Abstract
The study of how engagement in enriching cognitive, physical and social activities in childhood impacts cognitive function decades later will advance our understanding of how modifiable lifestyle activities promote cognition across the lifespan. 88 healthy older adults (aged 60–80 years) returned a retrospective questionnaire regarding their participation in seven lifestyle activities (musical instrument playing, language learning, sport participation, art/dance lessons, scouting, volunteering, family vacations) before age 13 years. After controlling for current age, educational attainment, socioeconomic status of the mother and current engagement in lifestyle activities, a greater number of activities were significantly associated with better vocabulary abilities, episodic memory and fluid intelligence. The relationships with vocabulary and fluid intelligence were mediated by educational attainment. We postulate that engagement in a higher number of enriching early life activities is a reflection of both one’s sociocontextual environment and engagement with that environment. This engagement leads to attributes relevant for educational aspirations/attainment, ultimately contributing to factors that have a lifespan impact on cognitive function.
Keywords: Executive function, Mediation Analysis, Life Course and Developmental Change, Plasticity
Introduction
Engagement in cognitively, physically and socially enriching lifestyle behaviors is associated with the promotion and maintenance of cognitive function with age (Erickson et al., 2011; Kotwal et al., 2016; Mansky et al., 2020; Voss et al., 2010). Importantly, cognitive decline and the development of pathological aging are not compulsory effects of aging (Pascual-Leone et al., 2011). Maintaining cognitive function with age is likely a function of both the brain’s preservation of efficient mechanisms of plasticity (i.e., the nervous system’s ability to make adaptations to constantly changing environmental inputs) (Pascual-Leone, 2006) and the brain’s expression of compensatory responses in the face of pathological changes (i.e., brain resilience / cognitive reserve) (Stern, 2012).
Cognitive reserve refers to the adaptability of cognitive processes to brain aging, pathology or insult (Stern et al., 2020). Individual differences in cognitive reserve are determined by existing cognitive or functional brain processes (Stern et al., 2020). A life course model of cognitive reserve put forward by Richards and Deary (Richards & Deary, 2005), proposes that (beyond genetics) early social and maternal environments, education and occupational attainment, socioeconomic status and degree of enriching lifestyle activities determine brain structure (white matter lesions, volume, vascularization) and function (neural network density, efficiency, processing capacity). Engagement in modifiable enriching lifestyle activities, such as learning a language or musical instrument, physical exercise and team sports, volunteering, family vacations and socialization, can promote these mechanism of plasticity and cognitive reserve across the lifespan (Bak et al., 2014; Hertzog et al., 2008; Mansky et al., 2020; Voss et al., 2010). A small number of recent studies have assessed the relationships between engagement in a variety of enriching activities in childhood/early adolescence and cognitive function later in life (Chan et al., 2019; Dik et al., 2003; Wilson et al., 2003). This is important because during development, the brain may be particularly susceptible to environmental and psychosocial processes that can positively affect the development of neural mechanisms of plasticity and cognitive reserve (Richards & Deary, 2005). It is possible therefore that engagement in a variety of enriching early life activities (EELA) during a critical period of brain development (before 13 years) may lead to enhanced mechanisms of plasticity that are still present decades later. Alternatively, engagement in EELAs may promote engagement in similar lifestyle activities across the lifespan, leading to a consistent upkeep of mechanisms of cognitive reserve (Richards & Deary, 2005; Schreiber et al., 2016). Lifespan studies have either looked at in-home activities, such as reading and writing (Wilson et al., 2003) or individual activities such as participation in physical activity (Dik et al., 2003). A lot of previous research has also focused on the frequency of engagement in a given activity, yet the variety of different activities may be just as important for the effects on cognitive function (Bielak et al., 2019). A recent study by Chan and colleagues (Chan et al., 2019) utilized a multimodal perspective of EELA involving not only cognitive but also social and physical stimulating experiences, and demonstrated a link between greater variety of EELA before age 13 and greater performance in a select few cognitive abilities (processing speed and set shifting ability), in a sample of older African American adults. Whether these results extend to other older populations who are not deemed to be at greater risk for cognitive decline (Mayeda et al., 2016) is not known (Chan et al., 2019). However, this knowledge would significantly improve both the scientific and public health perspectives on how lifestyle activities can promote brain health maintenance across the lifespan.
Educational attainment, often measured as years in formal education is also a strong proxy of cognitive reserve (Lövdén et al., 2020), with numerous studies demonstrating a relationship between more educational attainment and higher cognitive function across the lifespan (Baldivia et al., 2008; Wilson et al., 2019). In a meta-analysis of cognitive reserve and cognitive function in older adults, (Opdebeeck et al., 2016) a relationship between education and fluid and crystalized abilities (vocabulary) was found, as well as with processing speed, with a smaller correlation being seen with episodic memory. The education-cognitive function relationship is, in part, a reflection of the sociocontextual environment during childhood and how one interacts with that environment (Lövdén et al., 2020). Consequently, educational attainment may be one pathway through which early life activities lead to the long-term promotion of mechanisms of plasticity and cognitive reserve, ultimately resulting in better later life cognitive function.
The objectives of this study were therefore to assess whether engagement in a greater variety of EELA before age 13 is associated with educational attainment and better later life (>60 years) cognitive function using a comprehensive battery of neuropsychological tasks in a sample of healthy older adults. We hypothesized that EELA would be associated with perceptual, executive, episodic and vocabulary abilities and that education attainment would mediate these relationships.
