ABSTRACT
The human gastrointestinal tract is home to trillions of microbes. Gut microbial communities have a significant regulatory role in the intestinal physiology, such as gut motility. Microbial effect on gut motility is often evoked by bioactive molecules from various sources, including microbial break down of carbohydrates, fibers or proteins. In turn, gut motility regulates the colonization within the microbial ecosystem. However, the underlying mechanisms of such regulation remain obscure. Deciphering the inter-regulatory mechanisms of the microbiota and bowel function is crucial for the prevention and treatment of gut dysmotility, a comorbidity associated with many diseases. In this review, we present an overview of the current knowledge on the impact of gut microbiota and its products on bowel motility. We discuss the currently available techniques employed to assess the changes in the intestinal motility. Further, we highlight the open challenges, and incorporate biophysical elements of microbes-motility interplay, in an attempt to lay the foundation for describing long-term impacts of microbial metabolite-induced changes in gut motility.
KEYWORDS: Microbiota, gut motility, bacterial metabolites, computational modeling
Introduction
Understanding gut motility is fundamental when addressing functional gastrointestinal disorders, including inflammatory bowel disease and constipation. A large-scale multinational study revealed that more than 40% of the population worldwide suffer from functional gastrointestinal disorders, which, consequently, have a negative impact on the quality of life and health care use1. Gastrointestinal motility is a key physiological parameter that governs digestion and absorption of nutrients, and is regulated by various factors, such as the enteric nervous system, immune system, gut hormones, as well as gut microbes.2–4 Nonetheless, the mechanisms that govern the complex and dynamic interrelationships between these factors remain unclear. To date, reports on the mechanisms underlying gut motility typically comes from three types of research methodologies; 1) in vivo investigations, including human or animal studies,4–7 2) in vitro/ex vivo experiments, which involve cell/organ culture, and organ bath studies,4,5,8 and 3) in silico models, which employ simulations and computational methods in an attempt to cover different aspects of interactions between motility, flow, transport, bacteria, and their metabolites. This review summarizes current literature available on the impact of the microbiota on gut motility and describes how in silico studies can be designed to complement the existing methods to allow deciphering the mechanisms underlying the microbiota-gut motility interplay.
Regulation of the gastrointestinal motility
Gastrointestinal motility refers to the digestive motor function and the transit of ingested material throughout the gastrointestinal tract.9 It involves the coordination of smooth muscle and nerve function to mix, triturate, and propel products of digestion. Digestion and motility are facilitated by the collaborative work of different parts of the digestive tract; the esophagus, stomach, small, and large intestine. Gut motility is regulated via a multitude of regulatory elements, including enteric neurons, smooth muscle cells, interstitial cells, hormones, and particular stimuli, such as gut bacteria and their metabolites.2–4,10,11 Additionally, there are various timescales that govern the regulation of the above-mentioned elements and gut motility. For example, changes in the innervation of the gastrointestinal tract, or signaling in the enteric nervous system and neurogenesis, occur in a timescale ranging from several minutes12 to several weeks,13 respectively. Induction of gene expression in response to receptor activation, however, happens in a timescale of seconds to microseconds.12 The unique architecture of the human gastrointestinal tract facilitates all the components necessary for the precise functioning of gut motility14 (Figure 1). For example, the gastrointestinal wall is composed of several layers protruded by enteric neurons, those are the mucosa (epithelium, lamina propria, and muscularis mucosa), the submucosa (submucosal plexus), the muscularis propria (circular layer of the smooth muscle, myenteric plexus, and longitudinal layer of the smooth muscle), and the serosa.14 The movement of the muscles underlying the propulsion of content is coordinated by the myenteric plexus, while the submucosal plexus is broadly involved in secretion and absorption.14 Unraveling the fundamental mechanisms underlying the regulation of gastrointestinal motility is required to understand the causes of bowel dysfunction.
Figure 1.
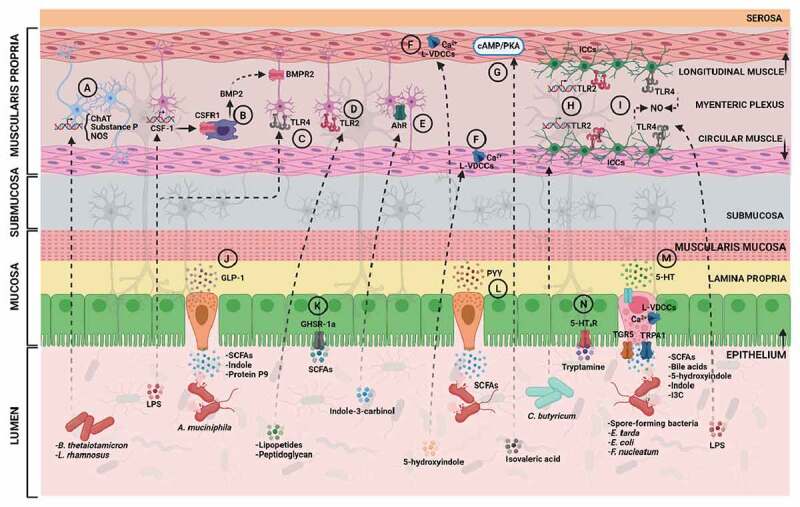
Gut microbiota, its metabolites and impact on gut motility. Potential routes by which the gut microbiota can influence intestinal motility via different mechanisms and pathways located in the gut epithelium, circular or longitudinal muscles, or myenteric plexus. Abbreviations: CSF-1, colony stimulatory factor 1; CSFR1, colony stimulatory factor 1 receptor; BMP2, bone morphogenetic protein 2; BMPR2, bone morphogenetic protein 2 receptor; AhR, aryl hydrocarbon receptor; ChAT, choline acetyltransferase; NOS, nitric oxide synthase; L-VDCCs, L-type voltage-dependent calcium channels; TLR2, toll-like receptor 2; ICCs, interstitial cells of Cajal; TLR4, toll-like receptor 4; NO, nitric oxide; 5-HT4R, serotonin receptor 4; SCFAs, short-chain fatty acids; GLP-1, glucagon-like peptide 1; TGR5, G-protein coupled bile acid receptor; TRPA1, transient receptor potential cation channel 1; 5-HT, serotonin; PYY, peptide YY; LPS, lipopolysaccharides. (Created with bioRender.com).
Why is the figure not in color?
The gastrointestinal tract is the only organ that has evolved with its independent nervous system, known as the enteric nervous system. The enteric nervous system was uncovered in 1755 by Albert Von Haller, who stated that “the intestines in this state after being deprived from all communication with the brain, preserve their peristaltic motion”.15 Nonetheless, although the enteric nervous system can function independent of the central nervous system, under physiological conditions, the bowel motility is also influenced by the central nervous system.16 In humans, the enteric nervous system comprises 200–600 million neurons, the great majority of which are found in the myenteric and submucosal plexus.17 Recently, profiling of the enteric nervous system in the colon of adult mice enabled the identification of 21 neuronal types.18 Among those 21 colonic neuronal types are: (1) sensory neurons (4 subsets), also referred to as intrinsic primary afferent neurons (IPANs), which sense and respond to chemical and mechanical stimuli in the intestine; (2) interneurons (3 subsets), which relay signals between neurons; (3) secretomotor/vasodilator neurons (2 subsets), which trigger secretions and fluid movement in other cell types; (4) excitatory motor neurons (5 subsets) and (5) inhibitory motor neurons (7 subsets), which together coordinate muscle contraction and relaxation and innervate longitudinal and circular muscles in the gastrointestinal tract.18 Interestingly, the neuronal types differ between various regions of the gastrointestinal tract as well as between various species.18 For example, mouse ileum contains 12 neuronal types, while human colon contains 14 neuronal types.18
The primary neurotransmitters of the excitatory neurons are the neuropeptides, acetylcholine and tachykinins, acetylcholine responses are mediated by the muscarinic receptors (M2 and M3), tachykinins (substance P and neurokinin A) bind to NK1 and NK2 receptors and activate pathways similar to those that involve acetylcholine.19–28 The inhibitory neurons constitute multiple transmitters, including nitric oxide, vasoactive intestinal peptide (VIP), and ATP-like transmitters.17,29 The nitric oxide is considered as the predominant inhibitory neurotransmitter, and deficits in transmission are observed in its absence as demonstrated in models where nitric oxide synthase is knocked out.2,30 The role of the enteric nervous system in the regulation of gut motility has been extensively reviewed elsewhere.3 Here, we will focus on the less well-studied role of the gut microbiota and its metabolites as a key regulator of the gut motility.
Gut motility is highly dependent on the microbiota
Studies investigating the role of microbiota in regulating the gut motility started decades ago, when researchers observed up to 10 times larger cecum in germ-free rats compared to conventional rats, in addition to delayed gastric emptying, suggesting a strong role for the microbiota in the development of a normal gut motility.31–34 Since then, germ-free animals have been used in many studies to investigate the influence of the microbiota on gut motility. For example, Husebye et al. demonstrated that germ-free rats displayed a significant delay in the intestinal transit and in the contractility of the small intestine compared to their conventional counterparts, and this delay was partially reversed after colonization with Lactobacillus acidophilus together with Bifidobacterium bifidum.35 Moreover, germ-free mice were found to have a reduced number of colonic neurons in the enteric nervous system compared to their conventional controls.36 Analogously, germ-free mice were reported to have lower excitability in the myenteric IPANs, which was normalized when these mice were conventionalized.37 Mice treated with antibiotics had significantly lower defecation frequency and slower total gut transit time.38,39 Ge et al. also showed that the contractility of the proximal colonic longitudinal muscles was inhibited in the antibiotics-treated mice compared to the untreated controls.38 Altogether, these studies demonstrate a significant role of gut microbes in controlling the gut motility.
Bacterial modulation of the gut motility via the enteric neurons and immune system
Emerging evidence is pointing to the impact of the gut microbiota on the complex signaling of the enteric nervous system.36,39–41 For example, the abundant human resident gut bacterium, Bacteroides thetaiotamicron, was shown to be critical for the enteric nervous system innervation in the colon.42 In that study, colonization of germ-free mice with Bacteroides thetaiotamicron restored the expression of excitatory and inhibitory motor neuron signaling enzymes, such as choline acetyltransferase (responsible for synthesis of acetylcholine), substance P, and nitric oxide synthase42 (Figure 1(A)). Moreover, when the authors used the pan-neuronal marker, PGP9.5 to investigate the changes in the neuronal innervation between germ-free and specific pathogen-free mice, they observed a reduction in the overall neuronal innervation in both the colonic mucosa and muscle layers (including the myenteric plexus) in germ-free mice.42 Similarly, when Lactobacillus rhamnosus GG was administered to mice, the expression of choline acetyltransferase was elevated43 (Figure 1(A)).
Since the enteric nervous system and gut microbiota reside in close proximity to the intestinal mucosal immune system, it is not surprising that the latter plays a role in modulating the bacterial influence on gut motility. Indeed, Muller et al. observed a role of a subtype of macrophages that reside in the muscularis mucosa, namely muscularis macrophages, in regulating the peristaltic activity of the colon in mice.40 Muscularis macrophages could change the pattern of colonic contractility by releasing a growth factor, BMP2, which activates BMP receptor expressed on the enteric neurons (Figure 1(B)). In response, the enteric neurons release a growth factor CSF-1 required for macrophages development40 (Figure 1(B)). Muller et al. showed that this immune-neuronal crosstalk is induced by intestinal microbial stimuli, in particular, the microbial cellular component, lipopolysaccharide (LPS), which regulates BMP2 and CSF-1 expression and may, in turn, alter intestinal motility40 (Figure 1(B)).
Similarly, two subtypes of the Toll-like receptors (TLRs), TLR2 and TLR4, were recently linked to the regulation of murine intestinal enteric neurons, and gut motility36,44 (Figures 1C, 1D). Yarandi et al. observed that the inhibition of endogenous signaling of TLR2 (which recognizes lipopeptides and peptidoglycan45), in vivo, resulted in inhibition of neurogenesis in healthy mice, and, in turn, in significant dysmotility and loss of colonic myenteric neurons.44 Anitha et al. reported that the lack of TLR4 signaling (which recognizes LPS) led to delayed gastrointestinal motility in mice as demonstrated by reduced fecal pellet frequency and delayed intestinal transit.36 Furthermore, the aryl hydrocarbon receptor (AhR), which is directly activated by gut microbial metabolites (discussed in more details in section Tryptophan metabolites),46 and is considered a key component of the immune response at barrier sites,47 has been recently shown to be involved in the regulation of murine total intestinal transit time in response to microbiota-derived metabolites39(Figure 1(E)). Indeed, microbiota-induced expression of AhR in the colonic neurons were shown to be involved in the induction of response of these neurons to the luminal environment, specifically to the microbiota-derived AhR agonist indole-3-carbinol.39 Overall, these studies provide invaluable information on the immunological control of intestinal motility and on the basis for understanding the pathophysiology of gastrointestinal motility disorders.
