Abstract
OBJECTIVE
The metabolic phenotype of youth-onset type 2 diabetes (T2D) differs from that of adult-onset T2D, but little is known about genetic contributions. We aimed to evaluate the association between a T2D genetic risk score (GRS) and traits related to glucose-insulin homeostasis among healthy youth.
RESEARCH DESIGN AND METHODS
We used data from 356 youth (mean age 16.7 years; 50% female) in the Exploring Perinatal Outcomes Among Children (EPOCH) cohort to calculate a standardized weighted GRS based on 271 single nucleotide polymorphisms associated with T2D in adults. We used linear regression to assess associations of the GRS with log-transformed fasting glucose, 2-h glucose, HOMA of insulin resistance (HOMA-IR), oral disposition index, and insulinogenic index adjusted for age, sex, BMI z score, in utero exposure to maternal diabetes, and genetic principal components. We also evaluated effect modification by BMI z score, in utero exposure to maternal diabetes, and ethnicity.
RESULTS
Higher weighted GRS was associated with lower oral disposition index (β = −0.11; 95% CI −0.19, −0.02) and insulinogenic index (β = −0.08; 95% CI −0.17, −0.001), but not with fasting glucose (β = 0.01; 95% CI −0.01, 0.02), 2-h glucose (β = 0.03; 95% CI −0.0004, 0.06), or HOMA-IR (β = 0.02; 95% CI −0.04, 0.07). BMI z score and in utero exposure to maternal diabetes increased the effect of the GRS on glucose levels.
CONCLUSIONS
Our results suggest that T2D genetic risk factors established in adults are relevant to glucose-insulin homeostasis in youth and that maintaining a healthy weight may be particularly important for youth with high genetic risk of T2D.
Introduction
The prevalence of type 2 diabetes (T2D) has been increasing worldwide in recent decades (1). While T2D was once a disease of adulthood, it now accounts for ∼20–50% of new-onset diabetes cases among youth in the U.S. (2), and it has been projected that there could be a fourfold increase in the prevalence of youth-onset T2D by 2050 (3). Youth-onset T2D is strongly associated with obesity and tends to be characterized by higher rates of complications, more aggressive progression of disease, and reduced responsiveness to treatment relative to adult counterparts (4). Therefore, there is an urgent need to improve our understanding of risk factors for T2D in youth, including genetic contributors, in order to more effectively prevent this condition.
Numerous risk factors have been associated with youth-onset T2D, most notably obesity, which is present in ∼85% of youth-onset T2D cases in the U.S. and Europe, although it is a less consistent feature of cases in Eastern countries (5). Genetics also contribute to T2D both in adults and youth (6,7). However, our understanding of the genetics of T2D is predominantly from adult populations (8). Large-scale genome-wide association studies (GWAS) have identified more than 300 single nucleotide polymorphisms (SNPs) associated with T2D in adults (6), and at least some of these SNPs have also been associated with T2D, glycemia, or insulin metabolism traits in youth (9,10). The first GWAS of youth-onset T2D was recently published (7), and this study identified seven genome-wide significant associations, one of which was novel while the remaining have previously been linked to adult-onset T2D. The relatively low prevalence of youth-onset T2D is a substantial challenge to understanding its genetic influences, as is the complex interplay between genetic, environmental, and behavioral factors. An improved understanding of the genetic influences on pediatric glucose-insulin homeostasis may help to inform personalized prevention approaches.
In this study, we calculated weighted and unweighted T2D genetic risk scores (GRS) using 271 SNPs established in adults (6) in order to quantify the cumulative effect of T2D-associated genetic variants. We applied these GRS to a population of 356 youth from the Exploring Perinatal Outcomes Among Children (EPOCH) study and examined the associations with five glycemic and insulin metabolism traits at adolescent age: fasting glucose, 2-h postchallenge glucose, HOMA of insulin resistance (HOMA-IR), oral disposition index, and insulinogenic index. We also evaluated whether BMI z score or Hispanic ethnicity modifies the effects of the GRS.
