose testing (8,9). RISE also demonstrated that in youth, β-cell dysfunction progressed despite glucose-lowering interventions aimed at reducing β-cell secretory demand but remained relatively stable in similarly treated adults (10). The basis for these important differences remains elusive and could include, in youth, epigenetic changes, impaired insulin-independent glucose uptake resulting in increased β-cell secretion to compensate and thus increase insulin-dependent glucose uptake, and α-cell dysfunction resulting in disordered regulation of glucagon. In this article we address the potential role of glucagon.
Glucagon, the primary α-cell peptide product, is a critical determinant of glucose metabolism. It can contribute to hyperinsulinemia via direct and indirect effects on the β-cell. Within the islet, glucagon has a direct paracrine stimulatory effect on the β-cell, resulting in immediate insulin release, the magnitude of which depends in part on the prevailing glucose level (11–14). Indirectly, glucagon increases hepatic glucose production resulting in an increase in the circulating glucose concentration, which in turn enhances insulin release; together they return glucose levels towards normal (15–17). These latter features appear to contribute to a hyperinsulinemic, insulin-resistant phenotype.
Given these known physiological effects of glucagon and prior data that obese and insulin-resistant youth have increased glucagon concentrations (18,19), we hypothesized that increased α-cell glucagon release in youth may underlie the β-cell hyperresponsiveness and insulin resistance that we observed in youth with IGT or recently diagnosed type 2 diabetes. Thus, we analyzed the glucagon response to intravenous and oral glucose to determine whether α-cell function was different in youth compared with adults in RISE and whether relationships among glucagon, β-cell function, and insulin sensitivity were similar or different in the two age-groups.
Research Design and Methods
Participants
Youth 10–19 years of age with pubertal development beyond Tanner stage I were eligible for the RISE Pediatric Medication Study if they had IGT or type 2 diabetes for <6 months, with the latter group being either drug naive or on metformin and meeting predefined HbA1c criteria. Adults were eligible for the RISE Adult Medication Study and RISE Adult Surgery (BetaFat) Study if they had IGT or type 2 diabetes, the latter group with known diabetes for <1 year and never having received glucose-lowering medications. Individuals were classified as having IGT or diabetes based on the 2-h glucose concentration during a screening oral glucose tolerance test (OGTT) (20).
Ninety-one participants were randomized into the Pediatric Medication Study, 267 into the Adult Medication Study, and 88 into the Adult Surgery Study. Of the 91 pediatric participants, 25 were either taking metformin at the time of randomization or had previously been exposed to it. These participants were excluded from the current analyses for avoidance of potential confounding by this exposure. Additional details on participant inclusion/exclusion criteria, procedures, and measurements have previously been published (7–9) and are provided in the three study protocols available from https://rise.bsc.gwu.edu/web/rise/collaborators.
All participants gave written informed consent/assent, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
Anthropometric Measurements
Waist circumference, height, and weight were measured with participants wearing light clothing without shoes (8). BMI was calculated as weight in kilograms divided by square of height in meters.
Procedures for Phenotyping Glucose Metabolism
Following a 10-h overnight fast, the following procedures were performed on separate days.
Hyperglycemic Clamp
A two-step hyperglycemic clamp was performed as previously described in detail (8). Briefly, with variable rate 20% dextrose infusions, glucose was clamped with use of a computerized algorithm combined with bedside blood glucose monitoring every 5–10 min. For the first step, a targeted, steady-state plasma glucose concentration of 11.1 mmol/L was achieved and maintained through 120 min. Blood samples were drawn through an indwelling intravenous catheter in a warmed hand prior to starting of the clamp and at 100, 110, and 120 min (steady state) after the dextrose infusion was commenced. Thereafter, the infusion rate was increased to achieve the second step target, a plasma glucose >25 mmol/L. After this target glucose had been attained for a minimum of 30 min after commencement of the second step, a bolus of l-arginine (5 g) was administered over 1 min. Blood samples for subsequent assays were drawn at −5, −1, 2, 3, 4, and 5 min relative to the arginine injection.
OGTT
A 3-h, 75-g OGTT was performed as previously described in detail (9). Blood samples were obtained at −10, −5, 10, 20, 30, 60, 90, 120, 150, and 180 min relative to glucose ingestion.
Assays
All blood samples were drawn in EDTA tubes, with those for glucagon measurement including nine parts of a protease inhibitor (P2714; Sigma-Aldrich) to one part (v/v) of a DPP-4 inhibitor (DPP4-010; Millipore). All tubes were immediately placed on ice, separated by centrifugation, and frozen at −80°C prior to shipment to the central biochemistry laboratory at the University of Washington for subsequent assay.
Plasma glucose concentrations were measured by the glucose hexokinase method with Roche reagent on a Roche cobas c 501 analyzer (Indianapolis, IN). C-peptide and insulin were measured by a two-site immunoenzymometric assay performed on the AIA-2000 Automated Immunoassay Analyzer (Tosoh Biosciences, Inc., South San Francisco, CA). Greater detail on the performance characteristics of these assays in the study’s central biochemistry laboratory has previously been published (8).
