Abstract
OBJECTIVE
To identify predictors of glycemic worsening among youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes in the Restoring Insulin Secretion (RISE) Study.
RESEARCH DESIGN AND METHODS
A total of 91 youth (10–19 years) were randomized 1:1 to 12 months of metformin (MET) or 3 months of glargine, followed by 9 months of metformin (G-MET), and 267 adults were randomized to MET, G-MET, liraglutide plus MET (LIRA+MET), or placebo for 12 months. All participants underwent a baseline hyperglycemic clamp and a 3-h oral glucose tolerance test (OGTT) at baseline, month 6, month 12, and off treatment at month 15 and month 21. Cox models identified baseline predictors of glycemic worsening (HbA1c increase ≥0.5% from baseline).
RESULTS
Glycemic worsening occurred in 17.8% of youth versus 7.5% of adults at month 12 (P = 0.008) and in 36% of youth versus 20% of adults at month 21 (P = 0.002). In youth, glycemic worsening did not differ by treatment. In adults, month 12 glycemic worsening was less on LIRA+MET versus placebo (hazard ratio 0.21, 95% CI 0.05–0.96, P = 0.044). In both age-groups, lower baseline clamp-derived β-cell responses predicted month 12 and month 21 glycemic worsening (P < 0.01). Lower baseline OGTT-derived β-cell responses predicted month 21 worsening (P < 0.05). In youth, higher baseline HbA1c and 2-h glucose predicted month 12 and month 21 glycemic worsening, and higher fasting glucose predicted month 21 worsening (P < 0.05). In adults, lower clamp- and OGTT-derived insulin sensitivity predicted month 12 and month 21 worsening (P < 0.05).
CONCLUSIONS
Glycemic worsening was more common among youth than adults with IGT or recently diagnosed type 2 diabetes, predicted by lower baseline β-cell responses in both groups, hyperglycemia in youth, and insulin resistance in adults.
Introduction
Type 2 diabetes has become increasingly common in youth and adults as the prevalence of overweight and obesity increases. Progressive deterioration of islet β-cell function in individuals with prediabetes typically leads to type 2 diabetes (1). The rate of progression to type 2 diabetes and further loss of β-cell function once hyperglycemia is established varies widely among individuals with prediabetes. In an observational longitudinal study of 77,000 adults with prediabetes defined by HbA1c, a small subset (5.2%) had a very high risk for development of type 2 diabetes within 2 years, while most (81.5%) were at lesser risk (2). Similarly, among obese youth with prediabetes based on HbA1c, up to 8% developed type 2 diabetes after 12–22 months of follow-up (3). In youth with established type 2 diabetes monitored for a mean of 3.86 years in the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study, insulin was required after oral treatments failed in 50% of participants, with a median time to treatment failure of 11.5 months, whereas other youth in TODAY maintained glycemic control on oral diabetes medications alone (4). Of note, youth in TODAY overall had a more rapid deterioration of β-cell function and glycemic control (4) than that previously reported in adults (5–8); however, no prior studies have directly compared youth and adults with type 2 diabetes longitudinally in the same study. Identification of factors that predispose to deterioration of β-cell function in youth and adults is essential to designing interventions to delay or prevent glycemic worsening in each age-group.
The Restoring Insulin Secretion (RISE) studies examined whether pharmacologic interventions could successfully restore or preserve β-cell function in youth and adults with impaired glucose intolerance (IGT) or recently diagnosed type 2 diabetes (9). As previously reported, none of the interventions resulted in sustained improvements in β-cell function after medication withdrawal in youth or adults (10,11). However, there was individual heterogeneity in responses both on and after withdrawal of therapy, ranging from normalization to rapid worsening of glycemia requiring study withdrawal; the latter was especially common among youth (10,11). In this analysis, we aimed to compare glycemic worsening between youth and adult RISE participants with IGT or recently diagnosed type 2 diabetes and identify baseline characteristics that predict glycemic worsening in each group, while on treatment (month 12 [M12]) and 9 months after treatment withdrawal (month 21, [M21]).
Research Design and Methods
Study Protocol
The RISE Pediatric and Adult Medication Studies were two of the three clinical trials performed as part of the RISE Consortium, funded by the National Institute of Diabetes and Digestive and Kidney Diseases (9). Both studies enrolled participants with IGT or recently diagnosed type 2 diabetes (12). The Pediatric Medication Study was a four-center, randomized, open-label clinical trial comparing 12-month interventions with insulin glargine for 3 months, followed by metformin (MET) for 9 months (G-MET) versus MET alone (9,10). The Adult Medication Study was a three-center, randomized, partially blinded clinical trial comparing 12-month interventions with 1) G-MET, 2) MET, 3) liraglutide plus MET (LIRA+MET), or 4) placebo. In the RISE Adult Medication Study, the placebo versus MET arms were double blinded. The rationale for and methods used in RISE were described previously in detail (9–11), and the study protocols can be found at https://rise.bsc.gwu.edu/web/rise/collaborators. Each center’s Institutional Review Board (IRB) approved the protocol. Written informed consent was obtained from every adult participant, and parental or participant consent and child assent (when age-appropriate) were obtained before study procedures, consistent with the Declaration of Helsinki and each center’s IRB guidelines.
Participants
A summary of the RISE Pediatric and Adult Medication Study participants and their rates of completion of oral glucose tolerance tests (OGTTs) during study visits are shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Supplementary Figs. 1 and 2). The pediatric study randomized 91 youth aged 10–19 years with BMI ≥85th percentile for age and sex but <50 kg/m2, with IGT (60%) or recently diagnosed (<6 months’ duration) type 2 diabetes (40%), negative GAD and islet antigen 2 autoantibodies, and Tanner stage ≥2 (using breast development for females and testicular volume >3 mL for males) (9,10). Youth were also required to have a fasting glucose of ≥90 mg/dL (5 mmol/L) and an OGTT 2-h glucose ≥140 mg/dL (7.8 mmol/L), and if they were drug naive, HbA1c ≤8.0% (64 mmol/mol). Youth with type 2 diabetes already taking metformin had to have HbA1c ≤7.5% (58 mmol/mol) if on metformin for <3 months or HbA1c ≤7.0% (53 mmol/mol) if on metformin for 3–6 months (9,10). Eligible youth underwent baseline evaluations, followed by random 1:1 treatment assignment by study site, stratified by a baseline diagnosis of IGT versus type 2 diabetes (9,10). Eligibility criteria for adults included age 20–65 years and BMI ≥25 but <50 kg/m2 (≥23 kg/m2 for Asian Americans), with IGT or drug-naive physician-diagnosed type 2 diabetes <12 months’ duration (9,11). Adults were required to have a fasting glucose 95–125 mg/dL (5.3–6.9 mmol/L), OGTT 2-h glucose ≥140 mg/dL (7.8 mmol/L), and HbA1c ≤7% (9,11). Eligible adults underwent baseline evaluations, followed by random 1:1:1:1 treatment assignment by study site, stratified by a baseline diagnosis of IGT versus type 2 diabetes (9,11).
