The ultrasound-mediated transient materials enable the management of biodegradation processes for implantable electronics.
Abstract
On-demand transient electronics, technologies referring subsequent material disintegration under well-defined triggering events and programmed time lines, offer exceptional clinical experiences in diagnosis, treatment, and rehabilitation. Despite potential benefits, such as the elimination of surgical device removal and reduction of long-term inimical effects, their use is limited by the nontransient conventional power supplies. Here, we report an ultrasound-mediated transient triboelectric nanogenerator (TENG) where ultrasound determines energy generation and degradation period. Our findings on finite element method simulation show that porous structures of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) play an essential role in the triggering transient process of our device under high-intensity ultrasound. Besides, the addition of polyethylene glycol improves triboelectric output performance; the voltage output increased by 58.5%, from 2.625 to 4.160 V. We successfully demonstrate the tunable transient performances by ex vivo experiment using a porcine tissue. This study provides insight into practical use of implantable TENGs based on ultrasound-triggered transient material design.
INTRODUCTION
More than 20 million people in the United States have so far benefitted from implantable medical devices (IMDs) intended for different purposes: therapeutic, diagnostic, and rehabilitation (1). Efforts to improve IMDs have seen the development and use of transient materials, which dissolve in the body after a programmed lifetime, therefore, eliminating the need for secondary surgery to remove the IMD (2). However, the conventional lithium-ion batteries are nontransient, therefore limiting the use of transient IMDs (3). Recent advances in energy harvesting technologies have provided transient material–based options with great potential to power transient IMDs.
There have been several attempts to develop batteryless IMDs by integrating energy harvesting technologies with transient materials. Photovoltaic and thermoelectric in vivo energy harvesters were introduced as sources of power for transient electronics, but their use is limited by lack of light sources and constant body temperature, respectively (4, 5). On the other hand, the biodegradability of piezoelectric catalysts [i.e., PbZrxTi1-xO3 (PZT) and barium titanate (BTO)] is unverified, limiting the use piezoelectric nanogenerators in transient electronics (6–8). Triboelectric nanogenerators (TENGs), on the basis of the coupling effect of contact electrification and electrostatic induction, are an emerging technology with great potential for powering transient IMDs. They offer a number of advantages including wide material selection and minimizable system scale (9–11). Material study, on the basis of nature-driven and artificially synthesized materials, has gained particular interest as a way to improve power-generating performance of transient TENGs (12–14). However, conventional transient TENGs exploit passive operation, referring the circumstances in which their transience starts when implanted and deployed inside the body. Because the long time scale from the loss of energy generation to the complete transience, undesirable burdens, and potential hazards can be induced to the patients. Active operation, the system of which function is terminated with the precisely manipulated stimuli that entails mechanical and/or chemical degradation, has been introduced as a promising approach toward the elimination of negative health consequences (15). Many efforts to take benefits of active operation for transient TENGs have been made using light sources and photothermal processes, but they are neither available deep in the body nor secured biological safety (16–18).
Here, we demonstrate a previously unidentified protocol by establishing a remotely stimulated transience mechanism using biologically certified ultrasound sources. To demonstrate the feasibility of ultrasound-triggered TENGs, we developed a fully biodegradable and implantable TENG (FBI-TENG) consisting of a magnesium (Mg) electrode layer, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) membranes, and a polyethylene glycol–based polymeric composite (PHBV/PEG) layer. We aimed to determine the efficiency of the FBI-TENG by solely altering the degree of ultrasound intensity. The level of energy generation, mainly at low ultrasound intensity, was enhanced through our PHBV/PEG membranes design. On the other hand, the ultrasound-triggered transient process, which originated from high ultrasound intensity, was initiated by the microporous structure of the PHBV encapsulation layer developed using finite element method (FEM) simulation that facilitates the distribution of intensified acoustic pressure inside the micropores. When the FBI-TENG was inserted into a porcine tissue, we observed a notable reduction in electrical output 20 min after applying high ultrasound intensity. Our findings support that ultrasound, a verified noninvasive and reliable power source, can be used for powering IMDs and for provoking device dissolution after a programmed lifetime.
