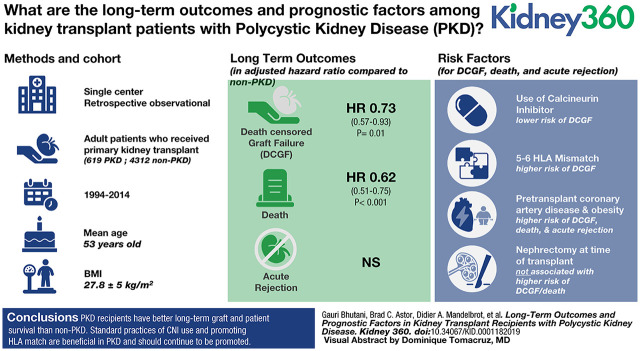
Visual Abstract
Keywords: transplantation, calcineurin inhibitor, coronary artery disease, death, graft survival, human leukocyte antigen (HLA), kidney transplant, nephrectomy, obesity, polycystic kidney disease, post-transplant diabetes mellitus
Abstract
Background
Polycystic kidney disease (PKD) accounts for approximately 15% of kidney transplants, but long-term outcomes in patients with PKD who have received a kidney transplant are not well understood.
Methods
In primary recipients of kidney transplants at our center (1994–2014), we compared outcomes of underlying PKD (N=619) with other native diseases (non-PKD, N=4312). Potential factors influencing outcomes in PKD were evaluated using Cox proportional-hazards regression and a rigorous multivariable model.
Results
Patients with PKD were older and were less likely to be sensitized or to experience delayed graft function (DGF). Over a median follow-up of 5.6 years, 1256 of all recipients experienced death-censored graft failure (DCGF; 115 patients with PKD) and 1617 died (154 patients with PKD). After adjustment for demographic, dialysis, comorbid disease, surgical, and immunologic variables, patients with PKD had a lower risk of DCGF (adjusted hazard ratio [aHR], 0.73; 95% CI, 0.57 to 0.93; P=0.01) and death (aHR, 0.62; 95% CI, 0.51 to 0.75; P<0.001). In our multiadjusted model, calcineurin-inhibitor (CNI) use was associated with lower risk of DCGF (aHR, 0.45; 95% CI, 0.26 to 0.76; P=0.003), whereas HLA mismatch of five to six antigens (aHR, 2.1; 95% CI, 1.2 to 3.64; P=0.009) was associated with higher likelihood of DCGF. Notably, both pretransplant coronary artery disease (CAD) and higher BMI were associated with increased risk of death (CAD, aHR, 2.5; 95% CI, 1.69 to 3.71; P<0.001; per 1 kg/m2 higher BMI, aHR, 1.07; 95% CI, 1.04 to 1.11; P<0.001), DCGF, and acute rejection. Nephrectomy at time of transplant and polycystic liver disease were not associated with DCGF/death. Incidence of post-transplant diabetes mellitus was similar between PKD and non-PKD cohorts.
Conclusions
Recipients with PKD have better long-term graft and patient survival than those with non-PKD. Standard practices of CNI use and promoting HLA match are beneficial in PKD and should continue to be promoted. Further prospective studies investigating the potential benefits of CNI use and medical/surgical interventions to address CAD and the immunologic challenges of obesity are needed.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/K360/2021_02_25_KID0001182019.mp3
Introduction
Polycystic kidney diseases (PKD) refer to genetic tubulointerstitial diseases that result in the formation of multiple cysts in the renal parenchyma, accompanied by gradual loss of renal function (1). Recent annual incidence rates of ESKD from PKD in the United States range between 11 and 12 per million population, making PKD the fourth most common cause of ESKD (2). Although new disease-modifying therapies for PKD are being developed (3,4), renal transplantation is the only alternative to dialysis after reaching ESKD (5,6). While PKD is responsible for 2%–3% of annual incident ESKD in the United States, it is the underlying disease for almost 12%–14% of the annual renal transplants performed (2,7).
Despite being a major primary renal disease leading to renal transplantation, many post-transplant outcomes specific to PKD remain unclear. Epidemiologic studies have reported graft and patient survival in kidney transplant recipients with PKD as equal (8–15), better (7,15–17), and worse (18) than recipients with other native diseases (non-PKD). Patients with PKD show better graft and patient outcomes than those with non-PKD in unadjusted reports from large transplant databases (7). Specific transplant or PKD-linked factors that influence post-transplant outcomes in patients with PKD have not assessed rigorously in prior epidemiologic studies (8–16,18,19). There is also no consensus on the timing of native nephrectomy and the risk of post-transplant diabetes mellitus (PTDM) in recipients with PKD (17). To optimize renal transplantation practices, protocols, and patient education in PKD, we analyzed data from our center for a better understanding of postrenal transplant outcomes and the factors affecting these outcomes in PKD.
Materials and Methods
The Wisconsin Allograft Recipient Database was initiated in 1984 to collect information on all solid organ transplants performed at the University of Wisconsin. All patients who received a primary kidney transplant at the University of Wisconsin between January 1, 1994 and June 30, 2014, and who were at least 18 years of age at the time of transplantation were eligible for inclusion in this study. Patients had follow-up through December 31, 2014. The University of Wisconsin Health Sciences Institutional Review Board approved this study. The clinical and research activities reported are consistent with the Principles of the Declaration of Istanbul, as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
We categorized recipients of kidney transplants by the cause of ESKD recorded in the database. Patients with ESKD from cystic kidney disease were classified as “PKD,” and the rest were grouped as non-PKD. We further categorized non-PKD as ESKD due to GN, diabetes mellitus, hypertension, or “other.” Information on baseline characteristics, including demographics, pretransplant dialysis, donor characteristics, comorbid diseases, immunosuppression, immunologic markers (HLA mismatch and calculated panel-reactive antibody >10%), prior kidney transplantation and native nephrectomy, and immediate graft function were obtained from the transplant database.
Post-transplant outcomes of death-censored graft failure (DCGF), death, and acute rejection were compared across the five causes of ESKD (PKD, GN, diabetes mellitus, hypertension, and other) by Kaplan–Meier curves and Cox proportional-hazards regression analyses. Acute rejection was defined as biopsy specimen–proven acute cellular and/or acute antibody-mediated rejection. The multivariable Cox proportional-hazards model was adjusted for age, sex, race, body mass index (BMI), pre-emptive kidney transplant, dialysis before transplant, duration of dialysis, pretransplant comorbidities (coronary artery disease, congestive heart failure, and polycystic liver disease), deceased donor, donor age, prior transplant, induction agent, calcineurin-inhibitor (CNI) and mycophenolate use, the degree of HLA mismatch (zero to two, three to four, and five to six), and nephrectomy at time of transplant.
Potential prognostic factors for the outcomes of DCGF, death, and acute rejection in patients with PKD after kidney transplant were investigated using Cox proportional-hazards regression analyses and the multivariable model described above within the PKD cohort. To get a complete picture of outcomes in recipients with PKD, the cause of graft failure and incidence rates of PTDM and post-transplant native kidney nephrectomy were also evaluated and compared with recipients with non-PKD using the chi-squared test (for graft failure) and the above multivariable model and Cox proportional-hazards regression analyses (for PTDM and post-transplant nephrectomy). All statistical analyses were performed using Stata 13.1 (stata.com).
Results
Baseline Characteristics
Our cohort included 619 patients with PKD and 4312 patients with non-PKD. Baseline demographic, comorbid disease, donor, dialysis, and immunologic characteristics and immunosuppression utilization are fully detailed in Table 1.
Table 1.
