Abstract
We performed an in-depth analysis of the virucidal effect of discrete wavelengths: UV-C (278 nm), UV-B (308 nm), UV-A (366 nm) and violet (405 nm) on SARS-CoV-2. By using a highly infectious titer of SARS-CoV-2 we observed that the violet light-dose resulting in a 2-log viral inactivation is only 104 times less efficient than UV-C light. Moreover, by qPCR (quantitative Polymerase chain reaction) and fluorescence in situ hybridization (FISH) approach we verified that the viral titer typically found in the sputum of COVID-19 patients can be completely inactivated by the long UV-wavelengths corresponding to UV-A and UV-B solar irradiation. The comparison of the UV action spectrum on SARS-CoV-2 to previous results obtained on other pathogens suggests that RNA viruses might be particularly sensitive to long UV wavelengths. Our data extend previous results showing that SARS-CoV-2 is highly susceptible to UV light and offer an explanation to the reduced incidence of SARS-CoV-2 infection seen in the summer season.
Keywords: UV light, Virus replication, FISH, Disinfection, Infectivity, Action spectrum, SARS-CoV-2
1. Introduction
At the end of 2019 a new coronavirus, SARS-CoV-2, was firstly described in Wuhan, China, as being responsible for pneumonia within the scenario of a new disease: COVID-19. COVID-19 rapidly became a worldwide pandemic which is still raging and has been responsible for dramatic and unforeseeable health, social and economic consequences [1]. The development of multiple SARS-CoV-2 vaccines made it possible to predict that the pandemics will eventually be defeated [2], [3], [4], but the implementation of disinfection and prevention strategies is still of the foremost importance to curb the spread of new infections [5], [6], [7].
The germicidal effect exerted by UV light on bacteria and viruses has been widely documented since the end of 1800 [8]. UV-C light produced from low pressure mercury tubes is commonly employed in the disinfection of wastewaters, drinking water and closed environments as well as of blood products, and represents a well-established, economical and safe technology [9], [10], [11], [12], [13], [14]. SARS-CoV-2 was shown to be highly sensitive to UV-C light [15], [16], [17], [18], although discrepancies in the results indicated that all the variables involved in the experimental setting have to be taken into account to obtain reliable and replicable data [15]. Other interesting new results, employing UV LEDs show that the UV-C inactivation kinetics of the virus is wavelength dependent in the 265nm – 285nm [16,[19], [20], [21]]. We notice again a slight scattering in the results, which can be ascribed to the test conditions. These outcomes are of paramount importance for the development of air and surface disinfecting devices, but do not explain the particular seasonal epidemiology of SARS-CoV-2 infection, which has been extensively investigated in different regions and countries during this year, suggesting a correlation with some key environmental factors [[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Concerning the UV light, the idea that viral epidemiology could be modulated by the intensity of the solar pump is rebutted by the observation that the UV-C light emitted by the Sun, the most important UV light source, is filtered and blocked by the ozone layer in the stratosphere. UV-A and UV-B radiations, on the other hand, reach the earth surface with different intensities, which depend on the season, the latitude, and the weather conditions. The effect of solar UV-A and UV-B radiation on microorganisms [31,32] and on the seasonal behavior of infectious diseases has been extensively discussed [33,34], and recent predictive models suggest that SARS-CoV-2 infection is indeed solar-sensitive [35,36]. Solid, convincing and reproducible experimental data on the possible virucidal effects of UV-A and UV-B and their correlation with SARS-CoV-2 epidemiology are nevertheless missing.
Here, we used the TCID50 approach to quantify SARS-CoV-2 inhibition upon irradiation, using wavelengths spanning form UV-C to violet light and we build the corresponding UV-action spectrum. Next, we used PCR (Polymerase chain reaction) and FISH (fluorescence in situ hybridization) measurements to identify the UV-A and UV-B doses that completely inhibit the viral concentration comparable to the one present in the sputum of SARS-CoV-2-infected patients. Finally, we compared the obtained UV action spectrum on SARS-CoV-2, with those reported in the literature on other viruses and bacteria.
Our data define the wavelengths and the doses of UV radiation that result in SARS-CoV-2 inactivation and show that SARS-CoV-2 infectivity can be completely blocked by UV-A and UV-B. These results offer an explanation to the seasonality of such infection, and provide fundamental information to build sterilization devices that avoid the toxicity of UV-C-based methods and that could be potentially effective in the inhibition of multiple RNA viruses.
2. Materials and Methods
2.1. Analyses of SARS-CoV-2 replication following UV-exposure
2 × 104 VeroE6 cells were cultured in DMEM (ECB20722L, Euroclone, Milan, Italy) with 2 % FBS medium, 100 U/ml penicillin, and 100 μg/ml streptomycin, in a 96-well plate for 24 hours before infection. To determine SARS-CoV-2 susceptibility to UV-irradiation, a viral stock (Virus Human 2019-nCoV strain 2019- nCoV/Italy-INMI1, Rome, Italy) at a concentration of 6 × 106 TCID50/mL in DMEM (ECB20722L, Euroclone, Milan, Italy) was placed under the LED lamp and irradiated with 3 different doses (D1, D2, D3) for each tested UV-wavelength (see Table 1 ). For each UV-wavelength viral titers were determined by TCID50 endpoint dilution assay. Briefly: serial ten-fold dilutions, from 106 to 10−4 TCDI50/mL (50ul), were plated onto 96-well plates, incubated at 37°C in 5% CO2 and checked daily to monitor the UV-induced cytopathic effect. Seventy-two hours post infection (hpi) viral titer was determined as previously described [37].
Table 1.