RESULTS
Ultrasound-triggered transient TENG materials design
Our FBI-TENG was designed to generate stable electrical outputs under low ultrasound intensity (0.5 W cm−2), while a higher intensity of 3.0 W cm−2 was intended to initiate its transient process (Fig. 1A). Figure 1B displays a conceptual diagram of the FBI-TENG system. The high-intensity ultrasound (HIU) plays an essential role for us to manipulate device lifetime on-demand by triggering mechanical disruption and chemical degradation of FBI-TENG. By contrast, if the FBI-TENG remains not HIU-triggered, then the extended and prolonged degradation process may cause abnormal health conditions. The inset images describe that the device is intact under 0.5 W cm−2 and mechanically disintegrated under 3.0 W cm−2. These ultrasound intensities were rationally chosen due to their human body biosafety with a frequency of 20 kHz (19, 20). The overall structure of an FBI-TENG is illustrated in Fig. 1C. FBI-TENG fabrication process is displayed in fig. S1, and we prepared a PHBV membrane with a thickness of 50 μm. A PHBV membrane with the dimension of 2 cm by 2 cm was used as a substrate layer, where a 100-nm-thick Mg electrode layer was deposited using electron beam (e-beam) evaporation. A PHBV/PEG membrane with a thickness of 50 μm was developed and placed on the Mg electrode, playing the role of a triboelectric layer that provokes electrical output as described in Fig. 1C (inset). Another piece of the 50-μm PHBV membrane was adopted as an encapsulation layer that protects the device from moisture and foreign materials. We cultured fibroblast cells and placed them on the polymer membranes for 7 days to investigate the constituent materials’ cytotoxicity (Fig. 1D). Figure S2 is their microscopic images showing cytoskeletal structures of fibroblast cells on different membranes. The PHBV membranes, a well-known biodegradable polymer, and our developed PHBV/PEG were evaluated for biocompatibility. The long-term [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] (MTT) assay results (after 7 days) showed that the PHBV/PEG membranes have high viability of 99.6% when compared to the control cells that were cultured without polymer membranes.
Fig. 1. Overall concept and material design of ultrasound-mediated FBI-TENG for defining transient performances.
(A) Schematic illustration of ultrasonic energy generation and degradation of FBI-TENG under the skin at different ultrasound probe powers. (B) Conceptual illustration of HIU-triggered transience mechanism. HIU defines the device lifetime on demand. The inset images of (i) an intact and (ii) a degraded FBI-TENG in response to different probe powers. Scale bars, 1 cm. Photo credit: Dong-Min Lee, SKKU. (C) Schematic device structure consisting of fully biodegradable materials: PHBV, PHBV/PEG, and Mg. The insets are field-effect scanning electron microscopy (FE-SEM) images that describe microscopic morphology and operation mechanism for generating triboelectric output of FBI-TENG. The scale bar inside FE-SEM images is 20 μm. (D) Cell viability test measured for 7 days to confirm biocompatibility of the constituent materials through MTT assay analysis. (E) Plotted root mean square roughness (Rq) and contact potential difference (CPD) values of pristine PHBV and various PHBV/PEG composite materials. (F) KPFM images showing the CPD of a PHBV/PEG (7:1) membrane before and after friction with Mg.
The effects of PEG contents on surface potential were explored to optimize the triboelectric output performance of a PHBV/PEG membrane. We conducted Kelvin probe force microscopy (KPFM) measurements to study the PEG role in a quantitative manner as a function of PHBV:PEG weight ratio (Fig. 1E and fig. S3). Bare PHBV membrane showed a surface potential of 480 mV, but the addition of PEG at a ratio of PHBV to PEG (7:1) increased the surface potential by 110 mV. This enhancement in surface potential is mainly resulted by the presence of hydroxyl group (―OH) in PEG (21). Whereas when a large amount of PEG was added (a ratio of PHBV to PEG, 3:1), the surface potential decreased to 237 mV, which may be attributed to the conformational change to the PEG (21). On the basis of these findings, we established a triboelectric series of PHBV and PHBV/PEG membranes to describe their triboelectric properties (fig. S5) (22). The PHBV/PEG (7:1) membrane shows the highest tribo-positive properties that assure the highest electric output performance when it contacts the Mg electrode. This membrane was further studied using contact potential difference (CPD) measurements to verify a triboelectric potential change before/after contact with an Mg electrode (Fig. 1F). The CPD value of the PHBV/PEG (7:1) membrane increased by 73 mV from 519 to 592 mV, demonstrating that electrons were transferred from the membrane to the Mg electrode surfaces when friction was made. We implemented device scale analysis to confirm whether it showed identical characteristics with microscopic scale analysis. We adopted a pushing tester that generates contact-separation motions to examine triboelectric performances (fig. S6). We concluded that the tendency of electrical outputs was equivalent to that of surface potentials displayed in Fig. 1E (fig. S7).
We rationally designed PHBV/PEG membranes that had a high susceptibility to moisture because, to function as transient electronics, the internal components should simultaneously collapse when a PHBV encapsulation layer is degraded. Because the transient performance is generally influenced by the material crystallinity, we investigated the crystallinity of the membranes through x-ray diffraction (XRD) (fig. S8) (23). On the basis of the knowledge that PHBV/PEG membranes have strong XRD characteristic peaks at 19.6° and 23.5°, we hypothesized that a higher content of PEG would show more amorphous characteristics when considered through the amplitudes of diffraction peaks (24, 25). In a quantitative manner, we calculated their crystallinity (XC) using the following equation (26)
| (1) |
where ACi is an area under each crystalline peak and At is a total area for both amorphous and crystalline regions. We confirmed that a PHBV/PEG (10:1) membrane exhibited relatively high crystallinity, 80.3%, whereas a PHBV/PEG (3:1) membrane was found to have a crystallinity of 45.7%. We further carried out differential scanning calorimetry (DSC) measurements to further confirm the crystallinity findings. We derived the crystallinity of the membranes using the following equation (27)
| (2) |
where is a theoretical value of melting enthalpy from 100% crystalline PHBV, is a theoretical value of melting enthalpy from 100% crystalline PEG, wPHBV is the weight fraction of PHBV, wPEG is the weight fraction of PEG, and ∆Hm is a melting enthalpy value of the membranes. We found that the crystallinity values derived from DSC measurements showed the same tendency as the values from XRD measurements. As shown in fig. S9, we used contact angle measurements to demonstrate their hydrophilicity in a visible way. The hydrophilicity of the membranes was enhanced by PEG in a concentration dependent manner. Because enhanced hydrophilicity secures an improved transient performance, on the basis of our results, we conclude that our designed PHBV/PEG membrane was suitable for the FBI-TENG triboelectric layer.