Not only gut commensals but also several pathogens such as Vibrio cholerae and Salmonella typhimurium, can influence gastrointestinal motility via distinct mechanisms.48,49 For example, V. cholerae use the syringe-like type VI secretion system to induce intestinal movements in zebrafish, which led to expulsion of the resident microbiota by the host.48 S. typhimurium-derived enterotoxin caused dramatic changes in intestinal myoelectric activity in white albino rabbits and substantial fluid production after injection of E. coli lysates containing the cloned S. typhimurium enterotoxin to the ileal loops.49
Bacterial interaction with the gastrointestinal smooth muscles
Throughout the gastrointestinal tract, smooth muscles are organized into two layers of circularly- and longitudinally-oriented muscle bundles to form electrical and mechanical junctions between cells that facilitate coordination of contractions2 (Figure 1). Excitation and contraction of gut muscles are regulated by membrane depolarization (influx of positively charged ions inside the cell), which activates voltage-dependent Ca2+ channels and triggers elevated levels of intracellular Ca.2+50–54 Gastrointestinal smooth muscles express mostly L-type voltage-dependent Ca2+ channels, encoded by the CACNA1C gene.55 However the T-type Ca2+ channels also contribute to the Ca2+ influx in these muscles.56 Recently, we showed how a novel gut bacteria-derived metabolite, 5-hydroxyindole, affects the total gut transit time in rats via modulating L-type voltage-dependent Ca2+ channels located on the colonic smooth muscle cells4 (Figure 1(F)). Ca2+ influx into smooth muscle cells also occurs via nonselective cation channels, such as transient receptor potential channels activated by muscarinic stimulation (as shown by gene deactivation in mice).19 Transient receptor potential channels are voltage-independent and are activated by either intracellular Ca2+ or by G-protein coupled receptors.57 Following an increase in intracellular Ca2+, Ca2+ binds to calmodulin (ubiquitous calcium-binding protein), which activates myosin light chain kinase and subsequent phosphorylation of myosin light chain kinase, which causes smooth muscle contraction.51 In contrast, dephosphorylation of myosin light chain kinase results in muscle relaxation.51 Muscle relaxation is also controlled by cAMP/PKA signaling pathway (reviewed in details in2).
Blakeney et al. demonstrated that the gut microbiota-produced branched-chain fatty acid, isovaleric acid, caused relaxation of colonic smooth muscles in mice via cAMP/PKA pathway using an ex vivo organ bath set-up and dispersed colonic muscle cells8 (Figure 1(G);discussed also in section Short-chain fatty acids). This microbiota-dependent relaxation was found to be concentration-dependent and to occur in whole colonic segments or in isolated colonic muscle cells.8 Other studies focusing on the effects of gut bacteria on the intestinal smooth muscle contractility either in human or rat colonic tissue observed inhibitory effects on the gut contractility.58,59 Overall, though the key role of the gut microbiota and its metabolites in regulating gastrointestinal smooth muscle cells starts to be unraveled, the molecular mechanisms by which the gut bacteria act remain largely unknown.
Gut bacterial signaling to interstitial cells
Interstitial cells of Cajal (ICCs) are the intestinal pacemaker cells responsible for the generation of electrical oscillatory activity (also known as the “slow waves”). Electrical oscillatory activity is the dominant omnipresent pacemaker activity of the intestine, that is electrically coupled to smooth muscle cells, providing it with cyclic changes in excitability.60 ICCs constitute the basis for peristalsis, which is then stimulated by migrating motor complexes (MMCs; intestinal motility pattern of the interdigestive state) or segmentation motility patterns (see section Contractility and flows below),2,60–62 and were first characterized by Cajal in 1893.63 The functional development of ICC networks depends on signaling via the Kit receptor pathway.64 In fact, mice with loss-of-function in c-Kit signaling failed to generate pacemaker activity64 indicating that ICCs serve as pacemakers.
Remarkably, a cocktail of four different lactic acid bacteria (Lactobacillus plantarum 2362, Lactobacillus casei ssp. paracasei 19, Leuconostoc raffinolactis 23 ~ 77:1, and Pediococcus pentosaceus 16:1), Synbiotic2000TM, fed to a traumatic brain injury (TBI) mouse model, which is prone to suffer from gastrointestinal motility abnormalities, was reported to improve the reduced contractile amplitudes, frequencies and tension forces in the small intestine of these mice compared to the control group.65 The TBI model also showed severe impairments in the number of ICCs and c-Kit protein concentrations. However, after administration of Synbiotic2000TM, the number of ICCs and c-Kit protein concentrations were significantly improved, though the concentrations did not reach the levels detected in the control group.65 Analogously, Sui et al. showed that the probiotic strain, Clostridium butyricum, promoted ICCs (isolated from murine small intestine) proliferation by their regulation of the TLR2 expressed on ICCs66 (Figure 1(H)). Another study suggested that signaling through TLR4 by LPS, activated murine ICCs to produce nitric oxide, and thus inhibited the pacemaker currents67 (Figure 1(I)). ICCs represent a potentially valuable therapeutic target. Further characterization of the ICCs cells and their interactions with the gut microbes and their metabolites may provide new insights into development of novel therapies for functional gastrointestinal disorders.
Modulation of bowel motility by bacterial-dependent production of gut hormones
Gut hormones are released from specialized intestinal epithelial cells, enteroendocrine cells, in response to meal-related stimuli. Subsequently, these gut hormones exert actions ranging from the local control of gut motility, to the regulation of glucose homeostasis, and food intake.68 Gastrointestinal motility is modulated by gut hormones during both the interdigestive (i.e. between meals; motilin and ghrelin), and postprandial (i.e. after meals; cholecystokinin (CCK), glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY)) periods.69 GLP-1, and PYY are key mediators of the shift from an interdigestive to a postprandial gastrointestinal motor pattern.69 Moreover, the gut hormones CCK, GLP-1, and PYY control blood nutrient levels by modulating digestion and absorption, as slowing either of these steps reduces the rate at which ingested nutrients enter the circulation.68
Gut microbiota has been shown to influence the levels of gut hormones secreted into the circulatory system.68,70,71 For example, using fast protein liquid chromatography and liquid chromatography coupled to mass spectrophotometry, it was shown that Akkermansia muciniphila secretes the protein P9, which signals to a subtype of the enteroendocrine cells, L cells, to produce GLP-172 (Figure 1(J)). P9-dependent GLP-1 production was associated with ameliorating thermogenesis, body weight, and glucose homeostasis in obese mice.72 In healthy individuals, GLP-1 induces insulin release, delays gastric emptying and increases satiety.73 Obese subjects had more rapid gastric emptying than their non-obese counterparts,74 thus GLP-1 agonists have become well-established therapies in obesity.73,75 Gut bacteria promoting GLP-1 production may help ameliorate obesity through reduction of gastric motility. Similarly, the production of other gut hormones was reported to be altered by the gut microbiota or their metabolites.71,76,77 For example, short-chain fatty acids (SCFAs, discussed in details in section Short-chain fatty acids), acetate, propionate, butyrate, and lactate, as well as bacterial supernatants of the Bifidobacterium and Lactobacillus genera, were shown to affect ghrelin signaling via internalization of ghrelin receptor stably expressed in human embryonic kidney cell line71(Figure 1(K)). In addition, physiological concentrations of propionate and butyrate were shown to induce PYY gene expression and production in human enteroendocrine cells76 (Figure 1(L)).
Gut bacteria, serotonin, and control of the gut motility
It is well accepted that the greatest quantity of serotonin (5-HT) in the human body is synthesized within a subtype of the enteroendocrine cells, enterochromaffin cells, in the intestinal mucosa, via the enzyme tryptophan hydroxylase 1 (TPH1).78 Enterochromaffin cells act as sensory transducers to release 5-HT in response to various mechanical and chemical stimuli.79 In 2017, enterochromaffin cells were recognized as specialized stimulus detectors that constitute a direct line of communication between the mouse gut epithelium and enteric nervous system.80 A year later, it was shown that, throughout the mouse gut, enterochromaffin cells release 5-HT in response to their sensing of nutrients and microbial metabolites, indirectly through the release of GLP-1 by neighboring cells.81
On a much smaller scale, 5-HT is also synthesized in approximately 1% of nerve cell bodies in the enteric nervous system, via a different enzyme called tryptophan hydroxylase 2 (TPH2).82 Despite the low levels of exogenous 5-HT (5-HT produced in the enteric nervous system), its role in stimulating gastrointestinal motility is well established.83 In contrast, the role of endogenous 5-HT (5-HT produced from enterochromaffin cells) has been very challenging to resolve with many contradicting reports.84–86 Several studies have shown that when TPH1 is either deleted in mice or pharmacologically inhibited, it alters the murine total gut transit time, gastric emptying, small bowel propulsion, or rate of expulsion of beads inserted into the rectum.84,85 Analogously, in vitro, the colonic emptying time, propulsion of fecal pellets and migrating motor complexes were compromised in mice lacking mucosal 5-HT (Tph1−/− mice).86 In contrast, in vivo studies on gut motility in the absence of the TPH1 did not lead to any inhibitory effects on the gastrointestinal transit in conscious mice.84,85 Therefore, it was suggested that while endogenous 5-HT is released in response to a number of stimuli and plays an important role in paracrine and endocrine systems,87,88 it acts only as a modulator, not as an initiator, of neurogenic motor patterns and gut transit.89
Numerous studies have reported that gut microbiota or their metabolites can regulate host serotonin biosynthesis from the enterochromaffin cells and thus may be involved in modulation, but not initiation of, gut motility. For example, Yano et al. showed that TPH1 inhibition in mice blocks the ability of the microbiota to promote colonic 5-HT production from enterochromaffin cells suggesting that the gut microbiota, specifically spore-forming microbes dominated by Clostridial species, requires host TPH1 activity to induce 5-HT synthesis90 (Figure 1(M)). Another study showed that heat-killed Lactobacillus casei subsp. casei has a potential to promote serotonin biosynthesis in the murine colonic mucosa.91 Employing 5-HT quantification in the colonic tissues and bead expulsion measurements after administration of heat-killed Lactobacillus casei subsp. casei showed significantly higher levels of 5-HT in the colonic tissue and significantly lower bead expulsion time. These data suggestthat promoting 5-HT synthesis could contribute to improving the bowel motility.91 Besides the role of TPH1 in the induction of 5-HT synthesis, 5-HT release is highly dependent on Ca2+ influx into the cells and enterochromaffin cells are endowed with L-type voltage-dependent Ca2+ channels.92In fact, the microbiota-produced metabolite 5-hydroxyindole, was shown to induce the production of 5-HT from an in vitro cell model of enterochromaffin cells, potentially, via the induction of L-type voltage-dependent Ca2+ channels and Ca2+ influx into the cells4 (Figure 1(M)). Taken together, the currently available data suggest that gut microbial luminal stimuli can promote 5-HT release, however what exact role the endogenous 5-HT plays in the modulation of gut motility remains to be resolved.
Gut bacteria regulate the bowel motility via their metabolic products
One of the different ways by which gut microbiota can influence the gut motility is via the release of metabolites or end products of bacterial fermentation (Table 1). Two main groups of bacterial products, which have been well studied in relation to altered gut motility, are SCFAs, and tryptophan metabolites. In addition, several other metabolites that belong to a wide range of chemical groups have been shown to alter gut motility. Nevertheless, we are only dealing with the tip of the iceberg as there are many more microbiota-related metabolites yet to be uncovered and investigated for their potential role in regulating the gut motility.
Table 1.