Research Design and Methods
Study Cohort
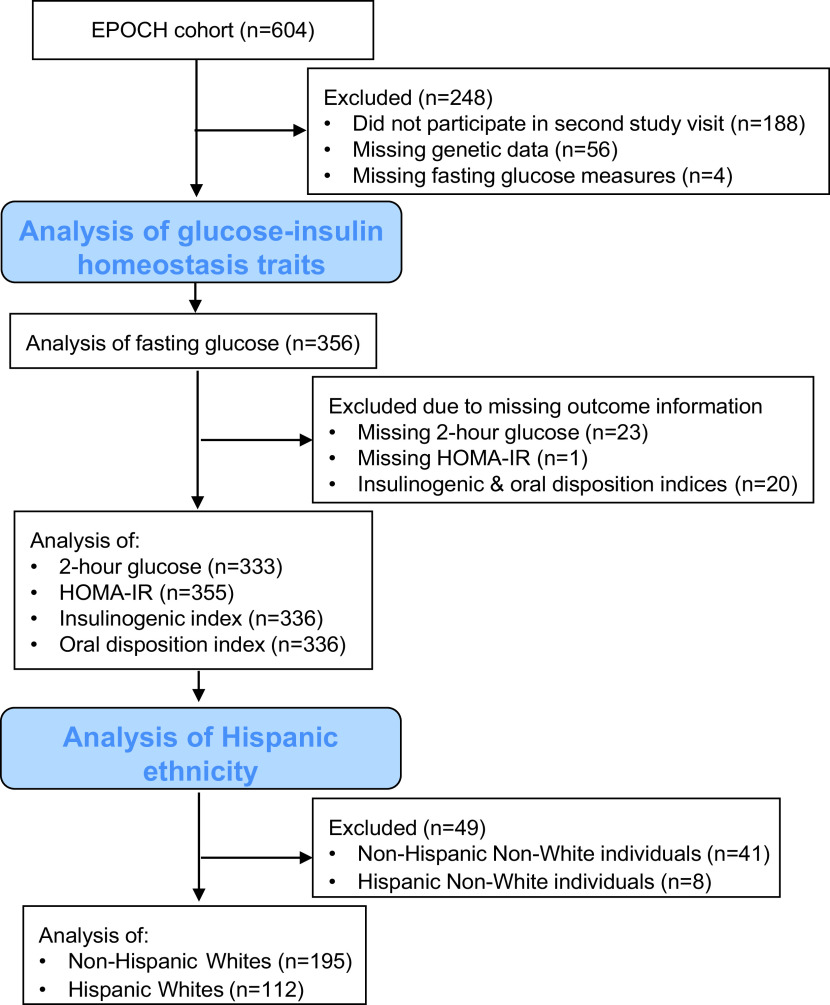
EPOCH is a historical prospective study of 604 mother/child pairs identified through the Kaiser Permanente of Colorado (KPCO) Perinatal Database based on presence or absence of maternal diabetes during gestation. The children included in the study were offspring of singleton pregnancies of biological mothers who were members of the KPCO Health Plan, and they were born at a single hospital in Denver between 1992 and 2002. Eligible children and their mothers were invited to participate in two childhood research visits, at average ages of 10.5 and 16.5 years. The sample used for this study includes the subset of children (n = 356) with genetic data and data on glucose-insulin homeostasis measured during the second in-person study visit when they were between 12 and 19 years of age (median age 16.9 years; interquartile range 15.9, 17.6). The inclusion and exclusion criteria for this study are shown in Fig. 1. Among the 416 individuals with data from this visit, the following individuals were excluded: missing genetic data (n = 56) and missing fasting glucose information (n = 4). In the analyses of other examined traits, additional individuals were excluded because of missing data: 2-h glucose (n = 23), HOMA-IR (n = 1), and insulinogenic index and oral disposition index (n = 20). The study was approved by the Colorado Multiple Institutional Review Board and the Human Participant Protection Program, and all participants provided written informed consent, and youth provided written assent.
Figure 1.
The inclusion and exclusion criteria for this study. This diagram shows the number of individuals in the EPOCH cohort and in each analysis in this study.
