Abstract
OBJECTIVE
To compare the risk of severe adverse pregnancy complications in women with preexisting diabetes.
RESEARCH DESIGN AND METHODS
Multinational, prospective cohort study to assess the prevalence of newborns free from major congenital malformations or perinatal or neonatal death (primary end point) following treatment with insulin detemir (detemir) versus other basal insulins.
RESULTS
Of 1,457 women included, 727 received detemir and 730 received other basal insulins. The prevalence of newborns free from major congenital malformations or perinatal or neonatal death was similar between detemir (97.0%) and other basal insulins (95.5%) (crude risk difference 0.015 [95% CI −0.01, 0.04]; adjusted risk difference −0.003 [95% CI −0.03, 0.03]). The crude prevalence of one or more congenital malformations (major plus minor) was 9.4% vs. 12.6%, with a similar risk difference before (−0.032 [95% CI −0.064, 0.000]) and after (−0.036 [95% CI –0.081, 0.009]) adjustment for confounders. Crude data showed lower maternal HbA1c during the first trimester (6.5% vs. 6.7% [48 vs. 50 mmol/mol]; estimated mean difference −0.181 [95% CI −0.300, −0.062]) and the second trimester (6.1% vs. 6.3% [43 vs. 45 mmol/mol]; −0.139 [95% CI −0.232, −0.046]) and a lower prevalence of major hypoglycemia (6.0% vs. 9.0%; risk difference −0.030 [95% CI −0.058, −0.002]), preeclampsia (6.4% vs. 10.0%; −0.036 [95% CI −0.064, −0.007]), and stillbirth (0.4% vs. 1.8%; −0.013 [95% CI −0.024, −0.002]) with detemir compared with other basal insulins. However, differences were not significant postadjustment.
CONCLUSIONS
Insulin detemir was associated with a similar risk to other basal insulins of major congenital malformations, perinatal or neonatal death, hypoglycemia, preeclampsia, and stillbirth.
Introduction
Pregnant women with preexisting diabetes are at an increased risk of adverse maternal and fetal outcomes if their diabetes is not well controlled.
Long-acting insulins such as insulin detemir (detemir) and other basal insulins such as insulin glargine provide a steady background control of blood glucose levels over a long period of time, optimizing blood glucose levels with minimal risk of low blood glucose. Detemir may be better than other basal insulins at stabilizing blood glucose levels during the night. Detemir and other basal insulins are used widely to treat pregnant women with diabetes, but limited data are available on their impact on the risk of birth defects or perinatal or neonatal death in a real-world setting.
The EValuation Of LeVEmir in Pregnancy (EVOLVE) study aimed to assess the effect of detemir compared with other long-acting insulins on the risk of these severe birth outcomes in women with preexisting diabetes in a real-world setting. The study also assessed maternal HbA1c levels, a measure of long-term blood-sugar control, during pregnancy and the risk of developing preeclampsia or episodes of very low blood glucose.
Pregnant women with diabetes are at an increased risk of serious complications in pregnancy, with congenital malformations or perinatal or neonatal death being the most severe (1–5). In Europe, the proportion of pregnancies, in women with diabetes, with severe congenital malformations is reported to be ∼4–6% and the rate of perinatal mortality ∼3% (2,3,5–9).
Optimal management of diabetes in pregnant women is required to decrease maternal hyperglycemia, while limiting hypoglycemia, in order to prevent pregnancy complications (1,5,10,11). Even small elevations in HbA1c levels in early pregnancy in women with diabetes can lead to a significantly increased risk of congenital malformations (11). Insulin detemir (detemir) is a long-acting basal insulin analog with slow absorption rates and a prolonged metabolic effect (12,13). It has a more consistent effect on fasting plasma glucose levels (less day-to-day variability in the glucose-lowering profile) than NPH insulin (12) or insulin glargine (14,15), which may improve the probability of reaching target HbA1c levels, lowering the risk of severe hypoglycemia and congenital malformations. Outside of pregnancy, long-acting insulin analogs improve glycemic control, with lower associated rates of hypoglycemia than NPH insulin (16,17). Among the basal insulin analogs, the European Union prescribing labels for both detemir and insulin glargine allow for consideration of use in pregnancy (18,19), and insulin analogs are now widely used in pregnant women (20,21).
There are limited prospective data available on the use of detemir and other basal insulins in pregnancy. A single randomized controlled trial (RCT) investigated the use of detemir versus NPH insulin, both in combination with insulin aspart, in pregnant women with type 1 diabetes (22). The data showed significant improvements in fasting pla-sma glucose with detemir compared with NPH insulin, with similar HbA1c levels and rates of hypoglycemia (22), and suggested that both insulins had a good safety profile with regard to perinatal outcomes (23). While RCTs give the best scientific evidence for more common outcomes, prospective cohort studies provide important real-world data for rare events such as congenital malformations and perinatal or neonatal mortality. Different insulin analogs have been shown to vary in their affinities to growth factor receptors, and the safety implications of this are unclear (24); whether this may relate to prevalence of congenital malformations has not previously been investigated in prospective studies. Stillbirths, which are the main component of perinatal mortality, are known to be related to poor glycemic control (7).
