Abstract
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for the ongoing COVID-19 pandemic, and causes many health complications, including major lung diseases. Besides investigations into the virology of SARS-CoV-2, understanding the immunological routes underlying the clinical manifestations of COVID-19 is important for developing effective therapeutic interventions. The clearance of SARS-CoV-2-infected apoptotic cells by professional efferocytes, through a process termed as 'efferocytosis', is essential for maintaining tissue homeostasis, and reducing the chances of health complications caused by SARS-CoV-2 infection. In this review, we focus on the cellular events leading to engagement of the SARS-CoV-2 with type 2 alveolar cells, and how SARS-COV-2 infection impairs the macrophage anti-inflammatory programming. We also discuss accounts of impaired efferocytosis, and the “cytokine storm” which occur concomitantly with the SARS-CoV-2 infection. Finally, we propose how targeting impaired efferocytosis, due to the SARS-CoV-2 infection, may be a beneficial therapeutic strategy to combat COVID-19, and its complications.
Abbreviations: ACE2, angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; AMP, adenosine monophosphate; ATP, adenosine triphosphate; BAI1, brain angiogenesis inhibitor 1; cAMP, cyclic adenosine monophosphate; COPD, Chronic obstructive pulmonary disease; COVID-19, coronavirus disease of 2019; COX2, cyclooxygenase 2; CXCL, chemokine (C-X-C motif) ligand; DAMP, damage associated molecular pattern; DC, dendritic cell; FDA, food and drug administration; GAS-6, Growth arrest specific gene 6; HCoV229E, human coronavirus 229E; HIV, human immunodeficiency virus; HMGB1, High mobility group box 1 protein; IL, interleukin; LC3, microtubule-associated protein 1 A/1B-light chain 3; LPC, lysophosphatidylcholines; , Macrophage, Macrophages; MARS-CoV, middle east respiratory syndrome-related coronavirus; MERTK, proto-oncogene tyrosine-protein kinase MER; MFG-E8, milk fat globule-EGF factor 8 protein; PS, Phosphatidyl serine; Rac-GEF, Rac guanine nucleotide exchange factor; RAGE, receptor for advanced glycation end products; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2-AC, Severe acute respiratory syndrome coronavirus 2 infected apoptotic cell; SOCS, Suppressor Of Cytokine Signalling; S1P, sphingosine-1-phosphate; TGF-β, transforming growth factor beta; TIM, T cell immunoglobulin mucin receptor; TMPRSS2, transmembrane serine protease 2; TNF-α, tumour necrosis factor α; TLR, toll like receptor; TUNNEL, Terminal deoxynucleotidyl transferase dUTP nick end labelling; WHO, World health organization
Keywords: Apoptosis, ARDS, Autoimmune disease, COVID-19, Cytokine storm, Diabetes, Phagocytosis, Efferocytosis, Inflammation, SARS-CoV-2
Graphical Abstract
1. Introduction
The Corona Virus Disease 2019 (COVID-19) has quickly emerged as one of the leading causes of death. The WHO has elevated the novel coronavirus (COVID-19) outbreak to the rank of a global pandemic [1]. The severe acute respiratory Corona Virus 2 (SARS-COV-2) is the cause of COVID-19 [2]. However, the pathologies associated with COVID-19 are still poorly defined. Patients affected by COVID-19 can develop different kinds of clinical manifestations, including pneumonia, acute respiratory distress syndrome (ARDS), septic shock, and multi-organ dysfunction [3]. The signs and symptoms of COVID-19 have been attributed to uncontrolled host immune response, and compromised functions of myeloid cells, which include dendritic cells, monocytes, tissue macrophages, and granulocytes, suggesting that both hyper-inflammation and unresolved tissue damages may contribute to the pathogenesis of severe COVID-19 infection [4], [5], [6].
The SARS-CoV-2 virus infects alveolar cells of the lungs, and other cells in various tissues, and triggers cell death along a variety of modes, such as apoptosis, necrosis, pyroptosis, autophagy-induced cell death, and ferroptosis [7]. The body, in turn, by a process known as efferocytosis, maintains tissue homeostasis by phagocytosis of the apoptotic bodies of dying cells. The efficient clearance of damaged or dead cells, through the process of efferocytosis, prevents further tissue dysfunction caused by the release of damage-associated molecular patterns (DAMP). The efferocytes, cells such as macrophages and monocytes, promote disease tolerance to maintain host fitness, without affecting the pathogen burden; they do so, upon recognising pathogenic signals, by resolving inflammation, by releasing anti-inflammatory cytokines, reducing pro-inflammatory cytokine production, and reorienting towards an anti-inflammatory phenotype [8]. Moreover, efferocytosis not only prevents the deleterious effect of secondary necrosis, and but also helps in and promotes the repair of damaged tissue to sustain physiological function [9], [10], [11]. On the contrary, impaired efferocytosis causes chronic inflammation, resulting in severe lung diseases and autoimmune disorders [12].
COVID-19 infection has been shown to hamper the proper function of alveolar macrophages, although the mechanism remains vague. The inadequate clearance of apoptotic cells generated due to the SARS-CoV-2 infection contributes to COVID-19-associated autoimmune disorders [13]. Thus, the clearance of apoptotic bodies by boosting efferocytosis in SARS-CoV-2-infected tissues can play an important role in the management of COVID-19 pathology by preventing inflammatory responses, such as a ‘cytokine storm’ [14]. Because of the consistent spread of the COVID-19 disease all over the world, it is of great significance to gain more in-depth knowledge of how to combat its possible side-effects. This review article aims to discuss the engagement of the SARS-CoV-2 with type 2 alveolar cells, and how SARS-CoV-2 infection impairs macrophage anti-inflammatory programming. Besides this, we will discuss and hypothesise the probable link between impaired efferocytosis and COVID-19 disease, and attempt to propose possible treatment options to combat COVID-19 side-effects.
2. The process of efferocytosis, and the role played by the macrophages
Efferocytosis is the process of phagocytosis of apoptotic and necrotic cells, in any damaged tissue, by professional efferocytes, such as macrophages and dendritic cells (DCs). Sometimes, non-professional efferocytes, such as epithelial cells, astrocytes, Sertoli cells, and neural progenitor cells, also participate in this process of phagocytosis [15], [16]. Apoptotic cells contain autoantigens, such as adenosine, heat-shock proteins, and high-mobility group box-1 proteins (HMGB1), and the release of these necrotic factors, during secondary necrosis, causes chronic inflammation, sepsis, and autoimmunity [17]. In healthy individuals, the uptake of apoptotic and necrotic cells by macrophages is so efficient that very few apoptotic cells escape efferocytosis. Efferocytosis, a highly orchestrated process, actively induces the macrophage phenotype that favours damaged tissue repair, and helps to suppress chronic inflammation [18]. Impaired efferocytosis is the common thread between a plethora of chronic health conditions, including cystic fibrosis, chronic obstructive pulmonary disease (COPD), asthma, and ARDS [19]. It has been proposed that providing a pharmacological fillip to the efferocytosis process might arrest lung disease progression [20].