Methods
Participants and study design
Participants in the study comprised a sub-sample of low active (< 3 days of physical activity per week) but healthy older adults aged between 60–80 years who participated in a randomized controlled trial of exercise (https://clinicaltrials.gov/ct2/show/NCT01472744). All outcome data are taken from baseline pre-intervention measurements. All participants provided informed consent and the University of Illinois Institutional Review Board approved all procedures used in the study. Inclusion criteria for the original study consisted of 1) >75% right-handed on the Edinburgh Handedness Questionnaire; (2) normal or corrected-to-normal vision of at least 20/40; (3) no color- blindness; (4) no history of stroke, transient ischemic attack, or head trauma; (5) >23 score on Mini-Mental State Examination (MMSE); (6) >21 score on Telephone Interview of Cognitive Status (TICS); (7) <10 score on Geriatric Depression Scale (GDS). 202 of the original study participants (out of 247) who had agreed to be recontacted were sent a letter gauging their interest in completing a short questionnaire about their early life experiences. Those interested were mailed a short, 10-item paper and pencil questionnaire (supplementary table 1), which were completed and returned via US postal service. At the same time, participants read and signed a new informed consent document pertaining to these new measures. Full demographic information about the included participants (N=88) are detailed in Table 1. No significant differences in demographics between the included sub-sample and the initial randomized control trial participants existed (supplementary table 1).
Table 1.
Participant Characteristics
| N | 88 |
| Current age (mean (SD) | 65.81 (4.72) |
| Gender: Female (%) | 63 (71.6) |
| Race (%) | |
| Caucasian | 79 (89.7) |
| African American | 7 (8) |
| Asian/Pacific Island | 2 (2.3) |
| Years of education (mean (SD) | 15.89 (2.66) |
| Mother’s SES (mean (SD) | 5.93 (2.01) |
| Mini mental status exam (mean (SD) | 28.65 (1.36) |
| EELA composite (mean (SD) | 4.16 (1.78) |
| EELA specific | |
| Musical instrument (%) | 58 (65.9) |
| Extracurricular lessons (%) | 62 (70.5) |
| Team sports (%) | 30 (34.1) |
| Foreign language (%) | 20 (22.7) |
| Volunteer at place of worship (%) | 72 (82.8) |
| Scouts (%) | 65 (73.9) |
| Family vacations (%) | 59 (67.0) |
| Current lifestyle activities (mean (SD) | 16.25 (3.58) |
Power analysis
We performed a power analysis (in R using the ‘pwr’ package) on our sample size to ensure sufficient power was gained to detect a true effect. Based on our sample size (N=88) and an assumed type I error rate of 0.05, we calculated an estimated 88% power to detect an effect size of 0.19 from multiple linear regression with five covariates in our main analysis (association between EELA and cognitive function).
Enriching early life activities
Enriching early life experiences were assessed through a retrospective questionnaire. The questionnaire asked for a “yes” or “no” answer to whether they participated in the following seven activities before the age of 13 years; “Did you play a musical instrument?” “Did you take art, dance or musical lessons?” “Did you play team sports?” “Did you study a foreign language?” “Did you volunteer at a place of worship?” “Did you participate in scouting?” “Did you take family vacations?” The main measure was comprised of the sum total of all “yes” answers with a resultant score ranging from 0-to-7, where a higher score meant more engagement in early life activities.
Outcome measures
Educational attainment was measured as the total number of years in formal education. Cognitive performance was assessed using a comprehensive battery of neuropsychological assessments taken from the Virginia Cognitive Aging Project (Salthouse & Ferrer-Caja, 2003). A detailed description of each task can be found in a previous open access publication (Baniqued et al., 2018). We grouped each task into four broad cognitive domains (sum of the standardized (z-score) score of each individual task) based on a previous principal component analysis performed using the same dataset (Baniqued et al., 2018). These included: word vocabulary, picture vocabulary and synonym-antonym, to create a ‘vocabulary’ domain; digit symbol, letter comparison and pattern comparison, to create a ‘perceptual speed’ domain; logical memory, paired associations and word recall, to create a ‘episodic memory’ domain; Shipley abstraction, foam boards, letter sets, matrix reasoning, paper folding and spatial relations, to create a ‘fluid intelligence’ domain. Composite scores for each domain were computed by averaging the z-scores of each task.
Covariates
Covariates included current age (in years), gender, the subjective socioeconomic status (SES) of the participants’ mother, per studies showing a relationship between early childhood SES and later life outcomes (Guralnik et al., 2006; Rahkonen et al., 1997) and engagement in current lifestyle activities. The mothers’ subjective socioeconomic status was measured using a ladder ranging from 1-to-10 asking “what did you consider the socioeconomic status of your mother?”, with 1 being ‘worst off’ and 10 being ‘best off’. This was measured in accordance with previous publications (Chan et al., 2019). Current engagement in lifestyle activities was measured as the number of later life activities via a 23-item questionnaire that probed participants engagement in a variety of activities ranging from volunteer work, gardening and cooking to crosswords, drawing and socialization. A detailed description of the questionnaire development can be found in a previous publication (Carlson et al., 2012). We used the sum of “yes” answers as our covariate.
Statistical analyses
All statistical analyses were performed in R Version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). The independent associations between EELA and cognitive performance/educational attainment were assessed using separate multiple linear regression, controlling for current age, gender, education (cognitive models), mother’s SES and current engagement in lifestyle activities. Model assumptions were checked using Q-Q and fitted vs. residual plots in R and the normality of the residuals was formally checked using Shapiro-Wilk tests of normality. The significant influence of outliers was checked using Cooke’s distance with a cut off of 0.5 (no outliers removed). Beta coefficients (β) with 95% confidence intervals are given and model fitness is presented as adjusted R2 values and significance is considered at the p < .05 level. To account for a violation of the model assumptions (normality of residuals) in the vocabulary model, a generalized linear model with a gaussian family and identity link function was fit (both for the multiple linear regression and subsequent mediation models). Multiple comparisons (for cognitive models) were corrected for using Bejamini and Hochberg’s false discovery rate (FDR) at a q value of 0.05, after pooling the p values from the regression analysis. FDR-adjusted p values (FDRpval) are presented. A table of all bivariate correlations between each independent and dependent variable is included in supplementary table 2.
