Abstract
Podocytes are critical components of the filtration barrier and responsible for maintaining healthy kidney function. An assault on podocytes is generally associated with progression of chronic glomerular diseases. Therefore, podocyte pathophysiology is a favorite research subject for nephrologists. Despite this, podocyte research has lagged because of the unavailability of techniques for culturing such specialized cells ex vivo in quantities that are adequate for mechanistic studies. In recent years, this problem was circumvented by the efforts of researchers, who successfully developed several in vitro podocyte cell culture model systems that paved the way for incredible discoveries in the field of nephrology. This review sets us on a journey that provides a comprehensive insight into the groundbreaking breakthroughs and novel technologic advances made in the field of podocyte cell culture so far, beginning from its inception, evolution, and progression. In this study, we also describe in detail the pros and cons of different models that are being used to culture podocytes. Our extensive and exhaustive deliberation on the status of podocyte cell culture will facilitate researchers to choose wisely an appropriate model for their own research to avoid potential pitfalls in the future.
Keywords: renal physiology, basic science, cell culture techniques, glomerulonephritis, podocytes, proteinuria
Introduction
Damage to the glomeruli, the renal filtration units, is a leading cause of CKD and ESKD, affecting almost 10% of the population in the Western world (1). Despite recent advances in the understanding of glomerular biology, strategies for combating these diseases remain extraordinarily challenging. Several factors that contribute to the complexity of kidney research are: (1) onset of these diseases is often undetected or poorly understood; (2) disease may be acute or chronic in nature; (3) the genetic makeup of the host adds to the variability of clinical symptoms; and (4) multiple organs are often involved simultaneously. Considering these bottlenecks and the complex cellular biology of kidney, it is of paramount importance to generate innovative tools and methodologies that will facilitate the study of renal biology, in particular the glomerulus. In this review, we attempt to describe different podocyte culture models, the conceptualization followed by their improvisation, pros, and cons of each system, and finally the review looks at the present-day novel innovations and their future (Figure 1).
Figure 1.
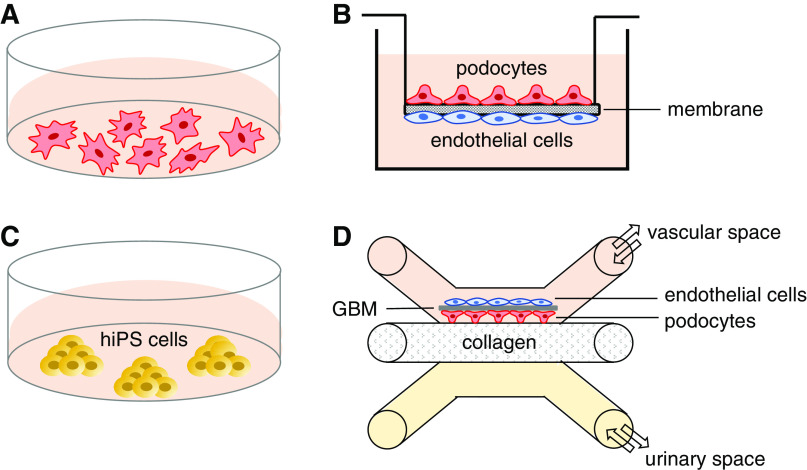
Diagram summarizing the podocyte-culturing models described in this review. (A) Podocytes (primary, immortalized, or urine derived) cultured on collagen in a dish with growth medium (pink). (B) Cross-section of a transwell insert showing the array of podocytes (above) and endothelial cells (below), which are cocultured with a membrane and recapitulate the glomerular filtration barrier in vitro. (C) Human-induced pluripotent stem (hiPS) cells, which are capable of differentiating into podocytes. These cells can be derived (i.e., reprogrammed) from either healthy controls or patients, and express a range of podocyte proteins, which might not be obtained through standard two-dimensional podocyte cell lines. (D) Glomerulus-on-a-chip design, where sequential seeding of podocytes and endothelial cells results in synthesis of the key components of the glomerular basement membrane (GBM) on the vascular space filled with growth medium. This microfluidic technology allows direct contact between podocytes and endothelial cells and free exchange of nutrients, gasses, and growth factors between vascular space and urinary space, where the filtrate is collected (yellow).
Podocytes: The Achilles Heel of Kidney Diseases
Anatomically, the glomerular filtration barrier (GFB) consists of three layers: fenestrated endothelial cells, the glomerular basement membrane (GBM), and podocytes (2,3). Although all three layers contribute substantially to the integrity and proper functioning of the GFB, podocytes are considered pivotal (4). Podocytes, also known as glomerular visceral epithelial cells, are highly specialized, terminally differentiated cells that are bestowed with a unique architecture characterized by multiple interdigitating foot processes (FPs) (5). These processes, along with the filtration slits that impart size- and charge-selective permeability to the GFB, constitute the two most prominent features of podocytes (6–8). Because podocytes are postmitotic cells with a limited capacity for self-renewal, they are irreplaceable and consequently cannot be compensated for their loss or dysfunction (9,10). Thus, depletion or damage to podocytes is decisively associated with most, if not all, glomerular diseases that result in glomerulosclerosis in humans and experimental animals (11,12).
Deal with the “Real World,” and not the “Ideal World”
To understand the disease etiology, susceptibility, mechanisms, prognosis, and potential therapies, studying human subjects would be the ideal gold standard. However, factors such as inadequate volunteers (and associated ethical issues), complexity of the disease (e.g., high level of heterogeneity in the causes, age of onset and rate of progression), and variability among the host (race/ethnicity, gender, and environment associated), have deterred the researchers from obtaining an archetype/prototype human model, thus making it impossible to rely solely on the human subjects for renal research. On a brighter side, with the advent of genomics and generation of null or transgenic mice (wherein the expression of a particular gene of interest can be either restricted to/deleted from a specific cell or enhanced to a greater extent), experimental animal models have proven quite valuable in providing crucial insights into several aspects of kidney disease. Although well-characterized animal models can provide a decent starting point for evaluating the efficacy of potential therapeutics for human diseases, they nevertheless suffer from limitations. This is because they do not always fully replicate their human counterparts and do not allow for mechanistic studies. Despite the introduction of cutting-edge technology of mouse genome editing that has deepened our understanding of renal physiology and pathology to a significant extent, studies involving animal models remain time consuming, with relatively long reproductive cycles, high maintenance cost, and strict regulatory and ethical guidelines and protocols. These factors make these model systems less flexible and cumbersome.
Moreover, attachment of podocyte FPs to the GBM makes direct isolation of podocytes technically difficult; therefore, in vitro studies of these cells depend largely on cell culture systems. Although cell culture systems do not completely recapitulate and mimic the in vivo environment, they afford several advantages, such as the ability to control the environment and perform multiple experiments to test the hypothesis. Therefore, it is reasonable to validate any hypothesis using a combination of different model systems (including cell lines, animal models, and human studies) to gain mechanistic insights and uncover a concept holistically.
Onset of a New Era: How and When did it Start?
For the initial characterization, researchers have relied primarily on nonpodocyte cell lines such as HEK, generated by the viral transformation of human embryonic kidney cells (13) or other cell lines with a kidney origin, such as MDCK, LLC-PK1, and A6 (14). However, they do not fully represent podocytes, which are terminally differentiated cells, and thus the results obtained from those studies may not be factual or entirely trustworthy, or skewed to some extent. This is because the techniques, such as viral or chemical transformation that lead to immortality in cell culture, unfortunately lead to loss of differentiation. Further, there is a fundamental incompatibility between the properties exhibited by continuously growing and differentiated cells. This necessitated generation of a cell-culture technology that can facilitate ex vivo cultivation of podocytes.
Primary Cell Culture
When cells are directly isolated from the tissue and propagated in vitro, they are referred to as primary cells. Thus, primary culture serves as a bridge between cell lines and cells in vivo. Krakower and Greenspon (15) were the pioneers who described a method for isolation and culture of primary podocyte cells in 1954. Overall, the procedure entails isolation of glomeruli by differential sieving, seeding them on a collagen-treated surface, then allowing the cells to grow in the nutrient medium. The sieving technique (passing the glomeruli through a series of stainless-steel sieves with decreasing pore sizes) is believed to remove the parietal sheet (decapsulate the glomeruli) and expose the visceral cells (podocytes). However, some groups have reported heterogeneity in the glomerular cells prepared using this classic protocol, questioning its validity (16). The original protocol involved using only two sieves, 250 μm and 150 μm, and Yaoita et al. (17) modified the protocol further, by using 250 μm and 75 μm instead, in an attempt to reduce podocyte damage during the sieving process. Further, to push the cell line toward a molecular and structural phenotype that resembles closely with the in vivo podocyte with FPs and slit diaphragm (SD), heparin and all-trans retinoic acid were used, and cells were grown on laminin-coated plates (18). This not only coaxed podocytes to project primary processes that further bifurcated and appeared to interdigitate with adjacent cells, but also the podocyte-specific gene expression pattern was remarkable. Using a combination of sieves with different mesh sizes, glomeruli are isolated from different species (e.g., sieves with 250, 150, and 75 μm pores are used for collecting rat glomeruli, and an additional 53 μm sieve is added to harvest mouse glomeruli) (19). Exploiting differential sieving technique with 149 μm and 70 μm sieves, primary culture of human fetal podocytes has also been derived (20,21). When a preparation contains 95% glomeruli and only few tubules as nonglomerular cells, it is considered good. Including a Ficoll-gradient centrifugation step has been shown to enrich glomeruli and reduce tubular contaminants in the cellular preparation (22). Once the glomeruli are isolated, two protocols are followed routinely. In the first, the cells are plated directly on the collagen-coated dish to allow cellular growth for 4–5 days, followed by passing the trypsinized cells through a 25 μm cell strainer to remove the residual mesangial and endothelial cells (23). The alternative is to subject the glomeruli to enzymatic dissociation, followed by passing them through a 25 μm cell strainer and use the cells in the filtrate for cultivation. There are different enzymes used for digestion, such as collagenase (0.1%) for human glomeruli, and trypsin (0.2%) or DNase (0.01%) along with collagenase for rat glomeruli (24). Because longer incubation with the enzymes has been shown to cause overdigestion of glomeruli, which effects cell viability and results in crosscontamination due to release of mesangial cells, modification of protocols that involve harvesting the glomeruli without enzymes is also adopted (25,26).