Overview of currently known bacterial metabolites, their effect on gastrointestinal motility, and experimental models used for the assessment of gut motility
| Bacterial metabolite | Effect on gut motility | Methods used | Model organism and effect size (N) | Reference |
|---|---|---|---|---|
| Lipopolysaccharides | -Regulation of BMP2 (growth factor produced by macrophages, which regulate peristaltic activity of the colon) and CSF-1 expression (a growth factor secreted by enteric nervous system) |
-Ex vivo organ bath model (colonic contractility measurements) Bead expulsion test (colonic transit analysis) -GI transit assay using carmine red in vivo (gut motility measurements) -Rhodamine B dextran fluorescence detection (gastric emptying and small intestinal transit) |
Mice (N = NS*) | 40 |
| -Signaling via TLR4 receptor leads to delayed gastrointestinal motility | -Fecal pellets collection (stool frequency) Bead expulsion test (colonic transit analysis) -Isometric muscle recordings of colonic longitudinal muscle strips |
Mice (N = 5-10) | 36 | |
| -Signaling through TLR4 activates ICCs to produce nitric oxide and inhibits the pacemaker currents of the gut contractility | -Whole-cell patch clamp (cultured ICCs measurements of membrane currents and potentials) -RT-PCR in cultured ICCs from small intestine |
Mice (N = 6-11) | 67 | |
| Lipopeptides and peptidoglycan | -Signaling via TLR2 receptor resulted in inhibition of neurogenesis leading to significant dysmotility and loss of colonic myenteric neurons | -GI transit assay using carmine red in vivo (gut motility measurements) -Bead latency test (distal colonic transit time) -Detection of fluorescein isothiocyanate-dextran (determination of colonic geometric center) |
Mice (N = NS) | 44 |
| Salmonella typhimurium-derived enterotoxin | -Causing dramatic changes in intestinal myoelectric activity and substantial fluid production | -Ex vivo organ bath (measurement of myoelectric activities in ileal loops) | Rabbits (N = 8) | 49 |
| Short-chain fatty acids | -Stimulation of PYY production in human enteroendocrine cells | -ELISA (PYY quantification) | NA | 76 |
| -Modulation of 5-HT release from model of enterochromaffin cells (butyrate, propionate) | -RIN14B cell line in vitro (5-HT release measurements) | NA | 90 | |
| -Increase of the proportion of cholinergic neurons translating to increased gut motility |
-Ex vivo organ bath model (colonic contractility measurements) -Bead expulsion test (colonic transit analysis) |
Rats (N = 5-6) | 41 | |
| -Stimulation of increase/decrease in colonic motility (butyrate, propionate, acetate) | -Ex vivo organ bath model (colonic contractility measurements) | Guinea pigs and rats (N = 4-9 and N = 4-6) | 93,94 | |
| -Stimulation of GLP-1 production (butyrate, propionate, acetate) |
-Ex vivo organ bath model (colonic contractility measurements) -Evans Blue dye detection (small intestinal transit) |
Mice (N = 4-7) | 95 | |
| -Modulation of ghrelin signaling (acetate, propionate and butyrate) | -Cell culture (activation of G protein coupled receptors using β-arrestin assay) | NA | 71 | |
| Tryptophan metabolites | -Acceleration of gastrointestinal transit by activation of epithelial 5-HT4 receptor in the proximal colon (tryptamine) | -Ussing Chamber (assessment of epithelial ionic flux) -GI transit assay using carmine red in vivo (gut motility measurements) |
Mice (N = 4-6) | 5 |
| -Modulation of secretion of GLP-1 (indole) | -Total GLP-1 assay (GLP-1 quantification) | NA | 96 | |
| -AhR signaling in the colonic neurons alters gut motility (indole-3-carbinol) | -GI transit assay using carmine red in vivo (gut motility measurements) -Live video imaging and spatiotemporal mapping of colonic motility |
Mice (N = 8-18) | 39 | |
| -Enhancement of the epithelial barrier functions by increasing of expression of genes involved in maintenance of epithelial cell structure and function (indole) | -Microarrays | NA | 97 | |
| -Regulation of intestinal barrier function in vivo by acting as a ligand for xenobiotic sensor, pregnane X receptor (IPA) | -Fluorescein isothiocyanate-dextran detection in serum (intestinal permeability assay in vivo) | Mice (N = 3-6) | 98 | |
| -Reduction of intestinal permeability in mice fed a high fat diet | -Fluorescein isothiocyanate-dextran detection in plasma (intestinal permeability assay in vivo) -TEER (colonic intestinal permeability assay in vitro) |
Mice (N = 7-9) | 99 | |
| Bile acids | -Modulation of 5-HT release from model of enterochromaffin cells (cholate, deoxycholate) | -RIN14B cell line in vitro (5-HT release measurements) | NA | 90 |
| -Bacterial bile salt hydrolase activity is associated with faster gastrointestinal transit in gnotobiotic mouse model | -GI transit assay using carmine red in vivo (gut motility measurements) | Mice (N = 5-6) | 100 | |
| -Promote gastrointestinal motility by activation TGR5 receptors located on enterochromaffin cells |
-Ex vivo organ bath model (colonic contractility measurements) -Evans Blue dye detection (gastrointestinal transit) -Bead expulsion test (colonic transit analysis) -Fecal pellets collection (stool frequency) |
Mice (N = NS) | 101 | |
| 5-hydroxyindole | -Modulation of gut motility via L-type voltage-dependent Ca2+ channels located on the colonic smooth muscle cells -Control of serotonin release from model of enterochromaffin cells |
-RIN14B cell line in vitro (5-HT release measurements) -GI transit assay using carmine red in vivo (gut motility measurements) -Ex vivo organ bath model (colonic contractility measurements) |
Rats (N = 6-10) | 4 |
| Protein P9 | -Production of protein P9 signals to L cells to produce GLP-1 | -ELISA (GLP-1 quantification) | NA | 72 |
| Indole and indole-3-carboxaldehyde | -Activation of TRPA1 in EECs, leads to production of 5-HT from enterochromaffin cells and thus modulate gut motility | -Real-time measurements of EECs in vivo in zebrafish (activation of TRPA1 and gut motility) -Amperometry (5-HT release) |
Zebrafish (N = 117-213) | 102 |
| Quercetin | -Improvement of the symptoms of constipation in rat loperamide-induced constipation model | -Charcoal propulsion test (gut motility) | Rats (N = 3) | 103 |
| Heptadecanoic and stearic acid (saturated long-chain fatty acids) | -Enhancement of colonic contractility ex vivo and stool frequency in vivo |
-Ex vivo organ bath model (colonic contractility measurements) -Fecal pellets collection (stool frequency) |
Rats (N = 6-8) | 104 |
| Isovaleric acid (branched-chain fatty acids) | -Causes contractile relaxation of colonic smooth muscles via cAMP/PKA pathway |
-Ex vivo organ bath model (colonic contractility measurements) -Isolated muscle cells culture (direct activation of PKA activity) |
Mice (N = 4-7) | 8 |
| Polyamines (spermidine, putrescine, spermine) and trace amines (isoamylamine, cadaverine) | -Modulation of intestinal peristalsis | -Ex vivo organ bath model (ileal and colonic contractility measurements) | Mice (N = 7) | 105 |
| Ferulic acid | -Acceleration of gastrointestinal transit and gastric emptying | -Charcoal propulsion test (gut transit) -Phenol red detection (gastric emptying) |
Rats (N = 8) | 106 |
| Histamine | -Promotion of colonic motility via activation of histamine receptors in the gut | -Fecal output assay | Mice (N = 3-5) | 107 |
| 3-(3,4-dihydroxyphenyl)propionic acid (DHPPA) | -Potent stimulation of ileal motility ex vivo | -Ex vivo organ bath model (ileal contractility measurements) | Mice (N = 4-6) | 108 |
| Dopamine | -Inhibition of longitudinal muscle contractility | -Ex vivo organ bath model (longitudinal ileal muscle contractility measurements) | Guinea pigs (N = 10) | 109 |
| -Decreased the duration of the MMCs in the small intestine (duodenum and jejunum) | -Implanted Ni/Cr electrodes | Dogs (N = 4) | 110 | |
| -Induced phase-III like MMCs in the duodenum | -Intestinal radiopaque tube | Humans, healthy (N = 14) | 111 |
*NS = not specified
**NA = not applicable.
Short-chain fatty acids
SCFAs, including, acetate, butyrate, and propionate112 originate from bacterial degradation of dietary fibers and are key energy source and signaling molecules in the mammalian colonocytes.113,114 SCFAs, in particular, butyrate, have been shown to modulate the activity of the enteric nervous system in rats.41 A resistant starch diet (in which starch reaches the colon and can be considered as a dietary fiber), intracecal butyrate infusion, and butyrate application to cultured myenteric ganglia were all shown to affect the rat enteric nervous system by increasing the proportion of cholinergic neurons translating to shorter colonic transit time and increased cholinergic-mediated colonic circular muscle contractile response.41 Hurst et al. showed that different SCFAs have different effects on the colonic contractility in guinea pigs.93 Butyrate caused an increase in colonic contractility, while propionate, and, to a lesser extent, acetate resulted in a decrease in contractility.93 Such a difference in the effect of the different types of SCFAs studied may lie in the different animal models used in the study since previous findings reported that, in the rat colonic tissue, all three SCFAs had stimulatory effects on the colonic peristalsis.93,94 Furthermore, butyrate, propionate and acetate, can indirectly affect the murine gut motility through their induction of colonic GLP-1 secretion (Figure 1(J)), which was reported to significantly prolong intestinal transit.95
Besides the main SCFAs, branched SCFA, isovaleric acid, has been shown to have an inhibitory effect on the murine colonic contractility.8 Isovaleric acid was shown to act directly on the isolated colonic smooth muscles in vitro and to cause muscle relaxation via the PKA pathway (Figure 1(G)).8 Overall, bacterial-produced SCFAs are key players in the regulation of gut motility.112,113 However, when the levels of SCFAs are chronically elevated, they may result in a detrimental increase in colonic transit rate, which could play a role in pathogenesis, where gut dysfunction is a comorbidity, as in irritable bowel syndrome.115
Tryptophan metabolites
Tryptophan metabolites generated exclusively by the gut microbiota are key contributors to the intestinal homeostasis.46 A growing number of evidence has associated various tryptophan metabolites with gut motility.116 Gut microbes can break down tryptophan to produce a variety of metabolites. For example, Clostridium sporogenes and other gut bacteria, such as Ruminococcus gnavus, harboring the tryptophan decarboxylase enzyme can produces tryptamine.117 Tryptamine was recently shown to accelerate gastrointestinal transit by activating epithelial serotonin receptor 4 (Figure 1(N)), and by increasing the anion-dependent fluid secretion in the proximal colon of mice.5 Moreover, both tryptophan and tryptamine were significantly increased in fecal samples from diarrhea-predominant irritable bowel syndrome (IBS-D) patients, suggesting that these metabolites might be responsible for the higher water content of feces in IBS-D.118 Another gut bacterial metabolite of tryptophan is indole, which is produced by gut bacteria harboring the tryptophanase enzyme.119 Indole has been observed to modulate the secretion of GLP-1 from immortalized and primary mouse colonic enteroendocrine L cells96 (Figure 1(J)). Indole, as well as another bacteria-derived tryptophan metabolite, indole-3-carboxaldehyde, produced by Edwardsiella tarda, were recently shown to activate the transient receptor potential ankyrin A1 (Trpa1) in enteroendocrine cells of zebrafish, mouse and human, and to induce the production of 5-HT (Figure 1(M)), thus accelerate intestinal motility.102 As mentioned above, AhR signaling has also been recently associated with the regulation of intestinal transit time in mice39 (Figure 1(E)). Bacterial-produced tryptophan metabolites, such as indole, tryptamine, skatole, indoleacetic acid, indoleacrylic acid, indole-3-carboxaldehyde and indolelactic acid are widely described as ligands of AhR,46 thus may be involved in the control of bowel motility. Moreover, indole was shown to enhance the intestinal epithelial barrier function in human colon-cancer cell line and in vivo in mice studies by increasing the expression of genes involved in maintenance of epithelial cell structure and function.97,120 Impaired intestinal permeability, which is a significant factor in several (gastrointestinal) diseases, such as inflammatory bowel disease, irritable bowel syndrome, celiac disease or colon carcinoma, has been linked to dysmotility.121 The intestinal barrier prevents loss of water and electrolytes and entry of antigens and microorganisms into the body, while allowing exchange of molecules between host and environment and absorption of nutrients in the diet.121 Therefore, normal functioning of gut barrier is necessary for healthy gastrointestinal transit. Similarly, indolepropionic acid (IPA) another bacterial metabolite of tryptophan produced via the phenyllactate dehydratase gene cluster122 was found to regulate the intestinal barrier function in vivo in mice by acting as a ligand for the xenobiotic sensor, pregnane X receptor, particularly in the presence of indole.98 In addition, IPA reduced intestinal permeability in mice fed a high fat diet.99 Lastly, another bacterial-produced indole derivative, 5-hydroxyindole, was shown to be a potent stimulant of rat intestinal contractility via its action on L-type Ca2+ channels (see also section Bacterial interaction with the gastrointestinal smooth muscles) (Figure 1(F)).4 5-hydroxyindole is a structural analogue of indole and is produced by bacterial degradation of 5-hydroxytryptophan, which is a chemical precursor and intermediate metabolite in the biosynthesis of 5-HT.4
Other bacterial metabolites
Studies on the effect of nutrients on regulating the gut motility have unraveled numerous compounds to affect the gut motility, several of which are the products of gut bacterial metabolization of these nutrients. For example, quercetin, an abundant flavonoid found in many fruits, vegetables and grains,123 but also produced by gut bacteria, specifically Fusobacteria species124,125 can improve the symptoms of constipation in rat loperamide-induced constipation model. The laxative effects of quercetin have been attributed to the interaction between quercetin and muscarinic receptor signaling pathway.103 Saturated long-chain fatty acids, heptadecanoic acid and stearic acid, produced by gut bacteria, specifically Prevotella, Lactobacillus and Alistipes genera, were observed to enhance the rat colonic contractility ex vivo and defecation frequency in vivo.104 Another study showed that polyamines (spermidine, putrescine, spermine) and trace amines (isoamylamine, cadaverine) derived from intestinal bacteria may act as chemosensors and thus modulate the rat intestinal peristalsis.105 Bile acids affect several gastrointestinal functions including absorption, gastric emptying, and small intestinal and colonic motility in mice.100 Bile acids are modified by gut microbiota and the bacterial bile salt hydrolases activity was correlated with faster gastrointestinal transit in gnotobiotic mouse model.100 Moreover, that study showed that commonly used spice, turmeric, affects gut motility through bacterial bile salt hydrolase activity and Ret signaling (Ret is a gene implicated in Hirschsprung’s disease, a development disorder associated with absent peristalsis in the distal colon) in the enteric nervous system.100 Deoxycholate is a secondary bile acid, produced by microbial biotransformation of cholate and was reported to inhibit the murine spontaneous contractility of colonic longitudinal muscle by mechanism that requires expression of TGR5 G protein-coupled receptors on enterochromaffin cells101 (Figure 1(M)). Furthermore, ferulic acid, a potent anti-inflammatory and antioxidant compound was shown to be produced by Lactobacillus fermentum126 and to accelerate the gastrointestinal transit and gastric emptying in a dose-dependent manner in rats.106 Another study showed that a shift in colonic metabolism from carbohydrate fermentation to protein catabolism, as reflected by higher urinary levels of potentially deleterious protein-derived metabolites, is associated with longer colonic transit time in humans.127 Additionally, gut microbiota is able to produce neurotransmitters involved in gut motility, such as γ-aminobutyric acid (GABA).128,129 GABA receptors are expressed in the enteric nervous system, where GABA may modulate intestinal motility.130 Lastly, the dietary histidine has been observed to be metabolized by Morganella morganii and Lactobacillus reuteri strains into histamine, which significantly increases fecal output (number of fecal pellets per hour) via activation of histamine receptors along the murine gut.107 Collectively, the interaction between dietary components and gut microbiota is a key element in the regulation of gut motility, which in turn, determines which microbes colonize the gut,131(see section Design of in silico studies to unravel the microbiota-gut motility interplay and Figure 2 below), entering a vicious circle.
