Abstract
OBJECTIVES
Patients with type 1 diabetes (T1D) exhibit modest lipid abnormalities as measured by traditional metrics. This study aimed to identify lipidomic predictors of rapid decline of kidney function in T1D.
RESEARCH DESIGN AND METHODS
In a case-control study, 817 patients with T1D from three large cohorts were randomly split into training and validation subsets. Case was defined as >3 mL/min/1.73 m2 per year decline in estimated glomerular filtration rate (eGFR), while control was defined as <1 mL/min/1.73 m2 per year decline over a minimum 4-year follow-up. Lipids were quantified in baseline serum samples using a targeted mass spectrometry lipidomic platform.
RESULTS
At individual lipids, free fatty acid (FFA)20:2 was directly and phosphatidylcholine (PC)16:0/22:6 was inversely and independently associated with rapid eGFR decline. When examined by lipid class, rapid eGFR decline was characterized by higher abundance of unsaturated FFAs, phosphatidylethanolamine (PE)-Ps, and PCs with an unsaturated acyl chain at the sn1 carbon, and by lower abundance of saturated FFAs, longer triacylglycerols, and PCs, PEs, PE-Ps, and PE-Os with an unsaturated acyl chain at the sn1 carbon at eGFR ≥90 mL/min/1.73 m2. A multilipid panel consisting of unsaturated FFAs and saturated PE-Ps predicted rapid eGFR decline better than individual lipids (C-statistic, 0.71) and improved the C-statistic of the clinical model from 0.816 to 0.841 (P = 0.039). Observations were confirmed in the validation subset.
CONCLUSIONS
Distinct from previously reported predictors of GFR decline in type 2 diabetes, these findings suggest differential incorporation of FFAs at the sn1 carbon of the phospholipids’ glycerol backbone as an independent predictor of rapid GFR decline in T1D.
Introduction
Diabetes is the leading cause of kidney failure in the U.S. and many parts of the world. Prevention of diabetic kidney disease (DKD) progression requires identification of high-risk patients at an early stage when the preventive strategies may provide the opportunities for preservation of kidney function. Historically, assessment of kidney function in clinical care of DKD has been limited to estimated glomerular filtration rate (eGFR) and urine albumin excretion, which have limited accuracy and predictive power early in diabetes when kidney function is normal. With the evolution of precision medicine and the application of high-throughput data-generating platforms, interrogation of molecular interplays is a promising approach to address this challenge. As such, some recent studies have identified the predictive role of inflammatory markers in renal function decline that are shared in both type 1 (T1D) and type 2 diabetes (T2D) (1–5).
Alteration of lipid metabolism is an integral manifestation of diabetes. In an earlier study, we showed that alterations of circulating free fatty acids (FFAs), acylcarnitines (ACs), and glycerolipids were significantly linked to transcriptional regulators of de novo lipogenesis and mitochondrial β-oxidation, suggesting a role for upregulation of de novo lipogenesis and impaired β-oxidation in DKD progression in T2D. In T2D, such lipid alterations are partly mediated by insulin resistance, which leads to upregulation of acetyl-CoA carboxylase, a key promoter of de novo lipogenesis (6). Because patients with T1D with normal kidney function and body weight are likely insulin sensitive, we anticipated that the lipidomic predictors of DKD progression in T1D would differ from those in T2D. We hypothesized that a unique pattern of circulating FFAs, ACs, and glycerophospholipids would discriminate the rapid from slow decliners of GFR in T1D and that this pattern is different from that in T2D.
Research Design and Methods
Cohorts
Cohort design and sample selection are presented elsewhere (7). In brief, 817 patients with a history of T1D and available fasting plasma samples were selected from three established cohorts, including the Steno Diabetes Center Copenhagen study (Steno, n = 398), the Epidemiology of Diabetes Complications study (EDC, n = 139) and the Coronary Artery Calcification in Type 1 Diabetes study (CACTI, n = 281), and were randomly split in to training and validation subsets, aimed at validating the findings of the training subset (Supplementary Fig. 1). Samples were gathered from participants examined between 1995 and 2011 and stored at −80°C. Inclusion criteria were eGFR ≥30 mL/min/1.73 m2 at baseline, follow-up of at least 4 years, at least 3 longitudinal eGFR measurements, and baseline plasma sample availability. Rapid decline of eGFR (case group) was defined as an annual decline in eGFR ≥3 mL/min/1.73 m2, and slow decline (control group) was defined as having no decline or <1 mL/min/1.73 m2 annual decline in eGFR.
Lipids Studied
Using plasma samples obtained at baseline, we applied an untargeted lipidomic platform to identify the lipid classes with the highest number of differentially quantified lipids between the slow and rapid eGFR decliners using previously published methods (6,8,9). Next, using a targeted lipidomic platform, we quantified 324 lipids, including free fatty acids (FFAs, n = 13), lysophosphatidylcholines (LPCs, n = 16), sphingomyelins (SMs, n = 14), phosphatidylcholines (PCs, n = 59), triacylglycerols (TAGs, n = 65), diacylglycerols (DAGs, n = 10), cholesteryl esters (n = 11), phosphatidylethanolamines (PEs, n = 40), PE-Os (PE with alkyl ether substitute at sn-1 carbon of the glycerol backbone, n = 21), PE-Ps (PE with alkenyl ether substitute at sn-1 carbon, n = 46), and ACs (n = 29) (Supplementary Table 1), using previously described mass spectrometry-based quantification methods (6,8–11), with details described in the Supplementary Materials and Methods.