Because podocyte damage was observed during the isolation of glomeruli using conventional sieving methods, techniques that entail gentle isolation without forced sieving were devised. Using spherical beads exhibiting magnetic properties for kidney perfusion and collagenase treatment, both glomeruli yield and purity were augmented. Further, the cellular outgrowths were identified as podocytes by either immunostaining (using antibodies against podocyte marker proteins) or gene expression analysis (27,28).
The quality and the purity of cells obtained generally depends on the first step, that is, isolation of decapsulated glomeruli, which are devoid of parietal epithelial cells (PECs), from the kidneys. This is because decapsulated glomeruli have been shown to generate large, arborized cells with the marker profile corresponding to podocytes (positive for WT-1, synaptopodin, and podocalyxin but not for pan-cadherin), PECs, mesangial cells (Thy-1), or endothelial cells (vWf, RECA-1). On the contrary, cells that outgrow from encapsulated glomeruli fall into two morphologically distinct types: (1) polygonal cobblestone-like cells (which are either dedifferentiated podocytes or PECs of Bowman’s capsule, and stained strongly positive for pan-cadherin but faintly for WT-1 and synaptopodin, and negative for podocalyxin) and (2) large irregularly shaped cells, which are weakly positive for synaptopodin and negative for podocalyxin (17). During the early days, glomerular outgrowths possessing nonspecific cobblestone-like appearance were mistakenly considered as podocytes (29). Later, stringent criteria were used to confirm their authenticity, such as staining for specific marker proteins, and by monitoring the in vitro developmental profile as observed during podocyte maturation in vivo.
Another major challenge that researchers faced was the rapid dedifferentiation of primary podocytes in cell culture, which was accompanied by the loss of their specific cellular architecture, namely, FPs, degeneration of podocytes into cobblestone-like morphology, and loss of expression of synaptopodin, a key marker of differentiated or postmitotic podocytes in vivo (30). This incited the researchers to direct and gear their efforts toward generation of a podocyte culture model that can closely mimic mature podocytes in vivo, especially the expression of synaptopodin. Seminal study by Mundel et al. (31) in 1997 devised a protocol that not only evaded the problem of spontaneous dedifferentiation of podocytes in primary cell culture, but also showed for the first time that podocytes can be differentiated effectively in vitro. Just by changing the standard culture conditions and avoiding repeated subcultivation, they were able to achieve remarkable differentiation of the cobblestone-like cells (un/dedifferentiated and proliferating) into arborized podocytes with some intermediate phenotypes. These podocytes exhibited growth arrest (cell cycle exit) and formation of FPs along with positive staining for WT-1, podocyte-specific O-acetylated ganglioside, and synaptopodin, something that was never detected in cobblestone-like cells. However, this process of conversion was shown to be independent of variability in culture conditions, such as contents of the serum, growth medium, and coating matrix used to improve the adherence; high plating density could delay this conversion (31).
Immortalized Podocytes: Constitutive and Conditional
After the landmark breakthrough provided by Dr. Mundel et al. (31), researchers had the availability of differentiated primary human and rat podocytes, which undoubtedly resembled the in vivo counterpart. However, the fact that differentiation had induced a growth arrest led to a significant hiatus in the research. Owing to the limited ability of these terminally differentiated cells to proliferate, the challenge was to increase their numbers with subsequent passages to obtain cells enough for assays.
Therefore, during that era, to provide the in vitro cultured cells with the ability to divide indefinitely, a plethora of immortalized cell lines were generated (32–34). This was accomplished by inserting an immortalizing gene encoding the simian virus 40 (SV40) large tumor antigen (TAg) into in vitro cultured cells by transfection or retroviral infection. Another approach was culturing the cells isolated from transgenic mice harboring the SV40 TAg (T-SV40) immortalizing gene. Both approaches were shown to suffer from severe drawbacks, including (1) uncontrolled expression of the immortalizing gene led to constitutive proliferation that altered cellular physiology; (2) a large number of cells were required for transfection to attain the desired number of cells; (3) random or nontargeted insertion of the immortalized gene; and (4) the transgenic mice bearing the immortalizing gene exhibited tumor formation, and aberrant growth and development patterns (35–37).
These challenges were circumnavigated by generating conditional immortalized cell lines. The concept is to switch the cells from the proliferative phase to the differentiating phase by exposing them to a particular condition that would inactivate the immortalized gene or degrade its product. Toward this end, an advanced strain of transgenic mouse, popularly known as “immortomouse”, was generated that harbored a “double” conditional immortalizing gene (38,39). Briefly, the transgenic animals were generated by the insertion of a chimeric construct consisting of the mouse MHC H-2Kb class I promoter (that is inducible by IFN-γ), and the immortalizing gene, tsA58 early region encoding the thermolabile TAg. Thus, exposure to IFN-γ would induce the activation of the H-2Kb promoter that drives the expression of the immortalizing protein tsA58 TAg. This protein is thermolabile so remains stable and functional only at 33°C, and previously accumulated tsA58 TAg protein rapidly degrades at higher temperatures, such as 37–39.5°C. Further, concomitant omission of IFN-γ prevents the de novo synthesis of the immortalizing tsA58 TAg protein. Therefore, in essence, the cells isolated from the immortomouse would proliferate only in the presence of IFN-γ under permissive temperatures, and cease to multiply with the removal of IFN-γ and undergo differentiation in nonpermissive temperature by exiting the cell cycle. Under these conditions, most of the cells had exhibited growth arrest within 6–14 days. Exploiting the transgenic mouse model generated by Jat et al., a research group led by Mundel et al.(40) spearheaded to develop a protocol to generate immortalized conditional murine podocyte lines (35–37). Although the original mouse podocyte cell line exhibited standard marker proteins of mature podocyte, spontaneous transformation of cells was observed occasionally in cultures that exceeded 30 passages. Therefore, their recommendation was to cryopreserve a bank of low passage stock and not use the podocytes in culture after 10–15 passages to avoid any alterations in growth rate, morphology, protein expression, and transfection efficiency.
Several modifications to the original protocol were adopted later, which includes the practices of (1) altering the IFN-γ concentrations, and (2) using 38 or 39°C as opposed to 37°C (the standard accepted) to match the body temperature of the mouse. This was tried despite the observation made by Mundel et al. (40) that an increase in the nonpermissive temperature led to an increased cell death. Finally, (3) the time podocytes are allowed to grow under nonpermissive temperature to attain differentiation, that is, either 7–14 or most often 10–14 days (24). It is also interesting that although podocytes were maintained in culture dishes coated with collagen type I, the main structural component of the GBM is collagen type IV (41). This prompted the investigators to assess the effect of various extracellular matrices on adhesion, proliferation, and differentiation of podocytes. The results indicated that although collagen IV is a physiologically better-suited attachment substrate, the podocytes still differentiated with a similar phenotype and expressed all podocyte-specific proteins regardless of the choice of plating matrix (31,42,43). However, the expression and localization pattern of SD protein CD2AP were dependent on the type of matrix used (41). Unlike type I, on a type IV collagen matrix, CD2AP was expressed by both undifferentiated and differentiated podocytes and its localization changed from diffuse cytoplasmic to cell periphery, which corresponds closely to its association with the SD structures.
Although several podocyte cell lines, and primary cultures of podocytes, have been established and utilized to date, dedifferentiation of podocytes is often observed in vitro. The typical dedifferentiation is characterized by rapid loss of specialized FPs and SDs, and attenuation of expression of marker proteins that indicate differentiation. Among several differentiation markers, the expression of nephrin is lost relatively easily (44). Some culture conditions have been reported to upregulate podocyte-specific gene expression. Addition of 1,25-dihydroxyvitamin D3, all-trans–retinoic acid (ATRA), and dexamethasone to DMEM/F12 was shown to be the most potent and suitable medium for the recovery of nephrin gene expression in podocytes (45). Expression of other podocyte markers such as P-cadherin and NEPH1 were also shown to be upregulated in this medium. Because of the dexamethasone-induced toxicity, which is well recognized in several cell types, this medium is not competent for long-term maintenance of podocytes in the differentiated state. Thus, DMEM/F12 supplemented with vitamin D3 and retinoic acid was found to be optimal for the maintenance of nephrin gene expression in prolonged cultures. It is interesting that DMEM/F12 and α-MEM could increase the nephrin gene expression to a greater extent compared with RPMI 1640, a conventional basal culture medium used for podocytes. These effects were not attributed to the substances enriched in the DMEM/F12 and α-MEM media, such as sodium pyruvate, D-pantothenic acid, folic acid, and vitamin B12, but to the other unknown factors including saccharides, inorganic salts, amino acids, and other vitamins. Short-term exposure of vitamin D3, ATRA, and dexamethasone to murine podocytes, cultured in the conventional RPMI 1640 medium, has been shown to synergistically increase the expression of nephrin mRNA and the activity of the nephrin gene promoter (46). Kabgani et al. (47) used different media such as RPMI or the endothelial cell growth medium, to demonstrate their impact on the morphology of primary podocytes. Culturing the primary podocytes in endothelial cell growth medium at low cellular densities was shown to preserve their characteristic morphology (large cells with intracytoplasmic extensions) even after nine passages. Yaoita et al. (48) found the long-arborized cell processes radiated extensively from the cell body only when podocytes were cultured in the presence of heparin and ATRA on laminin-coated dishes with decreasing concentrations of FBS (18).
