Abstract
OBJECTIVE
To investigate glucose variations associated with glycated hemoglobin (HbA1c) in insulin-treated patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patients included in Diabetes and Lifestyle Cohort Twente (DIALECT)-2 (n = 79) were grouped into three HbA1c categories: low, intermediate, and high (≤53, 54–62, and ≥63 mmol/mol or ≤7, 7.1–7.8, and ≥7.9%, respectively). Blood glucose time in range (TIR), time below range (TBR), time above range (TAR), glucose variability parameters, day and night duration, and frequency of TBR and TAR episodes were determined by continuous glucose monitoring (CGM) using the FreeStyle Libre sensor and compared between HbA1c categories.
RESULTS
CGM was performed for a median (interquartile range) of 10 (7–12) days/patient. TIR was not different for low and intermediate HbA1c categories (76.8% [68.3–88.2] vs. 76.0% [72.5.0–80.1]), whereas in the low category, TBR was higher and TAR lower (7.7% [2.4–19.1] vs. 0.7% [0.3–6.1] and 8.2% [5.7–17.6] vs. 20.4% [11.6–27.0], respectively; P < 0.05). Patients in the highest HbA1c category had lower TIR (52.7% [40.9–67.3]) and higher TAR (44.1% [27.8–57.0]) than the other HbA1c categories (P < 0.05), but did not have less TBR during the night. All patients had more (0.06 ± 0.06/h vs. 0.03 ± 0.03/h; P = 0.002) and longer (88.0 [45.0–195.5] vs. 53.4 [34.4–82.8] minutes; P < 0.001) TBR episodes during the night than during the day.
CONCLUSIONS
In this study, a high HbA1c did not reduce the occurrence of nocturnal hypoglycemia, and low HbA1c was not associated with the highest TIR. Optimal personalization of glycemic control requires the use of newer tools, including CGM-derived parameters.
Introduction
Glycated hemoglobin (HbA1c), the clinical accepted standard for monitoring glycemic control, has a linear correlation with average blood glucose concentration of the past 2 to 3 months and is a useful tool to monitor long-term glycemic control in people with diabetes (1). Prevention of microvascular complications, which are related to chronic hyperglycemia, is a major treatment goal. In both type 1 and in type 2 diabetes, there is a clear relationship between HbA1c and the risk of microvascular complications (2,3). The risk of developing such complications is limited when the HbA1c is 53 mmol/mol (7.0%) or lower, corresponding with an approximate mean blood glucose concentration of 8.6 mmol/L (4,5). However, the use of HbA1c has important limitations. First, its assessment does not contribute to reduction of hypoglycemic episodes, the second aim of glycemic regulation. This is especially relevant in people treated with insulin, as lower HbA1c may be accompanied by an increased risk of hypoglycemia (6).
Second, HbA1c does not reflect blood glucose fluctuations. For day-to-day glucose regulation, and in particular minimizing hypoglycemic episodes, patients perform self-monitoring. However, self-monitoring provides, at best, one to seven measurements per day, and a large part of the glucose fluctuations remains unknown (7,8). Consequently, individualized HbA1c target levels are applied without actual confirmation that, for example, a permitted higher HbA1c translates in a reduction of time spent in hypoglycemia (9).
Currently, continuous glucose monitoring (CGM) technology, either real-time or intermittently viewed (iCGM), is becoming available for an increasing number of patients, also with type 2 diabetes. In the Netherlands, for example, iCGM is reimbursed by insurance companies for patients on multiple daily injection therapy with insulin. So far, CGM technology is primarily used to act on day-to-day variations. However, it would be logical to also continuously capture the data generated with this technology and to translate these into relevant parameters for evaluation of long-term glycemic control in clinical practice. Such parameters have been evaluated previously in a research setting using classic real-time CGM technology in patients with type 1 diabetes (i.e., a highly selected population) (10,11). The main parameter derived from CGM is the time in range (TIR), usually defined as the percentage of time per day spent within glucose range 3.9–10.0 mmol/L (12). As sensor techniques are becoming more available to broader populations, including patients with type 2 diabetes, evaluation of TIR may become a main parameter to guide blood glucose regulation in clinical practice. In addition to TIR, there are other relevant parameters (i.e., time below range [TBR], time above range [TAR], glucose variability measures, and data for glucose metrics reported in sleep and wake time blocks), all adding to a complete picture of glycemic control (12,13).
In this study, we investigated the glucose variations behind HbA1c in a real-world setting in insulin-treated patients with type 2 diabetes. We investigated the differences in TIR, TBR, and TAR between different HbA1c categories used in clinical practice, and we investigated whether there are differences in glucose variability. Because hypo- and hyperglycemic episodes are of special interest, we also evaluated the frequency, duration, and start time of the TBR and TAR episodes in different HbA1c categories. Furthermore, we analyzed which patient characteristics are related to TIR, TBR, or TAR.
