Abstract
Several behavioral and physiological adaptations have been developed in evolution of Pinnipeds allowing them to sleep both on land and in water. To date sleep has been examined in detail in eared and true seals (the families of Otariidae and Phocidae). The aim of this study was to examine sleep in another semiaquatic mammal – the walrus, which is the only extant representative of the family Odobenidae. Slow wave and paradoxical sleep (SWS and PS) in the examined walrus (2 year old female, weight 130 kg) averaged 19.4 ± 2.0 and 6.9 ± 1.1% of 24-h when on land, and 20.5 ± 0.8% of 24-h and 1.1 ± 0.6% when in water, respectively. The average duration of PS episode was 6.4 ± 0.6 min (maximum 23 min) when on land and 1.8 ± 0.1 min (maximum 3.3 min) when in water. In water, sleep occurred predominantly while the walrus submerged and lay on the bottom of the pool (89% of total sleep time). The walrus usually woke up while emerging to the surface for breathing. Most often EEG slow waves developed synchronously in both cortical hemispheres (90% of SWS time when on land and 97% when in water). Short episodes of interhemispheric EEG asymmetry usually coincided with brief opening of one eye. The pattern of sleep in the walrus was similar to the pattern of sleep in the Otariidae seals while on land (predominantly bilateral SWS, accompanied by regular breathing) and to the pattern of sleep in the Phocidae while in water (sleep during apneas both in depth and at the surface, interrupted by brief arousal when emerging for breathing).
The pattern of sleep in aquatic (cetaceans) and semiaquatic (pinnipeds) mammals is substantially different from that in terrestrial mammals. Studies of sleep in cetaceans have led to the discovery of an unusual type of sleep called unihemispheric slow wave sleep (SWS). The essence of this phenomenon is that only one cortical hemisphere can be asleep while the other hemisphere is awake at any given time [1]. Moreover, all cetaceans can sleep while swimming and they don’t display paradoxical sleep (PS; [1–3]). Sleep in fur seals (the most extensively studied species of eared seals; the Otariidae family) when on land is very similar to sleep of terrestrial mammals. They display both sleep stages (SWS and PS) with slow waves developing in the electroencephalogram (EEG) in two cortical hemispheres most often synchronously. The breathing pattern of fur seals during SWS is regular. When in water, fur seals sleep on their sides in a characteristic posture holding their nostrils above the surface. They paddle with one front flipper to maintain the posture. The ratio of SWS with interhemispheric EEG asymmetry greatly increases when fur seals sleep in water, while the amount of REM sleep substantially decreases [4]. True seals (the Phocidae family) are motionless while asleep both on land and in water. As in terrestrial mammals, SWS in the Phocids always develops synchronously in both brain hemispheres. Breathing in sleeping true seals is interrupted: periods of regular breathing are alternated with long breathing pauses (BP) – apneas, exceeding 10 min [5–7]. When in water this pattern of breathing allows seals to sleep both at the surface and at depth while holding their breath. The objective of this study was to examine sleep in the walrus, another species of Pinnipeds, and the only extant representative of the family Odobenidae.
Experiments were conducted at the Utrish Marine Station of the Severtsov Institute of Ecology and Evolution RAS (Small Utrish, Krasnodar district). The subject was a subadult female walrus (age about 2 years, weight 130 kg). Under general anesthesia (isoflurane) she was implanted with electroencephalogram (EEG) electrodes positioned bilaterally in two cortical hemispheres. The walrus was also implanted with electrodes for electromyogram (EMG), electrooculogram (EOG) and electrocardiogram (ECG) recording. The animal was housed in a 5 × 5 m indoor pool and the two experimental conditions—“on land” (the walrus slept on the platform, positioned above the water) and “in water” (the walrus slept in water, the water level was 1.6 m and the platform was removed from the pool). When on land, polygrams (continuous records of EEG, EMG EOG and ECG) were recorded via an amplifier (Medicor, Hungary) and then digitally sampled and stored (CED, UK). The recording electrodes were connected to a polygraph via flexible cables. When in water, all parameters were recorded using a custom 8–channel portable (weight was 200 g) recorder of our design [8, 9], positioned on the walrus’s back in a harness. The walrus’s behavior was videotaped via several video cameras. The data collected during 5 days of recording while on land and 3 days of recording while in water were scored visually in 20-s epochs and synchronized with behavior. The EEG recorded from the symmetrical left and right cortical fronto-occipital derivations was visually scored as low–voltage desynchronization or low and high voltage slow wave activity (1.2–4.0 Hz). The EEG power in the range of slow waves (1.2–4.0 Hz) was calculated for the same epochs. After that, each epoch was scored as waking, SWS or PS. SWS was further subdivided into bilateral or asymmetrical SWS. Surgical procedures and data analysis have been described in detail in our previous publications (e.g., [4, 10]).
