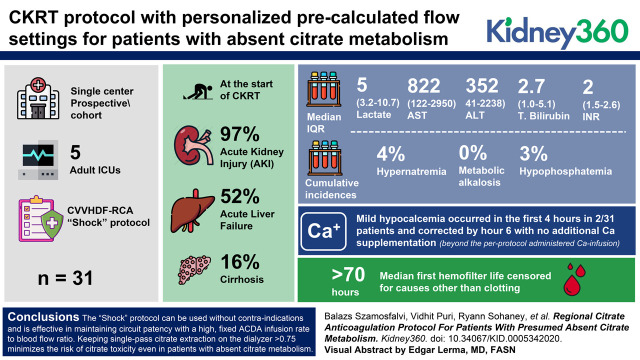
Visual Abstract
Keywords: acute kidney injury and ICU nephrology, citrate anticoagulation, citrate toxicity, CKRT, CRRT, filter life, hypocalcemia, lactic acidosis, liver failure, veno-venous hemodiafiltration
Abstract
Background
Regional citrate anticoagulation (RCA) is not recommended in patients with shock or severe liver failure. We designed a protocol with personalized precalculated flow settings for patients with absent citrate metabolism that abrogates risk of citrate toxicity, and maintains neutral continuous KRT (CKRT) circuit calcium mass balance and normal systemic ionized calcium levels.
Methods
A single-center prospective cohort study of patients in five adult intensive care units triaged to the CVVHDF-RCA “Shock” protocol.
Results
Of 31 patients included in the study, 30 (97%) had AKI, 16 (52%) had acute liver failure, and five (16%) had cirrhosis at the start of CKRT. The median lactate was 5 mmol/L (interquartile range [IQR], 3.2–10.7), AST 822 U/L (IQR, 122–2950), ALT 352 U/L (IQR, 41–2238), total bilirubin 2.7 mg/dl (IQR, 1.0–5.1), and INR two (IQR, 1.5–2.6). The median first hemofilter life censored for causes other than clotting exceeded 70 hours. The cumulative incidence of hypernatremia (Na >148 mM), metabolic alkalosis (HCO3- >30 mM), and hypophosphatemia (P<2 mg/dl) were one out of 26 (4%), zero out of 30 (0%), and one out of 30 (3%), respectively, and were not clinically significant. Mild hypocalcemia occurred in the first 4 hours in two out of 31 patients, and corrected by hour 6 with no additional Ca supplementation beyond the per-protocol administered Ca infusion. The maximum systemic total Ca (tCa; mM)/ionized Ca (iCa; mM) ratio never exceeded 2.5.
Conclusions
The Shock protocol can be used without contraindications and is effective in maintaining circuit patency with a high, fixed ACDA infusion rate to blood flow ratio. Keeping single-pass citrate extraction on the dialyzer >0.75 minimizes the risk of citrate toxicity even in patients with absent citrate metabolism. Precalculated, personalized dosing of the initial Ca-infusion rate from a table on the basis of the patient’s albumin level and the filter effluent flow rate maintains neutral CKRT circuit calcium mass balance and a normal systemic iCa level.
Introduction
The Kidney Disease Improving Global Outcomes 2012 guidelines recommend regional citrate anticoagulation (RCA) in continuous KRT (CKRT), but only in patients who do not have shock or severe liver failure. This guidance is problematic for providers as in many intensive care units (ICUs), patients with shock or liver failure may comprise ≥10% of those on CKRT. Consequently, unfractionated heparin remains a commonly used CKRT-anticoagulation method worldwide (1), despite increased hemorrhagic complications and transfusion requirements compared with RCA (2–4).
To empower ICU providers to use CKRT-RCA without contraindications in patients with absent systemic citrate clearance (normal is 42 L/h) (5), we developed a postdilution continuous venovenous hemodiafiltration (CVVHDF)-RCA Shock protocol for the Prismaflex. This novel protocol has been in use at our institution since January 2018, and utilizes CKRT-RCA flow settings selected from precalculated tables for a customized effluent flow goal that is very high relative to circuit blood flow resulting in >0.75 single-pass fractional removal of citrate (ECit) on the dialyzer preventing citrate toxicity. Personalized Ca-infusion rates are obtained from a precalculated table on the basis of effluent flow and systemic albumin level for a neutral Ca balance on the CKRT circuit. We triage patients to the Shock protocol if their citrate metabolism is presumed to be severely impaired, or if they develop citrate toxicity with our CVVHDF-RCA NonShock protocol.
Here we report on the design, efficacy, and safety of the CVVHDF-RCA Shock protocol. The approach is on the basis of principles of CKRT-RCA protocols designed for near-automated delivery we have described before (6), specifically adapted for use on the Prismaflex and in patients with absent citrate metabolism.
Materials and Methods
Study Design and Participants
This was a prospective study of patients triaged to the CVVHDF-RCA Shock protocol in five adult ICUs at the University of Michigan between March and September 2018. The study was approved by the Ethics Committee of the University of Michigan (Institutional Review Board # HUM00029545) with a waiver of informed consent.
CKRT Procedure
The Prismaflex CKRT machine is used in postdilution CVVHDF mode with low blood flow (QB). Commercially available acid citrate dextrose anticoagulant flow (QACDA) is delivered by the preblood pump (PBP) at a fixed high flow ratio relative to QB. Bicarbonate-buffered dialysate flow (QD) and postdilution replacement fluid flow (QRF) are delivered by their respective pumps at a 5:2 flow ratio to maximize ECit without causing immediate postfilter hematocrit (Hct) values >50. Calcium chloride solution (136 mM CaCl2 in 0.9% saline) is infused at the end of the return limb of the blood circuit by a separate infusion pump.
CKRT prescribing is designed to support providers without special expertise in solute kinetic analysis and consists of mechanistically following several steps. We use RCA in all CKRT sessions. First, patients are triaged to the citrate metabolism presumed absent pathway if one or more of the following criteria are satisfied: systemic lactate ≥10 mM in patients with shock, or requirement of either a dextrose drip to prevent hypoglycemia or fresh frozen plasma drip to keep the INR <3 in patients with cirrhosis, or a diagnosis of acute liver failure/shock liver close to the time of CKRT-RCA start. These arbitrary criteria are aimed at putting every patient with a significant chance of severely reduced citrate metabolism on the Shock protocol, at the cost of including some patients with sufficient citrate metabolism in this pool, because there is no risk of treating such patients with the Shock settings. Second, we select the flows QB, QACDA, QD, and QRF from Table 1 on the basis of body weight (10 kg increments) for an effluent dose about 35–40 ml/kg per hour (this relatively high effluent dose is often needed to improve and maintain acceptable pH in patients with lactic acidosis). Table 1 settings were precalculated by us to achieve circuit iCa <0.4 mM and to maximize ECit. The detailed solute kinetic analysis underpinning Table 1 is beyond the scope of this manuscript and has been presented in American Society of Nephrology Kidney Week 2019 (Szamosfalvi and Yessayan, Posters FR-PO076 and FR-PO079: accessed at www.asn-online.org). Third, the initial Ca-solution flow rate (QCa) is selected from Table 2 on the basis of the patient’s systemic albumin level and the total effluent flow rate predicted from the selected Table 1 flow settings. Finally, during CVVHDF-RCA therapy, the QCa is adjusted in increments of +/−10%–20% of the current flow rate on the basis of how the systemic iCa measured every 6 hours compares to the goal systemic iCa (either 1.05–1.25 mM, see Table 3 or 1.2–1.4 mM, see Table 4).
Table 1.