Research Design and Methods
Patient Inclusion
For this study, we selected all patients treated with insulin included in the Diabetes and Lifestyle Cohort Twente (DIALECT)-2 between March 2017 and May 2019. DIALECT is an observational study in adult patients with type 2 diabetes who are treated in the Ziekenhuisgroep Twente Hospital, Almelo and Hengelo, the Netherlands. The only exclusion criteria were end-stage kidney disease and inability to understand the informed consent procedure. The study consists of two subcohorts, DIALECT-1 and DIALECT-2. The study procedures of DIALECT-1 have been described in detail previously (14). In DIALECT-2, the data collection is more extensive and includes iCGM registration, as detailed below. The study was performed in accordance with the Declaration of Helsinki and the guidelines of good clinical practice. It has been approved by the local institutional review boards (METC-registration numbers NL57219.044.16 and 1009.68020) and is registered in the Netherlands Trial Register (https://www.trialregister.nl, trial code NTR5855). Prior to participation, all patients signed informed consent.
Baseline Data
DIALECT-2 consisted of three hospital visits in a period of 2 weeks. Information about medical condition and medication was obtained from electronic patient files and verified with the patient. Information about smoking and diet was collected through questionnaires. Anthropometric measurements and presence of diabetic polyneuropathy were obtained by physical examination. Polyneuropathy was assessed by touch test (Semmes-Weinstein monofilament) and vibration sense test (VibraTip). Fat percentage and predicted muscle mass were determined by bioimpedance using the noninvasive Tanita BC418MA (15). Venous blood samples were taken, and blood pressure was measured (Dinamap; GE Medical Systems, Milwaukee, WI).
CGM-Derived Parameters
iCGM data were collected using a FreeStyle Libre sensor (Abbott Diabetes Care, Alameda, CA), applied to the patient’s upper arm. The sensor measures individual glucose levels at 15-min intervals for 2 weeks and can store up to 8 h of data. Using the FreeStyle Libre reader, blinded for the patients with a custom-made three-dimensional printed case, the data were stored. Data from the reader were uploaded in MATLAB (2019a; The MathWorks, Inc., Natick, MA). Patients’ data were considered valid when ≥3 days of measurements, each with ≥90% of data, were available. The TIR, TAR, and TBR (percent and minutes) were calculated based on the target glycemic range defined between 3.9 and 10.0 mmol/L. TBR and TAR were subclassified. Level 1 TBR (TBR1) ranges from 3.0 to 3.9 mmol/L, and level 2 TBR (TBR2) represents values <3.0 mmol/L. Level 1 TAR (TAR1) ranges from 10.0 to 13.9 mmol/L and level 2 TAR (TAR2) represents values >13.9 mmol/L (12,16).
SD (in millimoles per liter) and coefficient of variation (CV; in percent) were calculated as standard glycemic variability parameters. Whereas SD reflects the spread around the average, the CV is the SD divided by the mean, providing a normalized glucose variability reducing influence of the mean value.
Low blood glucose index (LBGI) and high blood glucose index (HBGI) were calculated to provide an index for the severity of hypo- and hyperglycemic episodes with a single positive number (7,17). These indices have been specifically developed to be sensitive for only the hypo- (LBGI) or hyperglycemic range (HBGI) and ignore the respective fluctuations in the opposite direction.
For the hypo- and hyperglycemic episodes, the starting time and the median duration of each TBR and TAR episode were determined. The starting time is defined to identify whether it is an episode started during the day (6:00–12:00 a.m.) or during the night (12:00–6:00 a.m.) (12). To enable comparison between day and night, the average number of TBR and TAR episodes for the day and night were calculated per hour (number per hour), as the nighttime is shorter (6 h) than daytime (18 h).
Data Analysis
To evaluate whether CGM-derived parameters differ according to HbA1c, the patients were divided into three commonly applied HbA1c categories: HbA1c ≤53 mmol/mol (7.0% [HbA1c ≤53]), HbA1c of 54–62 mmol/mol (7.1–7.8% [HbA1c 54–62]), and HbA1c ≥63 mmol/mol (7.9% [HbA1c ≥63]) (18–22). In addition, we determined for each HbA1c category how many patients fulfilled the criteria of having TIR >70%, while TBR should remain <5%, and severe hypoglycemia should be <1%, as these were previously suggested for optimal prevention of diabetes complications (13).