Plasma glucagon was measured with the Mercodia Glucagon ELISA (cat. no. 10-1271-01, lot no. 28689; Winston-Salem, NC). The assay was performed with a sequential protocol, which includes an extra washing step prior to the incubation with detection antibody, rather than the standard simultaneous protocol. This approach had been reported to reduce cross-reactivity to glicentin and oxyntomodulin, two alternative posttranslational products of the glucagon gene (21,22). Further details on the assay and comparison of results with the standard versus extended wash approach are provided in Supplementary Material.
The performance characteristics of the sequential protocol were established by the central biochemistry laboratory. The standard curve for glucagon had a range of 1.3–126 pmol/L, with an assay sensitivity of 1.1 pmol/L. Linearity of the assay was determined by serial dilution and was demonstrated to be between 89 and 129% up to a dilution of 1:64. Analyte recovery, determined with use of spiked human plasma samples, ranged from between 92 and 107%. Intra-assay coefficient of variation was 6.9%, 4.0%, and 1.9% on samples with average values of 1.9, 3.1, and 30.9 pmol/L, respectively. Interassay coefficient of variation was 8.3%, 7.5%, and 5.7% on samples with average values of 4.4, 13.9, and 41.6 pmol/L. Glucagon values ≤1.0 pmol/L (below lower limit of quantification) were assigned a value of 0.5 pmol/L for data analysis.
Calculations for Clamp-Derived Measurements
Insulin Sensitivity
Insulin sensitivity (M/I) was quantified as the mean of the glucose infusion rate (M) at 100, 110, and 120 min of the clamp corrected for urinary glucose loss, divided by the mean steady-state plasma insulin concentration at these same time points (I). This measure was expressed per kilogram of body weight (8).
Glucagon and C-peptide Responses
From the hyperglycemic clamp, the following measures were calculated. Fasting concentrations were calculated as the average of the two samples drawn prior to glucose administration. Steady-state glucagon and C-peptide concentrations were calculated as the mean of the respective measurements at 100, 110, and 120 min. The increment in C-peptide and decrement in glucagon concentrations during the first 120 min of the clamp were calculated as the difference between the steady-state and fasting measurements. The incremental acute glucagon response (AGR) and C-peptide response (ACPRmax) to arginine at maximal glycemic potentiation (>25 mmol/L) were calculated as the mean concentrations in samples drawn 2, 3, 4, and 5 min after arginine injection minus the average concentration of the samples drawn 1 and 5 min prior to arginine (23).
Calculations for OGTT-Derived Measurements: Glucose and Peptide Responses
The trapezoidal method was used to calculate the decremental area under the curve (dAUC) for the glucagon response relative to the fasting concentration over the entire 3-h sampling period. The glucose and C-peptide responses were calculated similarly as the incremental area under the curve (iAUC). The ratio of the dAUC response for glucagon was expressed relative to the iAUC of glucose as a measure of the α-cell response to the prevailing glucose. The ratio of iAUC of C-peptide expressed relative to the iAUC of glucagon was used as a measure of the β-cell response to glucagon.
Data Management and Statistical Analyses
The SAS analysis system (SAS Institute, Cary, NC) and R (The R Foundation) were used for all statistical analyses. Descriptive statistics are presented as percentages, mean ± SD, and geometric means and 95% CIs for non–normally distributed data; for the latter, P values from the log-transformed data are presented. Comparisons between groups were computed with χ2 tests or Student t tests. Nominal P values are presented. Except where noted, P values <0.05 were considered nominally statistically significant, with no adjustments made for multiple tests.
Mean levels between groups of clamp and OGTT summary measures were calculated with ANCOVA models, before and after adjustment for insulin sensitivity, sex, and race/ethnicity. Linear regression models were used to evaluate the relationship of islet cell responses with each other and with BMI and insulin sensitivity. Models used natural logarithmically transformed measures for M/I, all C-peptide responses, and steady-state glucagon due to the skewed distribution of these data.
We were interested in whether the relationship between glucagon responses and insulin sensitivity (M/I) differed between youth and adults; however, we anticipated differences between those with IGT and diabetes at baseline. Therefore, a three-way interaction for M/I by youth/adult and IGT/type 2 diabetes was run first to assess whether the four separate slopes of the glucagon response on M/I differed, i.e., whether the slopes for adults with IGT, adults with diabetes, youth with IGT, and youth with diabetes were significantly different. No difference was observed, so subsequent models were built including two-way interaction terms for [diabetes status * insulin sensitivity] and [adult/youth status * insulin sensitivity]. If neither two-way interaction test was significant (i.e., the slopes in the two age-groups or diabetes status groups were not significantly different), a simple model was constructed, including terms for diabetes status and adults/youth without any interaction variables. Slopes in these regression models that do not differ indicate that β-cell responses in youth and adults are proportionate across the range of insulin sensitivity. The same approach was used to examine the relationship between glucagon responses and C-peptide responses.