Figure 2.
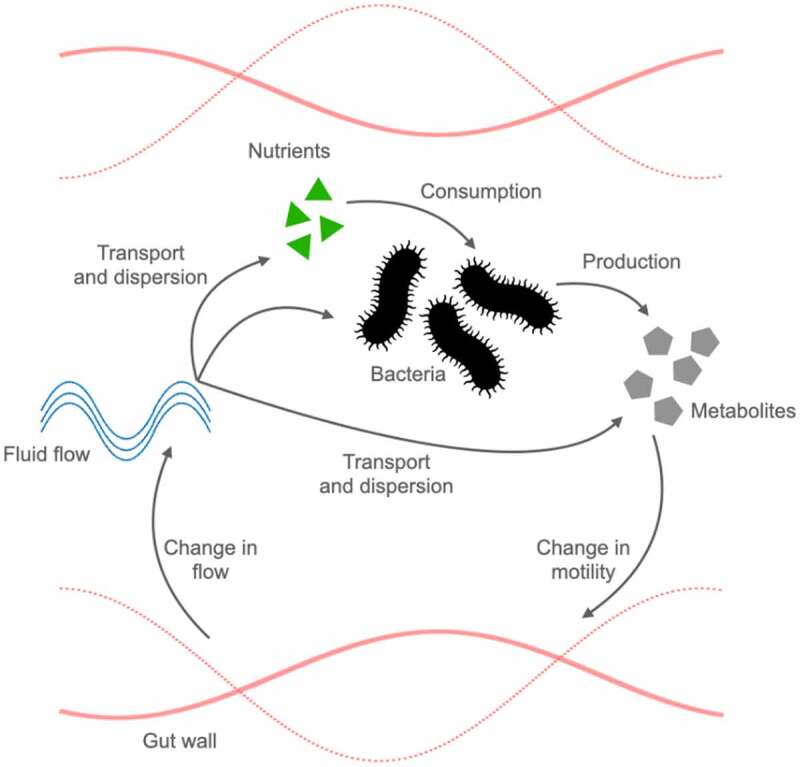
A comprehensive in silico model of bacterial-produced metabolites and motility interactions. Bacterial-produced metabolites can directly influence gut motility, leading to a cascade of effects and feedback mechanisms impacting the production of the metabolite itself. Gut bacteria replicate and produce their metabolites based on the availability of nutrients. Metabolites impact gut motility, which, in turn, modifies the fluid flow. The flow is directly responsible for the transport and dispersion of nutrients, bacteria, and their metabolites, closing the feedback loop.
Besides the ability of gut microbiota to take up dietary small molecules, they can also sequester medical drugs with consequences on interpersonal variation in drug efficacy and toxicity.132 For example, we showed that levodopa, the primary treatment of Parkinson’s disease, in particular, its unabsorbed residues, is deaminated by C. sporogenes to 3-(3,4-dihydroxyphenyl)propionic acid (DHPPA), a potent inhibitor of mouse ileal contractility ex vivo.108 Moreover, bacterial species, such as Enterococcus faecalis, Enterococcus faecium and Lactobacillus brevis, can produce luminal dopamine.133 Dopamine has been shown to affect gut motility in several model organisms, including rodents, dogs and humans.109–111 For example, in guinea pigs, it was shown that dopamine inhibits longitudinal muscle contractions.109 In dogs, dopamine decreased the duration of the migrating motor complexes in the small intestine 1 h prior a meal compared to controls110 and in fasted human subjects, intravenous administration of dopamine induced phase-III like migrating motor complexes (last phase in the MMC cycle, which consists of strong contractions to completely occlude the lumen) in the duodenum.111 This, and similar studies, underpin the importance of the metabolic pathways of the gut microbiome involved in drug metabolism not only to preserve drug effectiveness, but also to avoid potential side effectsof bacterial breakdown products of the unabsorbed residue of medication, such as inhibition of gut motility.
Models used to decipher the mechanisms underlying bacterial regulation of the gut motility
Deciphering the mechanisms by which gut bacteria and their metabolites influence gut motility is crucial to understand the role of these microbes in bowel dysfunction and its targeting by the design of microbiota-based interventions. To date, experimental research investigating the effect of microbiota on gut motility is directed along three main types of methodologies; in vivo human or animal studies,4–7 in vitro and ex vivo experiments (Tables 1 and 2), the latter two focus on unraveling the mechanisms underlying the observations made in vivo.4,5,8 In vivo studies in humans and animals include magnetic resonance measurements, manometry measurements, mini-capsules or ingestion of surrogate markers, such as carmine red dye or fluorescein isothiocyanate.4,99,136–139 These measurements allow the functional scanning of gut motility (e.g., water flux and water content),136 the acquisition of intraluminal pressure patterns,137,139 the determination of gastrointestinal transit time,4,140 as well as intestinal permeability.99 Transit time can also be measured with other techniques, such as evaluation of the Bristol Stool-Scale score135 and recently, a new method for gut transit time measurements in humans was introduced, namely the blue dye method.134 The blue dye method provides a novel, inexpensive and scalable assessment of gut transit time compared to traditional measures of stool frequency.134
Table 2.
Overview of gut bacterial taxa currently described to impact gastrointestinal motility, and experimental models used for motility assessment
| Gut bacterial species | Effect on gut motility | Methods used | Model organism and effect size (N) | Reference |
|---|---|---|---|---|
|
-Lactobacillus acidophilus -Bifidobacterium bifidum |
-Administration of L. acidophilus and B. bifidum to germ-free rats displayed improved intestinal transit and contractility of the small intestine | -Myoelectric recording in vivo -Measurements of a radioactive marker (Na251CrO4) distribution along the small intestine |
Rats (N = 5-18) | 35 |
| -Bacteroides thetaiotamicron | -Critical for enteric nervous system innervation -Administration of B. thetaiotamicron (Bt) restored the expression of excitatory and inhibitory motor neurons signaling enzymes |
-Colonic manometry in vitro (colonic motility measurements in the colonic tissue of SPF, GF and Bt-conventionalized mice) -Immunohistochemistry (neuronal cell populations determination) -qPCR (expression of ENS signaling enzymes) |
Mice (N = 3-5) | 42 |
| -Vibrio cholerae | -Use of the syringe-like type VI secretion system for induction of intestinal movements, which leads to expulsion of the resident microbiota by the host | -In vivo imaging of fluorescently-labeled bacterial populations and dynamics of unlabeled intestinal tissue (motility) | Zebrafish (N = 5-6) | 48 |
| -Lactobacillus rhamnosus GG | -Increase of choline acetyltransferase expression (responsible for synthesis of main metabolite involved in gut motility, acetylcholine) in the ENS after administration of L. rhamnosus GG | -RT-PCR -Immunoblotting -Immunostaining |
Mice (N = 3-5) | 43 |
| -Escherichia coli Nissle 1917 | -Inhibitory effect on the smooth muscle contractility | -Ex vivo organ bath model (contractility measurements after application of E. coli Nissle 1917 bacterial supernatant) | Rats (N = 5) | 59 |
|
-Bifidobacterium longum -Lactobacillus acidophilus -Streptococcus thermophilus -Enterococcus faecalis |
Inhibitory effect on human colonic smooth muscle in vitro | -Ex vivo organ bath model (contractility measurements after application of sonicated cell fractions and bacterial supernatants) | Humans (N = 25) | 58 |
|
Akkermansia muciniphila Bacteroides spp -Alistipes |
Modulation of longer gut transit time in humans | -Blue dye method (measurements of gut transit time in humans) | Humans (N = 863) | 134 |
|
-Ruminococcus -Bacteroides -Prevotella |
-Abundance of Ruminococcus and Bacteroides is linked to shorter transit times -Abundance of Prevotella is linked to longer transit times |
-Bristol Stool Scale score (measurements of colonic transit times in humans) | Humans (N = 53) | 135 |
| -Lactic acid bacteria (Lactobacillus plantarum 2362, Lactobacillus casei ssp. paracasei 19, Leuconostoc raffinolactis 23~77:1, and Pediococcus pentosaceus 16:1) | -Amelioration of the small intestinal contractile impairment in traumatic brain injury mouse model fed with probiotic mixture | -Ex vivo organ bath model (contractility measurements) | Mice (N = 6) | 65 |
| -Clostridium butyricum | -Promotion of ICCs proliferation and intestinal motility by the regulation of TLR2 expression on ICCs after stimulation with C. butyricum suspensions | -Cell culture (culture of ICCs) RT-PCR (expression of TLR2) -Western Blot (protein levels of TLR2) |
NA | 66 |
| -Bifidobacterium -Lactobacillus | -Modulation of ghrelin signaling (acetate, propionate and butyrate) | -Cell culture (activation of G protein coupled receptors using β-arrestin assay stimulated with bacterial supernatants) | NA | 71 |
| -Spore-forming bacteria | -Modulation of metabolites that promote transit time | -GI transit assay using carmine red in vivo (gut motility measurements after colonization of germ-free mice with fecal samples from spore-forming conventionalized mice) | Mice (N = 4-8) | 90 |
| -Lactobacillus casei subsp. casei | -Administration of heat killed L. casei subsp. casei increases levels of 5-HT in the colonic tissue and lowers bead expulsion time | -HPLC (5-HT levels) Bead expulsion test (colonic transit analysis) |
Mice (N = 6) | 91 |
| -Escherichia coli -Fusobacterium nucleatum | -Modulation of gut motility via L-type voltage-dependent Ca2+ channels located on the colonic smooth muscle cells -Control of serotonin release from model of enterochromaffin cells |
-RIN14B cell line in vitro (5-HT release measurements after application of 5-hydroxyindole produced from E. coli and F. nucleatum derived)) -GI transit assay using carmine red in vivo (gut motility measurements after application of 5-hydroxyindole produced from E. coli and F. nucleatum derived) -Ex vivo organ bath model (colonic contractility measurements after application of 5-hydroxyindole produced from E. coli and F. nucleatum derived-) |
Rats (N = 6-10) | 4 |
| -Akkermansia muciniphila | -Production of protein P9 signals to L cells to produce GLP-1 | -ELISA (GLP-1 quantification after stimulation with bacterial pellets or supernatants) | NA | 72 |
| -Edwardsiella tarda | -Activation of TRPA1 in EECs, leads to production of 5-HT from enterochromaffin cells and thus modulate gut motility | -Real-time measurements of EECs in vivo in zebrafish (activation of TRPA1 and gut motility after oral gavage of indole or indole-3-acetaldehyde produced from E. tarda to zebrafish) -Amperometry (5-HT release after application of indole or indole-3-acetaldehyde produced from E. tarda to the mouse or human small intestinal tissue) |
Zebrafish (N = 117-213) | 102 |
| -Fusobacteria | -Improvement of the symptoms of constipation in rat loperamide-induced constipation model | -Charcoal propulsion test (gut motility measurements after oral administration of quercetin derived from Fusobacteria genera to rats) | Rats (N = 3) | 103 |
|
-Prevotella -Lactobacillus -Alistipes |
-Enhancement of colonic contractility ex vivo and stool frequency in vivo |
-Ex vivo organ bath model (colonic contractility measurements after application of saturated long-chain fatty acids derived from Prevotella, Lactobacillus and Alistipes genera to the rat colonic tissue) -Fecal pellets collection (stool frequency measurements after conventionalization of germ-free rats) |
Rats (N = 6-8) | 104 |
| -Lactobacillus fermentum | -Acceleration of gastrointestinal transit and gastric emptying | -Charcoal propulsion test (gut transit measurements after oral administration of ferulic acid derived from L. fermentum to rats) -Phenol red detection (gastric emptying measurements after oral administration of ferulic acid derived from L. fermentum to rats) |
Rats (N = 8) | 106 |
| -Morganella morganii -Lactobacillus reuteri | -Promotion of colonic motility via activation of histamine receptors in the gut | -Fecal output assay (after individual mice were fed with L-histamine) | Mice (N = 3-5) | 107 |
| -Clostridium sporogenes | -Potent stimulation of ileal motility ex vivo | -Ex vivo organ bath model (ileal contractility measurements after application of 3-(3,4-dihydroxyphenyl)propionic acid derived from C. sporogenes to the ileal rat tissue) | Mice (N = 4-6) | 108 |
Yet, gut bacterial distribution is not accessible in vivo. In vitro studies involve cell culturing techniques, such as isolation of smooth muscle cells,8 neuronal cell populations42 or ICCs.66,67 Among others, cell line models of enterochromaffin cells are used to study 5-HT release upon stimulation of microbial stimuli.4,90 Electrophysiology studies, such as whole-cell patch clamp are employed to measure membrane currents and potentials.67 Other molecular biology techniques such as RT-PCR, qPCR and immunohistochemistry are used in gene expression studies and detection of specific proteins in the intestinal tissue, respectively.42,43,66,67 Ex vivo organ bath systems remain the most frequently used method to unravel the effects of gut bacteria and their metabolites on gut contractility.4,8,38,39,42,58 An organ bath set-up employs suspending a section of intestinal tissue in a bath, oxygenated with carbogen gas mixture (5% CO2, balanced with O2) in a Krebs-Henseleit solution, and the application of a pressure transducer and data-acquisition software.4,58,108 The organ bath systems are designed to imitate in vivo situation. Although an organ bath clearly misses the biological complexity of an organism, organ bath-based studies provide the possibility to elucidate the mechanisms underlying the effects of gut bacteria or their metabolites on the intestinal contractility.4,8,40 In recent years, mini-guts or guts on chip141–143 models have emerged as very promising tools to investigate the complexities of the gut motility system. Analogously, the Ussing chamber system can be utilized as a valuable tool for measuring gut integrity and intestinal permeability,5,117 both of which have their impact on the gut motility as discussed earlier.