Data Collection
Child traits related to glucose-insulin homeostasis were evaluated during an in-person research visit at the University of Colorado Anschutz Medical Campus, as described previously (11). Blood samples were obtained after an overnight fast. The participants completed a 2-h, 75-g oral glucose tolerance test with venous blood measurements of glucose and insulin at 0, 30, and 120 min. HOMA-IR, a marker of insulin resistance, was calculated as (fasting glucose [mmol/L] × fasting insulin [μU/mL])/22.5. The insulinogenic index, a marker of early insulin response, was calculated in SI units as Δinsulin0–30/Δglucose0–30. The oral disposition index, which reflects β-cell function adjusted for insulin resistance, was calculated by dividing this value by the fasting insulin: (Δinsulin0–30/glucose0–30) × (1/insulin0) (12). Impaired fasting glucose was defined as ≥5.6 mmol/L, impaired glucose tolerance was defined as ≥7.8 mmol/L, and prediabetes was defined as meeting either of these thresholds (13).
Height was measured by SECA stadiometer, and weight was measured using an electronic SECA scale, as described previously (11). Age- and sex-specific BMI z scores were calculated using Centers for Disease Control and Prevention reference standards (14), and BMI categories were based on BMI percentiles defined as normal weight (≤85th percentile), overweight (85th–95th percentiles), or obese (≥95th percentile) (15). Waist circumference was measured according to the National Health and Nutrition Examination Survey protocol (16). Child age was calculated from the date of delivery. Pubertal development was assessed by child self-report with a diagrammatic representation of Tanner staging adapted from Marshall and Tanner (17). Race and ethnicity were self-reported and categorized as White, Black/African American, and other; ethnicity was dichotomized as Hispanic (yes or no). Maternal gestational diabetes status was physician diagnosed using a standard two-step screening protocol (18) and ascertained from the KPCO Perinatal Database, an electronic database linking the neonatal and perinatal medical records.
Genetic Data
A sample of peripheral venous blood was collected from children at the study visit and stored at −80°C. DNA was extracted using the QIAamp Kit (Qiagen, Germantown, MD). DNA samples were quantified and purity assessed using a NanoDrop spectrophotometer and a Qubit fluorometer (Thermo Scientific, Wilmington, DE). Genotyping occurred in two batches: the first batch (n = 226 included in this study) using the Illumina Infinium Omni2.5–8 (version 1.1) BeadChip, and the second batch (n = 130 in this study) using the Illumina Multi-Ethnic Global Array (version 1.0). Quality control processes and filtering were performed using PLINK (version 1.9) (https://www.cog-genomics.org/plink/1.9), as previously described in detail (19,20). Briefly, individuals with >5% missing genotypes and SNPs with >2% missing genotypes were excluded. Genetic data were imputed to the 1000 Genomes Phase 3 (version 5) multiethnic reference panel. Principal components (PCs) for global ancestry, genotyping batch effects, and any residual relatedness were calculated using SNPs that were directly genotyped and passed quality control on both BeadChips.
SNP Selection
The first GWAS of youth-onset T2D was recently published and identified seven genome-wide significant SNPs (7). Given the vastly larger sample sizes of available GWAS in adult populations, we chose to focus on the substantially larger set of established T2D-associated SNPs from studies of adults. Among the 318 genetic SNPs associated with T2D in a recent multiethnic GWAS in adults, we used the subset of 272 previously established, validated SNPs (6). This set of SNPs includes six of the seven variants associated with youth-onset T2D either directly or through close proxy (7). All of the SNPs had adequate imputation quality with R2 > 0.3, except rs3816605, which was excluded. We calculated a GRS as the weighted sum of the number of risk alleles at each of the 271 loci, with weights based on the previously reported transethnic effect sizes for association with T2D (6). We also calculated an unweighted GRS as the sum of the number of risk alleles at each of the 271 risk loci. The GRS were standardized to have a mean of zero and SD of one, so each unit change in the GRS corresponds to one SD.
Statistical Methods
We used linear regression to model the glycemic and insulin metabolism traits at the second visit as a function of the weighted GRS, controlling for age, sex, BMI z score, in utero exposure to maternal diabetes, and the first five genetic PCs (see above). The outcomes were log transformed to meet assumptions of normality. In order to evaluate the potential effect of pubertal development, we performed a sensitivity analysis including Tanner stage as an additional covariate; this was inclu-ded as a linear term, because all individuals were pubertal (Tanner stage ≥2).