Interventions
Complete details of the interventions were previously published (9–11), and the study timeline is shown in Supplementary Fig. 3. Youth and adult participants in the MET group both received 500 mg metformin that was titrated up to 1,000 mg twice daily over 4 weeks or to the maximal tolerated dose. The G-MET group in both youth and adults received 3 months of glargine insulin, titrated by study staff based on daily self-monitored fasting blood glucose. After 3 months, insulin glargine was stopped, and MET was initiated and titrated up to 1,000 mg twice daily (or the maximal tolerated dose) for the remainder of the 9-month intervention. For the adult LIRA+MET group, liraglutide was started first, with weekly titration from 0.6 to 1.2 to a final dose of 1.8 mg daily as tolerated. After a tolerated liraglutide dose over the first 4 weeks was established, MET (unblinded) was added and titrated up to a goal of 1,000 mg twice daily (or the maximal tolerated dose) for the remainder of the intervention period (11). The adult placebo group received tablets that were identical in appearance to the metformin tablets and were titrated up in the same manner as the metformin and continued for 12 months.
The study medication for all participants in the Pediatric and Adult Medication Studies was withdrawn after 12 months of treatment (9–11). Study measurements in the present analysis included evaluations at baseline, M3, M6, M9, and M12 (on treatment) and M15, M18, and M21 (through 9 months after medication withdrawal). Measurements included in this analysis were baseline anthropometric measures, fasting and 2-h OGTT glucose, HbA1c, and hyperglycemic clamp- and OGTT-derived measures of β-cell response and insulin sensitivity, as well as HbA1c at M3, M6, M9, M12, M15, M18, and M21, and fasting and 2-h OGTT glucose at M6, M12, M15, and M21.
If protocol-specified HbA1c safety thresholds were exceeded, outcome measures were promptly performed, if possible, and the participant was then withdrawn from the study and referred for additional clinical diabetes treatment. In addition to the IRB and the investigators, the study was regularly monitored by an independent National Institute of Diabetes and Digestive and Kidney Diseases-appointed Data and Safety Monitoring Board.
Procedures and Calculations
HbA1c was measured at all quarterly visits. A two-step hyperglycemic clamp with target glucose concentrations of 200 mg/dL (11.1 mmol/L) and >450 mg/dL (>25 mmol/L), the latter paired with the insulin secretagogue arginine, was performed at baseline as previously described to assess pancreatic β-cell function (9,13–15). The hyperglycemic clamp simultaneously quantified insulin sensitivity (M/I) and three β-cell response measures: 1) acute (first-phase) C-peptide and insulin responses to glucose (ACPRg, AIRg), 2) steady-state (second-phase) C-peptide (SSCP), and 3) acute C-peptide and insulin responses to arginine at maximal glycemic potentiation (ACPRmax and AIRmax) achieved by glucose >450 mg/dL (16–19). M/I was calculated as the mean glucose infusion rate (M) at 100, 110, and 120 min of the clamp divided by the corresponding mean steady-state plasma insulin concentration. ACPRg and AIRg were calculated as the mean incremental response above baseline for the first 10 min after the glucose bolus. Mean SSCP was calculated at 100, 110, and 120 min (16–19). ACPRmax and AIRmax were calculated as the mean incremental response above concentrations before the arginine bolus.
A 3-h 75-g OGTT was performed at baseline to determine fasting and 2-h glucose, and fasting and 2-h insulin and C-peptide concentrations. From these, 1/fasting insulin (I/FI) was calculated to estimate insulin sensitivity, and OGTT-derived β-cell response measures were computed, including the C-peptide index (CPI, nmol/mmol; ΔC-peptide30–0/Δglucose30–0) and insulinogenic index (IGI, Δinsulin30–0/Δglucose30–0) (14,20). The hyperglycemic clamp-derived insulin and C-peptide responses were adjusted for M/I, and OGTT-derived responses were adjusted for 1/FI.
Laboratory assessments were performed at the study’s central biochemistry laboratory at the University of Washington (13,14). Glucose was measured using the glucose hexokinase method on a Roche c501 autoanalyzer (Roche Diagnostics, Indianapolis, IN). C-peptide and insulin were measured by a two-site immunoenzymometric assay performed on the Tosoh 2000 autoanalyzer (Tosoh Biosciences, South San Francisco, CA). Interassay coefficients of variation on quality control samples with low, medium, medium-high, and high concentrations were ≤2.0% for glucose, ≤4.3% for C-peptide, and ≤3.5% for insulin. HbA1c was measured on a Tosoh G8 analyzer, under Level 1 NGSP certification. The interassay coefficients of variation, as measured on quality control samples with low and high HbA1c levels, were 1.9% and 1.0%, respectively (9–11).
Statistics
Glycemic worsening was defined as an absolute increase in HbA1c by ≥0.5% from baseline to M12 and from baseline to M21 (e.g., an increase from a baseline HbA1c of 6.0% to 6.5% or from baseline of 7.7% to 8.2%, etc.). We additionally examined glycemic worsening defined as ≥5% and ≥10% relative increases from baseline HbA1c to M12 and to M21 (e.g., a 5% relative increase from a baseline of 6.0% would be 6.3%, and a 10% relative increase from a baseline of 6% would be 6.6%). Cox proportional hazards models were used to identify baseline factors that were associated with time to glycemic worsening. Baseline characteristics used as predictors included age, sex, race/ethnicity, BMI, HbA1c, fasting and 2-h OGTT glucose, and measures of β-cell response and insulin sensitivity from the OGTT and hyperglycemic clamp. Results from continuous variables in the Cox models are presented per 1 SD. Cox models were adjusted for treatment arm. β-Cell function measures were further adjusted for insulin sensitivity as assessed during the procedure, using M/I for hyperglycemic clamp-derived measures (ACPRg, AIRg, SSCP, ACPRmax, and AIRmax) and using 1/FI for OGTT-derived measures (CPI and IGI). When the analyses regarding β-cell responses were repeated without adjustment for insulin sensitivity, there was no significant impact on the results, so only the data that were adjusted are presented. Measures from the hyperglycemic clamp were log transformed before analyses to normalize the distributions. Additionally, progression from IGT to type 2 diabetes based on American Diabetes Association criteria for fasting and 2-h glucose concentrations (12) was evaluated at M06, M12, M15, and M21, and the impact of pharmacologic interventions on progression from IGT to type 2 diabetes was examined using Cox models.