Prismaflex fixed flow settings for severe Shock patients are selected according to weight and/or total effluent flow goal
| Weight | Effluent Flow, QEFF ml/h | Blood Flow, QB ml/min | Citrate Flow, ACDA ml/h | Dialysate Flow, QD ml/h | Postdilution Flow, QRF ml/h |
| ≤50 kg | 1900+ | 50 | 125 | 1250 | 500 |
| 51–60 kga | 2300+ | 60 | 150 | 1500 | 600 |
| 61–70 kg | 2650+ | 70 | 175 | 1750 | 700 |
| 71–80 kg | 3050+ | 80 | 200 | 2000 | 800 |
| 81–90 kg | 3450+ | 90 | 225 | 2250 | 900 |
| 91–100 kga | 3800+ | 100 | 250 | 2500 | 1000 |
| 101–110 kg | 4200+ | 110 | 275 | 2750 | 1100 |
| 111–120 kg | 4550+ | 120 | 300 | 3000 | 1200 |
| 121–130 kg | 4950+ | 130 | 300 | 3250 | 1300 |
| 131–140 kg | 5300+ | 140 | 300 | 3500 | 1400 |
| ≥141 kga | 5650+ | 150 | 300 | 3750 | 1500 |
Table 1 flow settings ensure >0.75 single-pass fractional removal of citrate (ECit) on the dialyzer limiting systemic citrate accumulation to ≤2.5 mM (CMax) even in the absence of citrate metabolism. Different rows yield a different hourly effluent flow; the prescriber may calculate the total effluent flow as a product of the dosing weight and desired ml/kg per hour dose, or may simply select the proper Table 1 row on the basis of dosing weight to deliver about 35–40 ml/kg per hour effluent dose. The fixed and high citrate-to-blood flow ratio is designed to achieve adequate citrate anticoagulation (circuit iCa <0.4 mM) irrespective of variable systemic hematocrit (Hct) level, and hence plasma flow rate at a fixed QB. Very high effluent flows relative to circuit plasma flow ensure >70% single pass citrate removal and CKRT dose 38–42 ml/kg per hour in severe shock. QEFF, effluent flow rate; QB, postdilution continuous venovenous hemodiafiltration (CVVHDF) mode with low blood flow; ACDA, acid citrate dextrose anticoagulant flow; QD, bicarbonate-buffered dialysate flow; QRF, postdilution replacement fluid flow; CKRT, continuous KRT; iCa, ionized Ca.
Patients included in this study were treated using one of these rows.
Table 2.
Initial infusion rate (ml/h) of 136 mM CaCl2 in 0.9% saline for goal systemic ionized Ca 1.15 mM
| Effluent Flow Rate (ml/hr) | 0.0–0.7 g/dl | 0.8–1.2 g/dl | 1.3–1.7 g/dl | 1.8–2.2 g/dl | 2.3–2.7 g/dl | 2.8–3.2 g/dl | 3.3–3.7 g/dl | 3.8–4.2 g/dl | 4.3–4.7 g/dl | 4.8–5.2 g/dl |
| ≤2100 | 28 | 29 | 30 | 31 | 32 | 32 | 33 | 34 | 35 | 36 |
| 2101–2500a | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 |
| 2501–2850 | 39 | 41 | 42 | 43 | 44 | 45 | 47 | 48 | 49 | 50 |
| 2851–3250 | 45 | 47 | 48 | 50 | 51 | 52 | 53 | 55 | 55 | 57 |
| 3251–3650 | 51 | 52 | 54 | 56 | 57 | 58 | 60 | 61 | 62 | 64 |
| 3651–4000a | 56 | 58 | 60 | 62 | 63 | 65 | 67 | 68 | 69 | 71 |
| 4001–4400 | 62 | 64 | 66 | 68 | 69 | 71 | 73 | 75 | 76 | 78 |
| 4401–4750 | 68 | 70 | 72 | 74 | 76 | 78 | 80 | 82 | 83 | 85 |
| 4751–5150 | 72 | 74 | 77 | 79 | 81 | 83 | 85 | 87 | 89 | 91 |
| 5151–5500 | 76 | 78 | 81 | 84 | 85 | 88 | 90 | 92 | 94 | 97 |
| 5501–5850a | 79 | 82 | 85 | 87 | 90 | 92 | 94 | 97 | 99 | 101 |
In patients with citrate metabolism presumed absent, the initial QCa is chosen from Table 2 on the basis of the systemic albumin level and the total effluent flow rate (≈QACDA+QD+QRF). The effect of any net ultrafiltration on QCa can be neglected. Precalculated, plasma clearance-based Ca-infusion dosing is largely independent of the intake blood Hct level if the systemic hemoglobin (Hb) <14 g/dl and the fixed post-CVVHDF-RCA flow settings are selected from Table 1. To target a higher systemic iCa of 1.3 mM (at the ICU team’s discretion) the initial Ca-infusion rate derived from Table 2 can be multiplied by 1.13. QCa, calcium infusion rate; QACDA, acid citrate dextrose anticoagulant infusion rate; QD, dialysate flow rate; QRF, replacement fluid flow rate.
Patients included in this study were treated using one of these rows.
Table 3.
Rate change of 136 mM CaCl2 in 0.9% saline based on systemic ionized Ca every 6 h: GOAL 1.15 (1.05–1.25) mM
| Current Ca Infusion Flow Rate, ml/h | The Patient’s Ionized Calcium Level Checked Every 6 h | ||||
| <0.95 mmol/L | 0.95–1.04 mmol/L | 1.05–1.25 mmol/L | 1.26–1.4 mmol/L | >1.4 mmol/L | |
| Increase Rate +20%; Notify ICU and Nephro Fellows | Increase Rate +10% | No Change | Reduce Rate −10% | Reduce Rate −20%; Notify ICU and Nephro Fellows | |
| ≤15 | +2 | +1 | No change | −1 | −2 |
| 16–25 | +4 | +2 | No change | −2 | −4 |
| 26–35 | +6 | +3 | No change | −3 | −6 |
| 36–45 | +8 | +4 | No change | −4 | −8 |
| 46–55 | +10 | +5 | No change | −5 | −10 |
| 56–65 | +12 | +6 | No change | −6 | −12 |
| 66–75 | +14 | +7 | No change | −7 | −14 |
| 76–85 | +16 | +8 | No change | −8 | −16 |
| 86–95 | +18 | +9 | No change | −9 | −18 |
| 96–105 | +20 | +10 | No change | −10 | −20 |
Systemic iCa is checked within 1 h before start of CKRT and at 2, 4, and 6 h, and every 6 h thereafter. If the iCa is outside the limits of the “no change” range at h 2, 4, and 6, the CKRT prescribing team is notified for advice but no titration per protocol is initiated by the nurse. Subsequently, the Ca rate is adjusted in increments of +/−10%–20% of the current rate on the basis of the systemic iCa value obtained every 6 h. Even with severe liver dysfunction and shock, most patients will have some citrate clearance in the range of 1–6 L/h, and will have systemic citrate levels in the 0.5–1.5 mM range. Therefore, it is expected the initial Ca rate will be titrated down 10%–25% in the first 24 h of CKRT-RCA according to Shock protocol unless citrate metabolism is completely absent. CKRT, continuous KRT; RCA, regional citrate anticoagulation; iCa, ionized Ca.
Table 4.