We assessed if BMI z score modified the association between the weighted GRS and each outcome by evaluating the significance of the interaction term between BMI z score and the GRS. Because we previously observed that Hispanic ethnicity modified the effects of a GRS for hepatic fat in this cohort (20), we likewise evaluated the significance of an interaction term between ethnicity and the GRS among the subset (n = 307) of non-Hispanic White (n = 195) and Hispanic White participants (n = 112). This cohort has increased prevalence of in utero exposure to maternal diabetes based on the EPOCH study design; therefore, we also evaluated the significance of an interaction term between the GRS and diabetes exposure status.
Weighted GRS can have variable accuracy across racial/ethnic groups, with lower accuracy among non-White individuals as a result of less representation in genetic cohorts (21); furthermore, the effect estimates for the weighted GRS reflect risk of T2D in adults and may not accurately represent the risk of traits related to glucose-insulin homeostasis among youth. Therefore, we repeated all of the above regression models using the unweighted GRS as the exposure of interest.
In order to evaluate which SNPs might be driving associations between the GRS and outcomes, and to assess the consistency of prior associations between individuals SNPs and outcomes, we additionally examined the individual main effects linear regressions for each SNP used in the GRS, assuming a genetic additive model and including the same covariates (age, sex, BMI z score, in utero exposure to maternal diabetes, and the first five genetic PCs). We used similar models to examine the seven SNPs recently associated with youth-onset T2D (7).
We used a statistical significance threshold of α = 0.05. We considered P values <0.05 as significant for the GRS analyses. We applied the Benjamini-Hochberg false discovery rate to correct for multiple testing in the regressions of individual T2D SNPs as predictors of the outcomes (22). All analyses were performed using R software (version 3.6.1) (23).
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
The characteristics of the study cohort are listed in Table 1. The population includes predominantly White individuals (86.2%), and one-third of the cohort is Hispanic White. Compared with children in the U.S. as a whole, this cohort has somewhat lower prevalence of overweight (12.4% vs. 16.1%), obesity (15.4% vs. 19.3%), and prediabetes (7.2% vs. 18.0%), despite the higher prevalence of gestational exposure to maternal diabetes (18.3% vs. ∼10%), because the EPOCH population was overselected for exposed youth (24–26).
Table 1.
Demographic and clinical characteristics of EPOCH participants at second study visit
| Characteristic | Overall |
|---|---|
| n | 356 |
| Age, years | 16.9 (15.9, 17.6) |
| Male sex | 178 (50.0) |
| Race | |
| White | 307 (86.2) |
| Black/African American | 29 (8.1) |
| Other | 20 (5.6) |
| Hispanic ethnicity | 120 (33.7) |
| Hispanic White | 112 (93.3) |
| Hispanic Black/African American | 5 (4.2) |
| Hispanic other | 3 (2.5) |
| Intrauterine exposure to maternal diabetes | 65 (18.3) |
| BMI, kg/m2 | 22.0 (19.8, 25.2) |
| BMI category | |
| Normal weight | 257 (72.2) |
| Overweight | 44 (12.4) |
| Obese | 55 (15.4) |
| BMI z score | 0.3 (−0.4, 1.2) |
| Weighted T2D GRS | 0.0 (−0.6, 0.7) |
| Unweighted T2D GRS | 0.0 (−0.7, 0.7) |
| Fasting glucose, mmol/L | 4.9 (4.7, 5.2) |
| 2-h glucose, mmol/L | 5.0 (4.3, 5.8) |
| HOMA-IR | 3.0 (2.3, 4.2) |
| Oral disposition index | 2.8 (1.9, 4.8) |
| Insulinogenic index | 5.8 (3.5, 9.8) |
| Prediabetes | 24 (7.2) |
| Impaired fasting glucose (≥5.6 mmol/L) | 18 (5.1) |
| Impaired glucose tolerance (≥7.8 mmol/L) | 9 (2.7) |
Values are presented as median (interquartile range) or n (%).