Results
Demographic, Physical, and Glucose Tolerance Characteristics
Glucagon data were available for all 66 youth and 350 of 355 adult participants. Their characteristics are presented in Table 1. Adults from the two adult protocols (Adult Medication and Adult Surgery) were combined for these analyses, as there were no relevant baseline differences between participants in the two protocols (8).
Table 1.
Select baseline demographic and glycemic characteristics for youth and adults
| Youth (n = 66) | Adults (n = 350) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 14.2 ± 2.0 | 52.7 ± 9.3 | <0.001 |
| Female, n (%) | 47 (71.2) | 182 (52.0) | 0.006 |
| Race/ethnicity n (%) | 0.001 | ||
| White | 19 (28.8) | 163 (46.6) | |
| Black | 14 (21.2) | 96 (27.4) | |
| Hispanic | 25 (37.9) | 67 (19.1) | |
| Other | 8 (12.1) | 24 (6.9) | |
| Weight (kg) | 98.9 ± 22.6 | 100.7 ± 18.3 | 0.538 |
| BMI (kg/m2) | 36.6 ± 6.0 | 35.1 ± 5.1 | 0.063 |
| Glycemic characteristics | |||
| IGT, n (%) | 53 (80.3) | 246 (70.3) | 0.131 |
| HbA1c (mol/mmol) | 38.5 ± 6.1 | 39.7 ± 4.4 | 0.151 |
| HbA1c (%) | 5.7 ± 0.6 | 5.8 ± 0.4 |
Data are means ± SD unless otherwise indicated. Other race/ethnicity includes mixed, Asian, American Indian, and other.
The youth cohort comprised a greater proportion of females and a lesser proportion of White participants than the adult cohort. Most youth were in the later stages of puberty; 78.8% were at Tanner stage IV or V and 21.2% were at Tanner stage II or III on physical examination. BMI tended to be higher in youth, despite the two age-groups having similar weights. The proportion of participants with IGT or recently diagnosed type 2 diabetes and overall glycemia control did not differ between the two age-groups.
Hyperglycemic Clamp–Derived Measurements in Youth Versus Adults
Metabolic characteristics from the hyperglycemic clamp are listed in Table 2. Fasting glucose concentrations were not different in youth and adults. Fasting glucagon concentrations were also not different, whereas fasting C-peptide concentrations were significantly higher in youth compared with adults.
Table 2.
Select metabolic characteristics from the hyperglycemic clamps and OGTTs in youth and adults
| Youth (n = 66) | Adults (n = 350) | P | |
|---|---|---|---|
| Hyperglycemic clamp parameters | |||
| Fasting glucose (mmol/L) | 5.99 ± 0.91 | 6.11 ± 0.63 | 0.303 |
| Fasting glucagon (pmol/L) | 7.63 ± 3.47 | 8.55 ± 4.47 | 0.063 |
| Fasting C-peptide (nmol/L) | 1.60 (0.88, 2.90) | 1.15 (0.57, 2.29) | <0.001 |
| Steady-state glucose (mmol/L) | 11.6 ± 0.7 | 11.7 ± 0.7 | 0.366 |
| Steady-state glucagon (pmol/L) | 2.24 ± 1.46 | 2.49 ± 1.96 | 0.234 |
| Steady-state C-peptide (nmol/L) | 5.19 (2.50, 10.74) | 3.83 (1.85, 7.96) | <0.001 |
| Steady-state insulin (I) (pmol/L) | 1,370.3 (298.6, 6,288.0) | 606.2 (1,461.1, 2,514.7) | <0.001 |
| AGR (pmol/L) | 14.1 ± 5.2 | 16.8 ± 8.8 | 0.001 |
| ACPRmax (nmol/L) | 7.85 (3.70, 16.66) | 4.83 (1.90, 12.25) | <0.001 |
| M/I (×10−5 mmol/kg/min per pmol/L) | 1.69 (0.37, 7.69) | 3.14 (0.76, 12.94) | <0.001 |
| ΔSteady-state glucagon − fasting glucagon (pmol/L) | −5.38 ± 3.12 | −6.05 ± 3.36 | 0.117 |
| Fasting C-peptide / fasting glucagon (nmol/pmol) | 0.29 ± 0.30 | 0.18 ± 0.10 | 0.003 |
| Steady-state C-peptide / steady-state glucagon (nmol/pmol) | 4.23 ± 3.78 | 2.83 ± 2.39 | 0.005 |
| ACPRmax / AGR (nmol/pmol) | 0.688 ± 0.379 | 0.410 ± 0.590 | <0.001 |
| OGTT parameters | |||
| Fasting glucose (mmol/L) | 5.93 ± 0.93 | 6.16 ± 0.65 | 0.064 |
| Fasting glucagon (pmol/L) | 8.73 ± 3.70 | 8.55 ± 4.73 | 0.739 |
| Fasting C-peptide (nmol/L) | 1.64 ± 0.59 | 1.24 ± 0.48 | <0.001 |
| 2-h glucose (mmol/L) | 9.89 ± 2.46 | 10.17 ± 2.36 | 0.396 |
| ΔGlucose 30–0 min (mmol/L) | 3.47 ± 1.31 | 3.47 ± 1.35 | 0.992 |
| ΔGlucagon 30–0 min (pmol/L) | −3.35 ± 2.94 | −2.12 ± 2.92 | 0.003 |
| ΔGlucagon 30–0 min / Δglucose 30–0 min (pmol/mmol) | −1.079 ± 1.041 | −0.759 ± 1.472 | 0.036 |
| iAUC glucose (mmol/L) | 591.5 ± 235.6 | 614.8 ± 250.4 | 0.468 |
| dAUC glucagon (pmol/L) | −950.1 ± 476.3 | −803.4 ± 499.2 | 0.030 |
| iAUC C-peptide (nmol/L) | 856.7 ± 283.8 | 669.4 ± 202.6 | <0.001 |
| dAUC glucagon / iAUC glucose (pmol/mmol) | −2.04 ± 2.11 | −1.54 ± 1.62 | 0.080 |
| iAUC C-peptide / dAUC glucagon (nmol/pmol) | −1.23 ± 0.99 | −1.19 ± 2.61 | 0.802 |
Data are means ± SD or geometric mean (95% CI) and P values for non–normally distributed data based on log-transformed values.
