Abstract
Background
Sickle cell disease includes a group of inherited haemoglobinopathies affecting multiple organs including the eyes. Some people with the disease develop ocular manifestations due to vaso‐occlusion. Vision‐threatening complications of sickle cell disease are mainly due to proliferative sickle retinopathy which is characterized by proliferation of new blood vessels. Laser photocoagulation is widely applicable in proliferative retinopathies such as proliferative sickle retinopathy and proliferative diabetic retinopathy. It is important to evaluate the efficacy and safety of laser photocoagulation in the treatment of proliferative sickle retinopathy to prevent sight‐threatening complications.
Objectives
To evaluate the effectiveness of various techniques of laser photocoagulation therapy in sickle cell disease‐related retinopathy.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. Date of last search: 21 September 2015.
We also searched the following resources (24 March 2015): Latin American and Carribean Health Science Literature Database (LILACS); WHO International Clinical Trials Registry Platforms (ICTRP); and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials comparing laser photocoagulation to no treatment in children and adults.
Data collection and analysis
Two authors independently assessed trial eligibility, the risk of bias of the included trials and extracted and analysed data. We contacted the trial authors for additional information.
Main results
Two trials (341 eyes of 238 children and adults) were included comparing efficacy and safety of laser photocoagulation to no therapy in people with proliferative sickle retinopathy. There were 121 males and 117 females with an age range from 13 to 67 years. The laser photocoagulation technique used was different in the two trials; one single‐centre trial employed sectoral scatter laser photocoagulation using an argon laser; and the second, two‐centre trial, employed feeder vessel coagulation using argon laser in one centre and xenon arc in the second centre. The follow‐up period ranged from a mean of 21 to 32 months in one trial and 42 to 47 months in the second. Both trials were at risk of selection bias (random sequence generation) because of the randomisation method employed for participants with bilateral disease. One study was considered to be at risk of reporting bias.
Using sectoral scatter laser photocoagulation, one trial (174 eyes) reported that complete regression of proliferative sickle retinopathy was seen in 30.2% in the laser group and 22.4% in the control group (no difference between groups). The same trial reported the development of new proliferative sickle retinopathy in 34.3% of laser‐treated eyes and in 41.3% of eyes given no treatment; again, there was no difference between treatment groups. The second trial, using feeder vessel coagulation, did not present full data for either treatment group for these outcomes.
There was evidence from both trials (341 eyes) that laser photocoagulation using scatter laser or feeder vessel coagulation may prevent the loss of vision in eyes with proliferative sickle retinopathy (at median follow up of 21 to 47 months). Data from both trials indicated that laser treatment prevented the occurrence of vitreous haemorrhage with both argon and xenon laser; with the protective effect being greater with feeder vessel laser treatment compared to scatter photocoagulation.
Regarding adverse effects, the incidence of retinal tear was minimal, with only one event reported. Combined data from both trials were available for 341 eyes; there was no difference between the laser and control arms for retinal detachment. In relation to choroidal neovascularization, treatment with xenon arc was found to be associated with a significantly higher risk, but visual loss related to this complication is uncommon with long‐term follow up of three years or more.
Data regarding quality of life and other adverse effects were not reported in the included trials.
Authors' conclusions
Our conclusions are based on the data from two trials conducted over 20 years ago. In the absence of further evidence, laser treatment for sickle cell disease‐related retinopathy should be considered as a one of therapeutic options for preventing visual loss and vitreous haemorrhage. However, it does not appear to have a significant different effect on other clinical outcomes such as regression of proliferative sickle retinopathy and development of new ones. No evidence is available assessing efficacy in relation to patient‐important outcomes (such as quality of life or the loss of a driving licence). There is limited evidence on safety, overall, scatter argon laser photocoagulation is superior in terms of adverse effects, although feeder vessel coagulation has a better effect in preventing vitreous haemorrhage. Further research is needed to examine the safety of laser treatment compared to other interventions such as intravitreal injection of anti‐vascular endothelial growth factors. In addition, patient‐important outcomes as well as cost‐effectiveness should be addressed.
Plain language summary
Laser therapy for retinopathy in sickle cell disease
Review question To evaluate the effectiveness of various techniques of laser photocoagulation in sickle cell disease‐related proliferative retinopathy (development of sight‐threatening complications due to excessive growth of blood vessels in the back of the eye).
Background Sickle cell disease is a genetic disorder affecting many organs including eyes. The back of the eye (retina) can develop problems due to sickle cell disease. A certain number of people with sickle cell disease develop sight‐threatening complications due to excessive blood vessel growth in the retina which is known as proliferative sickle retinopathy. Laser therapy is used to control the growth of new blood vessels in affected eyes. There are different types and techniques of laser used in treatment. However, it is not known whether these various laser treatments offer advantages compared to no treatment or other interventions with regards to effectiveness and safety.
Search date The evidence is current to: 21 September 2015.
Study characteristics We included two randomised trials with 341 eyes of 238 participants comparing laser treatment to no intervention. There were 121 males and 117 females with an age range from 13 to 67 years. The trials employed different types of laser treatment. One trial employed scatter laser treatment in which lasers were applied to the retina near the new blood vessels using argon laser. Another employed feeder vessel laser coagulation in which lasers were applied directly to feeding blood vessels using xenon arc as well as argon laser. Participants were followed up for an average of 21 to 47 months.
Key results There is mixed evidence on the benefits of using laser therapy in people with retinopathy related to sickle cell disease. For instance, the effect of laser therapy on stopping the progression of new blood vessels and the development of new lesions did not differ greatly between the groups. However, there is evidence that laser therapy may prevent loss of vision and sight‐threatening complications. Patient‐important outcome data, such as quality of life, were not reported.
The safety of laser treatment is acceptable, particularly scatter laser treatment using an argon laser. Although xenon arc lasers are associated with a higher number of complications, a loss of vision is not common. However, given that there are few trials with relatively low quality evidence, results should be treated with caution. Further research is needed to examine the safety of laser treatment compared to other interventions. In addition, patient‐important outcomes (such as quality of life and loss of driving licence) as well as cost‐effectiveness should be addressed.
Quality of the evidence Both trials were at risk of bias due to the way participants were selected for groups (especially since treatment may be required for both eyes). One study was considered to be at risk of reporting bias as some results were only presented for one of the two treatment groups.
Background
See: appendices for glossary (Appendix 1).
Description of the condition
Sickle cell disease (SCD) is common genetic disorder affecting millions of people worldwide. It is characterised by the presence of haemoglobin S in which the glutamic acid in position 6 of the β chain of adult haemoglobin is replaced by valine. It is most endemic in tropical regions, mainly sub‐Saharan Africa, India and the Middle East (Weatherall 2001). It has become a global issue due to the migration of population from these areas to Europe and other parts of the world, particularly over the last few decades (Roberts 2007). Sickle cell disease includes homozygous sickle cell diseases, also known as sickle cell anaemia (Hb SS), sickle cell‐haemoglobin C disease (Hb SC), sickle cell‐β thalassaemia (Sβ0 Thal and Sβ+ Thal) and other less prevalent double heterozygous conditions (Serjeant 2001). It is a systemic disease that affects almost all the organs and leads to neurological, cardiac, pulmonary, hepatic, renal, ophthalmic, musculoskeletal and dermatological manifestations (Ballas 2010).
The main pathophysiology associated with ophthalmic manifestations in SCD is vaso‐occlusion that occurs in any vascular bed of ocular structures including conjunctiva, anterior segment, choroid, retina and optic nerve with potential visual impairment (Emerson 2005). Sight‐threatening problems in SCD are mainly due to proliferative sickle retinopathy (PSR), which is secondary to occlusion of the peripheral retinal vasculature, which in turn leads to retinal ischaemia and proliferation of new blood vessels with characteristic sea fans appearance. The incidence of PSR is more common in Hb SC disease and sickle cell‐β+ thalassaemia, being approximately 33% and 14% respectively, compared to 3% in Hb SS (Lutty 1994). The incidence of PSR increases with age, it is relatively common between 15 and 29 years of age (Condon 1972), but there were reported studies in which PSR was detected in children as young as seven to 13 years (Abiose 1978; Condon 1974a; Erachulu 2006). The peak prevalence of PSR in people with Hb SS occurs between 25 and 39 years in both men and women, whereas in the Hb SC genotype it occurs earlier, from 15 to 24 years in men and 20 to 39 years in women (Elagouz 2010).
Goldberg developed a classification of PSR according to the severity of fundus changes (Table 1) (Goldberg 1971). Subsequently, researchers from a Jamaican sickle cohort study proposed a new classification for early peripheral retinal vascular changes in SCD, based on fundus fluorescein angiographic changes (Table 2) (Penman 1994).