Rate change of 136 mM CaCl2 in 0.9% saline based on systemic ionized Ca every 6 h: GOAL 1.3 (1.2–1.4) mM
| Current Ca-Infusion Flow Rate, ml/h | The Patient’s Ionized Calcium Level Checked Every 6 h | ||||
| <1.1 mmol/L | 1.1–1.19 mmol/L | 1.2–1.4 mmol/L | 1.41–1.55 mmol/L | >1.55 mmol/L | |
| Increase Rate +20%; Notify ICU and Nephro Fellows | Increase Rate +10% | No Change | Reduce Rate −10% | Reduce Rate −20%; Notify ICU and Nephro Fellows | |
| ≤15 | +2 | +1 | No change | −1 | −2 |
| 16–25 | +4 | +2 | No change | −2 | −4 |
| 26–35 | +6 | +3 | No change | −3 | −6 |
| 36–45 | +8 | +4 | No change | −4 | −8 |
| 46–55 | +10 | +5 | No change | −5 | −10 |
| 56–65 | +12 | +6 | No change | −6 | −12 |
| 66–75 | +14 | +7 | No change | −7 | −14 |
| 76–85 | +16 | +8 | No change | −8 | −16 |
| 86–95 | +18 | +9 | No change | −9 | −18 |
| 96–105 | +20 | +10 | No change | −10 | −20 |
Solutions Used with the Postdilution CVVHDF-RCA Shock Protocol
In the United States there is no commercially available citrate solution with Food and Drug Administration (FDA) approval for CKRT-RCA. To avoid the cost and uncertain availability of compounded citrate solutions, we use USP ACDA. ACDA is FDA approved for anticoagulation during plasmapheresis and has a published record of clinical use in CKRT-RCA off label.
The default CKRT fluid (dialysate and replacement) bicarbonate (HCO3) level is 35 mM, potassium (K) is 4 mM and phosphate (P) is 1.36 mM; these can be adjusted as needed; see Table 5. Solutions for the Shock protocol were spiked from a commercial CKRT fluid (BBraun Duosol 4553: 136 mM sodium, Na; 2 mM potassium, K; 25 mM HCO3; 0.75 mM magnesium, Mg; and 0 mM calcium, Ca) using K-phosphate or K-chloride or Na-phosphate to final K 2, 3, or 4 mM and phosphate 0, 2.1, or 4.2 mg/dl (0, 0.68, or 1.36 mM), and 8% NaHCO3 (1 mEq/ml) in most prescriptions to final Na 146 and HCO3 35 mM. For severe lactic acidosis, we added further NaHCO3 up to a final CKRT fluid HCO3 45 mM and Na 156 mM. This resulted in moderately hypernatric CKRT fluids. A 5% dextrose solution was administered at rates between 30 and 80 ml/h for neutral CKRT glucose balance (unless glucose was present as the 5% dextrose water part of a vasopressor or isotonic bicarbonate solution). The calcium chloride solution was produced in the local hospital compounding pharmacy from 10% CaCl2 (6.8 mmol Ca/10 ml), with 125 ml added to 500 ml of 0.9% saline. Table 5 shows a modified CKRT fluid strategy we would recommend with the Shock protocol on the basis of a glucose-containing base fluid (NxStage RFP-403), which will also have 6 mM less sodium for the same HCO3 level achieved.
Table 5.
Solutions used with the postdilution continuous venovenous hemodiafiltration with regional citrate anticoagulation (CVVHDF-RCA) Shock protocol
| Solute (mM) | ACDA Citrate, 113 mM | CKRT Fluid 1 NxStage RFP-403 2K/35Bic | CKRT Fluid 2 (Fluid 1 Spiked with 3M KHPO4) 4K/35Bic | 136 mM CaCl2 in 0.9% Saline |
| Calcium | 0 | 0 | 136 | |
| Magnesium | 0.75 | 0.75 | 0 | |
| Chloride | 108.5 | 108.5 | 395 | |
| Glucose | 124 | 5.5 | 5.5 | 0 |
| Sodium | 225 | 140 | 140 | 123 |
| Citric acid | 38 | |||
| Citrate3− | 75 | |||
| Potassium | 2 | 4 | ||
| Bicarbonate | 35 | 35 | ||
| Phosphate1.3− | 1.5 |
Ideally, CKRT Fluid 1 (NxStage RFP-403) and CKRT Fluid 2 could be used with the Shock protocol as these have 140 mM Na level (instead of 146) for the same 35 mM HCO3 level provided. CKRT fluid 1 can be spiked with K-phosphate or K-chloride or Na-phosphate to final K 2, 3, or 4 mM and phosphate 0, 2.1, or 4.2 mg/dl (0, 0.68, or 1.36 mM). The CKRT Fluid one or two glucose level of 5.5 mM ensures a mildly positive glucose balance on the CKRT circuit. The 136 mM calcium chloride solution was compounded in the hospital pharmacy by adding 125 ml of 10% CaCl2 (6.8 mmol Ca/10 ml) to 500 ml of 0.9% saline. Commercially available glucose-containing CKRT Fluid 1; 3M K-phosphate spiked CKRT Fluid 2 and compounded Ca infusion. ACDA, acid citrate dextrose anticoagulant; CKRT, continuous KRT.
Systemic Citrate Level Simulations and CVVHDF-RCA Ca Clearance Calculations
The plasma clearance of citrate and calcium on the Prismaflex circuit with the HF1400 (QB ≥80 ml/min) and HF1000 (QB <80 ml/min) dialyzer was calculated by adapting post-CVVHDF clearance equations described in the literature and using a Microsoft Excel clearance calculator (Tables 1 and 2) (7). We used a single-pool, fixed-volume kinetic equation as described by Szamosfalvi et al. in US Patent Application 20080015487 and validated by Zheng et al. (8) to model systemic citrate levels with 12-minute resolution during the design of the Shock protocol.
Data Sources
Demographics, clinical variables, and laboratory data during the first 96 hours of the CVVDF-RCA treatment were collected from the electronic medical records. Filter clotting data and reasons for disconnection were extracted from data recorded by the ICU nurse in the electronic health record. Data was collected by two research fellows and transcribed into Excel files by a research resident.
Study Variables
RCA effectiveness in decreasing clotting was measured in terms of time to first hemofilter loss due to clotting as recorded by the ICU nurse, and by the established surrogate variable, the circuit iCa levels. Hemofilter life was defined as the time elapsed between the start of the blood flow through the filter and the time when blood was unable to pass through the filter due to clot formation or obstruction of the filter (9).
Electrolyte complications after CKRT initiation were attributable to CVVHDF-RCA if the following criteria were met for each of the following variables: iCa <0.9 mM; iCa >1.5 mM in the absence of exogenous calcium administration beyond dictated by the protocol; serum Na >148 mM in the absence of hypertonic intravenous Na infusion; HCO3− >30 mM with pH >7.45 in the absence of exogenous intravenous bicarbonate administration; and P<2.0 mg/dl.
Study Outcomes
The primary outcome was hemofilter life. Secondary outcomes were surrogate of citrate accumulation (tCa/iCa ratio), prevalent electrolyte and acid-base trends, the cumulative incidence of electrolyte disturbances in general and those attributable to CVVHDF-RCA.
Statistical Analyses
Statistical analysis was performed using MedCalc Statistical Software version 19.1.5 (MedCalc Software by Ostend, Belgium; https://www.medcalc.org; 2020). Categorical data were reported as frequencies±percentages and continuous data as mean±SD for normally distributed data and as median (interquartile range [IQR]) for non-normally distributed data. The 96-hour clotting/clogging-free hemofilter survival rates were calculated using the Kaplan-Meier product limit estimator. Quantitative data trends for select solute levels are presented in boxplots.
Results
A total of 31 patients with up to 96-hour sessions of CVVHDF-RCA satisfied the inclusion criteria. Demographics and primary reason for admission are shown in Table 6. Four patients were started on blood flow of 60 ml/min, 24 on blood flow of 100 ml/min, and three on blood flow 150 ml/min (from the CKRT setting rows noted in Table 1). Characteristics of the initial prescriptions are shown in Table 7.
Table 6.