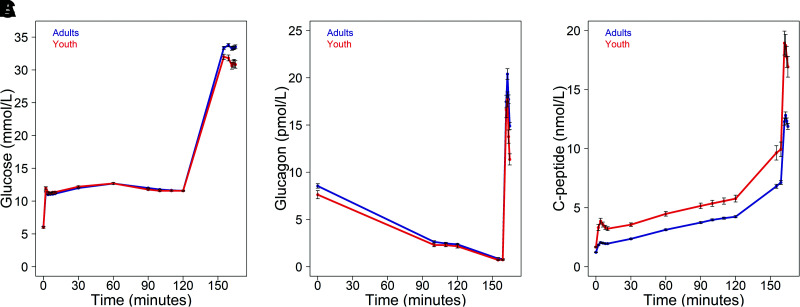
During the clamp, the targeted glucose levels were achieved and maintained (Fig. 1A). The increase in glucose to ∼11.1 mmol/L was associated with suppression of glucagon in both youth and adults, with no difference in the achieved steady-state glucagon concentrations between groups (Fig. 1B). The decrement in glucagon from fasting to this steady-state step of the clamp was not different between youth and adults. Raising the glucose concentration to >25 mmol/L resulted in further suppression of glucagon, again with no difference in the resultant glucagon concentrations between the groups. Following arginine stimulation, glucagon increased briskly in both groups. However, the mean values were lower in youth at 2, 3, 4, and 5 min after arginine; thus, the AGR was significantly lower in youth than adults.
Figure 1.
Plasma glucose (A), glucagon (B), and C-peptide (C) concentrations during the hyperglycemic clamps in 66 youth (red) and 350 adults (blue). Data are mean ± SEM. Peak values were observed 3 min after the arginine bolus (163 min: youth, 17.7 ± 0.79 pmol/L, and adults, 20.4 ± 0.57 pmol/L), with this difference being similar at the subsequent time points.
As reported previously (8) and listed in Table 2, insulin sensitivity was lower and the C-peptide responses were all significantly higher in youth (Fig. 1C). When C-peptide concentrations were expressed relative to glucagon, they were higher in youth in the fasting state, at the steady-state glucose of ∼11.1 mmol/L and in response to arginine (AGR) (Table 2).
Adjusting the above analyses for insulin sensitivity, sex, and race/ethnicity did not appreciably alter the differences in youth and adults for glucagon or C-peptide (data not shown).
OGTT-Derived Measurements in Youth Versus Adults
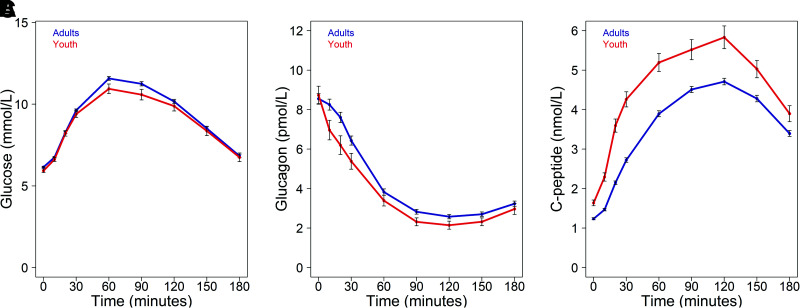
The profiles of glucose, glucagon, and C-peptide responses during the 3-h test are illustrated in Fig. 2A–C, respectively, with select metabolic measures provided in Table 2.
Figure 2.
Plasma glucose (A), glucagon (B), and C-peptide (C) concentrations during the OGTT in 66 youth (red) and 350 adults (blue). Data are means ± SEM.
The fasting glucagon concentrations did not differ between the two age-groups on the day of the OGTT. With the increase in glucose following ingestion, glucagon concentrations decreased in both youth and adults. The absolute glucagon concentrations were lower in youth at multiple time points during the test. Notably, much of this difference was due to a more rapid decline in glucagon in the first 30 min following glucose ingestion in youth than adults. However, iAUC C-peptide relative to dAUC glucagon across the 3-h OGTT was not significantly different in youth and adults.