Electrical output characterization of FBI-TENG
According to our previous work, metallic materials have a prominent ultrasound reflecting feature, while polymers have a remarkable characteristic in transmitting acoustic energy (28). On the basis of this knowledge, we hypothesized that acoustic energy is able to reach the Mg electrode after penetrating the PHBV encapsulation and PHBV/PEG triboelectric layer. Then, this energy will undergo reflection on the surface of the Mg electrode and back to the PHBV/PEG membrane. Because of this phenomenon, mechanical vibration takes place on the PHBV/PEG triboelectric layer, defining the operating mechanism of ultrasound-driven triboelectrification (fig. S10). According to the previous study, an adhesive layer plays an essential role in the structure and principle of the ultrasound-driven TENG (28). However, because of its unverified biodegradable function, our FBI-TENG was fabricated using a hot pressing method to eliminate the need for the adhesive layer. Because the PHBV/PEG layer was softly placed on the PHBV substrate and the layer has its surface roughness of 125 nm, the air gap still exists for the ultrasound-driven mechanical vibration in the area between the Mg electrode and the PHBV/PEG triboelectric layer. The sinusoidal waveform of electrical signals is evidence that the contact-separation motions take place between a PHBV/PEG membrane and an Mg electrode.
On the basis of our findings on the triboelectric output performances of PHBV/PEG membranes, we chose a PHBV/PEG (7:1) membrane as a triboelectric layer of our FBI-TENG. To investigate the electrical output performances, we immersed the FBI-TENG into deionized water at 5 mm beneath an ultrasound probe (Fig. 2A). We adopted an ultrasound with a frequency of 20 kHz and an intensity of 0.5 W cm−2 for safety. Under these experimental conditions, we observed that the FBI-TENG (dimension of 2 cm by 2 cm) generates a voltage output of 4.51 V and a current output of 27.86 μA (Fig. 2, B and C). The electrical outputs can also be expressed in root mean square values as follows: voltage output of 1.33 V and current output of 7.10 μA. We further investigated the effects of electrical impedance on current density values of an FBI-TENG to identify the maximum value of its power density (Fig. 2D). We plotted the current density values at different electrical impedance ranging from 10 kilohms to 1 gigaohms. The FBI-TENG achieved a maximum power density value of 17.24 μW cm−2 when the impedance was 20 kilohms. We also evaluated the electric outputs at different probe power (fig. S11). Because a high level of probe power could trigger transient processes of an FBI-TENG (details will be discussed later), we captured an initial value of electric outputs when the probe power was increased. We observed that both the voltage and current outputs were proportional to the probe power, featuring their maximum values, 6.05 V and 42.17 μA, respectively.
Fig. 2. Electrical characterization of the FBI-TENG.
(A) Experimental setup for measuring ultrasound-driven electric output of FBI-TENG in water. Scale bar, 1 cm. Photo credit: Dong-Min Lee, SKKU. (B and C) Triboelectric voltage output (B) and current output (C) measured in water at 5 mm from an ultrasound probe to the FBI-TENG, with a setup of 20 kHz and 0.5 W cm−2. (D) Measurement of power and current density depending on electrical impedance from 10 kilohms to 1gigaohms. The units of “k,” “M,” and “G” represent “kilo-,” “mega-,” and “giga-,” respectively.