Demographics and baseline characteristics
| Demographics and Baseline Characteristics | Value |
| Age, yr, mean±SD | 51±17 |
| Male, n (%) | 21 (67.7) |
| Cause of admission, n (%) | |
| Medical | 27 (87.1) |
| Surgical | 4 (12.9) |
| ESKD, n (%) | 1 (3.2) |
| CKD, n (%) | 7 (22.6) |
| Total AKI, n (%) | 30 (96.8) |
| ATN | 26 (83.9) |
| Cardiorenal | 3 (9.7) |
| HRS | 1 (3.2) |
| PVD, n (%) | 2 (6.5) |
| CHF, n (%) | 12 (38.7) |
| Hyperlipidemia, n, % | 9 (29.0) |
| CAD, n (%) | 5 (16.1) |
| HTN, n (%) | 11 (35.5) |
| COPD, n (%) | 5 (16.1) |
| DM, n (%) | 7 (22.6) |
| Cirrhosis, n (%) | 5 (16.1) |
| Acute liver failure, n (%) | 16 (51.6) |
| Cancer, n (%) | 4 (12.9) |
| AST (U/L), median (IQR) | 822 (122–2950) |
| ALT (U/L), median (IQR) | 352 (41–2238) |
| Total bilirubin (mg/dl), median (IQR) | 2.7 (1.0–5.1) |
| INR (U), median (IQR) | 2 (1.5–2.6) |
| Lactate (mmol/L), median (IQR) | 5 (3.2–10.7) |
| Albumin (g/dl), median (IQR) | 2.7 (2.4–2.9) |
ATN, acute tubular necrosis; HRS, hepatorenal syndrome; PVD, peripheral vascular disease; CHF, congestive heart failure; CAD, coronary artery disease; HTN, hypertension; COPD, chronic obstructive pulmonary disease; DM, diabetes; AST, aspartate aminotransferase; IQR, interquartile range; ALT, alanine aminotransferase; INR, international normalized ratio.
Table 7.
Initial prescription settings and calcium flow rate at 24 h
| Citrate Flow Rate Initiation (ml/h) | Dialysate Fluid Flow Rate (ml/h) | Replacement Fluid Flow Rate (ml/h) | Calcium Flow Rate (ml/h) at Initiation | Calcium Flow Rate (ml/h) at 24 h |
| Blood flow rate initiation 60 ml/min (n=4) | ||||
| 150±0 | 1500±0 | 600±0 | 39.5±1.7 | 33.7±5.5 |
| Blood flow rate initiation 100 ml/min (n=24) | ||||
| 252±28 | 2500±0 | 1000±0 | 63.3±6.9 | 57.0±9.1 |
| Blood flow rate initiation 150 ml/min (n=3) | ||||
| 300±0 | 3500±433 | 1333±289 | 85.3±11.7 | 82.7±32.5 |
Data is presented as mean±SD.
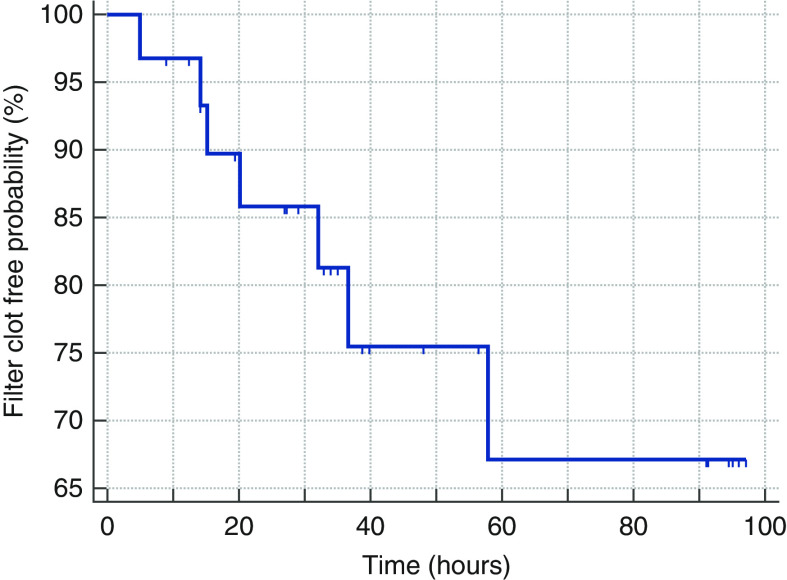
The median first CKRT circuit duration was 35.00 hours (IQR, 19.63–82.69 hours). The first hemofilter clotting free probability for 31 patients censored for other causes of interruption is shown in Figure 1, with a median first hemofilter life exceeding 70 hours. The mean circuit iCa level was 0.35±0.05 mmol/L. Filter clotting was the documented reason for interruption in six of 31 patients (19%). Clotting occurred in one of four hemofilters at QB 60 ml/min, five of 24 at QB 100 ml/min, and one of three at QB 150 ml/min. Other causes of CKRT interruption included the need for procedures (seven out of 31, 23%), catheter dysfunction (three out of 31, 10%), death or withdrawal of care (two out of 31, 6%), recirculation for physical therapy (one out of 31, 3%), or discontinued per physician order for a variety of reasons (six out of 31, 19%).
Figure 1.
Kaplan-Meier survival curve for hemofilter life.
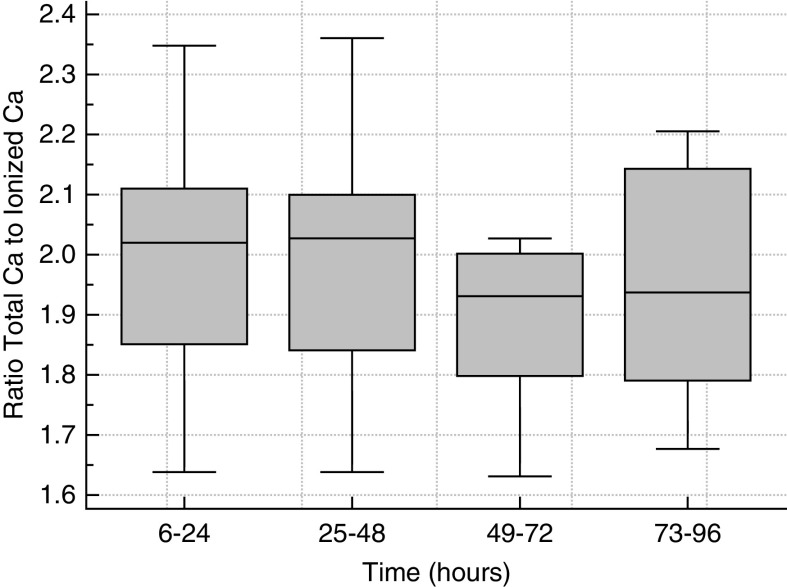
Standard boxplots for sodium, HCO3, phosphorus, and iCa are shown in Figure 2, and tCa/iCa ratio over time is shown in Figure 3. The cumulative incidence of hypernatremia, metabolic alkalosis, hypophosphatemia, hypocalcemia, and hypercalcemia were one out of 26 (4%), zero out of 30 (0%), one out of 30 (3%), two out of 31 (6%), and zero out of 27 (0%), respectively, and were not clinically significant. Hypernatremia (Na 149 mM) occurred in one patient receiving hypertonic CKRT solution (Na 156 mM); this was due to how we generated high-bicarbonate CKRT fluids by spiking and was not unexpected. Hypocalcemia occurred in two patients and in three out of 251 of samples collected between hours 2 and 6 from CKRT initiation. The iCa was 0.86 mmol/L at 2 hours in one patient, and 0.88 and 0.87 mmol/L at hours 2 and 4 in another patient. These were corrected by hour 6 with no additional Ca supplementation beyond the per-protocol administered Ca infusion. All samples with iCa >1.5 mM between hours 2 and 96 occurred in a single patient who was hypercalcemic at baseline. This patient had calcium channel blocker poisoning, and iCa was deliberately being maintained at levels higher than 1.6 mmol/L before and during CKRT. Table 8 shows numeric values of highest tCa/iCa ratio and magnesium as median, IQR, minimum, and maximum at select time intervals. The maximum tCa/iCa ratio never exceeded 2.5, consistent with limiting systemic citrate accumulation with the Shock protocol to a clinically acceptable level. The maximum observed value of 2.36 implies a systemic citrate level of about 2 mM and near-absent systemic citrate metabolism when considering the >0.75 ECit prescribed with the Table 1 Shock CVVHDF-RCA settings. Total magnesium IQR was 1.8–2.1 mg/dl, consistent with normal total Mg and likely low normal ionized Mg (assuming 0.5–2 mM citrate accumulation) with CKRT fluid Mg level 0.75 mM (1.5 mEq/L).