Statistical Analysis
Statistical analyses to determine differences of TIR, TBR, TAR, CV, SD, LBGI, and HBGI between the HbA1c groups and differences between day and night were performed using MATLAB. Normally distributed variables (assessed by visual inspection of frequency histograms) were presented as mean ± SD, skewed variables as median (interquartile range [IQR]), and dichotomous variables as numbers (percentage). Differences among the three HbA1c groups were tested with one-way ANOVA test with post hoc Tukey honest significant difference (normally distributed parameters), with Kruskal-Wallis test with post hoc Tukey honest significant difference (skewed distribution) or χ2 test (categorical parameters). Holm–Bonferroni corrections were applied for secondary analyses to reduce the risk of type I error with the threshold set at α = 0.05. Best fit regression analyses with 95% prediction intervals and R2 were calculated to determine the relationship of HbA1c to TIR, TAR, and TBR. Differences between day and night were compared using the Student t test for the number of TBR and TAR episodes and the Mann-Whitney U test for the duration of the episodes. To give visual insight in the amount of the episodes during the day, the hours of the day parameters were plotted in circular (24-h) histograms.
Univariate analysis among TIR, TBR, and TAR (%) and the potentially contributing variables were evaluated with IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY) using Pearson or Spearman correlation coefficients as appropriate. The tested potentially contributing variables were age, sex, diabetes duration, BMI, fat percentage, predicted muscle mass, waist-to-hip ratio, systolic blood pressure, diastolic blood pressure, heart rate, alcohol intake, pack-years (if current or former smoker), and presence of microvascular disease or macrovascular disease. Variables with a P level <0.15 were selected. Multiple linear regression analyzes for the association among TIR, TAR, TBR, and the selected variables were carried out, making three models, one each for TIR, TAR, and TBR. The variables were backward removed from the model when P was >0.05, starting with the highest P value. Variables that were skewed were transformed before the linear regression analysis was carried out.
Results
Patient Characteristics
In the selected period, 87 patients were included in the DIALECT-2 cohort. A total of 79 of these patients fulfilled the inclusion criteria. Reasons for exclusion were “missing iCGM data” (n = 2), and “no 3 days with at least 90% data” (n = 8). Of the included patients, a median (IQR) of 10 (7–12) days with a minimum of 90% iCGM data were available for analysis. Of all patients, 21 had an HbA1c ≤53 mmol/mol (≤7.0%), 22 had an HbA1c of 54–62 mmol/mol (7.1–7.8%), and 36 had an HbA1c ≥63 mmol/mol (≥7.9%) (Table 1). Mean age was 67 ± 10 years, and 54% of the patients were men. Mean diabetes duration was 17 ± 10 years and mean BMI was 31.6 ± 4.5 kg/m2. The prevalence of long-term diabetes-related complications was high. Overall, approximately two-thirds of the patients had microvascular complications. The proportion of patients with microvascular complications increased gradually, but without statistical significance, across the HbA1c categories. Slightly over one-third of the patients had macrovascular disease, which was not statistically different across the HbA1c categories. Only smoking behavior was statistically different among the HbA1c groups, with highest pack-years in the HbA1c ≤53 group.
Table 1.
Clinical characteristics of the total population and according to HbA1c categories
| Characteristic | N | Total population | HbA1c ≤53 | HbA1c 54–62 | HbA1c ≥63 | P |
|---|---|---|---|---|---|---|
| Number of patients, n | 79 (100) | 21 (26.6) | 22 (27.8) | 36 (46.6) | ||
| Age, years | 79 | 67 ± 10 | 67 ± 8 | 68 ± 10 | 68 ± 11 | 0.94 |
| Sex, n of men | 79 | 43 (54) | 13 (62) | 14 (64) | 16 (44) | 0.10 |
| Diabetes duration, years | 79 | 17 ± 10 | 14.2 ± 12.0 | 17.4 ± 8.8 | 18.1 ± 10.2 | 0.39 |
| BMI, kg/m2 | 78 | 31.6 ± 4.5 | 30.7 ± 4.3 | 31.5 ± 4.9 | 32.2 ± 4.4 | 0.48 |
| Basal regimen, n | 79 | 23 (29) | 7 (33) | 4 (18) | 12 (33) | 0.18 |
| Basal bolus/plus regimen, n | 79 | 34 (43) | 8 (38) | 9 (41) | 17 (47) | 0.48 |
| Mixed regimen, n | 79 | 14 (18) | 4 (19) | 6 (27) | 4 (11) | 0.12 |
| Bolus only regimen, n | 79 | 8 (10) | 2 (10) | 3 (14) | 3 (8) | 0.51 |
| Total daily units of insulin, units/day | 79 | 64 ± 41 | 54 ± 40 | 56 ± 33 | 74 ± 44 | 0.11 |
| Metformin, n | 79 | 59 (75) | 15 (71) | 18 (82) | 26 (72) | 0.36 |
| Sulfonylureas, n | 79 | 13 (16) | 3 (14) | 4 (18) | 6 (17) | 0.73 |
| Fat percentage, % | 74 | 34.8 ± 9.0 | 33.5 ± 8.6 | 32.3 ± 8.4 | 36.9 ± 9.3 | 0.14 |
| Predicted muscle mass, kg | 75 | 55.0 ± 13.2 | 55.3 ± 8.4 | 55.2 ± 15.3 | 54.7 ± 14.4 | 0.99 |
| Waist-to-hip ratio | 79 | 1.00 ± 0.07 | 1.0 ± 0 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.38 |
| SBP, mmHg | 78 | 127 ± 13 | 123.0 ± 12.0 | 129.3 ± 9.5 | 127.3 ± 14.8 | 0.27 |
| DBP, mmHg | 78 | 73 ± 10 | 70.8 ± 9.1 | 76.2 ± 8.7 | 71.3 ± 11.7 | 0.15 |
| Heart rate, bpm | 76 | 73 ± 12 | 71.8 ± 12.0 | 72.6 ± 10.6 | 73.2 ± 12.7 | 0.91 |
| Alcohol intake, units/month | 78 | 2 (0–24) | 0 (0–20) | 7.5 (0–42) | 2 (0–8) | 0.22 |
| Pack-years, packs/day × year | 72 | 6 (0–22) | 14 (0–62) | 9 (0–16.5) | 0 (0–18.5) | 0.049 |
| Microvascular disease, n | 79 | 50 (63) | 10 (48) | 14 (64) | 26 (72) | 0.06 |
| Macrovascular disease, n | 79 | 30 (38) | 10 (48) | 8 (36) | 12 (33) | 0.28 |
Data are mean ± SD or median (IQR). bpm, beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure.