As opposed to the colossal amount of effort directed toward generation of murine podocyte cell lines, there have been feeble attempts to generate an immortalized human podocyte cell line. The very first attempt to generate and establish a human cell line involved harvesting glomeruli from a month-old baby’s kidney, followed by isolation of podocytes and subsequent transfection of the cells with two immortalizing genes, T-SV40 and Ha-Ras (49). However, only T-SV40 was expressed at the protein level and Ha-Ras, although was integrated into the genome, failed to transcribe. This cell line was designated 56/10A1, and exhibited some morphologic abnormalities (smaller cells with leaky junctions and large intercellular spaces), enhanced proliferation, and a limited ability to differentiate. Despite these drawbacks, 56/10A1 expressed several determinant marker proteins specific for differentiated podocytes, such as PHM-5, common acute lymphoblastic leukemia antigen, cytokeratin, and WT-1, and retained the phenotype for over 50 passages. Acknowledging the need and scope for improvement, glomerulus from a nephrectomy specimen of a 3-year old child was isolated to develop a conditional immortalized human podocyte cell line (50). This was developed in collaboration with Dr. Mundel et al. (31) using the same strategy as described above to generate conditionally immortalized murine cell lines.
Both cell lines were generously shared, and thus became the leading workhorses for researchers worldwide to study podocyte biology. However, caution must be exercised and following facts must be considered when translating experimental findings from cultured podocyte cell lines. These immortalized cell lines are convenient compared with the primary cell lines, but they remain artificial and are an insufficient surrogate because of the way they are derived, via an insertion of an unnatural gene into their genome. Moreover, podocytes in culture are grown on petri dishes as a monolayer without the presence of their natural neighbors, mesangial and endothelial cells. Some alternatives that are adopted to address these issues coculture podocytes with glomerular endothelial cells to better study the permeability, or grow podocytes in a Matrigel to attain a three-dimensional effect.
Another bottleneck is that the capillaries in the renal glomerulus are formed by three cell types, mesangial cells, endothelial cells, and podocytes, and in natural environment, all three are exposed to the mechanical load arising from glomerular capillary pressure and glomerular filtration. Among these glomerular cell types, the effect of mechanical stress has been studied first in cultured mesangial cells. The podocytes in culture encounter neither the conventional mechanical stretch nor the flow of primary urine filtrate. Thus, it is not surprising that they do lack SDs between neighboring cells (51), and express only a limited amount of specific marker proteins, such as nephrin, podocin, or transient receptor potential cation channel 6 (52). To test if these conditionally immortalized podocytes can tolerate the physiologic intraglomerular conditions, several mechanical and fluid shear stress models were generated to imitate the in vivo conditions (53–55). The findings report that the immortalized podocytes were mechanosensitive, that is, they were extremely sensitive to the fluid shear stress. In response to the stress, they underwent unique alterations in their shape and cytoskeletal architecture; however, these changes were reversible. More specifically, the transversal F-actin stress fibers were diminished, vinculin distribution was altered significantly, enhanced localization of cortactin at their cell periphery, and frequent appearance of lamellipodia were observed, which overall is indicative of a highly motile podocyte phenotype. Further, as the shear forces arising from the fluid flow increased, a progressive loss of podocytes was also observed (53–55).
Other pitfalls encountered with these conditionally immortalized cell lines include sensitivity to even minimal deviations in temperatures as low as 1°C, deterring complete differentiation under nonpermissive conditions, careful handling of the cells, and maintaining sterile aseptic conditions are required, extra added step of coating culture dishes with collagen, aliquoting, and maintaining single-use, high-quality IFN-γ frozen stocks, maintaining cultures at no more than 80% confluence to prevent overgrowth, loss of contact inhibition and multilayering that has been shown to negatively affect differentiation. Further, despite the availability of state-of-the-art tools for manipulating cell lines through gene expression or gene silencing, the biggest hindrance that has crippled the podocyte field is the compromised ability of podocytes to uptake and incorporate foreign DNA, which is similar to the other postmitotic cells, such as neurons or cardiomyocytes. Even with the introduction of myriad of effective transfection reagents, podocytes still suffer from relatively low transfection efficiency, ranging between only 10%–20% in proliferating cells. Although transient transfection requires less labor and could be time intensive, it does not lead to incorporation of the gene of interest into the genome, thereby making it vulnerable to loss on subsequent cycles of cell division. Stable transfection, in contrast, affords advantages such as incorporation of the gene into the genome, allowing for transmission of expression to the daughter cells and a growth-selection process that allows for continuous expression of transgene from virtually all of the cells. Therefore, although the selection process and expansion of the stable transfectants (i.e., survivors postselection) usually take 2–3 months, viral transduction (by means of retroviral, adenoviral or lentiviral gene transfers) has emerged as the ideal method for alteration of gene expression in podocyte cell lines. Moreover, viral transduction can also be performed in growth-arrested differentiated podocytes maintained under nonpermissive temperature to conduct overexpression or knockdown studies.
Union is Strength: Custom-Built Complexes by Podocyte Coculture Systems
It is overly simplistic to see GFB as comprising one cell type. In fact, it is a tripartite structure with podocytes, endothelial cells (both of which are postmitotic, highly specialized, interdependent cell populations), and an intervening GBM in between. Proteinuria, a common symptom of glomerular diseases, is caused when proteins leak from the GFB. Despite enormous advances made in the pathogenesis of kidney diseases, the underlying steps leading to proteinuria remains to be completely uncovered. To gain a full insight into the proteinuric disease etiology and to conduct functional studies in vitro, generation of a three-dimensional model of the GFB became in need. The year 2002 marked the first attempt for generating a sandwich model in which immortalized primary rat podocytes were grown on collagen-coated coverslips overlaid with Matrigel (56,57). Then the HUVECs were seeded on top of the Matrigel. However, this model suffered from several limitations because the podocytes adhered to the matrix proteins on both the sides, and the assembly did not create the intended/necessary separation between podocytes and endothelial cells. Subsequently, in 2008, another method was proposed wherein the conditionally immortalized mouse podocytes were grown on cell culture inserts hung into wells and the endothelial cells were seeded at the bottom of the wells (58). With this improvised procedure, spatial separation between podocytes, extracellular matrix on the membrane, and endothelial cells was achieved. Later, Slater et al. (59) devised another coculture model in which the human conditionally immortalized podocytes and endothelial cells were grown on the opposite sides of a nanofiber membrane, which was obtained by electrospinning collagen type I and polycaprolactone on nickel micromeshes. Bruggeman et al. (60) described the production of thin films of hydrogel suitable for coculturing podocyte and endothelial cells on the opposite sides of the film. Hitherto, these were the only two models that could mimic a correct and close relationship between podocytes and endothelial cells. However, both methods were not adopted widely by the scientific community, most likely due to their specialized requirements for complex biomaterial synthesis. Consequently, coculturing podocyte-endothelial cell types without a need for any specialized instruments or devoted biomaterials was established and patented (61). This coculture system is composed of an isoporous membrane that is coated with type IV collagen on both sides. Because type IV collagen is the physiologic collagen present in the GBM to which both podocytes and endothelial cells adhere in vivo (62), an optimal adherence and growth of both cell populations were achieved. Podocytes were attached or adhered to the upper side of the membrane and endothelial cells on the lower side. Additionally, this methodology allowed researchers to use a third cell type at the bottom of the well, thereby multiplying the possibility to study more complex glomerular intercellular signaling events. Because the coculture can be assembled with podocyte cell lines and with primary podocytes, extending their use to cells derived from transgenic mice, this three-dimensional model of the GFB is thus considered quite versatile over other existing methods. Although this type of three-dimensional coculture system facilitates studies on intercellular signaling, an obvious limitation of this model is the extreme simplification of the filtration barrier, as represented by the absence of SDs in podocyte FPs as well as the absence of the hemodynamic component.