Figure 2.
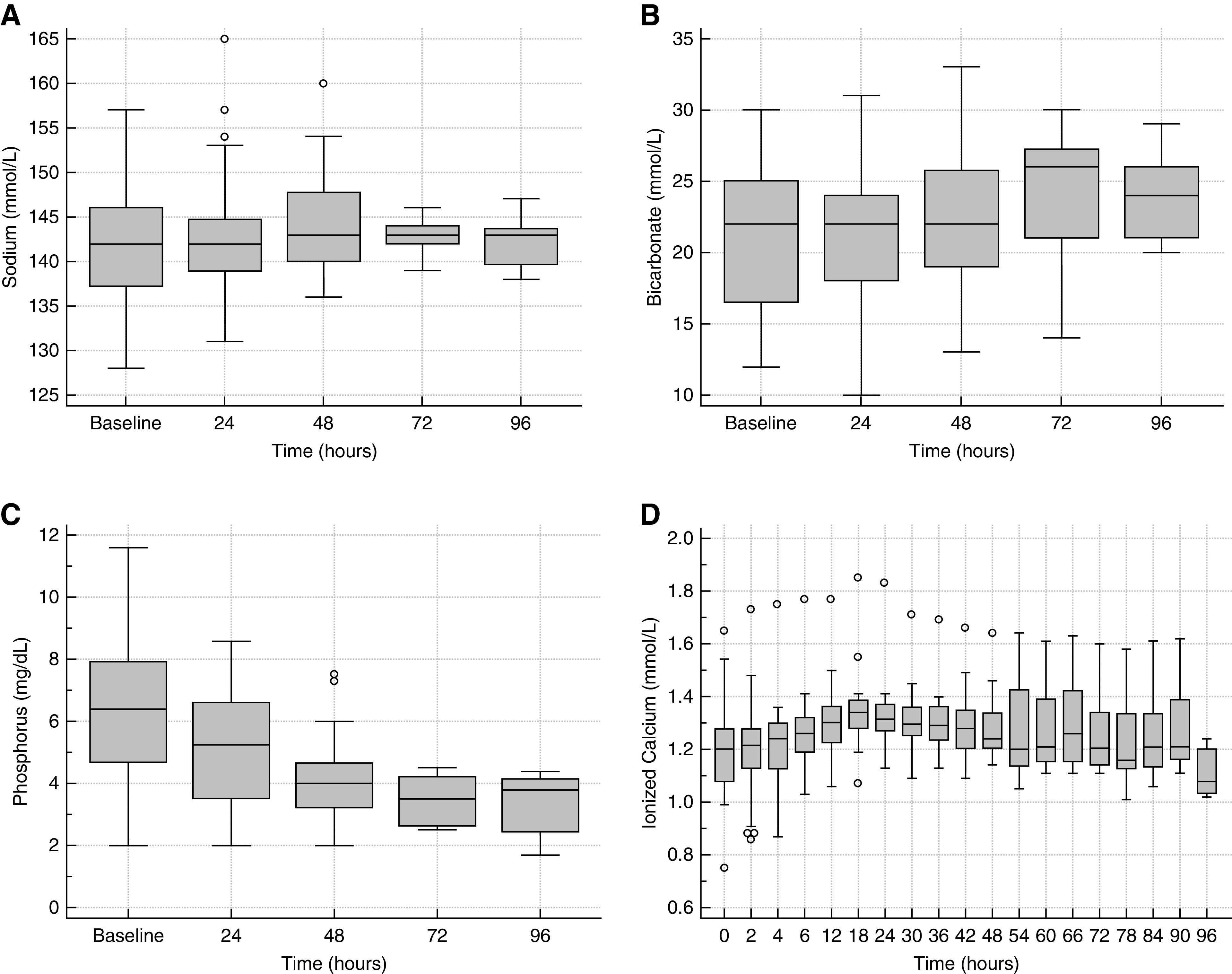
Sodium, bicarbonate, phosphorus and ionized calcium levels over time during continuous venovenous hemodiafiltration with regional citrate anticoagulation. Standard boxplots of (A) plasma sodium, (B) bicarbonate, (C) phosphorus, and (D) systemic ionized calcium.
Figure 3.
Total calcium to ionized calcium ratio over time during continuous venovenous hemodiafiltration with regional citrate anticoagulation.
Table 8.
Measures of central tendency and spread every 24 h for total Ca/ionized Ca ratio and magnesium while on postdilution continuous venovenous hemodiafiltration with regional citrate anticoagulation (CVVHDF-RCA) Shock protocol
| Variable | N | Median | IQR | Minimum | Maximum |
| Max tCa/iCa | |||||
| First 24 h | 31 | 2.02 | 1.85–2.11 | 1.64 | 2.35 |
| 25–48 h | 17 | 2.028 | 1.84–2.10 | 1.64 | 2.36 |
| 49–72 h | 8 | 1.931 | 1.80–2.00 | 1.63 | 2.03 |
| 73–96 h | 8 | 1.938 | 1.79–2.14 | 1.68 | 2.21 |
| Magnesium (mg/dl) | |||||
| Baseline | 30 | 2.3 | 2.1–2.5 | 1.3 | 3.5 |
| 24 h | 27 | 2 | 1.9–2.2 | 1.7 | 2.4 |
| 48 h | 17 | 2 | 1.8–2.1 | 1.6 | 2.8 |
| 72 h | 8 | 1.85 | 1.8–1.9 | 1.7 | 2.1 |
| 96 h | 7 | 1.9 | 1.7–2.0 | 1.6 | 2.1 |
IQR, interquartile range; tCa, total Ca; iCa, ionized Ca.