Statistical Methods
We used mean ± SD and count with relative frequency (%) to describe normally distributed continuous and categorical variables, respectively. When describing skewed variables, we used median and interquartile range. We applied the Student t test to compare normally distributed continuous variables, the Kolmogorov-Smirnov test to compare skewed continuous variables, and the χ2 test to compare categorical variables between two groups. We applied an estimation maximization algorithm using the patients’ characteristics and laboratory values for imputation of clinical covariates, which imputed 15% of the missing data on background variables.
After quantification, the lipids were intraclass sum normalized, logit transformed, and z-score standardized for downstream analyses. To identify the top differentially measured lipids, we applied a compound-by-compound t test with false discovery rate correction using the Benjamini-Hochberg procedure (12). We applied principal component analysis (PCA) to reduce the number of lipids of the FFA, TAG, PC, PE, PE-O, PE-P, and SM to subclasses using orthogonal varimax rotation. The rationale for use of PCA was to build secondary variables with increased coverage of total variance predictive power compared with that of individual lipids as well as to decrease multiplicity and false discovery (6,8,13).
To identify independent lipidomic predictors of rapid eGFR decline, we used separate logistic regression models including 1) top differentially regulated lipids that were validated in the validation subset as predictors, and 2) principle components as predictors. Both models were built without and with adjusting for clinical variables (age, sex, race, duration of diabetes, history of hypertension, use of antihypertensive and lipid-lowering medications, baseline hemoglobin A1c [HbA1c], eGFR, and albumin-to-creatinine ratio [ACR]), with deletion of nonsignificant covariates in the adjusted models. We compared the C-statistic of a panel of lipids predicting rapid decline when added to clinical variables and replicated the finding in the validation subset with coefficients that were developed in the training subset using the Wilk likelihood ratio test to prognostic gain. Subgroup analysis was performed by categories of eGFR and albuminuria (ACR cutoff of 30 mg/g). In a subgroup analysis, we compared the alteration of lipid factors by eGFR categories and their interaction terms using a two-way ANOVA and the likelihood ratio tests in both the training and the validation subsets. We compared the abundance of lipid factors in rapid versus slow decliners of eGFR stratified by eGFR categories in both the training and the validation subsets. We used a linear mixed model to test the significance of lipid alteration in fast eGFR decline by eGFR categories. Statistical analyses were performed separately on both the training and validation subsets. Using a meta-analysis, we showed the pooled effect of alterations in lipid factors associated with rapid eGFR decline.
Results
Baseline Characteristics
Overall, the distribution of baseline characteristics in both the training and the validation subsets were comparable. In the training subset, rapid eGFR decliners were younger, had a higher baseline eGFR, and were more likely to have a urine ACR >30 mg/g, a pattern that was replicated in the validation subset (Table 1). In the validation subset, the rapid decliners were younger and more likely to be women, White, have hypertension, higher baseline eGFR, and use an ACE inhibitor at baseline. The rest of the baseline clinical characteristics were not significantly different between the rapid and slow eGFR decliners in the two subsets.
Table 1.
Comparing baseline characteristics of slow and rapid decline of eGFR in patients with type 1 diabetes in the training and validation subsets
| Training subset | Validation subset | |||
|---|---|---|---|---|
| Variables | Slow decline (n = 284) | Fast decline (n = 123) | Slow decline (n = 283) | Fast decline (n = 127) |
| Age, years | 47 ± 13 | 39 ± 12* | 46 ± 13 | 41 ± 11* |
| Male sex | 152 (53.5) | 57 (46.3) | 156 (55.1) | 46 (36.2)* |
| White race | 281 (98.9) | 119 (96.7) | 278 (99.3) | 118 (93.7)* |
| Duration of diabetes, years | 29 ± 14 | 26 ± 11 | 28 ± 13 | 28 ± 11 |
| Height, m | 1.72 ± 0.09 | 1.72 ± 0.08 | 1.73 ± 0.09 | 1.71 ± 0.09 |
| Weight, kg | 76 ± 16 | 77 ± 15 | 77 ± 15 | 73 ± 15 |
| BMI, kg/m2 | 25.6 ± 4.1 | 26.1 ± 4.3 | 25.8 ± 3.8 | 25.8 ± 4.8 |
| Smoking | 39 (13.7) | 28 (22.8) | 34 (12.0) | 19 (15.0) |
| Blood pressure, mmHg | ||||
| Systolic | 128 ± 19 | 125 ± 19 | 126 ± 18 | 127 ± 20 |
| Diastolic | 75 ± 10 | 76 ± 10 | 75 ± 10 | 76 ± 11 |
| Hypertension | 88 (31.0) | 48 (39.0) | 72 (25.4) | 61 (48.0)* |
| Proliferative retinopathy | 158 (55.6) | 58 (47.2) | 151 (53.4) | 67 (52.8) |
| Use of ACE inhibitors | 96 (33.8) | 45 (36.6) | 70 (22.7) | 48 (37.8)* |
| Use of lipid-lowering agents | 39 (13.7) | 17 (13.8) | 39 (13.8) | 13 (10.2) |
| HbA1c, % | 8.4 ± 1.0 | 8.6 ± 1.6 | 8.3 ± 0.9 | 8.7 ± 1.5 |
| eGFR, mL/min/1.73 m2 | 89 ± 19 | 100 ± 27* | 89 ± 18 | 98 ± 27* |
| ΔeGFR, mL/min/year | 0.6 ± 1.7 | −4.9 ± 2.2* | 0.8 ± 1.9 | −6.0 ± 7.2* |
| Urine ACR >30 mg/g | 63 (22.2) | 60 (48.8)* | 64 (22.6) | 63 (49.6)* |
| eGFR categories | ||||
| ≥90 mL/min/1.73 m2 | 139 (48.9) | 83 (67.5)* | 142 (50.2) | 86 (67.7)* |
| 60–89 mL/min/1.73 m2 | 131 (46.1) | 29 (23.6) | 129 (45.6) | 29 (22.8) |
| <60 mL/min/1.73 m2 | 14 (4.9) | 11 (8.9) | 12 (4.2) | 12 (9.4) |
Data are presented as mean ± SD or n (%).
