Abstract
OBJECTIVE
To compare effects of medications and laparoscopic gastric band surgery (LB) on α-cell function in dysglycemic youth and adults in the Restoring Insulin Secretion (RISE) Study protocols.
RESEARCH DESIGN AND METHODS
Glucagon was measured in three randomized, parallel, clinical studies: 1) 91 youth studied at baseline, after 12 months on metformin alone (MET) or glargine followed by metformin (G/M), and 3 months after treatment withdrawal; 2) 267 adults studied at the same time points and treated with MET, G/M, or liraglutide plus metformin (L+M) or given placebo (PLAC); and 3) 88 adults studied at baseline and after 12 and 24 months of LB or MET. Fasting glucagon, glucagon suppression by glucose, and acute glucagon response (AGR) to arginine were assessed during hyperglycemic clamps. Glucagon suppression was also measured during oral glucose tolerance tests (OGTTs).
RESULTS
No change in fasting glucagon, steady-state glucagon, or AGR was seen at 12 months following treatment with MET or G/M (in youth and adults) or PLAC (in adults). In contrast, L+M reduced these measures at 12 months (all P ≤ 0.005), which was maintained 3 months after treatment withdrawal (all P < 0.01). LB in adults also reduced fasting glucagon, steady-state glucagon, and AGR at 12 and 24 months (P < 0.05 for all, except AGR at 12 months [P = 0.098]). Similarly, glucagon suppression during OGTTs was greater with L+M and LB. Linear models demonstrated that treatment effects on glucagon with L+M and LB were largely associated with weight loss.
CONCLUSIONS
Glucagon concentrations were reduced by L+M and LB in adults with dysglycemia, an effect principally attributable to weight loss in both interventions.
Introduction
Glycemic control is critically dependent on both β- and α-cells, given the importance of insulin and glucagon in regulating metabolism (1,2). These cells interact in a paracrine manner, with their respective products modulating each other’s function (3–6). There is currently expanded interest in the potential beneficial effects of interventions on α-cell function, as modulating glucagon release could contribute to better glycemic control.
In the Restoring Insulin Secretion (RISE) study, medical and surgical interventions were used in adults and youth with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes to determine whether it was possible to durably improve β-cell function. Aside from impacting the β-cell, the choice of interventions was also built around the potential for effects on weight and α-cell function, which could also contribute to alleviating the workload on the β-cell. We previously reported that in adults, 12 months of the medication interventions metformin alone (MET), glargine followed by metformin (G/M), and liraglutide plus metformin (L+M) produced weight loss and improvements in glucose-stimulated β-cell responses, which reverted to baseline following treatment withdrawal (7). In contrast, in youth neither MET nor G/M slowed the progressive loss of β-cell function, which proceeded at a rate that well exceeded that observed in adults using the same two regimens (8). Finally, laparoscopic gastric band surgery (LB) in adults reduced body weight and had beneficial effects on β-cell function (9).
These observations in the three RISE protocols prompted us to address three hypotheses related to α-cell effects. First, as β-cell products regulate the α-cell, and in youth β-cell function deteriorated despite intervention, we hypothesized that the progressive β-cell dysfunction that we observed would be accompanied by a parallel worsening of α-cell function. Second, since GLP-1 suppresses glucagon release in vitro (10) and GLP-1 receptor agonists reduce body weight (10), we hypothesized that L+M in adults would improve α-cell function beyond any potential effect of weight loss. Third, as weight loss improves glucose metabolism (11,12), we hypothesized that weight reduction with LB would improve α-cell function.
Research Design and Methods
Study Protocols
The three RISE study protocols (Pediatric Medication, Adult Medication, and Adult Surgery [BetaFat]) were randomized, partially blinded clinical trials. The rationale, methods, and primary outcomes have previously been described in detail (13–15), and study protocols are available from https://rise.bsc.gwu.edu/web/rise/collaborators. The institutional review board at each center approved the protocol. Written informed assent and/or consent was obtained from each participant, consistent with the Declaration of Helsinki and each center’s institutional review board guidelines.
Details of participants, interventions, procedures, and assays along with portions of the β-cell response data have previously been published (7–9,13–15). The following description provides a brief summary of these.
Participants
Pediatric Medication Study
Eligible youth were 10–19 years of age, at Tanner stage ≥II in pubertal development, had a BMI ≥85th percentile for age and sex but <50 kg/m2, and had IGT or type 2 diabetes on an oral glucose tolerance test (OGTT). Those with diabetes who were drug naive were eligible if HbA1c was ≤64 mmol/mol (≤8%). If known to have diabetes and taking metformin, they were eligible if HbA1c was ≤58 mmol/mol (≤7.5%) if on metformin for <3 months and ≤53 mmol/mol (≤7.0%) if on metformin for 3–6 months.
Adult Medication Study and Adult Surgery Study
Adults were eligible if they were 20–65 years of age and had IGT or physician-diagnosed type 2 diabetes (drug naive) for <12 months with HbA1c ≤53 mmol/mol (7.0%). For the Adult Medication Study, participants had a BMI ≥25 and <50 kg/m2 (≥23 kg/m2 for Asian Americans), while for the Adult Surgery Study BMI was 30–40 kg/m2 despite at least 2 months on a diet, exercise, and lifestyle modification program.
Interventions and Timing of Measurements
The interventions used in the three protocols and timing of hyperglycemic clamp and OGTT measurements are summarized below, have previously been described in detail (7–9,13), and are illustrated in Supplementary Fig. 1.
Pediatric Medication Study
Youth were randomized to receive either MET for 12 months or glargine for 3 months followed by metformin for 9 months (G/M). After 12 months of active intervention, treatment was withdrawn. Measurements were made at baseline, at the end of 12 months of intervention and 3 months after treatment withdrawal.
Adult Medication Study
Adults were randomized to one of four interventions. The MET and G/M arms were identical to those in youth. The third intervention was L+M for 12 months (L+M). The fourth group received placebo tablets for 12 months (PLAC). As in youth, treatment was withdrawn for 3 months after 12 months of intervention, with measurements made at the same time points.
Adult Surgery Study
Adults were randomized to MET or LB for 24 months. For ethical reasons, the lap band could not be removed; thus, the interventions in this protocol continued for the full 24 months with measurements made at baseline, 12, and 24 months. This approach allowed for an evaluation of the sustained effect of LB—in contrast to stopping the interventions at 12 months in the medication protocols.
Procedures and Calculations
Anthropometric Measurements
Height and weight, measured with participants wearing light clothing without shoes, were used to calculate BMI.
Hyperglycemic Clamps and OGTTs
A two-step hyperglycemic clamp (∼11.1 mmol/L [200 mg/dL] and >25 mmol/L [450 mg/dL]) was performed to quantify α- and β-cell responses (14). The second step included a bolus injection of arginine to acutely stimulate both glucagon and C-peptide release.
Following a 10-h overnight fast, a 75-g, 3-h OGTT was performed as previously described (15). Blood samples were obtained −10, −5, 10, 20, 30, 60, 90, 120, 150, and 180 min relative to glucose ingestion.
If the participant was on liraglutide, the last dose of the medication was taken on the morning of the month 12 visit. If they were taking metformin or placebo, the last dose was taken the night before the month 12 or 24 visit.
Calculations
Insulin sensitivity (M/I) was calculated as the mean of the glucose infusion rate (M) at 100, 110, and 120 min of the hyperglycemic clamp, corrected for urinary glucose loss, divided by the mean steady-state plasma insulin concentration at these same time points (I). This measure was expressed per kilogram body weight (14).
The two fasting samples drawn prior to glucose administration were used to calculate the average of the fasting peptide concentrations. Steady-state glucagon and C-peptide concentrations (at glucose ∼11.1 mmol/L) were calculated as the mean of their respective measurements at 100, 110, and 120 min. The acute glucagon response (AGR) and C-peptide response (ACPRmax) to arginine at maximal glycemic potentiation (>25 mmol/L) were calculated as the mean increment in peptide concentrations from samples drawn 2, 3, 4, and 5 min after arginine injection minus the average concentration of the samples drawn 1 and 5 min prior to arginine (16).