Against this background, the European Medicines Agency (EMA) therefore finds a need for large observational studies using real-world data to assess the effect of long-acting basal insulin analogs on the risk of these severe complications in pregnant women with diabetes. Data on maternal safety, including rare events such as severe hypoglycemia and development of preeclampsia, are also needed.
The EVOLVE cohort study aimed to evaluate the risk of having offspring with major congenital malformations or perinatal or neonatal death, when using detemir versus other basal insulins, in pregnant women with preexisting diabetes. It also set out to examine other important maternal end points, including HbA1c levels during pregnancy and the incidence of major hypoglycemia and preeclampsia.
Research Design and Methods
The EVOLVE study design has been published previously (25). In brief, the EVOLVE study (ClinicalTrials.gov, NCT01892319) was a large, multinational, prospective, noninterventional, multicenter study in pregnant women with preexisting type 1 or type 2 diabetes designed, in collaboration with the EMA, to assess the safety of detemir compared with other basal insulins during pregnancy. Women were enrolled in early pregnancy across 92 sites in 17 countries (including Europe, Israel, and Malaysia) (Table 1). Baseline data covering the prepregnancy period were collected at enrollment, after which the participants underwent a series of standard routine visits throughout pregnancy (frequency determined by the study site and data collected in the context of routine practice), up until delivery. After delivery, there was a 1-month follow-up (or within a month of the delivery date) and an additional 1-year follow-up (within 4 months of the 1-year follow-up date). Postpartum follow-up was performed using questionnaires and telephone interviews (25).
Table 1.
Baseline characteristics and glucose-lowering treatment of pregnant women with preexisting diabetes using detemir vs. other basal insulins at enrollment
| Detemir (n = 727) | Other basal insulins (n = 730) | |
|---|---|---|
| Country, n (%) | ||
| Croatia | 238 (32.7) | 47 (6.4) |
| Denmark | 126 (17.3) | 257 (35.2) |
| Finland | 57 (7.8) | 50 (6.8) |
| France | 9 (1.2) | 15 (2.1) |
| Germany | 4 (0.6) | 9 (1.2) |
| Greece | 4 (0.6) | 7 (1.0) |
| Ireland | 28 (3.9) | 31 (4.2) |
| Israel | 53 (7.3) | 8 (1.1) |
| Italy | 15 (2.1) | 21 (2.9) |
| Malaysia | 5 (0.7) | 14 (1.9) |
| The Netherlands | 11 (1.5) | 6 (0.8) |
| Norway | 4 (0.6) | 20 (2.7) |
| Poland | 19 (2.6) | 38 (5.2) |
| Portugal | 3 (0.4) | 12 (1.6) |
| Romania | 22 (3.0) | 1 (0.1) |
| Spain | 27 (3.7) | 87 (11.9) |
| U.K. | 102 (14.0) | 107 (14.7) |
| Type 1 diabetes, n (%) | 595 (81.8) | 654 (89.6) |
| Type 2 diabetes, n (%) | 132 (18.2) | 76 (10.4) |
| Age, years, mean (SD) | 31.1 (5.1) | 30.6 (5.3) |
| BMI, kg/m2, mean (SD) | 25.8 (5.7) | 26.6 (5.7) |
| Systolic blood pressure, mmHg, mean (SD) | 117.4 (13.5) | 119.5 (13.9) |
| Diabetes duration, years, mean (SD) | 13.1 (8.1) | 13.6 (8.5) |
| History of retinopathy, n (%) | 155 (21.4) | 158 (21.9) |
| History of nephropathy, n (%) | 34 (4.7) | 30 (4.1) |
| Hypertension, n (%) | 64 (8.8) | 60 (8.2) |
| HbA1c, % [mmol/mol], mean (SD) | 7.0 (1.3) [53 (14)] | 7.2 (1.4) [55 (15)] |
| White, n (%) | 688 (96.0) | 649 (90.8) |
| University degree, n (%) | 203 (30.2) | 154 (23.2) |
| Current smoker, n (%) | 66 (9.3) | 67 (9.5) |
| Alcohol consumption, n (%) | 7 (1.0) | 8 (1.2) |
| Gestational age of current pregnancy at enrollment, weeks, mean (SD) | 8.7 (2.5) | 8.8 (2.6) |
| Folic acid taken before and during first trimester, n (%) | 459 (63.9) | 579 (80.1) |
| Number of previous pregnancies, n (%) | ||
| 0 | 229 (31.5) | 260 (35.6) |
| 1 | 268 (36.9) | 247 (33.8) |
| 2 | 114 (15.7) | 116 (15.9) |
| 3 | 59 (8.1) | 58 (7.9) |
| ≥4 | 57 (7.8) | 49 (6.7) |
| Number of previous live births, n (%) | ||
| 0 | 325 (46.0) | 290 (43.9) |
| 1 | 283 (40.1) | 276 (41.8) |
| 2 | 74 (10.5) | 77 (11.6) |
| 3 | 13 (1.8) | 17 (2.6) |
| ≥4 | 11 (1.6) | 1 (0.2) |
| Previous pregnancy complications, n (%) | 375 (54.0) | 351 (54.0) |
| Previous preeclampsia, n (%) | 28 (3.9) | 32 (4.4) |
| Previous perinatal deaths, n (%) | 13 (1.8) | 12 (1.6) |
| Previous preterm delivery, n (%) | 47 (6.5) | 55 (7.5) |
| Previous spontaneous abortion, n (%) | 185 (25.4) | 162 (22.2) |
| Previous cesarean section, n (%) | 172 (23.7) | 119 (16.3) |
| Previous major malformations, n (%) | 12 (1.7) | 9 (1.2) |
| Previous minor malformations, n (%) | 2 (0.3) | 6 (0.8) |
| Glucose-lowering treatment, n (%) | ||
| Basal insulin | 727 (100) | 730 (100) |
| Detemir | 727 (100) | — |
| Insulin glargine | — | 594 (81.4) |
| NPH insulin | — | 95 (13.0) |
| Insulin degludec | — | 40 (5.5) |
| Unknown type | — | 1 (0.1) |
| Bolus insulin | 714 (98.2) | 713 (97.7) |
| Insulin aspart | 605 (83.2) | 530 (72.6) |
| Insulin lispro | 92 (12.7) | 120 (16.4) |
| Insulin glulisine | 11 (1.5) | 39 (5.3) |
| Regular human insulin | 5 (0.7) | 22 (3.0) |
| Unknown type | 1 (0.1) | 2 (0.0) |
| Metformin | 36 (5.0) | 32 (4.4) |
Data for other concomitant diseases not shown.