TIR Across HbA1c Categories
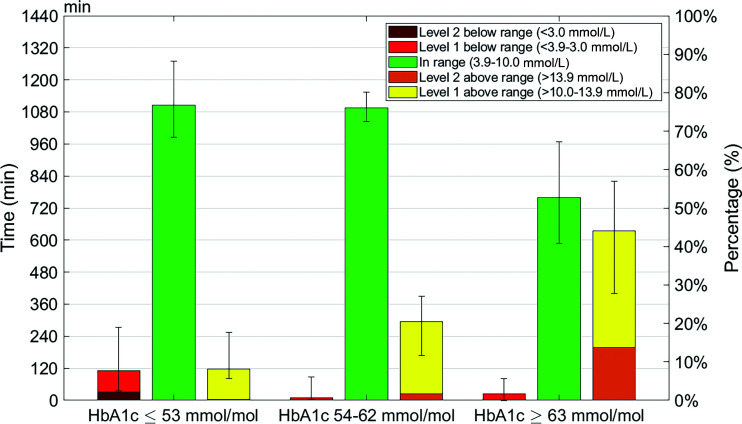
The distribution of time spent in different ranges of blood glucose across the three HbA1c categories is shown in Fig. 1. As expected, TBR decreased progressively across increasing HbA1c categories, whereas TAR increased progressively. All three glucose range variables (i.e., TIR, TBR, and TAR) differed significantly between the two extreme HbA1c categories (HbA1c ≤53 76.8% [68.3–88.2], 7.6% [2.4–19.1], and 8.2% [5.7–17.6] vs. HbA1c ≥63 52.7 [40.9–67.3], 1.8% [0–5.6], and 44.1% [27.8–57.0], respectively; all P < 0.05).
Figure 1.
Median TIR, TBR1, TBR2, TAR1, and TAR2 values binned into HbA1c ≤53 mmol/mol, HbA1c 54–62 mmol/mol, and HbA1c ≥63 mmol/mol. The values are shown in time in minutes per day (left y-axis) and percentage of day (right y-axis). The IQRs of TIR, TAR, and TBR are represented by the error bars.
Interestingly, Fig. 1 shows that the mean TIR was not different between the first two categories (76.8% [68.3–88.2] vs. 76.0% [72.4–80.1]; P = 0.84). However, patients in the lowest HbA1c category spent more TBR than patients in the intermediate category. This was the case for both degrees of severity of TBR (TBR1 is 5.5% [1.9–11.8] and TBR2 is 2.2% [0–7.8] in HbA1c ≤53 vs. TBR1 is 0.7% [0.3–4.3] and TBR2 is 0% [0–2.5] in HbA1c 54–62; both P < 0.05). For TAR, the reverse was true: patients in the intermediate category spent more in TAR than patients in the lowest HbA1c category. This applied to both degrees of severity of TAR (TAR1 is 7.9 [5.6–15.7] and TAR2 is 0.3 [0–1.9] in HbA1c ≤53 vs. TAR1 is 18.8 [11.4–23.7] and TAR2 is 1.7 [0.5–4.6] in HbA1c 54–62; both P < 0.05). With respect to differences between the intermediate and high HbA1c categories, TIR, TAR1, and TAR2 differed significantly (TIR is 76.8 [72.5–80.1], TAR1 is 18.8 [11.4–23.7], and TAR2 is 1.7 [0.5–4.6] in HbA1c 54–62 vs. TIR is 52.7 [40.9–67.3], TAR1 is 30.5 [20.8–33.0], and TAR2 is 13.7 [6.2–21.7] in HbA1c ≥63; all P < 0.05), whereas TBR was not different.