1. Clinical classification of PSR.
| Stage | Staging of proliferative sickle retinopathy (PSR) |
| Stage I | Peripheral arteriolar occlusion |
| Stage II | Vascular remodelling, formation of arteriovenous anastomoses |
| Stage III | Peripheral retinal neovascularization |
| Stage IV | Vitreous haemorrhage |
| Stage V | Retinal detachment |
PSR: proliferative sickle retinopathy
2. Angiographic classification of PSR.
| Types | Angiographic appearances of peripheral retinal capillary beds |
| Type 1 | Qualitatively similar to normal, may be displace posteriorly with loss of capillary beds. |
| Type 2 ‐ Type 2A ‐ Type 2B |
Qualitatively abnormal, with abrupt termination of small or medium‐sized vessels. ‐ presence of unstable border with capillary buds or stumps extending into non perfused retina. ‐ absence of capillary buds or stumps. |
| Type 3 | Indeterminate because recent acute arteriolar occlusion involving the vascular border gave rise to a type II pattern that reverted to normal following subsequent reperfusion of the vascular bed. |
PSR ‐ Proliferative sickle retinopathy
Early stages of PSR (stage I and II) may not need any intervention, as these early changes are asymptomatic or may even resolve due to auto‐infarction. Spontaneous regression is seen in 32% of eyes with PSR without any blinding complications (Downes 2005). Regression of PSR is more common in the eyes of people with Hb SS disease, seen in 40% compared to 20% of Hb SC; and complete non‐perfusion of PSR lesion is observed in 20% of SS and 7% of Hb SC (Fox 1991). Although permanent visual loss is rare, incidence of visual loss among people with Hb SS and Hb SC has been reported as 31 per 1000 eyes affected by PSR compared to 1.4 per 1000 eyes without PSR over a mean follow‐up period of 6.9 years (Moriaty 1988). Visual loss in PSR is commonly due to vitreous haemorrhage (stage IV) and tractional retinal detachment (stage V) (Moriaty 1988) and affects relatively younger people, indicating that early detection with timely effective treatment of stage III PSR is necessary to prevent such visual loss.
Description of the intervention
Various treatment options, such as diathermy, cryotherapy and transpupillary or transscleral diode laser photocoagulation, have been proven to be effective treatments of PSR (Condon 1974b; Goldbaum 1979; Seiberth 2001). Transpupillary laser photocoagulation is the safest and the preferred method among the available techniques, as cryotherapy is associated with adverse effects like retinal detachment (Goldbaum 1979). Transscleral diode laser photocoagulation is considered as an alternative in cases only when transpupillary laser coagulation is not applicable due to media opacities (Seiberth 2001).
Given the favourable chances of spontaneous regression, indication for the treatment of PSR varies among clinicians. Treatment is usually indicated in cases with peripheral neovascularization of more than 60° of circumference. This is particularly the case in eyes with bilateral involvement, spontaneous vitreous haemorrhage, large and elevated sea fans, rapid progression of new blood vessels, or precious eye in which the fellow eye has been lost due to PSR (Emerson 2006). The aim of treatment is to induce regression in stage III PSR prior to complications to prevent visual loss (Goldberg 1983). The different types of laser mainly used to achieve these goals are white xenon arc or blue/green argon.
The specific methods of laser in PSR include feeder vessel coagulation and scatter laser coagulation, either localized or 360° peripheral scatter coagulation (Ballas 2012). Scatter laser photocoagulation is considered to be the preferred method for PSR due to low rate of complications (Castro 1999). There are two types of scatter laser photocoagulation, the first being sectoral or localised and the second being 360° or circumferential laser treatment. In sectoral ablation, laser burns are applied only to the localised area around new blood vessels whereas in circumferential or 360° scatter laser, burns are applied circumferentially to entire peripheral retina (Cruess 1983; Kimmel 1986). The latter is usually indicated in those who are non‐compliant (Ballas 2012). Laser therapy is most effective when peripheral lesions are diagnosed early before involving the central retina (Castro 1999).
How the intervention might work
Laser photocoagulation has been considered safe as well as effective in the treatment of PSR, as it maintains quality of life and preserves the vision by preventing vision‐threatening complications in affected population (Goldbaum 1979; Goldberg 1983).
The mechanism of laser treatment in feeder vessel coagulation is to occlude the feeding vessels by applying direct, heavy laser burns to feeding arterioles leading to closure of neovascular fronds. Ocular media should be clear enough over the feeder vessels for successful photocoagulation (Goldbaum 1979). Both xenon arc and argon laser photocoagulation are used for feeder vessel coagulation; however, currently argon is more commonly used by clinicians as xenon has a higher complication rate compared to argon (Emerson 2005). Scatter laser coagulation has an indirect effect, as it destroys the ischemic retina responsible for production of vascular endothelial growth factor (VEGF) that triggers the proliferation of new blood vessels (Ballas 2012). This technique is primarily used to treat proliferative diabetic retinopathy. The fact that laser photocoagulation to ischaemic retina results in regression of new blood vessels in eyes with proliferative diabetic retinopathy has led to this technique being adapted for treatment of PSR. To achieve this goal, blue/green argon laser burns are applied to the retina with laser setting of 500 micrometre (µm) spot size and 0.1 second duration.
Studies have demonstrated that laser treatment for PSR has been accepted for several decades (Cruess 1983; Kimmel 1986; Rednam 1982). Timely, successful treatment avoids the need for surgical interventions with their potential complications and morbidities (Cohen 1986; Goldberg 1983).
Why it is important to do this review
Proliferative sickle retinopathy is a leading cause of visual impairment in people with SCD. Cochrane reviews of randomised controlled trials have been published for prophylaxis and treatment in other organs affected by SCD (Hirst 2012; Marti‐Carvajal 2012), but none to date for ocular involvement. Cochrane reviews evaluating the effects of laser photocoagulation in other proliferative retinopathies, such as proliferative diabetic retinopathy and neovascular age‐related macular degeneration, have found that laser treatment has beneficial effects in preventing visual loss (Evans 2014; Virgili 2009). Despite the well‐known clinical applications of laser photocoagulation in PSR, it is imperative to identify the treatment effect in people with SCD, given the potentially complication of blinding due to PSR if treatment is delayed.
Even though laser photocoagulation in PSR is a relatively simple and safe treatment, there is a lack of summarised safety and efficacy data comparing this treatment to no treatment or to other treatment options in people with PSR. Furthermore, various techniques of laser photocoagulation have been practised among clinicians based on preference and facilities. It is therefore essential to perform a systematic review to evaluate the evidence for the effectiveness and potential adverse effects of different laser photocoagulation therapies in people with PSR for preventing visual loss and ocular morbidity.
Objectives
To evaluate the effectiveness of various techniques of laser photocoagulation therapy in SCD‐related proliferative retinopathy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We also planned to include quasi‐RCTs if there had been sufficient evidence that the intervention and control groups were similar at baseline.
Types of participants
Children and adults diagnosed with SCD and PSR, irrespective of phenotype, age, gender, race, ethnic origin and setting.
Types of interventions
All types of laser photocoagulation therapy to the retina compared to no intervention or to other forms of treatment such as cryotherapy or intravitreal anti‐VEGF injection.
Types of outcome measures
Primary outcomes
Regression of PSR (change in number and size (in clock hours or in degrees of retinal circumference) of new blood vessels)
Development of new PSR (proliferation of blood vessels at a new area after treatment)
Secondary outcomes
Quality of life (using any validated measures)
Change in visual loss associated with PSR (visual loss is defined by the deterioration of visual acuity of three lines (post hoc) or more with the Snellen chart)
Occurence of vitreous haemorrhage (post hoc)
-
Adverse effects, such as:
retinal breaks or tears;
retinal detachment;
retinal haemorrhage;
choroidal haemorrhage;
choroidal neovascularization.
We planned to tabulate all adverse effects related to laser photocoagulation for the treatment of PSR that are reported in the included studies.
Search methods for identification of studies
Electronic searches
We identified relevant studies from the Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND retinopathy.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Health Research Council Meetings; and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of last search of Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 21 September 2015.
We also searched the following resources: Latin American and Caribbean Health Science Literature Database (LILACS) (http://lilacs.bvsalud.org/en/); WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) and ClinicalTrials.gov (www.clinicaltrials.gov). We did not restrict the electronic searches for trials by date or language.
See: appendices for details of search strategy for LILACS (Appendix 2); ICTRP (Appendix 3); and ClinicalTrials.gov (Appendix 4).
Date of last search of LILACS, ICTRP, ClinicalTrials.gov: 24 March 2014.
Searching other resources
We also searched the reference lists of review articles for details regarding the relevant publication. We contacted laser manufacturers by email for information on ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (KTM, SS) independently assessed trial eligibility by screening the titles and abstracts of all RCTs identified during the search process. We contacted the trial author for missing information in the published studies and studies published in abstract form only. The same two authors independently reviewed full texts of all potentially relevant trials and assessed the eligibility according to the specific criteria for inclusion of studies. We tried to resolve any disagreements by discussion and we requested opinion of third review author (HN) if necessary. We recorded the excluded studies in the 'Characteristics of excluded studies' table in Review Manager 5 software with reasons for exclusion (RevMan 2014).