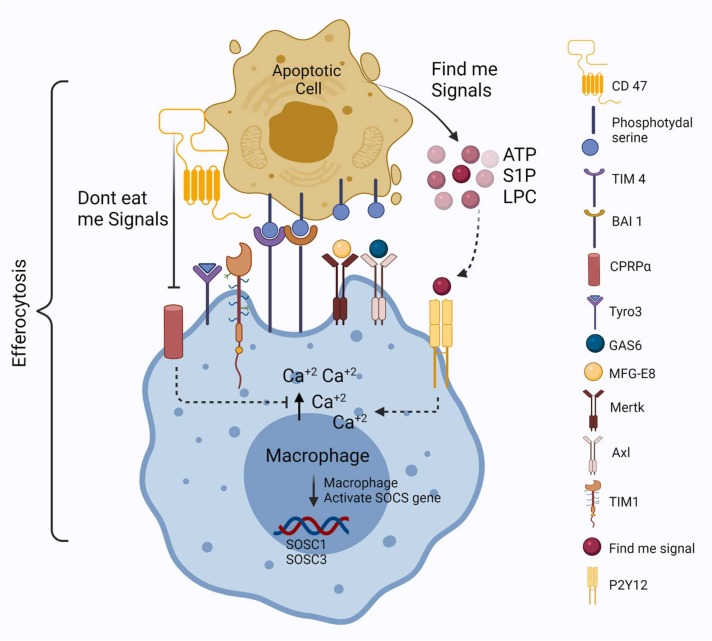
The process of efferocytosis is systematically different from many other types of phagocytosis, and involves several phases, including apoptotic cell finding, apoptotic cell binding, apoptotic cell internalisation, and apoptotic cell digestion [21]. Apoptotic cells actively release soluble factors to attract the mononuclear phagocytic cells (macrophages and DCs). Key “find-me” signals involved in this process are: chemokines, such as C-X-C motif chemokine ligand 1 (CX3CL1) [22], lysophosphatidylcholine (LPC) [23], sphingosine-1-phosphate (S1P) [24], [25], [26], [27], and triphosphate nucleotides [28], [29], such as ATP [30]. Apoptotic cells release these “find-me” signals through caspase 3/7-activated hexameric pannexin-1 channels [31] ( Fig. 1). Apart from such secreted molecules, apoptotic cells also display phosphatidylserine (PS) on the outer surface of the plasma membrane, to bind with the “find-you” signals [32]. Thus, the “find-me” and “find-you” signals both help in the binding of the professional efferocytes with apoptotic cells. Professional efferocytes digest apoptotic bodies through the activation of autophagy-related gene product, Microtubule-associated protein 1A/1B light chain 3 (LC3) [33], and induce specific signal transduction pathways that up-regulate efferocytotic receptors and opsonins [18].
Fig. 1.
Schematic representation of efferocytosis mechanism performed by professional macrophage efferocyte. Phosphatidylserine, which is generally present on the inner layer of the plasma membrane, is exposed to the outside after receiving the apoptotic signals and release find-me signals in the form of nucleotides (ATP), sphingosine-1-phosphate (S1P), and lipophosphatidyl choline (LPC). TIM-4 and BAI-1 directly bind with phosphatidylserine (PS), and all the TAM receptors such as Mertk, Axl, TIM-1–3, and Tyro-3 bind with PS through the bridging molecules like MFG-E8 and GAS-6 proteins. Find-me signals bind with the P2Y12 receptor present on the plasma membrane of macrophages and help to create a Ca+ gradient, which promotes the movement of macrophages towards the apoptotic cells. Suppressors of cytokine signalling 1 and 3 genes (SOCS1 and SOCS3) are activated for the maintenance of inflammatory programming. Apoptotic cells also express the don't eat me signal through the binding of CD-47 with CPRPα present on the macrophage cell membrane.
There are 12 types of PS efferocytotic receptors found to date, which form a heterogeneous group of surface proteins that bind to PS directly, , such as, eor via soluble PS-binding opsonins [34], [35], [36]. Four major families of PS efferocytosis receptors have been studied so far, which include (1) Brain-specific angiogenesis inhibitor (BAI), (2) T-cell immunoglobulin and mucin domain-containing (TIM), (3) Stablin, and (4) CD300. Macrophages and DCs bind PS directly through the receptors: BAI1 and TIM-4 [37]. The adhesion G-protein coupled receptor BAI1 directly binds to the PS with the activation of the Elmo-Dock bipartite Rac-GEF [38]. BAI1 expression is restricted to gastric and intestinal phagocytes, and important for the process of efferocytosis [39]. Lee et al. first demonstrated that BAI1-deficient mice display increased colonic inflammation and tissue damages [40]. It has also been reported that BAI1 deletion in macrophage and colonic epithelial cells leads to an increase in the level of pro-inflammatory cytokines, such as such as, eor IL-1α, IL-6, and TNF-α [40]. These findings suggest that inflammatory gene expression is regulated and influenced by the activation of Elmo-Dock signalling [41].
Whereas BAI1 is directly involved in juxtacrine signalling, the TIMs: TIM-1, TIM-3, and TIM-4; and the receptor tyrosine kinases TYRO3, AXL, and MERTK, collectively known as TAMs, are involved in the process of efferocytosis, mediated by mononuclear efferocytes, through bridging molecules, serum proteins such as GAS6 and ProS1 [37] (Fig. 1). The TIM family receptors, such as TIM1 and TIM3, are required to bind with PS to suppress NF-κβ activation, and the production of pro-inflammatory regulators, such as TNF, IL-6, and CCL5, in proximal tubular cells [42], [43]. It has recently been reported that TIM-4-mediated corpse engulfment can trigger LC3-associated autophagy [44]. The activation of TIM and TAM receptors inhibits pro-inflammatory cytokine signalling through the induction of Suppressor of cytokine signalling 1 and 3 (SOCS1 and SOCS3) [45], [46]. Moreover, recent reports have suggested that A2A and A2B receptors, present on macrophages, mediate the suppression of pro-inflammatory chemokines, such as CXCL1 and CXCL2, and up-regulate the pro-resolution factors, such as Nuclear Receptor 4A1 (NR4A1) and Thrombospondin 1 (THBS1), during the process of efferocytosis [47], [48]. Moreover, TAM deficiency disrupts apoptotic cell clearance by the macrophages, without disturbing other phagocytic properties [49], [50]. TAM receptor MERTK binds with PS through bridging molecules that inhibit NF-κβ signalling, thus reducing the release of pro-inflammatory cytokines [51], [52].
Some bridging molecules, including complement components, Milk fat globule-epidermal growth factor 8 (MFG-E8), Cellular communication network factor 1 (CCN1), Growth arrest-specific 6 (GAS6), and Protein S (PROS), are also involved in the process of recognition and binding of apoptotic cells with efferocytes [35], [36] (Fig. 1). Binding of these bridging molecules to the PS, through αvb3/5 integrin/vitronectin receptors on phagocytes, promotes Rac activation and corpse internalisation [53], [54]. Thus, αv integrin plays a key role in macrophage adhesion and performs an important role in the process of efferocytosis.
Direct interaction of stabilin surface receptors (Stab1 and Stab2), present on macrophage, with PS improves the efficacy of efferocytosis or apoptotic cell clearance [55], [56], [57]. In vitro analyses have revealed that the expression of the Stab2 receptor induces the production of anti-inflammatory cytokine TGF-β [56]. On the other hand, Stab1/Stab2 double knock-out mice have a shorter life span, and increased witho tissue inflammation [58], [59].
Another “find-me” signalling molecule, S1P also provokes anti-apoptotic and anti-inflammatory gene expression in macrophages. These gene expressions are ncreaseincreased characterised by the suppression of TNF-α and IL-2, along with the increased production of IL-10, vascular endothelial growth factor (VEGF), and prostaglandin E2 (PGE2). The S1P receptor (S1PR), present on the plasma membrane of macrophage, prompts the conversion to an M2-like anti-inflammatory phenotype inducing the production of cAMP and cyclooxygenase-2, and the suppression of NF-κβ signalling [60]. S1P binding with also increases intra-macrophage Ca2+ concentration, which promotes the movement of macrophage towards the apoptotic cells [61].