Theoretical and empirical study on ultrasound-triggered transient processes
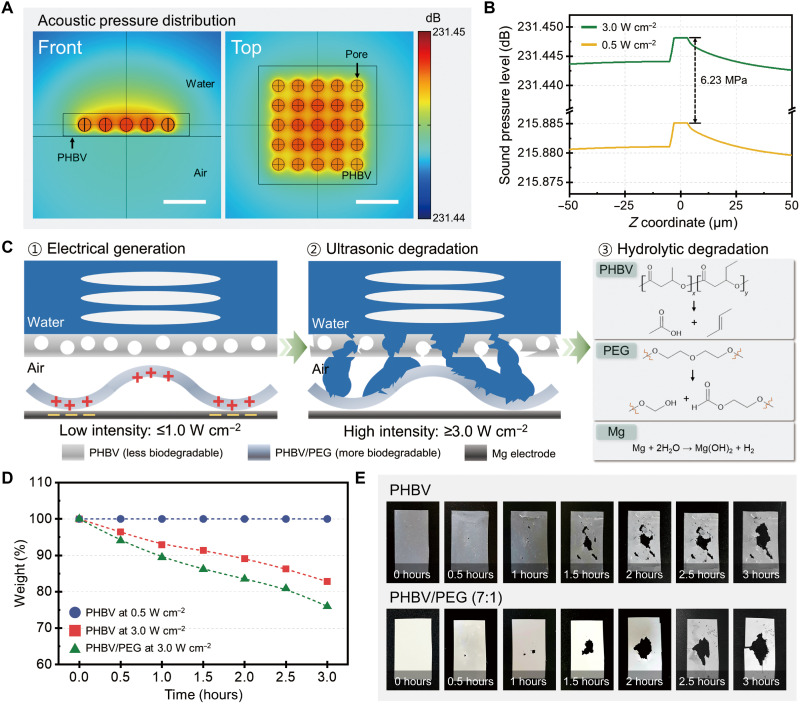
A fundamental study was implemented to demonstrate ultrasound as a triggering event of the transient processes. Because we designed a PHBV/PEG triboelectric layer with higher vulnerability to moisture for a faster biodegradation rate, the PHBV encapsulation layer plays an essential role in determining the transient performance of FBI-TENG. We described a microscopic environment of a PHBV encapsulation layer using FEM simulation. We introduced water on the upper surface of the PHBV; meanwhile, the air component was used under the encapsulation layer. Figure 3A shows sound pressure distribution displayed at a viewpoint of the front and top, respectively. To confirm sound pressure level at different probe power, we analyzed its distribution along the z axis as described in fig. S12A. To enable comprehension of the sound pressure level, we converted the simulated outcome in decibel into pascal units using the following equation (29)
| (3) |
where LP stands for standard pressure level in decibels, P is sound pressure in pascals, and P0 is a reference sound pressure of which the value is equivalent to 2 × 10−5 Pa. Taking into account the maximum value of sound pressure, a probe power of 3.0 W cm−2 exhibited a significant enhancement by 6.23 MPa when compared to that of 0.5 W cm−2 (Fig. 3B). Our FBI-TENG is designed to benefit from this magnificent contrast in sound pressure that enables distinct responsiveness to varied ultrasound intensity: generating electrical outputs at a low intensity (0.5 W cm−2) and device degradation at a high intensity (3.0 W cm−2). We also found that the porous structure in a PHBV membrane offers a favorable condition for initiating the transient processes (also described in Supplementary Text). Figure S12B displays a distribution of sound pressure level on a horizontal plane, showing that a high degree of pressure is mainly expressed on the inner space of the pores. This was confirmed in a quantitative way, as shown in fig. S12C. On the basis of our findings, we can conclude that locally intensified pressure resulting from porosity may promote mechanical disintegration when a higher acoustic energy is applied.
Fig. 3. Theoretical and experimental studies of transient performances for FBI-TENG under ultrasound stimulation.
(A) FEM simulation describing the disintegration mechanism of the PHBV encapsulation layer. Porosity in a PHBV membrane intensifies transient performances with higher sound pressure level (decibels). Scale bars, 10 μm. (B) Graph of calculated sound pressure level represents a significant difference of 6.23 MPa at different probe power of 0.5 and 3.0 W cm−2, which results in contrasting transient performances. (C) Schematic illustration of HIU-triggered transience mechanism. HIU triggers the mechanical disintegration of the PHBV encapsulation layer. Then, the hydrolytic degradation can be facilitated by the enlarged surface area. (D) Measurement of weight loss with at a time interval of 30 min under different ultrasound probe powers at a probe distance of 5 mm. The membranes are stacked to mimic an FBI-TENG structure, as shown in fig. S14. (E) Real images of a pristine PHBV and a PHBV/PEG (7:1) membrane at different degradation stages when HIU applied. Photo credit: Dong-Min Lee, SKKU.
Figure 3C describes the conceptual illustration of the HIU-triggered transience mechanism. Because PHBV membranes are less biodegradable and mechanically stable under the low-intensity ultrasound (≤1.0 W cm−2), they prevent water content from the penetration into the FBI-TENG. Thus, the low-intensity ultrasound ensures the continuous energy generation without triggering the transience mechanism of FBI-TENG. By contrast, given our findings on the FEM simulation results, HIU (≥3.0 W cm−2) induces locally reinforced acoustic pressure inside the pores of PHBV membranes, which subsequently causes mechanical fracture around the pores. With the increased degree of disintegration upon the HIU treatment, the enlarged surface area facilitates the molecular level degradation (i.e., hydrolysis) of the PHBV encapsulation layer (fig. S13) (30, 31). Meanwhile, the PHBV/PEG triboelectric layer and the Mg electrode that have relatively high biodegradation rate easily undergo hydrolysis when it encounters the water permeated through the degraded sites (32). Notably, the electric output performance of FBI-TENG begins to be reduced nearly upon the initial treatment of HIU, followed by the complete loss of energy-generating function within several minutes.