When on land, the walrus slept while lying on its belly. The majority of sleep occurred during the nighttime (20:00–08:00; on average 94% of total sleep time or 355 min per day). Over the 5 day recording period the walrus spent on average 6 h per day asleep (26% of 24 h). SWS comprised 3/4, and PS—1/4 of total sleep time (table). EEG slow waves developed usually synchronously in the cortical hemispheres (Fig. 1a). The EEG stages of two cortical hemispheres were similar at that time. This type of SWS (also called bilateral SWS) accounted for 85–96% (on average 90%) of total SWS time. Both eyes were typically closed during this state. Episodes of interhemispheric EEG asymmetry were occasionally recorded in the walrus. Those episodes were characterized by substantial differences in the amplitude of EEG slow waves and the EEG power between the two hemispheres. At these times, one walrus’s eye would briefly open while the other was closed. The closed eye was always contralateral to the hemisphere with higher-voltage EEG slow waves, and the open eye was opposite to the hemisphere with low-voltage slow waves (Fig. 2). As sleep progressed (as indicated by an increase in the amplitude of slow waves), the degree of EEG asymmetry gradually decreased and both eyes closed. PS followed SWS and was characterized by a decreased muscle tone, intense vibrissae and head jerks, and rapid eye movements. From 11 to 21 PS episodes per day were recorded in the walrus (table). The majority (90%) of the episodes were shorter than 13 min but several episodes lasted longer than 20 min. Therefore, when on land the pattern of sleep in the walrus did not differ from that in fur seals [4, 10].
Characteristic of sleep and wakefulness in a walrus
| Parameter | Sleep on land, N = 5 | Sleep in water, N = 3 |
|---|---|---|
| Total sleep time, % of 24-h | 26.3 ± 1.9 (21.9, 31.3) | 21.6 ± 1.4 (19.2, 23.9) |
| Slow wave sleep (SWS), % of 24-h | 19.4 ± 2.0 (15.5, 24.4) | 20.5 ± 0.8 (19.0, 21.8) |
| Paradoxical sleep (PS), % of 24-h | 6.9 ± 1.1 (5.5, 7.8) | 1.1 ± 0.6 (0.1, 2.1) |
| Duration of bilateral SWS, % total SWS | 90.2 ± 2.1 (85.6, 96.1) | 96.7 ± 0.9 (95.8, 98.5) |
| Number of PS episodes during the 24-h period | 15 ± 2 (11, 21) | 13 ± 4* (9, 16) |
| Duration of PS episodes, min** | 6.4 ± 0.6 (0.3, 22.7) | 1.8 ± 0.1 (0.7, 3.3) |
Note: The data presented as mean ± SEM. Minimal and maximal values are given in parentheses. N—number of recording sessions (days).
The mean values were calculated for the 2nd and 3rd recording days. Only one episode lasting 2.0 min was recorded on the first day.
The durations of PS episodes on land and in water were calculated for all recorded episodes—76 episodes when on land and 26 episodes when in water.
Fig. 1.