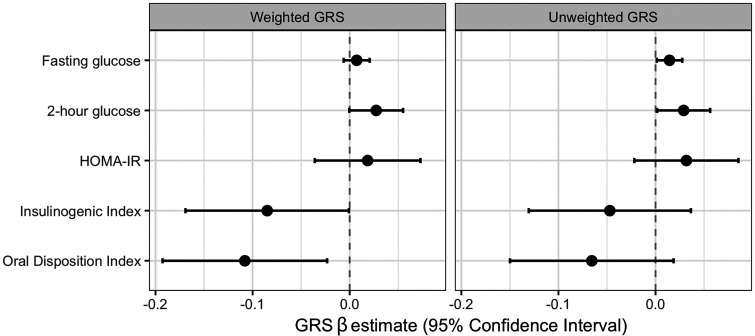
Higher weighted T2D GRS was associated with lower insulinogenic index (β = −0.08; 95% CI −0.17, −0.001; P = 0.048) (Fig. 2) and oral disposition index (β = −0.11; 95% CI −0.19, −0.02; P = 0.01), but not with fasting glucose (β = 0.01; 95% CI −0.01, 0.02; P = 0.29), 2-h glucose (β = 0.03; 95% CI −0.0004, 0.06; P = 0.053), or HOMA-IR (β = 0.02; 95% CI −0.04, 0.07; P = 0.51), when adjusting for age, sex, BMI z score, exposure to in utero maternal diabetes, and genetic PCs. The relationships with unweighted GRS showed similar patterns (Fig. 2), but different traits showed statistically significant associations. Higher unweighted T2D GRS was associated with higher fasting glucose (β = 0.01; 95% CI 0.001, 0.03; P = 0.03) and 2-h glucose (β = 0.03; 95% CI 0.002, 0.06; P = 0.04), but not with HOMA-IR (β =0.03; 95% CI −0.02, 0.09; P = 0.24), insulinogenic index (β = −0.07; 95% CI −0.15, 0.02; P = 0.13), or oral disposition index (β = −0.05; 95% CI −0.13, 0.04; P = 0.27). These results were consistent when controlling for Tanner stage as a measure of pubertal status (Supple-mentary Table 1). In the single-variant analyses of the T2D SNPs, none showed significant associations with any of the traits after accounting for multiple comparisons (Supplementary Table 2); ∼3% of the models showed nominally significant associations with the traits in the expected direction. The SNPs associated specifically with youth-onset T2D in a recently published GWAS (7) showed no significant associations with these traits (Supplementary Table 3).
Figure 2.
The association between standardized T2D GRS and traits related to glucose-insulin homeostasis among youth in the EPOCH study. These plots show the β estimates and CIs from linear regression models in the cohort of weighted (left) and unweighted (right) T2D GRS as predictors of five log-transformed traits: fasting glucose, 2-h glucose, HOMA-IR, insulinogenic index, and oral disposition index. Models controlled for age, sex, BMI z score, exposure to in utero maternal diabetes, and five genetic PCs. β estimates are reported per SD increase in GRS.
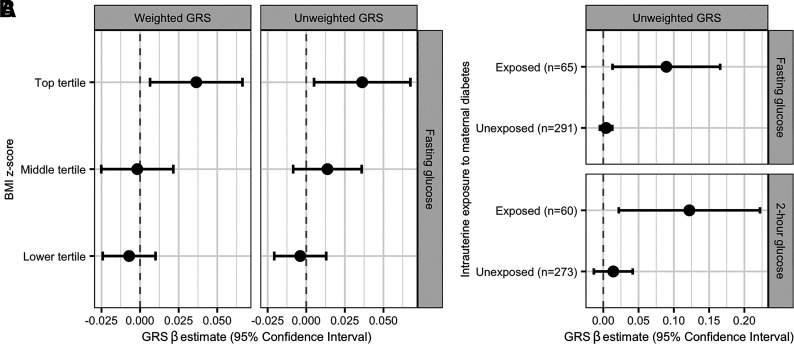
The associations between the weighted and unweighted GRS and fasting glucose were modified by BMI z score (Pinteraction = 0.01 and 0.03, respectively) such that higher GRS was more strongly associated with higher fasting glucose among individuals with higher BMI z scores. Regression results stratified by tertile of BMI z score are shown in Fig. 3A, adjusting for age, sex, BMI z score, exposure to in utero maternal diabetes, and genetic PCs. The significant associations between the unweighted GRS and both fasting glucose and 2-h glucose were dependent on in utero exposure to maternal diabetes (Pinteraction < 0.01), with higher GRS associated with higher glucose levels only among exposed youth (Fig. 3B), controlling for age, sex, BMI z score, and genetic PCs. We did not observe evidence that Hispanic ethnicity modified the association between the GRS and any of the examined traits (all Pinteraction> 0.05).