Mediation analysis using the R package ‘mediation’ (Tingley et al., 2014) was performed to assess whether educational attainment mediated the associations between EELA and later life cognitive performance, taking into account all covariates (current age, gender, mother’s SES, current lifestyle activities; see figure 1 for graphical representation of the model). The total effects (effect of X on Y), direct effects (effect of X on Y taking into account M (ADE)) and indirect effects (or ‘mediation effect’, the total effect minus the direct effect (ACME)) are reported. The presence of statistical mediation was determined through nonparametric bootstrap confidence intervals via 1000 bootstrap resamples of the estimated indirect effect. The estimated indirect (ACME) effect corresponds to the reduction in the independent variable effect on the dependent variable when adjusted for the mediator. For the direct and total effects, the estimate is interpreted as per 1-unit (1 EELA activity) increase.
Figure 1.
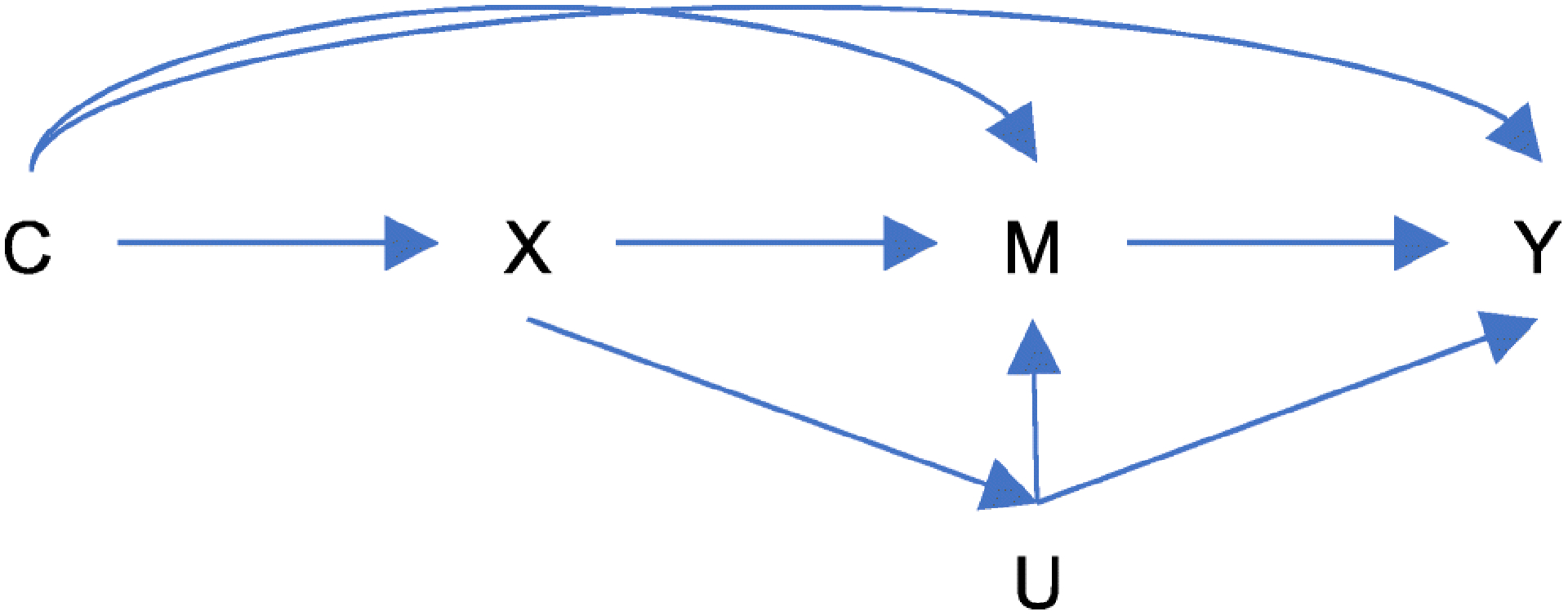
Conceptual model for the mediation analysis. X being the exposure (EELA), M the mediator (educational attainment), the outcome Y (cognitive performance), covariates C and the unmeasured mediator-outcome confounder, U, which itself can be affected by X.
To formally assess the robustness of the mediation findings to potential violations of the key assumption to mediation (sequential ignorability (SI) (Albert & Wang, 2015), i.e., that no unmeasured confounders effect X on M nor M on Y), we used sensitivity analyses per Imai et al. (Imai et al., 2010). The sensitivity parameters are presented as the correlation between the two error terms for the outcome and mediation models (rho) at the point at which the ACME becomes 0. That is, how large does this correlation need to be for mediation to disappear? Alternatively, we also express the sensitivity parameter as the function of the coefficient of determination (R2) for the proportion of original variance explained by the unobserved confounder (R^2_M*R^2_Y*) and the proportion of the previously unexplained variance explained by the unobserved confounder (R^2_M ~ R^2_Y~). That is, how large must the unobserved confounder be (relative to the observed pretreatment covariates in the model) for mediation to disappear (Imai et al., 2010).
Results
Participant characteristics
Table 1 presents the participant characteristics. At the group level, participants engaged in multiple early life activities, where a majority of participants learned to play a musical instrument, took extracurricular lessons, volunteered at a place of worship, engaged in the scouts and went on family vacations.
Relationship between EELA and later life cognitive performance
We examined whether EELA were associated with later life cognitive performance, controlling for current age, gender, mother’s SES and current engagement in lifestyle activities (figure 2). Episodic memory (β =.15, 95%CI = 0.04, 0.25, p = .005, aR2=.15, FDRpval = .01), fluid intelligence (β =.12, 95%CI = 0.03, 0.21, p = .008, aR2=.16, FDRpval = .011) and vocabulary (β =.20, 95%CI = 0.08, 0.32, p = <.001, aR2=.14, FDRpval = .002), were all significantly associated with EELA, which remained significant after multiple comparison correction. Perceptual speed was not associated with EELAs (β =.04, 95%CI = −0.05, 0.14, p = .369, aR2=.33).
Figure 2.