Results
Baseline demographic and metabolic characteristics of youth and adults and the primary outcome of the RISE studies have been reported (10,11) and are summarized in Supplementary Table 1. In brief, youth had a higher percentage of females, more racial/ethnic diversity, similar weight, and slightly but statistically higher BMI, and were markedly more insulin resistant than the adults. Furthermore, the proportion of youth with type 2 diabetes at baseline (40.7%) was higher than adults (26.2%) (P = 0.0410) (Supplementary Table 1). However, despite these differences, both groups had similar baseline HbA1c and fasting and 2-h glucose concentrations (Supplementary Table 1).
Glycemic Worsening
At both M12 and M21, significantly more youth than adults developed glycemic worsening as defined by an absolute increase of ≥0.5% in HbA1c (M12: 17.8% vs. 7.5%, P = 0.008; M21 36.7% vs. 20%, respectively, P = 0.002) (Table 1, Fig. 1, top panel). Findings were similar even when analyses were restricted to the interventions common to youth and adults; i.e., MET and G-MET groups, both at M12 (P = 0.011) and M21 (P = 0.005, data not shown). Additionally, we evaluated the percentage of youth and adults who had a ≥5% relative increase in HbA1c from baseline to M12 and M21. Overall, 27.8% of youth and 22% of adults had a ≥5% relative increase in HbA1c at M12 (P = 0.26), as did 54.4% of youth and 42% of adults at M21 (P = 0.041) (Fig. 1, bottom panel). Using a ≥10% relative increase in HbA1c, we determined 14.4% of youth and 3.9% adults had such an increase in HbA1c at M12 (P < 0.001), as did 31.3% of youth and 14.9% of adults at M21 (P < 0.001) (data not shown).
Table 1.
Glycemic worsening in youth and adults defined as an absolute increase of 0.5% in HbA1c from baseline to M12 and M21
| M12 | M21 | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||
| n | % | n | % | n | % | n | % | |
| Youth | ||||||||
| G-MET | 35 | 79.5 | 9 | 20.5 | 30 | 68.2 | 14 | 31.8 |
| MET | 39 | 84.8 | 7 | 15.2 | 27 | 58.7 | 19 | 41.3 |
| Total | 74 | 82.2 | 16 | 17.8 * | 57 | 63.3 | 33 | 36.7 † |
| Adult | ||||||||
| G-MET | 64 | 95.5 | 3 | 4.5 | 53 | 79.1 | 14 | 20.9 |
| MET | 57 | 91.9 | 5 | 8.1 | 51 | 82.3 | 11 | 17.7 |
| LIRA+MET | 61 | 96.8 | 2 | 3.2 | 54 | 85.7 | 9 | 14.3 |
| Placebo | 54 | 85.7 | 9 | 14.3 | 46 | 73.0 | 17 | 27.0 |
| Total | 236 | 92.5 | 19 | 7.5 | 204 | 80.0 | 51 | 20.0 |
Bold values are statistically significant comparing youth to adults.
P = 0.008 youth compared with adults at M12.
P = 0.002 youth compared with adults at M21.
Figure 1.
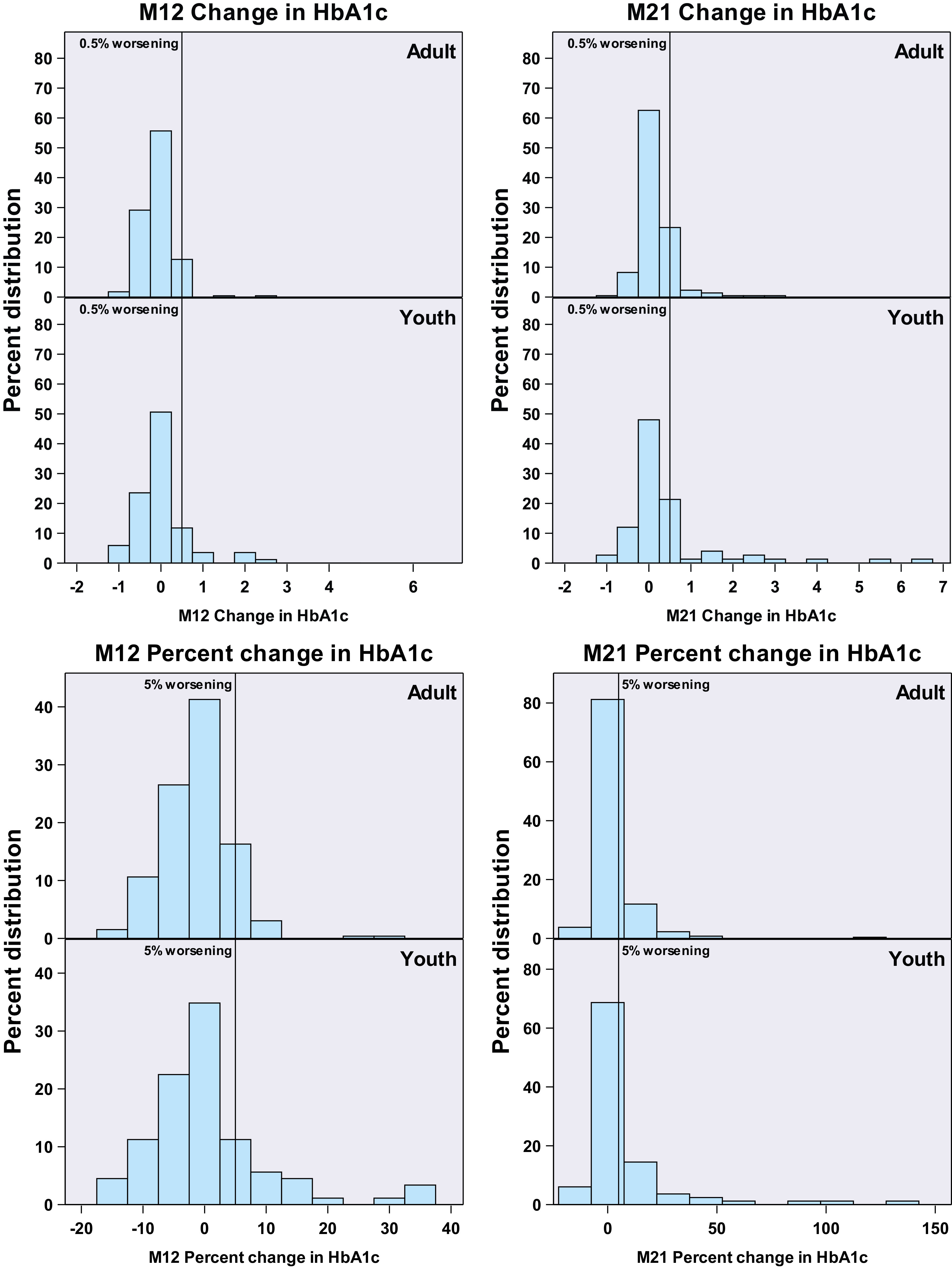
Change from baseline in glycemia at M12 (on treatment) and M21 (9 months after treatment withdrawal). Top panel: Glycemic worsening based on absolute increase in HbA1c in adults and youth from baseline to M12 (left panel) and M21 (right panel). The vertical lines depict 0.5% worsening in absolute HbA1c. On the left, there are no significant differences between adults and youth at M12. On the right, youth experienced greater glycemic worsening at M21 (P = 0.041). Bottom panel: Glycemic worsening based on the percentage (relative) increase in HbA1c in adults and youth from baseline to M12 (left panel) and M21 (right panel). The vertical lines are shown at 5% worsening. Youth experienced greater glycemic worsening when defined as the percentage increase in HbA1c at M12 (P < 0.001) and at M21 (P < 0.001).