Baseline characteristics of recipients with polycystic kidney disease and other native kidney disease
| Variable | PKD (N=619)a | Non-PKD (N=4312)a | P Value |
| Age (yr) | 52.9 (9.13) | 48.7 (13.4) | <0.001 |
| Female (%) | 46 | 39 | 0.002 |
| Non-White race (%) | 7 | 17 | <0.001 |
| BMI (kg/m2) | 27.8 (5.04) | 27.2 (5.43) | <0.001 |
| Any dialysis before transplantation (%) | 60 | 78 | <0.001 |
| Dialysis duration (mo) | 14.1 (24) | 22 (27.24) | <0.001 |
| Deceased donor (%) | 58 | 61 | 0.11 |
| Comorbid diseases (%) | |||
| Diabetes | 0.3 | 17 | <0.001 |
| Hypertension | 98 | 98 | 0.88 |
| Coronary artery disease | 22 | 35 | <0.001 |
| Congestive heart failure | 7 | 12 | 0.001 |
| Polycystic liver disease | 32 | 0 | <0.001 |
| Induction treatment (%) | <0.001 | ||
| Alemtuzumab | 14 | 16 | |
| Basiliximab | 61 | 49 | |
| Thymoglobulinb | 18 | 22 | |
| Other | 6 | 8 | |
| None | 11 | 16 | |
| CNI maintenance (%) | 0.88 | ||
| Tacrolimus | 52 | 53 | |
| Cyclosporine | 39 | 38 | |
| No CNI | 9 | 9 | |
| Mycophenolate maintenance (%) | 93 | 90 | 0.02 |
| HLA mismatch (%) | 0.02 | ||
| 0–2 | 24 | 29 | |
| 3–4 | 42 | 41 | |
| 5–6 | 34 | 30 | |
| cPRA >10% (%) | 23c | 31c | 0.009 |
| Prior kidney transplantation (%) | 10 | 23 | <0.001 |
| Native nephrectomy at transplantation (%) | 27 | 0.9 | <0.001 |
| Donor age (yr) | 43.3 (14.2) | 42.2 (14.5) | 0.04 |
PKD, polycystic kidney disease; non-PKD, other native kidney disease; BMI, body mass index; CNI, calcineurin inhibitor; cPRA, calculated panel-reactive antibody.
All values are expressed in means (±SD) or percentages, as noted in the variable column.
Thymoglobulin denotes rabbit anti-thymocyte globulin.
N=296 for PKD and 2088 for non-PKD.
Incidence of Graft Failure, Death, and Acute Rejection
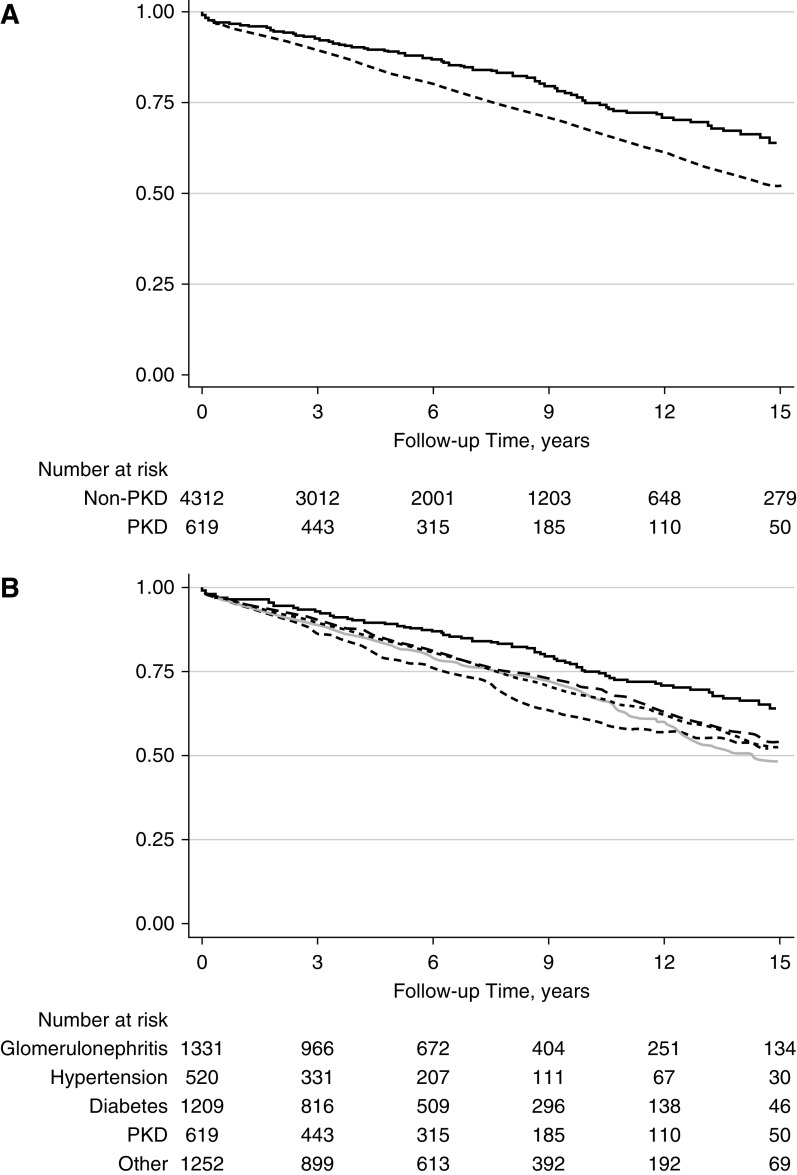
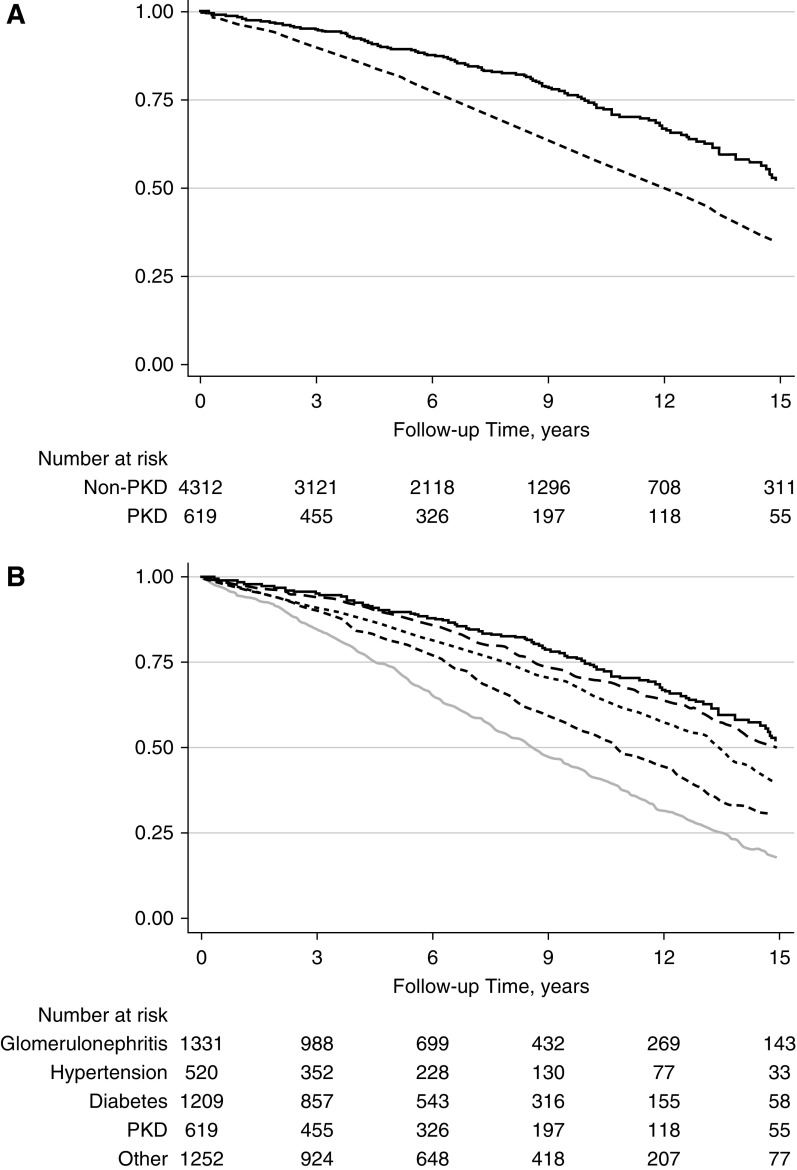
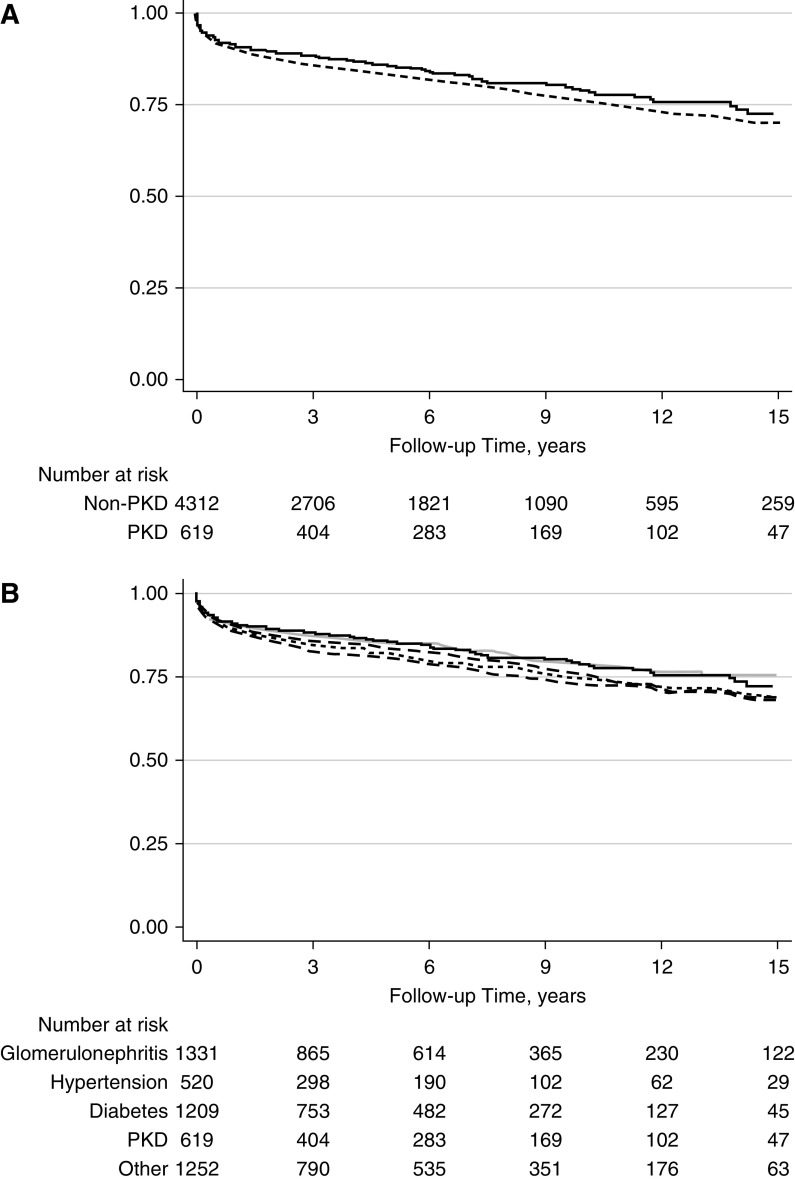
Delayed graft function (DGF) was less common in the recipients with PKD (15% [91/617] versus 19% [824/4312] in those with non-PKD; P<0.01). The incidence rate of DCGF was also lower in patients with PKD (2.72 per 100 person-years in patients with PKD and 4.11 per 100 person-years in patients with non-PKD; P<0.001; adjusted hazard ratio [aHR] for PKD versus non-PKD, 0.73; 95% CI, 0.57 to 0.93; P=0.01; Figure 1A). The incidence rate of death was lower in the PKD cohort (3.51 per 100 person-years for patients with PKD and 5.55 per 100 person-years in patients with non-PKD; P<0.01; aHR for PKD versus non-PKD, 0.62; 95% CI, 0.51 to 0.75; P<0.001; Figure 2A). The incidence of acute rejection was similar between patients with PKD and non-PKD (Figure 3A). Detailed comparison of these outcomes in PKD and specific causes of non-PKD ESKD is shown in Figures 1B, 2B, and B3B, and Table 2.
Figure 1.
Death censored graft failure in PKD and non-PKD kidney transplant recipients. (A) PKD (N=619; solid line) versus all non-PKD (N=4312; dashed line); P<0.001. (B) PKD (N=619; black solid line) versus GN (N=1331; gray long dashed line; P=0.001), diabetes (N=1209; light gray solid line; P<0.001), hypertension (N=520; gray dotted line; P<0.001), and other (N=1252; black dashed line; P=0.001) causes of ESKD.