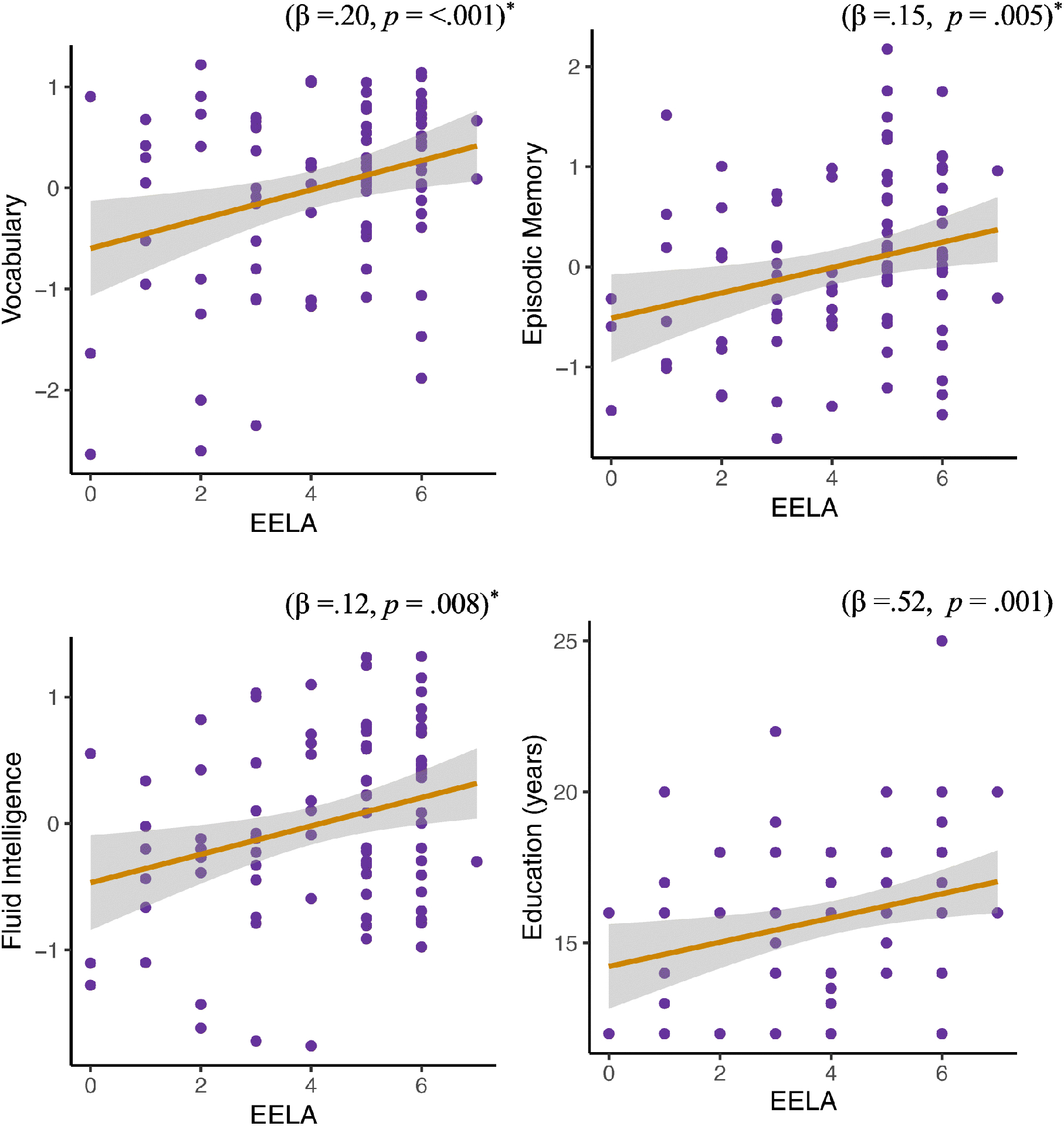
Scatter plots illustrating the significant relationships between EELA and our outcome measures. Standardized beta coefficients (β) are presented. * signifies statistical significance after multiple comparison corrections (FDR), for the cognitive models only.
Relationship between EELA and educational attainment and later life activities
We further asked if EELA were associated with educational attainment after controlling for mother’s SES. We found that the greater engagement in EELA, the more education was attained (β =.52, 95%CI = 0.20, 0.85, p = .001, aR2=.10). Furthermore, a trending but non-significant association between the variety of EELAs and the variety of later life lifestyle activities was seen (β =.46, 95%CI = −0.005, 0.92, p = .052, aR2=.03).
Mediation results and sensitivity analyses
Given EELA (prior to age 13) were significantly associated with later life cognitive performance and educational attainment, we asked whether the number of years of education mediated the relationship between EELA and later life cognitive performance. Table 2 displays the results of mediation analyses, where education significantly mediated the relationship between EELA and later life fluid intelligence and vocabulary, after controlling for current age, gender, mother’s SES and current engagement in lifestyle activities.
Table 2.
Mediation analysis results
| Outcomes | Total effect | ADE | ACME |
|---|---|---|---|
| Beta(95%CI) | Beta(95%CI) | Beta(95%CI) | |
| Episodic memory | .15(0.03,0.26)* | .12(0.02,0.24)* | .02(−0.02,0.07) |
| Fluid intelligence | .12(0.01,0.22)* | .08(−0.01,0.19) | .03(0.003,0.07)* |
| Vocabulary | .2(0.06,0.34)* | .13(−0.002,0.26) | .07(0.01,0.14)* |
| Perceptual speed | .05(−0.06,0.14) | .02(−0.08,0.13) | .02(−0.01,0.06) |
Each model was adjusted for age, gender, mother socioeconomic status and engagement in current lifestyle activities. ADE = average direct effect; ACME = average causal mediation effect.
Denotes statistical significance at p < 0.05 and 95% CI not including 0.
Sensitivity analyses suggest that the EELA-Fluid intelligence relationship appears more sensitive to violations of the SI assumption than the EELA-vocabulary relationship, with a moderate correlation between error terms (.25) and a moderate amount of variance required by an unobserved confounder to see null result (6%) (table 3).
Table 3.