Data extraction and management
Two review authors (KTM, HN) independently extracted data from eligible trials using standard data collection forms for optimal reliability. We checked for any errors and inconsistencies. We tried to resolve any disagreements by discussion and consensus. We maintained a record regarding any disagreement related with the extracted data. One review author (KTM) entered data into Review Manager 5 (RevMan 2014) and a second review author (AWT) checked for any errors or discrepancies.
We extracted the following data.
Participants' characteristics: demographic data (age, sex, race); eligibility (inclusion and exclusion criteria); total number in comparison groups; sickle cell types (SS, SC, Sβ‐thalassemia); withdrawals or dropouts and losses to follow up with reasons.
Methods: trial design; time and duration of trial; randomisation; allocation concealment method, blinding of participants.
Characteristics of PSR: location in retinal quadrants (superotemporal, superonasal, inferotemporal, inferonasal); extent in number of clock hours or degree in circumference of the retina; surface (raised or flat).
Interventions: method of laser (feeder vessel coagulation, generalised scattered or sectoral scattered coagulation); types of laser (argon, xenon or other); laser setting (laser power or intensity, spot size, duration of laser photocoagulation; number of laser sessions.
Outcomes: outcomes mentioned above with time of assessment and length of follow up.
Although we planned to report our outcomes at up to one month, over one month to six month, over six months to one year and over one year in our protocol (Myint 2013), we were only able to report over one year, according to the what was available in the included trials.
Assessment of risk of bias in included studies
Two review authors (KT, SM) independently assessed the risk of bias in the included trials and followed the domain‐based evaluation according to the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We evaluated the following six domains as 'low risk'; 'unclear risk'; or 'high risk' of bias.
Random sequence generation
Concealment of allocation
Blinding of participants, personnel and outcome assessors
Incomplete outcome data
Selective outcome reporting
Other sources of bias
We evaluated the assessments and discussed any inconsistencies between the review authors in the interpretation of risk of bias. We resolved any disagreement by discussion with a third author (HN). We recorded results on the above six domains in the relevant risk of bias tables in Review Manager 5 (RevMan 2014).
Measures of treatment effect
In this version of the review, we have been unable to enter data into the 'Data and analyses' section, given the unit of analysis issue referred to below. For future updates, when possible, we plan to analyse extracted data using Review Manager 5 (RevMan 2014). Specifically, for future updates, if we identify new eligible trials, we will assess the treatment effect as follows:
for dichotomous data (regression of PSR, development of new PSR, changes in visual loss associated with PSR, occurrence of vitreous haemorrhage and adverse reactions) we will calculate the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome.
for continuous outcome data (quality of life), if the outcomes are measured by the same scale within the trials, we will use the mean difference (MD) and corresponding CIs. If different scales are used to measure the same outcome we will use the standardized mean difference (SMD) and corresponding 95% CIs.
Unit of analysis issues
We assessed the included trials to determine the unit of analysis reported, which may be the eye or the participant. The unit of analysis reported in both of the included trials was the eye (rather than the individual), making standard data analysis not possible given that these data were not independent; therefore we presented these results narratively.
Dealing with missing data
We requested any missing data from the original investigators of the included trials. For each selected trial, we assessed the number of dropouts, withdrawals or losses to follow up. The reasons for missing data were well documented in the included trials and we conducted the analysis based on participants with complete data. We contacted authors for any missing information.
Assessment of heterogeneity
We intended to use Chi² test and I² statistic to evaluate statistical heterogeneity between the trials. For future updates, when more trials are included, we will assess statistical heterogeneity between trials using the Chi² test. We will consider results to be statistically significant if the P value is less than 0.1. We will use the I² statistic to quantify heterogeneity and interpret the values of this as follows: 0% to 40% as not significant heterogeneity; 30% to 60% as moderate heterogeneity; 50% to 90% as substantial heterogeneity; 75% to 100% as considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We performed comprehensive searches including search of abstracts and contacting manufacturer of laser machines to minimise publication and reporting bias. Within the trials, we considered selective outcome reporting as part of the risk of bias assessment. We compared the 'Methods' section to the 'Results' section of full published paper to ensure that all the outcomes which were measured, were reported. We did not use funnel plots to assess publication bias as there were insufficient number of trials (i.e. less than 10) and we could only include two trials in this review.
Data synthesis
In both of the included trials, the main comparison was between laser photocoagulation and no intervention. We did not perform meta‐analysis for this review given the unit of analysis issue referred to above.
For future updates, if there are eligible trials we will perform meta‐analysis using fixed‐effect model for combining data if there is an absence of significant heterogeneity, both statistical and clinical, amongst included studies. We will use a random‐effects model if substantial or considerable heterogeneity is identified (I² value of 50% or more).
Subgroup analysis and investigation of heterogeneity
For future updates of the review, if statistically significant heterogeneity is identified for the primary outcomes, we plan to conduct subgroup analyses as follows:
different types of laser photocoagulation (argon, xenon);
different methods of laser photocoagulation (feeder vessel coagulation, sectoral scattered coagulation, circumferential scattered coagulation);
types of sickle cell disease (Hb SS, Hb SC disease, SβThal).
Sensitivity analysis
We planned to perform a sensitivity analysis to determine the robustness of results regarding the risk of bias. However, we were not able to do so since there were only two trials included in this initial version of the review.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
The searches of the registers and databases identified 13 references. We excluded four references and retrieved the full text of nine references. Seven references representing two trials assessing the effects of laser treatment versus no intervention were selected for inclusion in the review (Farber 1991; Jampol 1983). We did not identify RCTs reporting other interventions, such as cryotherapy and anti‐VEGF. Three trials with one reference each were added to the excluded studies section. Two references representing one trial are currently awaiting assessment (Sayag 2008). See an additional figure for details of the screening and selection process (Figure 1).
1.
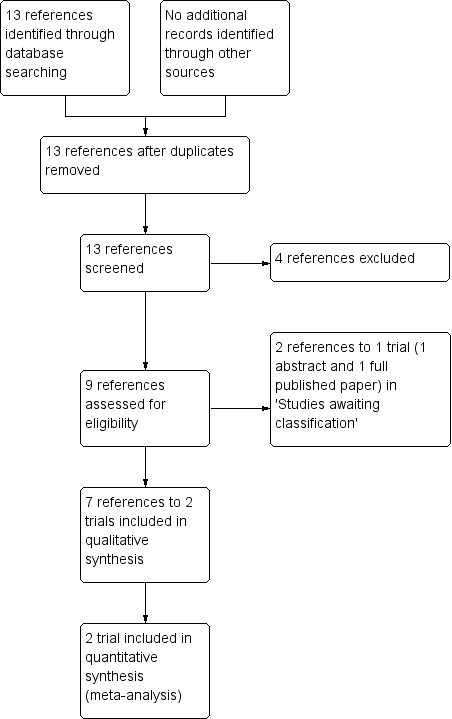
Study flow diagram.
Included studies
Methods and participants
Both trials used a parallel group design. One trial was conducted at single centre in Jamaica (Farber 1991), while the second trial was conducted at two centres, one in the USA (Chicago) and the other in Jamaica (Kingston) (Jampol 1983). Both trials were conducted by the same research group. The Farber trial was funded by a Comprehensive Sickle Cell Centre Grant from the National Heart, Lung, Blood Institute and National Eye Institute, National Institutes of Health, Bethesda, Maryland and by Research to Prevent Blindness Inc, New York, NY (Farber 1991). The Jampol trial was funded by a Comprehensive Sickle Cell Center grant from the National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, Maryland (Jampol 1983).
The trials included 238 participants with PSR (13 years to 67 years, 121 males and 117 females) but the unit of randomisation in both trials were the eyes. Farber randomised 174 eyes of 116 participants in Jamaica (Farber 1991); Jampol randomised a total of 167 eyes of 122 participants in Chicago and Kingston (Jampol 1983).
In both trials, participants with bilateral disease had their right eye randomised to either treatment or no treatment, with the other eye receiving the opposite modality. Participants with only one eye eligible for the trial were randomised to treatment or control for that eye. If the second eye became eligible later that eye received the opposite modality of treatment from the first eye.
In the Farber trial, there were 93 participants with Hb SC, 21 with Hb SS and two with Sβ Thal (Farber 1991); Jampol recruited people with Hb SS, Hb SC and Sβ Thal, but did not provide details on the proportions with each type (Jampol 1983). Both trials reported the extent of PSR in four groups according to circumferential extent of neovascularization; 1° to 30°, 31° to 60°, 61° to 90° and more than 91°.
Interventions
Both trials compared the effects of laser with no intervention. An additional table gives details of the laser treatment employed in the trials (Table 3). Farber employed sectoral scatter laser photocoagulation using argon laser in 99 eyes, with 75 eyes assigned into the control group (Farber 1991). Jampol employed feeder vessel coagulation using argon in Chicago, USA and xenon arc in Kingston, Jamaica (Jampol 1983). Among the 87 eyes assigned to the feeder vessel coagulation group, 34 eyes from the Chicago centre received argon laser photocoagulation, whereas the 53 eyes from the Kingston centre received xenon arc coagulation (Jampol 1983).