Scatterplots showed a relationship among HbA1c and TIR, HbA1c and TAR, and HbA1c and TBR, with the best curve fit being exponential relationship of second order between HbA1c and TIR (R2 = 0.65), linear between HbA1c and TAR (R2 = 0.68), and exponential of second order between HbA1c and TBR (R2 = 0.29) (Supplementary Fig. 1).
It has been suggested that, using CGM parameters, an optimal glycemic regulation implies a TIR >70%, while TBR1 and TBR2 are <4% and <1%, respectively (13). The >70% TIR criterion was reached in 38 patients of the total study population. Almost half of these patients (n = 17) also reached the TBR criteria. Of these 17 patients, the mean HbA1c was 56.6 ± 4.9 mmol/mol (7.4%). A total of 23.5% of these patients originated from the low, 58.9% from the intermediate, and 17.6% from the high HbA1c category.
Glycemic Variability Across HbA1c Categories
As expected, the variation in glucose readings reflected by the SD increased progressively across increasing HbA1c categories (Table 2). However, no significant differences of the CV were found.
Table 2.
CGM-derived parameters of the total population and according to HbA1c categories
| N | Total population | HbA1c ≤53 | HbA1c 54–62 | HbA1c ≥63 | P | |
|---|---|---|---|---|---|---|
| Number of patients, n | 79 (100) | 21 (26.6) | 22 (27.8) | 36 (46.6) | ||
| iCGM data, days | 79 | 10 (7–12) | 11 (8–11.3) | 8 (6–11) | 10 (7–12) | 0.53 |
| Glucose value, mmol/L | 79 | 8.7 ± 2.2 | 6.8 ± 0.9 | 8.1 ± 0.9 | 10.1 ± 2.4 | <0.001 |
| SD | 79 | 2.6 ± 0.8 | 2.1 ± 0.5 | 2.2 ± 0.4 | 3.1 ± 1.0 | <0.001 |
| CV (%) | 79 | 30 ± 7 | 32 ± 7 | 28 ± 5 | 31 ± 8 | 0.18 |
| LBGI | 79 | 1.4 ± 1.7 | 2.7 ± 2.1 | 1.0 ± 1.1 | 0.9 ± 1.3 | <0.001 |
| HBGI | 79 | 7.1 ± 6.8 | 2.6 ± 1.6 | 4.6 ± 1.8 | 11.3 ± 8.1 | <0.001 |
| TIR, min | 79 | 939.9 (784.1–1,110.5) | 1,106.2 (984.2–1,269.8) | 1,094.9 (1,043.5–1,154.2) | 758.5 (588.4–968.8) | <0.001 |
| TBR episodes, day, n | 62 | 0.03 ± 0.03 | 0.04 ± 0.03 | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.007 |
| TBR episodes, night, n | 62 | 0.06 ± 0.06 | 0.08 ± 0.08 | 0.04 ± 0.04 | 0.05 ± 0.05 | 0.06 |
| TAR episodes, day, n | 79 | 0.11 ± 0.05 | 0.08 ± 0.04 | 0.13 ± 0.04 | 0.12 ± 0.05 | 0.001 |
| TAR episodes, night, n | 79 | 0.04 ± 0.04 | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.06 ± 0.05 | <0.001 |
| TBR duration, day, min | 62 | 53.4 (34.4–82.8) | 46.9 (34.4–100.3) | 50.6 (29.0–86.2) | 55.0 (47.7–77.0) | 0.18 |
| TBR duration, night, min | 62 | 88.0 (45.0–195.5) | 162.9 (69.4–227.1) | 63.0 (39.4–157.8) | 126.0 (81.5–185.3) | 0.93 |
| TAR duration, day, min | 79 | 102.0 (62.5–149.0) | 81.9 (66.8–122.1) | 107.0 (79.7–154.5) | 207.5 (150.6–275.6) | <0.001 |
| TAR duration, night, min | 79 | 120.5 (50.0–232.0) | 39.7 (23.5–122.5) | 70.9 (27.6–168.5) | 232.0 (142.8–337.8) | <0.001 |
Data are mean ± SD or median (IQR). duration, median duration episode per day or night; episode, average number of episodes started per hour during daytime (6:00–12:00 a.m.) or nighttime (12:00–6:00 a.m.).
The findings of the LBGI and HBGI (Table 2) were in line with the TBR and TAR findings. The lowest HbA1c category had the highest LBGI value, and the highest HbA1c category had the highest HBGI value. The LBGI and HBGI were both statistically different between the two extreme HbA1c categories (HbA1c ≤53 LBGI of 2.7 ± 2.1 and HBGI of 2.6 ± 1.6 vs. HbA1c ≥63 LBGI of 0.9 ± 1.3 and HBGI of 11.3 ± 8.1; both P < 0.001). Also, the LBGI was higher in the lowest HbA1c category than in the intermediate category (1.0 ± 1.1), and the HBGI was higher in the highest HbA1c category than in the intermediate category (4.6 ± 1.8, all: P < 0.001).