Wealth Out of Waste: Podocyte Culture from Urine
The idea that the podocyte fragments or complete podocytes can be shed into urine stemmed from the observations of Pascual et al. (63) and Hara et al. (64). They detected cells that stained positive for the C3b complement receptor 1 and podocalyxin, in the urinary sediments of patients with kidney disease, although they are not specific markers for podocytes. A few years later, two other groups confirmed their findings (65,66), establishing a premise that during certain glomerulopathies, it is possible that injured podocytes can detach from the GBM and appear in the urine. However, the evidence that these podocalyxin-positive cells were truly podocytes still remained elusive, until Vogelmann et al. (67) successfully cultured these cells ex vivo, and demonstrated unequivocally that podocytes do exist in the urine of both healthy subjects and patients with active and inactive glomerulopathies. Although the growth pattern of urinary podocytes in cell culture was quite similar to both the primary and immortalized cells, they only expressed some (podocalyxin, WT-1, synaptopodin, P-cadherin) but not all (GLEPP-1) of the markers of podocytes. Not only that, the WT-1 expression was disappeared within a few weeks, which was not observed in the podocyte culture of nonurine origin, suggesting a strong likelihood of dedifferentiation of urinary podocytes in cell culture. To add further complexity, the cells positive for podocyte markers were also found to coexpress markers specific to other cell types, such as cytokeratin-8 (tubular epithelial cells) and α–smooth muscle actin (mesangial cells), indicative of transdifferentiation (67). On the basis of their findings, authors also defined a functional working model, that is, in active glomerular disease or in response to an assault, viable podocytes undergo dedifferentiation, which endows them with an ability to proliferate, thereby causing them to detach from the glomerular tuft; whereas in healthy individuals, mostly senescent podocytes are shed. This hypothesis is intriguing because it offers a paradigm shift in our understanding that adult podocytes either retain or regain (during some damage or insult) the potential for replication and thus may not be completely terminally differentiated as is conventionally assumed.
Subsequently, few other groups also isolated, studied, and compared urinary podocytes from healthy rats with several models of experimental rat nephropathies (68–70). Contrary to the findings of Vogelmann et al., no viable podocytes were found in the urine from healthy rats. But the urinary cells harvested from rats with membranous nephropathy (MN) expressed a large variety of podocyte-specific markers, including synaptopodin, nephrin, podocin, WT-1, and GLEPP1, although the latter was not detected in human urinary podocytes. Further, they did not observe coexpression of podocyte markers with the markers of other cell types (68). Continuing studies using other models of rat nephropathies substantiated most, if not all, of the findings of Vogelmann et al. (69,70). Hitherto, culturing podocytes from urine to study podocyte pathophysiology has not gained much popularity; rather, detection of podocytes in the urine has only served as a promising marker for assessing the severity of glomerular diseases (71).
Podocytes or Pseudocytes?
Confirmation of the identity and authenticity of the desired cell type is always essential in any in vitro culture system. The original and main criterion for the identification of cultured podocytes was their cellular morphology, which tends to differ grossly from their factual in vivo phenotype. This is because of variations in many factors such as handling, incubation, and storage of cells and culture components. The accepted standard is that the small, polygonal cells with a cobblestone-like appearance are proliferating or dedifferentiated podocytes and the large branched binucleated arborized cells are mature podocytes (15). Another common, yet obsolete, method to confirm podocyte identity was by testing their selective toxicity to puromycin aminonucleoside (PAN) (72). The downside of this methodology was that these puromycin-damaged podocytes were useless for further experiments. Therefore, a nondestructive method routinely used to identify podocytes in cell culture is immunofluorescence or immunocytochemistry; both detect the expression of podocyte-specific marker proteins. However, one of the most difficult conundrums faced by the experts in the field was to generate a toolbox that contains proteins exclusively and unambiguously expressed by a true podocyte. Several proteins continued to be added and later removed from the list, but overall majority of the researchers now use a palette of three to five podocyte-specific marker proteins to test and classify a cell as a podocyte. Krtil et al. (24) tabulated an array of proteins that are most commonly used as markers of podocytic phenotype. To conclude accurately, their recommendation is to include a cocktail of markers that are expressed in different podocytic compartments (i.e., a protein of apical membrane such as GLEPP1, podocalyxin, or PHM-5 antigen, a protein of FPs and SD, including nephrin, podocin, CD2AP, cytoskeleton such as synaptopodin, secreted protein) along with WT-1 as a general marker for both mature and immature podocytes.
It Is No More a Flight of Fancy
The GBM is sandwiched between podocytes and endothelial cells, which constitute the trilaminar GFB. Recapitulating such an arrangement in vitro is ultimately the goal and dream of bioengineers, warranting development of more optimal and sophisticated in vitro models. The ideal model would require not only the two highly differentiated cellular components (podocytes and endothelial cells) but also a matrix support that can withstand the pressure and sheer forces in a manner that mimics the biophysical properties of the GFB in vivo. Therefore, it is of paramount importance to improve the cell culture techniques and develop complex systems that closely represent the physiologic environment; the implications of this will fuel the discovery pipeline.
To find alternatives, Ronconi et al. were the first to identify and isolate CD133+ CD24+ renal progenitor cells (RPCs) from the PECs of the Bowman’s capsule of adult human kidney. Although this cell population represents only 1%–4% of all renal cells, it exhibited remarkable potential to differentiate into different renal cells. Within this cell population, a subset of CD133+ CD24+ cells that are Podocalyxin− displayed the potential to differentiate into podocytes and tubular cells in vitro (73). However, the next question was to identify an efficient source for RPCs. Interestingly, Da Sacco et al. (74) pioneered isolation and characterization of a novel cell population from human amniotic fluid, which possessed the characteristics of podocyte precursors (called amniotic fluid kidney progenitor cell podocytes). Their findings demonstrated that differentiated amniotic-fluid kidney progenitor cell podocytes indeed possessed characteristics similar to the immortalized human podocyte cell lines, not only morphologically, but also expressed major podocyte proteins such as GLEPP1, podocin, synaptopodin, nephrin, and collagen type IV along with sensitivity to PAN. Unlike existing immortalized cell lines, the benefits this system offers are: (1) this cell population can be cultured from any mammalian model system and propagated for many passages without immortalization, (2) it has a more nearly normal cell cycle regulation, and (3) there is a clear developmental pattern of specific protein expression. Isolation of RPCs from urine was considered another potential noninvasive alternative approach, wherein the progenitor cells were shown to indeed differentiate in vitro toward podocyte-like cells (75,76). Thus, although culture systems derived from these unique progenitor cells represent an attractive alternative to current immortalized cell lines, the low and variable number of progenitor cells present in these biologic samples has stymied the success of these systems.
To overcome this hurdle, recent progress in stem cell biology and the ability to reprogram any somatic cell type into pluripotent stem cells (PSCs) has opened a new dimension, because it essentially allows the generation of, in principle, any desired cell type (77). The induction of kidney organoids from PSCs including mouse/human embryonic stem cells (ESCs) and human- or patient-induced PSCs (iPSCs) has emerged as a promising approach. A kidney organoid is a three-dimensional kidney-like tissue that contains podocytes, renal tubular epithelial cells, and other types of cells. Deriving renal cells using iPSCs offers an inestimable tool to study “kidney diseases in a dish”, or podocytopathies. Additionally, this approach is advantageous because it promotes generation of renal cells via re-creation and mimicry of all of the in vivo stages of embryonic kidney development. The procedure begins with differentiation of iPSC into intermediate mesoderm and metanephric mesenchyme, followed by generation of nephron progenitor cells that are later induced to become multiple cell types on exposure to certain specific growth conditions (78). Since then, robust protocols have been developed and modified to push the cells to commit toward a particular lineage or subset of cells (79). For example, using either two- or three-dimensional culture approaches, and varying the choice and/or timing of growth factors, one can achieve induction of either one renal cell type (e.g., podocyte-like cells) (80,81) or self-organizing kidney organoids consisting of segmented nephrons (82–85). Using a gene-editing system (CRISPR/Cas9), the kidney lineage markers SIX2 and NPHS1 were fluorescently tagged to monitor the maturation of podocytes and progenitors in real time as indicators of nephron commitment (SIX2) and podocyte health (NPHS1) (86). Morizane et al. (87) also developed a protocol to induce human ESCs and iPSCs toward glomeruli and renal tubules via nephron progenitor cells with high efficiency. The iPSC-derived podocytes were confirmed by their ability to express podocyte-specific markers, endocytic internalization of albumin, and the disappearance of pluripotent markers (88). Further, they exhibited transcriptomic and protein expression profiles that matched those of mature podocytes, a feat that no other method has been able to achieve so far (89). Not only the expression levels of NPHS1 and NPHS2 genes in these induced podocytes were incredibly higher than that of the immortalized podocyte cell line, but also expressed abundant SD-related proteins with functional responsiveness to PAN-induced injury. The induced podocytes, however, still lacked typical interdigitated structures of FPs and expression of some important genes when compared with adult human podocytes (90). To assess how close podocytes in kidney organoids are to genuine podocytes, Wu et al.(91) performed a detailed evaluation using single cell RNA sequencing of kidney organoids generated using the protocols developed by Takasato and Morizane. Although the latter contained more podocytes (29%) than the former, podocytes derived from both the organoids displayed incomplete differentiation and a lack of expression of many transcription factors compared with human adult podocytes, suggesting the immaturity of the organoid podocytes. Nevertheless, these significant advancements not only afforded a valuable tool to generate an unlimited and renewable pool of podocytes for clinical research, but also opened an avenue for regenerative medicine, wherein these podocytes have a potential for use in cell-based therapies for combating kidney diseases associated with podocyte loss or dysfunction.
Although current protocols induce transformation of iPSCs into kidney organoids that resemble first-trimester kidney development (83,92), a fully functional “kidney in a dish” has not yet been established. This was due to lack of glomerular microcirculation, peritubular vascularization, and hemodynamic flow throughout the entire organoid tissue; all of which led to a stunted glomerular and tubular maturation in vitro. Therefore, to improve this model system and favor maturation, organoids were transplanted in mouse kidneys. This led to extensive graft vascularization, which was accompanied by a fenestrated glomerular endothelium, a GFB including GBM protein deposition, and polarized podocytes (93–95).