3. Characteristics of intervention and control in each study.
| Study ID | Intervention (Laser type) | Parameters | Control |
| Farber 1991 | Sectoral scatter laser photocoagulation by Argon blue/green (Britt 3250) in Kingston, Jamaica |
500 µm spot size, 0.1 second duration | Observation |
| Jampol 1983 | Feeder vessel photocoagulation Chicago ‐ Argon laser Kingston, Jamaica ‐ Xenon arc |
500 µm spot size, 0.2 second duration, 300‐600mW power. Intensity 5 ‐ 10, Size 3 ‐ 6 and 0.5 ‐ 2 second duration. |
Observation |
µm: micrometre
Outcomes
Farber reported data for this review's primary outcomes, 'regression of PSR' and 'development of new PSR' (Farber 1991). The time point reported in the trial was a mean follow up of 47 months for treated eyes and 42 months for the control eyes.
Jampol only reported data for 'regression of PSR' in the laser group (mean follow up of 21 months for Chicago and 32 months for Jamaica); data for the control group were not reported (Jampol 1983). We contacted the original investigators but data were not available. Over the long‐term follow‐up period of nine years, 29 participants from the Chicago centre in Jampol trial reported the development of new PSR.
Regarding the secondary outcomes for this review, both trials reported changes in visual loss associated with PSR, the incidence of vitreous haemorrhage, and the adverse event of retinal detachment (Farber 1991; Jampol 1983). Jampol reported the incidence of choroidal neovascularization (Jampol 1983).
The trials did not assess quality of life and some adverse effects like retinal haemorrhage or choroidal haemorrhage (Farber 1991; Jampol 1983).
We planned to report at one month, over one month to six months, over six months to one year and over one year. But in this review we reported outcome as mean and median duration as reported by original investigators.
Excluded studies
See: Characteristics of excluded studies.
A total of four trials which did not meet our inclusion criteria were excluded (Acheson 1991; Condon 1974; Lemaire 2013; Osuji 2003). None of the four trials were RCTs, one assessed the effect of scatter laser in iatrogenic choriovitreal neovascularization (CNV) (Acheson 1991), the second assessed the effect of photocoagulation and diathermy in eyes with PSR (Condon 1974), the third assessed the blood hyperviscosity in people with severe PSR (Lemaire 2013) and the last assessed the screening methods using panoramic fundus camera (Osuji 2003).
Studies awaiting classification
Two references representing one trial are awaiting classification (Sayag 2008). One reference is an abstract and one a full paper; however, the paper provided insufficient information to fully assess eligibility and the trial authors have been contacted for additional information.
Risk of bias in included studies
We assessed the risk of bias of the included trials according to the six domains outlined in the Assessment of risk of bias in included studies section of the review. Two figures demonstrate the overall assessment of risk of bias in the included trials (Figure 2; Figure 3).
2.
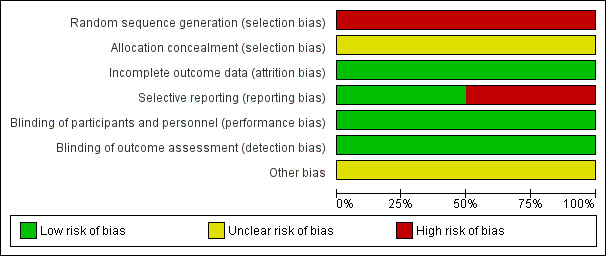
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
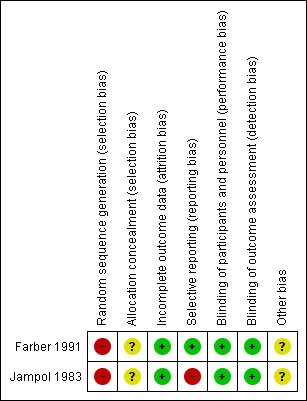
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Sequence generation
Both trials reported the use of computer‐generated randomisation; however, due to the randomisation method in participants with bilateral disease, we have assessed both of these trials as having a high risk of bias for this domain (Farber 1991; Jampol 1983).
Allocation concealment
Neither trial described the method of allocation concealment and we have therefore assessed these as having an unclear risk of bias (Farber 1991; Jampol 1983).
Blinding
Neither of the included trials mentioned blinding, but performance bias is unlikely with this type of intervention and the reported outcomes were objective outcomes which were not likely to be influenced by assessor bias. Therefore, we rated both trials as having a low risk of bias (Farber 1991; Jampol 1983).
Incomplete outcome data
The included trials had incomplete outcome data but adequately described the number and reason for withdrawals or dropouts and analysed all eyes as randomised (Farber 1991; Jampol 1983). Therefore, the risk of bias rating is assessed as low for both trials.
Farber reported "11 patients moved after average of 24 months (range 11 to 60 months) and one patient died after 15 months" (Farber 1991).
Jampol reported "Chicago: Two patients lost to follow‐up after 8 and 44 months and one refuse to cooperate. Kingston: eight patients lost to follow‐up due to emigration after average 12 months of follow‐up and two refuse to cooperate" (Jampol 1983).
Selective reporting
The trial protocols were not available (although not expected given the age of the trials) and we were not able to establish whether they had been prospectively registered in a publicly accessible database. The methods section of two included trials did not mention pre‐stated outcomes but the expected outcomes were reported in the results section of the Farber trial and therefore we considered as low risk of bias (Farber 1991). In Jampol trial, the outcome 'regression of new blood vessel' was reported only for the treatment group, not for the control group, we therefore assessed this trial as having a high risk of bias for this domain (Jampol 1983).
Other potential sources of bias
The trials were possibly under‐powered to be able to demonstrate non‐inferiority of laser photocoagulation for primary outcomes such as complete regression of PSR and development of new PSR with reasonable follow‐up duration of 32 to 47 months.
Effects of interventions
Given the unit of analysis issue referred to above, we were not able to perform meta‐analysis for this version of review. We have therefore presented the data in the additional tables (Table 4).
4. Effect of intervention.
| Outcome |
Farber 1991 (Randomised 174 eyes) Mean follow up of 42 to 47 months |
Jampol 1983 (Randomised 167 eyes) Mean follow up of 21 to 32 months |
|||
| Laser (Argon Scatter) | Control | Laser (Feeder vessel) | Control | ||
| 1 | Complete regression of PSR Partial egression of PSR |
30/99 (30.2%) 51/99 (51.0%) |
17/75 (22.4%) 18/75 (23.7%) |
78/87 (89.6%) | Not reported |
| 2 | Development of new PSR | 34/99 (34.3%) | 31/75 (41.3%) | Not reported | |
| 3 | Visual loss | 3/99 (3.0%) | 9/75 (8.3%) | 1/87 (1.14%) | 4/80 (5.0 %) |
| 4 | Occurrence of vitreous haemorrhage | 12/99 (12.0%) | 19/75 (25.3%) | 3/87 (3.4%) | 22/80 (27.5%) |
| 5 | Adverse effect: Retinal tear | Not reported | |||
| 6 | Adverse effect: Retinal detachment | 3/99 (3.0%) | 8/75 (10.6%) | 5/87 (5.74%) | No event |
| 7 | Adverse effect: Choroidal neovascularization | Not reported | 41/87 (47.12%) | No event | |
Unit of analysis is the eye.
PSR: proliferative sickle retinopathy.
Farber 1991: Mean follow up of 42 months for control group and 47 months for treatment group.
Jampol 1983: Mean follow up of 21 months for Chicago centre and 32 months for Kingston, Jamaica (overall participants).
Two trials (341 eyes of 238 participants (children and adults)) were included comparing efficacy and safety of laser photocoagulation to no therapy in participants with proliferative sickle retinopathy (Farber 1991; Jampol 1983).
Primary outcomes
1. Regression of PSR
Both trials reported on this outcome. Faber reported that, at mean follow up of 47 months (for treatment group) and 42 months (for control group), complete regression of PSR was seen in 30 out of 99 eyes (30.2%) in laser group and in 17 out of 75 eyes (22.4%) in the control group (Farber 1991). Partial regression is seen in 51 out of 99 eyes (51%) in the laser group and 18 out of 75 eyes (23.7%) in the control group. Data for long‐term follow up of participants with Hb SC disease from the Farber trial were presented in a separate paper to avoid the differences in behaviour between genotypes which reported that seven out of 74 (9.45%) treated eyes and two out of 60 (3.33%) control eyes had complete infarction of PSR over the median follow up of 2.9 years (P = 0.3) (Fox 1993).
Farber also reported that treated eyes had more regression than control eyes, but it was only significant in participants younger than 25 years at enrolment (P < 0.001), not in older participants (P = 0.6) and in small (less than 15°) PSR lesions as well as flat rather than elevated PSR (Fox 1993).