TBR and TAR During Day- and Nighttime
A large majority of the 62 (78%) patients experienced TBR episodes. In the lowest HbA1c category, only 5% of the patients did not experience any TBR episodes, whereas this number was somewhat higher in the intermediate (23%) and high (31%) HbA1c categories.
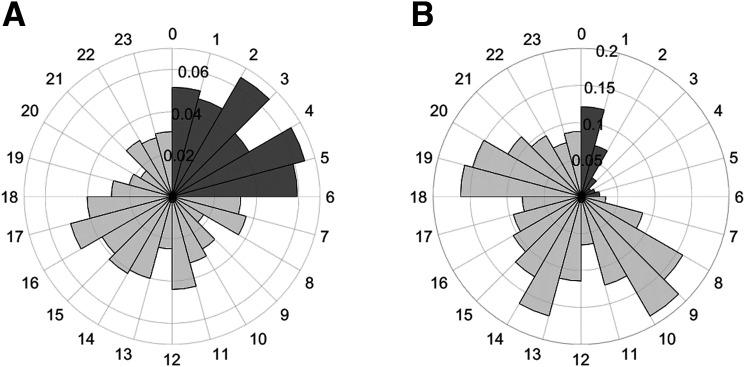
The timing of TBR and TAR episodes of the 79 patients is detailed in Fig. 2. Figure 2A shows that most TBR episodes started during nighttime, and the lowest number of TBR episodes developed between 8:00 and 9:00 a.m. and between 8:00 and 9:00 p.m. During nighttime, patients developed twice as many TBR episodes per hour compared with daytime (0.06 ± 0.06/h vs. 0.03 ± 0.03/h; P = 0.002). On average, a nighttime TBR episode started once every 3 days.
Figure 2.
Circular histogram of the number of TBR (A) and TAR (B) episodes that started on average per hour per patient. The circular axis shows the hour of day, and the radial axis shows the number of TBR and TAR episodes, which started, on average, in that hour.
Differences of the TBR episodes among the HbA1c categories during daytime were found between the lowest and highest category, with 2.0 times more started TBR episodes in HbA1c ≤53 (0.04 ± 0.03) compared with HbA1c ≥63 (0.02 ± 0.02; P < 0.01). No differences were found among the HbA1c categories in the started TBR episodes during the night.
The duration of the TBR episodes starting during night hours was longer than those of daytime episodes (median 88.0 [45.0–195.5] vs. 53.4 [34.4–82.8]; P < 0.001). The duration of daytime and nighttime TBR episodes was not different among HbA1c categories.
Figure 2B shows patients developed more TAR episodes per hour during daytime compared with nighttime (0.11 ± 0.05/h vs. 0.04 ± 0.04/h; P < 0.001). TAR episodes mostly occurred between 8:00 and 9:00 a.m., between 12:00 and 2:00 p.m., and between 6:00 and 7:00 p.m., following common mealtimes in the Netherlands.
Patients of the highest HbA1c category had more TAR episodes during the day (0.12 ± 0.05/h; P = 0.004) and night (0.06 ± 0.05/h; P < 0.001) than patients of the lowest HbA1c category (0.08 ± 0.04/h vs 0.02 ± 0.02/h, respectively). Also, during daytime, patients of HbA1c 54–62 had more TAR episodes (0.13 ± 0.04/h) than the lowest HbA1c category (P = 0.002).
Median duration of nighttime TAR episodes, 120.5 (50.0–232.0) minutes, did not differ from duration during the day, 102.0 minutes (62.5–149.0) (P = 0.66). However, the TAR duration of the episodes of the highest HbA1c category (day 207.5 [150.6–275.6] and night 232.0 [142.8–337.8]) was higher than of the lowest (day 102.0 [62.5–149.0] and night 120.5 [50.0–232.0]) and intermediate (day 107.0 [79.7–154.5] and night 70.9 [27.6–168.5]) category during the day as well as during the night (P < 0.001). An overview can be found in Table 2.
Factors Contributing to TIR, TAR, and TBR
An association of P < 0.15 was found between TIR and BMI, TBR and pack-years, fat percentage, sex and predicted muscle mass, and TAR and pack-years and microvascular complications.
Multiple linear regression was carried out for TIR, TBR, and TAR and their associating variables. No contributing factors (P < 0.05) for TIR were found. Pack-years has a weak positive association with TBR and a weak negative association with TAR, with R2 values of 0.052 and 0.045, respectively (Supplementary Table 1).
Conclusions
To the best of our knowledge, this is one of the few studies that investigated the glucose regulation beyond HbA1c in insulin-using patients with type 2 diabetes. We performed 2-week blinded iCGM measurements and made a number of remarkable observations.