Macrophages, involved in the innate immune system, play a crucial role in the antiviral response, tissue repair, and fibrosis. They can phagocytose virus, bacteria, as well as apoptotic cells to trigger an immune response, maintain tissue homeostasis, and promote regeneration [62]. Monocyte-derived macrophages co-exist as one of two sub-types, first, the classically activated or M1-like, and another, the alternatively activated or M2-like; cytokine signalling causes their phenotypic programming, and recruitment to the inflamed tissue [62], [63], [64]. Cytokine secretion profile sometimes varies between M1- and M2-like macrophages. M1-like macrophages are associated with microbial activity, pro-inflammatory cytokine production, and immune responses, while M2-like macrophages are recruited to heal the damaged tissue after viral infection. Therefore, the recruitment of macrophages into the damaged tissue is crucial for a balanced and controlled antiviral immune response, and for the clearance of dead cells caused by viral infection.
In the lungs, efferocytosis is predominantly orchestrated by not only the epithelial cells, but also the alveolar macrophages, the professional efferocytes that are well-distributed in the alveolar space, interspersed throughout the mucus layer and the interstitial space [65]. There are a few notable differences between alveolar macrophages and their counterparts in other tissues. The weaker efferocytotic capacity of alveolar macrophages, compared to other macrophages in different tissues, is compensated by a higher capacity for self-renewal and repair, which, thus, allows them to maintain homeostasis in the respiratory tract [66], [67], [68]. Moreover, alveolar macrophages require lower activation energy to activate inflammatory genes, against bacteria or viruses, than their counterparts in other tissues [69], [70]. Alveolar macrophages undertake the release of anti-inflammatory cytokines, transforming growth factor-β (TGF-β), and interleukin 10 (IL-10), while inhibiting the secretion of pro-inflammatory cytokines, including Tumour necrosis factor-α (TNF-α), IL-1, IL-8, and leukotriene C4 (LTC4) [71], [72] ( Fig. 2). Interestingly, impaired efferocytosis has been detected in the airways of patients suffering from lung diseases due to the increased burden of apoptotic cells, which culminates in chronic inflammation. In fact, the impaired clearance of apoptotic cells by professional efferocytes is the upstream event to sustained chronic lung inflammation [73].
Fig. 2.
Alveolar macrophages maintain lung tissue homeostasis through the process of efferocytosis. First alveolar macrophages find the apoptotic cells and bind with the help of TIM and TAM receptors. In the process of efferocytosis, apoptotic cell finding, apoptotic cell binding, and apoptosis of cells are internalised done by professional efferocytes such as macrophages. This process helps to maintain tissue homeostasis by the inhibition of pro-inflammatory cytokines release. Apoptotic cells convert into necrotic cells due to impaired efferocytosis and cause inflammation. The main reason for inflammation is the release of pro-inflammatory cytokines from necrotic cells such as TNF-α, IL-8, IL-1, and IL-6. TAM receptors, after binding with the ligands present on apoptotic cells, get activated and induced SOCS genes. Induction of SOCS1 and SOCS 3 genes helps to release anti-inflammatory cytokines and inhibit the release of pro-inflammatory cytokines. Anti-inflammatory cytokines such as TGF-β and IL-10 inhibit inflammation caused by necrotic cells and help to maintain lung tissue homeostasis.
3. The impact of SARS-CoV-2 infection on the respiratory system and its associated immune milieu
In a healthy individual, the airway epithelium acts as a barrier against the routine onslaught of viruses, bacteria, and different particles, to prevent infection and tissue injury. SARS-CoV-2 generally enters the human body through the respiratory tract, and compromises the airway epithelium, alveolar epithelium, and vascular endothelium [74], [75] ( Fig. 3). According to in vitro analyses, it has been observed that ciliated airway epithelial cells are the primary site of SARS-CoV-2 infection [76]. Like with other types of coronaviruses, the SARS-CoV-2 infection occurs through, initially, the binding of the viral S (spike) protein to the Angiotensin-converting enzyme 2 receptors (ACE2), abundantly present on the surface of lung alveolar epithelial cells, followed by the cleavage of the spike protein by Transmembrane serine protease 2 (TMPRSS2) [77], [78], [79], [80], [81]. SARS-CoV-2, after infiltrating the nasal mucosa, replicates and triggers the repertoire of immune and inflammatory responses that constitute the signs and symptoms of COVID-19 infection [82].
Fig. 3.
Macrophage activation syndrome due to SARS-CoV-2 infection. After the infection, SARS-CoV-2 enters through the respiratory tract and binds to the ACE2 receptor present on type II pneumocytes. Spike protein (S-protein) present on SARS-CoV-2 is cleaved by transmembrane protease TMPRSS2. This cleavage helps SARS-CoV-2 to bind with ACE2 receptor and exocytosis into pneumocyte cell. After exocytosis, SARS-CoV-2 enhances their replication and targets to attack alveolar macrophage present in the alveolar space. Subsequently, infected with SARS-CoV-2, alveolar macrophages, monocytes, neutrophils, and other immune cells release inflammatory cytokines like IL-6, IL-8, IL-1β, TNF-α, IFN-γ, IL-10 as well as several chemokines. The incidence of rapid release of different inflammatory cytokines is called ‘cytokine storm’, which attacks own cells and tissue rather than fighting off the virus. Inflammatory cytokines released from different immune cells bind to their specific receptor present on type I pneumocyte cells and the endothelial cells. After binding inflammatory cytokines to their specific receptors, it activates NF-κβ signalling, which rapidly promotes apoptosis of type I epithelial and endothelial cells. 'Cytokine storm', which is generated by the infection with SARS-CoV-2, causes endothelial cell activation, and damage associated with cytokine storm is triggered through multiple pathways, including the release of pro-inflammatory cytokines and complement activation. As a result of this activation, endothelial cells release ultra-large VWF (von Willebrand factor) and p-selectin, which binds to platelets, monocyte, and neutrophil to develop microvascular thrombosis. D-dimer and fibrin are released in the alveolar space by the infection with SARS-CoV-2, bind to TLR and RLR present on alveolar macrophages and hinders the function of macrophages in the process of efferocytosis by enhancing SARS-CoV-2 replication. SARS-CoV-2 binds to ACE2 receptors present on endothelial cells, stimulates the release of pro-inflammatory cytokines such as IL-6 and TNF-α, and causes activation of caspase-1. Activated caspase-1 is responsible for endothelial cell death or apoptosis.
The upper respiratory tract epithelium, upon being affected by SARS-CoV-2, suffers from impaired gas exchange and respiratory failure. Triggering a chain reaction, the virions produced by the infected epithelial cells also infect other adjacent cells, such as neighbouring epithelial cells, endothelial cells, and macrophages, which are, in fact, the active participants in the process of efferocytosis [83]. Interestingly, macrophages frequently interact with ACE2-expressing cells present in the lung tissue, dialogues which could help them in sensing, and orienting towards defensive and destructive functions. [84]. Chan et al. have reported that the viral proteins expressed in the hamster model upon SARS-CoV-2 infection can cause widespread cell apoptosis [85]. The vascular endothelium is also an important site for COVID-19 infection [86]. Infection of endothelial cells occurs in the luminal or interstitial site, and this infection triggers the release of cytokines and chemokines. The 'cytokine storm', caused due to the infection with SARS-CoV-2, impairs macrophage function, and hinders the process of efferocytosis or apoptotic cell clearance [87].