However, the complexity of the different processes that occur during digestion and absorption of nutrients, the vast interindividual variability in terms of their microbiota composition and the limitations of the currently available techniques,137 make gaining such a mechanistic insight very challenging. The complexity further arises from the fact that multiple physical, biological and chemical aspects interact to determine the dynamics of the gut. For example, gut bacteria, their metabolites and the nutrients on which the bacteria feed upon are transported, mixed and dispersed by the fluid flow of the gut, and thus, the stability of the bacterial population is strongly influenced by the flow. At the same time, the fluid flow is determined by gut motility, which is affected by bacterial metabolites, as well as by external factors such as the feeding state, circadian rhythm, meal content and viscosity (Figure 2). Therefore, to understand the long-term consequences of bacterial metabolites-host interactions, one has to take into account the biophysics of the gut as an interconnected system.
Currently, in silico models are employed to address some of the above-mentioned limitations, and focus on some of the key components of the system. For example, in silico models allow the integration of gut motility with an exact fluid flow calculation, solute and bacterial transport, and feedback of bacteria and components on motility and flow . These systems can accommodate exact organ geometries and motility patterns, which cannot be mimicked in an in vitro/ex vivo system. Moreover, such systems are flexible, and can help to explore the impact of many different, experimentally unknown, parameters in a systematic way. Three main areas of research have been addressed so far in silico and in theory: 1) motility and its effects on fluid flows, 2) transport and absorption of solutes, as well as digestion, and 3) bacterial population. Each of these areas is essential to build a complete picture to understand the effect of the microbiota and its metabolites on motility via the ensuing fluid flow that transport them. Despite being promising, in silico modeling is still in its infancy and an in silico model including all the interactions between motility, flow, transport, bacteria and metabolites is yet lacking. To the best of our knowledge, no in silico study including the feedback of bacterial metabolites onto motility has been developed yet. However, combining modeling with experimental data may lead the way to address many unresolved questions with respect to the microbiota-host interplay.144 Herein, we review currently available information that may help researchers in the field to structure a comprehensive model accounting for motility-induced flows, flow-associated transport of microbes and metabolites, as well as feedback of metabolites on motility (see Supplementary information for more information on on flow and transport in tubes providing background information for our review on existing in silico studies).
Design of in silico studies to unravel the microbiota-gut motility interplay
While how bacteria regulate motility via their produced metabolites has been studied extensively, unraveling the full interplay of microbiota and gut motility also requires understanding of the feedback of gut motility on the microbiota (Figure 2). Metabolites that change motility impact flows and thus nutrients absorption, indirectly affecting the host wellbeing. Moreover, changes in the flow will impact the microbiota population and growth, and thus the metabolites produced. In silico models are the most indicated models to take into account all of these aspects of the microbiota-gut motility interplay. So far, a comprehensive model that combines all these aspects is still lacking. To build a comprehensive description, the first challenge is to quantitatively model the metabolite-motility interaction, which is widely unexplored in in silico models. Although this proves to be difficult, one can still use effective descriptions of flow and transport, as outlined above, to assess how metabolite induced-changes in motility impact the long-term distribution of bacteria and nutrients. In the following section, we outline how to describe motility, fluid flows and particle dispersion to pave the way for a comprehensive model of microbiota-gut motility interplay. We further highlight challenges in describing the metabolite-motility interaction first and foremost.
Contractility and flows
Flows are determined by the gut motility and the gut geometry (see the Supplementary Information for more theoretical details); thus, any impact of metabolites onto motility affects the flow. One strategy for in silico studies to account for the effect of bacterial metabolites on motility is to employ motility patterns from experimental observation, as provided by exact motility maps (i.e. video recordings of the gut contractions) of ex vivo models,61,62,145–147 magnetic resonance imaging (MRI) measurements in vivo,136–138 pressure measurements in vivo with manometers137,139 or mini capsules.137,138,140 The observed motility patterns can be also generalized into theoretical maps from ex vivo motility maps.61,62 So far, only the most basic motility patterns have been studied, but the approach can be extended to metabolite-affected patterns. Another approach to model the effect of metabolites on gut motility is by engineering the contraction patterns using in silico models of the gut wall. The gut wall is viewed as an elastic or viscoelastic membrane representing the intestinal muscle layer, which is activated by the ICCs network through electrical stimuli (taken by ex vivo recordings), and thus produces a motility pattern.148–151 The underlying challenge is to understand and describe the effect of bacterial metabolites onto the ICCs network and the muscular contractions. Yet the advantage is the potential for direct comparison of simulated contraction patterns with experimental recording for model verification. To date, three types of contractions have been modeled in silico under normal conditions or after nutrients stimulation: peristalsis, segmentation/local stationary contraction, and pendular activity/longitudinal contractions. For the gut, multiple definitions of peristalsis have been described:152 the peristaltic reflex describes the bolus movement, where circular and longitudinal muscles combined, produce a constriction upward and a dilation downward;152–154 or a sine-like wave or a train of sine waves (as for example in the coordinated contractions of the migrating motor complexes phase III, see also the Supplementary Information)).61,151,152,155–158 Segmentation is also subjected to multiple definitions, from stationary local contractions induced exclusively by the circular muscles145,154,159–163 to Canon’s segmentation,164 which can be described as modulation of sine waves.61,62 Finally, pendular activity is connected to the contraction of the longitudinal muscles.145,159–161,165–168 Segmentation and pendular activity are mainly postprandial,61,62,169 while peristalsis happens both for the bolus transport in the peristaltic reflex,152,169 thus postprandial, and during migrating motor complexes, thus during fasting.152,158 If the bacterial metabolite-induced changes in motility correspond to or are similar to one the above-mentioned well-known patterns, then existing literature outlined above would help in modeling which type of flow is produced by the motility pattern and how it affects the host and microbiota composition. In fact, once motility is known together with the inflow within the gut, both the flow in the axial (i.e. along the tube) and the radial direction are determined . Radial flows impact metabolite solute mixing, its availability at the wall for absorption, and its radial distribution; longitudinal flows impact the longitudinal dispersion of the solute and its transit times, with longer transit times correlated to higher absorption.
Different types of motility have different impact on the radial and longitudinal flows. For the longitudinal flow, it was shown that peristalsis is mainly propelling with a high net longitudinal flow forward associated with cleansing,61,151,169,170 while segmentation and pendular activity are slowing the content down to increase absorption,61,169 with strong longitudinal flows forward and backward (increasing dispersion) but a low net-flow movement forward. For the colon, where absorption of water is important, pumping of water decreases the longitudinal velocity along the tube.171 As for radial effects, the moving walls produce radial flows promoting the solute’s radial displacement.156,160,163,171,172 Often vortices are reported for low viscosity environments and water-like content, although increasing viscosity decreases such vortices, so in a real system where viscosity is higher due to the food content or the presence of fibers, vortices should play a minor role.139,153,154,157,160,163,165,167 Vortices are connected to mixing effects156,173 (discussed in the next section).
Viscosity has also been discussed in relation to the mucus layer of the intestine elsewhere.153,171,174–177 Not only macro flow (i.e., flow across the lumen and along the tube), but also microflow due to the villi movement are considered. Villi movement produces a local flow in the fluid layer close to the wall that increases absorption and mixing.166,172,173,178–181 Together, the gut, by changing its motility, changes the radial flows, turbulence, mixing, and net flow forward, thus transit time. The gut can also switch between fast and slow flows varying parameters like occlusion (higher occlusion means more mixing and faster flows), wavelength and frequency (higher frequencies means higher flows).182,183 Thus, any impact from the bacterial metabolite on motility pattern or on motility parameters will consequently change flows, which in turn will impact nutrients and bacterial-produced metabolites diffusion, but also bacterial colonization (Figure 2).
Transport and absorption of solutes
Describing solute transport (see Supplementary Information), secretion, degradation and absorption are essential to model the bacterial metabolite effect on motility. Solutes can be gases like oxygen or nutrients (such as glucose and amino acids), drugs, bacteria, bacterial metabolites, or antibodies. In general, solutes are diffusing, and advected by the flow, thus enhancing their apparent diffusivity due to the Taylor dispersion effect (see Supplementary Information).184 Solutes and particles can be mixed by the flow vortices, react with other solutes (through chemical reactions, degradation, or consumption by the microbiota), and be secreted or absorbed at the wall. To investigate the effect of fluid flows on solutes, transit times, absorption, and mixing are generally addressed. Absorption by the gut walls (for example of glucose in the small intestine) is increased if the solute remains in the gut a sufficiently long time,173,178 which can be achieved by slow flows thus long transient times, by long tubes, and an extended absorption surface (which is increased by the villi). Mixing and enhanced diffusion at the macro and micro levels due to flow, contractions and villi movement are also improving absorption,173 since the solute in this way is radially transported more efficiently to the wall. Mixing also helps to make the nutrients come in contact in the bulk with the enzymes secreted by the gut.173 It is well established that local circular muscle contractions, pendular activity and segmentation increase mixing and that increasing parameters such as occlusion, wavelength and frequency of contraction also increase mixing.173 Increasing chyme viscosity (for example, by increasing dietary fiber content) decreases mixing, since vortices which could arise in water-like environment decrease in duration and intensity or disappear,173 and decreases particle diffusivity (which enables particles to escape the flow streamlines and move radially to the walls for absorption, even in the absence of vortices). Accordingly, a higher content in dietary fiber can impact nutrients and drugs absorption.138,177,185 In conclusion, motility will impact flows and solute transport; this influences the solutes dynamics and interactions. Therefore, bacterial-produced metabolites, acting on motility, have an indirect effect on the solutes, thus on their own diffusion and advection (Figure 2). This type of feedback can determine the fate of the bacterial metabolite itself, e.g., whether the metabolite-induced motility aims at increasing or decreasing the metabolite concentration, which, in turn, affects the growth and colonization of the microbiota, and eventually impacts host health. Only by considering the impact of the fluid flow can these points be addressed.
Bacteria and their interactions with the flow
Bacteria can be considered as particles transported and mixed by the flow, similar to solutes. Moreover, their dynamics is coupled to the nutrient and oxygen availability. Bacterial metabolites are also subjected to the same flow effects, while also being connected to the bacteria dynamics. Therefore, to assess the impact of metabolites on the gut system, the relationship between bacteria and fluid flows should be addressed. Nevertheless, there are few studies that relate flow and bacterial washout. This section focuses on in silico studies which can be used as future basis to explore the flow effect on bacteria, which in turn, will help simulate the impact of bacterial-produced metabolites on motility (Figure 2). For the small intestine, Ishikawa et al. focused on the washout conditions, the influence of flow, nutrients, and oxygen availability for aerobic and anaerobic bacteria, for peristalsis only.186 The study showed that, even with peristaltic flow, a stable bacterial population can be found in the small intestine. Specifically, the flow field enhances the radial variation of nutrients and bacterial concentration. The authors observed a longitudinal bacterial distribution coherent with experimental data, with aerobes in higher numbers on the upstream side, and anaerobes on the downstream side. For the colon, bacterial washout was also discussed by Cremer et al. for peristalsis.141 Motivating their in silico model on mini-gut data, the authors showed, in vitro and in silico, that repeated contraction is crucial to avoid washout, even though a strong longitudinal flow is present. In fact, mixing helps to overcome flow. In their following study, Cremer et al., showed how pH and water absorption are crucial in shaping the bacterial distribution in the colon.187
Nonetheless, most of the in silico studies for the colon aim to represent digestion in the most complete way. Usually, these models include a high number of equations and parameters, describing different bacterial species, their interactions, degradation of fibers, and production of metabolites.137,171,174,176,188–191 In these studies, the flow is discussed mainly as an effective transit time, influenced by viscosity, the mucus layer, and water absorption. Interestingly, Labarthe et al. incorporated specific radial wall motility into the model by effective radial flows, and showed that radial gradients of viscosities due to mucus layer and water absorption, together with chemotaxis and radial flows, help the presence of bacteria at the walls: bacteria find a niche where they can prosper, helping their adhesion to the mucus layer.171 These bacteria can then detach and seed the lumen, contributing to the colonization of bacteria. This result calls for the need of incorporating such type of radial dynamics when describing the bacterial presence and their produced metabolites in a realistic way. Still, it is not clear how to implement different types of motility in these models, although varying longitudinal and radial mean flow intensity can be used as a proxy for different motilities.