Figure 2.
Mortality in PKD and non-PKD kidney transplant recipients. (A) PKD (N=619; solid line) versus all non-PKD (N=4312; dashed line); P<0.001. (B) PKD (N=619) versus GN (N=1331; gray long dashed line; P=0.68), diabetes (N=1209; light gray solid line; P<0.001), hypertension (N=520; gray dotted line; P<0.001), and other (N=1252; black dashed line; P=0.001) causes of ESKD.
Figure 3.
Acute rejection n PKD and non-PKD kidney transplant recipients. (A) PKD (N=619; solid line) versus all non-PKD (N=4312; dashed line); P=NS. (B) PKD (N=619) versus GN (N=1331; gray long dashed line; P=0.14), diabetes (N=1209; light gray solid line; P=0.77), hypertension (N=520; gray dotted line; P=0.005), and other (N=1252; black dashed line; P=0.05) causes of ESKD.
Table 2.
Outcomes in polycystic kidney disease and other native kidney disease groups
| Outcomes | Polycystic Kidney Disease (N=619) | GN (N=1331) | Diabetes (N=1209) | Hypertension (N=520) | Other (N=1252) |
| Death-censored graft failure (N=1256) | |||||
| No. of events | 115 | 371 | 304 | 143 | 323 |
| Incidence rate (per 100 person-yr) | 2.72 | 2.98a | 4.27a | 4.82a | 3.87a |
| Adjusted hazard ratio (95% CI), P valueb | 1.0 | 1.28 (0.98 to 1.66) | 1.51 (1.15 to 1.98) | 1.47 (1.09 to 1.97) | 1.32 (1.02 to 1.72) |
| Reference | P=0.07 | P=0.003 | P=0.01 | P=0.04 | |
| Death (N=1617) | |||||
| No. of events | 154 | 363 | 656 | 199 | 399 |
| Incidence rate (per 100 person-yr) | 3.21 | 3.74 | 8.68a | 6.21a | 4.59a |
| Adjusted hazard ratio (95% CI), P valueb | 1.0 | 1.28 (1.02 to 1.59) | 2.23 (1.80 to 2.76) | 1.50 (1.18 to 1.91) | 1.61 (1.29 to 1.99) |
| Reference | P=0.03 | P<0.001 | P=0.001 | P<0.001 | |
| Acute rejection (N=937) | |||||
| No. of events | 107 | 274 | 189 | 106 | 261 |
| Incidence rate (per 100 person-yr) | 2.76 | 3.23 | 2.84 | 3.9c | 3.51c |
| Adjusted hazard ratio (95% CI), P valueb | 1.0 | 1.16 (0.86 to 1.54) | 0.99 (0.73 to 1.35) | 1.29 (0.92 to 1.79) | 1.18 (0.88 to 1.57) |
| Reference | P=0.33 | P=0.95 | P=0.14 | P=0.27 |
P<0.01 versus PKD.
The model was adjusted for all variables noted in Methods: age, sex, race, body mass index, pre-emptive kidney transplant, dialysis duration, comorbid disease (coronary artery disease, congestive heart failure, polycystic liver disease), deceased donor, prior transplant, induction, calcineurin-inhibitor use, mycophenolate use, HLA mismatch, and nephrectomy at time of transplant.
P<0.05 versus PKD.
Risk Factors for Graft Failure, Death, and Acute Rejection in PKD
Graft loss in those with PKD was from acute rejection in 14% (16/114) and chronic rejection in 54% (62/114) of patients. The frequency of recurrent primary disease was significantly lower in patients PKD (0% [0/114] versus 5% [60/1156], P=0.01; Figure 4). Several factors were expectedly associated with DCGF in the PKD cohort, including deceased donor (aHR, 1.62; 95% CI, 1.01 to 2.58; P=0.05), donor age (aHR, 1.36 per decade; 95% CI, 1.16 to 1.58; P<0.001), and HLA mismatch (for mismatch of five to six versus zero to two antigens, aHR, 2.1; 95% CI, 1.20 to 3.64; P=0.009) (Table 3).