Features of the illumination conditions. Transmittance through a 1mm thick DMEM layer at the selected wavelengths; the three doses provide to the virus stock for the same wavelengths.
| LED | Peak Wavelength (nm) | DMEM Transmittance | Dose (mJ/cm2)* | |||
|---|---|---|---|---|---|---|
| Min | Ave | D1 | D2 | D3 | ||
| UV-C | 278 | 0.647 | 0.811 | 2 | 4 | 12 |
| UV-B | 308 | 0.867 | 0.932 | 100 | 200 | 600 |
| UV-A | 366 | 0.901 | 0.950 | 2000 | 4000 | 12000 |
| Violet | 405 | 0.897 | 0.948 | 4000 | 8000 | 24000 |
as explained in the text, this is the fluence provided to the samples
The same UV-wavelengths were used to irradiate a SARS-CoV-2 viral stock at a concentration of 1.5 × 103 TCID50/mL. VeroE6 cell cultures were incubated with the virus inoculum in quadruplicate for one hour at 37°C and 5% CO2 and viral replication was assessed by qPCR, as previously described [15], at 24, 48 and 72 hpi. SARS-CoV-2 that was not exposed to UV light was used as control.
2.2. Fluorescence in-situ hybridization (FISH)
It was performed using previously described 96 smiFISH probes [38] that target the whole (positive-strand) genome of the virus. According to the smiFISH protocol [39], a FLAP sequence is appended at the 3’ of each primary probe. Prehybridization with a complementary Cy3-labelled Cy3 probe was performed as using a PCR machine, as described [38].
A SARS-CoV-2 viral stock (1.5 × 103 TCID50/mL) was exposed to the UV-doses reported in Table 1 and an in vitro infection assay was performed on 1 × 105 Vero E6 cells grown on a 13mm glass coverslip (0.17mm thickness) as described in the previous section. Twenty-four hpi the supernatant was collected to quantify viral replication by qPCR, while cells were fixed by 4% PFA for 10 minutes, washed twice with PBS, and then stored until labeling in 70% ethanol at -20°C (minimum ethanol incubation: overnight). The day of the labeling, the coverslips were brought to room temperature, washed twice with 10% SSC-20 × in RNase-free water (Buffer I) for 5 minutes, followed by a wash in 10% SSC-20 × and 20% formamide in nuclease-free water (Buffer II). Cells were incubated overnight with the hybridized FLAP-duplex in a humidified chamber at 37°C. The probes were diluted 1:100 in the hybridization buffer (10% (w/v) of dextran sulfate, 10% of SSC-20 × buffer and 20% formamide in Rnase-free water). Following hybridization, cells were washed twice in Buffer II in the dark for 30 min at 37°C, then washed in PBS for 10 min and stained with 1μg/ml Hoechst 33342 in PBS. The coverslips were then mounted on glass slides using Vectashield (Vector Laboratories, Peterborough, UK).
Images were acquired on a custom-built epifluorescence microscope, equipped with a led source for illumination (Excelitas Xcite XLED1, Qioptiq, Rhyl, UK), an Olympus LUCPlanFLN 20x/0.45NA objective (Olympus Life Science Segrate, IT) and a Hamamatsu Orca Fusion sCMOS detector (Hamamtsu Photonics Italia S.R.L, Arese, Italy), resulting in a pixel size equal to 324.6nm. By using a motorized stage (Mad City Labs GmbH, Schaffhauserstrasse, CH), mosaic of 6 × 6 fields of views were collected and then stitched using the Image Stitching ImageJ plugin [40]. The resulting large fields of view (approx. 3mm of side) were then scaled by a factor 0.25 in FiJi [41] and then imported in Matlab for image analysis using a custom-written routine. The code segments the nuclei in the Hoechst channel – discarding objects smaller than 40 pixels or larger than 400 pixels – and then computes the average intensity in the FISH probes channel, in a 3-pixel wide ring surrounding each individual nucleus. The distribution of the single-cell FISH signal intensity is then plot as a histogram. The distribution of the single-cell FISH signal intensity is then plot as a histogram and the fraction of positive cells is calculated by counting those cells with a FISH signal intensity 23 times higher than background intensity.
2.3. LED illumination conditions
To irradiate the virus we placed the above mentioned virus titers in 6-well plates, which were then irradiated using LEDs with the spectral features reported in Fig. 1 A. While these sources are not monochromatic as in the case of low-pressure mercury lamps, they show a bandwidth of about 10 nm, similar to the sources used to generate the action spectrum on other viruses [42]. The sources were calibrated using an Ocean Optics HR2000+ spectrometer (Ocean Optics Inc., Dunedin, USA). The spectrometer was calibrated against a reference deuterium–halogen source (Ocean Optics Inc. Winter Park, Winter Park, Florida) and in compliance with the National Institute of Standards and Technology (NIST) practices recommended in NIST Handbook 150-2E, Technical guide for Optical Radiation Measurements. The last calibration was performed in March 2019. During irradiation the virus is suspended in a 1mm thick layer of DMEM medium: in the UV-range, medium absorption is non-negligible and needs to be accounted to calculate the real dose delivered to the virus [15]. It is important to note that the virus itself does not contribute significantly to the source attenuation instead, as its concentration is too low to have a significant effect (see results). Consequently, we irradiate the virus stock with a defined fluence which is different from the dose, as it implies to know the absorption by the virus itself. On the other hand, the term “dose” is commonly used, instead of fluence, in most of the literature related to the UV tests on microorganism, that's why we decided to adopt it in this paper. To compute the fraction of UV light that is absorbed by the medium we used the absorption spectrum of DMEM reported in Fig. 1B and calculated the transmittance trend as function of the liquid thickness (Fig. 1C). The resulting fraction of UV light delivered on average in the well (average) and at the bottom of the well (minimum) as function of the wavelength is reported in Table 1.
Fig. 1.
Features of the illuminated medium. A) Normalized emission spectra of the LEDs used in the experiments. B) Transmission spectrum of the DMEM used in the experiment (1 mm thick) with the dotted line corresponding to the LED peak wavelength. C) Fraction of transmitted UV light as function of the liquid thickness.
As expected from the transmittance spectrum, for UV-A and UV-B light the minimum and average values were comparable (approx. 90-95%), indicating that all the virions suspended in the solution do receive the same UV dose. At 278 nm (UV-C), the discrepancy between the minimum and average value became more evident. The consequence is that the viruses at the bottom of the well receive 20% less UV photons than the average.