Conclusions
Even though the knowledge gap between the microbiota and bowel function is narrowing, enormous efforts are undoubtedly needed to fully unravel the underlying mechanisms, thereby potentiating the application of microbiota-targeted therapies in clinical practice and in a personalized manner. Future ex vivo and in vivo studies should focus on unraveling the basic mechanisms required to feed the in silico models. The latter should focus on the representation of the different elements that regulate the interaction between gut microbes and bowel movement, as well as feedbacks, including physics elements like fluid flow. Predictions from in silico and ex vivo can be then verified in in vivo models, to evaluate if all gut motility-regulatory elements are taken into consideration. Altogether, combining in silico, ex vivo, and in vivo outcomes will provide novel mechanistic insights in the microbiota-gut motility interplay.
Supplementary Material
Funding
S.E.A. is supported by Rosalind Franklin Fellowships https://www.rug.nl/about-ug/work-with-us/rff/, co-funded by the European Union and University of Groningen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
B.W., K.A. and A.C. researched data for the article, designed the figures, and wrote the manuscript. S.E.A reviewed/edited the manuscript before submission. All authors approved the final version of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160(1):99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Sanders KM, Koh SD, Ro S, Ward SM.. Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 2012;9(11):633–26. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020;17(6):338–351. doi: 10.1038/s41575-020-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waclawiková B, Bullock A, Schwalbe M, Aranzamendi C, Nelemans SA, van Dijk G, El Aidy S. Gut bacteria-derived 5-hydroxyindole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLOS Biol. 2021;19(1):e3001070. doi: 10.1371/journal.pbio.3001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, et al. Gut microbiota-produced tryptamine activates an Epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23(6):775–785.e5. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang S, Park MY, Brooks I, Lee J, Kim SH, Kim JY, Oh B, Kim JW, Kwon O. Spore-forming Bacillus coagulans SNZ 1969 improved intestinal motility and constipation perception mediated by microbial alterations in healthy adults with mild intermittent constipation: a randomized controlled trial. Food Res. Int. 2021;146:110428. doi: 10.1016/j.foodres.2021.110428. [DOI] [PubMed] [Google Scholar]
- 7.Krammer HJ, Von Seggern H, Schaumburg J, Neumer F. Effect of lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology. 2011;33(2):109–113. doi: 10.1007/s00053-011-0177-0. [DOI] [Google Scholar]
- 8.Blakeney BA, Crowe MS, Mahavadi S, Murthy KS, Grider JR. Branched short-chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig. Dis. Sci. 2019;64(5):1171–1181. doi: 10.1007/s10620-018-5417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surjanhata BC, Kuo B. Gastrointestinal motility and enteric neuroscience in health and disease. Ref Modul Biomed Sci. 2014. doi: 10.1016/B978-0-12-801238-3.00051-9. [DOI] [Google Scholar]
- 10.El Aidy S, van Baarlen P, Derrien M, Lindenbergh-Kortleve DJ, Hooiveld G, Levenez F, Doré J, Dekker J, Samsom JN, Nieuwenhuis EES, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567–579. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 11.El Aidy S, Kunze W, Bienenstock J, Kleerebezem M. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Benef Microbes. 2012;3(4):251–259. doi: 10.3920/BM2012.0042. [DOI] [PubMed] [Google Scholar]
- 12.Phillips R, Kondev J, Theriot J, and Garcia H. Physical biology of the cell (New York City: Garland Science; ). 2013. [Google Scholar]
- 13.Anderson RB, Newgreen DF, Young H. Neural crest and the development of the enteric nervous system. 2013. [DOI] [PubMed]
- 14.Rao JN, Wang J-Y. Intestinal architecture and development. 2010.
- 15.Von Haller AA. Dissertation on the sensible and irritable parts of animals. Bulletin of the Institute of the History of Medicine (1755). [Google Scholar]
- 16.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. [DOI] [PubMed] [Google Scholar]
- 17.Furness JB. The enteric nervous system (Oxford: Blackwell Publishing; ). 2006. [Google Scholar]
- 18.Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182(6):1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsvilovskyy VV, Zholos, AV, Aberle, T, Philipp, SE, Dietrich, A, Zhu, MX, Birnbaumer, L, Freichel, M, and Flockerzi, V . Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HK, Shuttleworth CW, Sanders KM. Tachykinins activate nonselective cation currents in canine colonic myocytes. Am. J. Physiol. - Cell Physiol. 1995;269(6):C1394–C1401. doi: 10.1152/ajpcell.1995.269.6.C1394. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Horowitz B, Buxton ILO. Muscarinic receptors in canine colonic circular smooth muscle. I. Coexistence of M2 and M3 subtypes. Mol Pharmacol. 1991;40:943–951. [PubMed] [Google Scholar]
- 22.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 2001. Elsevier Inc.;68(22–23):2573–2578. doi: 10.1016/S0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 23.Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- 24.Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in Guinea pig ileum through a G protein. Am. J. Physiol. - Cell Physiol. 1990;258(6):C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- 25.Grady EF, Baluk, P, Böhm, S, Gamp, PD, Wong, H, Payan, DG, Ansel, J, Portbury, AL, Furness, JB, and McDonald, D, . Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 1996;16(21):6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W, Pricolo VE, Zhang L, Behar J, Biancani P, Kirber MT. Gq-linked NK2 receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology. 2000;119(1):51–61. doi: 10.1053/gast.2000.8552. [DOI] [PubMed] [Google Scholar]
- 27.Lecci A, Capriati A, Altamura M, Maggi CA. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Autonomic Neuroscience: Basic and Clinical. 2006;126–127:232–249. doi: 10.1016/j.autneu.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Mulè F, Amato A, Vannucchi MG, Faussone-Pellegrini MS, Serio R. Role of NK 1 and NK 2 receptors in mouse gastric mechanical activity. Br. J. Pharmacol. 2006;147(4):430–436. doi: 10.1038/sj.bjp.0706645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of β-NAD + and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol. Motil. 2013;25(3):e194–e204. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera LR, Poole DP, Thacker M, Furness JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterology and Motility. 2011;23(11):980–988. doi: 10.1111/j.1365-2982.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 31.Wostmann B, Bruckner-Kardoss E. Development of cecal distention in germ-free baby rats. Am. J. Physiol. 1959;197(6):1345–1346. doi: 10.1152/ajplegacy.1959.197.6.1345. [DOI] [PubMed] [Google Scholar]
- 32.Abrams GD. Microbial effects on mucosal structure and function. Am. J. Clin. Nutr. 1977;30(11):1880–1886. doi: 10.1093/ajcn/30.11.1880. [DOI] [PubMed] [Google Scholar]
- 33.Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc. Soc. Exp. Biol. Med. 1967;126(1):301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 34.Iwai H, Ishihara Y, Yamanaka J, Ito T. Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn. J. Exp. Med. 1973;43:297–305. [PubMed] [Google Scholar]
- 35.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol - Gastrointest Liver Physiol. 2001;280:368–380. [DOI] [PubMed] [Google Scholar]
- 36.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. 2012;143(4):1006–1016.e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mcvey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013;25(2):183–190. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 38.Ge X, Ding, C, Zhao, W, Xu, L, Tian, H, Gong, J, Zhu, M, Li, J, and Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017;15(1):1–9. doi: 10.1186/s12967-016-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578(7794):284–289. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 40.Muller PA, Koscsó B, Rajani G, Stevanovic K, Berres M-L, Hashimoto D, Mortha A, Leboeuf M, Li X-M, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772–1782.e4. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 42.Aktar R, Parkar N, Stentz R, Baumard L, Parker A, Goldson A, Brion A, Carding S, Blackshaw A, Peiris M, et al. Human resident gut microbe bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes. 2020;11(6):1745–1757. doi: 10.1080/19490976.2020.1766936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandrasekharan B, Saeedi BJ, Alam A, Houser M, Srinivasan S, Tansey M, Jones R, Nusrat A, Neish AS. interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology. 2019;157(1):179–192.e2. doi: 10.1053/j.gastro.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarandi SS, Kulkarni S, Saha M, Sylvia KE, Sears CL, Pasricha PJ. Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology. 2020;159(1):200–213.e8. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Roager HM, Licht TR, Harvey TA, Prum RO. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018. Accepted;9(1):1–10. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11(4):1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 48.Logan SL, Thomas, J, Yan, J, Baker, RP, Shields, DS, Xiavier, JB, Hammer, BK, and Parthasarathy, R . The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts PNAS 115 16 . 2018. doi: 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeves-Darby VG, Turner JA, Prasad R, Chopra AK, Chary P, Clench MH, Peterson JW, Mathias JR. Effect of cloned Salmonella typhimurium enterotoxin on rabbit intestinal motility. FEMS Microbiol. Lett. 1995;134(2–3):239–244. doi: 10.1111/j.1574-6968.1995.tb07944.x. [DOI] [PubMed] [Google Scholar]
- 50.Somlyo AP, Somlyo AV. Ca 2+ sensitivity of smooth muscle and nonmuscle Myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 51.Taylor DA, Stull JT. Calcium dependence of myosin light chain phosphorylation in smooth muscle cells. J. Biol. Chem. 1988;263(28):14456–14462. doi: 10.1016/S0021-9258(18)68241-9. [DOI] [PubMed] [Google Scholar]
- 52.Mitra R, Morad M. Ca2+ and Ca2+ -activated K + currents in mammalian gastric smooth muscle cells. Science (80-). 1985;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- 53.Droogmans G, Callewaert G. Ca2+-channel current and its modification by the dihydropyridine agonist BAY k 8644 in isolated smooth muscle cells. Pflügers Arch. Eur. J. Physiol. 1986;406(3):259–265. doi: 10.1007/BF00640911. [DOI] [PubMed] [Google Scholar]
- 54.Vogalis F, Publicover NG, Hume JR, Sanders KM. Relationship between calcium current and cystolic calcium in canine gastric smooth muscle cells. Am. J. Physiol. - Cell Physiol. 1991;260(5):C1012–C1018. doi: 10.1152/ajpcell.1991.260.5.C1012. [DOI] [PubMed] [Google Scholar]
- 55.Wegener JW, Schulla, V, Koller, A, Klugbauer, N, Feil, R, and Hofmann, F . Control of intestinal motility by the Cav 1.2 L-type calcium channel in mice. FASEB J. 2006;20(8):1260–1262. doi: 10.1096/fj.05-5292fje. [DOI] [PubMed] [Google Scholar]
- 56.Gibbons SJ, Strege, PR, Lei, S, Roeder, JL, Mazzone, A, Ou, Y, Rich, A, and Farrugia, G . The α1HCa2+channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J. Cell. Mol. Med. 2009;13(11–12):4422–4431. doi: 10.1111/j.1582-4934.2008.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76(1):387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong J, Bai T, Zhang L, Qian W, Song J, Hou X. Inhibition effect of Bifidobacterium longum, Lactobacillus acidophilus, Streptococcus thermophilus and Enterococcus faecalis and their related products on human colonic smooth muscle in vitro. PLoS One. 2017;12(12):1–15. doi: 10.1371/journal.pone.0189257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalziel JE, Mohan V, Peters J, Anderson RC, Gopal PK, Roy NC. The probiotic Escherichia coli Nissle 1917 inhibits propagating colonic contractions in the rat isolated large intestine. Food Funct. 2015;6(1):257–264. doi: 10.1039/C4FO00831F. [DOI] [PubMed] [Google Scholar]
- 60.Yin J, Chen JDZ. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J Cell Mol Med. 2008;12(4):1118–1129. doi: 10.1111/j.1582-4934.2008.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huizinga JD, Chen J-H, Fang Zhu Y, Pawelka A, McGinn RJ, Bardakjian BL, Parsons SP, Kunze WA, Wu RY, Bercik P, et al. The origin of segmentation motor activity in the intestine. Nat. Commun. 2014;5(1):1–11. 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huizinga JD, Parsons SP, Chen J-H, Pawelka A, Pistilli M, Li C, Yu Y, Ye P, Liu Q, Tong M. Motor patterns of the small intestine explained by phase-amplitude coupling of two pacemaker activities: the critical importance of propagation velocity. Am. J. Physiol. - Cell Physiol. 2015;309(6):C403–C414. doi: 10.1152/ajpcell.00414.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.CAJAL & SR . Sur les ganglions et plexus nerveux de l’intestin. CR Soc Biol Paris. 1893;45:217–223. [Google Scholar]