Figure 3.
Interactions with T2D GRS in relation to glucose levels. A: This plot shows the β estimates and CIs from linear regression models of log-transformed fasting glucose as a function of a weighted (left) and unweighted (right) T2D GRS stratified by tertile of BMI z score. Models controlled for age, sex, exposure to in utero maternal diabetes, and five genetic PCs. β estimates are reported per SD increase in GRS. B: This plot shows the β estimates and CIs from linear regression models of log-transformed fasting glucose and 2-h glucose as a function of an unweighted T2D GRS among youth exposed and unexposed in utero to maternal diabetes. Models controlled for age, sex, BMI z score, and five genetic PCs. β estimates are reported per SD increase in GRS.
Conclusions
In this study, we applied GRS based on SNPs associated with T2D in adults to a cohort of youth. We observed associations between the T2D GRS and several glycemic and insulin metabolism traits, including fasting glucose, 2-h glucose, oral disposition index, and insulinogenic index. We also observed an interaction between BMI z score and the GRS such that the GRS was more strongly associated with higher fasting glucose at higher BMI z scores. Similarly, the unweighted GRS was more strongly associated with fasting glucose and 2-h glucose among children who had been exposed to maternal diabetes during gestation. Our results confirm that GRS based on T2D risk variants established in adults are also related to some glycemic and insulin metabolism traits among youth and that BMI and gestational exposure to maternal diabetes may influence these associations.
Most of the prior genetic work related to pediatric diabetes has focused on individual genetic risk variants (4), but there have been some studies of GRS for T2D-related traits in young populations. A study using the Danish Childhood Obesity Biobank found that a GRS of 53 insulin resistance SNPs identified in adults was associated among children/adolescents with overweight/obesity with numerous traits, including higher HOMA-IR, fasting glucose, and systolic blood pressure and lower HDL cholesterol, total fat mass, and leg fat mass (27). The inverse associations between this insulin resistance GRS and total and leg fat mass were likewise seen in adults (28), as was an inverse association with BMI. On the basis of these results, we conducted a post hoc analysis in our cohort of the relationship between BMI and the T2D GRS, and there was no evidence of association (P = 0.80). We also observed no associations between the T2D GRS and HOMA-IR. While HOMA-IR is a valid measure of insulin resistance among adolescents (29,30), the validity has been shown to vary with sex and pubertal status, with lower correlation among females than males and higher correlation among pubertal than pre- or postpubertal adolescents (31). Thus, it is possible than the association between the T2D GRS and HOMA-IR would be stronger among subsets of our cohort. It is also possible that a GRS specific for insulin resistance would yield different results. T2D is a heterogeneous condition with heterogeneous pathophysiology, and prior research supports that GRS representing different components of the T2D pathophysiology, such as a primary β-cell function versus a primary insulin resistance abnormality, may be associated with different phenotypic presentations (32). Further work in this area is warranted, particularly among youth.
We observed interactions between the T2D GRS and both BMI z score and in utero exposure to maternal diabetes, such that the GRS was more strongly associated with glucose levels in the presence of obesity and among children who had been exposed to maternal diabetes. The interaction with BMI contrasts with prior findings in adults, which show the opposite direction of effect modification, with greater relative risk of incident T2D associated with a T2D GRS among leaner individuals, even though the absolute risk of T2D among individuals with obesity is far greater than that among lean individuals, at any level of genetic risk (33). Among older individuals, individual risk factors, such as genetics or obesity, would have a longer time span during which to lead to metabolic impairments and T2D, whereas the presence of multiple risk factors may be a key component for the development of metabolic impairments among younger individuals.
A recent study using the U.K. EarlyBird cohort examined the association between several candidate loci and the course of metabolic traits in healthy children 5–16 years of age and assessed interactions between SNPs and numerous hormones (9). While we used several of the same SNPs (or proxies) in calculating our GRS, most were not associated with the examined traits in our single-variant analyses. One exception was rs1111875 in the HHEX-IDE gene region, which was associated in the EarlyBird study with trajectories of fasting glucose and HOMA-B and showed strong associations with numerous pubertal hormones (9). In our study, this SNP was inversely associated with oral disposition index, although the association was only nominally significant (P = 0.01) before adjustment for multiple testing. This SNP was also nominally associated with lower 2-h glucose levels (P = 0.01). The EarlyBird study also examined phenotype-specific GRS and found associations with trajectories over time of fasting glucose, fasting insulin, and HOMA-B (9). We did not examine hormones or their interactions with genetic risk factors in our study, but this is a potential area for further research, especially because the interactions that we observed between the GRS and BMI z score with regard to fasting glycemia could be mediated through hormones (34).