Statistical significance of P < 0.01 when comparing fast vs. slow decline subgroups.
Compound-by-Compound Analysis
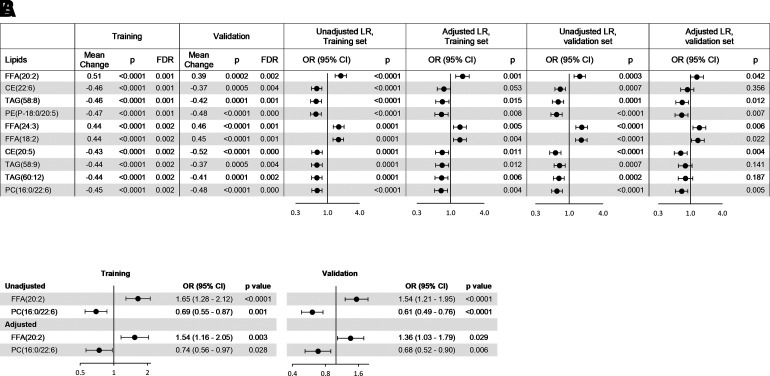
The median and interquartile range of the lipids (in micromoles per liter) in rapid and slow decliners in the entire subsets as well as stratified by eGFR categories and presence or absence of albuminuria (urine ACR>30 mg/g) in both the training and validation subsets are shown in Supplementary Table 2. In total, 47 lipids were significantly different between the rapid and slow decliners, passed the false discovery rate threshold of <0.05 in the training set, and were replicated in the validation subset based on a univariate compound-by-compound analysis (Supplem-entary Fig. 5). The top 10 are shown in Fig. 1A. Plasma concentrations of FFA(20:2), FFA(24:3), and FFA(18:2) were higher at baseline in rapid decliners, and plasma concentrations of other complex lipids were lower compared with slow decliners. Unadjusted logistic regression models showed each 1-SD higher concentration of FFA(20:2), FFA(24:3), or FFA(18:2) was associated with significantly higher odds of rapid decline (P < 0.0003) in the training subset, an observation which was replicated in the validation subset (P ≤ 0.007) (Fig. 1A). In contrast, each 1-SD higher concentration of other complex lipids was associated with lower odds of rapid decline. After adjusting for age, sex, duration of diabetes, baseline mean arterial pressure, HbA1c levels, eGFR, and ACR, the significant association of most lipids with rapid decline disappeared or decreased toward null. Only FFA(20:2), FFA(24:3), and FFA(18:2) continued to remain associated with a significantly higher odds of fast progression and PE(P-18:0/20:5), cholesteryl ester(20:5), PC(16:0/22:6), PE(P-18:1/20:5), and PC(16:0/20:5), with a significantly lower odds of fast progression for each 1-SD increase in their levels both in the training and the validation subsets (Fig. 1A).
Figure 1.
A: The top 10 lipids with differential levels in patients with fast vs. slow progression of kidney disease in the training set and replicated in the validation set. Mean change is shown as standardized mean difference. Logistic regressions (LR) are unadjusted or adjusted univariate models for each one lipid at a time, but listing all of the covariates listed below. B: Individual lipids predictors of fast progression independent of the top seven significant validated lipids are shown unadjusted and adjusted with clinical covariates. Adjustment was for age, sex, race, duration of diabetes, hypertension, use of ACE inhibitor or angiotensin receptor blocker, use of lipid-lowering agents, baseline mean arterial pressure, HbA1c, eGFR, and urine ACR. CE, cholesteryl ester; FDR, false discovery rate; OR, odds ratio.
Next, we used a multiple logistic regression model that included all three FFAs and five complex lipids associated with fast progression in both the training and validation subsets independent of clinical covariates. Only FFA(20:2) and PC(16:0/22:6) remained significantly associated with fast progression independent of the other lipids, so that each 1-SD increase in FFA(20:2) was associated with a 1.65-fold higher odds of fast progression (95% CI 1.28–2.12; P < 0.0001), and each 1-SD increase in PC(16:0/22:6) was associated with a 0.69-fold (95% CI 0.55–0.87; P = 0.001) lower odds of rapid decline. After adjusting for clinical covariates, these values were 1.54 (95% CI 1.16–2.05; P = 0.003) and 0.74 (95% CI 0.56–0.97; P = 0.028), respectively. Similarly, each 1-SD increase in FFA(20:2) in the validation subset was associated with a 1.54-fold higher odds of rapid decline (95% CI 1.21–1.95; P = 0.0004), and each 1-SD increase in PC(16:0/22:6) was associated with 0.61-fold (95% CI 0.49–0.76; P < 0.0001) lower odds of rapid decline. After adjusting for clinical covariates, these odds remained significant at 1.36 (95% CI 1.03–1.79; P = 0.029) and 0.68 (95% CI 0.52–0.90; P = 0.006), respectively (Fig. 1B).