Jampol reported the regression of PSR only for the treatment group (Jampol 1983). At mean follow up of 26 (Chicago group) to 32 (Kingston group) months, using feeder vessel coagulation, 78 out of 87 eyes (89.6%) showed complete closure of neovascularization. The data for the control group were not provided and we contacted the trial author who was not able to provide relevant data due to the time elapsed since the trial was conducted.
2. Development of new PSR
Both trials reported on this outcome. Farber reported that new PSR developed in 34 out of 99 laser‐treated eyes (34.3%) and in 31 out of 75 eyes (41.3%) in the control group at mean follow up of 42 to 47 months (P = 0.3) (Farber 1991).
Jampol did not report this outcome in the initial publication, but did follow up the participants for mean period of nine years (range 5.75 to 12 years) in the Chicago centre and 29 participants (45 eyes with 25 eyes in laser group and 20 eyes in control group) completed this follow up (Jampol 1983). Development of new sea fan was reported in 12 out of 25 eyes (48.0%) in the treated group and nine out of 20 eyes (45.0%) in the control group. There was no statistical significance (P = 0.64).
Secondary outcomes
3. Quality of life
This outcome was not assessed in the included trials (Farber 1991; Jampol 1983).
4. Change in visual loss associated with PSR
Visual loss, defined as deterioration of visual acuity three lines or more from the Snellen chart, was reported in both trials.
Farber reported that, at a mean follow up of 42 and 47 months for the control and treatment group respectively, visual loss in seen in three out of 99 eyes (3.0%) treated by scatter laser photocoagulation compared to nine out of 75 eyes (12.0%) from control group (Farber 1991). Kaplan‐Meier survival analysis (two‐year survival curves for visual loss) showed a significant difference between the treated and control groups (P = 0.019).
Jampol reported that visual loss was seen in one out of 87 eyes (1.14%) treated by feeder vessel coagulation group and six out of 80 eyes (7.5%) in the control group at a mean follow up of 26 (in Chicago) to 32 months (in Kingston) (P = 0.07) (Jampol 1983).
5. Occurence of vitreous haemorrhage
Both trials reported this outcome. In the Farber trial, vitreous haemorrhage was detected in 12 out of 99 (12.0%) laser‐treated eyes and in 19 out of 75 (25.3%) control eyes in mean follow up of 42 (treatment) to 47 months (control) (Farber 1991). They performed analysis controlling vitreous haemorrhage and the amount of neovascularization at entry which had a significant difference between the treated and control eyes (P ≤ 0.5).
Jampol reported that three out of 87 (3.4%) eyes treated with feeder vessel coagulation, compared to 22 out of 80 (27.5%) controlled eyes, were complicated by vitreous haemorrhage at mean follow up of 26 (treatment) to 32 months (control). With long‐term mean follow up of nine years in the Chicago centre, vitreous haemorrhage occurred in one out of 25 eyes (4.0%) in the treatment group and nine out of 20 eyes (45.0%) in the control group (P = 0.002) (Jampol 1983).
6. Adverse effects
(a) Retinal tear
Farber did not report this adverse effect (Farber 1991). There was no report of retinal tear at initial follow up of the Jampol trial. With long‐term follow up of 5.75 to 12 years, it was reported that in one of the 25 eyes that completed the follow up (Chicago centre), retinal tear developed at the base of the treated sea fan in the feeder vessel coagulation group (Jampol 1983).
(b) Retinal detachment
Both trials reported retinal detachment (Farber 1991; Jampol 1983).
At mean follow up of 42 (treatment) to 47 months (control), retinal detachment was reported in three out of 99 (3.0%) eyes in the scatter laser group and eight out of 75 (10.6%) in the control group (Farber 1991).
At mean follow up of 26 (in Chicago) to 32 months (in Kingston), retinal detachment was seen in five out of 87 (5.74%) eyes treated by feeder vessel coagulation (confined to argon laser group) and not at all in the control group (Fisher's exact test, P = 0.07; Pearson's chi square, P = 0.03) (Jampol 1983).
(c) Choroidal neovascularization
Choroidal neovascularization, which is solely an adverse effect of laser therapy, particularly with xenon arc, was reported in the Jampol trial (Jampol 1983).
At follow up of 21 to 32 months, 41 out of 87 (47.12%) treated eyes were reported to have developed this adverse effect. It was seen in 38 out of 53 xenon‐treated eyes and three out of 34 argon lasered‐eyes. There was no such event in the control group (Jampol 1983). The incidence of choroidal neovascularization was significantly higher in the xenon group (P < 0.0001).
Other complications, such as retinal haemorrhage and choroidal haemorrhage, were not reported in either trial (Farber 1991; Jampol 1983).
Discussion
Summary of main results
Only two trials contributed data to this systematic review. One trial, involving 174 eyes with proliferative sickle retinopathy (PSR), compared the effect of scatter argon laser photocoagulation and no treatment over a follow up of 42 to 47 months; it revealed that there was a higher rate of regression of PSR in the laser group but no significant difference in complete regression (Farber 1991). Spontaneous regression was also noted without treatment. Treated eyes had more regression than control eyes but it was only significant in people younger than 25 years at enrolment, in small (less than 15°) PSR lesions as well as flat rather than elevated PSR (Fox 1993). Neither sectoral scatter argon laser photocoagulation or feeder vessel coagulation exhibited a significant protective effect against the development of new vessels (Farber 1991; Jampol 1983).
Neither of the trials reported on the outcome of quality of life.
Changes in visual loss measured by the deterioration of three lines or more from the Snellen chart was reported by both trials including 341 eyes (Farber 1991; Jampol 1983). Data from these studies suggested that laser photocoagulation probably prevented visual loss at mean follow up of over one year. It also reported that laser therapy had a protective effect for the occurrence of vitreous haemorrhage. Data from both trials indicated that laser treatment prevented the occurrence of vitreous haemorrhage with both argon and xenon laser; with the protective effect being greater with feeder vessel laser treatment compared to scatter photocoagulation (Farber 1991; Jampol 1983).
As for adverse effects, the incidence of retinal tear was very minimal with only one event reported from the long‐term follow up of the Jampol trial (Jampol 1983). Farber did not report this adverse effect (Farber 1991). Regarding retinal detachment, there was no statistical difference between the laser and the control arms in the Farber trial (Farber 1991); whereas borderline statistical significance was seen in the Jampol trial (Jampol 1983). As for choroidal neovascularization, xenon arc treatment was found to be associated with a significantly higher risk, but visual loss related to this complication is uncommon with long term‐follow up of three years or more (Jampol 1983).
The results of this review suggest that laser photocoagulation therapy for eyes with PSR may prevent visual loss and occurrence of vitreous haemorrhage. It also shows a positive effect in relation to the regression of PSR, but it may not be sufficient to prevent development of new lesions.
Overall completeness and applicability of evidence
We did not perform meta‐analyses because the units of analysis in the included trials were not independent (i.e. eyes, not participants). Moreover, different time points were used to present data and different methods of laser therapy were employed in the trials.
Few data contributed to the primary outcomes of this review, only one (174 eyes of 116 participants with PSR receiving either laser treatment or no treatment) out of two included trials assessed regression of PSR and development of new PSR (Farber 1991). A clinically meaningful difference for the complete regression of PSR and the development of new PSR, has not yet been demonstrated in this population. As mentioned above, different types and techniques of laser photocoagulation were used to induce the regression of new blood vessel in eyes with PSR (Background). The regression is largely dependent on the size, extent and surface of the PSR lesions, therefore, drawing any conclusion about this outcome would be difficult.
Two trials provided evidence on the effect of laser photocoagulation in preventing visual loss and vitreous haemorrhage in 341 eyes of 238 participants with PSR (Farber 1991; Jampol 1983). One trial reported no significant differences between lasered and control eyes for retinal detachment (Farber 1991); whereas a second trial reported borderline statistical significance for retinal detachment in the laser group treated by argon feeder vessel coagulation (Jampol 1983). Choroidal neovascularization is major complication of xenon arc and occurred in 80% of participants treated by xenon over the follow up of three years or more (Jampol 1983). It is also seen in eyes treated by argon but the incidence was significantly higher with xenon‐treated eyes. However, none of the trials reported quality of life and other adverse effects, such as retinal haemorrhage or choroidal haemorrhage.
Overall, the evidence is applicable to individuals presenting with stage III PSR. However, evidence is of limited quality, due to the fact that only two trials were included, and these were conducted several years ago.
Currently, argon laser scatter photocoagulation is commonly used in practice in most countries, whereas feeder vessel coagulation is of limited use due to a higher rate of complications. Feeder vessels coagulation by argon laser was associated with retinal detachment while xenon arc xenon arc is almost obsolete due to the higher rate of complications, particularly choroidal neovascularization.