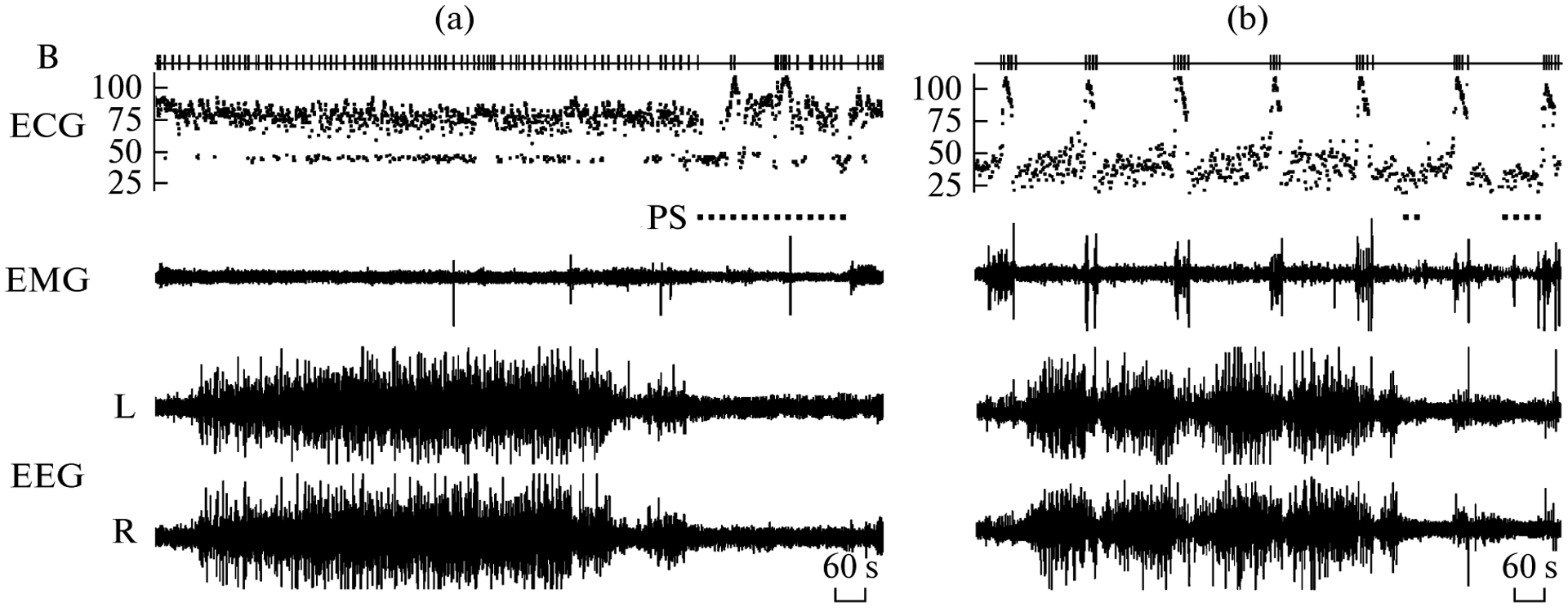
Polygrams of main behavioral states in a walrus while on land (a) and in water (b). EEG—electroencephalogram of the left (L) and right (R) hemispheres. EMG—electromyogram of neck muscles. ECG—instantaneous heart rate (beats per min). B – respiratory acts (breaths). PS—paradoxical sleep. During breath holdings (apneas) the walrus submerged and lay on the bottom of pool.
Fig. 2.