PCA
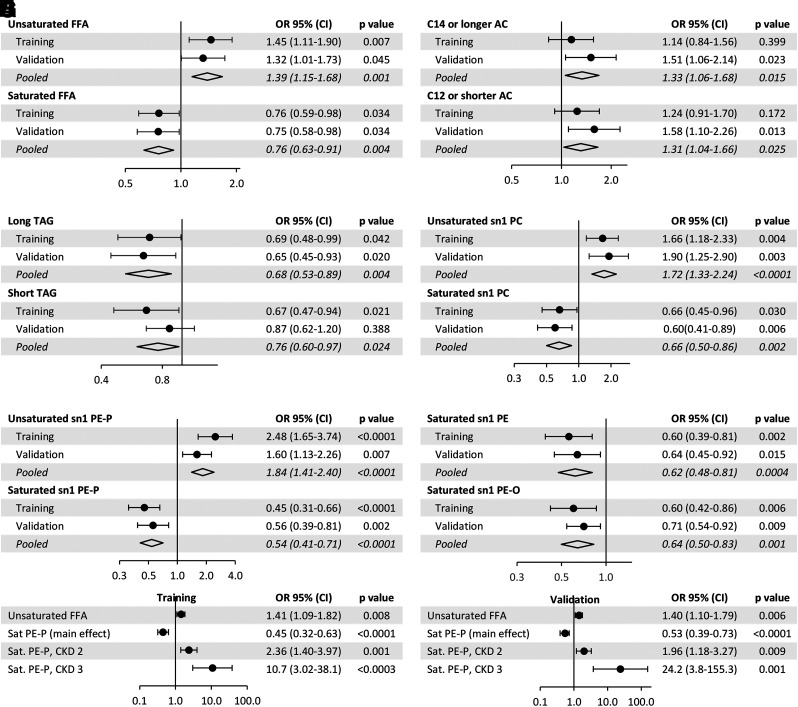
We reduced the lipids to secondary variables composed of primarily saturated versus unsaturated FFAs, AC ≤12 carbons versus ≥14 carbons, shorter TAGs versus longer TAGs, and saturated complex phospholipids at the sn1 carbon versus unsaturated complex phospholipids at the sn1 carbon (Supplementary Fig. 6). Using multiple logistic regression models adjusted for clinical covariates, we found that each 1-SD decrease in saturated FFAs and increase in unsaturated FFAs was independently associated with rapid eGFR decline (Fig. 2A). Neither the short nor the long ACs were inversely associated with rapid decline (Fig. 2B). An abundance of longer TAGs was inversely and independently associated with rapid decline (Fig. 2C). Each 1-SD increase in unsaturated sn1 PCs (PCs with an unsaturated acyl at the sn1 carbon) and unsaturated sn1 PE-Ps, and each 1 SD decrease in saturated sn1 PCs, sn1 PEs, sn1 PE-Ps, and sn1 PE-Os was associated with higher odds of DKD progression independent of clinical covariates (Fig. 2D–F).
Figure 2.
A–F: Odds of eGFR decline by each 1-SD difference in the level of the corresponding lipids in single-lipid panels using multiple logistic regression models adjusted for baseline eGFR, urine album-creatinine ratio, HbA1c, age, sex, race, duration of diabetes, hypertension, and use of ACE inhibitor and lipid-lowering agents, with elimination of nonsignificant covariates from the model, and interaction of the lipid with eGFR categories. G and H: Odds of eGFR decline by each 1-SD difference in a multilipid panel using multiple logistic regression with a main effect of saturated PE-P and interaction effects of saturated PE-P and unsaturated FFA by eGFR categories with eGFR ≥90 mL/min/1.73 m2 as the reference category. OR, odds ratio.
Using a multilipid panel, defined as 12 principal components (Fig. 2A–F) in a logistic regression model, we found unsaturated FFAs and saturated sn1 PE-Ps were associated with rapid decline independent of other lipids (Fig. 2G and H). In the training set, each 1-SD increase in unsaturated FFA was associated with 1.41-fold higher odds of rapid decline (95% CI 1.09–1.82; P = 0.008), and each 1-SD increase in saturated sn1 PE-P (the main effect variable) was associated with 0.45-fold lower odds of rapid decline (95% CI 0.32–0.63; P < 0.0001).