According to the spectral and irradiance calibrations and the DMEM transmittances, the exposure times were calculated to provide the doses reported in Table 1.
2.4. Statistical Analyses
In the bar plots in Fig. 3, the mean ± standard error of the mean (SEM) are indicated. Data were analyzed using two-way ANOVA test by GRAPHPAD PRISM version 5 (Graphpad software, La Jolla, Ca, USA), and p-values of 0.05 or less were considered to be significant.
Fig. 3.
SARS-CoV-2 action spectrum according to the TCID50 measurements. The plot reports the spectrum normalized at 1 for the UV-C (red curve) and the dose required for a 2-Log inactivation (blue curve).
3. Results and Discussion
We first applied the TCID50 approach to measure how effective UV light of different wavelengths is in inactivating the virus. To achieve this result, we infected cells with an initial viral concentration (6 × 106 TCID50/mL), which is significantly higher than that observed in infected patients. Next, we performed experiments on lower initial viral loads, comparable to the ones present in the sputum of COVID-19 samples [43]. Here, we used qPCR to evaluate the capability of the irradiated virus to replicate and FISH to quantify the ability of the different UV doses to impair SARS-CoV-2 infectivity at the single-cell level. According to the experimental conditions reported in 2.1, the virus is suspended in DMEM and the absorption of the medium is taken into account. This shrewdness allows to verify the direct effect exerted by UV light on the virus and to mimic the condition of the virus in aerosol form. The results can be directly used in predictive models of virus seasonality (at least if the concentration effect is not considered). Conversely, they cannot be employed directly to monitor the UV-effect on fomites with a screening film on the virus deposit. Indeed, in this situation the evaluation of the screening factor depends on the chemical-physical properties of the film itself.
3.1. Wavelength-dependent inhibition of SARS-CoV-2 infectivity
Results reported in Fig. 2 describe the trend of the viral titer as function of the dose for the four wavelengths considered.
Fig. 2.
Virus titer trend as function of the light doses. The trend is reported for the four different wavelengths tested (UV-C,-B,-A stand for 278, 308, and 366 nm respectively, violet for 405 nm).
For all the analyzed wavelengths, we observed a dose-response relationship between the administered UV-dose and viral titer, indicating that virus is susceptible to different extents to a wide range of UV wavelengths. Notably, the decreasing trend was observed to be exponential, as predicted for such kind of mechanism [44]. Concerning the UV-C light, our results are in line with those reported in the same spectral range (265 nm – 280 nm). Indeed, Shimoda et al. find a 2-Log reduction with a dose of 10 mJ/cm2 at 265 nm [21]; Ma et al., using 270 nm and 282 nm LEDs found the same Log reduction with doses equal to 2 mJ/cm2 for the low wavelength and 4 mJ/cm2 for the other one [19]. In another paper, 265 nm, 280 nm and 300 nm LEDs were employed and a 3-Log inactivation of virus replication was obtained with values of 1.8 mJ/cm2, 3.0 mJ/cm2 and 23 mJ/cm2 respectively, with an important increase at 300 nm [20]. Going back to the results here reported, while a few mJ/cm2 of UV-C light are sufficient to cause a several order of magnitude drop in the effective viral concentration, 100x to 1000x higher doses were needed to observe a similar viral load reduction following UV-B and UV-A irradiation.
These data confirm earlier findings indicating that UV-C is more effective in virus inactivation compared to both UV-B and UV-A, and reinforce the possibility of using UV-C irradiation as an efficient method for a fast inactivation of SARS-CoV-2. Further, the data indicate that UV-B and UV-A also have virucidal potential, although at higher doses than UV-C.
3.2. Action spectrum
To better characterize these results, we built the action spectrum at a 2-Log inactivation of SARS-CoV-2. We extrapolated the data fitting the effective concentration curves of Fig. 2 with a simple single exponential trend, normalized the action spectrum by setting at 1 the inhibition efficiency at 278 nm and calculated the relative effectiveness at the other wavelengths (Table 2 and Fig. 3 ).
Table 2.
Extrapolated doses resulting in a 2-log reduction. The data are derived according to the TCID50 measurements and the relative efficiency as function of the wavelength.
| Wavelength (nm) | 2-Log dose (mJ/cm2) | Relative effectiveness |
|---|---|---|
| 278 | 2.07 | 1 |
| 308 | 309.07 | 4.2 × 10−3 |
| 366 | 9719.65 | 2.0 × 10−4 |
| 405 | 13224.56 | 1.1 × 10−4 |
The data and the plot recapitulate the results obtained by TCID50, namely that the measured relative effectiveness of UV irradiation on SARS-CoV-2 reaches a plateau at longer wavelengths, with UV-C being 104 times more effective than violet light.
Such a substantial difference is expected given that the absorption spectrum of both proteins and nucleic acids peak in the UV-C region. Nevertheless, the result that higher doses of UV-A and violet light can still have a virucidal effect on SARS-CoV-2 is noteworthy, as it supports the idea of a disinfecting effect of solar radiation on the virus.
3.3. UV-dependent virus inhibition of SARS-CoV-2
To examine the efficacy of UV-inactivation in a real-world scenario, we used the UV-doses reported in Table 1 on a SARS-CoV-2 viral concentration equivalent to the one found in the sputum of SARS-CoV-2 infected patients (1.5 × 103 TCID50/ml) [43]. qPCR was employed to quantify viral replication over time (Fig. 4 ).
Fig. 4.
Viral replication of UV-irradiated SARS-CoV-2 (1,5 × 106 TCID50) in the supernatant of in vitro infected VeroE6 cells. Vero E6 cells were infected with SARS-CoV-2 irradiated with different doses of UV-A (D1 = 2000, D2 = 4000, D3 = 12000 mJ/cm2), -B (D1 = 100, D2 = 200, D3 = 600 mJ/cm2), -C (D1 = 2, D2 = 4, D3 = 12 mJ/cm2) and violet light (D1 = 4000, D2 = 8000, D3 = 24000 mJ/cm2). Culture supernatants were harvested at the indicated times (24, 48 and 72 hpi) and virus titers were measured by absolute copy number quantification (Real-Time PCR). All cell culture conditions were seeded in quadruplicate. Mean values ±SEM are shown.