The epithelial cells which are infected by SARS-CoV-2 express inflammatory mediators, such as CXCL10, and interferon [88], [89]. In vitro analyses have shown that type II alveolar cells, upon infection with influenza virus or coronavirus, secrete pro-inflammatory cytokine mediators, such as IL-6, IL-8, IL-29, CCL5, CXCL9, CXCL10, and CXCL1152 [90].
The brutality of SARS-CoV-2 infection can be stratified based on the chemokine level present in the SARS-CoV-2 infected patients. Higher levels of CCL7, CCL3, and CXCL9 have been found in severe cases of COVID-19, compared with mild and moderate cases [91]. According to Huang et al., the plasma levels of CXCL10, CCL2, and CCl3 are reported to be higher in ICU patients than non-ICU patients, affected by SARS-CoV-2 [92]. Another study has revealed that the plasma levels of homeostatic chemokines, such as CXCL12, neutrophil-targeted CXCL1, eosinophil-targeted CCL11, and the memory T-cell-homing chemokine CCL27, stayed high during infection [93]. COVID-19 patients suffering from pneumonia and hypoxia show a higher level of CCL3 and CXCL10 [94]. Higher levels of chemokine secretion due to SARS-CoV-2 infection in lung tissue cause the release of higher levels of pro-inflammatory cytokines, and may hinder the process of apoptotic cell clearance, which may lead to different kinds of lung-related disorders and several complications.
4. Impaired efferocytosis and the consequent pro-inflammatory programming linked with SARS-CoV-2 infection
Given that viruses can take over and down-regulate antiviral immune responses prompted by macrophages to enhance their own replication and pathogenesis, it has been postulated that there is a similarity between HCoV-229E, SARS-CoV, MARS-CoV, and SARS-CoV-2 in their ability to cause respiratory tissue damage and several types of respiratory disorders associated with high morbidity and mortality. Development of these respiratory disorders may be linked to imbalanced macrophage populations during SARS-CoV-2 infection and, consequently, impaired efferocytosis [13], [95], [96]. Like other SARS-CoV, SARS-CoV-2 impairs the function of the macrophages, the professional efferocytes involved in the process of efferocytosis. Non-classical macrophages, such as monocyte, M1-like macrophage, and M2-like macrophage, have also been detected in the peripheral blood of COVID-19 patients [97]. The infection of the ACE2+ macrophage by SARS-CoV-2 enhances the secretion of IL-6 from the macrophage [98]. The massive secretion of IL-6 from the macrophage induces the apoptosis of lymphocytes through Fas signalling [98]. A disproportionately large ratio of apoptotic cells to macrophages, engendered due to SARS-CoV-2 infection, may be the reason for impaired efferocytosis.
Associated with macrophage activation and the development of respiratory distress syndrome, is the release of inflammatory cytokines, such as IL-6, TNF-α, IL-1β, and IFN-γ, directly [99]. Macrophage activation syndrome and dysregulated immune response have been found in COVID-19 patients suffering from severe respiratory failure [100]. Wang et al., have shown, in two severe cases of COVID-19, that the lung cavities were filled with macrophages and neutrophils, along with increased IL-6, TNF-α, and PD-L1; these might be responsible for ‘cytokine storm’ and pulmonary fibrosis [101]. Besides pulmonary fibrosis and failure, other complications such as hyper-coagulation or clot formation have been found in COVID-19 patients [102], [103], [104]. A recent finding has suggested that the spleen macrophages are responsible for the viral spread, and exacerbate the subsequent ‘cytokine storm’ [105]. Multiple research groups have proposed that patients suffering from SARS-CoV-2 infection have increased plasma levels of fibrinogen and D-dimer [102], [103], [104]; in fact, correlation studies have revealed that 71% of non-survivors had a higher level of D-dimer and fibrin degradation products [106]. Interestingly, increased levels of fibrin and fibrinogen may modulate the function of macrophages by triggering the release of the pro-inflammatory cytokine TNF-α [107], and also engage with Toll-like receptors (TLRs), which boosts the release of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-6, and IL-1β [108] ( Fig. 4). These reports suggest that there may be a strong relationship between disrupted macrophage function and the hypercoagulability found in COVID-19 patients.
Fig. 4.
Impaired efferocytosis signals concomitant with cytokine storm due to SARS-CoV-2 infection. SARS-CoV-2 infection alters two main signalling pathways (Find-me and Eat-me signalling) involved in the process of efferocytosis. SARS-CoV-2 infection hampers the binding of find-me signalling molecules such as ATP/AMP to their receptor-like A2a and down-regulates cyclic AMP production. Down-regulation of cAMP induces NF-κβ signalling. Stimulation of NF-κβ signalling up-regulates the production of chemokines and pro-inflammatory cytokines like CXCL1, CXCL2, and IL-1 and again down-regulates the synthesis of anti-inflammatory cytokines such as IL-10 and TGF-β. This up and downregulation of inflammatory cytokines troubles immune regulation by impaired efferocytosis. SARS-CoV-2 infection also helps to release HMGB1, which binds to TLR2, TLR4, and Receptor for advanced glycation endproducts (RAGE), thus shows a positive regulation towards NF-κβ signalling. Increased chemokines such as CCL3 and CXCL10 are directly correlated with impaired efferocytosis by the production of pro-inflammatory cytokines. Mutation or downregulation of S1P signalling also down-regulates the production of cAMP and COX2 and stimulates NF-κβ signalling. Stimulation of NF-κβ signalling inhibits the expression of TAM receptor protein MERTK and bridging molecule MFG-E8, causes impaired efferocytosis. SARS-CoV-2 infection also down-regulates the expression of TIM and TAM receptors associated with Eat-me signallings such as BAI1, TIM1–3, MERTK and produces cytokine storm, which is connected with inflammation and impaired efferocytosis. Some proposed drugs are mentioned for targeting impaired efferocytosis concomitant with cytokine storm.
Recently, it has been reported that epithelial cell lines (both human lung epithelial Calu-3 and simian kidney epithelial Vero CCL-81), infected with SARS-CoV-2, show apoptotic features [109], [110]. Moreover, Habib et al. have already linked COVID-19 infections with ferroptosis-mediated cell death [7]. Exposure of phosphatidylserine (PS) on the outer surface of SARS-CoV-2 infected cells leads to induction of programmed cell death, or apoptosis, through annexin V binding [111], [112]. Other groups, through extensive imaging and staining of the spike protein of the virus, have shown that macrophages efficiently engulf SARS-CoV-2-infected dying cells [13], [113]. It has also been proposed that the engulfment of SARS-CoV-2-AC (SARS-CoV-2 infected apoptotic cell) by macrophage significantly increases the production of IL-6 and IL-1β, and switches the latter from an anti-inflammatory, resolutive programming to an inflammatory phenotype [13] (Fig. 4).
It has also been reported that efferocytosis of SARS-CoV-2 infected dying cells might hamper the routine clearance of apoptotic cells. Since the primary aspect of efferocytosis is the engulfment of multiple apoptotic cells within a short period of time, this capability of professional efferocytes, such as macrophages, is essential when the ratio of apoptotic cells to efferocytes is higher after events which trigger acute inflammatory responses [114], [115], [116]. Therefore, continual efferocytosis is important, after an infection, to maintain tissue homeostasis and controlled inflammation [117].