Sensitivity analyses
| Outcomes | ||
|---|---|---|
| Fluid intelligence | Vocabulary | |
| Rho at which ACME = 0 | .25 | .4 |
| R^2_M*R^2_Y* at which ACME = 0: | .06 | .16 |
| R^2_M ~ R^2 Y~ at which ACME = 0: | .03 | .08 |
Discussion
Cognitive decline and onset of neurodegenerative disease is not an obligatory consequence of aging (Pascual-Leone et al., 2011; Yaffe et al., 2009). That is, certain individuals are capable of maintaining their cognitive function with age (Josefsson et al., 2012; Yaffe et al., 2009). A corpus of research has focused on the concept of cognitive or brain reserve (Barulli & Stern, 2013) and how it can promote cognitive function with advancing age, even in the face of neuropathology (Katzman et al., 1988). Educational attainment and occupational complexity have been used as proxies for cognitive reserve (Baldivia et al., 2008; Richards & Deary, 2005; Wilson et al., 2019) and have shown robust and replicable associations with cognitive performance in older adults (Lövdén et al., 2020; Opdebeeck et al., 2016). Further, interest in how modifiable lifestyle factors can promote and maintain cognitive function across the lifespan has increased. A wealth of literature demonstrates that social (Kok et al., 2018; Kotwal et al., 2016), intellectual (Bak et al., 2014; Mansens et al., 2018) and physical activities (Erickson et al., 2011; Kramer & Erickson, 2007) are beneficial for cognitive function in childhood (Donnelly et al., 2016; Hillman et al., 2011), midlife (Chang et al., 2010) and older healthy adults (Kotwal et al., 2016; Kramer & Colcombe, 2018). In our study, we demonstrated that educational attainment is one pathway through which early modifiable lifestyle activities (< 13 years of age) promote later life (over 60 years of age) cognitive function.
A small number of studies have taken a lifespan approach to study how early engagement in enriching activities affect later life cognitive function (Chan et al., 2019; Dik et al., 2003; Oveisgharan et al., 2020). This may be highly important as during childhood, the brain may be particularly sensitive to environmental and psychosocial processes that can beneficially affect the development of neural mechanisms of plasticity (Richards & Deary, 2005), key to the maintenance of cognitive function in older adults (Barulli & Stern, 2013; Scarmeas & Stern, 2003). For example, early engagement in physical exercise (15 to 25 years) was shown to be positively associated with later life processing speed (Dik et al., 2003), a result which was partially replicated in another study by Chan and colleagues (Chan et al., 2019). In Chan et al., (Chan et al., 2019) rather than focusing on a single activity the authors demonstrated that a variety of EELAs (using an identical retrospective questionnaire to the present study) were associated with higher later life processing speed (patten comparison) and set-shifting (trail-making test-B), in a sample of African American older adults. A key discrepancy between Chan and colleagues and our results is that we failed to see a relationship between EELA and processing speed. The use of empirically-derived construct-level cognitive abilities is often preferred to characterize cognitive function than individual tasks owing to their greater construct validity (Caemmerer et al., 2020), which may in part provide some explanation as to the discrepancies in the finding between studies. However it is more likely that the inconsistencies are reflective of a difference in the populations’ samples, with the study by Chan and colleagues consisting of mostly African American participants with greater risk factors for cognitive decline (Mayeda et al., 2016), where in our study, participants with certain risk factors were screened out and consisted of only healthy older adults. This interpretation is perhaps strengthened by a recent study (Oveisgharan et al., 2020) that found the relationship between early cognitive enrichment (without social or physical activities) and later life cognitive decline was attenuated when including Alzheimer’s disease indices into the model, concluding that lower pathology may explain some part of the relationship between early life enrichment and later life cognitive function (Oveisgharan et al., 2020).
Our results further demonstrated that education attainment mediated the effects of EELAs on later life fluid intelligence and vocabulary abilities, but not perceptual speed or episodic memory. At the global level, the associations between education attainment and cognitive function likely reflect both sociocontextual (environmental) factors throughout development as well as how an individual interacts (through their own actions and behaviors) with the contextual opportunities available (Lövdén et al., 2020). In this scenario, EELAs are likely, in part, a reflection of the sociocontextual environment (higher parental and financial resources, greater likelihood of family vacations/art and music lesson, for example) that lead to greater development of psychological and behavioral characteristics relevant for scholastic performance and educational aspirations (Lövdén et al., 2020; Sewell et al., 1970). The two ultimately contribute to increased cognitive function decades later. Importantly, this relationship does not appear to be unique to our sample of healthy older and mostly Caucasian adults with Chan and colleagues demonstrating a similar relationship in a sample of mostly African American adults (Chan et al., 2019).
There was no association between EELA and processing speed and education did not mediate this nor the relationship with episodic memory. In a meta-analysis of cognitive reserve and cognitive function in older adults (Opdebeeck et al., 2016), episodic memory had the smallest correlation with education compared to reasoning abilities (fluid intelligence) and vocabulary abilities (even though a relationship did exist, as did one between education and processing speed). However across studies it appears that the relationship between cognitive reserve proxies of education and cognitively-stimulating activities with cognition (Baldivia et al., 2008) differs depending on which cognitive domain is assessed (Opdebeeck et al., 2016). Whilst the immediate effects of education on fluid and crystallized abilities appear robust (Ritchie & Tucker-Drob, 2018), the direct effects of educational attainment on later life cognitive function or decline appear to be marginal (Lövdén et al., 2020; Protzko, 2015). Numerous studies do suggest that educational attainment affects later life cognitive function indirectly via a number of pathways related to self-selection of environmental demands across the lifespan (e.g., occupational complexity, lifestyle choices) that can reduce the fade-out of education’s effects on later life cognition (Lövdén et al., 2010, 2020). It is of note that the result that education mediated the association between EELA and fluid intelligence and vocabulary (a form of crystallized knowledge) is in line with the mutualism model put forward by Van der Mass and colleagues (van der Maas et al., 2006) that suggests their exits a bidirectional coupling of fluid and crystallized abilities. Cattell (Cattell & Cattell, 1971) hypothesized that those with higher fluid abilities would acquire more crystallized knowledge (via effortful cognitive processing) and more recently it has been shown that this relationship is likely bidirectional (Ferrer et al., 2010; Ferrer & McArdle, 2004; Kievit et al., 2017) and fundamentally facilitated by education (Lövdén et al., 2020). This is then perhaps one theory as to why we see an EELA-education-fluid/crystallized ability mediation in our study.