Again, adjustment for insulin sensitivity, sex, and race/ethnicity did not appreciably alter the differences in youth and adults for glucagon or C-peptide (data not shown).
Relationship of BMI and Fasting Glucose With α-Cell Responses in Youth Versus Adults
Fasting glucagon was not significantly associated with BMI in either age-group (r = 0.047, P = 0.708, in youth; r = 0.059, P = 0.274, in adults), while AGR and BMI were related in adults (r = 0.109, P = 0.042) but not youth (r = 0.048, P = 0.706). There were somewhat different relationships for fasting glucose. In adults, fasting glucagon concentrations were higher with higher fasting glucose concentrations (r = 0.133, P = 0.012), while in youth this relationship was inverse, though not significant (r = −0.143, P = 0.251). In both youth and adults, AGR was higher with higher fasting glucose, although this relationship was only significant in adults (r = 0.107, P = 0.397, in youth; r = 0.129, P = 0.016, in adults).
Relationship of Insulin Sensitivity and β-Cell Responses With α-Cell Responses in Youth Versus Adults
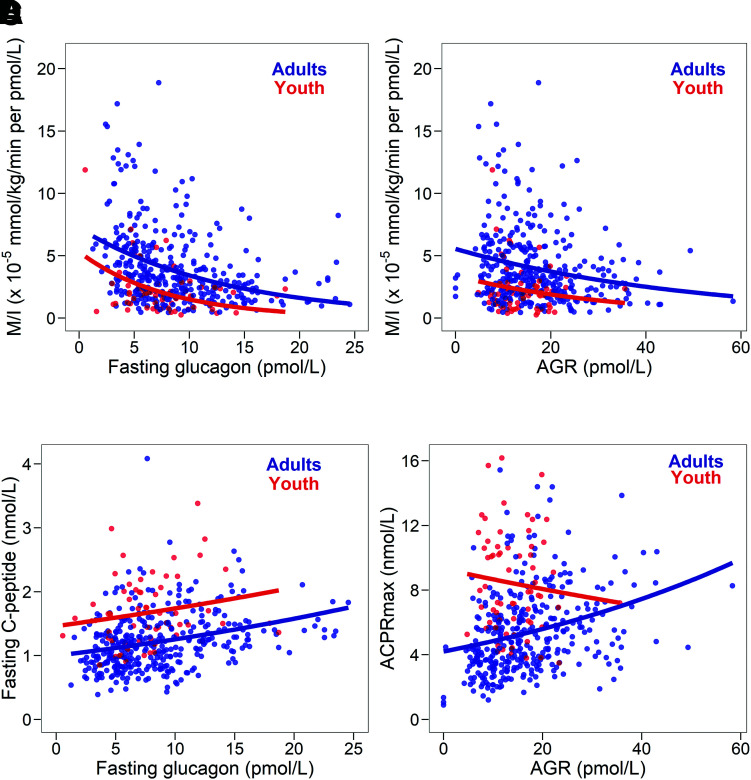
Higher fasting glucagon concentrations were associated with lower insulin sensitivity in both age-groups (youth r = −0.228, P = 0.066; adults r = −0.324, P < 0.001). Similarly, inverse relationships of AGR and insulin sensitivity were seen but achieved significance in adults only (youth r = −0.069, P = 0.588; adults r = −0.222, P < 0.001). In youth, across the range of fasting glucagon and AGR, insulin sensitivity was significantly lower than in adults (P < 0.001 for both) (Fig. 3A and B).
Figure 3.
Relationship of fasting glucagon and insulin sensitivity (M/I) (A), the AGR and M/I (B), fasting glucagon and fasting C-peptide (C), and AGR and ACPRmax (D) in 66 youth (in red) and 350 adults (in blue). The correlation of fasting glucagon with M/I was similar in youth (r = −0.228, P = 0.066) and adults (r = −0.324, P < 0.001); the slopes did not differ between youth and adults (Pinteraction = 0.944), while the intercepts did (P < 0.001). The correlation of AGR with M/I was significant in adults (r = −0.222, P < 0.001) but not in youth (r = −0.069, P = 0.588); again, the slopes were not significantly different in youth and adults (Pinteraction = 0.680), but the intercepts differed (P < 0.001). The relation of fasting glucagon to fasting C-peptide was similar in adults (r = 0.335, P < 0.001) and youth (r = 0.209, P = 0.091), with no significant difference in the slopes in youth and adults (Pinteraction = 0.514) and a significant difference in the intercepts (P < 0.001). The correlation of AGR and ACPRmax was significant in adults (r = 0.355, P < 0.001), but not in youth (r = −0.101, P = 0.423), with the interaction of the slopes being significant (P = 0.014).