The trapezoidal method was used to calculate responses during the OGTT as areas under the curve (AUC) above/below the fasting concentration. For glucose and C-peptide these were increments (iAUC) and for glucagon a decrement (dAUC).
Assays
Blood samples were immediately placed on ice, separated by centrifugation, and frozen at −80°C prior to shipment to the central laboratory at the University of Washington. Samples for glucagon were drawn into tubes containing EDTA to which was added nine parts of a protease inhibitor (P2714; Sigma-Aldrich) to one part (v/v) of a DPP-4 inhibitor (DPP4-010; Millipore). Samples for all other analytes were drawn in tubes containing EDTA.
Plasma glucose, C-peptide, and insulin were measured as previously described (14). Plasma glucagon was measured with the Mercodia Glucagon ELISA (cat. no. 10-1271-01, lot no. 28689; Winston-Salem, NC) using a sequential protocol that includes an extra washing step prior to incubation with the detection antibody. This approach reduces cross-reactivity by glicentin and oxyntomodulin (17,18). Performance characteristics of these assays in our hands have previously been published in detail (14,19). Glucagon values ≤1.0 pmol/L (lower limit of quantification) were assigned a value of 0.5 pmol/L for data analysis.
Data Management and Statistical Analyses
The SAS analysis system (SAS Institute, Cary, NC) and R (The R Foundation) were used for all statistical analyses. All analyses were completed within each of the three studies except where described. Descriptive statistics at baseline are presented as percentages, mean ± SD, and geometric means and 95% CIs for non–normally distributed data; for the latter, P values from the log-transformed data are presented. Comparisons between treatment groups were computed with ANOVA, χ2 tests, or Student t tests. Paired t tests were used to assess within-treatment changes from baseline to follow-up visits. Linear regression models adjusted for baseline were used to evaluate change from baseline in glucagon measures across treatment arms within each study. As three of the interventions (metformin, liraglutide, and LB) are known to reduce body weight, we adjusted for baseline weight and change in weight during the 12 months of the intervention to determine whether any differential effect on glucagon across the intervention arms was due to weight change. In youth we also adjusted for BMI, as some of their weight change may have been due to their development. In addition, we adjusted for insulin sensitivity in separate models using M/I or fasting insulin—the latter as an alternate measure of insulin sensitivity (20).
Except where noted, P values <0.05 were considered nominally statistically significant, with no adjustments made for multiple tests.
Results
Demographic, Physical, and Glucose Tolerance Characteristics at Baseline by Study and Intervention Group
Baseline characteristics of participants in the three study protocols are listed in Table 1. Aside from age in the youth, the randomized allocation of participants to the different intervention arms resulted in no important differences in sex, race/ethnicity, weight, BMI, and glycemic characteristics among intervention groups within each study protocol. The proportion of participants with IGT was greater among adults in the medication protocol than in the youth medication and adult surgery protocols. Despite these differences, glycemic and obesity measures were similar in the two age-groups.
Table 1.
Select baseline physical and demographic characteristics for youth and adults by treatment group in the three protocols
| Youth | Adult medication | Adult surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| G/M (n = 44) | MET (n = 47) | G/M (n = 67) | L+M (n = 68) | MET (n = 65) | PLAC (n = 62) | LB (n = 44) | MET (n = 44) | |
| Demographic characteristics | ||||||||
| Age (years) | 14.9 ± 2.0 | 13.9 ± 2.1* | 53.5 ± 9.3 | 54.0 ± 8.1 | 55.2 ± 8.2 | 52.9 ± 9.9 | 47.5 ± 10.2 | 50.6 ± 9.2 |
| Female, n (%) | 27 (61.4) | 38 (80.9) | 23 (34.3) | 29 (42.6) | 37 (56.9) | 24 (38.7) | 34 (77.3) | 35 (79.5) |
| Race/ethnicity, n (%) | ||||||||
| White | 13 (29.5) | 12 (25.5) | 37 (55.2) | 40 (58.8) | 34 (52.3) | 27 (43.5) | 15 (34.1) | 10 (22.7) |
| Black | 14 (31.8) | 9 (19.1) | 21 (31.3) | 20 (29.4) | 19 (29.2) | 20 (32.3) | 7 (15.9) | 9 (20.5) |
| Hispanic | 14 (31.8) | 20 (42.6) | 5 (7.5) | 6 (8.8) | 6 (9.2) | 10 (16.1) | 19 (43.2) | 21 (47.7) |
| Other | 3 (6.8) | 6 (12.8) | 4 (6.0) | 2 (2.9) | 6 (9.2) | 5 (8.1) | 3 (6.8) | 4 (9.1) |
| Weight (kg) | 102.0 ± 25.7 | 97.7 ± 23.0 | 104.4 ± 20.0 | 104.2 ± 21.0 | 98.1 ± 18.6 | 101.3 ± 19.6 | 97.5 ± 12.1 | 96.1 ± 10.9 |
| BMI (kg/m2) | 36.5 ± 6.4 | 36.9 ± 6.4 | 35.0 ± 5.9 | 35.6 ± 5.8 | 35.0 ± 5.1 | 34.4 ± 6.1 | 35.7 ± 2.8 | 35.0 ± 2.9 |
| Glycemic characteristics | ||||||||
| IGT, n (%) | 26 (59.1) | 28 (59.6) | 50 (74.6) | 49 (72.1) | 49 (75.4) | 44 (71.0) | 26 (59.1) | 28 (63.6) |
| Fasting glucose (mmol/L) | 5.96 ± 0.78 | 6.06 ± 1.09 | 6.22 ± 0.74 | 6.11 ± 0.50 | 6.21 ± 0.67 | 6.10 ± 0.57 | 6.19 ± 0.75 | 6.10 ± 0.70 |
| HbA1c (mol/mmol) | 39.2 ± 6.5 | 38.6 ± 6.3 | 39.9 ± 3.6 | 38.6 ± 4.3 | 39.5 ± 4.3 | 39.3 ± 4.8 | 41.2 ± 4.6 | 40.1 ± 4.5 |
| HbA1c (%) | 5.73 ± 0.60 | 5.68 ± 0.57 | 5.80 ± 0.33 | 5.69 ± 0.39 | 5.77 ± 0.40 | 5.75 ± 0.44 | 5.92 ± 0.42 | 5.82 ± 0.41 |
Data are mean ± SD unless otherwise indicated. Other for race/ethnicity includes mixed, Asian, American Indian, and other.
P < 0.05 MET vs. G/M.