The first important finding was that patients in the low HbA1c category (HbA1c ≤53 mmol/mol [7.0%]) did not have a higher TIR than those in the intermediate category (HbA1c 54–62 mmol/mol [7.1–7.8%]). The TIR ∼75%, as found in both categories, is considered as good glycemic control according to current consensus (13). Of note, these figures are higher than previously found in type 1 and type 2 diabetes, in which patients with a mean HbA1c of 58 mmol/mol (7.5%), comparable with our intermediate HbA1c category, had a mean TIR of 58% and 56%, respectively (10,23).
Whereas, in the lowest HbA1c category, glucose levels outside the target range were more often due to TBR, TAR was more common in the intermediate HbA1c group. Currently, it is unknown which distribution pattern of glycemic range is preferable for quality of life and long-term outcome. An expert panel previously suggested to target for TIR >70%, while TBR1 should remain <4% and severe hypoglycemia (TBR2) <1% (13). This was achieved in only a minority of the patients (22%) in our study, and most of these patients were in the intermediate HbA1c category and not the lowest HbA1c category as one might have expected.
A second important finding was that there were no differences in glucose variability between the low and intermediate HbA1c categories. The CV of <36% found in both categories is consistent with rather low variability (24), which is smaller than previously found in type 1 diabetes (25). This is of interest, because glucose fluctuation itself could be detrimental in the development of diabetes complications, and it might be speculated that similar HbA1c categories carry different risk between type 1 and type 2 diabetes (26,27). The lack of difference between HBGI values might seem unexpected based on TAR results, but can be explained by the hyperglycemia burden in the intermediate HbA1c category being almost exclusively limited to the 10.0–13.9 mmol/L range. Of all glycemic variability parameters, the only significant difference between the low and intermediate HbA1c category was found for the LBGI, being higher in the lowest HbA1c group, consistent with the TBR results. Additionally, the glucose variability expressed as SD and HBGI of the high HbA1c category were higher compared with the other two HbA1c categories.
A third important finding is the burden of nocturnal TBR episodes across the HbA1c categories, which occurred in almost two-thirds (61%) of all patients during the 2-week measurement period. TBR episodes were more frequent and of longer duration during nighttime than during daytime. Interestingly, the frequency of nocturnal TBR episodes was not lower in patients of the highest HbA1c category compared with the other categories, despite a high TAR of ∼45%. This strongly suggests that accepting a higher HbA1c is not effective to eradicate or even reduce nocturnal TBR episodes. Of note, these episodes had not previously been recognized in routine care, presumably because nocturnal blood glucose measurements are not regularly performed (28,29). It would be important to reduce these nocturnal hypoglycemic episodes as they are implicated with reduced quality of life and long-term complications (28,30,31).
This study showed that smoking had a positive association with TBR and inverse association with TAR. This corresponds with previous data in which a higher fasting blood glucose, postprandial blood glucose, and HbA1c were found in smoking as compared with nonsmoking patients with diabetes (32).
Unique in this are the real-world data we collected in a cohort of insulin-using patients with type 2 diabetes, representative for a secondary health care population. The patients did not use sensor-technology themselves and were blinded for the results. The data quality was high, with a median of 10 days of data available with a minimal coverage of 90% of the time per day. A limitation is that we cannot establish which distribution of glycemic range is optimal for quality of life and long-term outcome. This would require a carefully designed prospective trial investigating prespecified targets for TIR. Secondly, some of the unobserved differences might be due to the relatively small sample size. Also, the results of this study cannot be extrapolated to other populations, such as patients with type 2 diabetes treated with only oral blood glucose-lowering therapy.
In addition to evaluating targets for TIR, future research should also investigate how CGM technology can be effectively applied in clinical practice. Besides continuous use, which carries the largest window of opportunity to improve glycemic control, a strategy of incidental 2-week unblinded measurements could also be a worthwhile, economical strategy to explore.
In summary, an HbA1c below the target of 53 mmol/mol (7.0%), thus far considered optimal glycemic control in most patients, was not accompanied by the highest TIR in our study of patients with type 2 diabetes treated with insulin. Also, an HbA1c ≥63 mmol/mol (7.9%) was not accompanied, as would be expected, with less and shorter nocturnal hypoglycemic episodes burden. Therefore, to establish individualized glycemic treatment requires new tools in addition to HbA1c. The use of CGM-derived parameters should be further explored in this respect.
Article Information
Acknowledgments. The authors thank Christina Gant, Milou Oosterwijk, Ilse Hagedoorn, Nicole Oosterom, Annis Jalving, and Roos Nijboer from the Division of Nephrology, Department of Internal Medicine, Ziekenhuisgroep Twente, Almelo and Hengelo, the Netherlands for the contributions to DIALECT-2, including patient inclusion.