Alteration of Lipid Factors by Albuminuria and Categories of eGFR
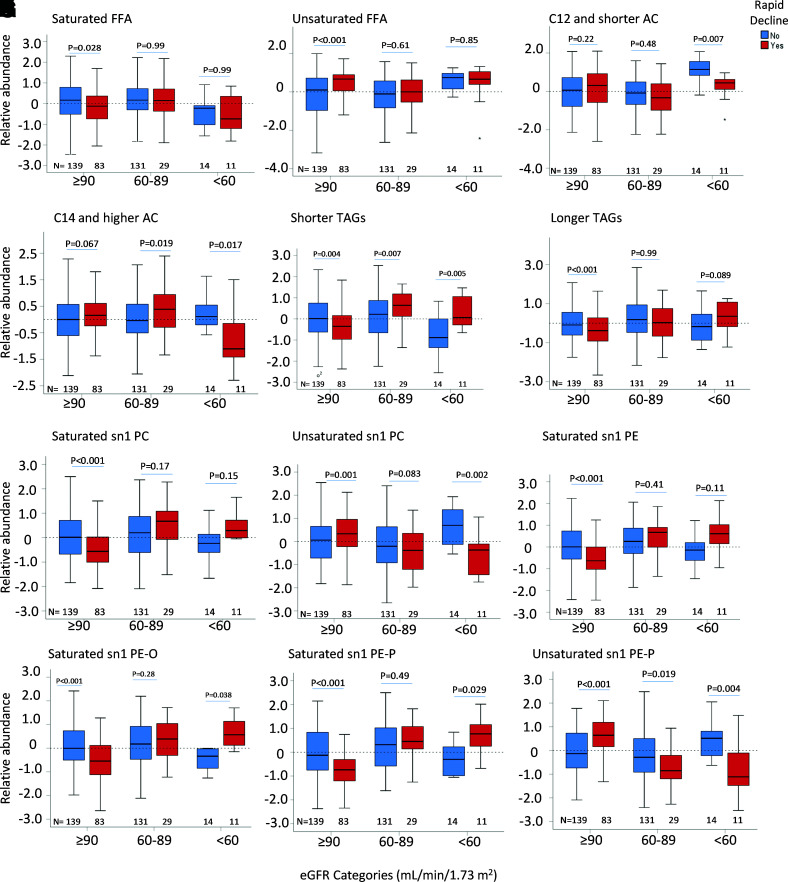
Overall, the lipid alterations were not different by the presence or absence of albuminuria in the training or validation subsets. In addition to stratified analysis by presence or absence of microalbuminuria, all of the downstream models were adjusted by ACR. In the training subset, although FFAs and ACs did not differ significantly in the rapid or slow decliners by eGFR categories (Fig. 3A–D), the rapid decliners at eGFR ≥90 mL/min/1.73 m2 had a higher abundance of unsaturated FFAs (P < 0.001) (Fig. 3B) and a relatively lower abundance of phospholipids containing a saturated acyl chain at the sn1 carbon (P < 0.001) (Fig. 3G and I–K). When the abundance of lipids in the two groups of fast and slow decliners was compared by categories of eGFR, we noted a higher abundance of both shorter (P < 0.001) (Fig. 3E) and longer (P = 0.007) (Fig. 3F) TAGs in rapid decliners at lower eGFR categories, while there were not any significant changes in TAGs by eGFR decline in slow eGFR decliners (Fig. 3E and F). Similarly, in rapid decliners, there was a higher abundance of saturated sn1 PCs (P < 0.001) (Fig. 3G), sn1 PEs (P < 0.001) (Fig. 3I), sn1 PE-Os (P < 0.001) (Fig. 3J), and sn1 PE-Ps (P < 0.001) (Fig. 3K), but a lower abundance of unsaturated sn1 PCs (P < 0.001) (Fig. 3H) and sn1 PE-Ps (P < 0.001) (Fig. 3L) by worsening categories of eGFR. There was no significant difference in the abundance of unsaturated sn1 PE by eGFR categories. The significance of changes in lipid abundance by eGFR categories was replicated in the validation subset (Supplementary Fig. 7A–L). When the interaction term of saturated sn1 PE-Ps by eGFR categories was tested and compared with eGFR ≥90 mL/min/1.73 m2 as the reference category, each 1-SD increase in saturated PE-P was associated with 2.36-fold higher odds of rapid decline in patients with eGFR of 60–89 mL/min/1.73 m2 (95% CI 1.40–3.97; P = 0.001) and 10.7-fold higher odds of rapid decline in patients with eGFR <60 mL/min/1.73 m2 (95% CI 3.02–38.1; P < 0.0003) (Fig. 2G). Similar findings were replicated in the validation subset (Fig. 2H).
Figure 3.
A–L: Comparing principal components representative of saturated and unsaturated FFA, AC, TAG, PC, and PE in rapid vs. slow eGFR decliners stratified by baseline eGFR categories in the training subset. Trend P values of lipids by eGFR categories were <0.001 for shorter TAGs, 0.007 for longer TAGs, and ≤0.001 for other phospholipids in rapid decliners.
System-Based Lipid Alterations
Supplemental Fig 8 illustrates systembased integrated alterations of individual lipids by lipid class and by acyl chain and saturation status in rapid and slow decliners across eGFR categories. In patients with eGFR >90 mL/min/1.73 m2, rapid decliners exhibited higher unsaturated FFAs and unsaturated PCs at the sn1 carbon but a low abundance of saturated phospholipids at the sn1 carbon. They also showed inverse correlations between unsaturated FFAs and phospholipids containing a saturated acyl chain at the sn1 carbon (P < 0.001) (Supplementary Fig. 8A). In patients with eGFR 60–89 mL/min/1.73 m2, rapid decliners exhibited a relatively higher abundance of saturated FFA and saturated phospholipids at their sn1 carbon but a lower abundance of unsaturated phospholipids at their sn1 carbon. They also showed inverse correlations between unsaturated FFAs and phospholipids containing saturated acyls at their sn1 carbon (Supplementary Fig. 8B).
Across the entire training subset, saturated lipids from different classes had highly significant direct correlations with each other. Similarly, we noticed highly significant direct correlations between different classes of unsaturated lipids. In contrast, saturated and unsaturated lipids were significantly and inversely correlated, a pattern that was replicated in the validation set (Supplementary Fig. 9A).
Classification
In the training set, the C-statistic of the FFA(20:2) and PC(16:0/22:6) was 0.610 (95% CI 0.555–0.664) (Supplementary Fig. 9B). On the other hand, the C-statistic of principal component-derived multipanel lipid factors consisting of unsaturated FFA and saturated PE-P (Fig. 2G) was 0.707 (95% CI 0.652–0.763) (Supplementary Fig. 9B). When added to a clinical model with C-statistics of 0.816 (95% CI 0.771–0.861), the full model had an incremental C-statistic up to 0.841 (95% CI 0.799–0.883) in the training set (P < 0.0001 compared with individual lipids, and P = 0.039 compared with clinical model). Using the coefficients developed in the training subset, we found the incremental C-statistic of the corresponding model was replicated in the validation subset (Supplementary Fig. 9B).