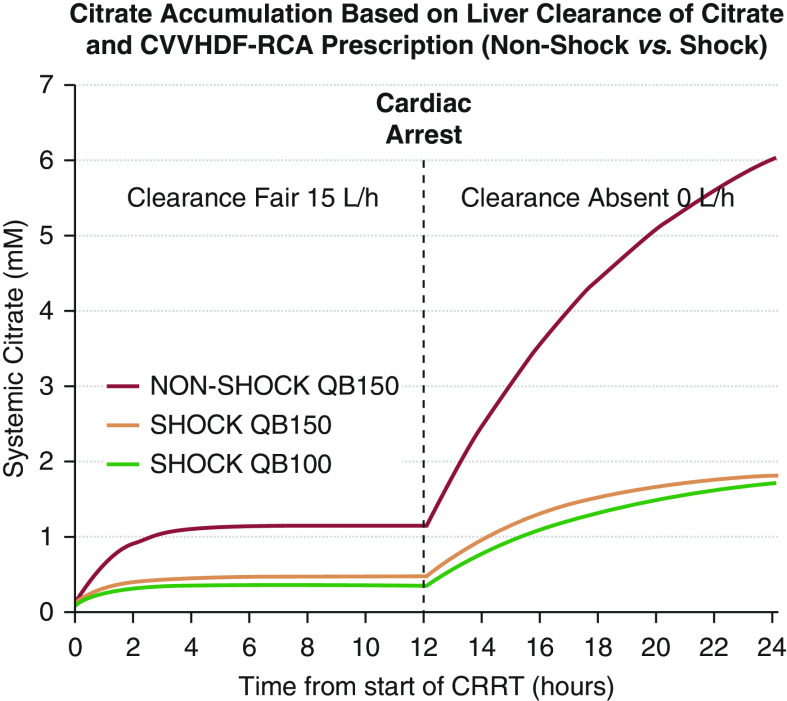
The results of simulations of systemic citrate levels as a function of time in a single pool, fixed volume kinetic model with variable body (liver) clearance of citrate (15 L/h during 0–12 hours and 0 L/h after 12 hours), and specific CVVHDF-RCA fixed flow settings with the NonShock protocol (QB 150 ml/min; QACDA 300 ml/h, QD/QRF 1.2/1.2 L/h) and the Shock protocol (QB 100 or 150 ml/min, Table 1 setting rows) are shown in Figure 4. Systemic citrate levels remain <1.5 mM with any of the CVVHDF-RCA protocols in the first 12 hours of simulation when body citrate clearance is set to 15 L/h (compensated liver cirrhosis). When body clearance of citrate is set to 0 L/h for the period of 12–24 hours, life-threatening systemic citrate accumulation will not occur with Shock setting prescriptions. The Shock settings, with high filter effluent flow relative to circuit plasma flow, ensure >75% single-pass dialyzer citrate removal and prevent systemic citrate accumulation ≥2.5 mM even in the complete absence of citrate metabolism.
Figure 4.
Simulated systemic citrate level curves with three different postdilution continuous venovenous hemodiafiltration with regional citrate anticoagulation (CVVHDF-RCA) prescriptions as a function of time and citrate metabolism. With precalculated Shock settings, very high filter effluent flows relative to circuit plasma flow ensure >0.75 single-pass fractional dialyzer citrate removal (ECit) and prevent maximum systemic citrate accumulation (CMax) ≥2.5 mM even in the absence of liver metabolism. CRRT, continuous RRT.
Discussion
ICU providers can simply prescribe postdilution CVVHDF-RCA without contraindications, with precalculated Shock protocol settings for the Prismaflex to critically ill patients suspected of having absent citrate metabolism. Because all Table 1 settings are fixed, the ICU nurse only titrates the Ca infusion, which simplifies bedside delivery of CVVHDF-RCA and troubleshooting of complications. The data show that, despite a 52% incidence of severe acute liver failure in our patients and a median lactate of five (IQR, 3.2–10.7 mmol/L), citrate toxicity was prevented, systemic iCa levels were maintained above 1 mM using a personalized initial Ca-dosing strategy, whereas circuit iCa levels were reduced below 0.4 mM. In our institution, every patient on CKRT receives RCA and all patients who qualify for the shock protocol are supposed to receive it without any deviations from the settings in Table 1 and the initial Ca dosing in Table 2. Therefore, we could not have excluded any patient with severe liver failure from receiving RCA. The median hemofilter life exceeded 70 hours in this study. The reported patients with CKRT related electrolyte abnormalities were rare and not clinically significant.
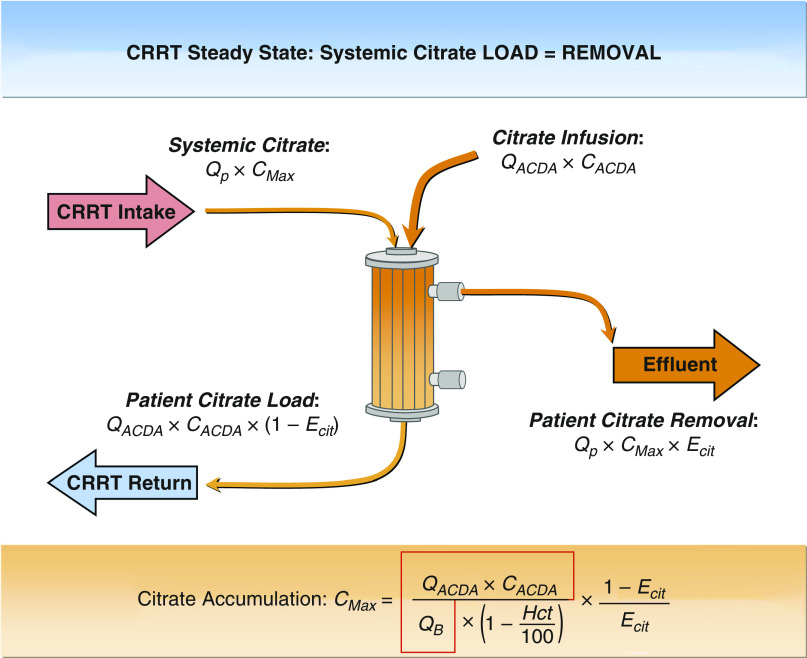
It is important to understand why systemic citrate accumulation and ionized hypocalcemia could be avoided using this protocol. Without systemic citrate clearance, the patient’s extracellular fluid space simply becomes a passive reservoir of citrate (Figure 5). The systemic citrate level in the extracellular fluid space will continue to rise until citrate generation from the ACDA infusion (QACDA × CACDA × [1-ECit]) and citrate removal (systemic citrate level × plasma flow × ECit) become equal, at which point the steady-state maximum systemic citrate level (CMax) is reached. The precalculated Table 1 flow settings ensure ECit in the 0.75–0.85 range and that the CMax value will always be <2.5 mM, even with absent citrate metabolism, as long as the dialyzer ECit does not decline markedly over the course of the CVVHDF-RCA therapy due to membrane fouling and/or fiber bundle partial clotting. To date, none of the authors observed a decline in ECit leading to citrate toxicity using the Shock protocol.
Figure 5.
Analysis of citrate fluxes in a patient with absent citrate metabolism. The expression in the red outline translates into how many mmol of citrate we add to each liter of blood entering the continuous kidney replacement therapy (CKRT) circuit; at a fixed value of 4.7 mmol/L for QB up to 120 ml/min, it ensures circuit ionized Ca (iCa) <0.4 mM even prefilter. Df, filter citrate clearance; QP, circuit plasma flow; QB, circuit blood flow (all L/h); QACDA, citrate flow rate (L/h); CACDA, ACDA citrate concentration (mM). ECit = Df/QP. The expression in the red outline is a constant (4.7 mmol/L) and ECit is 0.75–0.85 range (increases with higher Hct). ACDA, acid citrate dextrose anticoagulant flow; CRRT, continuous RRT; ECit, single-pass fractional removal of citrate.
The personalized initial Ca-infusion rate in this protocol ensures neutral Ca mass balance on the CKRT circuit (Ca infused = plasma volume cleared of tCa × systemic tCa goal) in almost all patients. The use of acidic ACDA at a high fixed ratio to QB disrupts Ca binding to albumin, making 90%–95% or the total Ca dialyzable or filterable and results in more predictable Ca losses on the filter and more precise calculation of the Ca-infusion rate needed for neutral CKRT Ca-mass balance at the targeted systemic total Ca level. This Ca-infusion rate will cause a slightly positive calcium balance in those who can metabolize citrate and those with systemic Hct >45, but will never underestimate the Ca rate needed to keep the systemic iCa >1.1 mM. Some cycling of the systemic iCa can occur if QCa adjustments are made beyond the scheduled every 6 hours iCa checks. A serum albumin measurement within 24 hours before CVVHDF-RCA start is sufficient to avoid underdosing the initial Ca rate in all patients. Even in those with frequent albumin infusions, the systemic iCa checks every 6 hours allow for Ca-rate adjustments without any concern for clinically significant ionized hypocalcemia. Finally, the Shock protocol uses about 10%–20% higher Ca rates than our Nonshock protocol for any specific effluent flow and albumin level to account for a maximum possible systemic citrate accumulation of approximately 2.5 mM.