Type IV collagen, an essential GBM component, forms three distinct networks by combining its six α-chains. Although, the trimers of α-1,1,2 predominate in early mouse glomerular development, the networks comprised of α-3,4,5 are most abundant in the mature GBM (96). The specific cues that initiate this isoform switch during GBM maturation are unknown. Although podocytes are known to produce the appropriate collagen IV isoforms, crosstalk between podocytes and adjacent cells is believed to provide essential cues for synthesis, secretion, and ultimate assembly of the matrix proteins (97). Matrisome analysis of glomeruli derived from organoids (OrgGloms) revealed enrichment of mature GBM components (98). Type IV collagen chains α-1 and α-2 were found abundantly, indicating the formation of basement membrane in OrgGloms. Additionally, the mature type IV collagen α-5 and α-6 chains were expressed in high abundance in the Bowman’s capsule as the α-5,6,5 network (98). However, α-3 and α-4 chains could not be detected, suggesting alternative cues such as blood supply might be required for this isoform switch and for the assembly of α-3,4,5 mature network (99). Although the OrgGloms suffer from this limitation, there are several glomerular proteins present within the OrgGloms, which are absent in the immortalized cultures, including the mature type IV collagen α-6. Therefore, OrgGloms certainly show greater congruence and resemblance to the human glomerular tissue, and offer higher-quality data when compared with the other in vitro systems.
Continuous efforts were made by the researchers to address and improve the limitation of these systems, that is, their inability to recapitulate assembly of mature type IV collagen trimers, which is a marker of true differentiation. A recent study by Bantounas et al. differentiated the hPSC lines into kidney progenitors and allowed them to undergo rudimentary morphogenesis in vitro, then implanted the differentiating cells from two-dimensional cultures into immunocompromised mice subcutaneously (in vivo). This technique was shown to generate more mature kidney structures than reported previously. Evidence for the maturity of these implanted glomeruli comes from the fact that they become substantially vascularized and express mature GBM proteins, such as type IV collagen α-3 chain with a fused trilaminar structure, and podocyte FPs and SDs (95).
Although transplantation of kidney organoids into murine models has substantially induced their maturation, experiments are still laborious, technically challenging, and expensive. Moreover, in future, the use of kidney organoids for disease modeling or drug testing would potentially reduce the use of animals, but organoid transplantation would still require their use. Therefore, generating mature kidney organoids in vitro was the next aspiration of the researchers, which prompted them to discover novel methods to stimulate organoid maturation without the involvement of protracted animal models. Toward this end, robust high-throughput screening platforms to study the effect of several factors on organoid maturation and differentiation were developed (86,100). Further, organoids also contain many cell types other than podocytes, which might obscure molecular details associated with this unique cell population. Yoshimura et al. (90) addressed this problem by tweaking organoid differentiation in a way to produce a podocyte population with a high degree of purity.
Along with the organoid vascularization that provides sufficient nutrients to the tissue for maturation, shear stress and hemodynamics are other crucial factors that stimulate organoid differentiation (76). Therefore, to improve the current model system, significant progress has been made over the past decade in generating “organs on a chip” by implementing flow using microfluidic systems (101). This technique was extrapolated successfully to engineer a “glomerulus on a chip” (GOAC) (89). Briefly, to reconstitute the podocyte-endothelial interface and imitate the microenvironmental cues, poly(dimethylsiloxane) (PDMS) chips consisting of two microchannels were used. The top channel contained podocytes and the bottom had glomerular endothelial cells separated by a porous extracellular matrix–coated membrane (made of polycarbonate, PDMS). The functionality of the GFB was demonstrated by albumin retention in the vascular channel and expression of podocyte-specific markers, such as nephrin, WT-1, podocin, and VEGF-A (89,101). Using GOAC, the first steps toward modeling of various diseases from hypertensive to diabetic nephropathy have already been initiated, with propitious results (102,103).
In most of the current glomerular chips, podocytes, and glomerular endothelial cells are separated by PDMS, which is a synthetic membrane. Although these synthetic membranes are equipped with pores that allow free exchange of media and growth factors, they still do not facilitate proper crosstalk between the glomerular cells, which is the key for efficient GFB function. Fairly recently, a GOAC was devised that is devoid of an artificial membrane separating the two monolayers (podocytes and glomerular endothelial cells) (104). The chip is a microfluidic layer sandwiched between two 175 μm glass organoplates (MIMETAS, Netherlands). On these microfluidic chips, the cells maintained their morphology, formed capillary-like structures, and expressed SD proteins, at least for a month. Proper interaction of glomerular cells also led to the de novo deposition of GBM extracellular matrix components comprising collagen IV trimer and laminin. Thus, this system not only afforded a greater resemblance to the structural components of the glomerulus but also allowed a broader window to perform long-term studies (104). Further, this system was shown to recapitulate the function of the glomerulus, including perm-selectivity. When the glomeruli on the chip were exposed to sera from patients with antipodocyte autoantibodies, the chips showed albuminuria proportional to patients’ proteinuria. Although the system is reproducible and versatile, it still suffers from some major limitations, such as (1) the bidirectionality of the flow (in vivo the flow in the vascular lumen is unidirectional, namely, the glomerulus receives its blood supply from afferent arterioles before exiting into the efferent arterioles and is never recirculated), and (2) the GOAC did not include mesangial cells, an important component of the normal glomeruli. This necessitates development of better models in future with four lane chips, allowing culture of three different cell types. Because the MIMETAS technology has already demonstrated the possibility of producing functional proximal tubules (105), the next step is to combine the GOAC and the tubules to generate a functional “nephron on a chip” where filtration and reabsorption can be studied simultaneously.
Are These Systems Equipped to Study Podocyte Health and Disease?
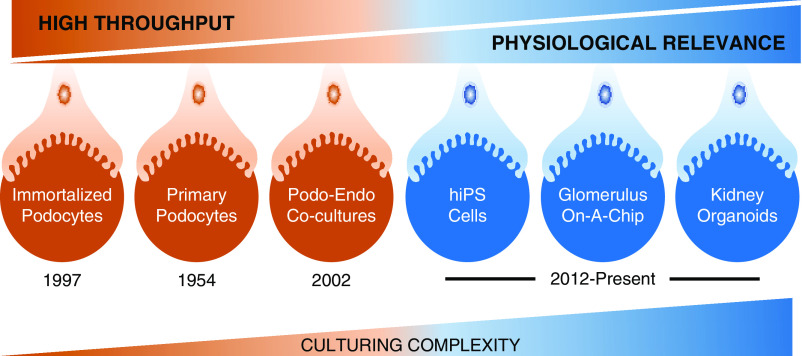
To develop podocentric therapies, robust and effective high-throughput screening assays have been developed to test compounds that afford either protection to the podocytes or are deleterious. However, the challenge lies in finding the right balance between high throughput model systems of limited physiologic context (immortalized or primary podocytes) and model systems with lower throughput but greater physiologic relevance (kidney organoids) (Figure 2).
Figure 2.
To study podocyte health, disease, and drug discovery, the challenge is to find a model system that is appropriately balanced. Although the high throughput reductionist assay systems suffer from limited physiologic relevance, the lower throughput systems afford greater physiologic relevance and opportunities for translational research. As we go to the right, the culturing complexity also increases substantially.
In addition to establishing a robust screening assay that allows for the classification of compounds into positive and negative hits, selecting a disease-relevant phenotype with a corollary to human disease is of utmost importance. Changes in the actin cytoskeleton and apoptosis have been considered congruent readouts to characterize diseased podocytes. To this end, high content–imaging technology that can capture such morphologic and cellular changes has been successfully used in drug-screening platforms with immortalized podocytes (106–109).
Besides using immortalized podocyte cell lines for drug screening, an alternate strategy uses glomeruli harvested from nephrin-EGFP knock-in mouse lines (110,111). This system functions on the principle that the podocytes that migrate away from the glomerular core undergo dedifferentiation with a concomitant loss of podocyte-specific markers, such as nephrin. On the basis of this, the compounds that maintain or promote GFP fluorescence are classified as putative factors that oppose podocyte dedifferentiation. This model system, unlike immortalized cultures, takes advantage of using glomeruli/podocytes ex vivo that closely mimics the in vivo situation.
Organoids as the “Avatars” for Precise and Personalized Medicine: Journey from Benchside to Bedside
To overcome the limitations of a reductionist approach using isolated podocytes in culture for target identification and validation, organoids are quite promising as a model system to study both nephrogenesis and nephropathies (83,92,99). Although high throughput for compound screening using the glomerulus-on-a-chip model system is limited and mostly used to study a structurally and functionally intact GFB, a recent study shows its applicability for modeling kidney diseases, including MN, diabetic nephropathy, and Alport syndrome (AS), a hereditary CKD characterized by mutations in the α-chains of COL4 genes (104). As a proof of principle, the chips generated using the AS podocytes were shown to exhibit impaired permselectivity to albumin, due to a dysfunctional assembly of the GBM, a hallmark of AS. Further, a podocyte-targeting drug such as alpha-melanocyte-stimulating hormone, clinically used to reduce proteinuria in MN patients, was shown to reverse the proteinuria induced by the MN sera in the GOAC, demonstrating the applicability of the system in drug screening. These remarkable and encouraging findings support the use of GOAC for drug screening studies, which is a major unmet need for research in nephrology.