Funding. This research was financially supported by an unrestricted research grant from the Pioneers in Health Care Innovation Fund established by the University of Twente, Medisch Spectrum Twente, and Ziekenhuisgroep Twente, the Ziekenhuisgroep Twente Hospital Research Fund, and Diabetes Fonds (grant 2017.30.005). N.d.B. is appointed by the grant Exceptional and Deep Intelligent Coach financed by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (grant 628.011.021).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.d.B., M.M.R.V.-H., and G.D.L. designed the study. N.d.B. and K.M.W. included patients and performed the measurements. N.d.B. analyzed the data. N.d.B., M.M.R.V.-H., and G.D.L. wrote the article. K.M.W., S.J.L.B., G.N., and B.-J.F.v.B. reviewed and edited the manuscript. G.D.L. is the principal investigator of DIALECT. G.D.L. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NTR5855, https://www.trialregister.nl
This article contains supplementary material online at https://doi.org/10.2337/figshare.14838837.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. World Health Organization . Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva, Switzerland, World Health Organization, 2011 [PubMed] [Google Scholar]
- 2. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 3. Reichard P, Pihl M, Rosenqvist U, Sule J. Complications in IDDM are caused by elevated blood glucose level: the Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia 1996;39:1483–1488 [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014;104:1–52 [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 7. Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick L, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther 2003;5:817–828 [DOI] [PubMed] [Google Scholar]
- 8. Pfützner A, Weissmann J, Mougiakakou S, Daskalaki E, Weis N, Ziegler R. Glycemic variability is associated with frequency of blood glucose testing and bolus: post hoc analysis results from the ProAct Study. Diabetes Technol Ther 2015;17:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rama Chandran S, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther 2018;20:353–362 [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019; 13:614–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersson J, Åkesson K, Sundberg F, Särnblad S. Translating glycated hemoglobin A1c into time spent in glucose target range: A multicenter study. Pediatr Diabetes 2019;20:339–344 [DOI] [PubMed] [Google Scholar]
- 12. Danne T, Nimri R, Battelino T, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gant CM, Binnenmars SH, Berg EVD, Bakker SJL, Navis G, Laverman GD. Integrated assessment of pharmacological and nutritional cardiovascular risk management: blood pressure control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017;9:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly JS, Metcalfe J. Validity and reliability of body composition analysis using the Tanita BC418-MA. J Exerc Physiol Online 2012;15:74–83 [Google Scholar]
- 16. Ratner RE. Hypoglycemia: new definitions and regulatory implications. Diabetes Technol Ther 2018;20(S2):S250–S253 [DOI] [PubMed] [Google Scholar]
- 17. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 18. McGuire H, Longson D, Adler A, Farmer A; Guideline Development Group . Management of type 2 diabetes in adults: summary of updated NICE guidance. BMJ 2016;353:i1575. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 [DOI] [PubMed] [Google Scholar]
- 20. Holmes VA, Young IS, Patterson CC, et al.; Diabetes and Pre-eclampsia Intervention Trial Study Group . Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care 2011;34:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu H, Hori A, Nishiura C, et al.; Japan Epidemiology Collaboration on Occupational Health Study Group . Hba1c, blood pressure, and lipid control in people with diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One 2016;11:e0159071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macisaac RJ, Jerums G, Weekes AJ, Thomas MC. Patterns of glycaemic control in Australian primary care (NEFRON 8). Intern Med J 2009;39:512–518 [DOI] [PubMed] [Google Scholar]
- 23. Beck RW, Riddlesworth TD, Ruedy K, et al.; DIAMOND Study Group . Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 24. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838 [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Yang D, Deng H, et al. Impacts of glycemic variability on the relationship between glucose management indicator from iPro™2 and laboratory hemoglobin A1c in adult patients with type 1 diabetes mellitus. Ther Adv Endocrinol Metab 2020;11:2042018820931664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes 2013;62:1405–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care 2008;31(Suppl. 2):S150–S154 [DOI] [PubMed] [Google Scholar]
- 28. Allen KV, Frier BM. Nocturnal hypoglycemia: clinical manifestations and therapeutic strategies toward prevention. Endocr Pract 2003;9:530–543 [DOI] [PubMed] [Google Scholar]
- 29. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 30. Frier BM. Living with hypoglycaemia. In Hypoglycaemia in Clinical Diabetes. 3rd ed. Frier BM, Heller S, McCrimmon R, Eds. Hoboken, NJ, Wiley-Blackwell, 2014, pp. 347–368 [Google Scholar]
- 31. King P, Kong MF, Parkin H, Macdonald IA, Tattersall RB. Well-being, cerebral function, and physical fatigue after nocturnal hypoglycemia in IDDM. Diabetes Care 1998;21:341–345 [DOI] [PubMed] [Google Scholar]
- 32. Sari MI, Sari N, Darlan DM, Prasetya RJ. Cigarette smoking and hyperglycaemia in diabetic patients. Open Access Maced J Med Sci 2018;6:634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]