A life course model of cognitive reserve put forward by Richards and Deary (Richards & Deary, 2005), proposes that (beyond genetics) early social and maternal environments, education and occupational attainment, socioeconomic status and degree of lifestyle activities determine brain structure (white matter lesions, volume, vascularization) and function (neural network density, efficiency, processing capacity). It is possible therefore that greater engagement in EELAs during a critical period of brain development (before 13 years) may lead to enhanced mechanisms of plasticity that are still present decades later. Alternatively, engagement in EELAs may promote engagement in similar lifestyle activities across the lifespan, leading to a consistent upkeep of mechanisms of cognitive reserve (Richards & Deary, 2005; Schreiber et al., 2016). Prior literature has demonstrated a link between childhood engagement in sports participation and physical exercise later in life (Telama et al., 2005), and Kagitcibasi and Colleagues (Kagitcibasi et al., 2009) found long lasting (22 years) beneficial effects of early enrichment on cognitive, social and educational achievement trajectories into young adulthood, compared to children who did not receive enrichment. Nevertheless, we did not find a significant correlation between EELA and later life activities.
Our results should be considered in light of their limitations. Firstly, the EELA questionnaire deployed, identical to that of previous studies (Chan et al., 2019; Moored et al., 2018) relied upon retrospective recall, which is susceptible to recall bias. Nevertheless, free from emotion and retrospective impact bias, such questionnaires that are designed to have low recall bias by asking simply whether one engaged or not in these actives has been shown to be very accurate, even after 50+ years (Berney & Blane, 1997). Notwithstanding, the questionnaire used did not capture duration of activity engagement nor intensity, which could provide greater information about engagement that we are unable to speculate upon. Additionally, our sample of healthy older adults was taken from a randomized clinical trial of exercise for cognitive and brain health study with explicit inclusion and exclusion criteria, not specific to the study of EELA and later life cognitive function, namely this sample included older adults who we deemed sedentary (spent less than 3 days per week performing any type of physical activity). Whilst this is a definite selection bias and should be considered, it is also perhaps reflective of the general US population with over a 3rd (34.8%) leading a sedentary lifestyle (Du et al., 2019), and of high-income Western countries on the whole (Guthold et al., 2018).
In light of our findings and of those in previous studies, strategies to increase childhood participation in a variety of enriching lifestyle activities should be developed to help maintain and promote cognitive function across the lifespan.
Supplementary Material
Statement of relevance.
Maintaining cognitive function with advancing age is fundamental to quality of life and independent living. Adherence to and participation in enriching activities, such as sport, dance, musical and art have beneficial effects on cognitive function. Engagement in these activities during a critical period in childhood may lead to optimal trajectories of brain development and lifestyle factors (educational attainment, occupational complexity, lifelong adherence to activities) which continue to have beneficial effects on cognitive function with advancing age. Our results demonstrate that the more variety of enriching early life activities (before age 13 years), the higher the educational attainment that is reached, leading to better cognitive function later in life (after age 60 years). Our results have wide ranging implications for both our understanding of how our interactions with our environment across the lifespan affect cognitive function and for the development of strategies to increase childhood engagement in enriching activities.
Acknowledgments
We would like to thank Anya Knecht, Susan Houseworth, Nancy Dodge, Hilly Tracy, Robert Weisshappel and all of the Lifelong Brain and Cognition and Exercise Psychology Laboratory graduate students and staff for their help in participant recruitment and data collection.
Funding
This work weas supported by the National Institute on Aging at the National Institutes of Health (R37 AG025667).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest
No authors declare any conflict of interest.
Ethics approval
The University of Illinois Institutional Review Board approved all procedures used in the study.
Consent to participate
All participants gave written informed consent before participation in any study procedures, all of which conformed to the Declaration of Helsinki for research involving human subjects.
Consent for publication
All authors agree to the contents of this manuscript and give consent for its publication.
Availability of data and materials
All data will be provided upon reasonable request to the corresponding author, without reservation.
Code availability
Code used in this manuscript includes R syntax for statistical analyses and will be shared upon request to the corresponding author.