5. Therapeutic strategies to combat SARS-CoV-2 infection: targeting impaired efferocytosis concomitant with the cytokine storm-induced complications
Impaired efferocytosis and huge production of pro-inflammatory cytokines due to SARS-CoV-2 infection have been observed in COVID-19-positive patients. The rapid surge of pro-inflammatory cytokines may be linked with the reduced expression of efferocytotic receptors. This ‘cytokine storm’ is one of the major causes of post-COVID-19 complications. Therefore, targeting ‘cytokine storms’ with potent antibodies or drugs could be a treatment option ( Table 1). In view of the present situation, the need of the hour is to find a therapeutic strategy to combat SARS-CoV-2 infection and SARS-CoV-2 infection-related complications. The ‘Big five’ lung diseases have seen a resurgence during the COVID-19 pandemic: according to Google Trends analyses, the incidence of the ‘Big five’ lung diseases, except for lung cancer, including Asthma, COPD, pneumonia, and ARDS has increased during the COVID-19 pandemic [118]. In the case of lung diseases, SARS-CoV-2 infection causes inflammation and bronchial cell apoptosis. The conversion of these massive numbers of apoptotic cells into necrotic ones, due to impaired efferocytosis, may result in the production and release pro-inflammatory cytokines in a large quantity, which could be the main reason behind COVID-19 complications. Therefore, targeting impaired efferocytosis could be a novel therapeutic strategy against COVID-19 infection.
Table 1.
Potential alternative approaches to treat cytokine storm induced complications in COVID-19 patients.
| Target | Drug | Mechanism | References | |
|---|---|---|---|---|
| IL-6 or IL-6 receptor | Tocilizumab (NCT04322773) Sarilumab (NCT04359901; NCT04357808) Clazakizumab (NCT04348500; NCT04381052; NCT04343989; NCT04381052) Olokizumab (NCT04380519) |
Neutralizing IL-6 or its receptor. | Ascierto et al. [136]; Buonaguro et al. [137]; Luo et al. [138]; Michot et al. [139]; Xu et al. [140]; Zhao [141]; Zhang et al. [142]. | |
| IL-1β | Canakinumab (NCT04362813; NCT04348448) | Targeting IL-1β by decreasing C-reactive protein through Anti-IL-1β antibodies | Ucciferri et al. [143]. | |
| TNF-α | Infliximab (NCT04425538; NCT04344249) |
evaluation of XPro1595, which prevents TNFs from binding to their receptors by forming heterotrimers with soluble TNF | Steed et al. [144]. | |
| Expression of pro-inflammatory cytokines like IL-6, IL-1β, and TNF-α | Trichostatin A | Decreases IL-6 production by modulating mRNA stability Decrease IL-1β, and TNF-α Decrease systemic inflammation |
DrugDrugMechaniGrabiec et al. [145]; Han and Lee [146]; Cui et al. [147]. |
|
| Cytokine storm | Methylprednisolone (NCT04244591, NCT04273321) Siltuximab (NCT04329650) Tocilizumab (NCT04377503, NCT04345445) Tacrolimus (NCT04341038) Colchicine (NCT04350320; NCT04375202) Anakinra (NCT04324021; NCT04443881) |
Immunosuppressive drugs targets NO (nitric oxide) and TNF-α, inhibits T cell activation, and decreases the extravasation of immune cells. | Sloka and Stefanelli [148]. | |
Recent studies have revealed that the engulfment of SARS-CoV-2-AC reduces the transcription of PS receptors, including that of the scavenger receptors CD36 and SRA-I, αVβ5 integrin (ITGB5), and T cell immunoglobulin mucin receptor 4 (TIM4), but not that of MER proto-oncogene tyrosine kinase (MERTK) [13]. Hence, it is clear that efferocytosis of SARS-CoV-2-infected dying cells depresses the expression of efferocytotic receptors, and impairs the continual elimination of apoptotic cells by macrophages. Since it has been found that activation of MERTK regulates immunosuppressive profiles, characterised by high IL-10 and TGF-β levels [119], Dexamethasone, a potent activator of the MERTK receptor, has been permitted, and is being broadly used, for the treatment of seriously ill COVID-19 patients [120].
It is assumed that the release of S1P, involved in the process of efferocytosis, is impaired by SARS-CoV-2 infection, and this can slow down the process of SARS-CoV-2-infected apoptotic cell clearance [121]. The immunomodulatory action of the orally available drug Fingolimod (FTY720), a modulator of S1PR, is being harnessed for the treatment of multiple sclerosis (MS) [122]. Several studies have reported that Fingolimod can potentially also be utilised for the treatment of the human immunodeficiency virus (HIV) disease [123], [124]. It has been reported that the case of a 57-year-old female, who was affected with SARS-CoV-2, upon a regime of Fingolimod treatment, had a positive outcome, thus, indicating that the immunomodulatory effect of Fingolimod might indeed prove to be beneficial to reduce the mortality caused due to the SARS-CoV-2 infection [125]. A clinical trial of Fingolimod (FTY720) in COVID-19 patients is already underway (NCT04280588). Moreover, thymoquinone, an anti-inflammatory agent, has also been shown to be effective against impaired S1P signalling system [126]. Therefore, both Thymoquinone and Fingolimod can be co-opted as therapeutic options to improve impaired efferocytosis through the modulation of S1P signalling.
Interestingly, the SARS-CoV-2 particle mimics apoptotic cells, by exposing PS on the viral envelope, which interacts with the phagocytic machinery of target cells, inducing its internalisation [127]. Morizono et al. were the first to report that TAM receptors are involved in viral molecular mimicry. Here, the GAS6 protein acts as a bridging molecule, connecting TAM receptors with the exposed PS, and prompts efficient entry and replication of the virus. Sustained TAM signalling is important for potent viral infection, thus TAM inhibitors may reduce viral infectivity [128], [129]. This study predicted that TAM receptors and bridging molecules, part of the process of efferocytosis, may be involved in COVID-19 viral entry. Moreover, the interaction of AXL, a TAM receptor, with SARS-CoV-2 has been shown to promote infection in epithelial cells [130]. Recently, the FDA has approved the use of Gilteritinib, an inhibitor of AXL, since it shows antiviral efficacy against SARS-CoV-2 infection in Vero E6 cells [131]. Another AXL inhibitor Bemcentinib (BGB324), has been fast-tracked towards phase II clinical trials by ACCORD (Accelerating COVID-19 Research and Development Platform) [131]. Therefore, GAS6/AXL targeting through Gilteritinib, Bemecentinib, and others AXL inhibitors could be a promising avenue for anti-COVID-19 treatment.
Lipoxin A4, belonging to a unique class of lipid mediators, possesses a wide spectrum of anti-inflammatory properties [132]. First reported by Wang et al., Lipoxin A4 stimulates the efferocytosis activity of alveolar macrophage [132]. Lipoxin A4 upregulates efferocytosis by blocking High mobility group box protein 1 (HMGB1), a well-known DAMP protein that acts as an “alarmin”, i.e., promotes inflammation along with impaired phagocytosis. HMGB1 binds to TLR2, TLR4, and RAGE to activate or upregulate the NF-κβ signalling pathway, which enhances the expression of leucocyte adhesion molecules, along with the production of pro-inflammatory cytokines [133]. Interestingly, HMGB1 expression has been shown, in the rat model, to be negatively correlated to that of the ACE2 receptor [134]. Moreover, Kuba et al. have reported that, in the mouse model, SARS-CoV infection downregulates ACE2 expression [135]. Therefore, it can be hypothesised that reduced ACE2 expression due to SARS-CoV-2 infection can increase HMGB1, thus contributing to the "cytokine storm", which is a hallmark of the worst COVID-19 infection scenarios. In fact, the elevated level of HMGB1 suppresses efferocytosis in patients suffering from ARDS, a major lung disease that has emerged as a post-COVID-19 complication. Thus, Lipoxin 4 could indeed be a novel therapeutic option against COVID-19 complications [132].