Results showed that all the wavelengths analyzed are capable to completely inhibit the amplification of the viral genome though, as expected, with different efficacy. In particular, a marked reduction of viral copy number was observed at 24 hour post infection (hpi) with a low dose of UV-C (278 nm) equal to 4 mJ/cm2. Even more important, the viral copy number did not increase over time (72 hpi), endorsing the complete inhibition of the virus. This is in line with the results reported with the 254 nm light [15] and confirms the high susceptibility of the virus to UV-C photons. Notably, and most interestingly, results indicated that a 200 mJ/cm2 UV-B dose, a 4000 mJ/cm2 UV-A dose and a 24000 mJ/cm2 violet dose were also sufficient to completely inactivate the virus.
All the results were further confirmed by 2-ANOVA statistical analyses performed on viral replication at extracellular level for UV-C (4 mJ/cm2 vs. untreated: N1, p = 0,0243; N2: p = 0,0119; 12 mJ/cm2 vs. untreated: N1, p = 0,0243; N2: p = 0,0119), UV-B (200 mJ/cm2 vs. untreated: N1, p = 0,0009; N2: p = <0,0001; 600 mJ/cm2 vs. untreated: N1, p = 0,0007; N2: p = <0,0001), UV-A (4000 mJ/cm2 vs. untreated: N1, p = 0,0283; N2: p = <0,0001; 12000 mJ/cm2 vs. untreated: N1, p = 0,0233; N2: p = <0,0001) and 405nm (4000 mJ/cm2 vs. untreated: N1, p = 0,0033; N2: p = 0,0056; 8000 mJ/cm2 vs. untreated: N1, p = <0,0001; N2: p = 0,0002; 24000 mJ/cm2 vs. untreated: N1, p = <0,0001; N2: p = <0,0001) (Fig. 4).
By comparing these results with those based on TCID50, we clearly observed that viral concentration is a key variable in the experimental setting. For example, a 200 mJ/cm2 UV-B dose is sufficient to totally inhibit viral replication at a viral concentration comparable to the one in the sputum of a COVID-19 patient, but results in just a 1-log reduction of the higher viral titer. This feature is crucial to properly compare different studies with different experimental conditions, in particular if these data should be used to develop models of sun irradiation on virus dispersed as aerosol in the atmosphere. It is important to note that the viral concentration in such case is expected to be much lower than those used in our experimental tests.
3.4. FISH experiments
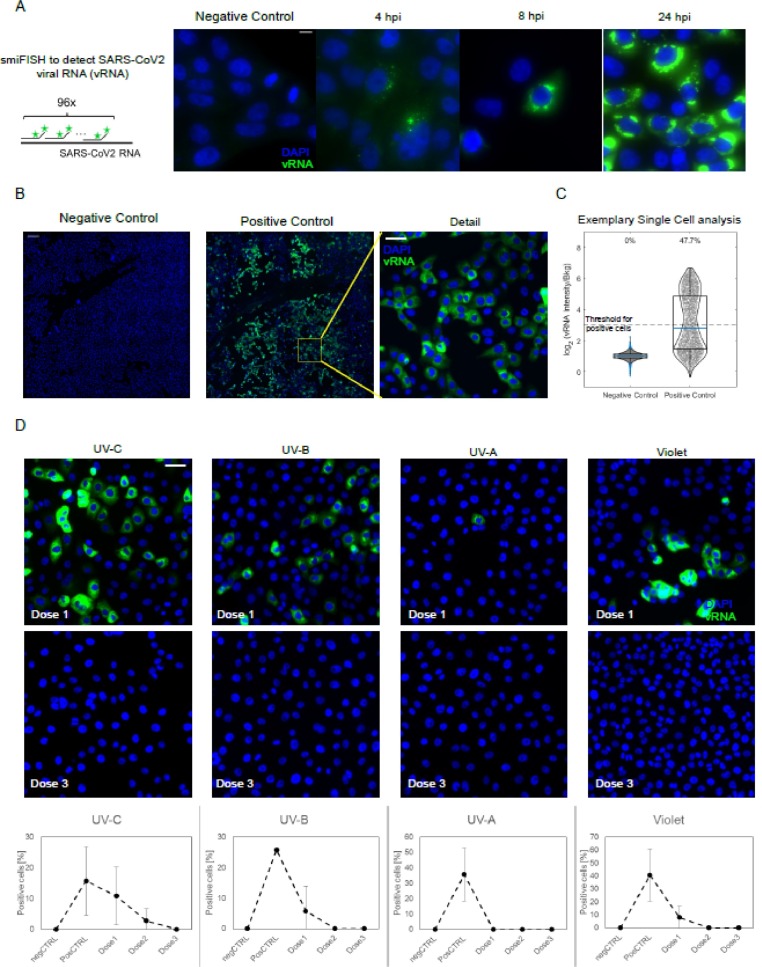
In order to directly visualize the capability of UV to interfere with the SARS-CoV-2 infection of host cells, we next performed an in vitro infection assay on VeroE6 cells following incubation with a 1.5 × 103 TCID50/ml virus titer that had or had not been irradiated with different UV wavelengths. FISH probes targeting the positive strand of the viral RNA (vRNA) were used in the experiments. Time-course analyses using non-irradiated virus showed that the FISH signal progressively increases in single cells over time due to the replication of the viral genome, and shifts from a punctate staining (representative of few vRNA copies/cell), which is observed at early time-points, to an intense cytosolic signal at 24hpi (Fig. 5 A). Such robust activation at late time points allows to detect SARS-CoV-2 positive cells with low magnification objectives, thereby enabling the analysis of thousands of cells per condition (Fig. 5B-C).
Fig. 5.