Empirical approaches were examined to verify our theoretical studies on ultrasound-triggered transient performances. To investigate the biodegradation process of the FBI-TENG, we placed a PHBV membrane on a PHBV/PEG (7:1) layer to imitate the circumstance of an FBI-TENG (fig. S14). We traced the extent of weight loss in a sequence of time while applying ultrasound (Fig. 3D). The weight of a PHBV membrane remained constant at a lower probe power (0.5 W cm−2), but it had a significant reduction in weight under a higher probe power (3.0 W cm−2) within several hours. Considering that it takes about 8 weeks for PHBV films to be degraded, the HIU offers a promising approach toward controlling the lifetime of our FBI-TENG (30). Besides, the PHBV/PEG (7:1) layer showed a faster transient rate even when it was enclosed by a PHBV layer. On the basis of the photographic images displayed in Fig. 3E, we confirmed that the composite material encountered degradation as the encapsulating membrane initiated a transient process in a simultaneous manner, a phenomenon that corresponds to our descriptions above. Surface morphology change of PHBV membranes was also observed using atomic force microscope (AFM) and optical microscope (OM) measurements (figs. S16 and S17). Considering the AFM images, the surface roughness (Rq) remained similar level upon the 0.5 W cm−2 of ultrasound intensity for 30 min. Meanwhile, the Rq of the HIU-triggered PHBV was increased by 89 nm than the pristine PHBV. The microscopic structure displayed on the OM images illustrates that the HIU-triggered PHBV has more abundantly distributed pores with a larger diameter scale. In addition, electrical output measurements allowed us to investigate transient performances of FBI-TENGs at diverse ultrasound conditions. We evaluated the performance by monitoring the time period until they produced negligible electrical outputs. We have the arranged degradation time at different probe powers and probe distances (fig. S18A). Because the increased probe powers endow intensified pressure on an encapsulation layer, the decreasing trend in degradation time is proportional to the probe powers. Meanwhile, a longer probe distance results in a reduced level of pressure on the encapsulating membrane; in the present study, we also observed that the probe distance is proportional to degradation time. Detailed voltage output for determining degradation time is shown in fig. S18B, implying that the FBI-TENG completely lost its function after 27 min under a probe power of 4.0 W cm−2 and a probe distance of 1 mm.
Ex vivo demonstration for potential use as an on-demand transient electronics
Owing to its anatomical structure, which is comparable with the human skin, a porcine tissue was used to evaluate the ultrasound-triggered transient performances of our FBI-TENG (Fig. 4A). Because IMDs for transdermal use are implanted at 0.5 to 1.0 cm under the epidermis, we inserted the FBI-TENG at the interface between porcine skin and fat layers (Fig. 4, B and C) (33). Figure S19 describes experimental methods for measuring electrical outputs generated from an ex vivo demonstration. We applied a commercial ultrasound gel on the porcine skin to minimize the loss of incident acoustic energy. When an ultrasound of 0.5 W cm−2 was used, our FBI-TENG generated 1.45 V of voltage output and 11.60 μA of current output. We found that they still had a typical sinusoidal waveform, indicating that they complied with the operating mechanism of ultrasound-driven triboelectricity illustrated in fig. S10.
Fig. 4. Ex vivo demonstration of ultrasound triggered biodegradation FBI-TENG.
(A and B) Schematics illustrating ex vivo experiments of an FBI-TENG implanted in a porcine tissue (A) and visualizing the location of an implanted FBI-TENG in a porcine tissue (B). (C) Photographic image of FBI-TENG implanted 0.5 cm under the porcine skin. Scale bar, 1 cm. (D and E) Voltage and current outputs generated by the FBI-TENG in the ex vivo experimental setup. The ultrasound probe frequency and power were set at 20 kHz and 0.5 W cm−2, respectively. (F) HIU-triggered transient performance of FBI-TENG and stable electrical output generation at lower probe power of 0.5 W cm−2. (G) The summation plots of (F). Photo credits: Dong-Min Lee, SKKU.
At first, we conducted long-term ex vivo experiments to investigate the FBI-TENG transient nature without any triggering events (fig. S20). We applied an ultrasound source of 0.5 W cm−2 on the FBI-TENG for only a few seconds to reduce the chances of device degradation. The FBI-TENG generated 544 mV of voltage output at the initial stage and remained implanted in the porcine tissue. During the repeated ex vivo experiments, we confirmed that the voltage output was between 400 and 500 mV for 5 days. Then, we observed a distinct decreasing trend in electrical output: a voltage output of 297 and 236 mV on days 7 and 14, respectively. The FBI-TENG was implanted in the porcine tissue for 21 days, and on the basis of our findings, its encapsulation layer was degraded, enough to provide merely trivial electric output performances. On the contrary, we adopted 3.0 W cm−2 of probe power, a higher intensity of ultrasound, as a triggering event for the transient processes (Fig. 4, F and G). Upon applying 0.5 W cm−2 of ultrasound probe power, the implanted FBI-TENG showed stable energy generation of voltage output ranging from 768 to 808 mV for 60 min. The sound pressure level was enhanced by adjusting the intensity to 3.0 W cm−2, and the electrical output was slightly increased to 896 mV because of improved incident energy. However, the voltage output was gradually diminished to a negligible output performance, indicating that the FBI-TENG lost its energy-generating ability.