Quality of the evidence
We found only two trials eligible for our review and those were conducted over 20 years ago when standards of reporting and trial conduct were not high. The trials were of open‐label, parallel design and aimed to demonstrate the effectiveness of laser therapy in preventing sight‐threatening complications in eyes with PSR. The overall risk of bias in the included trials was fairly unclear due to the inadequate reporting of methods and results of the included trials. Risk of bias related to sequence generation was high due to the randomisation method of participants with bilateral disease. The risk of bias related to incomplete outcome data was considered low in both trials. There is an unclear risk of bias in relation to allocation concealment and blinding, as none of the trials described these adequately. However, we judged both performance and detection bias to be low, given that the type of intervention and measurement of objective outcomes were unlikely to be influenced by blinding. One trial did not report the event data in the control group for the primary outcome 'regression of PSR' which we regard as a high risk of bias for selective reporting (Jampol 1983). We contacted the original investigators of this trial but were unable to retrieve the appropriate data which is likely due to the time length since trial completion. Although there is evidence that laser photocoagulation therapy is effective in preventing visual loss and vitreous haemorrhage, the sample size was small. It also induced regression of new blood vessels, but it is significant only in younger participants with small flat lesions. The trials were possibly under‐powered to be able to demonstrate non‐inferiority of laser photocoagulation for primary outcomes, such as complete regression of PSR and development of new PSR with reasonable follow‐up duration of 32 to 47 months.
Potential biases in the review process
Despite retinopathy being a sight‐threatening condition in a substantial population affected by sickle cell disease (SCD), there are few randomised controlled trials (RCTs) eligible for inclusion in our review. This may suggest that we were unable to identify and retrieve small trials, particularly those with inconclusive results, indicating the possibility of publication or retrieval bias. However, we believe this to be unlikely, given we searched multiple sources with no date or language restrictions. The small numbers of trials included in this review may introduce publication bias, but we were unable to perform funnel plot assessment for which more trials are needed. We were also unable to identify ongoing trials that met our inclusion criteria for SCD‐related retinopathy. A more credible explanation is that argon laser photocoagulation has become standard treatment for stage III PSR in many countries and RCTs, especially as a trial with no intervention or a placebo used as a control, may be unethical.
Agreements and disagreements with other studies or reviews
We are unaware of any similar reviews on this topic. Available evidence comes from small prospective non‐randomised studies (Cruess 1983; Kimmel 1986; Rednam 1982). One prospective non‐randomised study presented the effect of scatter circumferential argon laser photocoagulation in 40 eyes with PSR and showed that 26% of eyes were associated with complete regression; there were no complications reported related to treatment (Cruess 1983). Three years later, Kimmel reported the effect of laser treatment in 70 eyes of 44 participants having a total of 220 sea fans. At average follow up of 3.3 years, 33% of pre‐existing sea fans were associated with complete regression, 46% with partial regression, 19% with no change and 2% with progression. Vitreous haemorrhage developed in only one participant (2%) over the period of follow up (Kimmel 1986). In one small prospective study, Rednam investigated the effect of localised scatter photocoagulation in 21 eyes of 19 individuals with PSR lesions (Rednam 1982). They reported that flat sea fans responded dramatically with complete regression in 24 of 28 (85.7%) PSR lesions. Elevated sea fans responded less rapidly with complete regression seen only in four out of 17 (23.5%) lesions (Rednam 1982).
Authors' conclusions
Implications for practice.
In the absence of further evidence, laser treatment for SCD‐related retinopathy should be considered as a current therapeutic option for preventing visual loss and vitreous haemorrhage. However, it does not appear to have a significantly different effect on other clinical outcomes, such as the regression of existing PSRs and the development of new ones. Overall, scatter argon laser photocoagulation therapy is superior in terms of adverse effects, although feeder vessel coagulation has a better effect in preventing vitreous haemorrhage. We judged the evidence to be of low quality due to the method of sequence generation and the reporting of trials which were performed over 20 years ago, after which scatter argon laser photocoagulation has become the mainstay of treatment for stage III PSR to prevent blinding complications.
Implications for research.
Sickle cell disease‐related retinopathy has vision threatening complications in certain populations and scatter argon laser photocoagulation is standard practice for stage III PSR in many countries, following recommendations by Farber (Farber 1991). Under these circumstances, it is unethical to conduct randomised, placebo‐controlled trials to answer the efficacy and safety of laser treatment. Since intravitreal anti‐vascular endothelial growth factor (VEGF) is becoming well known to control the proliferation of new blood vessel in the treatment of various proliferative retinopathies, trials involving comparisons between laser and intravitreal injection of anti‐VEGF are needed to inform clinical decisions. There have been case reports of effectiveness of anti‐VEGF in treatment of PSR in recent decades (Shaikh 2008; Siqueira 2006). However, uncertainties in the progression and variation in PSR lesions in these individuals and problems with identifying stage III PSR may pose logistic problems in the implementation and interpretation of such trials.
Acknowledgements
We would like to acknowledge Miss Tracey Remmington, Managing Editor for Cochrane Cystic Fibrosis and Genetic Disorder Group (CFGD) for her assistance throughout the development of this review. We thank Mrs Natalie Hall, Trial Search Co‐ordinator for CFGD for running electronic searches. We are grateful to Editors of CFGD group and peer reviewers for improving quality of this review. We also like to express our gratitude to authorities of respective institutions and of Julius Centre University of Malaya, for their support and encouragement. Last but not the least, our thanks go to Professor Prathap Tharyan, Director of South Asian Cochrane Center and Network for his invaluable guidance for this review.
Appendices
Appendix 1. Glossary
| Term | Explanation |
| Anti‐ Vascular endothelial growth factor (Anti‐VEGF) | The drug that suppress or inhibit the effect of VEGF. |
| Cryotherapy | Cryotherapy, also called cryoablation is a minimally invasive treatment that uses extreme cold to freeze and destroy diseased tissue. During cryotherapy, liquid nitrogen or argon gas flows into a needle‐like applicator (a cryoprobe) creating intense cold that is placed in contact to diseased tissue. |
| Diathermy | Using high‐frequency electrical current to produce deep heating of tissue. |
| Intravitreal anti‐VEGF | Injection of anti‐VEGF into vitreous cavity. |
| Laser photocoagulation | Using laser light to treat certain disorders at the back of the eye. |
| New vessels | This term is used to signify the abnormal growth of vessels in the eye in response to a need for more oxygen. On the optic disc ‐ new vessels disc 'NVD', on the retina ‐ new vessels elsewhere 'NVE'. Generally, these new blood vessels do not have the normal integrity of blood vessel and tends to bleed. |
| Regression | New vessels stop growing or obliterated. |
| Retinal detachment | The retina has fallen away from its correct position at the back of the eye, which leads to a defect in the field of vision and ultimately loss of vision. |
| Retinopathy | Disease of the retina, for example, diabetic retinopathy is disease of the retina secondary to diabetes, sickle retinopathy is disease of retina secondary to sickle cell disease. |
| Vitrectomy | Surgical removal of the vitreous. |
| Vitreous | Soft gelatinous material that fills the back of the eye and sits behind the lens. |
| Vitreous haemorrhage | Bleeding into the vitreous cavity. |
| Vascular endothelial growth factor (VEGF) | A substance produced from retina particularly when the oxygen supply is insufficient. It causes growth of new blood vessels in the retina. |
Appendix 2. Search Strategy for LILACS
(tw:(sickle cell )) AND (tw:(haemoglobinopathies)) AND (tw:(retinopath*)) AND (tw:(laser OR photocoagulation))
Appendix 3. Search strategy for ICTRP
sickle cell OR haemoglobinopathies AND retinopathy AND laser or photocoagulation
Appendix 4. Search strategy for ClinicalTrials.gov
sickle cell OR haemoglobinopathies AND retinopathy AND laser OR photocoagulation
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farber 1991.
| Methods | RCT, single centre, parallel, open‐label design. | |
| Participants | 174 eyes of 116 participants. Males: 61 (52.6%); females: 55 (47.4%). Hb SC = 93 (80.2%), Hb SS = 21 (18.1%), Sβ thalassaemia = 2 (1.7%). Age: 16 to 60 years. |
|
| Interventions | Unit of allocation was the eye. Sectoral scatter laser photocoagulation (n = 99) using Argon blue‐green laser (Britt model 3250) through 3 mirror lenses. Spot size 500 µm, 0.1 second duration. Burns were placed approximately one burn diameter apart, placed from 1 disc diameter anterior and posterior to sea fan, 1 clock hour to each sides without treating sea fans or feeder vessels directly. Additional laser administered one week later if necessary. Control group: (n = 75) no laser photocoagulation. |
|
| Outcomes | 1. Regression of neovascularization. 2. Development of new sea fan. 3. Visual acuity decreased by 3 lines of the Snellen chart 4. Vitreous haemorrhage 5. Retinal detachment Trial period: February 1982 to January 1989, follow up; mean 47.4 months (5 ‐ 99 months), for treatment group, 42.4 months (5 ‐ 75 months) for control group. |
|
| Notes | This trial was funded by Comprehensive Sickle Cell Center grant and by training grant and core grant from National Eye Institute, National Institute of Health, Bethesda and by unrestricted grants from Research to Prevent Blindness Inc, New York, NY. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " patients with bilateral disease had their right eye randomized to either scatter photocoagulation or no treatment, with other eye receiving opposite modality". " ..we randomized three of every four eyes in unilateral cases to treatment group". "computer‐ generated randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "..174 eyes were included in the study (99 for treatment and 75 for control) and 11 patients moved after average of 24 months (range 11‐60 months) and one patient died after 15 months". Trial analysed all eyes as randomised although 11 participants moved out and 1 died. |
| Selective reporting (reporting bias) | Low risk | Trial did not clearly state the pre‐specified outcomes under the methods section, but all the expected outcomes were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned in the methods section, it is an open‐label trial but performance bias is unlikely with this type of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes reported were objective. |
| Other bias | Unclear risk | The trial was possibly under powered to be able demonstrate non‐inferiority of laser photocoagulation for the primary outcomes. |
Jampol 1983.