Participants were required to have a positive pregnancy test (gestational age ≤16 weeks at enrollment), type 1 or type 2 diabetes diagnosed prior to conception (no gestational diabetes), treatment at the time of enrollment with detemir or other injectable antidiabetic treatments, and unchanged basal insulin type or other injectable antidiabetic treatment products from 4 weeks prior to conception until study enrollment. For the results presented in this study, only women treated with detemir and other basal insulins and who did not change the type of basal insulin during early pregnancy were included. Women could participate in the study more than once. Recruitment took place between September 2013 and September 2018, and informed consent was given.
End Points
The primary end point was a composite of the proportion of pregnancies completing ≥22 weeks of gestation in women treated with detemir versus other basal insulins, without any of the following events: major congenital malformations, perinatal death (from 22 weeks before to 7 days after birth), or neonatal death (death of a liveborn infant from 0 to 28 days after delivery). A major congenital malformation was defined as a life-threatening structural anomaly or an abnormality likely to cause significant impairment of health or functional capacity and that needs medical or surgical treatment (26). The number of pregnancies resulting in abortions, stillbirth (from 22 weeks and until birth), perinatal death, or neonatal death was determined. The number of fetuses with major and/or minor congenital malformations was also assessed, in which minor malformation was defined as not fulfilling the criteria for major malformation.
Maternal end points included HbA1c levels and safety end points, namely the incidence of major hypoglycemia and preeclampsia during pregnancy. Major hypoglycemia was defined as a hypoglycemic episode in which the patient is not able to treat herself and oral carbohydrates, glucagon, or intravenous glucose must be administered to the patient by another person because of severe central nervous system dysfunction.
Statistical Methods
All statistical tests were performed as two-sided tests with a significance level of 0.05, and all statistical analyses were performed for all countries combined. In case of missing information on required variables, patients were excluded from respective analyses. For all binary end points (yes/no), n and percent were reported for the detemir group and for the “other basal insulins” group and the absolute risk difference between these proportions calculated, along with the 95% CI and the P value.
For all primary and secondary end points, both crude and confounder-adjusted analyses were performed. Potential confounding was adjusted for by propensity score matching (binary end points, using the Newcombe Method [27] or multiple regression analysis [continuous end points (HbA1c)]).
The primary end point was adjusted for country, maternal age, gestational age, diabetes type, duration of diabetes, history of any diabetes complications (diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, or macrovascular complications), history of hypertension, folic acid taken before and during the first trimester, history of spontaneous abortion, history of cesarean section, history of preterm delivery, history of major malformations, number of previous pregnancies, BMI, HbA1c, tobacco and alcohol consumption, and education.
Descriptive statistics of observed HbA1c around conception, end of first trimester, end of second trimester, and end of third trimester were reported. The association between treatment group at enrollment and HbA1c during pregnancy was examined by a linear mixed model for repeated measurements. An unstructured covariance matrix was used, and both crude and adjusted models were evaluated. Women were included as random effects to adjust for within-women clustering, and fixed factors of country, age, gestational week, type of diabetes, diabetes duration, history of diabetes complications, history of fetal pregnancy complications, BMI, tobacco and alcohol consumption, education, and bolus insulin and oral antidiabetic drug use were included. An interaction term between time and basal insulin treatment group was also evaluated to test (type 3 test) for differences in development of HbA1c over time. Differences in HbA1c between basal insulin treatment groups were estimated around conception and at the end of the first, second and third trimesters. The OBSMARGINS option, in the SAS/STAT 14.2 software PROC MIXED LSMEANS statement, was used when estimating mean HbA1c during pregnancy, to ensure that estimates were weighted to represent the original sample and not an artificially balanced sample. Similar analyses as described for the primary end point were performed for the secondary safety end points (major hypoglycemia or preeclampsia during pregnancy, perinatal death, neonatal death, stillbirth, induced abortion due to major malformation, spontaneous abortion, and major or minor congenital malformations).