- 64.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Zeng, J, Ma, Y, Tan, M, Fang, H, Bengmark, S, and Zhu, J . Effects of Synbiotic2000TM forte on the intestinal motility and interstitial cells of Cajal in TBI mouse model. Probiotics Antimicrob Proteins. 2017;9:172–181. [DOI] [PubMed] [Google Scholar]
- 66.Sui SJ, Tian, ZB, Wang, QC, Chen, R, and Nie, JLi, JS, and Wei, LZ . Clostridium butyricum promotes intestinal motility by regulation of TLR2 in interstitial cells of Cajal. Eur. Rev. Med. Pharmacol. Sci. 2018;22(14):4730–4738. doi: 10.26355/eurrev_201807_15533. [DOI] [PubMed] [Google Scholar]
- 67.Zuo DC, Choi, S, Shahi, PK, Kim, MY, Park, CG, Kim, YD, Lee, J, Chang, IY, Lee, HS, Yeom, SC. et al. Action of lipopolysaccharide on interstitial cells of cajal from mouse small intestine. Pharmacology. 2012;90(3–4):151–159. doi: 10.1159/000340018. [DOI] [PubMed] [Google Scholar]
- 68.Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019;15(4):226–237. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 69.Wu T, Rayner CK, Young RL, Horowitz M. Gut motility and enteroendocrine secretion. Curr Opin Pharmacol. 2013;13(6):928–934. doi: 10.1016/j.coph.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192(2):227–240. doi: 10.1007/BF00220741. [DOI] [PubMed] [Google Scholar]
- 71.Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, El Aidy S, Ross P, Roy BL, Stanton C, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019;33(12):13546–13559. doi: 10.1096/fj.201901433R. [DOI] [PubMed] [Google Scholar]
- 72.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim J-H, Han D, Cha KH, Moon SH, Lee K, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021;6(5):563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 73.Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14(7):390–403. doi: 10.1038/s41574-018-0016-2. [DOI] [PubMed] [Google Scholar]
- 74.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology. 1983;84(4):747–751. doi: 10.1016/0016-5085(83)90141-5. [DOI] [PubMed] [Google Scholar]
- 75.Anitha M, Reichardt F, Tabatabavakili S, Nezami BG, Chassaing B, Mwangi S, Vijay-Kumar M, Gewirtz A, Srinivasan S. Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice. CMGH. 2016;2(3):328–339. doi: 10.1016/j.jcmgh.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018;8(1):1–9. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee EY, Zhang X, Miyamoto J, Kimura I, Taknaka T, Furusawa K, Jomori T, Fujimoto K, Uematsu S, Miki T, et al. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J. Endocrinol. 2018;239(3):267–276. doi: 10.1530/JOE-18-0241. [DOI] [PubMed] [Google Scholar]
- 78.Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010;153(1–2):47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexe. Neurogastroenterol. Motil. 2004;16(s1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 80.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells e A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keating DJ, Peiris H, Kyloh M, Brookes SJH, Spencer NJ. The presence of 5-HT in myenteric varicosities is not due to uptake of 5-HT released from the mucosa during dissection: use of a novel method for quantifying 5-HT immunoreactivity in myenteric ganglia. Neurogastroenterol. Motil. 2013;25(10):849–853. doi: 10.1111/nmo.12189. [DOI] [PubMed] [Google Scholar]
- 83.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Yadav VK, Balaji, S, Suresh, PS, Liu, XS, Lu, X, Li, Z, Guo, XE, Mann, JJ, Balapure, AK, Gershon, MD. et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med. 2010;16(3):308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Chalazonitis A, Huang -Y-Y, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 2011;31(24):8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heredia DJ, Gershon, MD, Koh, SD, Corrigan, RD, Okamoto, T, and Smith, TK . Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J. Physiol. 2013;591(23):5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJH, Sia TC, Wattchow DA, Spencer NJ, Keating DJ. Identification of unique release kinetics of serotonin from Guinea-pig and human enterochromaffin cells. J. Physiol. 2013;591(23):5959–5975. doi: 10.1113/jphysiol.2013.259796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin AM, Young, RL, Leong, L, Rogers, GB, Spencer, NJ, Jessup, CF, and Keating, DJ . The diverse metabolic roles of peripheral serotonin. Endocrinology. 2017;158:1049–1063. [DOI] [PubMed] [Google Scholar]
- 89.Keating DJ, Spencer NJ. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol Res. 2019;140:50–55. [DOI] [PubMed] [Google Scholar]
- 90.Yano JM, Yu, K, Donaldson, GP, Shastri, GG, Ann, P, Ma, L, Nagler, CR, Ismagilov, RF, Mazmanian, SK, and Hsiao, EY . Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hara T, Mihara T, Ishibashi M, Kumagai T, Joh T. Heat-killed Lactobacillus casei subsp. casei 327 promotes colonic serotonin synthesis in mice. J Funct Foods. 2018;47:585–589. doi: 10.1016/j.jff.2018.05.050. [DOI] [Google Scholar]
- 92.Racké K, Reimann A, Schwörer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1995;73(1–2):83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 93.Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the Guinea pig colon. Neurogastroenterol. Motil. 2014;26(11):1586–1596. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am. J. Physiol. - Gastrointest. Liver Physiol. 2007;292(1):429–437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 95.Wichmann A, Allahyar A, Greiner T, Plovier H, Lundén G, Larsson T, Drucker D, Delzenne N, Cani P, Bäckhed F, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14(5):582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Chimerel C, Emery E, Summers D, Keyser U, Gribble F, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9(4):1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet A, Qiu Z, Maher L, Redinbo M, Phillips R, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity. 2014;41(2):296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jennis M, Cavanaugh, CR, Leo, GC, Mabus, JR, Lenhard, J, and Hornby, PJ . Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2017;30:1–12. [DOI] [PubMed] [Google Scholar]
- 100.Dey N, Wagner, VE, Blanton, LV, Cheng, J, Fontana, L, Haque, R, Ahmed, T, and Gordon, JI . Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alemi F, Poole, DP, Chiu, J, Schoonjans, K, Cattaruzza, F, Grider, JR, Bunnett, NW, and Corvera, CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye L, Bae, M, Cassilly, CD, Jabba, SV, Thorpe, DW, Martin, AM, Lu, HY, Wang, J, Thompson, JD, Lickwar, CR, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2020;1–18. doi: 10.1101/2020.06.09.142133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JE, Lee, MR, Park, JJ, Choi, JY, Song, BR, Son, HJ, Choi, YW, Kim, KM, Hong, JT, Hwang, DY. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm. Biol. 2018;56(1):309–317. doi: 10.1080/13880209.2018.1474932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao L, Huang Y, Lu L, Yang W, Huang T, Lin Z, Lin C, Kwan H, Wong HLX, Chen Y, et al. Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome. 2018;6(1):1–16. doi: 10.1186/s40168-018-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sánchez M, Suárez, L, Andrés, MT, Flórez, BH, Bordallo, J, Riestra, S, Cantabrana, B. Modulatory effect of intestinal polyamines and trace amines on the spontaneous phasic contractions of the isolated ileum and colon rings of mice. Food Nutr. Res. 2017;61(1):1321948. doi: 10.1080/16546628.2017.1321948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Badary OA, Awad AS, Sherief MA, Hamada FMA. In vitro and in vivo effects of ferulic acid on gastrointestinal motility: inhibiton of cisplatin-induced delay in gastric emptying in rats. World J Gastroenterol. 2006;12:5363–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen H, Nwe P-K, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, Crawford JM, et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. 2019;177(5):1217–1231.e18. doi: 10.1016/j.cell.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Kessel SP, de Jong, HR, Winkel, SL, van Leeuwen, SS, Nelemans, SA, Permentier, Hjalmar, Keshavarzian, A, El Aidy, S. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol. 2020;18(1):1–14. doi: 10.1186/s12915-020-00876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zar MA, Ebong OO, Bateman DN. Effect of metoclopramide in Guinea-pig ileum longitudinal muscle: evidence against dopamine-mediation. Gut. 1982;23(1):66–70. doi: 10.1136/gut.23.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fioramonti J, Fargeas M-J, Honde C, Bueno L, J, F., MJ, F., C, H. & L, B . Effects of central and peripheral administration of dopamine on pattern of intestinal motility in dogs. Dig. Dis. Sci. 1984;29(11):1023–1027. doi: 10.1007/BF01311254. [DOI] [PubMed] [Google Scholar]
- 111.Marzio L, Neri M, Pieramico O, Donne MD, Peeters TL, Cuccurullo F. Dopamine interrupts gastrointestinal fed motility pattern in humans. Effect on motilin and somatostatin blood levels. Dig Dis Sci. 1990;35(3):327–332. doi: 10.1007/BF01537410. [DOI] [PubMed] [Google Scholar]
- 112.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 114.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol Part B Comp Biochem. 1987;86:439–472. [DOI] [PubMed] [Google Scholar]
- 115.Shaidullov IF, Sorokina DM, Sitdikov FG, Hermann A, Abdulkhakov SR, Sitdikova GF. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021;21(1):1–12. doi: 10.1186/s12876-021-01613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benech N, Rolhion N, Sokol H. Tryptophan metabolites get the gut moving. Cell Host Microbe. 2021;29(2):145–147. doi: 10.1016/j.chom.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 117.Williams BB, Van Benschoten A, Cimermancic P, Donia M, Zimmermann M, Taketani M, Ishihara A, Kashyap P, Fraser J, Fischbach M, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mars RAT, Yang Y, Ward T, Houtti M, Priya S, Lekatz HR, Tang X, Sun Z, Kalari KR, Korem T, et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell. 2020;182(6):1460–1473.e17. doi: 10.1016/j.cell.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Snell EE. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–329. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- 120.Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8(11):1–10. doi: 10.1371/journal.pone.0080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bischoff SC, Te Morsche RHM, van Heumen BWH, Nagengast FM, Peters WHM. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:1–25. doi: 10.1186/1471-230X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hertog MGL, Katan MB, Hollman PCH, Hertog MGL, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992;40(12):2379–2383. doi: 10.1021/jf00024a011. [DOI] [Google Scholar]
- 124.Park SY, Kim JH, Kim DH. Purification and characterization of quercitrin-hydrolyzing α-L-rhamnosidase from Fusobacterium K-60, a human intestinal bacterium. J Microbiol Biotechnol. 2005;15:519–524. [Google Scholar]
- 125.Kim D-H, Kim S-Y, Park S-Y, Han MJ. Metabolism of Quercitrin by human intestinal bacteria and its relation to some biological activities. Biol. Pharm. Bull. 1999;22(7):749–751. doi: 10.1248/bpb.22.749. [DOI] [PubMed] [Google Scholar]
- 126.Westfall S, Lomis N, Prakash S. Ferulic acid produced by lactobacillus fermentum influences developmental growth through a dTOR-mediated mechanism. Mol Biotechnol. 2019;61:1–11. [DOI] [PubMed] [Google Scholar]
- 127.Roager HM, Hansen, LBS, Bahl, MI, Frandsen, HL, Carvalho, V, Gøbel, RJ, Dalgaard, MD, Plichta, DR, Sparholt, MH, Vestergaard, H. et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol<. 2016;1(9):1–9. doi: 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- 128.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 129.Cui Y, Miao K, Niyaphorn S, Qu X. Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int. J. Mol. Sci. 2020;21(3):1–21. doi: 10.3390/ijms21030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Auteri M, Zizzo MG, Serio RGABA. GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11–21. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Falony G, Joossens, M, Vieira-Silva, S, Wang, J, Darzi, Y, Faust, K, Kurilshikov, A, Bonder, MJ, Valles-Colomer, M, Vandeputte, D, et al. Population-level analysis of gut microbiome variation. Science (80-). 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 132.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science (80-). 2019;363(6427):eaat9931. doi: 10.1126/science.aat9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Kessel SP, Frye, AK, El-Gendy, AO, Castejon, M, Keshavarzian, A, van Dijk, G, and El Aidy, S. Gut bacterial tyrosine decarboxylases restrict the bioavailability of levodopa, the primary treatment in Parkinson’s disease. Nat Commun. 2019;31:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asnicar F, Leeming, ER, Dimidi, E, Mazidi, M, Franks, P, Khatib, HA, Valdes, AN, Davies, R, Bakker, E, Francis, L, et al. Blue poo: impact of gut transit time on the gut microbiome using a novel marker. Gut. 2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65(1):57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ailiani AC, Neuberger, T, Brasseur, JG, Banco, G, Wang, Y, Smith, NB, and Webb, AG. Quantitative analysis of peristaltic and segmental motion in vivo in the rat small intestine using dynamic MRI. Magn. Reson. Med. 2009;62(1):116–126. doi: 10.1002/mrm.21982. [DOI] [PubMed] [Google Scholar]