Effect of Medication Treatment and Withdrawal on Glucagon Concentrations During the Hyperglycemic Clamp and OGTT in the RISE Pediatric Medication Study
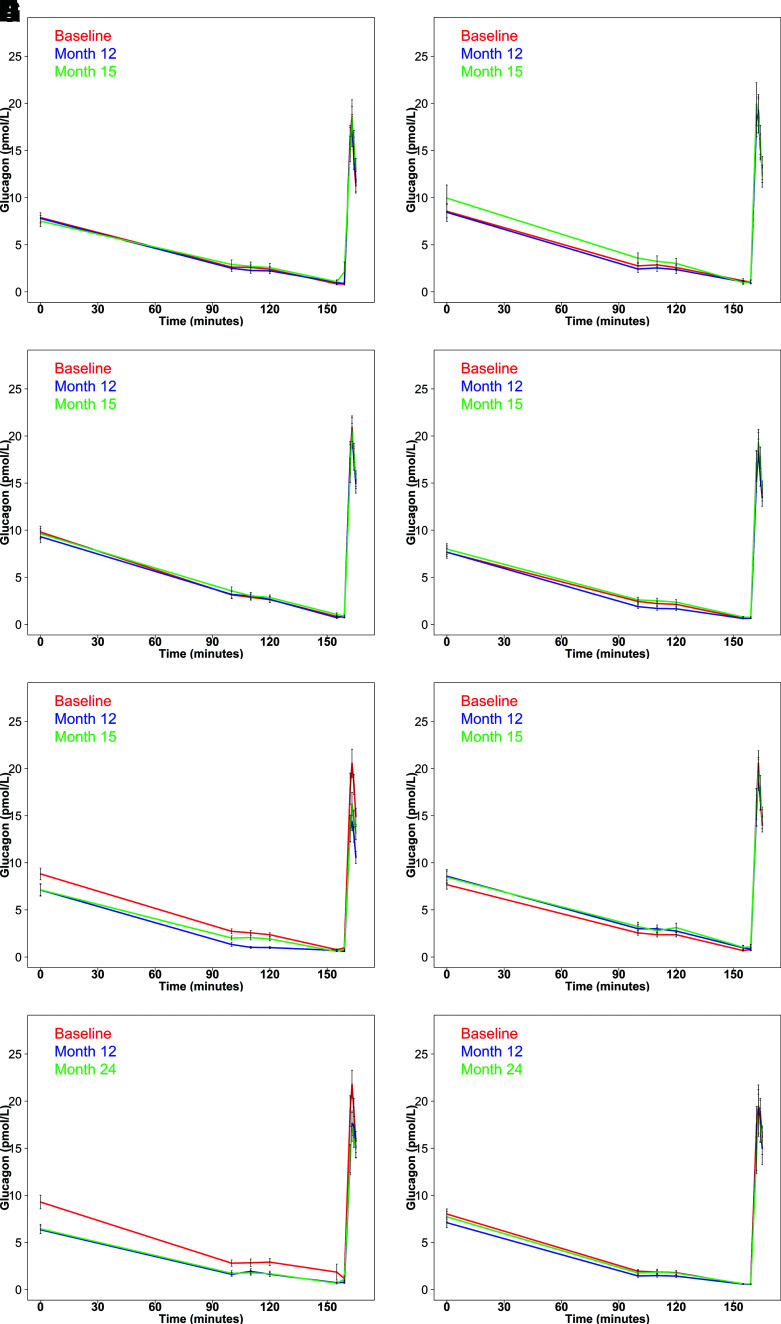
Figure 1A and B depict glucagon concentrations in youth during the hyperglycemic clamps performed at baseline, after 12 months on the intervention, and at 15 months, which was 3 months after withdrawal of the intervention. Glucose and C-peptide profiles at the same time points are illustrated in Supplementary Fig. 2. Glucagon concentrations decreased as glucose was increased to a steady state of ∼11.1 mmol/L between 100 and 120 min. There were no significant differences in fasting or glucose-suppressed glucagon concentrations across the two intervention arms at the three time points, with the exception of the MET arm, where the fasting (P = 0.047) and steady-state glucagon (P = 0.006) concentrations were higher and AGR (P = 0.039) was greater off treatment at 15 months than at 12 months on treatment (Table 2). The effects of the interventions on these glucagon measures are illustrated as box-and-whisker plots in Supplementary Fig. 3.
Figure 1.
Plasma glucagon concentrations during the hyperglycemic clamps in youth and adults in the three RISE protocols. Pediatric Medication Study: G/M (A) and MET (B) at baseline (red), after 12 months of intervention (blue), and after 3 months of intervention withdrawal (green). Adult Medication Study: G/M (C), MET (D), L+M (E), and PLAC (F) at baseline (red), after 12 months of intervention (blue), and after 3 months of intervention withdrawal (green). Adult Surgery Study: LB (G) and MET (H) at baseline (red) and after 12 months of intervention (blue) and 24 months of intervention (green). Data are displayed as means ± SEM.
Table 2.
Effect of medication interventions in youth on select metabolic characteristics from the hyperglycemic clamp and OGTT
| G/M | MET | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 44) | 12 months (n = 40) | 15 months (n = 41) | Baseline (n = 47) | 12 months (n = 39) | 15 months (n = 40) | ||
| Anthropometric parameters | |||||||
| Weight (kg) | 102.0 ± 25.7 | 106.0 ± 28.2@ | 107.8 ± 28.1*# | 97.7 ± 23.3 | 99.6 ± 23.7 | 99.6 ± 20.2*# | |
| BMI (kg/m2) | 36.5 ± 6.4 | 37.0 ± 7.3 | 37.7 ± 7.2*# | 36.9 ± 6.4 | 36.7 ± 7.0 | 37.1 ± 6.4# | |
| Hyperglycemic clamp parameters | |||||||
| Fasting glucose (mmol/L) | 5.97 ± 0.75 | 6.06 ± 1.69 | 6.48 ± 1.65*# | 6.11 ± 1.10 | 5.94 ± 1.36 | 6.10 ± 1.06# | |
| Fasting glucagon (pmol/L) | 7.88 ± 3.34 | 7.79 ± 3.69 | 7.51 ± 3.82 | 8.55 ± 4.84 | 8.44 ± 6.02 | 9.95 ± 8.79# | |
| Fasting C-peptide (nmol/L) | 1.54 (0.80, 2.97) | 1.22 (0.44, 3.41)@ | 1.34 (0.58, 3.07)* | 1.74 (0.96, 3.14) | 1.45 (0.68, 3.08)@ | 1.57 (0.90, 2.77)*# | |
| Fasting insulin (pmol/L) | 211.1 (54.8, 813.5) | 160.4 (45.9, 560.3)@ | 178.7 (44.1, 724.2)* | 247.4 (74.1, 825.4) | 195.9 (42.8, 895.9)@ | 228.0 (72.6, 716.0) | |
| Steady-state glucose (mmol/L) | 11.5 ± 0.6 | 11.6 ± 0.7 | 11.5 ± 0.6 | 11.6 ± 0.8 | 11.5 ± 0.6 | 11.5 ± 0.6 | |
| Steady-state glucagon (pmol/L) | 2.53 ± 1.67 | 2.33 ± 1.89 | 2.73 ± 2.85 | 2.72 ± 2.48 | 2.48 ± 2.35 | 3.27 ± 3.42# | |
| Steady-state C-peptide (nmol/L) | 5.22 (2.44, 11.15) | 4.37 (1.75, 10.94)@ | 4.18 (1.74, 10.08)* | 5.09 (2.32, 11.19) | 4.71 (2.02, 10.98)@ | 4.96 (2.33, 10.54)* | |
| AGR (pmol/L) | 14.3 ± 5.2 | 14.0 ± 7.9 | 13.9 ± 8.4 | 15.2 ± 7.9 | 15.4 ± 7.6 | 15.7 ± 9.5# | |
| ACPRmax (nmol/L) | 7.29 (3.29, 16.16) | 5.79 (2.49, 13.46)@ | 5.95 (2.17, 16.37)* | 8.12 (3.42, 19.26) | 6.97 (3.09, 15.73)@ | 7.16 (3.14, 16.31)* | |
| M/I (×10−5 mmol/kg/min per pmol/L) | 1.60 (0.35, 7.38) | 1.93 (0.30, 12.50) | 1.70 (0.25, 11.54) | 1.54 (0.35, 6.76) | 1.52 (0.16, 14.62) | 1.52 (0.29, 7.97) | |
| OGTT parameters | |||||||
| Fasting glucose (mmol/L) | 5.96 ± 0.78 | 5.92 ± 1.11 | 6.52 ± 1.87*# | 6.06 ± 1.09 | 5.98 ± 1.34 | 6.13 ± 1.13# | |
| Fasting glucagon (pmol/L) | 9.39 ± 3.91 | 8.41 ± 4.33 | 8.76 ± 5.84 | 9.52 ± 6.23 | 9.62 ± 5.11 | 10.82 ± 7.34# | |
| Fasting C-peptide (nmol/L) | 1.611 ± 0.662 | 1.372 ± 0.526@ | 1.445 ± 0.604* | 1.805 ± 0.541 | 1.595 ± 0.587@ | 1.740 ± 0.587# | |
| iAUC glucose (mmol/L) | 633.9 ± 233.9 | 577.5 ± 250.2@ | 579.8 ± 298.6 | 620.5 ± 248.5 | 679.3 ± 315.6 | 662.3 ± 312.3 | |
| dAUC glucagon (pmol/L) | −1021.9 ± 460.5 | −854.0 ± 546.5 | −821.0 ± 532.9 | −892.3 ± 603.4 | −957.2 ± 627.9 | −1117.3 ± 832.0 | |
| iAUC C-peptide (nmol/L) | 524.2 ± 178.4 | 451.8 ± 185.1@ | 422.0 ± 215.4* | 558.3 ± 273.4 | 545.0 ± 247.4 | 527.6 ± 250.7# | |
There were no differences between treatments at 12 months. Data are mean ± SD for normally distributed and geometric means (95% CIs) for non–normally distributed data; for the latter, P values from the log-transformed data are presented.