Figure 4.
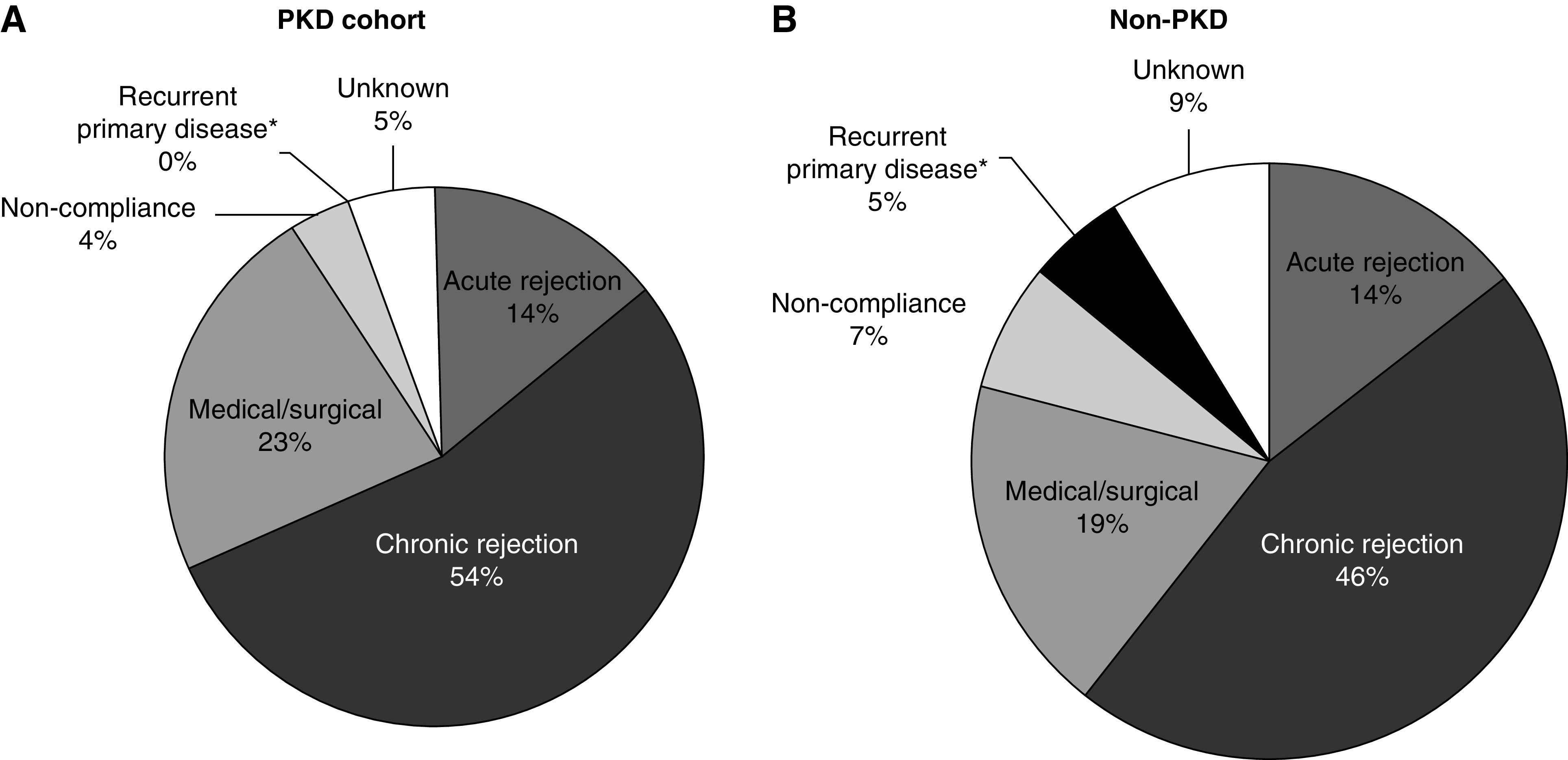
Causes of graft failure in PKD and non-PKD kidney transplant recipients. (A) Causes of graft loss in PKD cohort. (B) Causes of graft loss in non-PKD cohort. *There was a significantly lower frequency of recurrent native renal disease in the allograft in recipients with PKD (P=0.01), whereas acute rejection and other causes of graft failure were similar between the PKD (N=114) and non-PKD cohorts (N=1156).
Table 3.
Risk factors for death-censored graft failure in recipients with polycystic kidney disease
| Variable | Hazard Ratio (95% CI) | P Value | |
| Age (per decade) | 0.76 (0.6 to 0.96) | 0.02a | |
| Female sex | 1.22 (0.82 to 1.81) | 0.33 | |
| Non-White race | 1.14 (0.51 to 2.55) | 0.76 | |
| BMI (per 1 kg/m2 higher BMI) | 1.04 (1.01 to 1.08) | 0.03a | |
| Dialysis before transplantation | 1.26 (0.77 to 2.06) | 0.35 | |
| Dialysis duration | 1.00 (0.99 to 1.01) | 0.42 | |
| Coronary artery disease | 1.68 (1.03 to 2.76) | 0.04a | |
| Congestive heart failure | 1.3 (0.61 to 2.74) | 0.50 | |
| Polycystic liver disease | 1.12 (0.72 to 1.74) | 0.61 | |
| Deceased donor | 1.62 (1.01 to 2.58) | 0.05a | |
| Donor age | 1.36 (1.16 to 1.58) | <0.001a | |
| Prior kidney transplantation | 1.15 (0.58 to 2.26) | 0.69 | |
| Induction treatment | |||
| Basiliximab | Reference | ||
| Thymoglobulinb | 0.81 (0.46 to 1.43) | 0.47 | |
| Alemtuzumab | 1.12 (0.66 to 1.92) | 0.65 | |
| Other | 1.32 (0.60 to 2.90) | 0.50 | |
| None | 0.67 (0.22 to 2.03) | 0.48 | |
| Calcineurin-inhibitor maintenance | |||
| Tacrolimus | Reference | ||
| Cyclosporine | 0.69 (0.42 to 1.11) | 0.12 | |
| None | 1.80 (0.99 to 3.27) | 0.06a | |
| Mycophenolate maintenance | 1.05 (0.49 to 2.28) | 0.89 | |
| HLA mismatch | |||
| 0–2 | Reference | ||
| 3–4 | 1.32 (0.77 to 2.27) | 0.31 | |
| 5–6 | 2.1 (1.20 to 3.64) | 0.009a | |
| Nephrectomy at transplantation | 1.00 (0.64 to 1.54) | 0.99a | |
BMI, body mass index.
P<0.05.
Rabbit anti-thymocyte globulin.
Interestingly, the use of CNIs was associated with a strong protection from DCGF (for CNI versus no CNI, aHR, 0.45; 95% CI, 0.26 to 0.76; P<0.01) and acute rejection (aHR, 0.52; 95% CI, 0.29 to 0.92; P=0.03) in patients with PKD. There was no significant difference between tacrolimus and cyclosporine for both DCGF and acute rejection rates (Tables 4 and 5). A higher BMI, on the other hand, was associated with increased DCGF (per 1 kg/m2 higher BMI, aHR, 1.04; 95% CI, 1.01 to 1.08; P=0.03; Table 3) and acute rejection in patients with PKD (per 1 kg/m2 higher BMI, aHR, 1.04; 95% CI, 1.01 to 1.08; P=0.02; Table 5). Moreover, a higher BMI, also associated with increased likelihood of death in patients with PKD (per 1 kg/m2 higher BMI, aHR, 1.07; 95% CI, 1.04 to 1.11; P<0.001; Table 4).
Table 4.