Several observational studies (10–13) and clinical trials have shown that filter lifespan with citrate was significantly higher compared with heparin (2,14–16). Reported filter lifetimes with citrate anticoagulation varied widely in these studies likely related to differences in protocol design. Most RCA protocols monitor circuit iCa postdialyzer, where the iCa is lowest after a blood purification step with Ca-free CKRT fluid(s). Many of these protocols may not achieve circuit iCa <0.4 mM prefilter by not adding enough citrate to the incoming circuit blood. This protocol overcomes this concern by using a high, fixed 1:24 ACDA:QB ratio <QB 120 ml/min, which is then allowed to drop to 1:30 (still high) gradually until QB 150 ml/min is reached. The Kd for Ca citrate was determined in the past (17) and predicts that with this ACDA prescribing method, even the prefilter iCa will be <0.4 mM as long as systemic iCa is <1.4 mM and albumin is <5 g/dl. The circuit iCa is then further lowered by Ca-free CKRT fluids. The use of acidic ACDA at a high fixed ratio to QB disrupts Ca binding to albumin, rendering 90%–95% or the total Ca dialyzable or filterabl and results in more predictable Ca losses on the filter and more precise calculation of the Ca-infusion rate needed for neutral CKRT Ca mass balance at the targeted systemic total Ca level. There were incident patients with filter clotting in a small number of the CKRT runs. Complete abrogation of clotting was probably limited as the Prismaflex software available in the United States frequently interrupts citrate delivery by the PBP while keeping the blood pump running for instance during effluent-, CKRT fluid–, and/or PBP-delivered ACDA bag changes. This results in periods of normal iCa in the blood circuit, which may allow clotting.
Hypernatremia is a potential complication of using ACDA hypertonic citrate solution during CKRT-RCA, often mitigated with low, 122–130 mM sodium CKRT fluids, or completely avoided by changing from ACDA to a dilute, isotonic citrate solution as a predilution replacement fluid. In the United States, such dilute citrate solutions are neither FDA approved, nor commercially available. When ACDA is used with the precalculated settings from Table 1, the systemic sodium level is predicted to be about equal to the CKRT fluid Na level +2 mM if the patient is not receiving nonisotonic intravenous fluids. In the Shock protocol, mild increases in systemic serum sodium were expected, because spiking of the CKRT fluid from 25 to 35 mM HCO3 resulted in a final CKRT fluid Na level of 146 mM. There were no incident patients with hypernatremia (defined by us as systemic Na >148 mM and >5 mM above the CKRT fluid Na level), possibly in part due to many of these patients receiving hypotonic intravenous fluids (e.g., catecholamines in D5W or N-acetylcysteine in D5W). It is empirically obvious that moderate hypernatremia could develop with CKRT fluid Na level >150 mM in the Shock protocol. This was actually desired initially in many of the acute liver failure patients included in this study, who were at an increased risk of developing brain edema. Table 5 shows a modified CKRT fluid strategy we recommend with this protocol, on the basis of a different, glucose-containing base fluid (NxStage RFP-403) that will have 6 mM less sodium for the same HCO3 level achieved than with the CKRT fluid spiking we utilized.
Systemic bicarbonate levels are the most difficult to predict and control with CKRT-RCA as they are variably affected by lactic acidosis and other systemic acidosis and alkalosis processes, by bicarbonate generation from citrate metabolism, and by bicarbonate fluxes on the CKRT circuit using citric acid–containing ACDA and commercial CKRT fluids with a broad range of HCO3 levels (20–35 mM) available. The Shock protocol simplifies this in several ways. First, the fixed ACDA to blood flow ratio makes the effect of ACDA on circuit HCO3 mass balance more predictable. Second, due to the ECit >0.75, almost no citrate reaches the patient with the CKRT return blood, therefore systemic bicarbonate generation from citrate metabolism is negligible. Third, during CKRT without RCA (and hence without bicarbonate generation from citrate metabolism), it has been established that CKRT fluids with a level of 35 mM HCO3 are usually optimal; it follows that the default CKRT fluid HCO3 level in the shock protocol with high ECit should also be around 35 mM to achieve systemic HCO3 levels around 22–25. Patients with severe lactic acidosis need higher CKRT fluid HCO3 levels. We usually added 5 mM extra HCO3 for each 5 mM of systemic lactate above normal, limited only by not wanting to exceed a CKRT fluid Na level of 156 mM. Metabolic alkalosis with high pH was easier to avoid; using a 35 or lower HCO3 CKRT fluid resulted in <25 mM systemic HCO3 levels. Acid-base control was excellent overall in most patients.
Total hypomagnesemia was avoided by using a 0.75 mM (1.5 mEq/L) Mg CKRT fluid. Magnesium is complexed by citrate similar to calcium. For a neutral circuit Mg balance, especially at albumin levels >3.5 g/dl 1 mM (2 mEq/L), Mg CKRT fluids would be optimal; such CKRT fluids are available in Europe. Hypophosphatemia was easily avoided by spiking commercial CKRT fluids to either 0.68 or 1.36 mmol/L phosphate level. The high normal systemic phosphate levels in most patients reflect the preference of most prescribers in our CKRT program for systemic phosphate levels >3 mg/dl and the use of 4.2 mg/dl phosphate CKRT fluids.
A limitation of this single center study is that our outcomes in part might have been due to greater local expertise with CKRT than possible to acquire in most smaller centers. However, the fundamental premise that all patients with absent citrate metabolism can be treated with this shock protocol is rooted in solute kinetic principles, and can be expected to hold up in any center using this protocol, as described. Some minor limitations with this approach are not inherent to the protocol, but to CKRT machine and fluid characteristics, and could be addressed with updated CKRT machine and disposables designs. First, unequal dialysate/replacement flows (5:2 QD:QRF ratio) are used in this protocol to avoid undue hemoconcentration with postdilution filtration. This approach uses both scales on the Prismaflex to deliver enough clearance to keep ECit >0.75 albeit with an uneven QD/QRF bag emptying rate, which is not ideal for ICU nurse convenience. This is one of the reasons we do not use the Shock protocol for patients with fair or better citrate metabolism. Our Nonshock protocol uses equal QD and QRF flow rates, which means the separate dialysate and preplacement fluid scales on the Prismaflex are utilized equally and empty at the same time, which decreases nurse workload. Due to electronic order sets and that the initial QB, ACDA, QRF, QD settings are not titrated, and the QCa usually requires minimal to no adjustment after the first 24 hours of CKRT-RCA using the same titration tables for both protocols, we have been able to run two RCA protocols successfully. However, CKRT programs with fewer patients with severe liver failure could treat all of their patients with the Shock protocol, because it will work equally well for patients with good citrate metabolism as long as timely CKRT fluid bag changes can be ensured with uneven dialysate and replacement fluid flows. Targeting a lower effluent dose of 25–30 ml/kg per hour is possible by using a Table 1 row selected by QB in ml/min that is about two thirds of the patient weight in kg (for example, in a 100 kg patient we would use the row with QB of 70 ml/min and effluent flow of about of 2650 ml/h for a delivered dose of 27 ml/kg per hour). Second, the use of low blood flows (QB <80 ml/min) on the Prismaflex yields low return pressure alarms. These alarms are mitigated with a five-inch long, small diameter extension tubing between the catheter and the CKRT blood circuit return end. Further, we only clinically tested the flow setting rows in Table 1 noted at QB 60, 100, and 150 ml/min. However, given the tested flow settings encompass the entire range of flows in Table 1, and each row was generated using the same solute kinetic principles, it is clinically reasonable to expect the not-tested rows would also result in similar small solute outcomes. Finally, interruptions of PBP ACDA delivery as detailed above may result in greater clotting risk at lower QB values <80 ml/min.