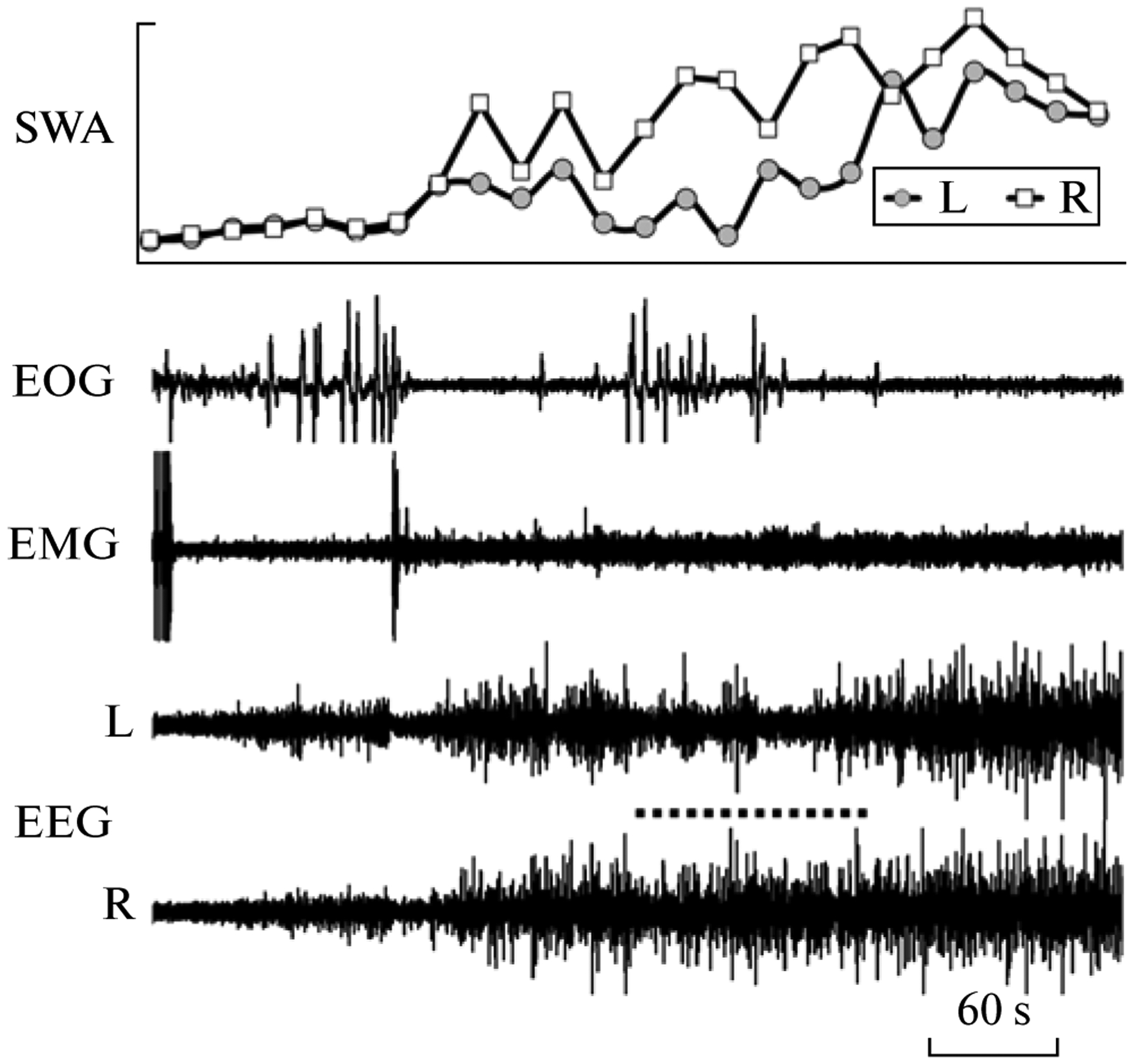
Interhemispheric EEG asymmetry during slow wave sleep in a walrus while on land. EEG—electroencephalogram of the left (L) and right (R) hemispheres. EMG—electromyogram of neck muscles. EOG—electrooculogram of the right eye. SWA—slow wave activity (EEG power in the range of 1.2–4.0 Hz calculated for 20 s epochs). The dotted line marks an episode of interhemispheric EEG asymmetry (a low voltage slow waves in the left hemisphere) coincided with brief opening (based on the EOG) of the right eye.
When on land the walrus’s breathing was regular during SWS. The majority (95%) of BP ranged between 10 and 30 s. During breath holding the instantaneous heart rate in the walrus decreased to 50 beats per min. Several seconds before inhalation, the heart rate accelerated to 70–80 beats per min. During PS breathing became irregular: apneas (BP longer than 60 s, maximum was 91 s) alternated with periods of regular breathing. The instantaneous heart rate during PS varied between 40 and 105 beats per min. In a prior study, we documented in walruses a similar breathing pattern during PS while scoring rest (SWS and quiet waking) and PS using behavioral criteria [11]. A similar pattern of breathing is characteristic of Otariidae seals when on land: in fur seals [4, 12], subadult Steller’s and southern sea lions [13].