P < 0.05 12 months vs. baseline;
P < 0.05 15 months vs. baseline;
P < 0.05 15 months vs. 12 months.
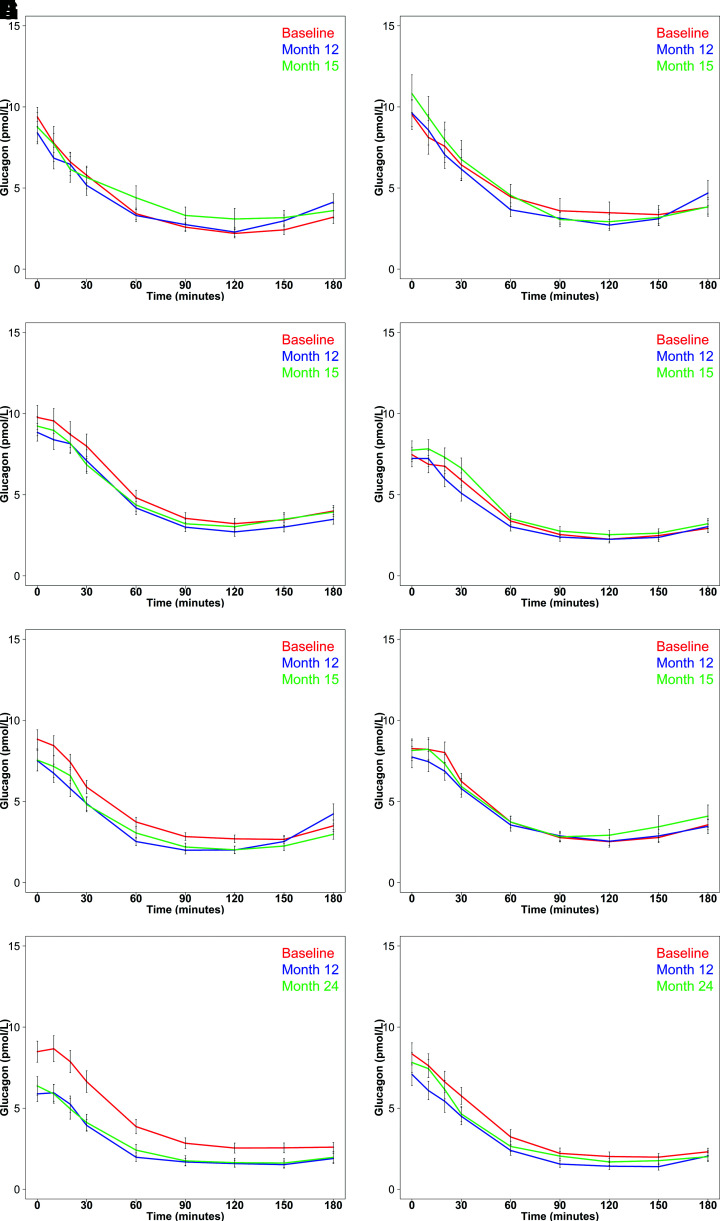
Glucagon concentrations during the OGTT in youth before, during, and after withdrawal of the medications are illustrated in Fig. 2A and B. At all three study time points, glucose ingestion resulted in reductions in glucagon concentrations, with no difference in the effect of MET or G/M. Thus, dAUC glucagon did not differ at the three time points during the study for either intervention (Table 2). While C-peptide increased with glucose ingestion (Supplementary Fig. 4), the iAUC C-peptide was lower at 12 and 15 months compared with baseline in the G/M group, as was that at 15 months compared with 12 months in the MET arm (Table 2).
Figure 2.
Plasma glucagon concentrations during the OGTTs in youth and adults in the three RISE protocols. Pediatric Medication Study: G/M (A) and MET (B) at baseline (red), after 12 months of intervention (blue), and after 3 months of intervention withdrawal (green). Adult Medication Study: G/M (C), MET (D), L+M (E), and PLAC (F) at baseline (red), after 12 months of intervention (blue), and after 3 months of intervention withdrawal (green). Adult Surgery Study: lap band surgery (G) and MET (H) at baseline (red) and after 12 months of intervention (blue) and 24 months of intervention (green). Data are displayed as mean ± SEM.
Effect of Medication Treatment and Withdrawal on Glucagon Concentrations During the Hyperglycemic Clamp and OGTT in the RISE Adult Medication Study
Glucagon concentrations in adults during the hyperglycemic clamp at each study time point for each medication intervention are illustrated in Fig. 1C–F, with data listed in Table 3. Profiles of glucose and C-peptide at the same time points are shown in Supplementary Fig. 5. In all four interventions, glucose suppressed glucagon concentrations. However, there were significant differences across the four medication interventions, due to larger effects with the combination of L+M (Fig. 1E). After 12 months of L+M, fasting glucagon had decreased by nearly 20% compared with baseline (P = 0.003), and this decrease persisted at 15 months (P = 0.001). This effect of L+M was also apparent in the nearly 60% lower (P < 0.001) steady-state glucagon concentrations at 12 months compared with baseline. While steady-state glucagon increased back toward pretreatment levels after withdrawal of L+M, it was still 22% lower at 15 months than baseline (P = 0.003). The pattern of change in AGR in the L+M arm mirrored that of steady-state glucagon: it was 28% lower at 12 months than baseline (P < 0.001), increasing at 15 months to be greater than at 12 months (P = 0.028), but still 19% lower than baseline (P = 0.004). The changes in these glucagon measures are illustrated as box plots in Supplementary Fig. 6 and were associated with concurrent lowering of fasting glucose at 12 months and fasting C-peptide at 12 and 15 months as well as an increase in steady-state C-peptide at 12 months (Supplementary Fig. 5).
Table 3.