Fluorescence In-situ hybridization (FISH) of the SARS-CoV-2 viral RNA (vRNA) in VeroE6 cells. (A) Scheme of the hybridization of the FISH probes (left) and timecourse of SARS-CoV-2 infection at different timepoints post infection, as collected with an high magnification/Numerical aperture (NA) objective (60 × 1.49NA). Scale bar 10 μm. (B) The bright signal of the smFISH probes at 24hpi allow to use a low NA objective (20x, 0.45 NA) to reliably identify infected cells among thousands of cells, by collecting 6 × 6 mosaics (total field of view approx. 3mm, scale bar in the ‘detail’ image 50 μm).(c) Positive cells are identified as those with a 8-fold higher intensity I than the background n the FISH channel. (D) Exemplary details of 6 × 6 mosaics upon irradiation with different wavelength and doses of UV/Violet light. UV doses are as indicated in Table 1. Quantification is provided as mean +/- standard deviation of the fraction of vRNA positive cells on at least two 6 × 6 mosaics. Scale bar: 10 μm.
Cell-by-cell distribution of the FISH signal displayed a bi-modal distribution, with 20-40% of cells being clearly infected at 24 hours post infection when non-irradiated virus was seeded on cells (Fig. 5C). In contrast with these data, all the analyzed UV-wavelength reduced the fraction of positive cells, as probed by smFISH, resulting in a complete inhibition of the virus when SARS-CoV-2 was irradiated with wavelength specific critical doses (4mJ/cm2 for UV-C, 200mJ/cm2 for UV-B, 4000 mJ/cm2 for UV-A and 24000 mJ/cm2 for violet light) (Fig. 5D). Notably at lower UV doses some cells with high levels of vRNA could be detected, suggesting that, rather than inhibiting the capability of the viral genome to replicate, UV-irradiation might affect either virus entry in the cells or the assembly of functional viral particles following the replication of the viral genome.
3.5. Action-spectrum comparison of the UV-susceptibility of different microorganisms
We finally compared the results obtained in the TCID50 assay with those reported in the literature for other viruses and bacteria by building the UV action spectra for each of the different pathogens (Fig. 6 ). Data were normalized to the UV-C inactivation dose (for SARS-CoV-2 it has been assumed the same level of inactivation at 253 and 278 nm).
Fig. 6.
Action spectra from UV-C to UV-A for different microorganisms (bacteria and viruses) and DNA. They're inferred by previous works with the results achieved in the present work for SARS-CoV-2 normalized to the UV-C inactivation dose (see text for details). The data refer to inactivation dose levels (between 1-Log and 2-Log).
The viruses we included in the analysis are influenza virus H1N1 [42] and the Bacteriophages MS2 [45,46], T1 [47] and T4 [46]. The “average” virus UV action spectrum inferred by Lytle and Sagripanti [33] and Horton et al.[48] and combining data achieved with different viruses (see also ref [48]) are shown as well. For bacteria, results for Escherichia Coli alone or combined with Salmonella [49, 50] are presented. DNA-damage action spectra are also displayed [51].
It should be noted that whereas for both bacteria and DNA viruses the inactivation observed in the UV-A is 10−5-10−6 times lower than that observed in the UV-C region, for RNA viruses the inactivation power is only marginally lower in the UV-A than in the UV-C region (just about 100-1000 times lower at 366 nm). Taking into account that the Solar illumination in the UV-A region is much larger than in the UV-B (95% of UV-A and 5% of UV-B), these results might justify the acknowledged seasonal behavior of the outbreaks of airborne viruses, including corona [35,52,53] and influenza viruses [54,55]. In this respect, we would like to stress how it is crucial to have the specific data of the target microorganism to develop reliable solar inactivation and/or seasonal models and how Lytle and Sagripanti action spectrum cannot be considered as a reference trend.
Concerning the reasons and mechanisms behind the UV-A and UV-B inhibitory effect on SARS-CoV-2, they go beyond the purpose of this study and not so many papers face this issue [42, 56]. It is known that the absorption of the nucleic acids is very weak in these spectral regions; therefore the direct damage should be almost absent. On the other hand, the fluence provided to the sample is very large and so the small probability is compensated by the large excess of photons. Nonetheless, the unexpected inhibitory effect exerted by UV-A and UV-B radiation on the RNA genome of SARS-CoV-2 may depend even on other molecular mechanisms, which deserve further investigations. However, a plausible explanation derives from a recently published paper analyzing the photostability of RNA nucleosides uridine, adenosine, cytidine, and guanosine. Indeed, according to this study uridine and uracil are considerably less stable compared to the other tested molecules [57].
Different is the case of the DNA for which to several information reported in the scientific literature it is possible to speculate on it. Thus: 1) UV-B/A radiation may cause DNA lesions, such as cyclobutane pyrimidine dimers (CPDs), pyrimidine 6-4 pyrimidone photoproducts (6-4PPs), and their Dewar isomers [58]; 2) UV-B radiation-induced modifications on DNA purine bases has been documented [59]; 3) UV-A may induce G to T transversions and Small Tandem base deletions [60].
Finally, an interesting observation is reported by Berstain et al.[61], who ascribed the different photostability of DNA and RNA mainly to the activation of repairing mechanisms.
4. Conclusions
The ability of UV light to inactivate SARS-CoV-2 replication was studied as function of the irradiation wavelengths. Results showed that irradiation of a viral stock with a high infectious titer irradiated with LEDs at 278, 308, 366 and 405 nm resulted in a significant decrease in the fraction of active virus proportional to the light dose for all the wavelengths. Considering a 2-Log decrease and fixing to 1 the efficiency at 278 nm, it was possible to build the action spectrum, which is characterized by an effectiveness ratio of 10−4 at 405 nm, indicating that even violet light has a non-negligible efficiency in inactivating SARS-CoV-2. While such a relatively flat action spectrum might appear surprising, our comparison of multiple UV-action spectra on diverse microbes revealed that RNA viruses, including SARS-CoV-2, might be more susceptible than other pathogens to long wavelength UV-light.