Most sleep in the walrus in water also occurred during the nighttime (on average 90% of total sleep time or 280 min per day). Most often the animal slept while submerged, lying on the bottom of the pool (on average 89% of total sleep time or 280 min per day). Occasionally sleep occurred while the walrus was floating at the surface (7% or 22 min) or positioning at an angle to the surface and touching the bottom with it’s flippers (3% or 9 min). Several sleep periods (episodes of SWS, interrupted by short episodes of arousal) were recorded in the walruses over the 24-h. They lasted from 40 min to 6.5 h and alternated with periods of active swimming. During the 3 days of recordings SWS accounted for 95% of total sleep time (table, Fig. 1b). The amount of asymmetrical SWS in water decreased but the difference between the pattern of SWS when in water and land was at the level of significance (T-test, p = 0.06, followed the test for normality). The amount of PS in water decreased significantly, to almost one-sixth of that on land (p ⪡ 0.001). The total sleep time did not differ between both conditions (T–test, p > 0.05).
As was stated above, the largest proportion of sleep in the walrus when in water occurred during repeated submergings to the bottom (varying between 160 and 250 s, on average 200 ± 6, n = 28) and surfacings for breathing (23–172 s, on average 63 ± 4, n = 46). The walrus always woke up when surfacing. Then she took several breaths (from 2 to 12, on average 6 ± 1; the average BP was 11 ± 1 s) and began slowly submerging. Up to 11 of such cycles were recorded in a row without transition to active wakefulness. Slow waves appeared in the EEG both during submerging and after she rested on the bottom. The walrus woke up several seconds before she started emerging for breathing. In the beginning of the sleep period the walrus opened its eyes and looked around at the time of emerging and was awake during all periods of regular breathing. As a sleep period continued, the duration of awakenings became shorter, slow waves reappeared in 10–15 s after surfacing and then progressed while the animal stayed at the surface. The eyes remained closed during all such periods of breathing.
When the walrus slept at the surface the nostrils were held above the water or immersed during each BP. Breathing could be regular or interrupted at these times but apneas were shorter (111–150 s, on average 131 ± 6, n = 6). SWS episodes while at the surface lasted up to 12 min and were not interrupted by arousal during respiratory acts.
When in water PS was recorded only when the walrus lay on the bottom. Usually 2–3 episodes of PS (one per submerging) followed a series of episodes of SWS. PS episodes started after the walrus had rested on the bottom (usually after a short episode of low voltage slow waves) and it had terminated sometime before the walrus started to surface for respiration. The data collected in this study corroborates the results of behavioral studies in nonimplanted walruses [11] which displayed a very similar sleep pattern.
To summarize, this study describes the pattern of sleep in the walrus—a member of the third family of Pinnipeds. When on land the pattern of sleep in the studied walrus resembled the pattern of sleep in fur seals, and when in water—the pattern of sleep in true seals. Similar to fur seals, SWS in the walrus was pre-dominantly bilateral while short episodes of asymmetrical SWS were correlated with brief openings of only one eye [14]. A similar association between asymmetrical eye state and asymmetrical slow wave development is characteristic of cetaceans [3, 14] but the degree of EEG asymmetry in dolphins and belugas is greater and the duration of such episodes is much longer than in fur seals and the walrus. Another similarity between fur seals and walruses is regular breathing while on land and particularly during SWS. Generally, the pattern of sleep in fur seals and walruses is rather the pattern of sleep of a “typical terrestrial mammal” (predominantly bilateral SWS and regular breathing).
The main features of sleep in the walrus (this study, [11]) and in true seals [5–7] when in water are long apneas and immobility. Sleep (bilateral SWS and PS) in these animals occur both at the surface and at depth. It is usually interrupted by awakenings during surfacing for breathing. Walruses and the majority of true seal species inhabit polar seas. Only such sleep patterns would allow them to sleep and survive in freezing conditions, especially since access to breathing holes is often limited and they are forced to spend much time under the ice. Fur seals inhabit a temperate climate zone. They avoid ice and sleep at the surface of water on their sides while holding the heads and three flippers in air. In this posture the eye directed into water is often seen to be open. This posture reduces heat loss via keeping 3 flippers out of the water, allows regular breathing and increases the probability of detecting approaching predators (killer whales and sharks). To keep this posture the fur seal needs to constantly paddle with one foreflipper and its movement stops only when PS develops.