6. Conclusions
This review presents various mechanisms of defective efferocytosis linked with pandemic COVID-19. We discuss how efferocytosis clears and digests apoptotic cells, maintains tissue homeostasis, and plays a pivotal role in rendering key immune functions. The entry of SARS-CoV-2 through the airway respiratory tract, and their binding to ACE2 receptors present on lung epithelial cells, allows them to exploit the host cells for their replication. Consequently, a huge number of cells suffer apoptosis due to SARS-CoV-2 infection. Macrophages, the professional efferocytes, are recruited to the infection or damaged site to clear SARS-CoV-2-AC. Although an efficient efferocytosis process is supposed clear the apoptotic load, as discussed above, the replicated SARS-CoV-2 enter the macrophages by hijacking the apoptotic process, and hamper the efferocytosis machinery. SARS-CoV-2 infection alters the expression level of efferocytotic receptors, which are involved in clearing SARS-CoV-2-AC, and is also responsible for triggering a 'cytokine storm' by causing the release of pro-inflammatory cytokines on a large scale. Thus, the ‘cytokine storm’ production, due to impaired efferocytosis, may be the reason for COVID-19 complications. Major lung diseases, caused by SARS-CoV-2 infection, are observed in COVID-19 patients as complications. Hence, it is critical to understand that the gravity of COVID-19 complications is no less than that of the SARS-CoV-2 infection itself. It is concerning that recent studies have shown that the SARS-CoV-2 virus may keep mutating frequently, and possibly into more virulent strains. In the foreseeable future, drug treatment holds promise of being a more effective option. However, research studies will continue to unearth new information, given that the pathophysiology of COVID-19 disease, and its relation to different diseases remain to be fully deciphered. This review underlines that targeting efferocytosis concomitant with the ‘cytokine storm’ can be an effective strategy against SARS-CoV-2 infection and its complications.
Funding
This work was financially supported by the University Grants Commission (UGC), New Delhi, India [F.4–2/2006 (BSR)/BL/19-20/0203], under the Dr. D. S. Kothari Post Doctoral Fellowship Scheme, and by the Council of Scientific and Industrial Research (CSIR), New Delhi, India [09/079(2787)/2018-EMR-I], under the Senior Research Fellowship Scheme.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Somit Dutta is supported by the Dr. D. S. Kothari Post Doctoral Fellowship. Amartya Mukherjee is supported by CSIR Senior Research Fellowship . The authors also acknowledge BioRender.com for rendering of the Figure images.
Declaration of competing interest
The authors report no declarations of interest.
Author contributions
The authors contributed equally to all aspects of the article.
Biographies

Somit Dutta, Ph.D., is a postdoctoral fellow in the Department of Molecular Reproduction, Development, and Genetics at the Indian Institute of Science, India. Dr. Dutta received his Master of Science degree, and earned his doctorate in Cellular Immunology from the University of North Bengal, Darjeeling. He currently works in the laboratory of Prof. Upendra Nongthomba, and focuses on Efferocytosis using the Zebrafish as the animal model. He will be interested to track macrophage function in different transgenic lines of Zebrafish. He has published 29 research articles in different peer-reviewed journals, and also acts as a reviewer of prestigious journals like Plos One, Frontiers in Pharmacology, Journal of Functional Food, Complementary and alternative medicine (BMC), etc. He has been awarded the Dr. D. S. Kothari Postdoctoral Fellowship, one of the prestigious fellowships in India, to conduct his postdoctoral study at the Indian Institute of Science.

Amartya Mukherjee, M.Sc., is a Ph.D. student in the Department of Molecular Reproduction, Development, and Genetics at the Indian Institute of Science, India. Mr. Mukherjee received his Master of Science degree in Zoology (with specialisation in Molecular & Human Genetics) from the Department of Zoology, Banaras Hindu University, Varanasi. He currently works in the laboratory of Prof. Upendra Nongthomba, and is studying early muscle developement using the fruit fly as the animal model. He is a recipient of the Senior Research Fellowship (SRF) from the Council of Scientific and Industrial Research (CSIR), to support his doctoral study at the Indian Institute of Science.

Prof. Upendra Nongthomba, Ph.D.. is a professor of Molecular Reproduction, Development, and Genetics Department at the Indian Institute of Science. Prof. Nongthomba is currently working on mechanisms of muscle and neuronal development, and their diseases; aging and longevity; epigenetic inheritance; freshwater ecology, drug screening, and understanding the science behind Ayurveda formulations, using small model organisms. The main focus of his group is to understand the molecular and cellular basis of myopathies and neurodegenerative disorders. His team use two genetically tractable model organisms, Drosophila melanogaster (fruit fly) and Danio rerio (zebrafish), to trace the etiology of these diseases, and dissect the associated mechanisms. He is also interested in identifying host factors responsible for host-pathogen interactions. He uses genetic tools and transgenic approaches to identify novel factors, and decipher pathways underlying normal development, and those which lead to the manifestation of diseased conditions. Experimental approaches include genetic, molecular, biochemical, and biophysical assays, electron and confocal microscopy, and behavioral tests. To this end, he has published near about 60 high-impact research articles.