Recently, organoid cultures from both normal kidney and clear cell renal cell carcinoma tissue were isolated and characterized (112). Although the organoids from healthy kidney present us with the model system for screening drug candidates for nephrotoxicity and studying drug-induced kidney diseases, deriving organoids from renal cancer patients represents generation of preclinical model system that can provide unprecedented opportunities for studying the molecular mechanisms underlying renal cell carcinoma, identifying new diagnostics, prognostic biomarkers, and personalized patient treatment (112). Because numerous reviews have already reported the applications of kidney organoids for regenerative medicine and as developmental, toxicity, and disease models, we are only illustrating their translational journey from benchside to bedside in Figure 3.
Figure 3.
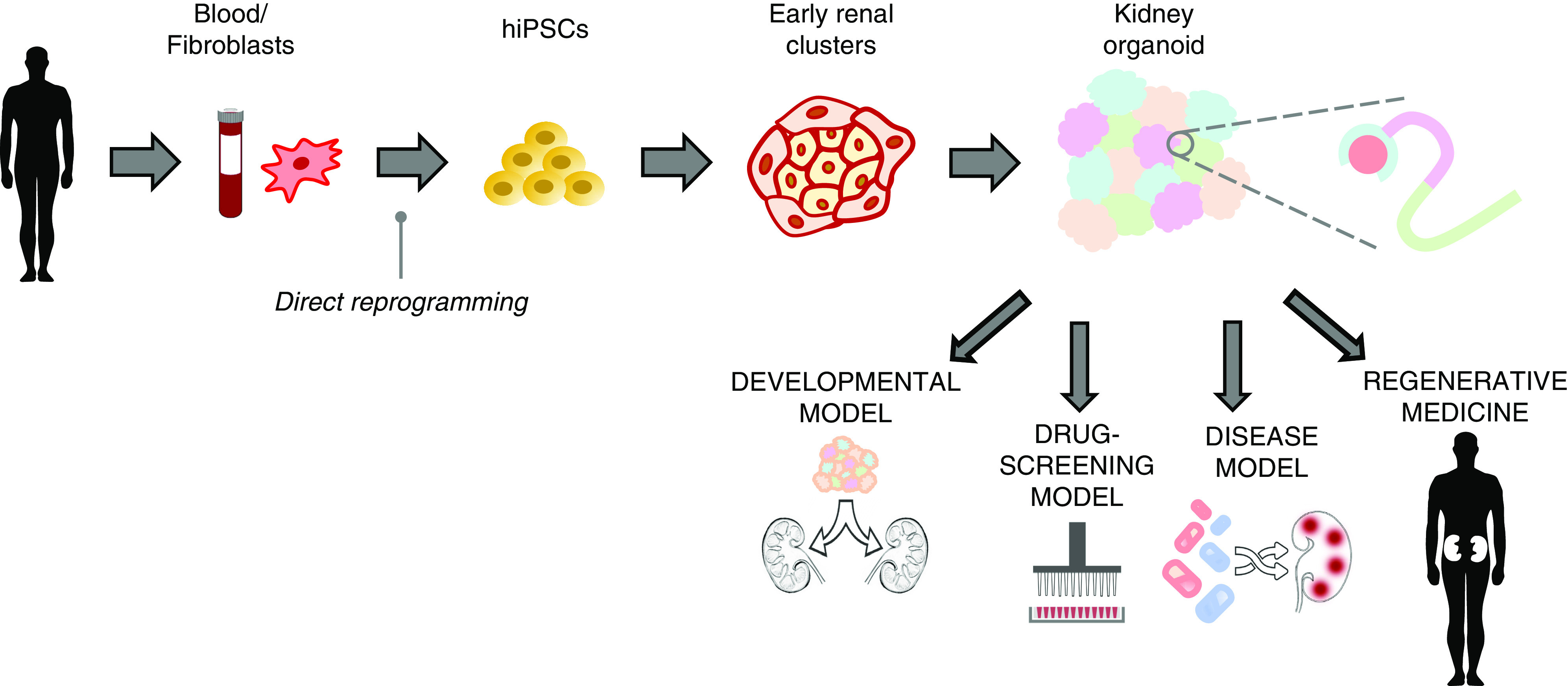
Translational journey of kidney organoids from benchside to bedside. Human-induced pluripotent stem cells (hiPSCs) can be isolated from healthy humans or even patients to create (patient-specific) kidney organoids to study specific nephropathies. The kidney organoids can also be used as developmental models to study nephrogenesis. Organoids can be used to generate disease models, either by introducing a genetic modification corresponding to a particular disease, or by exposing the organoids to certain disease-inducing compounds. Organoids as disease models then present us with the opportunity to perform high throughput drug screening and toxicity tests, thereby opening the avenue for development of podocentric therapies. Finally, kidney organoids have a potential to contribute to regenerative medicine by facilitating recreation of a functioning kidney.
To summarize, recent advancement in stem cell biology and microfluidic platforms is envisioned to overcome some of the challenges posed by canonical culture models such as primary or immortalized cell lines. Three-dimensional kidney organoids that recapitulate the GFB could therefore become a valuable tool to unravel molecular mechanisms underlying kidney diseases and develop effective therapies. Indeed, iPSC-derived kidney organoids are used actively in podocyte research for the past 5 years. However, many factors have dampened the use of this model, including maturation, functional properties, and molecular and physical interactions between podocytes and other cell populations. Deeper understanding of podocytes using a combination of several techniques, including kidney organoids, single cell RNA sequencing, and microdevices is envisaged to accelerate scientific advances toward the generation of genuine and effective podocyte culture model in vitro.
Disclosures
J. Reiser has patents on novel strategies for kidney therapeutics and stands to gain royalties from their commercialization. He is the cofounder of Walden Biosciences (Cambridge, MA), a biotechnology company in which he has financial interest, including stock. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
J. Reiser was responsible for conceptualization, funding acquisition, project administration, resources, and supervision, and reviewed and edited the manuscript; M.M. Altintas was responsible for conceptualization, investigation, project administration, resources, and supervision, wrote the original draft, and reviewed and edited the manuscript; S. Agarwal wrote the original draft and reviewed and edited the manuscript; and R. Sudhini wrote the original draft.
References
- 1.El Nahas AM, Bello AK: Chronic kidney disease: The global challenge. Lancet 365: 331–340, 2005. 10.1016/S0140-6736(05)17789-7 [DOI] [PubMed] [Google Scholar]
- 2.Kretzler M: Regulation of adhesive interaction between podocytes and glomerular basement membrane. Microsc Res Tech 57: 247–253, 2002. 10.1002/jemt.10083 [DOI] [PubMed] [Google Scholar]
- 3.Farquhar MG: The glomerular basement membrane: Not gone, just forgotten. J Clin Invest 116: 2090–2093, 2006. 10.1172/JCI29488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiser J, Sever S: Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 64: 357–366, 2013. 10.1146/annurev-med-050311-163340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser J, Altintas MM: Podocytes. F1000Res, 5, 2016. 10.12688/f1000research.7255.1 [DOI] [PMC free article] [PubMed]
- 6.Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG: Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20: 1491–1503, 2009. 10.1681/ASN.2008101117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. 10.1152/physrev.00020.2002 [DOI] [PubMed] [Google Scholar]
- 9.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012. 10.1146/annurev-physiol-020911-153238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata M: Podocyte injury and its consequences. Kidney Int 89: 1221–1230, 2016. 10.1016/j.kint.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 11.Assady S, Benzing T, Kretzler M, Skorecki KL: Glomerular podocytes in kidney health and disease. Lancet 393: 856–858, 2019. 10.1016/S0140-6736(18)33000-9 [DOI] [PubMed] [Google Scholar]
- 12.Matovinović MS: 3. Podocyte injury in glomerular diseases. EJIFCC 20: 21–27, 2009 [PMC free article] [PubMed] [Google Scholar]
- 13.Graham FL, Smiley J, Russell WC, Nairn R: Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36: 59–74, 1977. 10.1099/0022-1317-36-1-59 [DOI] [PubMed] [Google Scholar]
- 14.Handler JS: Studies of kidney cells in culture. Kidney Int 30: 208–215, 1986. 10.1038/ki.1986.173 [DOI] [PubMed] [Google Scholar]
- 15.Krakower CA, Greenspon SA: Factors leading to variation in concentration of nephrotoxic antigen(s) of glomerular basement membrane. AMA Arch Pathol 58: 401–432, 1954 [PubMed] [Google Scholar]
- 16.Weinstein T, Cameron R, Katz A, Silverman M: Rat glomerular epithelial cells in culture express characteristics of parietal, not visceral, epithelium. J Am Soc Nephrol 3: 1279–1287, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Yaoita E, Kurihara H, Sakai T, Ohshiro K, Yamamoto T: Phenotypic modulation of parietal epithelial cells of Bowman’s capsule in culture. Cell Tissue Res 304: 339–349, 2001. 10.1007/s004410100380 [DOI] [PubMed] [Google Scholar]
- 18.Yaoita E, Yoshida Y, Nameta M, Takimoto H, Fujinaka H: Induction of interdigitating cell processes in podocyte culture. Kidney Int 93: 519–524, 2018. 10.1016/j.kint.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 19.Holdsworth SR, Glasgow EF, Atkins RC, Thomson NM: Cell characteristics of cultured glomeruli from different animal species. Nephron 22: 454–459, 1978. 10.1159/000181513 [DOI] [PubMed] [Google Scholar]
- 20.Bridgewater DJ, Ho J, Sauro V, Matsell DG: Insulin-like growth factors inhibit podocyte apoptosis through the PI3 kinase pathway. Kidney Int 67: 1308–1314, 2005. 10.1111/j.1523-1755.2005.00208.x [DOI] [PubMed] [Google Scholar]
- 21.Bridgewater DJ, Matsell DG: Insulin-like growth factor binding protein-2 modulates podocyte mitogenesis. Pediatr Nephrol 18: 1109–1115, 2003. 10.1007/s00467-003-1242-x [DOI] [PubMed] [Google Scholar]
- 22.Norgaard JO: A new method for the isolation of ultrastructurally preserved glomeruli. Kidney Int 9: 278–285, 1976. 10.1038/ki.1976.30 [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi A, Yoshizawa N, Yamamoto M, Sawasaki Y, Oda T, Senoo A, Niwa H, Fuse Y: Basic fibroblast growth factor promotes proliferation of rat glomerular visceral epithelial cells in vitro. Am J Pathol 141: 107–116, 1992 [PMC free article] [PubMed] [Google Scholar]
- 24.Krtil J, Pláteník J, Kazderová M, Tesar V, Zima T: Culture methods of glomerular podocytes. Kidney Blood Press Res 30: 162–174, 2007. 10.1159/000102520 [DOI] [PubMed] [Google Scholar]
- 25.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE: The activated mesangial cell: A glomerular “myofibroblast”? J Am Soc Nephrol 2[Suppl]: S190–S197, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Quigg RJ, Cybulsky AV, Jacobs JB, Salant DJ: Anti-Fx1A produces complement-dependent cytotoxicity of glomerular epithelial cells. Kidney Int 34: 43–52, 1988. 10.1038/ki.1988.143 [DOI] [PubMed] [Google Scholar]
- 27.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T: An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006. 10.1038/sj.ki.5000398 [DOI] [PubMed] [Google Scholar]
- 28.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002. 10.1016/S0002-9440(10)64239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreisberg JI, Hoover RL, Karnovsky MJ: Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int 14: 21–30, 1978. 10.1038/ki.1978.86 [DOI] [PubMed] [Google Scholar]
- 30.Mundel P, Gilbert P, Kriz W: Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem 39: 1047–1056, 1991. 10.1177/39.8.1856454 [DOI] [PubMed] [Google Scholar]