Ethics Statement
All participants gave their written informed consent to participate in this observational cohort study. This study was conducted in accordance with International Society for Pharmaceutical Engineering guidelines for Good Pharmacoepidemiology Practice and the Declaration of Helsinki and was approved separately in each of the participating countries by national health authorities, local institutional review boards, or independent ethics committees.
Results
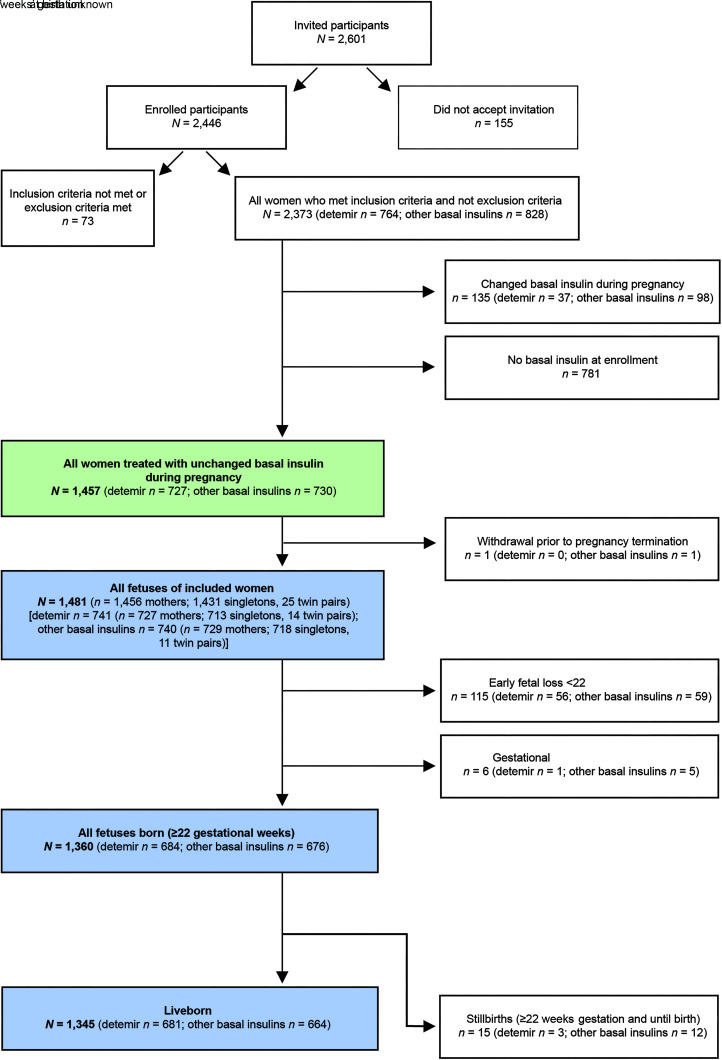
Overall, 2,373 women met the inclusion criteria for the study and, of these, 1,457 were receiving basal insulin at enrollment and were treated with unchanged basal insulin type during early pregnancy. Women who changed type of basal insulin during pregnancy (135) or who were not receiving basal insulin at enrollment, likely due to pump treatment (781), were not included in this analysis (Fig. 1). In total, 727 women were using insulin detemir as a basal insulin, and 730 women were using other basal insulins (Fig. 1). One woman on other basal insulin withdrew from the study prior to pregnancy termination. The remaining 1,456 mothers included in the study were pregnant with 1,431 singletons and 25 twin pairs (1,481 fetuses), 713 singletons and 14 twin pairs (741 fetuses) in the 727 mothers receiving detemir, and 718 singletons and 11 twin pairs (740 fetuses) in the 729 mothers on other basal insulins (Fig. 1). Of 1,481 fetuses, 1,360 were born (≥22 gestational weeks), with 1,345 being liveborn (Fig. 1).
Figure 1.
Patient disposition. N, number of cases.
Baseline characteristics of the women included in the study were comparable between treatment groups, with a few differences (Table 1). The majority of participants (detemir, 81.8%; other basal insulins, 89.6%) had type 1 diabetes. A lower percentage (63.9%) of women using detemir had folic acid supplementation before and/or during the first trimester than those using other basal insulins (80.1%). Of those using other basal insulins at enrollment, 81.4% were using insulin glargine. In both treatment groups, the majority of participants were using insulin aspart as bolus insulin (detemir group, 83.2%; other basal insulin group, 72.6%).
The unadjusted prevalence of newborns without major congenital malformations, perinatal or neonatal death was similar between detemir (97.0% [n = 647/667]) and other basal insulins (95.5% [n = 633/663]) (crude risk difference 0.015 [95% CI −0.01, 0.04], P = 0.1518; adjusted risk difference −0.003 [95% CI −0.03, 0.03], P = 0.8575).