In prior work in this cohort, we observed that genetic associations with hepatic fat varied significantly with ethnicity, with stronger associations among Hispanic than non-Hispanic White individuals (20). Like nonalcoholic fatty liver disease, youth-onset T2D is also more common among Hispanic than non-Hispanic White individuals (4,5). Furthermore, GRS tend to have variable accuracy across populations with diverse genetic ancestry because of differences in genetic architecture and prevalence of genetic risk factors, often with lower accuracy among populations of non-European ancestry resulting from their lack of adequate representation in many of the genetic cohorts used for GWAS (21). Therefore, we examined whether the GRS relationships observed in the overall cohort differed among non-Hispanic White versus Hispanic participants in the EPOCH cohort, but we did not observe evidence of significant effect modification by ethnicity.
While we observed numerous associations of clinical interest in this study, there are notable limitations. The cohort is relatively small and more metabolically healthy than youth in the U.S. generally and those with youth-onset T2D specifically (5,35), which limits our power to detect associations with glycemic and insulin metabolism traits, particularly for analyses of the individual T2D-associated SNPs. While this cohort is multiethnic, most participants are Hispanic or non-Hispanic White; an important area for future work is to examine other racial/ethnic groups, particularly Native American and Black individuals, who are disproportionately affected by youth-onset T2D (36). We used the largest transethnic T2D GWAS to date to construct the GRS (6). Most of these SNPs are from studies of T2D in adult populations; however, six of the seven SNPs associated with youth-onset T2D (7) were included either directly in our GRS or through close proxies. We chose to examine T2D GRS, because they have been previously associated with glucose and insulin traits in adults (37); however, it is possible that trait-specific GRS would show stronger associations. Numerous environmental factors, such as diet, physical activity, and socioeconomic status, may also interact with genetic risk factors and contribute to dysglycemia (38), but we limited our analyses of genetic interactions because of the small sample size and data availability.
In summary, we have demonstrated that GRS based on SNPs associated with T2D in adults also show some associations with glycemic and insulin metabolism traits in a multiethnic population of youth. We also report interactions of the T2D GRS with BMI z score and in utero exposure to maternal diabetes in relation to glucose levels. These findings help to elucidate the genetic etiology of T2D in youth.
Article Information
Acknowledgments. The authors thank the study team staff and participants in the EPOCH study.
Funding. This work was funded by National Institute of Diabetes, Digestive and Kidney Disease (NIDDK), National Institutes of Health (NIH), grant R01-DK068001. S.R. is supported by award P30DK116073 from the NIDDK, NIH, and by the Boettcher Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.S., S.R., E.M.L., D.D., and L.A.L. contributed to the study design. M.A.S., E.L., and J.S. analyzed the data. M.A.S. wrote the manuscript. All authors reviewed/edited the manuscript and contributed to the discussions. M.A.S. and L.A.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14731452.