Effect of medication interventions in adults on select metabolic characteristics from the hyperglycemic clamps and OGTTs
| G/M | L+M | MET | PLAC | P for comparisons between treatments at 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 67) | 12 months (n = 63) | 15 months (n = 64) | Baseline (n = 68) | 12 months (n = 54) | 15 months (n = 53) | Baseline (n = 65) | 12 months (n = 54) | 15 months (n = 55) | Baseline (n = 62) | 12 months (n = 54) | 15 months (n = 49) | ||
| Anthropometric parameters | |||||||||||||
| Weight (kg) | 104.4 ± 20.0 | 101.4 ± 20.7@ | 103.4 ± 21.5*# | 104.2 ± 21.0 | 97.4 ± 20.7@ | 101.2 ± 20.7*# | 98.1 ± 18.6 | 96.0 ± 19.4@ | 96.7 ± 19.9*# | 101.3 ± 19.6 | 99.2 ± 19.6 | 98.3 ± 20.3 | |
| BMI (kg/m2) | 35.0 ± 5.9 | 34.0 ± 6.1@ | 34.5 ± 6.2*# | 35.6 ± 5.8 | 33.6 ± 5.9@ | 34.9 ± 5.9*# | 35.0 ± 5.1 | 33.9 ± 5.7@ | 34.3 ± 5.8*# | 34.4 ± 6.1 | 33.9 ± 6.1 | 33.4 ± 5.8 | |
| Hyperglycemic clamp parameters | |||||||||||||
| Fasting glucose (mmol/L) | 6.08 ± 0.54 | 5.96 ± 0.55 | 6.31 ± 1.08# | 6.02 ± 0.54 | 5.44 ± 0.53@ | 6.06 ± 0.55# | 6.14 ± 0.60 | 5.92 ± 0.79@ | 6.30 ± 1.09# | 6.11 ± 0.55 | 6.16 ± 0.73 | 6.33 ± 0.82*# | 1, 5 |
| Fasting glucagon (pmol/L) | 9.80 ± 5.15 | 9.31 ± 5.13 | 9.63 ± 4.45 | 8.82 ± 5.11 | 7.12 ± 4.80@ | 7.13 ± 4.38* | 7.66 ± 3.51 | 7.67 ± 4.82 | 8.00 ± 4.19 | 7.68 ± 3.82 | 8.58 ± 5.12 | 8.47 ± 4.56 | |
| Fasting C-peptide (nmol/L) | 1.16 (0.55, 2.43) | 1.05 (0.47, 2.35)@ | 1.12 (0.52, 2.41) | 1.18 (0.63, 2.24) | 1.03 (0.50, 2.13)@ | 1.06 (0.50, 2.23)* | 1.13 (0.57, 2.27) | 1.02 (0.45, 2.28)@ | 1.08 (0.50, 2.34)# | 1.07 (0.50, 2.31) | 1.00 (0.49, 2.06) | 1.04 (0.52, 2.05) | |
| Fasting insulin (pmol/L) | 109.2 (40.2, 296.8) | 91.7 (27.7, 303.7)@ | 103.0 (26.6, 398.2) | 109.9 (41.9, 287.9) | 85.6 (27.6, 265.1)@ | 90.2 (30.3, 268.0)* | 102.9 (36.8, 287.8) | 86.7 (26.5, 284.1)@ | 94.1 (29.6, 299.5)# | 90.1 (28.2, 288.3) | 82.7 (28.3, 241.4) | 90.6 (30.5, 269.4)* | |
| Steady-state glucose (mmol/L) | 11.9 ± 0.7 | 11.8 ± 0.7 | 11.8 ± 0.8 | 11.9 ± 0.6 | 11.7 ± 0.6 | 11.7 ± 0.7 | 11.9 ± 0.6 | 11.8 ± 0.7 | 11.8 ± 0.7 | 11.9 ± 0.5 | 11.8 ± 0.7 | 11.8 ± 0.6 | |
| Steady-state glucagon (pmol/L) | 2.88 ± 2.41 | 2.94 ± 2.88 | 3.14 ± 2.41 | 2.56 ± 1.89 | 1.11 ± 0.94@ | 2.00 ± 1.58*# | 2.26 ± 1.65 | 1.75 ± 1.40@ | 2.49 ± 1.99# | 2.43 ± 1.82 | 2.91 ± 2.94 | 3.15 ± 3.16# | 1, 2, 4, 5 |
| Steady-state C-peptide (nmol/L) | 4.01 (1.91, 8.42) | 3.85 (1.85, 8.02) | 3.58 (1.54, 8.33)*# | 4.06 (2.09, 7.89) | 7.04 (3.09, 16.08)@ | 3.73 (1.87, 7.44)*# | 3.86 (1.98, 7.55) | 3.86 (1.87, 8.00) | 3.65 (1.94, 6.87)* | 3.80 (1.82, 7.95) | 3.56 (1.67, 7.59)@ | 3.49 (1.68, 7.24)* | 4, 5 |
| AGR (pmol/L) | 17.1 ± 8.3 | 16.7 ± 8.9 | 16.6 ± 7.7 | 17.0 ± 9.7 | 12.2 ± 6.0@ | 13.7 ± 7.1*# | 16.0 ± 8.4 | 15.0 ± 7.3 | 16.3 ± 8.3 | 16.2 ± 8.5 | 16.0 ± 8.7 | 16.0 ± 9.7 | |
| ACPRmax (nmol/L) | 4.78 (1.83, 12.52) | 4.68 (1.91, 11.44) | 4.32 (1.58, 11.80)* | 4.93 (2.27, 10.73) | 3.38 (1.18, 9.67)@ | 4.58 (2.21, 9.48)# | 4.83 (1.98, 11.74) | 4.48 (1.94, 10.37) | 4.61 (2.00, 10.66) | 4.90 (2.01, 11.97) | 4.46 (1.67, 11.93)@ | 4.42 (1.54, 12.69)* | 4 |
| M/I (×10−5 mmol/kg/min per pmol/L) | 2.81 (0.65, 12.14) | 3.07 (0.61, 15.52) | 3.38 (0.61, 18.84) | 2.92 (0.75, 11.31) | 2.33 (0.52, 10.50)@ | 3.49 (0.90, 13.54)*# | 3.26 (0.81, 13.07) | 3.89 (0.86, 17.64)@ | 3.53 (0.67, 18.49) | 3.38 (0.68, 16.69) | 3.79 (0.89, 16.05) | 3.93 (1.13, 13.60) | 4, 5 |
| OGTT parameters | |||||||||||||
| Fasting glucose (mmol/L) | 6.22 ± 0.74 | 5.96 ± 0.57@ | 6.32 ± 0.68# | 6.11 ± 0.50 | 5.44 ± 0.59@ | 5.98 ± 0.47*# | 6.21 ± 0.67 | 5.95 ± 0.68@ | 6.35 ± 0.96# | 6.10 ± 0.57 | 6.31 ± 0.69 | 6.29 ± 0.90 | 3, 4, 5, 6 |
| Fasting glucagon (pmol/L) | 9.77 ± 6.01 | 8.85 ± 4.24 | 9.24 ± 4.83 | 8.84 ± 4.74 | 7.51 ± 4.69 | 7.55 ± 4.86* | 7.47 ± 3.38 | 7.24 ± 3.81 | 7.75 ± 4.21 | 8.26 ± 4.65 | 7.74 ± 4.87 | 8.14 ± 4.41 | |
| Fasting C-peptide (nmol/L) | 1.339 ± 0.689 | 1.134 ± 0.428@ | 1.235 ± 0.533# | 1.249 ± 0.428 | 1.086 ± 0.416@ | 1.108 ± 0.451* | 1.234 ± 0.416 | 1.116 ± 0.534@ | 1.192 ± 0.577# | 1.133 ± 0.437 | 1.121 ± 0.397 | 1.108 ± 0.412# | |
| iAUC glucose (mmol/L) | 610.5 ± 260.4 | 692.3 ± 238.0 | 622.3 ± 269.1 | 577.4 ± 243.5 | 464.7 ± 202.7@ | 583.1 ± 251.8# | 601.9 ± 248.0 | 638.5 ± 232.1 | 626.7 ± 319.0 | 635.0 ± 237.9 | 602.1 ± 279.1 | 628.5 ± 270.2 | 3, 4, 6 |
| dAUC glucagon (pmol/L) | −848.4 ± 560.8 | −797.0 ± 492.7 | −833.3 ± 507.5 | −860.7 ± 510.4 | −774.5 ± 553.1 | −773.3 ± 573.0 | −697.2 ± 412.5 | −698.8 ± 392.3 | −686.7 ± 481.2 | −751.2 ± 520.0 | −742.9 ± 582.6 | −719.1 ± 534.7 | |
| iAUC C-peptide (nmol/L) | 454.2 ± 174.6 | 437.6 ± 154.5@ | 417.0 ± 165.9* | 439.3 ± 140.9 | 516.6 ± 161.0@ | 438.0 ± 144.8# | 447.1 ± 141.6 | 454.3 ± 145.3 | 406.6 ± 141.9*# | 444.7 ± 146.7 | 422.6 ± 139.1 | 413.0 ± 136.0* | 4, 5 |
Data are means ± SD for normally distributed and geometric means (95% CI) for non–normally distributed data; for the latter, P values from the log-transformed data are presented. Within-treatment comparisons:
P < 0.05 12 months vs. baseline;
P < 0.05 15 months vs. baseline;
P < 0.05 15 months vs. 12 months. Between-treatment comparisons adjusted for weight at baseline and 12 months; if P < 0.05 for comparison across four treatment arms, P < 0.05 for between-treatment comparisons: 1, G/M vs. L+M; 2, G/M vs. MET; 3, G/M vs. PLAC; 4, L+M vs. MET; 5, L+M vs. PLAC; 6, MET vs. PLAC.