Predictors of Glycemic Worsening in Youth and Adults
In youth, treatment group did not affect glycemic worsening defined by an absolute increase of ≥0.5% in HbA1c, with glycemic worsening at M12 and M21 being associated with higher baseline HbA1c (P = 0.0241 and P = 0.008, respectively) and higher baseline 2-h glucose (P = 0.045 and P = 0.042, respectively) (Table 2). Additionally, glycemic worsening at M21 in youth was associated with higher baseline fasting glucose (P = 0.044). In youth, glycemic worsening at M12 and M21 was also associated with lower baseline first-phase responses assessed by hyperglycemic clamp-derived ACPRg (P = 0.006 and P < 0.001, respectively) and AIRg (P = 0.014 and P < 0.001, respectively) and lower baseline second-phase β-cell response by clamp-derived SSCP (P = 0.004 and P = 0.001, respectively). Glycemic worsening at M21 was also associated with lower baseline maximal β-cell response by clamp-derived ACPRmax (P = 0.015) and AIRmax (P = 0.007). In youth, lower baseline OGTT-derived β-cell responses, including IGI (P = 0.017) and CPI P = 0.006), were associated with glycemic worsening at M21, and lower baseline CPI was also associated with glycemic worsening at M12 (P = 0.031).
Table 2.
Cox regression models of predictors of glycemic worsening (an absolute increase of 0.5% in HbA1c) in youth
| M12 hazard ratio† | Lower CI | Upper CI | P value | M21 hazard ratio† | Lower CI | Upper CI | P value | |
|---|---|---|---|---|---|---|---|---|
| G-MET vs. MET | 1.37 | 0.51 | 3.68 | 0.532 | 0.81 | 0.41 | 1.62 | 0.560 |
| Sex (male vs. female) | 1.52 | 0.54 | 4.31 | 0.432 | 0.94 | 0.41 | 2.13 | 0.878 |
| Race/ethnicity | ||||||||
| White vs. other | 0.35 | 0.08 | 1.56 | 0.170 | 0.54 | 0.22 | 1.32 | 0.177 |
| Black vs. other | 1.86 | 0.66 | 5.25 | 0.238 | 0.88 | 0.38 | 2.05 | 0.769 |
| Hispanic vs. other | 1.75 | 0.64 | 4.75 | 0.274 | 1.41 | 0.70 | 2.82 | 0.336 |
| Baseline continuous measures | ||||||||
| Age (years) | 0.98 | 0.59 | 1.63 | 0.932 | 0.99 | 0.70 | 1.41 | 0.956 |
| BMI (kg/m2) | 1.14 | 0.68 | 1.92 | 0.614 | 1.14 | 0.79 | 1.64 | 0.496 |
| HbA1c (%) | 1.67 | 1.07 | 2.60 | 0.024 | 1.56 | 1.12 | 2.16 | 0.008 |
| Fasting glucose (mg/dL) | 1.43 | 0.93 | 2.19 | 0.100 | 1.33 | 1.01 | 1.77 | 0.044 |
| 2-h glucose (mg/dL) | 1.49 | 1.01 | 2.20 | 0.045 | 1.33 | 1.01 | 1.75 | 0.042 |
| Log M/I | 0.83 | 0.50 | 1.38 | 0.472 | 0.93 | 0.65 | 1.34 | 0.704 |
| Log ACPRg | 0.45 | 0.25 | 0.79 | 0.006 | 0.47 | 0.32 | 0.69 | <0.001 |
| Log AIRg | 0.57 | 0.36 | 0.89 | 0.014 | 0.61 | 0.45 | 0.81 | <0.001 |
| Log SSCP | 0.45 | 0.26 | 0.78 | 0.004 | 0.54 | 0.37 | 0.78 | 0.001 |
| Log ACPRmax | 0.61 | 0.34 | 1.11 | 0.105 | 0.61 | 0.41 | 0.91 | 0.015 |
| Log AIRmax | 0.59 | 0.32 | 1.09 | 0.090 | 0.57 | 0.38 | 0.85 | 0.007 |
| Log 1/FI | 0.78 | 0.47 | 1.28 | 0.321 | 0.85 | 0.59 | 1.22 | 0.385 |
| IGI | 0.46 | 0.20 | 1.04 | 0.061 | 0.56 | 0.34 | 0.90 | 0.017 |
| CPI | 0.45 | 0.22 | 0.93 | 0.031 | 0.53 | 0.34 | 0.83 | 0.006 |
Bold P values are statistically significant (P < 0.05). All models included a term for the treatment arm. CI, 95% confidence interval.