Prior to this study unihemispheric (asymmetrical) SWS has been recorded in cetaceans and otariids but not in phocids. The need for cetaceans and fur seals to be half-awake and half—asleep at the same time was associated with the need to be in motion and to visually monitor the environment while being asleep [2, 3, 14]. Short episodes of asymmetrical SWS simultaneously with one eye brief opening were documented in several avian species and this type of sleep is also associated with the need to be vigilant while asleep [15]. The fact that asymmetrical SWS in the walrus was recorded while sleeping on land and coincided with the asymmetrical eye state supports the hypothesis that unihemispheric SWS serves to allow monitoring of the environment. In spite of the obvious correlation between these two phenomena (unilateral cortical activation and one eye opening) in cetaceans, otariids, walruses and also in birds, the relationship between them is still obscure. One eye briefly opening during USWS may be due to a more activated (waking) state of one cortical hemisphere which allows animals a multi-sensory monitoring of the environment [3, 14]. Walruses may need to monitor the environment (1) to detect predators, and (2) due to a high level of their sociality. Our data suggests that unihemispheric sleep appears to have evolved several times in phylogenetically different lineages of mammals and birds. It cannot be excluded that in future studies this type of sleep will be found in other animal species which live under pressure to be constantly vigilant to monitor the environment or be in motion while being asleep at the same time.
Presented by Academician D.S. Pavlov February 7, 2012
The article was translated by the authors.
REFERENCES
- 1.Mukhametov LM, Supin AY, and Polyakova IG, Brain Res, 1977, vol. 134, pp. 581–584. [DOI] [PubMed] [Google Scholar]
- 2.Mukhametov LM, Oleksenko AI, and Polyakova IG, in The Black Sea Bottle Nosed Dolphin, Moscow: Nauka, 1997, pp. 492–512. [Google Scholar]
- 3.Lyamin OI, Manger PR, Ridgway SH, et al. , Neurosci. Biobehav. Rev, 2008, vol. 32, pp. 1451–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyamin OI and Mukhametov LM, in The Northwern Fur Seal: Systematics, Morphology, Ecology, and Behavior, Moscow: Nauka, 1998, pp. 280–302. [Google Scholar]
- 5.Mukhametov LM, Supin A.Ya., and Polyakova IG, Zh. Vyssh. Nervn. Deyat, 1984, vol. 34, pp. 259–264. [PubMed] [Google Scholar]
- 6.Lyamin OI, J. Sleep Res, 1993, vol. 2, pp. 170–174. [DOI] [PubMed] [Google Scholar]
- 7.Castellini MA, Milsom WK, Berger RJ, et al. , Am. Physiol, 1994, vol. 266, pp. R863–R869. [DOI] [PubMed] [Google Scholar]
- 8.Lyamin OI, Kosenko PO, Lapierre JL, et al. , in XVI Biennial Conf. on the Biology of Marine Mammals, San Diego, 2005, p. 174. [Google Scholar]
- 9.Vyssotski AL, Serkov AN, Itskov PN, et al. ,J. Neurophysiol, 2006, vol. 95, pp. 1263–1273. [DOI] [PubMed] [Google Scholar]
- 10.Lyamin OI, Lapierre JL, Kosenko OP, et al. ,J. Sleep Res, 2008, vol. 17, pp. 154–165. [DOI] [PubMed] [Google Scholar]
- 11.Pryaslova JP, Lyamin OI, Siegel JM, et al. , Behav. Brain Res, 2009, vol. 201, pp. 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyamin OI, Kibalnikov AS, Kosenko PO, et al. , Sleep, 2011, vol. 33, p. 2011. [Google Scholar]
- 13.Lyamin OI, Mukhametov LM, Chetyrbok IS, et al. , Behav. Brain Res, 2002, vol. 128, pp. 129–138. [DOI] [PubMed] [Google Scholar]
- 14.Lyamin OI, Mukhametov LM, and Siegel JM, Arch. Ital. Biol, 2004, vol. 142, pp. 557–568. [PMC free article] [PubMed] [Google Scholar]
- 15.Rattenborg NC, Amlaner CJ, and Lima SL, Neurosci. Biobehav. Rev, 2001, vol. 24, pp. 817–842. [DOI] [PubMed] [Google Scholar]