Risk factors for death post–kidney transplant in recipients with polycystic kidney disease
| Variable | Hazard Ratio (95% CI) | P Value | |
| Age (per decade) | 1.80 (1.46 to 2.22) | <0.001a | |
| Female sex | 0.85 (0.60 to 1.21) | 0.37 | |
| Non-White race | 1.54 (0.73 to 3.27) | 0.26 | |
| BMI (per 1 kg/m2 higher BMI) | 1.07 (1.04 to 1.11) | <0.001a | |
| Dialysis before transplantation | 1.36 (0.88 to 2.12) | 0.17 | |
| Dialysis duration | 1.00 (1.00 to 1.01) | 0.53 | |
| Coronary artery disease | 2.50 (1.69 to 3.71) | <0.001a | |
| Congestive heart failure | 0.92 (0.44 to 1.94) | 0.83 | |
| Polycystic liver disease | 1.02 (0.66 to 1.56) | 0.94 | |
| Deceased donor | 1.35 (0.89 to 2.03) | 0.16 | |
| Donor age | 1.24 (1.09 to 1.41) | 0.001a | |
| Prior kidney transplantation | 1.40 (0.78 to 2.48) | 0.26 | |
| Induction treatment | |||
| Basiliximab | Reference | ||
| Thymoglobulinb | 0.80 (0.50 to 1.27) | 0.34 | |
| Alemtuzumab | 0.86 (0.49 to 1.54) | 0.62 | |
| Other | 0.46 (0.22 to 0.96) | 0.04a | |
| None | 0.41 (0.15 to 1.12) | 0.08 | |
| Calcineurin-inhibitor maintenance | |||
| Tacrolimus | Reference | ||
| Cyclosporine | 0.83 (0.54 to 1.27) | 0.39 | |
| None | 1.30 (0.70 to 2.40) | 0.41 | |
| Mycophenolate maintenance | 0.94 (0.46 to 1.91) | 0.86 | |
| HLA mismatch | |||
| 0–2 (reference) | Reference | ||
| 3–4 | 1.23 (0.81 to 1.86) | 0.34 | |
| 5–6 | 1.10 (0.69 to 1.76) | 0.69 | |
| Nephrectomy at transplantation | 0.83 (0.54 to 1.28) | 0.40 | |
BMI, body mass index.
P<0.05.
Rabbit anti-thymocyte globulin.
Table 5.
Risk factors for biopsy specimen–proven acute rejection after kidney transplant in recipients with polycystic kidney disease
| Variable | Hazard Ratio (95% CI) | P Value | |
| Age (per decade) | 0.79 (0.62 to 1.01) | 0.06 | |
| Female sex | 1.64 (1.07 to 2.50) | 0.02a | |
| Non-White race | 1.26 (0.65 to 2.47) | 0.49 | |
| BMI (per 1 kg/m2 higher BMI) | 1.04 (1.01 to 1.08) | 0.02a | |
| Dialysis before transplantation | 1.27 (0.79 to 2.10) | 0.33 | |
| Dialysis duration | 1.00 (0.99 to 1.01) | 0.82 | |
| Coronary artery disease | 2.37 (1.47 to 3.84) | <0.001a | |
| Congestive heart failure | 0.74 (0.32 to 1.72) | 0.48 | |
| Polycystic liver disease | 1.25 (0.79 to 1.84) | 0.39 | |
| Deceased donor | 0.92 (0.59 to 1.44) | 0.72 | |
| Donor age | 1.00 (0.85 to 1.16) | 0.96 | |
| Prior kidney transplantation | 2.59 (1.38 to 4.87) | 0.003a | |
| Induction treatment | |||
| Basiliximab | Reference | ||
| Thymoglobulinb | 0.82 (0.45 to 1.49) | 0.50 | |
| Alemtuzumab | 1.38 (0.81 to 2.33) | 0.23 | |
| Other | 0.13 (0.03 to 0.62) | 0.01a | |
| None | 0.64 (0.19 to 2.14) | 0.46 | |
| Calcineurin-inhibitor maintenance | |||
| Tacrolimus | Reference | ||
| Cyclosporine | 0.86 (0.53 to 1.37) | 0.52 | |
| None | 1.79 (0.97 to 3.32) | 0.06a | |
| Mycophenolate maintenance | 0.33 (0.14 to 0.73) | 0.006a | |
| HLA mismatch | |||
| 0–2 | Reference | ||
| 3–4 | 2.30 (1.21 to 4.37) | 0.01a | |
| 5–6 | 4.07 (2.10 to 7.92) | <0.001a | |
| Nephrectomy at transplantation | 1.17 (0.74 to 1.84) | 0.49 | |
BMI, body mass index.
P<0.05.
Rabbit anti-thymocyte globulin.
Pretransplant coronary artery disease strongly associated with death, as expected (aHR, 2.5; 95% CI, 1.69 to 3.71; P<0.001; Table 4). Interestingly, it also associated with increased risk of both DCGF (aHR, 1.68; 95% CI, 1.03 to 2.76; P=0.04; Table 3) and acute rejection (aHR, 2.37; 95% CI, 1.47 to 3.84; P<0.001; Table 5) the PKD cohort. Recipient age, on the other hand, strongly associated with death (per decade increase, aHR, 1.8; 95% CI, 1.46 to 2.22; P<0.001; Table 4), which was expected, but a paradoxic protective effect for DCGF (per decade increase, aHR, 0.76; 95% CI, 0.6 to 0.96; P=0.019; Table 3) was noted.
Additional prognostic factors that associated with death in PKD (Table 4) were donor age (aHR, 1.24; 95% CI, 1.09 to 1.41; P=0.001) and the use of the “other” subgroup of induction immunosuppression (in comparison to basiliximab subgroup, aHR, 0.46; 95% CI, 0.22 to 0.96; P=0.04). The latter consisted of only a small fraction of patients among the PKD cohort (34/619), and mainly consisted of patients who received Muromonab-CD3 (OKT-3; N=31).
Other than as noted above (lack of CNI maintenance, higher BMI, and pretransplant coronary artery disease), acute rejection also strongly associated with HLA mismatch, lack of mycophenolate maintenance, and female sex in the PKD cohort (Table 5).
It is noteworthy that two PKD-specific factors, native nephrectomy at time of transplant and the presence of polycystic liver disease, were not associated with increased DCGF, death, or acute rejection in our PKD cohort (Tables 3–5).
Other Post-Transplant Outcomes in PKD
Recipients with underlying PKD underwent native nephrectomy more often than those in the non-PKD cohort at the time of transplant (27% versus 0.9%; P<0.001; Table 1) and in the post-transplant period (18% versus 0.1%; P<0.001). The incidence rate of nephrectomy post-transplant in patients with PKD was 2.09 per 100 person-years (versus non-PKD, aHR, 4.57; 95% CI, 3.09 to 6.76; P<0.001; Table 6). The incidence of PTDM in the PKD cohort (2.86 per 100 person-years), on the other hand, was similar to non-PKD causes of ESKD (Table 6).
Table 6.
Incidence of post-transplant events in PKD versus non-PKD
| Post-transplant event | PKD | Diabetes | Hypertension | GN | Other |
| Post-transplant diabetes | |||||
| No. of events | 103 | N/A | 110 | 187 | 186 |
| Incidence rate (per 100 person-yr) | 2.86 | 4.55 | 2.27 | 2.54 | |
| Adjusted hazard ratio (95% CI), P valuea | 1.0 (Reference) | 1.12 (0.82 to 1.54) | 0.82 (0.62 to 1.10) | 0.94 (0.7 to 1.25) | |
| P=0.48 | P=0.19 | P=0.66 | |||
| Post-transplant nephrectomy | |||||
| No. of events | 59 | 20 | 11 | 31 | 48 |
| Incidence rate (per 100 person-yr) | 2.09 | 0.28 | 0.38 | 0.34 | 0.61 |
| Adjusted hazard ratio (95% CI), P valuea | 1.0 (Reference) | 0.14 (0.08 to 0.25) | 0.22 (0.11 to 0.43) | 0.18 (0.11 to 0.29) | 0.33 (0.21 to 0.51) |
| P<0.001 | P<0.001 | P<0.001 | P<0.001 |
PKD, polycystic kidney disease; non-PKD, other native kidney disease; N/A, not applicable.