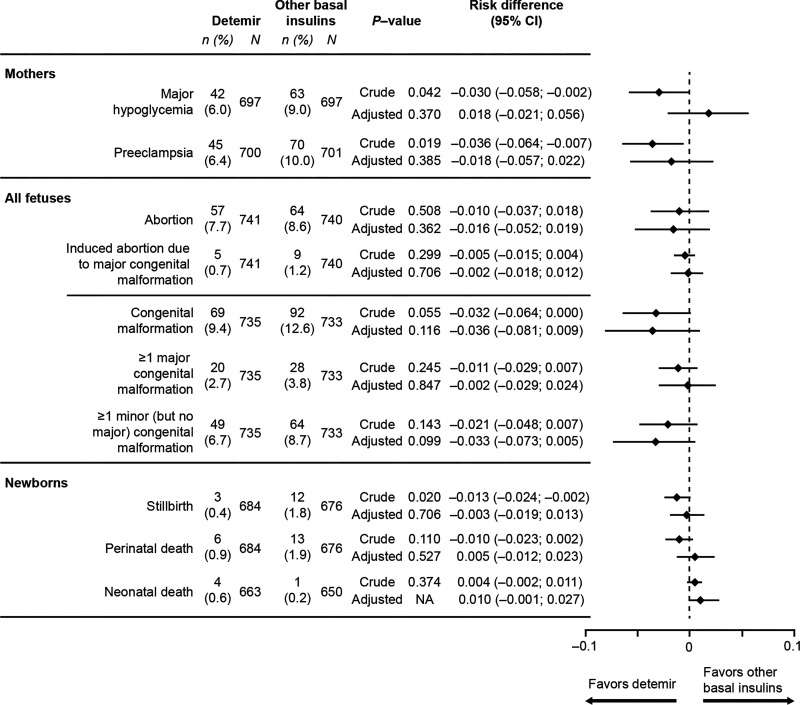
The crude prevalence of a fetus with at least one major or minor congenital malformation was lower with detemir versus other basal insulins (9.4% vs. 12.6%), as was the case for the prevalence of at least one major congenital fetal malformation (2.7% vs. 3.8%) or at least one minor malformation (6.7% vs. 8.7%). However, differences were not significant, either before or after adjustment for confounders (Fig. 2).
Figure 2.
Secondary pregnancy outcomes using detemir vs. other basal insulin, Absolute numbers shown for data before propensity score matching (crude data). Total n in analyses differs due to missing information on some of the end points. Adjusted values were adjusted by propensity score matching. NA, not available.
A total of 20 fetuses had at least one major congenital malformation in the detemir group versus 28 in the other basal insulin group. The most common major congenital malformations were related to the cardiovascular system (10 reported in the detemir group vs. 9 with other basal insulins), the genitourinary system (4 vs. 9), and the nervous system (7 vs. 8). A total of 49 fetuses in the detemir group had at least one minor, but no major, congenital malformations, versus 64 in the other basal insulin group. The most common minor congenital malformation was also related to the cardiovascular system (16 vs. 29 reported), followed by the genitourinary system (16 vs. 13).
The crude prevalence of perinatal death was lower with detemir versus other basal insulins (0.9% vs. 1.9%), as was the case for stillbirth (0.4% vs. 1.8%) or induced abortion of fetuses with major congenital malformations (0.7% vs. 1.2%) (Fig. 2). Although this difference was significant for stillbirth prior to adjustment, differences were not significant for any of these variables following adjustment for confounders (Fig. 2).
Estimated mean maternal HbA1c levels during pregnancy gradually decreased from conception (7.3% [56 mmol/mol] in patients using detemir and 7.5% [58 mmol/mol] with other basal insulins) to the end of the first (6.5% [48 mmol/mol] and 6.7% [50 mmol/mol]) and second trimesters (6.1% [43 mmol/mol] and 6.3% [45 mmol/mol]), after which levels rose slightly again at the end of the third trimester (6.3% [45 mmol/mol] and 6.4% [46 mmol/mol]) (Table 2). The crude estimated mean difference was significant at the end of the first (−0.181 [95% CI (−0.300, −0.062]; P = 0.003) and second (−0.139; [95% CI −0.232, −0.046]; P = 0.003) trimesters. However, no significant difference in estimated means was observed after adjustment for confounders (first trimester −0.070 [95% CI −0.201, 0.061]; and second trimester −0.013 [95% CI −0.121, 0.094]).
Table 2.
Maternal HbA1c with detemir vs. other basal insulin
| Detemir | Other basal insulins | Crude mean difference (95% CI),* P value | Adjusted mean difference (95% CI),* P value | |
|---|---|---|---|---|
| All mothers (n = 727) | All mothers (n = 730) | |||
| Estimated mean HbA1c, % (mmol/mol) | ||||
| Around conception | 7.3 (56) | 7.5 (58) | −0.145 (−0.350, 0.059), 0.163 | −0.051 (−0.280, 0.178), 0.665 |
| End of first trimester | 6.5 (48) | 6.7 (50) | −0.181 (−0.300, −0.062), 0.003 | −0.070 (−0.201, 0.061), 0.296 |
| End of second trimester | 6.1 (43) | 6.3 (45) | −0.139 (−0.232, −0.046), 0.003 | −0.013 (−0.121, 0.094), 0.805 |
| End of third trimester | 6.3 (45) | 6.4 (46) | −0.085 (−0.184, 0.014), 0.093 | 0.015 (−0.101, 0.132), 0.796 |
The association between treatment group and HbA1c during pregnancy was examined by a linear mixed model for repeated measurements. An unstructured covariance matrix was used. Estimated mean HbA1c values are shown for crude data. Women were included as random effects to adjust for within-women clustering, and fixed factors of country, age, gestational week, type of diabetes, diabetes duration, history of diabetes complications, history of severe hypoglycemia, history of fetal pregnancy complications, BMI, tobacco use, alcohol consumption, education, and bolus insulin and oral antidiabetic drug use were included. An interaction term between time and basal insulin treatment group was also evaluated to test (type 3 test) for differences in development of HbA1c over time. The OBSMARGINS option was used when estimating mean HbA1c.