Conclusions
At individual lipids, higher FFA(20:2) and lower PC(16:0/22:6), also known as PC(38:6), were independently associated with higher odds of rapid eGFR decline. At the class level, higher levels of unsaturated FFAs, and PCs and PE-Ps with an unsaturated acyl at the sn1 carbon, and lower saturated FFAs and PCs and PEs (PE, PE-P, and PE-O) with a saturated acyl at sn1 were associated with higher odds of rapid eGFR decline. We also observed low or unchanged levels of shorter TAGs and low levels of longer-chain TAGs in rapid decliners without any evidence of decrease in short- or long-chain ACs. When stratified by eGFR categories, rapid decliners with an eGFR ≥90 mL/min/1.73 m2 had a higher abundance of unsaturated FFA and a lower abundance of phospholipids containing saturated sn1 acyl chains. Conversely, rapid decliners with an eGFR of 60–89 mL/min/1.73 m2 had a relatively lower abundance of unsaturated FFAs and a higher abundance of phospholipids containing saturated sn1 acyl chains. With worsening eGFR categories, rapid decliners showed increased abundance of longer-chain TAGs and phospholipids (PC, PE, PE-O, and PE-P) containing a saturated acyl at the sn1 position and decreased abundance of phospholipids (PC and PE-P) containing an unsaturated acyl at sn1 (Supplementary Fig. 10A).
We also noted that the rapid decliners were younger. One explanation might be that younger age was a surrogate of a more severe disease that started earlier in life and contributed to faster decline of eGFR in this age group. Although, lipiduria is a known complication of nephrotic range proteinuria or nephrotic syndrome, urinary lipid loss in microalbuminuria is minimal. In this study, all patients with albuminuria had microalbuminuria. Furthermore, multivariate adjustments by ACR did not alter the association of lipids with fast eGFR decline, suggesting that associations were independent of ACR and microalbuminuria. Dysregulation of glycerophospholipids in patients destined to develop T1D precedes the appearance of islet cell autoantigens, T1D, or kidney dysfunction as diagnosed by eGFR (14–20). For example, La Torre et al. (14) reported decreased abundance of PEs and PCs, including PC(38:6), one of the top two independent candidates in our study, in the cord blood of children who subsequently developed T1D compared with a control group. In another study among individuals with T1D from Denmark, the serum, PC(O-34:2), PC(O-34:3), SM(d18:1/24:0), SM(d40:1), and SM(d41:1) were associated with a lower risk of the combined kidney end points of ≥30% decline in eGFR, end-stage kidney disease, and all-cause mortality (21). All of these studies observe associations at a single lipid level, while the current study provides further insight on lipids at a system biology level. Overall, the pattern of reduced TAGs and PCs along with increased polyunsaturated phospholipids associated with T1D reported by other studies (14–20) is aligned with our findings where a similar pattern was associated with eGFR decline.
At the early stage (eGFR >90 mL/min/1.73 m2) and in the absence of insulin resistance, increased incorporation of unsaturated FFAs in construct of phospholipids at the sn1 carbon in fast decliners might be explained by differential upregulation of phospholipase A1 (PLA1) activity, downregulation of PLA2 activity, or both. Our earlier study in T2D suggested that upregulation of de novo lipogenesis and impaired mitochondrial β-oxidation mediated by insulin resistance when GFR is >90 mL/min/1.73 m2 might be a putative mechanism of DKD progression (7). As T1D is characterized by a state of insulin deficiency, such a mechanism is unexpected to be the driving force of DKD progression at the early stage. In contrast, a relatively higher abundance of unsaturated FFAs and polyunsaturated phospholipids with an unsaturated acyl chain at the sn-1 carbon in rapid decliners suggests intact elongation and desaturation processes for FFAs coupled with their use in the construction of polyunsaturated phospholipids. Circulating TAGs are suppressed as a result diminished adipose tissue sensitivity to insulin and hence diminished lipolysis (22). Chronic kidney disease (CKD) progression is an independent risk factor for insulin resistance (23,24); therefore, with decline of eGFR, the likelihood of insulin resistance increases. Increased insulin resistance can potentially upregulate kidney and systemic de novo lipogenesis (6), as evidenced by an increased relative abundance of saturated FFAs and TAGs in rapid decliners in patients with eGFR <90 compared with those with eGFR ≥90 mL/min/1.73 m2. In parallel, a relatively lower abundance of unsaturated FFAs and a relatively higher abundance of phospholipids with saturated acyl chains at the sn1 carbon suggests decreased incorporation of unsaturated FFAs in the construction of unsaturated phospholipids at the sn1 carbon, which may be due to differential downregulation of PLA2 activity, upregulation of PLA1 activity, or both, in rapid decliners in the context of worsening insulin resistance with worsening stage of eGFR category.