Per 1 SD for continuous measures. Treatment arm is adjusted for insulin sensitivity by M/I, clamp-derived β-cell function responses are adjusted for treatment arm and insulin sensitivity by M/I, and all OGTT-derived β-cell function responses are adjusted for treatment arm and insulin sensitivity by 1/FI.
Unlike youth, in adults, overall treatment group was borderline significant in predicting glycemic worsening defined by an absolute increase of ≥0.5% in HbA1c by M12 (P = 0.089) but not at M21 (P = 0.233) (Table 3). However, treatment with LIRA+MET attenuated glycemic worsening at M12 compared with placebo (hazard ratio, 0.21; 95% CI 0.05–0.96, P = 0.044), although this beneficial effect was only borderline significant at M21 after withdrawal of LIRA+MET (hazard ratio 0.46, 95% CI 0.20–1.02, P = 0.057). Unlike youth, glycemic worsening in adults was not associated with baseline glycemic measures such as HbA1c and fasting or 2-h glucose, but M12 and M21 glycemic worsening was associated with lower baseline insulin sensitivity by clamp-derived M/I (P = 0.016 and P = 0.003, respectively) and OGTT-derived 1/FI (P = 0.010 and P = 0.029, respectively). In addition, similar to youth, glycemic worsening in adults at M12 and M21 was associated with lower baseline clamp-derived first-phase responses, including ACPRg (P = 0.010 and P = 0.011) and AIRg (P = 0.001 and P = 0.007). Like in youth, a lower baseline IGI on the OGTT was also associated with glycemic worsening at M21 in adults (P = 0.034).
Table 3.
Cox regression models of predictors of glycemic worsening (an absolute increase of 0.5% in HbA1c) in adults
| M12 hazard ratio† | Lower CI | Upper CI | P value | M21 hazard ratio† | Lower CI | Upper CI | P value | |
|---|---|---|---|---|---|---|---|---|
| Treatment group | 0.089 | 0.233 | ||||||
| G-MET vs. LIRA+MET | 1.32 | 0.22 | 7.90 | 0.763 | 1.35 | 0.58 | 3.12 | 0.485 |
| G-MET vs. MET | 0.49 | 0.12 | 2.04 | 0.325 | 1.03 | 0.47 | 2.27 | 0.939 |
| G-MET vs. placebo | 0.27 | 0.07 | 1.01 | 0.051 | 0.61 | 0.30 | 1.25 | 0.179 |
| LIRA+MET vs. MET | 0.37 | 0.07 | 1.91 | 0.234 | 0.77 | 0.32 | 1.87 | 0.551 |
| LIRA+MET vs. placebo | 0.21 | 0.05 | 0.96 | 0.044 | 0.46 | 0.20 | 1.02 | 0.057 |
| MET vs. placebo | 0.56 | 0.19 | 1.67 | 0.296 | 0.60 | 0.28 | 1.27 | 0.181 |
| Sex (male vs. female) | 1.96 | 0.69 | 5.53 | 0.207 | 1.75 | 0.95 | 3.25 | 0.074 |
| Race/ethnicity | ||||||||
| White vs. other | 1.04 | 0.42 | 2.58 | 0.925 | 1.04 | 0.60 | 1.81 | 0.893 |
| Black vs. other | 0.75 | 0.27 | 2.09 | 0.581 | 0.95 | 0.52 | 1.73 | 0.870 |
| Hispanic vs. other | 1.68 | 0.48 | 5.88 | 0.418 | 0.89 | 0.32 | 2.51 | 0.831 |
| Baseline continuous measures | ||||||||
| Age (years) | 0.78 | 0.51 | 1.18 | 0.239 | 1.11 | 0.82 | 1.49 | 0.514 |
| BMI (kg/m2) | 1.03 | 0.64 | 1.66 | 0.915 | 0.80 | 0.59 | 1.08 | 0.150 |
| HbA1c (%) | 1.05 | 0.66 | 1.65 | 0.842 | 0.94 | 0.70 | 1.27 | 0.695 |
| Fasting glucose (mg/dL) | 1.21 | 0.81 | 1.81 | 0.356 | 1.03 | 0.80 | 1.33 | 0.827 |
| 2-h glucose (mg/dL) | 1.09 | 0.68 | 1.76 | 0.714 | 1.21 | 0.92 | 1.60 | 0.179 |
| Log M/I | 0.59 | 0.39 | 0.91 | 0.016 | 0.67 | 0.52 | 0.88 | 0.0034 |
| Log ACPRg | 0.49 | 0.29 | 0.85 | 0.010 | 0.67 | 0.49 | 0.91 | 0.011 |
| Log AIRg | 0.47 | 0.30 | 0.75 | 0.001 | 0.68 | 0.52 | 0.90 | 0.007 |
| Log SSC-P | 0.89 | 0.49 | 1.61 | 0.691 | 0.86 | 0.59 | 1.24 | 0.415 |
| Log ACPRmax | 0.94 | 0.59 | 1.48 | 0.778 | 0.90 | 0.68 | 1.20 | 0.476 |
| Log AIRmax | 0.82 | 0.51 | 1.33 | 0.426 | 0.79 | 0.59 | 1.05 | 0.108 |
| Log 1/FI | 0.57 | 0.37 | 0.87 | 0.010 | 0.75 | 0.57 | 0.97 | 0.029 |
| IGI | 0.49 | 0.22 | 1.08 | 0.078 | 0.65 | 0.43 | 0.97 | 0.034 |
| CPI | 0.60 | 0.31 | 1.15 | 0.122 | 0.72 | 0.51 | 1.02 | 0.062 |
Bold P values are statistically significant (P < 0.05). All models included a term for treatment arm. CI, 95% confidence interval.