Expressed in units of percent.
The crude analysis showed a significantly lower risk of major hypoglycemia (6.0% vs. 9.0%) and preeclampsia (6.4% vs. 10.0%) during pregnancy in women using detemir compared with other basal insulins (Fig. 2). However, these differences were no longer significant following adjustment for confounders (Fig. 2).
Conclusions
EVOLVE was a large prospective observational study evaluating the incidence of severe pregnancy complications in women with preexisting diabetes treated with detemir or other basal insulins in a real-world setting. The chance of having a newborn without major congenital malformations or perinatal or neonatal death was comparable between insulin detemir and other basal insulins. Crude analysis demonstrated a lower level of HbA1c and lower prevalence of major hypoglycemia, preeclampsia, and stillbirth in women using detemir, but these differences did not persist after adjustments.
Evidence for the effective use of basal insulin analogs in pregnant women with diabetes has largely come from retrospective studies (20,28,29). A large population-based cohort study, which included 1,661 women with pregestational diabetes from seven European regions, has previously demonstrated a lower risk of major congenital malformations when using insulin analogs compared with human insulin (30). Our previously published RCT investigated the use of detemir versus NPH insulin, both in combination with insulin aspart, in 310 pregnant women with type 1 diabetes (22). Data presented in the current prospective real-world cohort expand the data set more than fourfold and are in agreement with the low risk of these pregnancy complications previously seen with detemir in the randomized clinical trial (22,23). Despite the difference in the number of congenital malformations in women using detemir compared with other basal insulins not being statistically significant, the findings presented in this study may still be of clinical importance. Further studies evaluating the occurrence of congenital malformations with different insulin treatments are warranted.
Data from this large, prospective, observational cohort provide further support for the HbA1c-lowering effect of detemir in pregnant women with preexisting diabetes. The current study has a number of strengths. It was designed in collaboration with the EMA and used real-world data with a large sample size, covering many nationalities, ethnicities, and health systems. Longitudinal data were collected prospectively, in the context of routine practice, from early pregnancy to the age of 1 year of the infant. Inclusion criteria were broad, with very few exclusion criteria, and hence the results should be more broadly applicable to the real-world population than those of typical RCTs. Finally, there were many covariates for confounder control. Study limitations included the fact that real-life studies are not as strictly controlled as RCTs, women were recruited at selected specialized diabetes sites, and adverse pregnancy outcomes very early in pregnancy may have been missed. There were, in general, few events, due to their rarity, that resulted in relatively low statistical power for some end points. It is also important to stress that the number of women and fetuses included in the adjusted analysis were reduced considerably (by ∼40%) after propensity score matching.
To prevent congenital malformations, such as neural tube defects (31), women are advised to take folic acid supplementation prior to conception and in early pregnancy (32–34). Folic acid intake was unlikely to be a contributor to the numerically lower number of congenital malformations seen among women using insulin detemir since the intake of folic acid was reported to be less frequent in the insulin detemir group.
The prevalence of other chronic diseases and medication that may have an impact on the development of congenital malformations (35–43), as well as late diabetic complications such as diabetic nephropathy (44), were similar in the two treatment groups. HbA1c during the first trimester and the prevalence of major hypoglycemia were lower in women using insulin detemir in the crude analysis, and whether this might contribute to the numerically lower numbers of congenital malformations remains speculative. The majority of patients included in EVOLVE had type 1 diabetes. However, the pathophysiology of increased prevalence of congenital malformations and perinatal death in women with diabetes is linked to elevated blood glucose and is therefore likely to be similar in type 1 and type 2 diabetes (6).
Stillbirths were less common in women using insulin detemir. Whether a lower HbA1c value during the development of the placenta, and a more stable fasting glucose, with insulin detemir in comparison with other basal insulins throughout pregnancy play a role in this finding remain speculative (7).
Several important clinical outcomes (i.e., lower HbA1c and lower prevalence of severe hypoglycemia, preeclampsia, and stillbirth, as well as a numerically lower number of congenital malformations) in women using detemir were seen in the crude analysis. These differences did not persist after adjustments, but the number of women and fetuses included in the adjusted analysis were reduced considerably after propensity score matching. Even larger studies are needed to make firm conclusions.
In conclusion, treatment with insulin detemir was associated with a comparable risk to other basal insulins of major congenital malformations, perinatal or neonatal death, hypoglycemia, preec-lampsia, and stillbirth.
Article Information
Acknowledgments. The authors thank all investigators, staff, and participants of this study. The authors also thank Rikke Baastrup Nordsborg and Usha Thamattoor for data analysis, Charlotte Højelse for study management, and Pranav Kelkar and Renuka Munikrishnappa, all from Novo Nordisk, for the input into the manuscript. Medical writing and editorial assistance were provided by Jane Blackburn, Amy Hepple, and Helen Marshall of Ashfield MedComms, an Ashfield Health company, funded by Novo Nordisk A/S. A complete list of the investigators in the EVOLVE study can be found in the Supplementary Material.