The model was adjusted for all variables noted in Methods: age, sex, race, body mass index, pre-emptive kidney transplant, dialysis duration, comorbid disease (coronary artery disease, congestive heart failure, polycystic liver disease), deceased donor, prior transplant, induction, calcineurin-inhibitor use, mycophenolate use, HLA mismatch, and nephrectomy at time of transplant.
Discussion
We present the largest published observational cohort study of patients with PKD who underwent kidney transplantation. Our findings confirm that kidney transplant recipients with PKD have better graft and patient survival than non-PKD recipients. Our study also reveals that two potentially modifiable variables, HLA mismatch of four or less antigens and CNI use, may be protective for graft failure in patients with PKD. Recipient age, BMI, and pretransplant coronary artery disease were associated with death in the PKD cohort, and are also potentially modifiable variables to some extent. Both native nephrectomy at the time of transplant and prior polycystic liver disease did not predict graft or patient survival.
Graft survival was better in the PKD cohort, mostly due to a significantly lower frequency of recurrent native renal disease in the allograft, whereas acute rejection and other causes of graft failure were similar between the PKD and non-PKD cohort. Recurrent disease is known to be a significant contributor to allograft loss (20) and, although recurrent native renal disease may be seen in most non-PKD causes of ESKD, PKD by its genetic pathophysiology is inherently safe from this.
Higher HLA mismatch (especially mismatch of five to six antigens) was associated with both worse allograft survival and higher acute rejection in our patients with PKD. Although the importance of HLA matching in the current era of immunosuppression has been questioned (21), it continues to be standard practice at most transplant centers, including ours. Our findings confirm the importance of HLA matching in appropriate donor selection for patients with PKD. Higher donor age and a deceased donor are well-reported risk factors for DCGF after renal transplantation overall (2,22). The apparent protective effect of higher recipient age for DCGF seen in our PKD cohort is likely due to confounding from longer survival in younger recipients, as described in other graft-survival reports (23,24). Our findings indicate that recipient-donor age matching and promoting living donation are important for optimizing graft survival in patients with PKD as well.
CNI-based therapy is still a cornerstone in renal transplantation, although long-term benefits of CNI to long-term allograft survival are questioned (25). CNI use associated with better allograft survival within our PKD cohort. This is helpful to know because both PKD and CNI have been individually linked to increased risk of post-transplant diabetes (19). In addition, inhibitors of mammalian target of rapamycin may theoretically seem preferable in patients with PKD because they have some efficacy in inhibiting both renal and liver cyst growth (5). CNI use was also associated with less acute rejection within our PKD cohort (Table 5), which suggests the observed benefit from CNI use in the PKD group could be from better immunologic performance of CNI. Given the retrospective nature of our study and selection bias (only approximately 10% were on non-CNI regimens), conclusions cannot be made. Prospective studies of CNI use in patients with PKD after transplantation are needed to understand the significance of this observation better, especially in the current era when new agents like belatacept are also available (26).
Our study also shows superior survival of patients with PKD, compared with those with non-PKD, who receive a kidney transplant. Most prior published studies show similar overall patient (8–15) and graft survival (8–14) in recipients with PKD versus those with non-PKD. One study (18) shows worse survival in patients with PKD compared with those with non-PKD. In this study, the PKD cohort was small (N=80) and age at transplant was significantly higher for patients with PKD (versus non-PKD). In fact, most of these prior reports used small numbers (8,9,11–14,18), lack of robust multivariable adjustment (8–15,18), and many included patients transplanted in the 1980s to 1990s, when the current standard maintenance immunosuppressants were not in use (8,9,12–15). Our findings of better graft and recipient survival in PKD are consistent with large transplant registry data (7) and one larger case-control study (16). Patients with PKD were also shown to have better survival rates compared with a demographic- and dialysis vintage–matched, nondiabetic control population in a retrospective study of patients on hemodialysis (17). It is possible that a residual effect from a lower chronic illness burden in PKD (as shown in Table 1) contributes to this improved survival despite statistical adjustments.
The association of recipient and donor age with survival was expected and is well reported in the post–kidney transplant population (27). Pretransplant coronary artery disease was highly associated with death in patients with PKD, which is not surprising because cardiovascular disease contributes heavily to post-transplant mortality (28). Notably, pretransplant coronary artery disease was also associated with worse DCGF and acute rejection in our PKD cohort. Coronary artery disease has not previously been linked with DCGF and rejection, to our knowledge, although post-transplant hypertension (29) and de novo congestive heart failure (30) have been. In addition, statin use has been linked with improved post-transplant outcomes, although not consistently for graft outcomes (31–34). Our findings suggest that coronary artery disease is an important, potentially modifiable, risk factor to improve multiple post-transplant outcomes in those with PKD.
Other than coronary artery disease, higher BMI was the only other risk factor that associated with all three outcomes of death, DCGF, and acute rejection. A higher BMI is well reported to associate with these outcomes in post–kidney transplant settings, likely due to the influence of increased cardiovascular risk, changes in pharmacokinetics/dynamics of immunosuppressive medications, and the inflammatory state linked with obesity (35,36). Although obesity—by itself—should not discourage transplantation in PKD or otherwise, because outcomes on dialysis are likely to be worse than with transplantation (37,38), prospective studies to understand the best interventions to address the cardiovascular and immunologic challenges in obesity are needed, because consistent improvements in BMI are not straightforward or easy to achieve (35,36). In addition, the contribution of the increased weight of the native kidneys and/or liver to the BMI in patients with PKD in our study is not known, but can be considerable (39), and how this factor contributes to outcomes certainly needs investigating as well.
Finally, we also found lack of mycophenolate agent and female sex to be risk factors for acute rejection, but not for DCGF. Similar findings have also been reported in the post–kidney transplant setting overall (40,41). Further studies of mycophenolate agent use and role of sex in patients with PKD are needed to better inform us about the significance of these findings. The apparently protective effect on rejection and survival with use of other induction agents (mainly OKT-3), on the other hand, was likely highly subject to selection bias and error due to the small number of patients who received this form of induction (N=34) and, therefore, this is likely not a reliable finding.
On the basis of our study findings, we recommend that general nephrologists educate their patients with PKD who have moderate-severe CKD (stages 3–4) about excellent post-transplant outcomes to help encourage timely kidney donation by family/friends. Given the excellent post-transplant outcomes, it may also be worthwhile for transplant centers to investigate a GFR cut off >20 ml/min per 1.73 m2 for deceased-donor listings for patients with high-risk PKD (in whom GFR decline can be as high as 5–7 ml/min per year) (42), who are otherwise healthy but do not have potential donors.
The timing of native nephrectomy (at or post-transplantation) did not significantly affect allograft or survival outcomes in our study. This is consistent with findings in some other prior smaller reports (43–45) and another recent report (46). Pretransplant nephrectomy is well established as undesirable due to increased surgical complications and the risk of losing native renal function (19,47). Nephrectomy at time of transplant, however, is still not a universally adopted practice due to concern of potential insults to the new allograft, the benefit of preserving residual renal function, and the avoidance of unnecessary surgical procedures (47). These are important considerations; however, our findings do strongly suggest safety of a simultaneous nephrectomy. An individualized approach to timing and need for nephrectomy on the basis of the patient’s needs, preference, and surgical risk would likely be the most optimal clinical practice (48).
Some studies (11,15,49,50) and meta-analyses (51,52) have showed increased risk of PTDM in recipients with PKD, but this has not been a consistent finding (53,54), and most of these studies have a small number of patients with PKD. Among the two largest cohorts, however, one study found a minimally increased risk of PTDM with PKD in an unadjusted analysis (15), whereas the other found none (54). Our findings (from the largest reported post-transplant PKD cohort to date) show no increase in risk of PTDM in recipients with PKD.
Limitations of our study include the observational nature of our study, the single-center population, and residual confounding due to differences in baseline characteristics, which may not have been completely accounted for by the statistical adjustments made. A potential era effect, due to the long observation period, and immortal time bias may affect outcomes in both PKD and non-PKD groups. There is also likely heterogeneity of the PKD group with regards to the underlying genetic mutation. Assessment of underlying genetic disease in PKD is not advocated as a routine clinical practice (5). This practice may change in the near future as disease-specific therapies become available. Assessing outcomes on the basis of genotype may provide novel genotype-specific information about post–renal transplantation outcomes and complications.