These observations were confirmed by experiments performed on a virus titer equivalent to that present in COVID-19 patients. Thus, in these experimental conditions it was possible to completely inhibit virus replication with only 4 mJ/cm2 at 278, 200 mJ/cm2 at 308 nm, 4000 mJ/cm2 at 366 nm and 24000 mJ/cm2 at 405 nm. FISH analysis confirmed that UV-C, UV-B, UV-A, and violet light irradiation can inhibit SARS-CoV-2 infection of target cells These results are possibly extremely relevant as they analyze the effects of different UV wavelengths on SARS-CoV-2 titers that relate to the real life scenario of the current pandemic. In particular, data herein indicate that solar irradiation reaching the surface of the Earth could completely inactivate a titer of isolated SARS-CoV-2 similar to the one found in the sputum of COVID-19 patients within minutes. In conclusion, for the first time, we have demonstrated that UV irradiation is effective in SARS-CoV-2 inhibition at multiple wavelengths including UV-A and violet light. These results support a role for solar irradiation in viral disinfection of external surfaces and might contribute in explaining the seasonality of this virus.
Funding
These experiments were supported by the following grants: Bando Regione Lombardia DG Welfare cod. RL_DG-WEL20MBIAS_01; CARIPLO - EXTRABANDO E PROGETTI TERRITORIALI cod. CAR_EXT20MBIAS_01, Progetto Regione Lombardia. POR FESR 2014‐2020. AZIONE I.1.B.1.3 ‘Artificial Intelligence – Sars Covid Risk Evaluation(AI-SCoRE) to CT; and by INAF in the context of the initiatives triggered by the Italian Ministero dell'Università e della Ricerca (MUR) to counter the COVID19 pandemic.
CRediT authorship contribution statement
Mara Biasin: Conceptualization, Funding acquisition. Sergio Strizzi: Conceptualization, Methodology, Investigation, Writing – original draft. Andrea Bianco: Conceptualization, Writing – original draft. Alberto Macchi: Conceptualization, Methodology. Olga Utyro: Methodology, Investigation. Giovanni Pareschi: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. Alessia Loffreda: Methodology, Investigation. Adalberto Cavalleri: Conceptualization, Methodology. Manuela Lualdi: Conceptualization, Methodology. Daria Trabattoni: Conceptualization, Investigation. Carlo Tacchetti: Conceptualization, Funding acquisition. Davide Mazza: Investigation, Writing – review & editing. Mario Clerici: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
They have participated in approval of the final version. The specific contribution is reported in the manuscript. This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue. They have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are grateful to De Sisti Lighting (ILT Italy s.r.l., Rome, Italy) for having provided a custom designed multi-LED UV lamp used in the experiments. We are grateful to Dr. F Mueller and Dr. C. Zimmer (Institut Pasteaur, Paris) for kindly providing the smiFISH probes. We acknowledge F. Nicastro, J. R. Brucato, P. Tozzi, I. Ermolli, G. Sironi, E. Antonello from INAF for many useful discussions.
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M. Bhatta, S. Nandi, S. Dutta, M.K. Saha, Coronavirus (SARS-CoV-2): a systematic review for potential vaccines, Human Vaccines & Immunotherapeutics. (2021) 1–18. 10.1080/21645515.2020.1865774. [DOI] [PMC free article] [PubMed]
- 3.Lipsitch M., Dean N.E. Understanding COVID-19 vaccine efficacy. Science. 2020;370:763–765. doi: 10.1126/science.abe5938. [DOI] [PubMed] [Google Scholar]
- 4.Forni G., Mantovani A., Forni G., Mantovani A., Moretta L., Rappuoli R., Rezza G., Bagnasco A., Barsacchi G., Bussolati G., Cacciari M., Cappuccinelli P., Cheli E., Guarini R., Bacci M.L., Mancini M., Marcuzzo C., Morrone M.C., Parisi G., Pasquino G., Patrono C., Curzio A.Q., Remuzzi G., Roncaglia A., Schiaffino S., Vineis P., R. on behalf of the COVID-19 Commission of Accademia Nazionale dei Lincei COVID-19 vaccines: where we stand and challenges ahead. Cell Death & Differentiation. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noorimotlagh Z., Mirzaee S.A., Jaafarzadeh N., Maleki M., Kalvandi G., Karami C. A systematic review of emerging human coronavirus (SARS-CoV-2) outbreak: focus on disinfection methods, environmental survival, and control and prevention strategies. Environmental Science and Pollution Research. 2021;28:1–15. doi: 10.1007/s11356-020-11060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemecha Obsu L., Feyissa Balcha S. Optimal control strategies for the transmission risk of COVID-19. Journal of Biological Dynamics. 2020;14:590–607. doi: 10.1080/17513758.2020.1788182. [DOI] [PubMed] [Google Scholar]
- 8.Reed N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Reports. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller S.L., Linnes J., Luongo J. Ultraviolet Germicidal Irradiation: Future Directions for Air Disinfection and Building Applications. Photochemistry and Photobiology. 2013;89:777–781. doi: 10.1111/php.12080. [DOI] [PubMed] [Google Scholar]
- 10.Wong T., Woznow T., Petrie M., Murzello E., Muniak A., Kadora A., Bryce E. Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C–emitting devices. American Journal of Infection Control. 2016;44:416–420. doi: 10.1016/j.ajic.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Dumyahn T., First M. Characterization of Ultraviolet Upper Room Air Disinfection Devices. American Industrial Hygiene Association Journal. 1999;60:219–227. doi: 10.1080/00028899908984439. [DOI] [Google Scholar]