Fasting glucagon and fasting C-peptide were positively related in both youth and adults but did not reach significance in youth (youth r = 0.209, P = 0.091; adults r = 0.335, P < 0.001), with the levels of C-peptide being greater in youth for any matched glucagon concentration (P < 0.001) (Fig. 3C). Regarding the arginine-stimulated responses, AGR and ACPRmax were positively related in adults but were not in youth (youth r = −0.101, P = 0.423; adults r = 0.355, P < 0.001) (Fig. 3D).
Conclusions
Given that glucagon can stimulate insulin release (11–14) and increase hepatic glucose production (15–17), we hypothesized that hyperglucagonemia may be making an important contribution to the enhanced β-cell responsiveness and insulin resistance that we previously reported in youth in RISE. The present analyses demonstrate that glucagon concentrations are not greater in youth and thus increased α-cell glucagon release is not the basis for the differences we observed in the two different age-groups.
In RISE we used two approaches to characterize glucose metabolism, namely, the hyperglycemic clamp and OGTT. Using these two methods we were able to interrogate different aspects of glucose metabolism, including α-cell function. First, it is known that glucagon release is suppressed with increasing glucose, by glucose per se and by the release of β-cell contents (24–30). We observed that raising glucose to ∼11.1 mmol/L during the hyperglycemic clamp resulted in the expected decrements from fasting glucagon concentrations, with this decrease not differing between age-groups. Similarly, in the OGTT we observed a suppression of glucagon with the maximal decline in both youth and adults occurring at ∼120 min and a small increase thereafter as the glucose concentration fell. Interestingly, the decrement in glucagon during the whole OGTT was greater in youth, with a different pattern early in the test, namely, a greater decline in glucagon over the first 30 min after glucose ingestion.
Second, it is well recognized that certain amino acids, including arginine, can stimulate the α-cell along with the β-cell (23,31,32), and we observed arginine-stimulated glucagon release in both youth and adults. Intriguingly, we found this glucagon response to be significantly less in youth than adults, despite the maximal glucose concentration being significantly lower in youth (33.7 ± 4.6 vs. 31.8 ± 3.6 pmol/L; P < 0.001). As the response to arginine is known to be modulated by the prevailing glucose concentration (23,33), this lower glucose concentration in youth would have, if anything, been expected to lead to a lesser suppression of the glucagon response. Since glucagon is extracted by the liver (34), we cannot entirely exclude the possibility that glucagon release is increased in youth but that their livers are extracting more of the peptide than those of adults and thus peripheral concentrations and responses are not different.
The α- and β-cells are known to regulate each other’s function. Glucagon release by the α-cell acts in a paracrine manner to enhance insulin secretion from the β-cell, acting via the glucagon and glucagon-like peptide 1 (GLP-1) receptors on the β-cell (35–37). Our data, based on peripheral glucagon measurements, suggest that the increase in β-cell peptide release in youth is not simply occurring as a result of increased glucagon stimulating the β-cell. This inference is based on our observation that although the glucagon concentrations were not higher overall in youth, with evaluation as a continuous relationship we observed that at matched glucagon concentrations youth exhibit greater C-peptide concentrations (Fig. 3C). To the extent that glucagon is contributing to the regulation of C-peptide secretion, this may suggest greater β-cell sensitivity to glucagon in youth. However, this relationship differs principally in the intercept rather than the slope relating glucagon and C-peptide, suggesting that factors other than glucagon may also be important in determining C-peptide concentrations.
The dynamics of the glucagon profile during the OGTT also demonstrated differences between youth and adults. In youth the rate of suppression was greater during the first 30 min following glucose ingestion, with no significant difference in the subsequent time period. This observation in youth would again imply that increased glucagon was not the basis for the increased C-peptide and insulin responses that we observed during the same time period (9). Interestingly, it was recently reported that glucagon suppression during this same early phase of the OGTT was more rapid in those who were more insulin sensitive and in individuals with IGT compared with those with type 2 diabetes (38). We did not observe similar profile differences and, if anything, observed a more rapid decline in youth who had lower insulin sensitivity. It is possible that this difference may be age related in part, as fasting and stimulated glucagon concentrations increase with age (39,40). Lastly, we measured glucagon responses to oral glucose and not a mixed meal. The latter is expected to increase glucagon concentrations more than an isolated glucose load because of the protein content (41). While we cannot be certain, we believe it likely that our conclusions regarding α-cell function in youth and adults would have been the same.
While the primary goal of these analyses was to examine the effect of glucagon on the β-cell response and insulin sensitivity, by inverting the independent and dependent variables, we gathered data that also provided an opportunity to address additional concepts related to the α-cell. We observed an inverse relationship between insulin sensitivity, quantified using the hyperglycemic clamp, and fasting glucagon concentration. Thus, in those participants who were more insulin resistant, plasma glucagon was higher. A similar observation has previously been reported with insulin sensitivity quantified as the Gutt index from the glucose and insulin measures during an OGTT (38) and in obese youth with an euglycemic-hyperinsulinemic clamp (18). Our findings extend these observations, as we have also compared youth and adults. We find that in both age-groups, decreasing insulin sensitivity is associated with increasing fasting glucagon, although, at similar degrees of insulin sensitivity, fasting glucagon is lower in youth. Further, examination of the relationship between insulin sensitivity and stimulated glucagon measured in response to arginine shows a similar relationship in adults but less so in youth.