DISCUSSION
On-demand transient electronics have attracted significant attention because of their efficiency and biodegradability while inside the body after a programmed lifetime. However, their use is limited due to their reliance on a commercial energy sources. In the present study, we described a FBI-TENG that uses ultrasound energy, a verified and noninvasive energy source for medical use, to generate electric power for transient electronics. To achieve an improved performance of energy generation at lower ultrasound intensity, we developed a PHBV/PEG (7:1) membrane with surface potential of 590 mV. Our FBI-TENG generated an electrical output of 4.61 V and 27.86 μA at 0.5 W cm−2 underwater. Our study demonstrated that the ultrasound can power the transient electronics and also regulate the transient process of FBI-TENG as a triggering event. At a higher ultrasound probe power, we evaluated FBI-TENG material disintegration. The porosity in a PHBV encapsulation layer enabled the localization of ultrasound-driven acoustic pressure. FEM simulation allowed us to display the distribution of sound pressure level for clear visibility, thereby promoting a clear comprehension of ultrasound-driven material disintegration. We found that the FBI-TENG did not generate energy under a high probe power (4.0 W cm−2) after 27 min. A porcine tissue, which is anatomically close to human tissue, was adopted to confirm our findings ex vivo. When exposed to 3.0 W cm−2 for 20 min, the FBI-TENG stopped generating electric power. Our ex vivo experiment findings demonstrate that our FBI-TENG can be easily triggered to initiate a transient process by tuning ultrasound power and therefore eliminating the need for surgical energy storage devices removal methods. We believe that our ultrasound-responsive transient FBI-TENG is a promising technology for powering transient electronics and, in addition, reducing surgical trauma and achieving better outcomes.
MATERIALS AND METHODS
Materials preparation
We commercially obtained PHBV (Goodfellow) and PEG powder (weight-average molecular weight, 20,000; Sigma-Aldrich). These polymer materials were mixed with the determined weight ratio and completely dissolved in chloroform solvent upon stirring it for 4 hours at 90°C to form 5 weight % solution. This solution was poured onto a glass substrate where a membrane was prepared by the solvent casting method. We left the membrane on the glass substrate for 6 hours at room temperature to evaporate the chloroform solution entirely.
Device fabrication
PHBV and PHBV/PEG membranes were peeled off from the glass substates after the complete evaporation of solvents. A PHBV membrane was used as the substrate for the FBI-TENG device. The Mg electrode with a thickness of 100 nm was directly deposited using an e-beam evaporator on the PHBV membrane. The PHBV/PEG composite membrane was stacked on the Mg-deposited PHBV substrate. Another PHBV membrane was placed on the top of the PHBV/PEG membrane as an encapsulation layer of the FBI-TENG. We carefully sealed all the edges of the FBI-TENG using a hot pressing method.
Materials characterization
We observed the surface morphology of our transient materials using field-effect scanning electron microscopy (Jeol Ltd., JSM-6701F). The material crystallinity and chemical structures were explored through XRD (Bruker D8 DISCOVER), Fourier transform infrared (Bruker IFS66/S, TENSOR27), and DSC (SEICO INST., DSC 7020) measurements. KPFM measurements were done using Park Systems XE-100 (NSC36, Cr/Au-coated silicon tips, tip radius of <35 nm, force constant of 1 N m−1, and resonance frequency of 90 kHz).
Electrical characterization
A commercially available ultrasound transducer and generator (Mirae MV100) was used to generate ultrasound in the water and on the porcine skin. The voltage outputs were measured using an oscilloscope (Tektronix DPO3052) with a voltage probe (Tektronix P5100A) of a 40-megaohm input impedance. The current outputs were measured using a low noise current amplifier (FEMTO, DLPCA-200). Triboelectric characterization of materials was done by a pushing tester (Z-Tech, ZPS-100) that applied mechanical forces regularly.
Acknowledgments
We thank others for any contributions.
Funding: This work was financially supported by Nano Material Technology Development Program (2020M3H4A1A03084600) and Basic Science Research Program (2021R1A2C2010990) through the National Research Foundation (NRF) of Korea grant.
Author contributions: Conceptualization: D.-M.L., N.R., and S.-W.K. Methodology: D.-M.L., N.R., I.H., W.K., and M.K. Investigation: D.-M.L., N.R., and I.H. FEM simulation: D.-M.L. and Y.-J.K. Visualization: D.-M.L. and N.R. Supervision: B.O.C. and S.-W.K. Writing—original draft: D.-M.L. and N.R. Writing—review and editing: D.-M.L, N.R., and S.-W.K.