Clamp glucagon concentrations in the other three treatment arms are illustrated in Fig. 1C, D, and F and listed in Table 3. In the MET arm, the steady-state glucagon concentration at 12 months was lower than at baseline (P = 0.014) but not at 15 months (P = 0.421). In the G/M arm, fasting and steady-state glucagon and AGR did not differ between the three time points. Likewise, in the PLAC arm, all glucagon concentrations and responses were similar at baseline and 12 and 15 months.
The glucagon responses during the OGTT in the four intervention groups are illustrated in Fig. 2C–F and provided in Table 3. As was seen with hyperglycemic clamps, the decrease in glucagon was greatest in the L+M group. However, in the L+M group dAUC glucagon was not significantly different at the three study time points. The iAUC C-peptide for the L+M group was higher at 12 months than at baseline (P < 0.001) but not at 15 months (P = 0.816). The net effect of these changes in the L+M group was a reduction in plasma glucose excursions from baseline at 12 months (P < 0.001) but not at 15 months (P = 0.570). In the other three intervention arms, glucagon suppression during the OGTT did not differ before, during, or after withdrawal of the intervention, while iAUC C-peptide was lower at 15 months compared with baseline (Supplementary Fig. 7).
Effect of LB Compared With That of MET on Glucagon Concentrations During the Hyperglycemic Clamp and OGTT in the RISE Adult Surgery Study
The glucagon profile during the hyperglycemic clamp in participants in the LB or MET arm at baseline and after 12 and 24 months of the interventions is illustrated in Fig. 1G and H, with measured and calculated values in Table 4. Concurrent glucose and C-peptide concentrations are illustrated in Supple mentary Fig. 8. In the LB group, fasting and steady-state glucagon concentrations were, respectively, 32% and 60% lower than baseline at 12 months and 31% and 60% lower than baseline at 24 months (P < 0.001 for all). AGR was 12% lower than baseline at 12 months (P = 0.098) and 15% lower than baseline at 24 months (P = 0.028). Box plots of these changes over time appear in Supplementary Fig. 9. In the MET group in the Adult Surgery Study, only the steady-state glucagon value at 12 months was significantly lower than baseline (P = 0.022).
Table 4.
Effect of MET or LB in adults on select metabolic characteristics from the hyperglycemic clamps and OGTTs
| LB | MET | P for comparison between treatments at 12 months | P for comparison between treatments at 24 months | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 44) | 12 months (n = 38) | 24 months (n = 34) | Baseline (n = 44) | 12 months (n = 30) | 24 months (n = 33) | |||
| Anthropometric parameters | ||||||||
| Weight (kg) | 97.5 ± 12.1 | 86.7 ± 12.0@ | 85.5 ± 13.5* | 96.1 ± 10.9 | 92.8 ± 11.0@ | 93.9 ± 10.5* | ||
| BMI (kg/m2) | 35.7 ± 2.8 | 32.0 ± 2.6@ | 31.4 ± 3.2* | 35.0 ± 2.9 | 33.8 ± 3.4@ | 34.2 ± 2.9* | ||
| Hyperglycemic clamp parameters | ||||||||
| Fasting glucose (mmol/L) | 6.30 ± 0.92 | 5.72 ± 0.73@ | 5.87 ± 1.03* | 6.05 ± 0.71 | 5.86 ± 0.60 | 5.95 ± 0.65 | ||
| Fasting glucagon (pmol/L) | 9.29 ± 4.78 | 6.35 ± 2.49@ | 6.45 ± 2.84* | 8.02 ± 3.61 | 7.10 ± 2.96 | 7.73 ± 3.12 | ! | |
| Fasting C-peptide (nmol/L) | 1.26 (0.75, 2.13) | 0.89 (0.50, 1.58)@ | 0.90 (0.52, 1.56)* | 1.09 (0.53, 2.24) | 0.91 (0.44, 1.86)@ | 1.02 (0.47, 2.20) | ! | |
| Fasting insulin (pmol/L) | 125.4 (49.0, 320.8) | 69.9 (26.6, 183.5)@ | 70.1 (27.3, 180.1)* | 101.6 (33.7, 305.9) | 81.4 (29.6, 223.9) | 94.1 (26.0, 340.7) | ||
| Steady-state glucose (mmol/L) | 11.1 ± 0.7 | 11.0 ± 0.6 | 11.0 ± 0.5 | 11.0 ± 0.6 | 10.9 ± 0.5@ | 11.6 ± 3.6 | ||
| Steady-state glucagon (pmol/L) | 2.86 ± 2.39 | 1.73 ± 1.29@ | 1.73 ± 1.55* | 1.87 ± 1.11 | 1.46 ± 0.95@ | 1.79 ± 1.51 | ||
| Steady-state C-peptide (nmol/L) | 3.74 (1.87, 7.47) | 3.18 (1.59, 6.35)@ | 3.07 (1.45, 6.49)* | 3.36 (1.40, 8.11) | 3.12 (1.25, 7.77) | 3.11 (1.20, 8.07)* | ||
| AGR (pmol/L) | 17.3 ± 8.4 | 15.2 ± 6.6 | 14.7 ± 6.5* | 17.4 ± 9.7 | 16.2 ± 11.5 | 15.9 ± 9.9 | ||
| ACPRmax (nmol/L) | 4.68 (1.48, 14.78) | 4.36 (1.72, 11.06) | 4.05 (1.40, 11.70) | 4.77 (1.72, 13.24) | 4.11 (1.45, 11.60) | 3.88 (1.57, 9.63)* | ||
| M/I (×10−5 mmol/kg/min per pmol/L) | 3.20 (1.17, 8.75) | 4.92 (1.39, 17.41)@ | 4.66 (1.36, 16.06) * | 3.46 (0.72, 16.68) | 4.99 (1.31, 18.93)@ | 4.12 (1.01, 16.88) | ! | |
| OGTT parameters | ||||||||
| Fasting glucose (mmol/L) | 6.19 ± 0.75 | 5.75 ± 0.88@ | 5.87 ± 1.08* | 6.10 ± 0.70 | 5.76 ± 0.55@ | 5.95 ± 0.62 | ||
| Fasting glucagon (pmol/L) | 8.48 ± 4.31 | 5.89 ± 2.96@ | 6.37 ± 3.33* | 8.35 ± 4.51 | 7.09 ± 3.78 | 7.82 ± 3.48 | ||
| Fasting C-peptide (nmol/L) | 1.258 ± 0.299 | 0.937 ± 0.250@ | 0.958 ± 0.298* | 1.190 ± 0.449 | 0.959 ± 0.410@ | 1.069 ± 0.482* | ||
| 2-h glucose (mmol/L) | 10.37 ± 2.66 | 9.58 ± 2.75@ | 10.03 ± 3.19 | 10.45 ± 2.54 | 10.52 ± 2.92 | 10.87 ± 3.30 | ||
| iAUC glucose (mmol/L) | 638.2 ± 258.5 | 560.6 ± 261.4@ | 600.9 ± 317.9 | 645.9 ± 263.1 | 665.9 ± 356.6 | 704.5 ± 347.9 | ||
| dAUC glucagon (pmol/L) | −793.8 ± 417.6 | −608.0 ± 405.9@ | −659.2 ± 472.3 | −885.3 ± 535.1 | −801.4 ± 488.4 | −865.0 ± 438.0 | ||
| iAUC C-peptide (nmol/L) | 455.6 ± 209.6 | 412.4 ± 118.4 | 425.4 ± 218.8 | 457.5 ± 147.6 | 419.4 ± 168.7 | 415.7 ± 131.8* | ||
Data are mean ± SD for normally distributed and geometric means (95% CIs) for non–normally distributed data; for the latter, P values from the log-transformed data are presented. Within-treatment comparisons:
P < 0.05, 12 months vs. baseline;
P < 0.05, 24 months vs. baseline;
P < 0.05, 24 months vs. 12 months.