References
- Albert JM, & Wang W (2015). Sensitivity analyses for parametric causal mediation effect estimation. Biostatistics (Oxford, England), 16(2), 339–351. 10.1093/biostatistics/kxu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak TH, Nissan JJ, Allerhand MM, & Deary IJ (2014). Does bilingualism influence cognitive aging? Annals of Neurology, 75(6), 959–963. 10.1002/ana.24158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldivia B, Andrade VM, & Bueno OFA (2008). Contribution of education, occupation and cognitively stimulating activities to the formation of cognitive reserve. Dementia & Neuropsychologia, 2(3), 173–182. 10.1590/S1980-57642009DN20300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EA, Aguiñaga S, McAuley E, Kramer AF, & D’Esposito M (2018). Brain Network Modularity Predicts Exercise-Related Executive Function Gains in Older Adults. Frontiers in Aging Neuroscience, 9. 10.3389/fnagi.2017.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, & Stern Y (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney LR, & Blane DB (1997). Collecting retrospective data: Accuracy of recall after 50 years judged against historical records. Social Science & Medicine, 45(10), 1519–1525. 10.1016/S0277-9536(97)00088-9 [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Mogle JA, & Sliwinski MJ (2019). Two sides of the same coin? Association of variety and frequency of activity with cognition. Psychology and Aging, 34(3), 457–466. 10.1037/pag0000350 [DOI] [PubMed] [Google Scholar]
- Caemmerer JM, Keith TZ, & Reynolds MR (2020). Beyond individual intelligence tests: Application of Cattell-Horn-Carroll Theory. Intelligence, 79, 101433. 10.1016/j.intell.2020.101433 [DOI] [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q-L, Rebok GW, Bandeen-Roche K, & Fried LP (2012). Lifestyle activities and memory: Variety may be the spice of life. The women’s health and aging study II. Journal of the International Neuropsychological Society: JINS, 18(2), 286–294. 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB, & Cattell RB (1971). Abilities: Their Structure, Growth, and Action. Houghton Mifflin. [Google Scholar]
- Chan T, Parisi JM, Moored KD, & Carlson MC (2019). Variety of Enriching Early-Life Activities Linked to Late-Life Cognitive Functioning in Urban Community-Dwelling African Americans. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 74(8), 1345–1356. 10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, Eiriksdottir G, Jonsdottir MK, Lopez OL, Harris TB, Gudnason V, & Launer LJ (2010). The Effect of Midlife Physical Activity on Cognitive Function Among Older Adults: AGES—Reykjavik Study. The Journals of Gerontology: Series A, 65A(12), 1369–1374. 10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik M, Deeg DJH, Visser M, & Jonker C (2003). Early life physical activity and cognition at old age. Journal of Clinical and Experimental Neuropsychology, 25(5), 643–653. 10.1076/jcen.25.5.643.14583 [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Hillman CH, Castelli D, Etnier JL, Lee S, Tomporowski P, Lambourne K, & Szabo-Reed AN (2016). Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Medicine and Science in Sports and Exercise, 48(6), 1197–1222. 10.1249/MSS.0000000000000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, & Bao W (2019). Trends in Adherence to the Physical Activity Guidelines for Americans for Aerobic Activity and Time Spent on Sedentary Behavior Among US Adults, 2007 to 2016. JAMA Network Open, 2(7), e197597–e197597. 10.1001/jamanetworkopen.2019.7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, & McArdle JJ (2004). An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Developmental Psychology, 40(6), 935–952. 10.1037/0012-1649.40.6.935 [DOI] [PubMed] [Google Scholar]
- Ferrer E, Shaywitz BA, Holahan JM, Marchione K, & Shaywitz SE (2010). Uncoupling of reading and IQ over time: Empirical evidence for a definition of dyslexia. Psychological Science, 21(1), 93–101. 10.1177/0956797609354084 [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Nesbitt D, Shafto M, Rowe JB, Price D, Gadie A, Cam-CAN, & Kievit RA (2019). Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiology of Aging, 74, 46–55. 10.1016/j.neurobiolaging.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Muñoz Maniega S, Valdés Hernández MC, Morris Z, Murray C, Royle NA, Starr JM, Deary IJ, & Wardlaw JM (2012). Neuroprotective lifestyles and the aging brain: Activity, atrophy, and white matter integrity. Neurology, 79(17), 1802–1808. 10.1212/WNL.0b013e3182703fd2 [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Butterworth S, Wadsworth MEJ, & Kuh D (2006). Childhood Socioeconomic Status Predicts Physical Functioning a Half Century Later. The Journals of Gerontology: Series A, 61(7), 694–701. 10.1093/gerona/61.7.694 [DOI] [PubMed] [Google Scholar]
- Guthold R, Stevens GA, Riley LM, & Bull FC (2018). Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. The Lancet Global Health, 6(10), e1077–e1086. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, Kivipelto M, Winblad B, Granholm A-C, & Mohammed AKH (2017). BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. Journal of Alzheimer’s Disease: JAD, 55(2), 645–657. 10.3233/JAD-160593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2008). Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychological Science in the Public Interest: A Journal of the American Psychological Society, 9(1), 1–65. 10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kamijo K, & Scudder M (2011). A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Preventive Medicine, 52, S21–S28. 10.1016/j.ypmed.2011.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Keele L, & Tingley D (2010). A general approach to causal mediation analysis. Psychological Methods, 15(4), 309–334. 10.1037/a0020761 [DOI] [PubMed] [Google Scholar]
- Josefsson M, de Luna X, Pudas S, Nilsson L-G, & Nyberg L (2012). Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. Journal of the American Geriatrics Society, 60(12), 2308–2312. 10.1111/jgs.12000 [DOI] [PubMed] [Google Scholar]
- Kagitcibasi C, Sunar D, Bekman S, Baydar N, & Cemalcilar Z (2009). Continuing effects of early enrichment in adult life: The Turkish Early Enrichment Project 22 years later. Journal of Applied Developmental Psychology, 30(6), 764–779. 10.1016/j.appdev.2009.05.003 [DOI] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, & Peck A (1988). Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology, 23(2), 138–144. 10.1002/ana.410230206 [DOI] [PubMed] [Google Scholar]
- Kievit RA, Lindenberger U, Goodyer IM, Jones PB, Fonagy P, Bullmore ET, Neuroscience in Psychiatry Network, & Dolan, R. J. (2017). Mutualistic Coupling Between Vocabulary and Reasoning Supports Cognitive Development During Late Adolescence and Early Adulthood. Psychological Science, 28(10), 1419–1431. 10.1177/0956797617710785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok R, Prinzie P, Bakermans-Kranenburg MJ, Verhulst FC, White T, Tiemeier H, & IJzendoorn M. H. van. (2018). Socialization of prosocial behavior: Gender differences in the mediating role of child brain volume. Child Neuropsychology, 24(6), 723–733. 10.1080/09297049.2017.1338340 [DOI] [PubMed] [Google Scholar]