As noted above, at matched glucagon concentrations the youth exhibited higher C-peptide concentrations (Fig. 3C), which may suggest a greater sensitivity of β-cells to glucagon in youth. Given that insulin and the β-cell products GABA and zinc can decrease glucagon release through a paracrine mechanism and/or by vascular flow (24–30), by inverting the variables in this relationship we were also able to evaluate the effects of β-cell products on glucagon concentrations. With this perspective we observed lower glucagon levels in youth at a given level of C-peptide (again, with a major difference in the intercept and essentially parallel slopes). Analogous to the evaluation above, this observation might suggest greater sensitivity of the α-cell to β-cell products. If insulin is the important regulator of α-cell function, in youth the α-cell may be more responsive to insulin than other tissues, an aspect of insulin action that has also been described for the liver and peripheral tissues (42).
It is apparent that problems continue with certain glucagon assays resulting in different characteristics and performance (43–45). We used a sequential protocol for the glucagon assay rather than the standard simultaneous protocol. The sequential protocol has been demonstrated to reduce the likelihood of cross-reactivity with glicentin and oxyntomodulin, alternative products of the glucagon gene (21,22). Thus, we are confident that our observations reflect excursions of glucagon itself in response to increased glucose and arginine stimulation rather than a combination of glucagon and related cross-reacting products.
Despite our study’s major strength of being the first to directly compare youth and adults with use of the same assays in a central laboratory, there are some limitations. First, the smaller sample size in youth affects statistical power for evaluating relationships within the youth group and for comparing youth and adults. Overall this did not confound interpretation of the current results. Second, the distribution of race/ethnicity and sex differed between youth and adults. However, adjustment for these did not change our findings, indicating that the differences (insulin sensitivity and C-peptide) or lack thereof (glucagon) between youth and adults are age related and not due to differences between the groups in the race/ethnicity and sex distributions. Third, the cross-sectional approach allowed us to address some potential mechanisms but not directly test them.
In conclusion, we made a number of novel observations related to α-cell function in obese, dysglycemic youth compared with adults. First, since glucagon concentrations were essentially equal in absolute terms and lower in youth with adjustment for relevant physiologic features, the β-cell hyperresponsiveness or insulin resistance that we identified in youth is not attributable to α-cell dysfunction resulting in increased glucagon release. Second, based on peripheral glucagon concentrations, α-cell function may be differentially regulated by insulin sensitivity in youth, with the cell being more sensitive to insulin (or as a marker of other β-cell products that may regulate the α-cell). And lastly, given that α-cell hypersecretion was not observed, further study is required to gain additional insights into what may be the basis for the increased response of the β-cell and insulin resistance in youth.
Article Information
Acknowledgments. The RISE Consortium acknowledges the support and input of the RISE Data and Safety Monitoring Board; Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Program Official for RISE; Ellen Leschek, the NIDDK Project Scientist for RISE; and Peter Savage, who served as the NIDDK Project Scientist for RISE prior to retirement. The RISE Consortium is also grateful for the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding. RISE is partly supported by grants from the NIDDK, National Institutes of Health (U01DK-094406, U01DK-094430, U01DK-094431, U01DK-094438, U01DK-094467, P30DK-017047, P30DK-020595, P30DK-045735, P30DK-097512, UL1TR-000430, UL1TR-001082, UL1TR-001108, UL1TR-001855, UL1TR-001857, UL1TR-001858, UL1TR-001863) and the Department of Veterans Affairs. Additional financial and material support from the American Diabetes Association, Allergan Corporation, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk A/S is gratefully acknowledged.
Duality of Interest. RISE is also supported by Kaiser Permanente Southern California. S.E.K. and S.A.A. serve as paid consultants on advisory boards for Novo Nordisk. S.E.K. is a member of the steering committee for a Novo Nordisk–sponsored clinical trial, and S.A.A is a participant in a Novo Nordisk–sponsored clinical trial. K.J.M. held an investigator-initiated research grant from Novo Nordisk. T.A.B. has received research support from Allergan Corporation and Apollo Endosurgery. No other potential conflicts of interest relevant to this article were reported.
The involvement of K.J.M. and S.M. in the research underlying this manuscript preceded their employment at Eli Lilly and Co. and Medpace Reference Laboratories, respectively, and their participation in data review and the writing of this manuscript were independent of their employment at Eli Lilly and Co. and Medpace Reference Laboratories.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. S.E.K. proposed the analysis, interpreted data, and wrote and edited the manuscript, which was also reviewed and edited by members of the writing group. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. S.E.K. and S.L.E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
K.J.M. is currently employed by Eli Lilly and Co.
S.M. is currently employed by Medpace Reference Laboratories.
A complete list of the RISE Consortium Investiga-tors appears in the supplementary material online.
Clinical trial reg. nos. NCT01779362, NCT01779375, NCT01763346, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14474022.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/the-RISE-study-more-insights-into-T2D-in-youth-and-adults.