In conclusion, renal allograft and patient survival is better in those with PKD than in other native kidney diseases (non-PKD) after kidney transplantation. Our study confirms that many currently used standard transplant practices are associated with better post-transplant outcomes in recipients with PKD. A better HLA match, living donor, recipient-donor age matching, and cardiovascular health should continue to be encouraged during pretransplant evaluations in this group. For maintenance immunosuppression, both CNI and mycophenolate appear to be associated with better allograft outcomes. Further prospective studies are needed to better inform about potential benefits of specific immunosuppressive agents, the potential medical/surgical interventions to address coronary artery disease, and the immunologic challenges in obesity in recipients with PKD. Our findings also strongly suggest that native nephrectomy at time of transplant is safe, and the risk of PTDM is not increased in recipients with PKD.
Disclosures
S. Wells reports consultancy for Ethicon Inc. T. Ziemlewicz reports consultancy and research funding with Neuwave and Histosonics. T. Ziemlewicz also has ownership interest in Histotonics. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
We acknowledge Ms. Dana Clark, MA, for her significant editorial assistance.
Each author takes responsibility that this study has been reported honestly, accurately and transparently, that no important aspects of the study have been omitted. Each author accepts accountability for the overall work and will ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Author Contributions
B.C. Astor and G. Bhutani were responsible for data curation and formal analysis; B.C. Astor, G. Bhutani, and A. Djamali were responsible for methodology; G. Bhutani wrote the original draft; A. Djamali conceptualized the study, was responsible for resources, and provided supervision; and all authors reviewed and edited the manuscript.
References
- 1.Wilson PD: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004. 10.1056/NEJMra022161 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2020. Annual Data Report: Vol. 2, Ch. 1, Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. Available at: https://adr.usrds.org/2020. Accessed December 17, 2020
- 3.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017. 10.1056/NEJMoa1710030 [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants: Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 88: 17–27, 2015. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O; Kidney Disease: Improving Global Outcomes: Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management--A KDIGO consensus report. Kidney Int 88: 676–683, 2015. 10.1038/ki.2015.28 [DOI] [PubMed] [Google Scholar]
- 7.Scientific Registry of Transplant Recipients : OPTN/SRTR 2012. annual report. Available at: https://srtr.transplant.hrsa.gov/annual_reports/2012/Default.aspx. Accessed December 17, 2020
- 8.Hadimeri H, Nordén G, Friman S, Nyberg G: Autosomal dominant polycystic kidney disease in a kidney transplant population. Nephrol Dial Transplant 12: 1431–1436, 1997. 10.1093/ndt/12.7.1431 [DOI] [PubMed] [Google Scholar]
- 9.Stiasny B, Ziebell D, Graf S, Hauser IA, Schulze BD: Clinical aspects of renal transplantation in polycystic kidney disease. Clin Nephrol 58: 16–24, 2002. 10.5414/CNP58016 [DOI] [PubMed] [Google Scholar]
- 10.Roozbeh J, Razmkon AR, Jalaeian H, Raiss-Jalali GA, Behzadi S, Sagheb MM, Salahi H, Bahador A, Nikeghbalian S, Davari HR, Salehipour M, Malek-Hosseini SA: Outcome of kidney transplantation in patients with polycystic kidney disease: A single center study. Saudi J Kidney Dis Transpl 19: 72–75, 2008 [PubMed] [Google Scholar]
- 11.Gonçalves S, Guerra J, Santana A, Abreu F, Mil-Homens C, Gomes da Costa A: Autosomal-dominant polycystic kidney disease and kidney transplantation: Experience of a single center. Transplant Proc 41: 887–890, 2009. 10.1016/j.transproceed.2009.01.069 [DOI] [PubMed] [Google Scholar]
- 12.Florijn KW, Chang PC, van der Woude FJ, van Bockel JH, van Saase JL: Long-term cardiovascular morbidity and mortality in autosomal dominant polycystic kidney disease patients after renal transplantation. Transplantation 57: 73–81, 1994. 10.1097/00007890-199401000-00014 [DOI] [PubMed] [Google Scholar]
- 13.Shiroyanagi Y, Tanabe K, Hashimoto Y, Okuda H, Oshima T, Tokumoto T, Ishikawa N, Tomq H: Kidney transplantation in the recipient with autosomal-dominant polycystic kidney disease: A single center experience. Transplant Proc 32: 1841–1843, 2000. 10.1016/S0041-1345(00)01457-3 [DOI] [PubMed] [Google Scholar]
- 14.Sanfilippo FP, Vaughn WK, Peters TG, Bollinger RR, Spees EK: Transplantation for polycystic kidney disease. Transplantation 36: 54–59, 1983. 10.1097/00007890-198307000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Jacquet A, Pallet N, Kessler M, Hourmant M, Garrigue V, Rostaing L, Kreis H, Legendre C, Mamzer-Bruneel MF: Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: A nationwide longitudinal study. Transpl Int 24: 582–587, 2011. 10.1111/j.1432-2277.2011.01237.x [DOI] [PubMed] [Google Scholar]
- 16.Johnston O, O’Kelly P, Donohue J, Walshe JJ, Little DM, Hickey D, Conlon PJ: Favorable graft survival in renal transplant recipients with polycystic kidney disease. Ren Fail 27: 309–314, 2005. 10.1081/JDI-56606 [DOI] [PubMed] [Google Scholar]
- 17.Perrone RD, Ruthazer R, Terrin NC: Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: Contribution of extrarenal complications to mortality. Am J Kidney Dis 38: 777–784, 2001. 10.1053/ajkd.2001.27720 [DOI] [PubMed] [Google Scholar]
- 18.Illesy L, Kovács DA, Szabó RP, Asztalos L, Nemes B: Autosomal dominant polycystic kidney disease transplant recipients after kidney transplantation: A single-center experience [published correction appears in Transplant Proc 49: 2233, 2017 10.1016/j.transproceed.2017.09.002]. Transplant Proc 49: 1522–1525, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Kanaan N, Devuyst O, Pirson Y: Renal transplantation in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 10: 455–465, 2014. 10.1038/nrneph.2014.104 [DOI] [PubMed] [Google Scholar]
- 20.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009. 10.1111/j.1600-6143.2008.02519.x [DOI] [PubMed] [Google Scholar]
- 21.Su X, Zenios SA, Chakkera H, Milford EL, Chertow GM: Diminishing significance of HLA matching in kidney transplantation. Am J Transplant 4: 1501–1508, 2004. 10.1111/j.1600-6143.2004.00535.x [DOI] [PubMed] [Google Scholar]
- 22.Snoeijs MG, Schaefer S, Christiaans MH, van Hooff JP, van den Berg-Loonen PM, Peutz-Kootstra CJ, Buurman WA, van Heurn LW: Kidney transplantation using elderly non-heart-beating donors: A single-center experience. Am J Transplant 6: 1066–1071, 2006. 10.1111/j.1600-6143.2006.01312.x [DOI] [PubMed] [Google Scholar]
- 23.Ferrari P, Lim W, Dent H, McDonald SP: Effect of donor-recipient age difference on graft function and survival in live-donor kidney transplantation. Nephrol Dial Transplant 26: 702–708, 2011. 10.1093/ndt/gfq383 [DOI] [PubMed] [Google Scholar]
- 24.Foley DP, Patton PR, Meier-Kriesche HU, Li Q, Shenkman B, Fujita S, Reed A, Hemming AW, Kim RD, Howard RJ: Long-term outcomes of kidney transplantation in recipients 60 years of age and older at the University of Florida. Clin Transpl: 101–109, 2005 [PubMed] [Google Scholar]
- 25.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015. 10.1681/ASN.2014040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, Marks WH, Agarwal M, Wu D, Dong Y, Garg P: Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 11: 66–76, 2011. 10.1111/j.1600-6143.2010.03338.x [DOI] [PubMed] [Google Scholar]