The underpinning mechanism of differential lipid alterations among rapid decliners by worsening categories of eGFR may be explained by graded worsening of CKD-mediated insulin resistance, and altered PLA1/2 activities. At the early stage when eGFR is ≥90 mL/min/1.73 m2 and insulin resistance is minimal, a compensatory upregulation of elongation and desaturation of fatty acids and intact or even upregulated mitochondrial β-oxidation of fatty acids manifest as relatively lower saturated fatty acids, higher unsaturated fatty acids, and their shift in the construct of polyunsaturated phospholipids. With CKD stage progression, worsening of CKD-mediated insulin resistance and impaired mitochondrial β-oxidation, relative abundance of saturated fatty acids and their saturated corresponding phospholipids increase. Early in the course, diet and hepatic de novo lipogenesis may be the main sources of saturated FFAs, but with continued insulin resistance, systemic upregulation of de novo lipogenesis may contribute to further insulin resistance.
Insulin resistance causes hypothalamic disruption of satiety and hunger signals, overconsumption of calories, and storage of saturated FFAs in adipose tissues. Saturated fatty acids in adipose tissues recruit macrophages and promote proinflammatory responses, which in turn impair insulin signaling in nonadipose tissues, along with further release of fatty acids in the blood stream (25). Sequestration of fatty acids in liver, kidney, pancreas, skeletal muscle, and gastrointestinal systems leads to lipotoxicity, impairment of energy metabolism, and further progression of kidney disease (26). Recent reports show that saturated fatty acids can also directly cause insulin resistance in podocytes (27) and in tubular epithelial cells (28) and directly contribute to their impaired energy metabolism and hence CKD progression. The clinical implication is that lipid alteration is dependent on the CKD stage, and its values should be interpreted in the context of the corresponding stage. This explanation is further supported by lipidomic alterations in T2D that mimic the patterns observed in lower eGFR categories in our T1D cohort, characterized by decreased unsaturated FFA, long-chain AC, and shorter TAGs, but increased saturated FFA, longer TAGs, and unsaturated PEs (Supplementary Fig. 10B) (6). Such a contrast may be partly due to the state of insulin deficiency at T1D and insulin resistance at T2D. The prop-osed pathophysiology of DKD progression is shown in Supplementary Fig. 10C.
This study has several strengths. It is a multicenter observational study from established cohorts of well-phenotyped patients with T1D, with a research protocol-based high-quality data collection from multiple cohorts increasing yield of replication in independent cohorts. The mass spectrometry-based targeted quantification in multiple reaction monitoring mode quantified diverse lipids with high specificity by their molecular characteristics (acyl chain and saturation status) and furthered pathophysiologic insight to clinical outcome. High resolution with ultrasensitive detection led to no missing data, excellent reproducibility, and minimal batch-to-batch variation. The large sample size provided adequate power for most of the analyses, and the availability of clinical covariates allowed proper multivariate adjustments. Replication of the findings in the randomly selected validation subset confirmed the validity of the observations.
This study also has limitations. Its observational nature does not allow inference of causal effects. Although a number of studies have shown the detrimental kidney effects of saturated FFAs (especially palmitate) (29–36), lipid changes in our study likely reflect a multitude of underpinning mechanisms, including differential PLA1/2 activities, elongation, desaturation, lipolysis, and insulin resistance in fast- versus slow-progressing DKD and at various stages of CKD.
The study was underpowered for subgroup analysis by eGFR categories or individual cohorts alone. To overcome this limitation, we tested the interaction term of lipids by eGFR categories. Up to 14% of the patients were taking lipid-lowering agents. However, fatty acids and phospholipids are not the direct target of these agents, and in absence of insignificant association with outcome in adjusted multivariate models, they are unlikely to have modified the association of lipids with fast eGFR decline. Because the cohorts are distinct, diets are highly likely to be different across various cohorts. To minimize lipid variability attributed to diet, fasting plasma samples were used.
While providing pathophysiologic insight, this study suggests that no individual lipid may adequately represent the complex interrelationships that define lipid systems biology. Hence the clinical applicability of the findings is contingent on feasible quantification of a large array of circulating lipids. Circulating lipid levels represented the net effect of systemic alterations of FFA and phospholipid metabolism, and distinct alterations in individual organs require further research. In particular, we did not have kidney tissue, and further research investigating parallel lipid alteration in the kidney would enhance pathophysiologic understanding of the disease process.
In this study, we relied on eGFR to stratify the patients, which suffers from lower accuracy compared with measured GFR (37,38). However, application of the Chronic Kidney Disease Epidemiology Collaboration equations instead of the MDRD formula makes the subgroups less vulnerable to misclassification, particularly in the early stage with preserved kidney function. Furthermore, the intraindividual longitudinal alteration of eGFR, which is used to define rapid decline, is more likely to reflect a similar change in measured GFR over time and less likely to suffer from misclassification. Correction of hyperfiltration can contribute to eGFR decline; however, stability after hyperfiltration correction would have precluded their classification as fast decliners by design (only those with sustained continuous decline of eGFR throughout the follow up were included as fast decliners).
In conclusion, these findings suggest that DKD progression at the early stage may involve differential incorporation of FFAs at the sn1 glycerol backbone of phospholipids, pointing to PLA1/2 differential activities as the underpinning mechanism, and that a panel of unsaturated FFAs and saturated PE-Ps representing this mechanism predicts rapid decline of GFR at the early stage in T1D.