- 31.Mundel P, Reiser J, Kriz W: Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Jat PS, Sharp PA: Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol 59: 746–750, 1986. 10.1128/JVI.59.3.746-750.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frederiksen K, Jat PS, Valtz N, Levy D, McKay R: Immortalization of precursor cells from the mammalian CNS. Neuron 1: 439–448, 1988. 10.1016/0896-6273(88)90175-4 [DOI] [PubMed] [Google Scholar]
- 34.Burns JS, Lemoine L, Lemoine NR, Williams ED, Wynford-Thomas D: Thyroid epithelial cell transformation by a retroviral vector expressing SV40 large T. Br J Cancer 59: 755–760, 1989. 10.1038/bjc.1989.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley AJ, Paterson HF, Noble M, Land H: Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J 7: 1635–1645, 1988. 10.1002/j.1460-2075.1988.tb02990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanopoulou E, Early A, Elliott J, Crispe N, Ladyman H, Ritter M, Watt S, Grosveld F, Kioussis D: Complex lymphoid and epithelial thymic tumours in Thy1-myc transgenic mice. Nature 342: 185–189, 1989. 10.1038/342185a0 [DOI] [PubMed] [Google Scholar]
- 37.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D: Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A 88: 5096–5100, 1991. 10.1073/pnas.88.12.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin AS Jr, Sharp PA: Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol 7: 305–313, 1987. 10.1128/MCB.7.1.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David-Watine B, Israël A, Kourilsky P: The regulation and expression of MHC class I genes. Immunol Today 11: 286–292, 1990. 10.1016/0167-5699(90)90114-O [DOI] [PubMed] [Google Scholar]
- 40.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997. 10.1006/excr.1997.3739 [DOI] [PubMed] [Google Scholar]
- 41.Perry J, Tam S, Zheng K, Sado Y, Dobson H, Jefferson B, Jacobs R, Thorner PS: Type IV collagen induces podocytic features in bone marrow stromal stem cells in vitro. J Am Soc Nephrol 17: 66–76, 2006. 10.1681/ASN.2005060586 [DOI] [PubMed] [Google Scholar]
- 42.Cybulsky AV, Bonventre JV, Quigg RJ, Wolfe LS, Salant DJ: Extracellular matrix regulates proliferation and phospholipid turnover in glomerular epithelial cells. Am J Physiol 259: F326–F337, 1990. 10.1152/ajprenal.1990.259.2.F326 [DOI] [PubMed] [Google Scholar]
- 43.Bijian K, Takano T, Papillon J, Khadir A, Cybulsky AV: Extracellular matrix regulates glomerular epithelial cell survival and proliferation. Am J Physiol Renal Physiol 286: F255–F266, 2004. 10.1152/ajprenal.00259.2003 [DOI] [PubMed] [Google Scholar]
- 44.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004. 10.1111/j.1523-1755.2004.00711.x [DOI] [PubMed] [Google Scholar]
- 45.Takano Y, Yamauchi K, Hiramatsu N, Kasai A, Hayakawa K, Yokouchi M, Yao J, Kitamura M: Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol 292: F1573–F1582, 2007. 10.1152/ajprenal.00423.2006 [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Takano Y, Kasai A, Hayakawa K, Hiramatsu N, Enomoto N, Yao J, Kitamura M: Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 70: 892–900, 2006. 10.1038/sj.ki.5001625 [DOI] [PubMed] [Google Scholar]
- 47.Kabgani N, Grigoleit T, Schulte K, Sechi A, Sauer-Lehnen S, Tag C, Boor P, Kuppe C, Warsow G, Schordan S, Mostertz J, Chilukoti RK, Homuth G, Endlich N, Tacke F, Weiskirchen R, Fuellen G, Endlich K, Floege J, Smeets B, Moeller MJ: Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One 7: e34907, 2012. 10.1371/journal.pone.0034907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaoita E, Yoshida Y, Nameta M, Zhang Y, Fujinaka H, Magdeldin S, Xu B, Yamamoto T: Heparin increasing podocyte-specific gene expressions. Nephrology (Carlton) 19: 195–201, 2014. 10.1111/nep.12207 [DOI] [PubMed] [Google Scholar]
- 49.Delarue F, Virone A, Hagege J, Lacave R, Peraldi MN, Adida C, Rondeau E, Feunteun J, Sraer JD: Stable cell line of T-SV40 immortalized human glomerular visceral epithelial cells. Kidney Int 40: 906–912, 1991. 10.1038/ki.1991.292 [DOI] [PubMed] [Google Scholar]
- 50.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Chittiprol S, Chen P, Petrovic-Djergovic D, Eichler T, Ransom RF: Marker expression, behaviors, and responses vary in different lines of conditionally immortalized cultured podocytes. Am J Physiol Renal Physiol 301: F660–F671, 2011. 10.1152/ajprenal.00234.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagmann H, Brinkkoetter PT: Experimental models to study podocyte biology: Stock-taking the toolbox of glomerular research. Front Pediatr 6: 193, 2018. 10.3389/fped.2018.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Friedrich C, Endlich N, Kriz W, Endlich K: Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006. 10.1152/ajprenal.00196.2005 [DOI] [PubMed] [Google Scholar]
- 55.Petermann AT, Hiromura K, Blonski M, Pippin J, Monkawa T, Durvasula R, Couser WG, Shankland SJ: Mechanical stress reduces podocyte proliferation in vitro. Kidney Int 61: 40–50, 2002. 10.1046/j.1523-1755.2002.00102.x [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Braet F, Brodsky S, Weinstein T, Romanov V, Noiri E, Goligorsky MS: VEGF-induced mobilization of caveolae and increase in permeability of endothelial cells. Am J Physiol Cell Physiol 282: C1053–C1063, 2002. 10.1152/ajpcell.00292.2001 [DOI] [PubMed] [Google Scholar]
- 57.Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS: VEGF expression in hypoxia and hyperglycemia: Reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol 13: 2027–2036, 2002. 10.1097/01.ASN.0000024436.00520.D8 [DOI] [PubMed] [Google Scholar]
- 58.Hirschberg R, Wang S, Mitu GM: Functional symbiosis between endothelium and epithelial cells in glomeruli. Cell Tissue Res 331: 485–493, 2008. 10.1007/s00441-007-0526-z [DOI] [PubMed] [Google Scholar]
- 59.Slater SC, Beachley V, Hayes T, Zhang D, Welsh GI, Saleem MA, Mathieson PW, Wen X, Su B, Satchell SC: An in vitro model of the glomerular capillary wall using electrospun collagen nanofibres in a bioartificial composite basement membrane. PLoS One 6: e20802, 2011. 10.1371/journal.pone.0020802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruggeman LA, Doan RP, Loftis J, Darr A, Calabro A: A cell culture system for the structure and hydrogel properties of basement membranes; Application to capillary walls. Cell Mol Bioeng 5: 194–204, 2012. 10.1007/s12195-012-0221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Corbelli A, Watanabe S, Armelloni S, Ikehata M, Parazzi V, Pignatari C, Giardino L, Mattinzoli D, Lazzari L, Puliti A, Cellesi F, Zennaro C, Messa P, Rastaldi MP: Three-dimensional podocyte-endothelial cell co-cultures: Assembly, validation, and application to drug testing and intercellular signaling studies. Eur J Pharm Sci 86: 1–12, 2016. 10.1016/j.ejps.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 62.Miner JH: Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol 26: 1413–1417, 2011. 10.1007/s00467-011-1785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pascual M, Steiger G, Sadallah S, Paccaud JP, Carpentier JL, James R, Schifferli JA: Identification of membrane-bound CR1 (CD35) in human urine: Evidence for its release by glomerular podocytes. J Exp Med 179: 889–899, 1994. 10.1084/jem.179.3.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, Yoshizumi A, Kawasaki K, Kihara I: Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron 69: 397–403, 1995. 10.1159/000188509 [DOI] [PubMed] [Google Scholar]
- 65.Nakamura T, Ushiyama C, Shimada N, Sekizuka K, Ebihara I, Hara M, Koide H: Effect of cyclophosphamide or azathioprine on urinary podocytes in patients with diffuse proliferative lupus nephritis. Nephron 87: 192–193, 2001. 10.1159/000045913 [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15: 1379–1383, 2000. 10.1093/ndt/15.9.1379 [DOI] [PubMed] [Google Scholar]
- 67.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV: Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003. 10.1152/ajprenal.00404.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, Shankland SJ: Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64: 1222–1231, 2003. 10.1046/j.1523-1755.2003.00217.x [DOI] [PubMed] [Google Scholar]
- 69.Petermann AT, Pippin J, Krofft R, Blonski M, Griffin S, Durvasula R, Shankland SJ: Viable podocytes detach in experimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron, Exp Nephrol 98: e114–e123, 2004. 10.1159/000081555 [DOI] [PubMed] [Google Scholar]
- 70.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J: Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16: 1733–1741, 2005. 10.1681/ASN.2005020159 [DOI] [PubMed] [Google Scholar]
- 71.Mundel P: Urinary podocytes: Lost and found alive. Kidney Int 64: 1529–1530, 2003. 10.1046/j.1523-1755.2003.00339.x [DOI] [PubMed] [Google Scholar]
- 72.Striker GE, Striker LJ: Glomerular cell culture. Lab Invest 53: 122–131, 1985 [PubMed] [Google Scholar]