References
- 1.Ghebreyesus T.A. Addressing mental health needs: an integral part of COVID‐19 response. World. Psychiatry. 2020;19:129. doi: 10.1002/wps.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O’Neil A., Athan E., Carvalho A., Manes M., Walder K., Berk M. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life. Sci. 2020;258 doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L., Almire C., Hénon C., Kosmider O., Darion N., Rameau P. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib H.M., Ibrahim S., Zaim A., Ibrahim W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins R., Carlos A.R., Braza F., Thompson J.A., Bastos-Amador P., Ramos S., Soares M.P. Disease tolerance as an inherent component of immunity. Annu. Rev. Immunol. 2019;37:405–437. doi: 10.1146/annurev-immunol-042718-041739. [DOI] [PubMed] [Google Scholar]
- 9.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosurgi L., Cao Y.G., Cabeza-Cabrerizo M., Tucci A., Hughes L.D., Kong Y., Weinstein J.S., Licona-Limon P., Schmid E.T., Pelorosso F., Ganglionic N. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry J.S., Morioka S., Medina C.B., Etchegaray J.I., Barron B., Raymond M.H., Lucas C.D., Onengut-Gumuscu S., Delpire E., Ravichandran K.S. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat. Cell Biol. 2019;21:1532–1543. doi: 10.1038/s41556-019-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boada-Romero E., Martinez J., Heckmann B.L., Green D.R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 2020;21:398–414. doi: 10.1038/s41580-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.dos-Santos D., Salina A.C., Rodrigues T.S., Rocha M.F., Freitas-Filho E.G., Alzamora-Terrel D.L., de Lima M.H., Nascimento D.B., Castro I., Silva C.M., Toller-Kawahisa J.E. Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory programming and continual clearance of apoptotic cells. MedRxiv. 2021 doi: 10.1101/2021.02.18.21251504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeldine J., Lord J.M. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front. Immunol. 2020;11:2381. doi: 10.3389/fimmu.2020.573662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arandjelovic S., Ravichandran K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morioka S., Maueröder C., Ravichandran K.S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity. 2019;50:1149–1162. doi: 10.1016/j.immuni.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwulst S.J., Muenzer J.T., Peck-Palmer O.M., Chang K.C., Davis C.G., McDonough J.S., Osborne D.F., Walton A.H., Unsinger J., McDunn J.E., Hotchkiss R.S. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock. 2008;30:127–134. doi: 10.1097/shk.0b013e318162cf17. [DOI] [PubMed] [Google Scholar]
- 18.Korns D.R., Frasch S.C., Fernandez-Boyanapalli R., Henson P.M., Bratton D.L. Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2021;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandivier R.W., Henson P.M., Douglas I.S. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 20.Henson P.M., Cosgrove G.P., Vandivier R.W. State of the art. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006;3:512–516. doi: 10.1513/pats.200603-072MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yurdagul A., Jr, Doran A.C., Cai B., Fredman G., Tabas I.A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. cardiovasc. Med. 2018;4:86. doi: 10.3389/fcvm.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truman L.A., Ford C.A., Pasikowska M., Pound J.D., Wilkinson S.J., Dumitriu I.E., Melville L., Melrose L.A., Ogden C.A., Nibbs R., Graham G. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 23.Mueller R.B., Sheriff A., Gaipl U.S., Wesselborg S., Lauber K. Attraction of phagocytes by apoptotic cells is mediated by lysophosphatidylcholine. Autoimmunity. 2007;40:342–344. doi: 10.1080/08916930701356911. [DOI] [PubMed] [Google Scholar]
- 24.Gude D.R., Alvarez S.E., Paugh S.W., Mitra P., Yu J., Griffiths R., Barbour S.E., Milstien S.E., Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine‐1–phosphate as a “come‐and‐get‐me” signal. FASEB. J. 2018;22:2629–2638. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauber K., Bohn E., Kröber S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., Marini P., Wiedig C., Zobywalski A., Baksh S., Xu Y. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 26.Weigert A., Johann A.M., von Knethen A., Schmidt H., Geisslinger G., Brüne B. Apoptotic cells promote macrophage survival by releasing the anti-apoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- 27.Gude D.R., Alvarez S.E., Paugh S.W., Mitra P., Yu J., Griffiths R., Barbour S.E., Milstien S., Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine‐1–phosphate as a “come‐and‐get‐me” signal. FASEB. J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott M.R., Chekeni F.B., Trampont P.C., Lazarowski E.R., Kadl A., Walk S.F., Park D., Woodson R.I., Ostankovich M., Sharma P., Lysiak J.J. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Y., Misaghi S., Newton K., Gilmour L.L., Louie S., Cupp J.E., Dubyak G.R., Hackos D., Dixit V.M. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 30.Elliott M.R., Chekeni F.B., Trampont P.C., Lazarowski E.R., Kadl A., Walk S.F., Park D., Woodson R.I., Ostankovich M., Sharma P., Lysiak J.J. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chekeni F.B., Elliott M.R., Sandilos J.K., Walk S.F., Kinchen J.M., Lazarowski E.R., Armstrong A.J., Panatela S., Laird D.W., Salvesen G.S., Isakson B.E. Pannexin 1 channels mediate ‘find-me’signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segawa K., Nagata S. An apoptotic ‘eat me’signal: phosphatidylserine exposure. Trends. Cell. Biol. 2015;25:639–650. doi: 10.15252/embj.2020107121. [DOI] [PubMed] [Google Scholar]
- 33.Han C.Z., Ravichandran K.S. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green D.R., Oguin T.H., Martinez J. The clearance of dying cells: table for two. Cell. Death. Differ. 2006;23:915–926. doi: 10.1038/cdd.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott M.R., Koster K.M., Murphy P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penberthy K.K., Ravichandran K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott M.R., Ravichandran K.S. The dynamics of apoptotic cell clearance. Dev. Cell. 2016;38:147–160. doi: 10.1016/j.devcel.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park D., Tosello-Trampont A.C., Elliott M.R., Lu M., Haney L.B., Ma Z., Klibanov A.L., Mandell J.W., Ravichandran K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 39.Das S., Sarkar A., Ryan K.A., Fox S., Berger A.H., Juncadella I.J., Bimczok D., Smythies L.E., Harris P.R., Ravichandran K.S., Crowe S.E. Brain angiogenesis inhibitor 1 is expressed by gastric phagocytes during infection with Helicobacter pylori and mediates the recognition and engulfment of human apoptotic gastric epithelial cells. FASEB. J. 2014;28:2214–2224. doi: 10.1096/fj.13-243238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C.S., Penberthy K.K., Wheeler K.M., Juncadella I.J., Vandenabeele P., Lysiak J.J., Ravichandran K.S. Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity. 2016;44:807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimazaki A., Kawamura Y., Kanazawa A., Sekine A., Saito S., Tsunoda T., Koya D., Babazono T., Tanaka Y., Matsuda M., Kawai K. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54:1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 42.Ichimura T., Asseldonk E.J., Humphreys B.D., Gunaratnam L., Duffield J.S., Bonventre J.V. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L., Brooks C.R., Xiao S., Sabbisetti V., Yeung M.Y., Hsiao L.L., Ichimura T., Kuchroo V., Bonventre J.V. KIM-1–mediated phagocytosis reduces acute injury to the kidney. J. Clin. Investig. 2015;125:1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez J., Almendinger J., Oberst A., Ness R., Dillon C.P., Fitzgerald P., Hengartner M.O., Green D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 47.Köröskényi K., Duró E., Pallai A., Sarang Z., Kloor D., Ucker D.S., Beceiro S., Castrillo A., Chawla A., Ledent C.A., Fésüs L. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. J. Immunol. 2011;186:7144–7155. doi: 10.4049/jimmunol.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi H., Maruyama T., Urade Y., Nagata S. Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. Elife. 2014;3:02172. doi: 10.7554/eLife.02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seitz H.M., Camenisch T.D., Lemke G., Earp H.S., Matsushima G.K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 50.Scott R.S., McMahon E.J., Pop S.M., Reap E.A., Caricchio R., Cohen P.L., Earp H.S., Matsushima G.K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 51.Sen P., Wallet M.A., Yi Z., Huang Y., Henderson M., Mathews C.E., Earp H.S., Matsushima G., Baldwin A.S., Jr, Tisch R.M. Apoptotic cells induce Mer tyrosine kinase–dependent blockade of NF-κB activation in dendritic cells. Blood. 2007;109:653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eken C., Martin P.J., Sadallah S., Treves S., Schaller M., Schifferli J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010;285:39914–39921. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 54.Jun J.I., Kim K.H., Lau L.F. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat. Commun. 2005;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kzhyshkowska J., Gratchev A., Goerdt S. Stabilin‐1, a homeostatic scavenger receptor with multiple functions, J. Cell. Mol. Med. 2006;10:635–649. doi: 10.1111/j.1582-4934.2006.