The Shock protocol CVVHDF-RCA on the Prismaflex with precalculated flow settings helps ICU providers safely prescribe CKRT-RCA to patients with compromised citrate metabolism (lactic acidosis or severe liver failure). The protocol is effective in maintaining circuit patency and keeps single-pass citrate extraction on the dialyzer >0.75, reducing the risk of citrate toxicity to only instances of human error in protocol delivery. Personalizing the initial Ca-infusion rate from a precalculated table on the basis of the patient’s albumin level and the filter effluent flow rate helps maintain neutral CKRT Ca-mass balance and a normal systemic iCa level. Bedside delivery of CVVHDF-RCA is also simplified for the ICU nurses because circuit iCa levels do not need to be monitored, and only the net UF settings and the Ca-infusion rate must be adjusted periodically. The presented Shock protocol will allow CKRT programs on the basis of the Prismaflex to use CVVHDF-RCA in all patients with suspected impaired citrate metabolism without contraindications.
Disclosures
M. Heung reports consultancy agreements with Baxter Inc, Potrero Medical Inc., and Wolters Kluwer (Lexicomp); reports receiving research funding from Centers for Disease Control, Patient-Centered Outcomes Research Institute, and Veterans Affairs; reports receiving honoraria from National Kidney Foundation; and is a scientific advisor or member of Associate Editor/Editorial board, Advances in CKD. D. Humes reports consultancy agreements with SeaStar Medical; having an ownership interest in Innovative BioTherapies, Inc. and SeaStar Medical, Inc.; reports receiving research funding from Innovative Biotherapies, Lowell Pharmaceuticals, Renal Research Institute, SeaStar Medical, and Sygin; reports being a scientific advisor or member of Innovative Biotherapies and SeaStar Medical. B. Szamosfalvi reports receiving research funding from Renal Research Institute. B. Wagner reports having an ownership interest in Able-Wagner, Inc. L. Yessayan reports receiving research funding from renal research institute and being a Section Editor for ASAIO Journal, Renal\Extracorporeal Blood Treatment. R. Sohaney reports being funded by a training grant from the National Institutes of Health (5T32DK007378-40). All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
A. Riddle, L. Napolitano, and S. Dickinson were responsible for resources; B. Szamosfalvi was responsible for conceptualization, methodology, project administration, supervision, and validation, and wrote the original draft; B. Szamosfalvi, L. Yessayan, M. Heung, and V. Puri were responsible for investigation; B. Wagner, L. Yessayan, M. Heung, R. Sohaney, and V. Puri were responsible for data curation; L. Yessayan was responsible for conceptualization, formal analysis, methodology, project administration, supervision, and validation, and wrote the original draft; M. Heung was responsible for conceptualization, methodology, and resources, and wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten H, Ronco C, Kellum JA: Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med 33: 1563–1570, 2007. 10.1007/s00134-007-0754-4 [DOI] [PubMed] [Google Scholar]
- 2.Kutsogiannis DJ, Gibney RT, Stollery D, Gao J: Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 67: 2361–2367, 2005. 10.1111/j.1523-1755.2005.00342.x [DOI] [PubMed] [Google Scholar]
- 3.Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P: Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: A prospective randomized study. Intensive Care Med 30: 260–265, 2004. 10.1007/s00134-003-2047-x [DOI] [PubMed] [Google Scholar]
- 4.Betjes MG, van Oosterom D, van Agteren M, van de Wetering J: Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: Similar hemofilter survival but significantly less bleeding. J Nephrol 20: 602–608, 2007 [PubMed] [Google Scholar]
- 5.Kramer L, Bauer E, Joukhadar C, Strobl W, Gendo A, Madl C, Gangl A: Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med 31: 2450–2455, 2003. 10.1097/01.CCM.0000084871.76568.E6 [DOI] [PubMed] [Google Scholar]
- 6.Szamosfalvi B, Frinak S, Yee J: Automated regional citrate anticoagulation: Technological barriers and possible solutions. Blood Purif 29: 204–209, 2010. 10.1159/000245648 [DOI] [PubMed] [Google Scholar]
- 7.Walther JL, Bartlett DW, Chew W, Robertson CR, Hostetter TH, Meyer TW: Downloadable computer models for renal replacement therapy. Kidney Int 69: 1056–1063, 2006. 10.1038/sj.ki.5000196 [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Xu Z, Zhu Q, Liu J, Qian J, You H, Gu Y, Hao C, Jiao Z, Ding F: Citrate pharmacokinetics in critically ill patients with acute kidney injury. PLoS One 8: e65992, 2013. 10.1371/journal.pone.0065992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin I: Nonanticoagulation strategies to optimize circuit function in renal replacement therapy. In: Continuous Renal Replacement Therapy, edited by Kellum JA, Bellomo R, and Ronco C, Oxford, Oxford University Press, 2013 [Google Scholar]
- 10.Hafner S, Stahl W, Fels T, Träger K, Georgieff M, Wepler M: Implementation of continuous renal replacement therapy with regional citrate anticoagulation on a surgical and trauma intensive care unit: Impact on clinical and economic aspects-an observational study. J Intensive Care 3: 35, 2015. 10.1186/s40560-015-0102-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morabito S, Pistolesi V, Tritapepe L, Zeppilli L, Polistena F, Strampelli E, Pierucci A: Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding: A continuous veno-venous hemofiltration protocol with a low concentration citrate solution. Crit Care 16: R111, 2012. 10.1186/cc11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J-S, Kim G-H, Kang CM, Lee CH: Regional anticoagulation with citrate is superior to systemic anticoagulation with heparin in critically ill patients undergoing continuous venovenous hemodiafiltration. Korean J Intern Med 26: 68–75, 2011. 10.3904/kjim.2011.26.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobe SW, Aujla P, Walele AA, Oliver MJ, Naimark DM, Perkins NJ, Beardsall M: A novel regional citrate anticoagulation protocol for CRRT using only commercially available solutions. J Crit Care 18: 121–129, 2003. 10.1053/jcrc.2003.50006 [DOI] [PubMed] [Google Scholar]
- 14.Gattas DJ, Rajbhandari D, Bradford C, Buhr H, Lo S, Bellomo R: A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med 43: 1622–1629, 2015. 10.1097/CCM.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 15.Hetzel GR, Schmitz M, Wissing H, Ries W, Schott G, Heering PJ, Isgro F, Kribben A, Himmele R, Grabensee B, Rump LC: Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: A prospective randomized multicentre trial. Nephrol Dial Transplant 26: 232–239, 2011. 10.1093/ndt/gfq575 [DOI] [PubMed] [Google Scholar]
- 16.Schilder L, Nurmohamed SA, Bosch FH, Purmer IM, den Boer SS, Kleppe CG, Vervloet MG, Beishuizen A, Girbes ARJ, Ter Wee PM, Groeneveld ABJ; CASH study group: Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: A multi-center randomized clinical trial. Crit Care 18: 472, 2014. 10.1186/s13054-014-0472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walser M: Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest 40: 723–730, 1961. 10.1172/JCI104306 [DOI] [PMC free article] [PubMed] [Google Scholar]