Per 1 SD deviation for continuous measures. Treatment arm is adjusted for insulin sensitivity by M/I, clamp-derived β-cell function responses are adjusted for treatment arm and insulin sensitivity by M/I, and all OGTT-derived β-cell function responses are adjusted for treatment arm and Insulin sensitivity by 1/FI.
Glycemic Worsening Based on Progression From IGT to Type 2 Diabetes
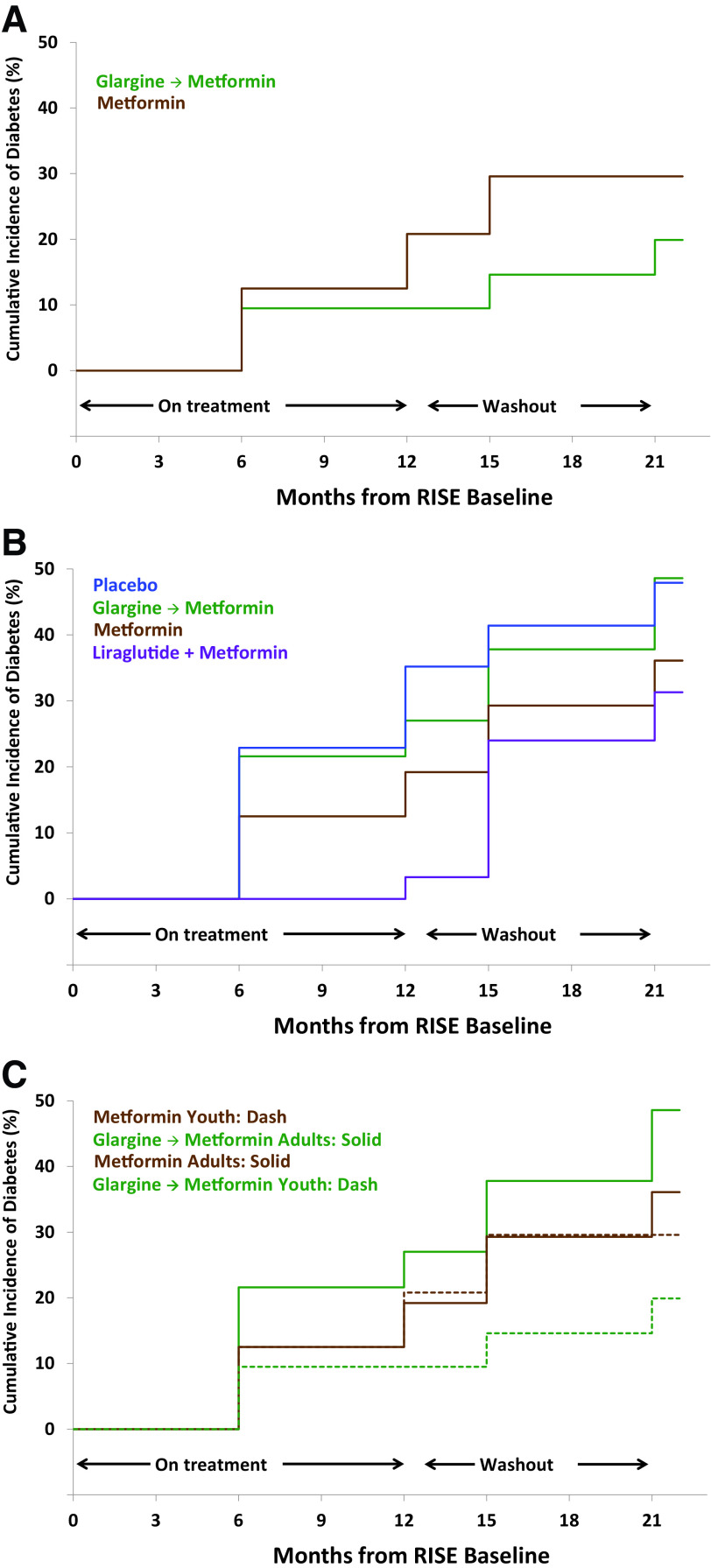
Among youth with IGT at baseline, 15.9% progressed to diabetes by M12 and 25% to diabetes by M21. In youth, treatment group did not affect progression from IGT to type 2 diabetes at M12 (P = 0.28) or M21 (P = 0.40) (Fig. 2A). Among adults with IGT at baseline, 21.2% progressed to diabetes by M12 and 39.1% by M21. In adults, treatment was borderline effective in reducing the progression from IGT to type 2 diabetes at M12 (P = 0.06 with the lowest rate in LIRA+MET) but not by M21 (P = 0.20) (Fig. 2B). The overall rate of progression from IGT to type 2 diabetes did not differ between youth and adults by M12 (P = 0.55) but was borderline significant by M21 (P = 0.089 with lower rates among youth). Limiting this comparison to include only adults randomized to MET or G-MET (to compare the interventions common to youth and adults) did not alter our findings (Fig. 2C).
Figure 2.
Life table estimate to progression from IGT to type 2 diabetes. A: Cumulative incidence of type 2 diabetes in youth over the duration of the study among youth with IGT at baseline. The progression to type 2 diabetes did not differ in youth between glargine, followed by metformin (G-MET) or metformin alone (MET) at M12 (P = 0.28) or M21 (P = 0.40). B: Cumulative incidence of type 2 diabetes in adults over the duration of the study among adults with IGT at baseline. The progression to type 2 diabetes was borderline significant between the four intervention arms at M12 (P = 0.06 for overall comparison) but not at M21 (P = 0.20 for overall comparison). C: Cumulative incidence of type 2 diabetes in youth and adults randomized to metformin (MET) alone vs. glargine, followed by metformin (G-MET), over the duration of the study, among youth and adults with IGT at baseline. The progression to type 2 diabetes did not differ between youth and adults in either intervention arm.
In youth, progression to diabetes at M12 and M21 was associated with lower baseline maximal β-cell responses by clamp-derived ACPRmax (P = 0.017 and P = 0.039, respectively, data not shown), at M12 by lower baseline AIRmax (P = 0.004, data not shown), and at M21 by lower baseline first-phase response by clamp-derived AIRg (P = 0.039, data not shown). Similar to youth, in adults, lower baseline clamp-derived first-phase responses (AIRg and ACPRg) also predicted progression to diabetes at M12 (P = 0.001 and P = 0.010, respectively; data not shown) and M21 (P = 0.007 and P = 0.010, respectively; data not shown), as did lower baseline OGTT-derived IGI at M21 P = 0.034; data not shown). Different from youth, progression to type 2 diabetes at M12 and M21 in adults was associated with lower baseline insulin sensitivity by clamp-derived M/I (P = 0.016 and P = 0.003, respectively; data not shown) and by OGTT-derived 1/FI (P = 0.010 and P = 0.029, respectively; data not shown).