P ≤ 0.05, LB vs. MET with adjustment for weight at baseline and follow-up.
Figure 2G and H illustrate the glucagon profiles during OGTTs, with corresponding summary data in Table 4. OGTT glucose and C-peptide profiles are illustrated in Supplementary Fig. 10. With the LB, fasting glucagon was below baseline at both 12 (P < 0.001) and 24 (P = 0.001) months and the dAUC for glucagon was significantly below baseline at 12 months (P = 0.025) but not at 24 months (P = 0.141). LB also reduced the fasting glucose (P = 0.002 and P = 0.005) and C-peptide (P < 0.001 and P < 0.001) at both 12 and 24 months, respectively, but iAUC glucose was significantly below baseline only at 12 months (P = 0.010). In the metformin arm, neither fasting glucagon nor dAUC glucagon changed with the intervention. Similarly, aside from the fasting C-peptide, which was lower at 12 (P = 0.002) and 24 (P = 0.004) months, glucose and C-peptide measures did not change with metformin.
Within-Protocol Comparisons of the Intervention Effects on Glucagon Responses and Impact of Changes in Body Weight and Insulin Sensitivity
In youth, weight increased significantly in the G/M arm (12 months, P = 0.005, and 15 months, P < 0.001, vs. baseline), while in the MET arm it was significantly increased at 15 months (P = 0.010) but not 12 months (P = 0.772). The weight difference between treatment arms was not significant (P = 0.17) (Table 2). Adjustment for baseline weight or BMI, the latter as youth were growing, and for changes in these measures over time did not impact the lack of difference in glucagon concentrations between the two interventions (data not shown). Further, accounting for baseline and changes in either measure of insulin sensitivity did not change the findings.
In adults in the medication study, weight was significantly lower than baseline at 12 and 15 months in all three active medication arms (P ≤ 0.05 for all). The greatest weight reduction was in the L+M arm (Table 3). Adjusting for baseline weight and change in weight at 12 months eliminated the statistical significance of differences in fasting glucagon (P = 0.526) and AGR (P = 0.081) across the four treatment arms, suggesting that changes in these measures were influenced by weight change. In contrast, steady-state glucagon remained significantly different across the four intervention arms after adjustment for baseline and change in body weight at 12 months (P = 0.001), suggesting a weight-independent effect of treatment; this difference was no longer present at 15 months (P = 0.177), when treatment effects had dissipated. In pairwise comparisons, at 12 months steady-state glucagon was significantly lower in the L+M arm compared with G/M (P < 0.001), MET (P = 0.008), and PLAC (P = 0.001) arms after adjustment for differences in weight. Adjustment for insulin sensitivity did not modify the differential effect of treatment on glucagon measures.
In the Adult Surgery Study, weight decreased at 12 and 24 months in both arms, with a greater decline in the LB group (Table 4). At 12 months, fasting glucagon was lower in the LB compared with MET group (P = 0.029), but this difference was no longer significant after baseline and change in body weight were accounted for (P = 0.673). Neither steady-state glucagon (P = 0.342) nor AGR (P = 0.575) differed between LB and MET at 12 months. At 24 months, fasting glucagon was lower, steady-state glucagon trended to be lower, and AGR was not lower in the LB compared with MET group (P = 0.001, P = 0.059, and P = 0.296, respectively). However, the significance of LB versus MET was eliminated by adjustment for change in body weight (P = 0.126, P = 0.678, and P = 0.959). Adjustment for insulin sensitivity did not modify the differential effect of LB versus MET on glucagon.
Conclusions
RISE provided an opportunity, with use of complementary intravenous and oral testing, to provide new insights related to the effects of medications and weight loss surgery on α-cell function in youth and adults. First, we observed that regulation of glucagon release was not impacted by either G/M or MET in youth or adults. Thus, while β- and α-cell function are known to be intimately related (3–6), the progressive and more rapid loss of β-cell function that we observed in youth (21) was not paralleled by a hypothesized deterioration in α-cell function. This observation suggests that progressive β-cell dysfunction may be more critical in the development of hyperglycemia in youth. Second, L+M improved α-cell function during the active intervention phase, with some of this beneficial effect retained 3 months after treatment withdrawal. This improved regulation of glucagon release by liraglutide appears to be principally attributable to its effect on weight rather than a possible direct effect on the α-cell (10). Third, while the expected weight loss with gastric band surgery improved insulin sensitivity (12,22,23), it was the weight change rather than insulin sensitivity that was associated with the improvement in α-cell function.
We previously reported that β-cell function in youth deteriorated more rapidly than in adults, evident in evaluation of the effects of equivalent interventions aimed at preserving or improving β-cell function (21). The current analyses with glucagon used as an estimate of α-cell function show that while β-cell function worsened, a parallel deterioration of α-cell function, namely, increased glucagon concentrations or impaired suppression by glucose, did not occur. These observations exclude an α-cell defect as a causative factor in the accelerated decline of β-cell function in youth. They also highlight an interesting lack of reciprocal changes in C-peptide and glucagon concentrations that, based on peripheral measurements of these peptides, suggests the β-cell does not have a major regulatory impact on the α-cell. Rather, the increase in glucose per se may be important in reducing glucagon release, given its ability to directly inhibit the α-cell (4). It is possible this effect of glucose may be compensating for the expected lack of α-cell suppression as β-cell secretory function deteriorates (3–6,24,25).
GLP-1 receptor agonists have been approved as glucose-lowering therapies for type 2 diabetes in adults and, more recently, in youth (10,26). These agents not only increase insulin secretion but also induce weight loss through their effects on satiety. Further, they can uniquely decrease glucagon release by suppressing α-cell function independent of an effect on the β-cell (10). Our observation of reduced fasting, steady-state, and arginine-stimulated glucagon responses are in keeping with such a direct mechanism of action of liraglutide. However, we made an additional important observation regarding the effect of liraglutide on α-cell function. Given the effects of GLP-1 receptor agonists to decrease body weight and our observation of weight loss with liraglutide, when we accounted for the change in body weight, the differences in fasting glucagon and AGR were no longer significant. The glucagon concentration at steady state during the clamp remained significant after adjustment for the weight change, suggesting a weight-independent effect of treatment that could be due to liraglutide having a direct suppressive effect on the α-cell or enhancing the α-cell’s sensitivity to glucose’s suppressive effect on glucagon release. Lastly, whether in youth liraglutide has similar effects on the α-cell will be of interest for future studies.