| Methods | Randomised, multicentre, parallel, open‐labelled design. | |
| Participants | 167 eyes of 122 participants with Hb SC, Hb SS and Sβ thalassaemia. Chicago: 64 eyes. Kingston: 103 eyes. Males = 60, females = 62. Age 13 to 67 years. |
|
| Interventions | Unit of allocation was the eye. Chicago Feeder vessel photocoagulation (n = 34) using Coherent Radiation Model 800 Argon laser through 3 mirror lens. 500 µm spot size, 0.2 second duration and 300‐800 mW power. Control group (n = 30): no photocoagulation. Kingston Feeder vessel photocoagulation (n = 53) using O'Malley Log 2 xenon arc photocoagulator with a direct ophthalmoscope delivery system without contact lens. intensity 5 ‐ 10, size 3 ‐ 6, duration 0.5 ‐ 2 seconds. Control group (n = 50): no photocoagulation. Between 1 to 5 laser sessions given in both centres. |
|
| Outcomes | 1. Complete closure of neovascularization 2. Visual acuity decreased by 3 lines of the Snellen chart 3. Vitreous haemorrhage 4. Retinal detachment 5. Choroidal neovascularization Chicago: trial period ‐ October 1977 to January 1982. Follow up ‐ mean 21.3 months (range 0 ‐ 52 months) Kingston: trial period ‐ April 1978 to September 1980. Follow up ‐ mean 32.0 months (range 0 ‐ 46 months) |
|
| Notes | Supported partly by Comprehensive Sickle Cell Center grant from National Heart, Lung and Blood institute and National Institute of Health, Bethesda, Maryland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " patient with unilateral neovascularization ... were randomly assigned (by computer randomisation) to either photocoagulation or the control group." "patient with bilateral neovascularization, right eye was randomized and left eye was given alternate modality". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Chicago: 2 participants were lost to follow up and 1 refused to co‐operate. Kingston: 8 participants were lost to follow up due to emigration and 2 refused to co‐operate. Trial analysed all eyes as randomised. |
| Selective reporting (reporting bias) | High risk | The trial did not clearly state the pre‐specified outcomes in methods section, and the outcome 'regression of new blood vessel' was reported only for the treatment group, not for the control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned in the methods section, it is an open‐labelled trial but performance bias is unlikely with an intervention of this nature. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes reported were objective. |
| Other bias | Unclear risk | The trial was possibly under‐powered to demonstrate non‐inferiority of laser photocoagulation for primary outcomes. |
RCT: randomised controlled trial Hb SC: sickle cell haemogloin C Hb SS: homozygous sickle cell disease µm: micrometre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acheson 1991 | Not an RCT or CCT . |
| Condon 1974 | Not an RCT or CCT. |
| Lemaire 2013 | Not an RCT or CCT. |
| Osuji 2003 | Not an RCTor CCT. |
CCT: controlled clinical trial RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Sayag 2008.
| Methods | "Consecutive series of patients presenting sickle cell disease were included in a prospective comparative clinical trial for argon laser photocoagulation." Described as randomised but no further information on the methods were reported. |
| Participants | 73 eyes of 101 participants. |
| Interventions | Peripheral focal scatter laser photocoagulation (n = 38) using argon green laser. Spot size 300 to 500 µm, 0.1 second duration with mild whitening of retina. Burns were placed around all sea fans without treating the sea fans or its feeder vessels directly, spaced approximately one burn diameter apart and usually placed from 2 disc diameters anterior to the sea fan and 2 clock hours to each of its sides. Control group = no intervention (n = 35) |
| Outcomes | 1. Disease progression defined by an increased size of existing lesion associated with leakage. 2. Disease regression defined by reduction of sea fan size, no perfusion or leakage on FFA. 3. Complications |
| Notes | In the 'Methods' section, it was stated that each participant was randomised to a treatment or no treatment group. However, no other information was provided, so we were not able to fully assess eligibility. |
FFA: fundus fluorescein angiography RCT: randomised controlled trial µm: micrometre
Differences between protocol and review
We changed definition of visual loss. In the protocol version of this review we defined visual loss as the deterioration of visual acuity two lines or more from the Snellen chart. However, trials report this outcome using a definition of visual loss as three lines or more (Farber 1991; Jampol 1983).
We removed the outcome 'Change in leakage in FFA (fundus fluorescein angiogram) after three months of treatment', we added 'Occurrence of vitreous haemorrhage' as a secondary outcome. The primary outcome of regression of PSR is basically assessed by reviewing FFA, hence we did not report this as a separate outcome. Both trials eligible for our review reported the outcome 'vitreous haemorrhage'. We considered this as important outcome since it is a complication of PSR itself and is associated with visual loss in the affected eye.
Contributions of authors
| Roles and responsibilities | Authors undertook the task |
| Protocol stage: draft the protocol | KTM, HN |
| Review stage: select which trials to include (2 + 1 arbiter) | KTM, SS |
| Review stage: extract data from trials (2 people) | KTM, HN |
| Review stage: enter data into RevMan | KTM, AWT |
| Review stage: carry out the analysis | AWT, SM |
| Review stage: interpret the analysis | KTM, AWT |
| Review stage: draft the final review | KTM, SM |
| Update stage: update the review | KTM, SS |
Sources of support
Internal sources
-
Melaka Manipal Medical College, Malaysia.
Salary and logistic support for SS, AWT and SM
-
SEGi University, Malaysia.
Salary and logistic support for KTM & HN
External sources
-
Julius Center, University of Malaya, Malaysia.
Cochrane workshop for review completion
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors: none known.