References
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bobo N, Evert A, Gallivan J, et al.; Diabetes in Children Adolescents Work Group of the National Diabetes Education Program . An update on type 2 diabetes in youth from the National Diabetes Education Program. Pediatrics 2004;114:259–263 [DOI] [PubMed] [Google Scholar]
- 3. Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Todd JN, Srinivasan S, Pollin TI. Advances in the genetics of youth-onset type 2 diabetes. Curr Diab Rep 2018;18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch JL, Barrientos-Pérez M, Hafez M, et al. Country-specific prevalence and incidence of youth-onset type 2 diabetes: a narrative literature review. Ann Nutr Metab 2020;76:289–296 [DOI] [PubMed] [Google Scholar]
- 6. Vujkovic M, Keaton JM, Lynch JA, et al.; HPAP Consortium; Regeneron Genetics Center; VA Million Veteran Program . Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 2020;52:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srinivasan S, Chen L, Todd J, et al. The first genome-wide association study for type 2 diabetes in youth: the Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 2021;70:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchsberger C, Flannick J, Teslovich TM, et al. The genetic architecture of type 2 diabetes. Nature 2016;536:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carayol J, Hosking J, Pinkney J, et al. Genetic susceptibility determines β-cell function and fasting glycemia trajectories throughout childhood: a 12-year cohort study (EarlyBird 76). Diabetes Care 2020;43:653–660 [DOI] [PubMed] [Google Scholar]
- 10. Miranda-Lora AL, Cruz M, Molina-Díaz M, Gutiérrez J, Flores-Huerta S, Klünder-Klünder M. Associations of common variants in the SLC16A11, TCF7L2, and ABCA1 genes with pediatric-onset type 2 diabetes and related glycemic traits in families: a case-control and case-parent trio study. Pediatr Diabetes 2017;18:824–831 [DOI] [PubMed] [Google Scholar]
- 11. Crume TL, Scherzinger A, Stamm E, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 2014;22:608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genuth S, Alberti KGMM, Bennett P, et al.; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . SAS program for CDC growth charts (ages 0 to <20 years). Accessed 6 January 2017. Available from https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 15. Centers for Disease Control and Prevention . Defining childhood obesity: BMI for children and teens. Accessed 14 March 2017. Available from https://www.cdc.gov/obesity/childhood/defining.html
- 16. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey (NHANES): anthropometry procedures manual. Accessed 16 April 2021. Available from https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf
- 17. Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med 1968;19:283–300 [DOI] [PubMed] [Google Scholar]
- 18. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia 2011;54:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stanislawski MA, Shaw J, Litkowski E, et al. Genetic risk for hepatic fat among an ethnically diverse cohort of youth: the Exploring Perinatal Outcomes among Children study. J Pediatr 2020;220:146–153.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 23. R Core Team . R: The R Project for Statistical Computing. Accessed 30 November 2018. Available from https://www.r-project.org/
- 24. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr 2020;174:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fryar C, Carroll M, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. Accessed 20 April 2021. Available from https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
- 26. Centers for Disease Control and Prevention . Diabetes during pregnancy. Accessed 20 April 2021. Available from https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy.htm
- 27. Graae A-S, Hollensted M, Kloppenborg JT, et al. An adult-based insulin resistance genetic risk score associates with insulin resistance, metabolic traits and altered fat distribution in Danish children and adolescents who are overweight or obese. Diabetologia 2018;61:1769–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lotta LA, Gulati P, Day FR, et al.; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium . Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 2004;27:314–319 [DOI] [PubMed] [Google Scholar]
- 30. da Silva CC, Zambon MP, Vasques ACJ, et al.; Brazilian Metabolic Syndrome Study (BRAMS) Investigators . Homeostatic model assessment of adiponectin (HOMA-Adiponectin) as a surrogate measure of insulin resistance in adolescents: comparison with the hyperglycaemic clamp and homeostatic model assessment of insulin resistance. PLoS One 2019;14:e0214081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rössner SM, Neovius M, Montgomery SM, Marcus C, Norgren S. Alternative methods of insulin sensitivity assessment in obese children and adolescents. Diabetes Care 2008;31:802–804 [DOI] [PubMed] [Google Scholar]
- 32. Udler MS, Kim J, von Grotthuss M, et al.; Christopher D. Anderson on behalf of META-STROKE and the ISGC . Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med 2018;15:e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langenberg C, Sharp SJ, Franks PW, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 2014;11:e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nokoff N, Thurston J, Hilkin A, et al. Sex differences in effects of obesity on reproductive hormones and glucose metabolism in early puberty. J Clin Endocrinol Metab 2019;104:4390–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stančáková A, Kuulasmaa T, Kuusisto J, et al. Genetic risk scores in the prediction of plasma glucose, impaired insulin secretion, insulin resistance and incident type 2 diabetes in the METSIM study. Diabetologia 2017;60:1722–1730 [DOI] [PubMed] [Google Scholar]
- 38. Arya R, Farook VS, Fowler SP, et al. Genetic and environmental (physical fitness and sedentary activity) interaction effects on cardiometabolic risk factors in Mexican American children and adolescents. Genet Epidemiol 2018;42:378–393 [DOI] [PMC free article] [PubMed] [Google Scholar]