Competing interests: D.-M.L., N.R., I.H., W.K., Y.-J.K., and S.-W.K. are inventors on a patent application (KR/10-2021-0078210) filed through the Sungkyunkwan University Research and Business Foundation that covers the management of ultrasonic intensity to control the biodegradable and implantable TENG. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The experimentally characterized data, graphical images, and simulation files (.mph) are deposited in the Dryad Digital Repository (https://datadryad.org/stash/share/4FjvqkJ59pIjPqgn75lnvZgVK3EOiBkcKTYr6CeLSLw) (34).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S24
REFERENCES AND NOTES
- 1.R.J. Narayan, “[Imaging biomaterial-associated inflammation]”, in Monitoring and Evaluation of Biomaterials and their Performance In Vivo (Woodhead Publishing, 2016). [Google Scholar]
- 2.Yu K. J., Kuzum D., Hwang S.-W., Kim B. H., Juul H., Kim N. H., Won S. M., Chiang K., Trumpis M., Richardson A. G., Cheng H., Fang H., Thompson M., Bink H., Talos D., Seo K. J., Lee H. N., Kang S.-K., Kim J.-H., Lee J. Y., Huang Y., Jensen F. E., Dichter M. A., Lucas T. H., Viventi J., Litt B., Rogers J. A., Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Zhao C., Wang X., Meng J., Zou Y., Noreen S., Zhao L., Liu Z., Ouyang H., Tan P., Yu M., Fan Y., Wang Z. L., Li Z., Fully Bioabsorbable capacitor as an energy storage unit for implantable medical electronics. Adv. Sci. 6, 1801625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu L., Yang Z., Meacham K., Cvetkovic C., Corbin E. A., Vazquez-Guardado A., Xue M., Yin L., Boroumand J., Pakeltis G., Sang T., Yu K. J., Chanda D., Bashir R., Gereau R. W. IV, Sheng X., Rogers J. A., Biodegradable monocrystalline silicon photovoltaic microcells as power supplies for transient biomedical implants. Adv. Energy Mater. 8, 1703035 (2018). [Google Scholar]
- 5.Han S., Jiao F., Khan Z. U., Edberg J., Fabiano S., Crispin X., Thermoelectric polymer aerogels for pressure–temperature sensing applications. Adv. Funct. Mater. 27, 1703549 (2017). [Google Scholar]
- 6.Piech D. K., Johnson B. C., Shen K., Ghanbari M. M., Li K. Y., Neely R. M., Kay J. E., Carmena J. M., Maharbiz M. M., Muller R., A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Dagdeviren C., Yang B. D., Su Y., Tran P. L., Joe P., Anderson E., Xia J., Doraiswamy V., Dehdashti B., Feng X., Lu B., Poston R., Khalpey Z., Ghaffari R., Huang Y., Slepian M. J., Rogers J. A., Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. U.S.A. 111, 1927–1932 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L., Lu G., Yang Y., Zeng Y., Sun Y., Li R., Humayun M. S., Chen Y., Zhou Q., Photoacoustic and piezo-ultrasound hybrid-induced energy transfer for 3D twining wireless multifunctional implants. Energ. Environ. Sci. 14, 1490–1505 (2021). [Google Scholar]
- 9.Fan F.-R., Tian Z.-Q., Wang Z. L., Flexible triboelectric generator. Nano Energy 1, 328–334 (2012). [Google Scholar]
- 10.Zhang S., Bick M., Xiao X., Chen G., Nashalian A., Chen J., Leveraging triboelectric nanogenerators for bioengineering. Matter 4, 845–887 (2021). [Google Scholar]
- 11.Choi Y. S., Kim S.-W., Kar-Narayan S., Materials-related strategies for highly efficient triboelectric energy generators. Adv. Energy Mater. 11, 2003802 (2021). [Google Scholar]
- 12.Chao S., Ouyang H., Jiang D., Fan Y., Li Z., Triboelectric nanogenerator based on degradable materials. EcoMat 3, e12072 (2021). [Google Scholar]
- 13.Pan R., Xuan W., Chen J., Dong S., Jin H., Wang X., Li H., Luo J., Fully biodegradable triboelectric nanogenerators based on electrospun polylactic acid and nanostructured gelatin films. Nano Energy 45, 193–202 (2018). [Google Scholar]
- 14.Suresh L., Vaghasiya J. V., Jones M. R., Tan S. C., Biodegradable protein-based photoelectrochemical cells with biopolymer composite electrodes that enable recovery of valuable metals. ACS Sustain. Chem. Eng. 7, 8834–8841 (2019). [Google Scholar]
- 15.Lee G., Choi Y. S., Yoon H.-J., Rogers J. A., Advances in physicochemically stimuli-responsive materials for on-demand transient electronic systems. Matter 3, 1031–1052 (2020). [Google Scholar]
- 16.Wu C., Jiang J., Guo H., Pu X., Liu L., Ding W., Kohl P. A., Wang Z. L., Sunlight-triggerable transient energy harvester and sensors based on triboelectric nanogenerator using acid-sensitive poly(phthalaldehyde). Adv. Electron. Mater. 5, 1900725 (2019). [Google Scholar]