Article Information
Funding. This work was supported by the JDRF Network Grant (to K.S.), the JDRF Center for Excellence (5-COE-2019-861-S-B), and National Institute of Diabetes and Digestive and Kidney Diseases grants K08DK106523, R03DK121941, R01DK034818, P30DK089503, P30DK081943, P30DK020572, and 1R01DK110541-01A1.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.A. secured funding, generated the lipidomic data, performed analysis, and wrote the first draft. T.M.R., C.H., and J.B. performed mass spectrometry and reviewed and edited the paper. D.M. and M.D. contributed to data and sample processing and reviewed and edited the paper. J.T., J.K., C.P.L., and I.H.d.B. critically reviewed and edited the paper. R.G.M., T.C., T.J.O., T.S.A., P.R., and J.K.S.-B. contributed to conduct and generation of the clinical data and reviewed and edited the paper. L.N. contributed to design, statistical analysis, and critically reviewed and edited the paper. G.M. contributed to data analysis. K.S. and S.P. contributed to design, secured funding, and critically reviewed and edited the paper. F.A. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
M.D. is currently affiliated with Metabolomics/Biomarkers at Janssen Pharmaceuticals of Johnson & Johnson, Boston, MA.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14696676.
References
- 1. Ihara K, Skupien J, Kobayashi H, et al. Profibrotic circulating proteins and risk of early progressive renal decline in patients with type 2 diabetes with and without albuminuria. Diabetes Care 2020;43:2760–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ihara K, Skupien J, Krolewski B, et al. A profile of multiple circulating tumor necrosis factor receptors associated with early progressive kidney decline in Type 1 Diabetes is similar to profiles in autoimmune disorders. Kidney Int 2021;99:725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019;25:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are +associated with early glomerular lesions in type 2 diabetes. Kidney Int 2016;89:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skupien J, Warram JH, Niewczas MA, et al. Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5-18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabetes Care 2014;37:2601–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afshinnia F, Nair V, Lin J, et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 2019;4:e130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Limonte CP, Valo E, Montemayor D, et al. A targeted multiomics approach to identify biomarkers associated with rapid eGFR decline in type 1 diabetes. Am J Nephrol 2020;51:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afshinnia F, Rajendiran TM, Soni T, et al.; Michigan Kidney Translational Core CPROBE Investigator Group . Impaired β-oxidation and altered complex lipid fatty acid partitioning with advancing CKD. J Am Soc Nephrol 2018;29:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afshinnia F, Rajendiran TM, Karnovsky A, et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int Rep 2016;1:256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–917 [DOI] [PubMed] [Google Scholar]
- 11. Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 2016;1:e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300 [Google Scholar]
- 13. Afshinnia F, Zeng L, Byun J, et al.; CRIC Study Investigators . Elevated lipoxygenase and cytoch-rome P450 products predict progression of chronic kidney disease. Nephrol Dial Transplant 2020;35:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. La Torre D, Seppänen-Laakso T, Larsson HE, et al.; DiPiS Study Group . Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes 2013;62:3951–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamichhane S, Ahonen L, Dyrlund TS, et al. Cord-blood lipidome in progression to islet autoimmunity and type 1 diabetes. Biomolecules 2019;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamichhane S, Ahonen L, Dyrlund TS, et al. Dynamics of plasma lipidome in progression to islet autoimmunity and type 1 diabetes–Type 1 Diabetes Prediction and Prevention Study (DIPP). Sci Rep 2018;8:10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oresic M, Simell S, Sysi-Aho M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008;205:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Overgaard AJ, Weir JM, Jayawardana K, Mortensen HB, Pociot F, Meikle PJ. Plasma lipid species at type 1 diabetes onset predict residual beta-cell function after 6 months. Metabolomics 2018;14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pflueger M, Seppänen-Laakso T, Suortti T, et al. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes 2011;60:2740–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sen P, Dickens AM, López-Bascón MA, et al. Metabolic alterations in immune cells associate with progression to type 1 diabetes. Diabetologia 2020;63:1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tofte N, Suvitaival T, Ahonen L, et al. Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci Rep 2019;9:16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 1989;38:1595–1601 [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Carrero JJ. Insulin resistance in chronic kidney disease. Nephrology (Carlton) 2017;22(Suppl. 4):31–34 [DOI] [PubMed] [Google Scholar]
- 24. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 2016;311:F1087–F1108 [DOI] [PubMed] [Google Scholar]
- 25. Funaki M. Saturated fatty acids and insulin resistance. J Med Invest 2009;56:88–92 [DOI] [PubMed] [Google Scholar]
- 26. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis 2015;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denhez B, Rousseau M, Spino C, et al. Saturated fatty acids induce insulin resistance in podocytes through inhibition of IRS1 via acti-vation of both IKKβ and mTORC1. Sci Rep 2020;10:21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei W, An XR, Jin SJ, Li XX, Xu M. Inhibition of insulin resistance by PGE1 via autophagy-dependent FGF21 pathway in diabetic nephr-opathy. Sci Rep 2018;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martínez-García C, Izquierdo-Lahuerta A, Vivas Y, et al. Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS One 2015;10:e0142291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant 2009;24:3288–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang XS, Chen XM, Wan JM, Gui HB, Ruan XZ, Du XG. Autophagy protects against palmitic acid-induced apoptosis in podocytes in vitro. Sci Rep 2017;7:42764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 2006;147:3398–3407 [DOI] [PubMed] [Google Scholar]
- 33. Lee E, Choi J, Lee HS. Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J Cell Physiol 2017;232:3209–3217 [DOI] [PubMed] [Google Scholar]
- 34. Sieber J, Lindenmeyer MT, Kampe K, et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 2010;299:F821–F829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu S, Nam SM, Kim JH, et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis 2015;6:e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yasuda M, Tanaka Y, Kume S, et al. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta 2014;1842:1097–1108 [DOI] [PubMed] [Google Scholar]
- 37. Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014;64:821–835 [DOI] [PubMed] [Google Scholar]
- 38. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]