- 73.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009. 10.1681/ASN.2008070709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Da Sacco S, Lemley KV, Sedrakyan S, Zanusso I, Petrosyan A, Peti-Peterdi J, Burford J, De Filippo RE, Perin L: A novel source of cultured podocytes. PLoS One 8: e81812, 2013. 10.1371/journal.pone.0081812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazzeri E, Ronconi E, Angelotti ML, Peired A, Mazzinghi B, Becherucci F, Conti S, Sansavini G, Sisti A, Ravaglia F, Lombardi D, Provenzano A, Manonelles A, Cruzado JM, Giglio S, Roperto RM, Materassi M, Lasagni L, Romagnani P: Human urine-derived renal progenitors for personalized modeling of genetic kidney disorders. J Am Soc Nephrol 26: 1961–1974, 2015. 10.1681/ASN.2014010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veissi S, Smeets B, van den Heuvel LP, Schreuder MF, Jansen J: Nephrotic syndrome in a dish: Recent developments in modeling in vitro. Pediatr Nephrol 35: 1363–1372, 2020. 10.1007/s00467-019-4203-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi K, Yamanaka S: A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol 17: 183–193, 2016. 10.1038/nrm.2016.8 [DOI] [PubMed] [Google Scholar]
- 78.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R: Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014. 10.1016/j.stem.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 79.Soo JY, Jansen J, Masereeuw R, Little MH: Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol 14: 378–393, 2018. 10.1038/s41581-018-0003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD: The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7: e46453, 2012. 10.1371/journal.pone.0046453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ciampi O, Iacone R, Longaretti L, Benedetti V, Graf M, Magnone MC, Patsch C, Xinaris C, Remuzzi G, Benigni A, Tomasoni S: Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res (Amst) 17: 130–139, 2016. 10.1016/j.scr.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taguchi A, Nishinakamura R: Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21: 730–746.e6, 2017. 10.1016/j.stem.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 83.Takasato M, Little MH: The origin of the mammalian kidney: Implications for recreating the kidney in vitro. Development 142: 1937–1947, 2015. 10.1242/dev.104802 [DOI] [PubMed] [Google Scholar]
- 84.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ: A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Reports 11: 470–484, 2018. 10.1016/j.stemcr.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lam AQ, Bonventre JV: Regenerating the nephron with human pluripotent stem cells. Curr Opin Organ Transplant 20: 187–192, 2015. 10.1097/MOT.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 86.Boreström C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslöw A, Svensson A, Söderberg M, Reznichenko A, Nyström J, Patrakka J, Hicks R, Maresca M, Valastro B, Collén A: A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int 94: 1099–1110, 2018. 10.1016/j.kint.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 87.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. 10.1038/nbt.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rauch C, Feifel E, Kern G, Murphy C, Meier F, Parson W, Beilmann M, Jennings P, Gstraunthaler G, Wilmes A: Differentiation of human iPSCs into functional podocytes. PLoS One 13: e0203869, 2018. 10.1371/journal.pone.0203869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE: Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc 13: 1662–1685, 2018. 10.1038/s41596-018-0007-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimura Y, Taguchi A, Tanigawa S, Yatsuda J, Kamba T, Takahashi S, Kurihara H, Mukoyama M, Nishinakamura R: Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol 30: 304–321, 2019. 10.1681/ASN.2018070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD: Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018. 10.1016/j.stem.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freedman BS, Steinman TI: iPS cell technology: Future impact on renal care. Nephrol News Issues 29: 18, 20–21, 2015 [PMC free article] [PubMed] [Google Scholar]
- 93.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ: Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018. 10.1016/j.stemcr.2018.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R: Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27: 1778–1791, 2016. 10.1681/ASN.2015010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bantounas I, Ranjzad P, Tengku F, Silajdžić E, Forster D, Asselin MC, Lewis P, Lennon R, Plagge A, Wang Q, Woolf AS, Kimber SJ: Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Reports 10: 766–779, 2018. 10.1016/j.stemcr.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey SJ, Zheng K, Sado Y, Naito I, Ninomiya Y, Jacobs RM, Hudson BG, Thorner PS: Role of distinct type IV collagen networks in glomerular development and function. Kidney Int 54: 1857–1866, 1998. 10.1046/j.1523-1755.1998.00188.x [DOI] [PubMed] [Google Scholar]
- 97.Abrahamson DR, St John PL, Stroganova L, Zelenchuk A, Steenhard BM: Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J Histochem Cytochem 61: 706–718, 2013. 10.1369/0022155413501677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL: Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20: 1471–1479, 2009. 10.1681/ASN.2008101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, Little MH: 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun 9: 5167, 2018. 10.1038/s41467-018-07594-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS: High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22: 929–940.e4, 2018. 10.1016/j.stem.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE: Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1: 0069, 2017. 10.1038/s41551-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou M, Zhang X, Wen X, Wu T, Wang W, Yang M, Wang J, Fang M, Lin B, Lin H: Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci Rep 6: 31771, 2016. 10.1038/srep31771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Tao T, Su W, Yu H, Yu Y, Qin J: A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip 17: 1749–1760, 2017. 10.1039/C7LC00134G [DOI] [PubMed] [Google Scholar]
- 104.Petrosyan A, Cravedi P, Villani V, Angeletti A, Manrique J, Renieri A, De Filippo RE, Perin L, Da Sacco S: A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier [published correction appears in Nat Commun 10: 4791, 2019 10.1038/s41467-019-12177-7]. Nat Commun 10: 3656, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vriend J, Nieskens TTG, Vormann MK, van den Berge BT, van den Heuvel A, Russel FGM, Suter-Dick L, Lanz HL, Vulto P, Masereeuw R, Wilmer MJ: Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip. AAPS J 20: 87, 2018. 10.1208/s12248-018-0247-0 [DOI] [PubMed] [Google Scholar]
- 106.Lal MA, Young KW, Andag U: Targeting the podocyte to treat glomerular kidney disease. Drug Discov Today 20: 1228–1234, 2015. 10.1016/j.drudis.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 107.Lee HW, Arif E, Altintas MM, Quick K, Maheshwari S, Plezia A, Mahmood A, Reiser J, Nihalani D, Gupta V: High-content screening assay-based discovery of paullones as novel podocyte-protective agents. Am J Physiol Renal Physiol 314: F280–F292, 2018. 10.1152/ajprenal.00338.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V: A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol 26: 2741–2752, 2015. 10.1681/ASN.2014090859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reiser J, Lee HW, Gupta V, Altintas MM: A high-content screening technology for quantitatively studying podocyte dynamics. Adv Chronic Kidney Dis 24: 183–188, 2017. 10.1053/j.ackd.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kindt F, Hammer E, Kemnitz S, Blumenthal A, Klemm P, Schlüter R, Quaggin SE, van den Brandt J, Fuellen G, Völker U, Endlich K, Endlich N: A novel assay to assess the effect of pharmaceutical compounds on the differentiation of podocytes. Br J Pharmacol 174: 163–176, 2017. 10.1111/bph.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsuchida J, Matsusaka T, Ohtsuka M, Miura H, Okuno Y, Asanuma K, Nakagawa T, Yanagita M, Mori K: Establishment of nephrin reporter mice and use for chemical screening. PLoS One 11: e0157497, 2016. 10.1371/journal.pone.0157497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grassi L, Alfonsi R, Francescangeli F, Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi A, Pizzi S, Bruselles A, Pallocca M, Simone G, Haoui M, Falchi M, Milella M, Sentinelli S, Di Matteo P, Stellacci E, Gallucci M, Muto G, Tartaglia M, De Maria R, Bonci D: Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis 10: 201, 2019. 10.1038/s41419-019-1453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]