Funding. The EVOLVE study, including study design, data collection, analysis, and interpretation, was funded by Novo Nordisk A/S. Medical writing and editorial assistance were also sponsored by Novo Nordisk A/S.
Duality of Interest. E.R.M. has received speakers fees from Novo Nordisk, Eli Lilly and Company, and Sanofi Aventis; has participated in steering committee tasks and guidance involving writing protocols for Novo Nordisk; is participating in several multinational clinical studies on the use of insulin in pregnant women with preexisting diabetes, in collaboration with Novo Nordisk. K.C. has received speaker honoraria from or has participated in advisory boards for Novo Nordisk, Eli Lilly and Company, Sanofi, AstraZeneca, Boehringer Ingelheim, and Servier. J.D. has received speaker honoraria from or has participated in advisory boards for Novo Nordisk, Eli Lilly and Company, Sanofi, Amgen, Abbott, AstraZeneca, Boehringer Ingelheim, Mundipharma, and Merck Sharp & Dohme. A.C.A., M.-A.G., and L.L.N.H. are employees of, and hold shares in, Novo Nordisk. S.D.G. is a consultant for Sanofi, Eli Lilly and Company, Novo Nordisk, Takeda Pharmaceutical Company, Merck Sharp & Dohme, Servier, and Theracos. H.P.H. has received lecturer and scientific advisor fees from Abbott, AstraZeneca, Eli Lilly and Company, Insulet, LifeScan, and Novo Nordisk and research grants from Abbott, LifeScan, and Novo Nordisk. H.-P.K. has received speaker honoraria from, or has participated in advisory boards for, Novo Nordisk, Eli Lilly and Company, Sanofi, AstraZeneca, and Servier. D.R.M. has received speaker honoraria from, or has participated in advisory boards for, Novo Nordisk. P.D. is participating in clinical studies on the use of insulin analogs in pregnant women with preexisting diabetes and participates in an expert committee on this topic, in collaboration with Novo Nordisk, no personal honorarium is involved. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.R.M., N.A., A.C.A., E.A., K.C., H.d.V., J.D., F.D., M.-A.G., S.D.G., H.P.H., L.L.N.H., M.I., H.-P.K., D.R.M., and P.D. all interpreted results and provided input, critical review, and approval for the manuscript. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. E.R.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented in poster form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020, and in oral form at the 56th Annual Meeting of the European Association for the Study of Diabetes, 21–25 September 2020.
Footnotes
Clinical trial reg. no. NCT01892319, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14769912.
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 9th edition, 2019. Accessed 12 November 2020. Available from: https://www.diabetesatlas.org/
- 2. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 2009;32:2005–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eidem I, Stene LC, Henriksen T, et al. Congenital anomalies in newborns of women with type 1 diabetes: nationwide population-based study in Norway, 1999–2004. Acta Obstet Gynecol Scand 2010;89:1403–1411 [DOI] [PubMed] [Google Scholar]
- 4. Eidem I, Vangen S, Hanssen KF, et al. Perinatal and infant mortality in term and preterm births among women with type 1 diabetes. Diabetologia 2011;54:2771–2778 [DOI] [PubMed] [Google Scholar]
- 5. Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 2004;27:2819–2823 [DOI] [PubMed] [Google Scholar]
- 6. Macintosh MC, Fleming KM, Bailey JA, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ 2006;333:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathiesen ER, Ringholm L, Damm P. Stillbirth in diabetic pregnancies. Best Pract Res Clin Obstet Gynaecol 2011;25:105–111 [DOI] [PubMed] [Google Scholar]
- 8. Colstrup M, Mathiesen ER, Damm P, Jensen DM, Ringholm L. Pregnancy in women with type 1 diabetes: have the goals of St. Vincent declaration been met concerning foetal and neonatal complications? J Matern Fetal Neonatal Med 2013;26:1682–1686 [DOI] [PubMed] [Google Scholar]
- 9. Mackin ST, Nelson SM, Kerssens JJ, et al.; SDRN Epidemiology Group . Diabetes and pregnancy: national trends over a 15 year period. Diabetologia 2018;61:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014;103:341–363 [DOI] [PubMed] [Google Scholar]
- 11. Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia 2000;43:79–82 [DOI] [PubMed] [Google Scholar]
- 12. Scheen AJ, Radermecker RP, Philips JC, Paquot N. [Insulin detemir (Levemir)]. Rev Med Liege 2005;60:814–819 [PubMed] [Google Scholar]
- 13. Goldman-Levine JD, Lee KW. Insulin detemir--a new basal insulin analog. Ann Pharmacother 2005;39:502–507 [DOI] [PubMed] [Google Scholar]
- 14. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
- 15. Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab 2007;9:290–299 [DOI] [PubMed] [Google Scholar]
- 16. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 2008;81:184–189 [DOI] [PubMed] [Google Scholar]
- 17. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab 2009;11:372–378 [DOI] [PubMed] [Google Scholar]
- 18. Novo Nordisk . Levemir Summary of Product Characteristics. Accessed 12 November 2020. Available from http://www.novonordiskmedical.com/our-products/levemir.html
- 19. Sanofi . Lantus Summary of Product Characteristics. Accessed 12 November 2020. Available from https://products.sanofi.us/lantus/lantus.html