- Kotwal AA, Kim J, Waite L, & Dale W (2016). Social Function and Cognitive Status: Results from a US Nationally Representative Survey of Older Adults. Journal of General Internal Medicine, 31(8), 854–862. 10.1007/s11606-016-3696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, & Colcombe S (2018). Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study-Revisited. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 13(2), 213–217. 10.1177/1745691617707316 [DOI] [PubMed] [Google Scholar]
- Kramer AF, & Erickson KI (2007). Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends in Cognitive Sciences, 11(8), 342–348. 10.1016/j.tics.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Küster OC, Laptinskaya D, Fissler P, Schnack C, Zügel M, Nold V, Thurm F, Pleiner S, Karabatsiakis A, von Einem B, Weydt P, Liesener A, Borta A, Woll A, Hengerer B, Kolassa I-T, & von Arnim CAF (2017). Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. Journal of Alzheimer’s Disease: JAD, 59(3), 1097–1111. 10.3233/JAD-170447 [DOI] [PubMed] [Google Scholar]
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin a. S., Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods a. J., McAuley E, Kramer AF, & Erickson KI (2014). BDNF mediates improvements in executive function following a 1-year exercise intervention. Frontiers in Human Neuroscience, 8(December), 1–12. 10.3389/fnhum.2014.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Bäckman L, Lindenberger U, Schaefer S, & Schmiedek F (2010). A theoretical framework for the study of adult cognitive plasticity. Psychological Bulletin, 136(4), 659–676. 10.1037/a0020080 [DOI] [PubMed] [Google Scholar]
- Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, & Tucker-Drob EM (2020). Education and Cognitive Functioning Across the Life Span. Psychological Science in the Public Interest: A Journal of the American Psychological Society, 21(1), 6–41. 10.1177/1529100620920576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansens D, Deeg DJH, & Comijs HC (2018). The association between singing and/or playing a musical instrument and cognitive functions in older adults. Aging & Mental Health, 22(8), 970–977. 10.1080/13607863.2017.1328481 [DOI] [PubMed] [Google Scholar]
- Mansky R, Marzel A, Orav EJ, Chocano-Bedoya PO, Grünheid P, Mattle M, Freystätter G, Stähelin HB, Egli A, & Bischoff-Ferrari HA (2020). Playing a musical instrument is associated with slower cognitive decline in community-dwelling older adults. Aging Clinical and Experimental Research. 10.1007/s40520-020-01472-9 [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(3), 216–224. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moored KD, Chan T, Varma VR, Chuang Y-F, Parisi JM, & Carlson MC (2018). Engagement in Enriching Early Life Activities is Associated with Larger Hippocampal and Amygdala Volumes in Community-Dwelling Older Adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 10.1093/geronb/gby150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck C, Martyr A, & Clare L (2016). Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging, Neuropsychology, and Cognition, 23(1), 40–60. 10.1080/13825585.2015.1041450 [DOI] [PubMed] [Google Scholar]
- Oveisgharan S, Wilson RS, Yu L, Schneider JA, & Bennett DA (2020). Association of Early-Life Cognitive Enrichment With Alzheimer Disease Pathological Changes and Cognitive Decline. JAMA Neurology. 10.1001/jamaneurol.2020.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone. (2006). Disrupting the brain to guide plasticity and improve behavior. Progress in Brain Research, 157, 315–329. 10.1016/s0079-6123(06)57019-0 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone Freitas, C., Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, & Rotenberg A (2011). Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topography, 24(3–4), 302–315. 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C (2017). Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plasticity, 2017, 3589271. 10.1155/2017/3589271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzko J (2015). The environment in raising early intelligence: A meta-analysis of the fadeout effect. Intelligence, 53, 202–210. 10.1016/j.intell.2015.10.006 [DOI] [Google Scholar]
- Rahkonen O, Lahelma E, & Huuhka M (1997). Past or present? Childhood living conditions and current socioeconomic status as determinants of adult health. Social Science & Medicine, 44(3), 327–336. 10.1016/S0277-9536(96)00102-5 [DOI] [PubMed] [Google Scholar]
- Richards M, & Deary IJ (2005). A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology, 58(4), 617–622. 10.1002/ana.20637 [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, & Tucker-Drob EM (2018). How Much Does Education Improve Intelligence? A Meta-Analysis. Psychological Science, 29(8), 1358–1369. 10.1177/0956797618774253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, & Ferrer-Caja E (2003). What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging, 18(1), 91–110. 10.1037/0882-7974.18.1.91 [DOI] [PubMed] [Google Scholar]
- Scarmeas N, & Stern Y (2003). Cognitive Reserve and Lifestyle. Journal of Clinical and Experimental Neuropsychology, 25(5), 625–633. 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, & Jagust W (2016). Impact of lifestyle dimensions on brain pathology and cognition. Neurobiology of Aging, 40, 164–172. 10.1016/j.neurobiolaging.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell WH, Haller AO, & Ohlendorf GW (1970). The Educational and Early Occupational Status Attainment Process: Replication and Revision. American Sociological Review, 35(6), 1014–1027. JSTOR. 10.2307/2093379 [DOI] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet. Neurology, 11(11), 1006–1012. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, & Vuoksimaa E (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telama R, Yang X, Viikari J, Välimäki I, Wanne O, & Raitakari O (2005). Physical activity from childhood to adulthood: A 21-year tracking study. American Journal of Preventive Medicine, 28(3), 267–273. 10.1016/j.amepre.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K (2014). mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software, 59(1), 1–38. 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- Valero J, Paris I, & Sierra A (2016). Lifestyle Shapes the Dialogue between Environment, Microglia, and Adult Neurogenesis. ACS Chemical Neuroscience, 7(4), 442–453. 10.1021/acschemneuro.6b00009 [DOI] [PubMed] [Google Scholar]
- van der Maas HLJ, Dolan CV, Grasman RPPP, Wicherts JM, Huizenga HM, & Raijmakers MEJ (2006). A dynamical model of general intelligence: The positive manifold of intelligence by mutualism. Psychological Review, 113(4), 842–861. 10.1037/0033-295X.113.4.842 [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, & Kramer AF (2010). Plasticity of Brain Networks in a Randomized Intervention Trial of Exercise Training in Older Adults. Frontiers in Aging Neuroscience, 2. 10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, & Bennett DA (2003). Assessment of Lifetime Participation in Cognitively Stimulating Activities. Journal of Clinical and Experimental Neuropsychology, 25(5), 634–642. 10.1076/jcen.25.5.634.14572 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, & Bennett DA (2019). Education and cognitive reserve in old age. Neurology, 92(10), e1041–e1050. 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB, & Health ABC Study. (2009). Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology, 72(23), 2029–2035. 10.1212/WNL.0b013e3181a92c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