Conclusions
The current analysis of outcomes in the RISE study of youth and adults with IGT or recently diagnosed type 2 diabetes demonstrates that 1) short-term pharmacologic interventions did not reduce glycemic worsening or progression from IGT to type 2 diabetes in youth; 2) LIRA+MET was effective in reducing glycemic worsening while on therapy in adults, but this effectiveness did not persist 9 months after treatment withdrawal, consistent with our previous findings (10,14); 3) glycemic worsening was progressive in both groups, although greater in youth as 36% of youth versus 20% of adults (approaching 30% in adults randomized to placebo) developed glycemic worsening by the end of the 21-month study period, despite 12 months of active treatment with pharmacologic agents; and 4) independent of pharmacologic intervention, β-cell dysfunction at baseline was a significant predictor of glycemic worsening in both youth and adults, both while they were on treatment and after treatment withdrawal, whereas while initial glycemia was predictive in youth, insulin sensitivity was predictive in adults.
By direct longitudinal comparison between youth and adults with a similar degree of dysglycemia at baseline, we clearly demonstrate for the first time that a greater fraction of youth with IGT or recently diagnosed type 2 diabetes had deterioration of glycemia compared with adults. Previous cross-sectional, observational, and randomized trials have suggested that youth have a more accelerated progression to type 2 diabetes and earlier development of diabetes complications compared with adults (8,10,11,21). The current study is also the first to directly compare youth and adults who were treated with a variety of medications, were monitored for nearly 2 years, and were studied in an identical manner across study sites using sensitive measures of insulin sensitivity and β-cell function. In both youth and adults, a lower baseline hyperglycemic clamp-derived first-phase β-cell response to glucose was associated with a rise in HbA1c on treatment (M12) and after treatment withdrawal (M21), and lower OGTT-derived β-cell responses were similarly predictive after treatment withdrawal in both youth and adults. Lower baseline hyperglycemic clamp-derived second-phase and maximal C-peptide responses were also associated with glycemic worsening after treatment withdrawal in youth. Interestingly, baseline measures of glycemia in youth, such as fasting and OGTT 2-h glucose and HbA1c, also predicted glycemic worsening after treatment withdrawal. In contrast to youth, in adults, reduced insulin sensitivity by hyperglycemic clamp and OGTT was a consistent additional predictor of glycemic worsening both on treatment and after treatment withdrawal. These data indicate potential differences in the pathophysiology of diabetes progression between youth and adults.
Our finding in youth with IGT and recently diagnosed type 2 diabetes compliment and extend findings from the TODAY Study in youth with established type 2 diabetes (4,22). In TODAY, baseline OGTT-based β-cell dysfunction and dysglycemia predicted a greater probability of glycemic failure (defined as HbA1c ≥8% for 6 months); i.e., baseline proximity to a glycemic threshold predicted crossing that threshold during follow-up. In RISE, we focused on deterioration, defined as an increase in HbA1c, rather than reaching a specific glycemic threshold. In RISE, baseline β-cell dysfunction and dysglycemia were the strongest predictors of glycemic worsening in youth; i.e., youth with the highest glucose levels and lowest β-cell function at baseline were deteriorating the fastest. This finding is consistent with a pattern of accelerating glycemia deterioration on a background of monotonous deterioration in β-cell-compensation (23). Further, RISE demonstrated that multiple β-cell responses (OGTT-based and first-phase, second-phase, and maximal glycemic potentiation responses during hyperglycemic clamps) predicted glycemic worsening in youth. In contrast to the adults in RISE, neither TODAY (4,22) nor RISE found insulin sensitivity to be predictive of treatment failure or rising glycemia in youth. These findings point to β-cell dysfunction occurring on a background of severe insulin resistance as the key defect in youth that determines glycemic worsening, even at the earlier stage of IGT studied in RISE. Possible explanations for these age-related differences are that youth with IGT and type 2 diabetes are typically uniformly markedly insulin resistant due to multiple potential factors (the physiologic insulin resistance of puberty, lower physical activity, poor sleep, higher rates of racial/ethnic minorities, etc.), causing β-cell function to be the key differentiating factor in youth. In contrast, adults with IGT and type 2 diabetes may show more heterogeneity in their insulin sensitivity and β-cell responsiveness, allowing both to be predictors.
MET and G-MET did not reduce glycemic worsening in youth on treatment or after treatment withdrawal. In adults, only treatment with LIRA+MET was effective in reducing glycemic worsening while on therapy, but this beneficial effect did not persist after treatment withdrawal. This result is consistent with our earlier report of lack of durable benefit regarding β-cell function once LIRA+MET was discontinued for 3 months (11). Similarly, there are reports in the literature that the beneficial impact of glucagon-like peptide 1 agonists in reducing progression to type 2 diabetes in individuals with IGT is lost after withdrawal of therapy (24,25). Worsening of glycemia has also been reported upon withdrawal from liraglutide therapy in adults with type 2 diabetes of longer duration (26). In the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study, insulin glargine was shown to reduce progression from IGT to type 2 diabetes in adults, although the effect was minimal (27). Similarly, a study from China demonstrated that intensive short-term glucose control with the use of multiple daily dose insulin injections or an insulin pump is associated with less progression of β-cell dysfunction in adults with newly diagnosed type 2 diabetes (28). In RISE, treatment with G-MET was not associated with a reduction in glycemic worsening in youth or adults with IGT or recently diagnosed type 2 diabetes while on treatment or after treatment withdrawal. Other reports in the literature are consistent with our observation that glargine does not prevent glycemic worsening (29). To date, thiazolidinediones have been the only pharmacologic class reported to durably prevent deterioration of β-cell function in adults (30–32) and in some youth (4,22).