- 12.Emilio A., Alba D., Bernab C., Hermosa R., Monte E. Microbiological Evaluation of the Disinfecting Potential of UV-C and UV-C Plus Ozone Generating Robots. Microorganisms. 2021;9:172. doi: 10.3390/microorganisms9010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao Z., Ye Y., Chang P.H., Thirunarayanan D., Wigginton K.R. Nucleic Acid Photolysis by UV254 and the Impact of Virus Encapsidation. Environmental Science and Technology. 2018;52:10408–10415. doi: 10.1021/acs.est.8b02308. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y., Choi Y. The effects of UV disinfection on drinking water quality in distribution systems. Water Research. 2010;44:115–122. doi: 10.1016/j.watres.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Biasin M., Bianco A., Pareschi G., Cavalleri A., Cavatorta C., Fenizia C., Galli P., Lessio L., Lualdi M., Tombetti E., Ambrosi A., Redaelli E.M.A., Saulle I., Trabattoni D., Zanutta A., Clerici M. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Scientific Reports. 2021;11:6260. doi: 10.1038/s41598-021-85425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with Deep-UV LED irradiation. Emerging Microbes and Infections. 2020;9:1744–1747. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., Zheng X., Sutter K., Trilling M., Alt M., Steinmann E., Krawczyk A. Susceptibility of SARS-CoV-2 to UV irradiation. American Journal of Infection Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raeiszadeh M., Adeli B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics. 2020;7:2941–2951. doi: 10.1021/acsphotonics.0c01245. [DOI] [PubMed] [Google Scholar]
- 19.Ma B., Gundy P.M., Gerba C.P., Sobsey M.D., Linden K.G. UV Inactivation of SARS-CoV-2 across the UVC Spectrum: KrCl* Excimer, Mercury-Vapor, and Light-Emitting-Diode (LED) Sources. Applied and Environmental Microbiology. 2021;87 doi: 10.1128/AEM.01532-21. e01532-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minamikawa T., Koma T., Suzuki A., Mizuno T., Nagamatsu K., Arimochi H., Tsuchiya K., Matsuoka K., Yasui T., Yasutomo K., Nomaguchi M. Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode. Scientific Reports. 2021;11:5070. doi: 10.1038/s41598-021-84592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimoda H., Matsuda J., Iwasaki T., Hayasaka D. Efficacy of 265-nm ultraviolet light in inactivating infectious SARS-CoV-2. Journal of Photochemistry and Photobiology. 2021;7 doi: 10.1016/j.jpap.2021.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erren T.C., Lewis P., Morfeld P. Factoring in Coronavirus Disease 2019 Seasonality: Experiences From Germany. The Journal of Infectious Diseases. 2021;224:1096. doi: 10.1093/infdis/jiab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogeveen M.J., Hoogeveen E.K. Comparable seasonal pattern for COVID-19 and flu-like illnesses. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danon L., Brooks-Pollock E., Bailey M., Keeling M. A spatial model of COVID-19 transmission in England and Wales: early spread, peak timing and the impact of seasonality. Philosophical Transactions of the Royal Society B: Biological Sciences. 2021;376 doi: 10.1098/rstb.2020.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N., Baschir L.A., Tenciu D.V. Exploring the linkage between seasonality of environmental factors and COVID-19 waves in Madrid, Spain. Process Safety and Environmental Protection. 2021;152:583–600. doi: 10.1016/j.psep.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Huang J., Li C., Zhao Y., Wang D., Huang Z., Yang K. The role of seasonality in the spread of COVID-19 pandemic. Environmental Research. 2021;195 doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byun W.S., Heo S.W., Jo G., Kim J.W., Kim S., Lee S., Park H.E., Baek J.-H. Is coronavirus disease (COVID-19) seasonal? A critical analysis of empirical and epidemiological studies at global and local scales. Environmental Research. 2021;196 doi: 10.1016/j.envres.2021.110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly. 2020;150:w20224. doi: 10.4414/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson T.J., Nickbakhsh S., Schernhammer E.S. Drivers of Infectious Disease Seasonality : Potential Implications for COVID-19. Journal of Biological Rhythms. 2021;36:35–54. doi: 10.1177/0748730420987322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merow C., Urban M.C. Seasonality and uncertainty in global COVID-19 growth rates. Proceedings of the National Academy of Sciences. 2020;117:27456–27464. doi: 10.1073/pnas.2008590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson K.L., Boehm A.B., Davies-Colley R.J., Dodd M.C., Kohn T., Linden Karl.G., Liu Y., Maraccini P.A., McNeill K., Mitch W.A., Nguyen T.H., Parker K.M., Rodriguez R.A., Sassoubre L.M., Silverman A.I., Wigginton K.R., Zepp R.G. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Environmental Science: Processes & Impacts. 2018;20:1089–1122. doi: 10.1039/C8EM00047F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K., Yeager J., Altamura L.A., Biryukov J., Yolitz J., Schuit M., Wahl V., Hevey M., Dabisch P. Simulated Sunlight Rapidly Inactivates SARS-CoV-2 on Surfaces. The Journal of Infectious Diseases. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lytle C.D., Sagripanti J.-L. Predicted Inactivation of Viruses of Relevance to Biodefense by Solar Radiation. Journal of Virology. 2005;79:14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber T.P., Stilianakis N.I. A note on the inactivation of influenza a viruses by solar radiation, relative humidity and temperature. Photochemistry and Photobiology. 2008;84:1601–1602. doi: 10.1111/j.1751-1097.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 35.Nicastro F., Sironi G., Antonello E., Bianco A., Biasin M., Brucato J.R., Ermolli I., Pareschi G., Salvati M., Tozzi P., Trabattoni D., Clerici M. Solar UV-B/A radiation is highly effective in inactivating SARS-CoV-2. Scientific Reports. 2021;11:14805. doi: 10.1038/s41598-021-94417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carleton T., Cornetet J., Huybers P., Meng K.C., Proctor J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proceedings of the National Academy of Sciences. 2021;118 doi: 10.1073/pnas.2012370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrow K.A., Rich L.M., Vanderwall E.R., Reeves S.R., Rathe J.A., White M.P., Debley J.S. Inactivation of Material from SARS-CoV-2-Infected Primary Airway Epithelial Cell Cultures. Methods and Protocols. 2021;4:7. doi: 10.3390/mps4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.E. Rensen, S. Pietropaoli, F. Mueller, C. Weber, S. Souquere, P. Isnard, M. Rabant, J.-B. Gibier, E. Simon-Loriere, M.-A. Rameix-Welti, G. Pierron, G. Barba-Spaeth, C. Zimmer, Sensitive visualization of SARS-CoV-2 RNA with CoronaFISH, BioRxiv. (2021) 2021.02.04.429604. 10.1101/2021.02.04.429604.