- 137.Muttakin S, Moxon TE, and Gouseti O. In vivo, in vitro, and in silico studies of the GI tract. In: Interdisciplinary approaches to food digestion. Switzerland: Springer Nature; 2019. 29–67. doi: 10.1007/978-3-030-03901-1_3. [DOI] [Google Scholar]
- 138.Spiller R, Marciani L. Intraluminal impact of food: new insights from MRI. Nutrients. 2019;11(5):1147. doi: 10.3390/nu11051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schütt M, Stamatopoulos K, Simmons MJH, Batchelor HK, Alexiadis A. Modelling and simulation of the hydrodynamics and mixing profiles in the human proximal colon using discrete multiphysics. Comput. Biol. Med. 2020;121:103819. doi: 10.1016/j.compbiomed.2020.103819. [DOI] [PubMed] [Google Scholar]
- 140.Every-Palmer S, Inns SJ, Grant E, Ellis PM. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81–91. doi: 10.1007/s40263-018-0587-4. [DOI] [PubMed] [Google Scholar]
- 141.Cremer J, Arnoldini, M, and Hwa, T. Effect of flow and peristaltic mixing on bacterial growth in a gut-like channel. Proc. Natl. Acad. Sci. U. S. A. 2016;113(41):11414–11419. doi: 10.1073/pnas.1601306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nikolaev M, Mitrofanova O, Broguiere N, Geraldo S, Dutta D, Tabata Y, Elci B, Brandenberg N, Kolotuev I, Gjorevski N, et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 2020;585(7826):574–578. doi: 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- 143.Marrero D, Pujol-Vila F, Vera D, Gabriel G, Illa X, Elizalde-Torrent A, Alvarez M, Villa R. Gut-on-a-chip: mimicking and monitoring the human intestine. Biosens Bioelectron. 2021;181:113156. doi: 10.1016/j.bios.2021.113156. [DOI] [PubMed] [Google Scholar]
- 144.Jansma J, El Aidy S. Understanding the host-microbe interactions using metabolic modeling. Microbiome. 2020;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loubens CDE, Lentle RG, Love RJ, Hulls C, Janssen PWM. Fluid mechanical consequences of pendular activity, segmentation and pyloric outflow in the proximal duodenum of the rat and the Guinea pig. J R Soc Interface. 2013;10(83):20130027. doi: 10.1098/rsif.2013.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kendig DM, Hurst NR, and Grider JR. Spatiotemporal mapping of motility in Ex Vivo preparations of the intestines. J Vis Exp. 2016;107:e53263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lentle RG, Hulls CM. Quantifying patterns of smooth muscle motility in the gut and other organs with new techniques of video spatiotemporal mapping. Front Physiol. 2018;9:338. doi: 10.3389/fphys.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carew EO, Pedley TJ. An active membrane model for peristaltic pumping: part I - periodic activation waves in an infinite tube. J. Biomech. Eng. 1997;119(1):66–76. doi: 10.1115/1.2796066. [DOI] [PubMed] [Google Scholar]
- 149.Miftahof RN, and Gil Nam H. Mathematical foundations and biomechanics of the digestive system. United Kingdom: Cambridge University Press; 2010. [Google Scholar]
- 150.Wei R, Parsons SP, Huizinga JD. Network properties of interstitial cells of Cajal affect intestinal pacemaker activity and motor patterns, according to a mathematical model of weakly coupled oscillators. Exp. Physiol. 2017;102(3):329–346. doi: 10.1113/EP086077. [DOI] [PubMed] [Google Scholar]
- 151.Palmada N, Cater JE, Cheng LK, and Suresh V. Modelling flow and mixing in the proximal small intestine. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 20-24 July 2020 Montreal, QC, Canada; , 2496–2499 doi: 10.1109/EMBC44109.2020.9176688 (, 2020). [DOI] [PubMed] [Google Scholar]
- 152.Huizinga JD, Lammers WJEP. Gut peristalsis is governed by a multitude of cooperating mechanisms. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2009;296(1):G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 153.Jeffrey B, Udaykumar HS, Schulze KS. Flow fields generated by peristaltic reflex in isolated Guinea pig ileum: impact of contraction depth and shoulders. Am. J. Physiol. - Gastrointest. Liver Physiol. 2003;285(5):G907–G918. doi: 10.1152/ajpgi.00062.2003. [DOI] [PubMed] [Google Scholar]
- 154.Love RJ, Lentle RG, Asvarujanon P, Hemar Y, Stafford KJ. An expanded finite element model of the intestinal mixing of digesta. Food Dig. 2013;4(1):26–35. doi: 10.1007/s13228-012-0017-x. [DOI] [Google Scholar]
- 155.Lin ASH, Buist ML, Smith NP, Pullan AJ. Modelling slow wave activity in the small intestine. J. Theor. Biol. 2006;242(2):356–362. doi: 10.1016/j.jtbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 156.Lentle RG, Janssen PWM. Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: a review. J Comp Physiol B. 2008;178(6):673–690. doi: 10.1007/s00360-008-0264-x. [DOI] [PubMed] [Google Scholar]
- 157.Takahashi T. Flow behavior of digesta and the absorption of nutrients in the gastrointestine. J Nutr Sci Vitaminol (Tokyo). 2011;57:265–273. [DOI] [PubMed] [Google Scholar]
- 158.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012;9(5):271–285. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- 159.Lentle RG, De Loubens C, Hulls C, Janssen PWM, Golding MD, Chambers JP. A comparison of the organization of longitudinal and circular contractions during pendular and segmental activity in the duodenum of the rat and Guinea pig. Neurogastroenterol. Motil. 2012;24(7):686–e298. doi: 10.1111/j.1365-2982.2012.01923.x. [DOI] [PubMed] [Google Scholar]
- 160.Fullard LA, Lammers WJ, Ferrua MJ. Advective mixing due to longitudinal and segmental contractions in the ileum of the rabbit. J Food Eng. 2015;160:1–10. doi: 10.1016/j.jfoodeng.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 161.Avvari RK. Effect of local longitudinal shortening on the transport of luminal contents through small intestine. Acta Mech Sin Xuebao. 2019;35(1):45–60. doi: 10.1007/s10409-018-0809-5. [DOI] [Google Scholar]
- 162.Avvari RK. Effects of stationary contraction of the small intestine on digestion. J. Mech. Med. Biol. 2021;21(2):2150021. doi: 10.1142/S0219519421500214. [DOI] [Google Scholar]
- 163.Zha J, Zou S, Hao J, Liu X, Delaplace G, Jeantet R, Dupont D, Wu P, Dong Chen X, Xiao J, et al. The role of circular folds in mixing intensification in the small intestine: a numerical study. Chem. Eng. Sci. 2021;229:116079. doi: 10.1016/j.ces.2020.116079. [DOI] [Google Scholar]
- 164.Cannon WB. The movements of the intestines studied by means of the röntgen rays. Am J Physiol Content. 1902;6(5):251–277. doi: 10.1152/ajplegacy.1902.6.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Janssen PWM, Lentle RG, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y. Characterization of flow and mixing regimes within the ileum of the brushtail possum using residence time distribution analysis with simultaneous spatio-temporal mapping. J. Physiol. 2007;582(3):1239–1248. doi: 10.1113/jphysiol.2007.134403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y, Brasseur, JG, Banco, GG, Webb, AG, Ailiani, AC, and Neuberger, T, et al. Development of a lattice-boltzmann method for multiscale transport and absorption with application to intestinal function. In: Computational modeling in biomechanics. Netherlands: Springer; 2010. p. 69–96. doi: 10.1007/978-90-481-3575-2_3. [DOI] [Google Scholar]
- 167.De Loubens C, Lentle, RG, Hulls, C, Janssen, PWM, Love, RJ, and Chambers, JP. Characterisation of mixing in the proximal duodenum of the rat during longitudinal contractions and comparison with a fluid mechanical model based on spatiotemporal motility data. PLoS One. 2014;9 e95000. doi: 10.1371/journal.pone.0095000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fullard L, Lammers W, Wake GC, Ferrua MJ. Propagating longitudinal contractions in the ileum of the rabbit-Efficiency of advective mixing. Food Funct. 2014;5(11):2731–2742. doi: 10.1039/C4FO00487F. [DOI] [PubMed] [Google Scholar]
- 169.Barrett KE, Barman SM, Boitano S, and Brooks HL. Ganong’s review of medical physiology (New York: McGraw Hill Education; ). 2010. [Google Scholar]
- 170.Kerlin P, Zinsmeister A, Phillips S. Relationship of motility to flow of contents in the human small intestine. Gastroenterology. 1982;82(4):701–706. doi: 10.1016/0016-5085(82)90314-6. [DOI] [PubMed] [Google Scholar]
- 171.Labarthe S, Polizzi, B, Phan, T, Goudon, T, Ribot, M, and Laroche, B. A mathematical model to investigate the key drivers of the biogeography of the colon microbiota. J Theor Biol. 2019;462:552–581. doi: 10.1016/j.jtbi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 172.Lentle RG, Janssen PWM, DeLoubens C, Lim YF, Hulls C, Chambers P. Mucosal microfolds augment mixing at the wall of the distal ileum of the brushtail possum. Neurogastroenterol. Motil. 2013;25(11):881–e700. doi: 10.1111/nmo.12203. [DOI] [PubMed] [Google Scholar]
- 173.Lentle RG, de Loubens C. A review of mixing and propulsion of chyme in the small intestine: fresh insights from new methods. J Comp Physiol B. 2015;185(4):369–387. doi: 10.1007/s00360-015-0889-5. [DOI] [PubMed] [Google Scholar]
- 174.Muñoz-Tamayo R, Laroche B, Walter É, Doré J, Leclerc M. Mathematical modelling of carbohydrate degradation by human colonic microbiota. J. Theor. Biol. 2010;266(1):189–201. doi: 10.1016/j.jtbi.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 175.Tripathi D, Pandey SK, Das S. Peristaltic transport of a generalized Burgers’ fluid: application to the movement of chyme in small intestine. Acta Astronaut. 2011;69(1–2):30–38. doi: 10.1016/j.actaastro.2010.12.010. [DOI] [Google Scholar]
- 176.El Bouti T, Goudon , T, Labarthe, S, Laroche, B, Polizzi, B, Rachah, A, Ribot, M, and Tesson, R. A Mixture Model for the Dynamic of the Gut Mucus Layer. ESAIM: Proceedings and Surveys. 2016;55:111–130. doi: 10.1051/proc/201655111. [DOI] [Google Scholar]
- 177.Beukema M, Faas MM, de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp Mol Med. 2020;52(9):1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brasseur JG, Banco, GG, Ailiani, AC, Wang, Y, Neuberger, T, Smith, NB, and Webb, AG. Motility and absorption in the small intestines: integrating mri with lattice boltzmann models. Proceedings - 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro 28 June-1 July 2009 Boston, MA, USA; 2009. doi: 10.1109/ISBI.2009.5193062 [DOI] [Google Scholar]
- 179.Wang Y, Brasseur JG, Banco GG, Webb AG, Ailiani AC, Neuberger T. A multiscale lattice Boltzmann model of macro-to micro-scale transport, with applications to gut function. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010;368(1921):2863–2880. doi: 10.1098/rsta.2010.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lim YF, De Loubens C, Love RJ, Lentle RG, Janssen PWM. Flow and mixing by small intestine villi. Food Funct. 2015;6(6):1787–1795. doi: 10.1039/C5FO00285K. [DOI] [PubMed] [Google Scholar]
- 181.Hua X, Zhang, Y, Dong, Z, Wang, Y, Chen, XD, and Xiao, J. Simulation and analysis of mass transfer and absorption process intensification by villi movement. CIESC J. 2020;71 5 :2024–2034 doi: 10.11949/0438-1157.20191101. [DOI] [Google Scholar]
- 182.Shapiro AH, Jaffrin MY, Weinberg SL. Peristaltic pumping with long wavelengths at low reynolds number. J. Fluid Mech. 1969;37(4):799–825. doi: 10.1017/S0022112069000899. [DOI] [Google Scholar]
- 183.Li M, Brasseur JG. Non-steady peristaltic transport in finite-length tubes. J Fluid Mech. 1993;248:129–151. doi: 10.1017/S0022112093000710. [DOI] [Google Scholar]
- 184.Taylor G. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc R Soc London Ser A Math Phys Sci. 1953;219:186–203. [Google Scholar]
- 185.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ishikawa T, Sato T, Mohit G, Imai Y, Yamaguchi T. Transport phenomena of microbial flora in the small intestine with peristalsis. J. Theor. Biol. 2011;279(1):63–73. doi: 10.1016/j.jtbi.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 187.Cremer J, Arnoldini M, Hwa T. Effect of water flow and chemical environment on microbiota growth and composition in the human colon. Proc. Natl. Acad. Sci. U. S. A. 2017;114(25):6438–6443. doi: 10.1073/pnas.1619598114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kettle H, Louis P, Holtrop G, Duncan SH, Flint HJ. Modelling the emergent dynamics and major metabolites of the human colonic microbiota. Environ. Microbiol. 2015;17(5):1615–1630. doi: 10.1111/1462-2920.12599. [DOI] [PubMed] [Google Scholar]
- 189.Moorthy AS, Brooks SPJ, Kalmokoff M, Eberl HJ, Larsen PE. A spatially continuous model of carbohydrate digestion and transport processes in the colon. PLoS One. 2015;10(12):e0145309. doi: 10.1371/journal.pone.0145309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ishikawa T, Omori T, Kikuchi K. Bacterial biomechanics - From individual behaviors to biofilm and the gut flora. APL Bioengineering. 2020;4(4):41504. doi: 10.1063/5.0026953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Le Feunteun S, Al-Razaz A, Dekker M, George E, Laroche B, van Aken G. Physiologically based modeling of food digestion and intestinal microbiota: state of the art and future challenges. An INFOGEST review. Annu Rev Food Sci Technol. 2021;12(1):149–167. doi: 10.1146/annurev-food-070620-124140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