Strengths of our study include the inclusion of both youth and adults of similar initial glycemia and inclusion of participants with IGT and recently diagnosed type 2 diabetes in protocols run in parallel at multiple sites using identical procedures and a central laboratory. Additional strengths include the use of measures of insulin sensitivity and β-cell responses by fasting, oral, and intravenous stimulation, inclusion of both glucose and arginine stimulation, as well as the racial/ethnic diversity among participants. The availability of longitudinal follow-up data both on treatment as well as after treatment withdrawal also contributes to the strength of our findings. Limitations include the lack of follow-up >21 months, baseline sex and racial/ethnic differences between youth and adults inherent to the affected populations, and the lack of a placebo and LIRA+MET group in youth.
In summary, in youth and adults with IGT or recently diagnosed type 2 diabetes, the incidence of glycemic worsening is high despite pharmacologic interventions, is worse in youth, and is predicted in both age-groups by baseline β-cell dysfunction with loss of the first-phase response. In adults, baseline insulin sensitivity is an additional predictor of glycemic worsening, whereas in youth, initial glycemia and β-cell dysfunction involving second-phase and maximal responses are also predictive. Although pharmacologic treatment in adults with LIRA+MET was not effective in reducing glycemic worsening after treatment withdrawal, it did decrease glycemic worsening while on therapy, arguing for long-term studies of β-cell function in youth receiving glucagon-like peptide 1 agonists. In addition, data on the impact of SGLT-2 inhibitors and of bariatric surgery on β-cell function are needed. Finally, our data also support the need for development of alternative approaches or therapies with a specific focus on preventing or improving insulin sensitivity and first-phase β-cell function in adults and all aspects of β-cell function in youth.
Article Information
Acknowledgments. The RISE Consortium thanks the RISE Data and Safety Monitoring Board, Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases Program Official for RISE, and Ellen Leschek and Peter Savage, National Institute of Diabetes and Digestive and Kidney Diseases Scientific Officers for RISE, for their support and guidance. The Consortium is also grateful to the participants who, by volunteering, are furthering its ability to reduce the burden of diabetes. The authors dedicate this paper in memory of Bridget Pierpont, research associate from Yale University, for all she did to enrich their lives and further research in youth with diabetes.
Funding. RISE is supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (U01DK-094406, U01DK-094430, U01DK-094431, U01DK-094438, U01DK-094467, P30DK-017047, P30DK-020595, P30DK-045735, P30DK-097512) and the National Center for Advancing Translational Sciences (UL1TR-000430, UL1TR-001082, UL1TR-001108, UL1TR-001855, UL1TR-001857, UL1TR-001858, and UL1TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support was received from the American Diabetes Association, Allergan Corporation, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk A/S.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. S.A.A. is a paid consultant on advisory boards for Novo Nordisk, and a participant in a Novo Nordisk-sponsored clinical trial. S.E.K. is a paid consultant on advisory boards for Novo Nordisk and a steering committee for a Novo Nordisk-sponsored clinical trial. At the time of publication, K.J.M. was a full-time employee of Eli Lilly and Company. The data collection was performed before this employment, and the data analysis and preparation of the manuscript were independent of Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.S., S.L.E., and K.J.N. wrote the first draft of the manuscript. S.S., S.L.E., K.J.N., S.A.A., E.B., T.A.B., S.C., D.A.E., T.S.H., A.H.T., S.E.K., K.J.M., M.T., K.M.U., and A.H.X. researched data, contributed to the discussion, and edited and approved the final version of the manuscript. S.L.E. is the guarantor of this work and performed all data analysis, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A complete list of centers, RISE Consortium Investigators, and staff can be found in the supplementary material online.
Clinical trial reg. nos. NCT01779362 and NCT01779375, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14368220.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/the-RISE-study-more-insights-into-T2D-in-youth-and-adults.
Contributor Information
Collaborators: RISE Consortium Investigators, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree-Green, Yesenia GarciaReyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2. Glauber H, Vollmer WM, Nichols GA. A simple model for predicting two-year risk of diabetes development in individuals with prediabetes. Perm J 2018;22:17–050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes 2018;19:199–204 [DOI] [PubMed] [Google Scholar]
- 4. Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner RC, Cull CA, Frighi V; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 6. Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010;33:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 8. Barrett T, Jalaludin MY, Turan S, Hafez M; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel . Rapid progression of type 2 diabetes and related complications in children and young people—a literature review. Pediatr Diabetes 2020;21:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RISE Consortium . Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. RISE Consortium . Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2019;42:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl. 1(Suppl. 1):S67–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hannon TS, Kahn SE, Utzschneider KM, et al.; RISE Consortium . Review of methods for measuring β-cell function: design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab 2018;20:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 17. Elahi D. In praise of the hyperglycemic clamp. A method for assessment of β-cell sensitivity and insulin resistance. Diabetes Care 1996;19:278–286 [DOI] [PubMed] [Google Scholar]
- 18. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 19. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomon TP, Malin SK, Karstoft K, et al. Determining pancreatic β-cell compensation for changing insulin sensitivity using an oral glucose tolerance test. Am J Physiol Endocrinol Metab 2014;307:E822–E829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 22. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 24. le Roux CW, Astrup A, Fujioka K, et al.; SCALE Obesity Prediabetes NN8022-1839 Study Group . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409 [DOI] [PubMed] [Google Scholar]
- 25. Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care 2014;37:3270–3278 [DOI] [PubMed] [Google Scholar]
- 26. Tran S, Kramer CK, Zinman B, Choi H, Retnakaran R. Effect of chronic liraglutide therapy and its withdrawal on time to postchallenge peak glucose in type 2 diabetes. Am J Physiol Endocrinol Metab 2018;314:E287–E295 [DOI] [PubMed] [Google Scholar]
- 27. Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 28. Weng JL, Li Y, Xu W, et al. Effects of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;24:1753–1760 [DOI] [PubMed] [Google Scholar]
- 29. Retnakaran R, Choi H, Ye C, Kramer CK, Zinman B. Two-year trial of intermittent insulin therapy vs metformin for the preservation of β-cell function after initial short-term intensive insulin induction in early type 2 diabetes. Diabetes Obes Metab 2018;20:1399–1407 [DOI] [PubMed] [Google Scholar]
- 30. DeFronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 31. Knowler WC, Hamman RF, Edelstein SL, et al.; Diabetes Prevention Program Research Group . Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tripathy D, Schwenke DC, Banerji M, et al. Diabetes incidence and glucose tolerance after termination of pioglitazone therapy: results from ACT NOW. J Clin Endocrinol Metab 2016;101:2056–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]