- 20. Mathiesen ER, Damm P, Jovanovic L, et al. Basal insulin analogues in diabetic pregnancy: a literature review and baseline results of a randomised, controlled trial in type 1 diabetes. Diabetes Metab Res Rev 2011;27:543–551 [DOI] [PubMed] [Google Scholar]
- 21. de Jong J, Garne E, Wender-Ozegowska E, Morgan M, de Jong-van den Berg LT, Wang H. Insulin analogues in pregnancy and specific congenital anomalies: a literature review. Diabetes Metab Res Rev 2016;32:366–375 [DOI] [PubMed] [Google Scholar]
- 22. Mathiesen ER, Hod M, Ivanisevic M, et al.; Detemir in Pregnancy Study Group . Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care 2012;35:2012–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hod M, Mathiesen ER, Jovanovič L, et al. A randomized trial comparing perinatal outcomes using insulin detemir or neutral protamine Hagedorn in type 1 diabetes. J Matern Fetal Neonatal Med 2014;27:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005 [DOI] [PubMed] [Google Scholar]
- 25. Mathiesen ER, Andersen H, Kring SI, Damm P. Design and rationale of a large, international, prospective cohort study to evaluate the occurrence of malformations and perinatal/neonatal death using insulin detemir in pregnant women with diabetes in comparison with other long-acting insulins. BMC Pregnancy Childbirth 2017;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. EUROCAT Central Registry . EUROCAT Guide 1.3 and reference documents: Instructions for the Registration and Surveillance of Congenital Anomalies, 2005. Accessed 12 November 2020. Available from https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/EUROCAT-Guide-1.3.pdf
- 27. Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 1998;17:2635–2650 [PubMed] [Google Scholar]
- 28. O’Neill SM, Kenny LC, Khashan AS, West HM, Smyth RM, Kearney PM. Different insulin types and regimens for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev 2017;2:CD011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Callesen NF, Damm J, Mathiesen JM, Ringholm L, Damm P, Mathiesen ER. Treatment with the long-acting insulin analogues detemir or glargine during pregnancy in women with type 1 diabetes: comparison of glycaemic control and pregnancy outcome. J Matern Fetal Neonatal Med 2013;26:588–592 [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Wender-Ozegowska E, Garne E, et al. Insulin analogues use in pregnancy among women with pregestational diabetes mellitus and risk of congenital anomaly: a retrospective population-based cohort study. BMJ Open 2018;8:e014972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2015;12:CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Institute for Health and Care Excellence . NICE guideline [NG3]: Diabetes in pregnancy: management from preconception to the postnatal period, 2015. Accessed 12 March 2019. Available from https://www.nice.org.uk/guidance/ng3 [PubMed]
- 33. Chitayat D, Matsui D, Amitai Y, et al. Folic acid supplementation for pregnant women and those planning pregnancy: 2015 update. J Clin Pharmacol 2016;56:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ringholm L, Mathiesen ER, Kelstrup L, Damm P. Managing type 1 diabetes mellitus in pregnancy--from planning to breastfeeding. Nat Rev Endocrinol 2012;8:659–667 [DOI] [PubMed] [Google Scholar]
- 35. Stubert J, Reister F, Hartmann S, Janni W. The risks associated with obesity in pregnancy. Dtsch Arztebl Int 2018;115:276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antza C, Cifkova R, Kotsis V. Hypertensive complications of pregnancy: a clinical overview. Metabolism 2018;86:102–111 [DOI] [PubMed] [Google Scholar]
- 37. Giacobbe AM, Grasso R, Triolo O, Tonni G, Granese R. Thyroid diseases in pregnancy: a current and controversial topic on diagnosis and treatment over the past 20 years. Arch Gynecol Obstet 2015;292:995–1002 [DOI] [PubMed] [Google Scholar]
- 38. Mongan D, Lynch J, Hanna D, et al. Prevalence of self-reported mental disorders in pregnancy and associations with adverse neonatal outcomes: a population-based cross-sectional study. BMC Pregnancy Childbirth 2019;9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonham CA, Patterson KC, Strek ME. Asthma outcomes and management during pregnancy. Chest 2018;153:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakhai-Pour HR, Rey E, Bérard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B Dev Reprod Toxicol 2010;89:147–154 [DOI] [PubMed] [Google Scholar]
- 41. Garne E, Vinkel Hansen A, Morris J, et al. Risk of congenital anomalies after exposure to asthma medication in the first trimester of pregnancy - a cohort linkage study. BJOG 2016;123:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomson T, Xue H, Battino D. Major congenital malformations in children of women with epilepsy. Seizure 2015;28:46–50 [DOI] [PubMed] [Google Scholar]
- 43. Bérard A, Zhao J-P, Sheehy O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec Pregnancy Cohort. BMJ Open 2017;7: e013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathiesen ER, Ringholm L, Feldt-Rasmussen B, Clausen P, Damm P. Obstetric nephrology: pregnancy in women with diabetic nephropathy--the role of antihypertensive treatment. Clin J Am Soc Nephrol 2012;7:2081–2088 [DOI] [PubMed] [Google Scholar]