- 27.Keith DS, Demattos A, Golconda M, Prather J, Norman D: Effect of donor recipient age match on survival after first deceased donor renal transplantation. J Am Soc Nephrol 15: 1086–1091, 2004. 10.1097/01.ASN.0000119572.02053.F2 [DOI] [PubMed] [Google Scholar]
- 28.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y: Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant 8: 593–599, 2008. 10.1111/j.1600-6143.2007.02101.x [DOI] [PubMed] [Google Scholar]
- 29.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ: Hypertension after kidney transplantation. Am J Kidney Dis 43: 1071–1081, 2004. 10.1053/j.ajkd.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 30.Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC: De novo congestive heart failure after kidney transplantation: A common condition with poor prognostic implications. Am J Kidney Dis 46: 720–733, 2005. 10.1053/j.ajkd.2005.06.019 [DOI] [PubMed] [Google Scholar]
- 31.Masterson R, Hewitson T, Leikis M, Walker R, Cohney S, Becker G: Impact of statin treatment on 1-year functional and histologic renal allograft outcome. Transplantation 80: 332–338, 2005. 10.1097/01.tp.0000168941.19689.cf [DOI] [PubMed] [Google Scholar]
- 32.Fellström B, Holdaas H, Jardine AG, Holme I, Nyberg G, Fauchald P, Grönhagen-Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambühl P, Olsson AG, Hartmann A, Logan JO, Pedersen TR; Assessment of Lescol in Renal Transplantation Study Investigators: Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int 66: 1549–1555, 2004. 10.1111/j.1523-1755.2004.00919.x [DOI] [PubMed] [Google Scholar]
- 33.Katznelson S, Wilkinson AH, Kobashigawa JA, Wang XM, Chia D, Ozawa M, Zhong HP, Hirata M, Cohen AH, Teraski PI, et al.: The effect of pravastatin on acute rejection after kidney transplantation--a pilot study. Transplantation 61: 1469–1474, 1996. 10.1097/00007890-199605270-00010 [DOI] [PubMed] [Google Scholar]
- 34.Co MLF, Agdamag AC, Co MZ, Hertl M, Mohamedali B: Intensity-dependent benefit of statins in survival among prospective kidney transplant patients. Am J Cardiol 123: 254–259, 2019. 10.1016/j.amjcard.2018.09.037 [DOI] [PubMed] [Google Scholar]
- 35.Potluri K, Hou S: Obesity in kidney transplant recipients and candidates. Am J Kidney Dis 56: 143–156, 2010. 10.1053/j.ajkd.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 36.Lentine KL, Delos Santos R, Axelrod D, Schnitzler MA, Brennan DC, Tuttle-Newhall JE: Obesity and kidney transplant candidates: How big is too big for transplantation? Am J Nephrol 36: 575–586, 2012. 10.1159/000345476 [DOI] [PubMed] [Google Scholar]
- 37.Khwaja A, El-Nahas M: Transplantation in the obese: Separating myth from reality. Nephrol Dial Transplant 27: 3732–3735, 2012. 10.1093/ndt/gfs406 [DOI] [PubMed] [Google Scholar]
- 38.Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC: Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int 63: 647–653, 2003. 10.1046/j.1523-1755.2003.00761.x [DOI] [PubMed] [Google Scholar]
- 39.Freise J, Tavakol M, Gao Y, Klein O, Lee BK, Freise C, Park M: The effect of enlarged kidneys on calculated body mass index categorization in transplant recipients with ADPKD. Kidney Int Rep 4: 606–609, 2019. 10.1016/j.ekir.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton PA, McDonald SP, Chapman JR, Chadban SJ: Mycophenolate versus azathioprine for kidney transplantation: A 15-year follow-up of a randomized trial. Transplantation 94: 152–158, 2012. 10.1097/TP.0b013e31825475a3 [DOI] [PubMed] [Google Scholar]
- 41.Meier-Kriesche HU, Ojo AO, Leavey SF, Hanson JA, Leichtman AB, Magee JC, Cibrik DM, Kaplan B: Gender differences in the risk for chronic renal allograft failure. Transplantation 71: 429–432, 2001. 10.1097/00007890-200102150-00016 [DOI] [PubMed] [Google Scholar]
- 42.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators: Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015. 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glassman DT, Nipkow L, Bartlett ST, Jacobs SC: Bilateral nephrectomy with concomitant renal graft transplantation for autosomal dominant polycystic kidney disease. J Urol 164: 661–664, 2000. 10.1016/S0022-5347(05)67276-X [DOI] [PubMed] [Google Scholar]
- 44.Fuller TF, Brennan TV, Feng S, Kang SM, Stock PG, Freise CE: End stage polycystic kidney disease: Indications and timing of native nephrectomy relative to kidney transplantation. J Urol 174: 2284–2288, 2005. 10.1097/01.ju.0000181208.06507.aa [DOI] [PubMed] [Google Scholar]
- 45.Veroux M, Zerbo D, Basile G, Gozzo C, Sinagra N, Giaquinta A, Sanfiorenzo A, Veroux P: Simultaneous native nephrectomy and kidney transplantation in patients with autosomal dominant polycystic kidney disease. PLoS One 11: e0155481, 2016. 10.1371/journal.pone.0155481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abrol N, Bentall A, Torres VE, Prieto M: Simultaneous bilateral laparoscopic nephrectomy with kidney transplantation in patients with ESRD due to ADPKD: A single-center experience [published online ahead of print September 16, 2020]. Am J Transplant 10.1111/ajt.16310 [DOI] [PubMed] [Google Scholar]
- 47.Chebib FT, Prieto M, Jung Y, Irazabal MV, Kremers WK, Dean PG, Rea DJ, Cosio FG, Torres VE, El-Zoghby ZM: Native nephrectomy in renal transplant recipients with autosomal dominant polycystic kidney disease. Transplant Direct 1: e43, 2015. 10.1097/TXD.0000000000000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argyrou C, Moris D, Vernadakis S: Tailoring the ‘perfect fit’ for renal transplant recipients with end-stage polycystic kidney disease: Indications and timing of native nephrectomy. In Vivo 31: 307–312, 2017. 10.21873/invivo.11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamer RA, Chow CL, Ong AC, McKane WS: Polycystic kidney disease is a risk factor for new-onset diabetes after transplantation. Transplantation 83: 36–40, 2007. 10.1097/01.tp.0000248759.37146.3d [DOI] [PubMed] [Google Scholar]
- 50.Ducloux D, Motte G, Vautrin P, Bresson-Vautrin C, Rebibou JM, Chalopin JM: Polycystic kidney disease as a risk factor for post-transplant diabetes mellitus. Nephrol Dial Transplant 14: 1244–1246, 1999. 10.1093/ndt/14.5.1244 [DOI] [PubMed] [Google Scholar]
- 51.Culliford A, Phagura N, Sharif A: Autosomal dominant polycystic kidney disease is a risk factor for posttransplantation diabetes mellitus: An updated systematic review and meta-analysis. Transplant Direct 6: e553, 2020. 10.1097/TXD.0000000000000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheungpasitporn W, Thongprayoon C, Vijayvargiya P, Anthanont P, Erickson SB: The risk for new-onset diabetes mellitus after kidney transplantation in patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. Can J Diabetes 40: 521–528, 2016. 10.1016/j.jcjd.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 53.Ruderman I, Masterson R, Yates C, Gorelik A, Cohney SJ, Walker RG: New onset diabetes after kidney transplantation in autosomal dominant polycystic kidney disease: A retrospective cohort study. Nephrology (Carlton) 17: 89–96, 2012. 10.1111/j.1440-1797.2011.01507.x [DOI] [PubMed] [Google Scholar]
- 54.Courivaud C, Ladrière M, Toupance O, Caillard S, Hurault de Ligny B, Ryckelynck JP, Moulin B, Rieu P, Frimat L, Chalopin JM, Chauvé S, Kazory A, Ducloux D: Impact of pre-transplant dialysis modality on post-transplant diabetes mellitus after kidney transplantation. Clin Transplant 25: 794–799, 2011. 10.1111/j.1399-0012.2010.01367.x [DOI] [PubMed] [Google Scholar]