- 17.Wang R., Gao S., Yang Z., Li Y., Chen W., Wu B., Wu W., Engineered and laser-processed chitosan biopolymers for sustainable and biodegradable triboelectric power generation. Adv. Mater. 30, 1706267 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Feng H., Zheng Q., Li H., Zhao C., Ouyang H., Noreen S., Yu M., Su F., Liu R., Li L., Wang Z. L., Li Z., Photothermally tunable biodegradation of implantable triboelectric nanogenerators for tissue repairing. Nano Energy 54, 390–399 (2018). [Google Scholar]
- 19.Ziskin M. C., Fundamental physics of ultrasound and its propagation in tissue. Radiographics 13, 705–709 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Barnett S. B., Ter Haar G. R., Ziskin M. C., Rott H.-D., Duck F. A., Maeda K., International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med. Biol. 26, 355–366 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Winterhalter M., Bürner H., Marzinka S., Benz R., Kaianowicz J. J., Interaction of poly(ethylene-glycols) with air-water interfaces and lipid monolayers: Investigations on surface pressure and surface potential. Biophys. J. 69, 1372–1381 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J., Ryu H., Lee J. H., Khan U., Kwak S. S., Yoon H.-J., Kim S.-W., High permittivity CaCu3Ti4O12 Particle-Induced internal polarization amplification for high performance triboelectric nanogenerators. Adv. Energy Mater. 10, 1903524 (2020). [Google Scholar]
- 23.Hwang S.-W., Tao H., Kim D.-H., Cheng H., Song J.-K., Rill E., Brenckle M. A., Panilaitis B., Won S. M., Kim Y.-S., Song Y. M., Yu K. J., Ameen A., Li R., Su Y., Yang M., Kaplan D. L., Zakin M. R., Slepian M. J., Huang Y., Omenetto F. G., Rogers J. A., A physically transient form of silicon electronics. Science 337, 1640–1644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M. H., Lee J., Jung S.-K., Kang D., Park M. S., Cha G. D., Cho K. W., Song J.-H., Moon S., Yun Y. S., Kim S. J., Lim Y. W., Kim D.-H., Kang K., A biodegradable secondary battery and its biodegradation mechanism for eco-friendly energy-storage systems. Adv. Mater. 33, 2004902 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Li X., Liu K. L., Wang M., Wong S. Y., Tjiu W. C., He C. B., Goh S. H., Li J., Improving hydrophilicity, mechanical properties and biocompatibility of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide). Acta Biomater. 5, 2002–2012 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Yoon H.-J., Lee D.-M., Kim Y.-J., Jeon S., Jung J.-H., Kwak S. S., Kim J., Kim S., Kim Y., Kim S.-W., Mechanoreceptor-inspired dynamic mechanical stimuli perception based on switchable ionic polarization. Adv. Funct. Mater. 31, 2100649 (2021). [Google Scholar]
- 27.Kim J., Lee J. H., Ryu H., Lee J.-H., Khan U., Kim H., Kwak S. S., Kim S.-W., High-performance piezoelectric, pyroelectric, and triboelectric nanogenerators based on P(VDF-TrFE) with controlled crystallinity and dipole alignment. Adv. Funct. Mater. 27, 1700702 (2017). [Google Scholar]
- 28.Hinchet R., Yoon H.-J., Ryu H., Kim M.-K., Choi E.-K., Kim D.-S., Kim S.-W., Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Y.-H. Kim, [Vibrations and Waves], in Sound Propagation: An Impedance Based Approach (Wiley, 2010). [Google Scholar]
- 30.Amaro L., Correia D. M., Martins P. M., Botelho G., Carabineiro S. A. C., Ribeiro C., Lanceros-Mendez S., Morphology dependence degradation of electro- and magnetoactive poly(3-hydroxybutyrate-co-hydroxyvalerate) for tissue engineering applications. Polymers 12, 953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H., Murakami R., Padermshoke A., Hirose F., Senda K., Noda I., Ozaki Y., Infrared spectroscopy studies of CH∙∙∙O hydrogen bondings and thermal behavior of biodegradable poly(hydroxyalkanoate). Macromolecules 37, 7203–7213 (2004). [Google Scholar]
- 32.Ulbricht J., Jordan R., Luxenhofer R., On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 35, 4848–4861 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Yoon H.-J., Kim S.-W., Nanogenerators to power implantable medical systems. Joule 4, 1398–1407 (2020). [Google Scholar]
- 34.D.-M. Lee, N. Rubab, I. Hyun, W. Kang, Y.-J. Kim, M. Kang, B.O. Choi, S.-W. Kim, Data for “Ultrasound-mediated triboelectric nanogenerator for powering on-demand transient electronics” (Dryad, 2021); https://datadryad.org/stash/share/4FjvqkJ59pIjPqgn75lnvZgVK3EOiBkcKTYr6CeLSLw. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S24