New
References
References to studies included in this review
Farber 1991 {published data only}
- Farber MD, Jampol LM, Fox P, Moriarty BJ, Acheson RW, Rabb MF, et al. A randomized clinical trial of scatter photocoagulation of proliferative sickle cell retinopathy. Archives of Ophthalmology 1991;109(3):363‐7. [] [DOI] [PubMed] [Google Scholar]
- Fox PD, Minninger K, Forshaw ML, Vessey SJ, Morris JS, Serjeant GR. Laser photocoagulation for proliferative retinopathy in sickle haemoglobin C disease. Eye (London, England) 1993;7(Pt 5):703‐6. [] [DOI] [PubMed] [Google Scholar]
- Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye (London, England) 1991;5:260‐3. [] [DOI] [PubMed] [Google Scholar]
Jampol 1983 {published data only}
- Condon P, Jampol LM, Farber MD, Rabb M, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. II. Update and analysis of risk factors. Ophthalmology 1984;91(12):1496‐8. [] [DOI] [PubMed] [Google Scholar]
- Jacobson MS, Gagliano DA, Cohen SB, Rabb MF, Jampol LM, Farber MD, et al. A randomized clinical trial of feeder vessel photocoagulation of sickle cell retinopathy. A long‐term follow‐up. Ophthalmology 1991;98(5):581‐5. [] [DOI] [PubMed] [Google Scholar]
- Jampol LM, Condon P, Farber M, Rabb M, Ford S, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. I. Preliminary results. Ophthalmology 1983;90(5):540‐5. [] [DOI] [PubMed] [Google Scholar]
- Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye (London, England) 1991;5:260‐3. [] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acheson 1991 {published data only}
- Acheson RW, Fox PD, Chuang EL, Serjeant GR. Treatment of iatrogenic choriovitreal neovascularisation in sickle cell disease. British Journal of Ophthalmology 1991;75(12):729‐30. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Condon 1974 {published data only}
- Condon PI, Serjeant GR. Photocoagulation in sickle cell retinopathy [abstract]. Commonwealth Caribbean Medical Research Council, 19th Scientific Meeting; 1974 April 26‐29; Mona, Jamaica. 1974:1‐2. []
Lemaire 2013 {published data only}
- Lemaire C, Lamarre Y, Lemonne M, Waltz X, Chahed S, Cabot F, et al. Severe proliferative retinopathy is associated with blood hyperviscosity in sickle cellhemoglobin‐C disease but not in sicklecell anemia. Clinical Hemorheology and Microcirculation 2013;55:205‐11. [DOI: 10.3233/CH-2012-1622] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osuji 2003 {published data only}
- Osuji NC, Majone B, Lumley H, Day A, O'Sullivan E, Alwis D. Screening for sickle retinopathy: a new, rapid and effective approach using the panoramic 200 panretinal camera [abstract]. British Journal of Haematology 2003;121 Suppl 1:43‐4. [] [Google Scholar]
References to studies awaiting assessment
Sayag 2008 {published data only}
- Sayag D, Binaghi M, Souied E, Pawlak D, Galacteros F, Coscas G, et al. Peripheral retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy: a prospective clinical trial with new sea fan classification. European Journal of Ophthalmology 2008;18(2):248‐54. [CENTRAL: 638176;CRS:5500050000000040; PUBMED: 18320518] [DOI] [PubMed] [Google Scholar]
- Sayag D, Binaghi M, Souied E, Pawlak D, Galacteros F, Coscas G, et al. Peripheral retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy: is it always indispensable?. IOVS 2004; Vol. 45:AVO E‐Abstract:5266. [CENTRAL: 598655;CRS: 5500050000000039]
Additional references
Abiose 1978
- Abiose A, Lesi FE. Ocular findings in children with homozygous sickle cell anaemia in Nigeria. Journal of Paediatric Ophthalmology and Strabismus 1978;15(2):92‐5. [DOI] [PubMed] [Google Scholar]
Ballas 2010
- Ballas SK, Leiff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, et al. Definitions of phenotypic manifestations of sickle cell disease. American Journal of Haematology 2010;85(1):6‐13. [DOI: 10.1002/ajh.21550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballas 2012
- Ballas SK, Kesen MR, Goldberg MF, Lutty GA, Dampier K, Osunkwo I, et al. Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. The Scientific World Journal http://www.hindawi.com/journals/tswj/2012/949535 (accessed 15 April 2013). [DOI: 10.1100/2012/949535] [DOI] [PMC free article] [PubMed]
Castro 1999
- Castro O. Management of sickle cell disease: recent advances and controversies. British Journal of Haematology 1999;107(1):2‐11. [DOI] [PubMed] [Google Scholar]
Cohen 1986
- Cohen SB, Fletcher ME, Goldberg MF, Jednock NJ. Diagnosis and management of ocular complications of sickle haemoglobinopathies: part IV. Ophthalmic Surgery 1986;17(5):312‐5. [PubMed] [Google Scholar]
Condon 1972
- Condon PI, Serjeant GR. Ocular findings in homozygous sickle cell anaemia in Jamaica. American Journal of Ophthalmology 1972;73(4):533‐4. [DOI] [PubMed] [Google Scholar]
Condon 1974a
- Condon PI, Gray R, Serjeant GR. Ocular findings in children with sickle cell haemoglobin C disease in Jamaica. British Journal of Ophthalmology 1974;58(7):644‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Condon 1974b
- Condon PI, Serjeant GR. Photocoagulation and diathermy in the treatment of proliferative sickle retinopathy. British Journal of Ophthalmology 1974;58(7):650‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruess 1983
- Cruess AF, Stephens RF, Magargal LE, Brown GC. Peripheral circumferential retinal scatter photocoagulation for treatment of proliferative sickle retinopathy. Ophthalmology 1983;90(3):272‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins JPT, Altman D (editors). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Downes 2005
- Downes SM, Hambleton IR, Chuang EL, Lois N, Serjeant GR, Bird AC. Incidence and natural history of proliferative sickle cell retinopathy: observation from a cohort study. Ophthalmology 2005;112(11):1869‐75. [DOI] [PubMed] [Google Scholar]
Elagouz 2010
- Elagouz M, Jyothi S, Gupta B, Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Survey of Ophthalmology 2010;55(4):359‐77. [DOI] [PubMed] [Google Scholar]
Emerson 2005
- Emerson GG, Lutty GA. Effects of sickle cell disease on eye: clinical features and treatment. Hematology/Oncology Clinics of North America 2005;19(5):957‐73. [DOI] [PubMed] [Google Scholar]
Emerson 2006
- Emerson GE, Harlan JB, Fekrat S, Lutty GA, Goldberg MF. Hemoglobinopathies. In: Ryan SJ editor(s). Retina. 4th Edition. Vol. 2, Edinburgh: Elsevier, 2006:1429‐45. [Google Scholar]
Erachulu 2006
- Erachulu UV, Pam VA, Akuse RM. Ocular findings in children with severe clinical symptoms of homozygous sickle cell anaemia in Kaduna, Nigeria. West African Journal of Medicine 2006;25(2):88‐91. [DOI] [PubMed] [Google Scholar]
Evans 2014
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 1991
- Fox PD, Vessey SJR, Forshaw ML, Serjearnt GR. Influence of genotype on the natural history of untreated proliferative sickle retinopathy. British Journal of Ophthalmology 1991;75(4):229‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 1993
- Fox PD, Minninger K, Forshaw ML, Vessey SJ, Morris JS, Serjeant GR. Laser photocoagulation for proliferative retinopathy in sickle haemoglobin C disease. Eye (London, England) 1993;7(Pt 5):703‐6. [] [DOI] [PubMed] [Google Scholar]
Goldbaum 1979
- Goldbaum MH, Fletcher RC, Jampol LM, Goldberg MF. Cryotherapy for proliferative sickle retinopathy,II: a triple freeze‐thaw cycle. British Journal of Ophthalmology 1979;63(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 1971
- Goldberg MF. Natural history of untreated proliferative sickle retinopathy. Archives of Ophthalmology 1971;85(4):428‐37. [DOI] [PubMed] [Google Scholar]
Goldberg 1983
- Goldberg MF, Jampol LM. Treatment of neovascularization, vitreous haemorrhage and retinal detachment in sickle cell retinopathy. Transactions of the New Orleans Academy of Ophthalmology 1983;31:58‐81. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins PT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Hirst 2012
- Hirst C, Owusu‐Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD003427.pub2] [DOI] [PubMed] [Google Scholar]
Kimmel 1986
- Kimmel AS, Magagal LE, Stephens RS, Cruess AF. Peripheral circumferential retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy. An update. Ophthalmology 1986;93(11):1429‐34. [DOI] [PubMed] [Google Scholar]
Lutty 1994
- Lutty GA, Goldberg MF. Ophthalmologic complications. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH editor(s). Sickle cell disease: Basic principle and clinical practice. New York: Raven Press, 1994:703‐24. [Google Scholar]
Marti‐Carvajal 2012
- Marti‐Carvajal AJ, Knight‐Madden JM, Martinez‐Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008394.pub2] [DOI] [PubMed] [Google Scholar]
Moriaty 1988
- Moriaty BJ, Acheson RW, Condon PI, Serjeant GR. Patterns of visual loss in untreated sickle cell retinopathy. Eye 1988;2(Pt 3):330‐5. [DOI] [PubMed] [Google Scholar]
Myint 2013
- Myint KT, Sahoo S, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penman 1994
- Penman AD, Talbot AF, Chuang EL, Thomas P, Serjeant GR, Bird AC. New classification of peripheral retinal vascular changes in sickle cell disease. Bnrtish Journal ofOphthalmology 1994;78:681‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rednam 1982
- Rednam KRV, Jampol IM, Goldberg MF. Scatter retinal photocoagulation for proliferative sickle cell retinopathy. American Journal of Ophthalmology 1982;93(5):594‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Roberts 2007
- Roberts I, Montalembert M. Sickle cell disease as a paradigm of immigration hematology: new challenges for haematologists in Europe. Haematologica 2007;92(07):865‐92. [DOI] [PubMed] [Google Scholar]
Seiberth 2001
- Seiberth V. Transscleral and transpupillary laser coagulation in proliferative sickle‐cell retinopathy. Ophthalmologe 2001;98(2):199‐202. [DOI] [PubMed] [Google Scholar]
Serjeant 2001
- Serjeant GR. The emerging understanding of sickle cell disease. British Journal of Haematology 2001;112(1):3‐18. [DOI] [PubMed] [Google Scholar]
Shaikh 2008
- Shaikh S. Intravitreal bevacizumab (Avastin) for the treatment of proliferative sickle retinopathy. Indian Journal of Ophthalmology 2008;56(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siqueira 2006
- Siqueira RC, Costa RA, Scott IU, Cintra LP, Jorge R. Intravitreal bevacizumab (Avastin) injection associated with regression of retinal neovascularization caused by sickle cell retinopathy. Acta Ophthalmologica Scandinavica 2006;84:834‐5. [DOI: 10.1111/j.1600-0420.2006.00779.x] [DOI] [PubMed] [Google Scholar]
Virgili 2009
- Virgili G, Bini A. Laser photocoagulation for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004763.pub2] [DOI] [PubMed] [Google Scholar]
Weatherall 2001
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problems. Bulletin of the World Health Organization 2001;79(8):1‐15. [PMC free article] [PubMed] [Google Scholar]