- 39.Tsanov N., Samacoits A., Chouaib R., Traboulsi A.-M., Gostan T., Weber C., Zimmer C., Zibara K., Walter T., Peter M., Bertrand E., Mueller F. smiFISH and FISH-quant – a flexible single RNA detection approach with super-resolution capability. Nucleic Acids Research. 2016;44:e165. doi: 10.1093/nar/gkw784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preibisch S., Saalfeld S., Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics (Oxford, England) 2009;25:1463–1465. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishisaka-Nonaka R., Mawatari K., Yamamoto T., Kojima M., Shimohata T., Uebanso T., Nakahashi M., Emoto T., Akutagawa M., Kinouchi Y., Wada T., Okamoto M., Ito H., ichi Yoshida K., Daidoji T., Nakaya T., Takahashi A. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. Journal of Photochemistry and Photobiology B: Biology. 2018;189:193–200. doi: 10.1016/j.jphotobiol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., Doan K., Bastien N., Li Y., Van Caeseele P.G., Poliquin G. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovalski W. Springer Science & Business Media; 2010. Ultraviolet germicidal irradiation handbook: UVGI for air and surface disinfection. [Google Scholar]
- 45.Mbonimpa E.G., Blatchley III E.R., Applegate B., Harper Jr W.F. Ultraviolet A and B wavelength-dependent inactivation of viruses and bacteria in the water. Journal of Water and Health. 2018;16:796–806. doi: 10.2166/wh.2018.071. [DOI] [PubMed] [Google Scholar]
- 46.HASHIMOTO A., MAWATARI K., KINOUCHI Y., AKUTAGAWA M., OTA N., NISHIMURA K., HIRATA T., TAKAHASHI A. Inactivation of MS2 Phage and Cryptosporidium parvum Oocysts Using UV-A from High-Intensity Light-Emitting Diode for Water Disinfection. Journal of Water and Environment Technology. 2013;11:299–307. doi: 10.2965/jwet.2013.299. [DOI] [Google Scholar]
- 47.Furusawa Y., Suzuki K., Sasaki M. Biological and Physical Dosimeters for Monitoring Solar UV-B Light. Journal of Radiation Research. 1990;31:189–206. doi: 10.1269/jrr.31.189. [DOI] [PubMed] [Google Scholar]
- 48.Horton L., Torres A.E., Narla S., Lyons A.B., Kohli I., Gelfand J.M., Ozog D.M., Hamzavi I.H., Lim H.W. Spectrum of virucidal activity from ultraviolet to infrared radiation. Photochemical & Photobiological Sciences. 2020;19:1262–1270. doi: 10.1039/D0PP00221F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madronich S., Björn L.O., McKenzie R.L. Solar UV radiation and microbial life in the atmosphere. Photochemical & Photobiological Sciences. 2018;17:1918–1931. doi: 10.1039/C7PP00407A. [DOI] [PubMed] [Google Scholar]
- 50.Coohill T.P., Sagripanti J.-L. Bacterial Inactivation by Solar Ultraviolet Radiation Compared with Sensitivity to 254 nm Radiation. Photochemistry and Photobiology. 2009;85:1043–1052. doi: 10.1111/j.1751-1097.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- 51.J. Van Geffen, M. Van Weele, M. Allaart, R. Van der A, TEMIS UV index and UV dose operational data products, version 2. Dataset., 2017. 10.21944/temis-uv-oper-v2.
- 52.Monto A.S., DeJonge P.M., Callear A.P., Bazzi L.A., Capriola S.B., Malosh R.E., Martin E.T., Petrie J.G. Coronavirus Occurrence and Transmission Over 8 Years in the HIVE Cohort of Households in Michigan. The Journal of Infectious Diseases. 2020;222:9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagripanti J.-L., Lytle C.D. Estimated Inactivation of Coronaviruses by Solar Radiation With Special Reference to COVID-19. Photochemistry and Photobiology. 2020;96:731–737. doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sagripanti J.-L., Lytle C.D. Inactivation of Influenza Virus by Solar Radiation. Photochemistry and Photobiology. 2007;83:1278–1282. doi: 10.1111/j.1751-1097.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- 55.Nicastro F., Sironi G., Antonello E., Bianco A., Biasin M., Brucato J.R., Ermolli I., Pareschi G., Salvati M., Tozzi P., Trabattoni D., Clerici M. Forcing Seasonality of Influenza-like Epidemics with Daily Solar Resonance. IScience. 2020;23 doi: 10.1016/j.isci.2020.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wurtmann E.J., Wolin S.L. RNA under attack: Cellular handling of RNA damage. Critical Reviews in Biochemistry and Molecular Biology. 2009;44:34–49. doi: 10.1080/10409230802594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkler M., Giuliano B.M., Caselli P. UV Resistance of Nucleosides—An Experimental Approach. ACS Earth and Space Chemistry. 2020;4:2320–2326. doi: 10.1021/acsearthspacechem.0c00228. [DOI] [Google Scholar]
- 58.Pfeifer G.P., Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochemical & Photobiological Sciences. 2012;11:90–97. doi: 10.1039/C1PP05144J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rastogi R.P., Kumar R.A., Tyagi M.B., Sinha R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. Journal of Nucleic Acids. 2010;2010 doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Besaratinia A., Synold T.W., Xi B., Pfeifer G.P. G-to-T Transversions and Small Tandem Base Deletions Are the Hallmark of Mutations Induced by Ultraviolet A Radiation in Mammalian Cells. Biochemistry. 2004;43:8169–8177. doi: 10.1021/bi049761v. [DOI] [PubMed] [Google Scholar]
- 61.H. Bernstein, C. Bernstein, Origin of DNA Repair in the RNA World, in: S.-Q. Branch (Ed.), DNA - Damages and Repair Mechanisms, IntechOpen, 2021. 10.5772/intechopen.93822.