Surgery-induced weight loss and the associated improvement in insulin sensitivity have been associated with changes in a number of gastrointestinal hormones (27,28). However, there is little information regarding weight loss surgery effects on glucagon, with studies frequently including only a small number of participants and not including sophisticated assessments of α-cell function (29–32). The current studies used careful physiologic measures: hyperglycemic clamps, which do not evoke gut hormone responses, and OGTTs, which stimulate gut hormone responses. With these methods we demonstrate improved α-cell function following LB, attributable to the change in weight and not the attendant change in insulin sensitivity. In designing our study, we chose the LB approach so that we could examine the effect of weight loss per se without the confounding effects of changes in intestinal anatomy and nutrient flow as well as off-target effects of medications. Of relevance to this choice, a recent report highlighted differences in peptide responses during a mixed meal when the anatomy of the stomach (vertical sleeve gastrectomy) or intestinal tract (Roux-en-Y gastric bypass) are altered but, interestingly, not with laparoscopic gastric banding (32). In that study, which had a small number of participants in each group, only when the anatomy was altered were differences in classical alternative products of the glucagon gene, namely, GLP-1, glicentin, and oxyntomodulin, observed. While we did not measure these alternative products, given that we did not alter intestinal anatomy, we would not expect them to have been increased. Further, the sequential approach that we used in the glucagon assay was intended to minimize the risk of potential cross-reactivity of glicentin and oxyntomodulin (17,18). Thus, given our larger sample size, we believe the reduction in glucagon concentrations that we observed with surgery in RISE reflects changes in α-cell function associated with weight loss.
The design of the RISE studies has given us unique insights into the pathogenesis of dysglycemia in youth and adults and the impact of interventions. However, there are some limitations. First, for ethical reasons we did not include a placebo arm in the study of youth. Without a placebo control we cannot definitively exclude the possibility that the interventions in youth had some salutary effect on the α-cell that persisted 3 months after treatment withdrawal. However, given the fact that the effects we observed were similar to the parallel treatment interventions in the adult study, which did not differ from PLAC, we doubt this was the case. Second, in adults the effects we have ascribed to liraglutide are based on the effects of the combination of L+M. Given we observed no effect of MET, it seems reasonable to attribute the outcome we observed to liraglutide per se. Third, for ethical reasons we did not deflate the laparoscopic band. Thus, we were unable to examine the effect of any potential weight regain on α-cell function. Lastly, while our analyses suggest that weight loss was an important contributor to the improvement in α-cell function, we are unable to provide insight into how this may be occurring at a cellular level.
In summary, these analyses reveal that α-cell function in both youth and adults is not improved by 12 months of metformin or by 3 months of insulin glargine followed by 9 months of metformin. Further, in youth, α-cell function did not deteriorate, while β-cell function did, suggesting that dysregulated glucagon secretion may be less critical for progression in adolescents. In contrast, in adults both L+M and LB reduced glucagon concentrations. This beneficial effect was related to weight loss but not insulin sensitivity, so it is possible that sustained weight loss will allow persistence of the beneficial effect on the α-cell.
Article Information
Acknowledgments. The RISE Consortium acknowledges the support and input of the RISE Data and Safety Monitoring Board; Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Program Official for RISE; Ellen Leschek, the NIDDK Project Scientist for RISE; and Peter Savage, who served as the NIDDK Project Scientist for RISE prior to retirement. The Consortium is also grateful for the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding. RISE is partly supported by grants from the National Institutes of Health, including the NIDDK (U01DK-094406, U01DK-094430, U01DK-094431, U01DK-094438, U01DK-094467, P30DK-017047, P30DK-020595, P30DK-045735, P30DK-097512, UL1TR-000430, UL1TR-001082, UL1TR-001108, UL1TR-001855, UL1TR-001857, UL1TR-001858, UL1TR-001863), and the Department of Veterans Affairs. Additional financial and material support from the American Diabetes Association, Allergan Corporation, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk A/S is gratefully acknowledged.
Duality of Interest. RISE is also supported by grants from Kaiser Permanente Southern California. S.E.K. and S.A.A. serve as paid consultants on advisory boards for Novo Nordisk. S.E.K. is a member of the steering committee for a Novo Nordisk–sponsored clinical trial, and S.A.A. is a participant in a Novo Nordisk-sponsored clinical trial. T.A.B. has received research support from Allergan Corporation and Apollo Endosurgery. K.J.M. held an investigator-initiated research grant from Novo Nordisk during the period the RISE studies were conducted. No other potential conflicts of interest relevant to this article were reported.
The involvement of S.M. and K.J.M. in the research underlying this manuscript preceded their employment at Medpace Reference Laboratories and Eli Lilly and Co., respectively, and their participation in data review and the writing of this manuscript were independent of their employment at Medpace Reference Laboratories and Eli Lilly and Co.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. S.E.K. proposed the analysis, interpreted data, and wrote and edited the manuscript, which was also reviewed and edited by members of the writing group. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. S.E.K. and S.L.E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
S.M. is currently employed by Medpace Reference Laboratories.
K.J.M. is currently employed by Eli Lilly and Co.
A complete list of the RISE Consortium Investigators appears in the supplementary material online.
Clinical trial reg. nos. NCT01779362, NCT01779375, NCT01763346, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14460195.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/the-RISE-study-more-insights-into-T2D-in-youth-and-adults.
Contributor Information
Collaborators: The Rise Consortium Investigators:, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2. Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyper glycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 3. Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 2005;54:1789–1797 [DOI] [PubMed] [Google Scholar]
- 5. Rorsman P, Berggren PO, Bokvist K, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 1989;341:233–236 [DOI] [PubMed] [Google Scholar]
- 6. Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 2003;5:330–335 [DOI] [PubMed] [Google Scholar]
- 7. RISE Consortium . Lack of durable improvements in β-cell function following withdrawal of pharma cological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2019;42:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiang AH, Trigo E, Martinez M, et al.; RISE Consortium . Impact of gastric banding versus metformin on β-cell function in adults with impaired glucose tolerance or mild type 2 diabetes. Diabetes Care 2018;41:2544–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab 2003;16:277–283 [PubMed] [Google Scholar]
- 12. Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab 2004;89:2704–2710 [DOI] [PubMed] [Google Scholar]
- 13. RISE Consortium . Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts GP, Kay RG, Howard J, Hardwick RH, Reimann F, Gribble FM. Gastrectomy with Roux-en-Y reconstruction as a lean model of bariatric surgery. Surg Obes Relat Dis 2018;14:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexiadou K, Cuenco J, Howard J, et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res Care 2020;8:e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kahn SE, Mather KJ, Arslanian SA, et al.; the Rise Consortium . Hyperglucagonemia does not explain the β-cell hyperresponsiveness and insulin resistance in dysglycemic youth compared with adults: lessons from RISE. Diabetes Care 2021;44:1961–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 21. RISE Consortium . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Letiexhe MR, Scheen AJ, Gérard PL, Desaive C, Lefèbvre PJ. Insulin secretion, clearance and action before and after gastroplasty in severely obese subjects. Int J Obes Relat Metab Disord 1994;18:295–300 [PubMed] [Google Scholar]
- 23. Weyer C, Hanson K, Bogardus C, Pratley RE. Long-term changes in insulin action and insulin secretion associated with gain, loss, regain and maintenance of body weight. Diabetologia 2000;43:36–46 [DOI] [PubMed] [Google Scholar]
- 24. Greenbaum CJ, Havel PJ, Taborsky GJ Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest 1991;88:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 1992;41:93–97 [DOI] [PubMed] [Google Scholar]
- 26. Tamborlane WV, Barrientos-Pérez M, Fainberg U, et al.; Ellipse Trial Investigators . Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 2019;381:637–646 [DOI] [PubMed] [Google Scholar]
- 27. Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016;77:28–37 [DOI] [PubMed] [Google Scholar]
- 28. Dimitriadis GK, Randeva MS, Miras AD. Potential hormone mechanisms of bariatric surgery. Curr Obes Rep 2017;6:253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swarbrick MM, Stanhope KL, Austrheim-Smith IT, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia 2008;51:1901–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 2011;21:896–901 [DOI] [PubMed] [Google Scholar]
- 32. Perakakis N, Kokkinos A, Peradze N, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. Metabolism 2019;101:153997. [DOI] [PubMed] [Google Scholar]