Contributor Information
Collaborators: The Rise Consortium Investigators:, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, et al.; IDF Diabetes Atlas Committee . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 3. TODAY Study Group, Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 5. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahn SE, Lachin JM, Zinman B, et al.; ADOPT Study Group . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. RISE Consortium . Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RISE Consortium . Metabolic contrasts betw-een youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. RISE Consortium; RISE Consortium Investigators . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 1965;2:415–416 [DOI] [PubMed] [Google Scholar]
- 12. Crockford PM, Porte D Jr, Wood FC Jr, Williams RH. Effect of glucagon on serum insulin, plasma glucose and free fatty acids in man. Metabolism 1966;15:114–122 [DOI] [PubMed] [Google Scholar]
- 13. Karam JH, Grasso SG, Wegienka LC, Grodsky GM, Forsham PH. Effect of selected hexoses, of epinephrine and of glucagon on insulin secretion in man. Diabetes 1966;15:571–578 [DOI] [PubMed] [Google Scholar]
- 14. Goldfine ID, Cerasi E, Luft R. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. J Clin Endocrinol Metab 1972;35:312–315 [DOI] [PubMed] [Google Scholar]
- 15. Sherwin RS, Hendler R, DeFronzo R, Wahren J, Felic P. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci U S A 1977;74:348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ward WK, Best JD, Halter JB, Porte D Jr. Prolonged infusion of somatostatin with glucagon replacement increases plasma glucose and glucose turnover in man. J Clin Endocrinol Metab 1984;58:449–453 [DOI] [PubMed] [Google Scholar]
- 17. Rizza RA, Gerich JE. Persistent effect of sustained hyperglucagonemia on glucose production in man. J Clin Endocrinol Metab 1979;48:352–355 [DOI] [PubMed] [Google Scholar]
- 18. Weiss R, D’Adamo E, Santoro N, Hershkop K, Caprio S. Basal α-cell up-regulation in obese insulin-resistant adolescents. J Clin Endocrinol Metab 2011;96:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manell H, Staaf J, Manukyan L, et al. Altered plasma levels of glucagon, GLP-1 and glicentin during OGTT in adolescents with obesity and type 2 diabetes. J Clin Endocrinol Metab 2016;101:1181–1189 [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 21. Roberts GP, Kay RG, Howard J, Hardwick RH, Reimann F, Gribble FM. Gastrectomy with Roux-en-Y reconstruction as a lean model of bariatric surgery. Surg Obes Relat Dis 2018;14:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexiadou K, Cuenco J, Howard J. et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res Care 2020;8:e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenbaum CJ, Havel PJ, Taborsky GJ Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest 1991;88:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 2005;54:1789–1797 [DOI] [PubMed] [Google Scholar]
- 27. Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metab 2009;9:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rorsman P, Berggren PO, Bokvist K, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 1989;341:233–236 [DOI] [PubMed] [Google Scholar]
- 29. Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 2003;5:330–335 [DOI] [PubMed] [Google Scholar]
- 30. Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 1992;41:93–97 [DOI] [PubMed] [Google Scholar]
- 31. Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest 1972;51:2346–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970;49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaspan JB, Ruddick J, Rayfield E. Transhepatic glucagon gradients in man: evidence for glucagon extraction by human liver. J Clin Endocrinol Metab 1984;58:287–292 [DOI] [PubMed] [Google Scholar]
- 35. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic β-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 1998;47:66–72 [DOI] [PubMed] [Google Scholar]
- 36. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 37. Capozzi ME, Svendsen B, Encisco SE, et al. β cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Færch K, Vistisen D, Pacini G, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 2016;65:3473–3481 [DOI] [PubMed] [Google Scholar]
- 39. Berger D, Crowther RC, Floyd JC Jr, Pek S, Fajans SS. Effect of age on fasting plasma levels of pancreatic hormones in man. J Clin Endocrinol Metab 1978;47:1183–1189 [DOI] [PubMed] [Google Scholar]
- 40. Ferrero E, Casale G, De Nicola P. Serum glucagon after arginine infusion in aged and young subjects. J Am Geriatr Soc 1980;28:285–287 [DOI] [PubMed] [Google Scholar]
- 41. Ruetten H, Gebauer M, Raymond RH, et al.; Foundation for the NIH Biomarkers Consortium Beta Cell Project Team . Mixed meal and intravenous L-arginine tests both stimulate incretin release across glucose tolerance in man: lack of correlation with β cell function. Metab Syndr Relat Disord 2018;16:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630–E639 [DOI] [PubMed] [Google Scholar]
- 43. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014;57:1919–1926 [DOI] [PubMed] [Google Scholar]
- 44. Wewer Albrechtsen NJ, Veedfald S, Plamboeck A, et al. Inability of some commercial assays to measure suppression of glucagon secretion. J Diabetes Res 2016;2016:8352957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. Impact of the glucagon assay when assessing the effect of chronic liraglutide therapy on glucagon secretion. J Clin Endocrinol Metab 2017;102:2729–2733 [DOI] [PubMed] [Google Scholar]