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park S.Y., Jung M.Y., Kim H.J., Lee S.J., Kim S.Y., Lee B.H., Kwon T.H., Park R.W., Kim I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell. Death. Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 57.Park S.Y., Jung M.Y., Lee S.J., Kang K.B., Gratchev A., Riabov V., Kzhyshkowska J., Kim I.S. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 58.Hirose Y., Saijou E., Sugano Y., Takeshita F., Nishimura S., Nonaka H., Chen Y.R., Sekine K., Kido T., Nakamura T., Kato S. Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis. Proc. Natl. Acad. Sci. 2012;109:4263–4268. doi: 10.1073/pnas.1117560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schledzewski K., Géraud C., Arnold B., Wang S., Gröne H.J., Kempf T., Wollert K.C., Straub B.K., Schirmacher P., Demory A., Schönhaber H. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and-2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J. Clin. Investig. 2011;121:703–714. doi: 10.1172/JCI44740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weigert A., Weis N., Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology. 2009;214:748–760. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Doran A.C., Yurdagul A., Tabas I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020;20:254–267. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 63.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujimori T., Grabiec A.M., Kaur M., Bell T.J., Fujino N., Cook P.C., Svedberg F.R., MacDonald A.S., Maciewicz R.A., Singh D., Hussell T. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal. Immunol. 2015;8:1021–1030. doi: 10.1038/mi.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 67.Mukherjee S., Subramaniam R., Chen H., Smith A., Keshava S., Shams H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS. One. 2017;12 doi: 10.1371/journal.pone.0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curtis J.L., Todt J.C., Hu B., Osterholzer J.J., Freeman C.M. The contribution of tyro3 family receptor tyrosine kinases to the heterogeneity of apoptotic cell uptake by mononuclear phagocytes. Front. Biosci. 2009;14:2631. doi: 10.2741/3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Summers K.M., Bush S.J., Hume D.A. Network analysis of transcriptomic diversity amongst resident tissue macrophages and dendritic cells in the mouse mononuclear phagocyte system. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emam M., Cánovas A., Islas-Trejo A.D., Fonseca P.A., Medrano J.F., Mallard B. Transcriptomic Profiles of Monocyte-Derived Macrophages in Response to Escherichia coli is Associated with the Host Genetics. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-019-57089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alciato F., Sainaghi P.P., Sola D., Castello L., Avanzi G.C. TNF‐α, IL‐6, and IL‐1 expression is inhibited by GAS6 in monocytes/macrophages. J. Leukoc. Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 72.Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit pro-inflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krysko O., Vandenabeele P., Krysko D.V., Bachert C. Impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis. 2010;15:1137–1146. doi: 10.1007/s10495-010-0504-x. [DOI] [PubMed] [Google Scholar]
- 74.Astuti I. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes. Metab. Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reyfman P.A., Walter J.M., Joshi N., Anekalla K.R., McQuattie-Pimentel A.C., Chiu S., Fernandez R., Akbarpour M., Chen C.I., Ren Z., Verma R. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis, Am. J. Respir. Crit. Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip M.S., Leung N.H.L., Cheung C.Y., Li P.H., Lee H.H.Y., Daëron M., Peiris J.S.M., Bruzzone R., Jaume M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 2014;11:1–11. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan J.F.W., Zhang A.J., Yuan S., Poon V.K.M., Chan C.C.S., Lee A.C.Y., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020;71:2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hancock A.S., Stairiker C.J., Boesteanu A.C., Monzón-Casanova E., Lukasiak S., Mueller Y.M., Stubbs A.P., Garcia-Sastre A., Turner M., Katsikis P.D. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J. Virol. 2018;92 doi: 10.1128/JVI.01325-18. e01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang N.L.S., Chan P.K.S., Wong C.K., To K.F., Wu A.K.L., Sung Y.M., Hui D.S.C., Sung J.J.Y., Lam C.W.K. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J., Nikrad M.P., Prang T., Gao B., Alford T., Ito Y., Edeen K., Travanty E.A., Kosmider B., Hartshorn K., Mason R.J. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 2011;45:582–591. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chi Y., Ge Y., Wu B., Zhang W., Wu T., Wen T., Liu J., Guo X., Huang C., Jiao Y., Zhu F. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J. Infect. Dis. 2020;222:746–754. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Z.S., Shu T., Kang L., Wu D., Zhou X., Liao B.W., Sun X.L., Zhou X., Wang Y.Y. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal, Transduct. Target. Ther. 2020;5:1–3. doi: 10.1038/s41392-020-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young B.E., Ong S.W., Ng L.F., Anderson D.E., Chia W.N., Chia P.Y., Ang L.W., Mak T.M., Kalimuddin S., Chai L.Y.A., Pada S. Viral dynamics and immune correlates of coronavirus disease 2019 (COVID-19) severity. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hazeldine J., Lord J.M. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front. Immunol. 2020;11:2381. doi: 10.3389/fimmu.2020.573662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.M. Soy, G. Keser, P. Atagündüz, F. Tabak, I. Atagündüz, and S. Kayhan, Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment, Clin. Rheumatol., 39, pp.2085–2094, https://doi.org/DOI: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed]
- 97.Gracia-Hernandez M., Sotomayor E.M., Villagra A. Targeting macrophages as a therapeutic option in COVID-19. Front. Pharmacol. 2020;11:1659. doi: 10.3389/fphar.2020.577571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y., Yuan Z. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes, MedRxiv. Preprint. 2020 doi: 10.1101/2020.03.27.20045427. [DOI] [Google Scholar]
- 99.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., Ntaganou Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell. Host. Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., Nie X., Zhou L., Liu Z., Ren Y., Yuan L. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., Navalesi P., Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020;120:998. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terpos E., Ntanasis‐Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID‐19. Am. J. Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat. Rev. Immunol. 2020;20 doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsieh J.Y., Smith T.D., Meli V.S., Tran T.N., Botvinick E.L., Liu W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta. Biomater. 2016;47:14–24. doi: 10.1016/j.actbio.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Foley J.H., Conway E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 109.Zhu N., Wang W., Liu Z., Liang C., Wang W., Ye F., Huang B., Zhao L., Wang H., Zhou W., Deng Y. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chu H., Chan J.F.W., Yuen T.T.T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C.Y., Tsang J.O.L., Huang X., Chai Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 112.Nagata S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 113.Yurdagul A., Jr, Subramanian M., Wang X., Crown S.B., Ilkayeva O.R., Darville L., Kolluru G.K., Rymond C.C., Gerlach B.D., Zheng Z., Kuriakose G. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell. Metab. 2020;31:518–533. doi: 10.1016/j.cmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arandjelovic S., Ravichandran K.S. Phagocytosis of apoptotic cells in homeostasis. Nature immunology. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bäck M., Yurdagul A., Tabas I., Öörni K., Kovanen P.T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019;16:389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greenberg S., Grinstein S. Phagocytosis and innate immunity. Curr. Opin. Immunol. 2002;14:136–145. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- 117.Han C.Z., Ravichandran K.S. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barbosa M.T., Morais-Almeida M., Sousa C.S., Bousquet J. The “Big Five” lung diseases in CoViD-19 Pandemic–a Google Trends analysis. Pulmonology. 2021;27:71. doi: 10.1016/j.pulmoe.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adomati T., Cham L.B., Hamdan T.A., Bhat H., Duhan V., Li F., Ali M., Lang E., Huang A., Naser E., Khairnar V. Dead cells induce innate anergy via mertk after acute viral infection. Cell. Rep. 2020;30:3671–3681. doi: 10.1016/j.celrep.2020.02.101. [DOI] [PubMed] [Google Scholar]
- 120.Lemke G., Silverman G.J. Blood clots and TAM receptor signalling in COVID-19 pathogenesis. Nat. Rev. Immunol. 2020;20:395–396. doi: 10.1038/s41577-020-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meacci E., Garcia-Gil M., Pierucci F. SARS-CoV-2 infection: a role for S1P/S1P receptor signaling in the nervous system? Int. J. Mol. Sci. 2020;21:6773. doi: 10.3390/ijms21186773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Bur P. tin, Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 123.Geffin R., Martinez R., de Las Pozas A., Issac B., McCarthy M. Fingolimod induces neuronal-specific gene expression with potential neuroprotective outcomes in maturing neuronal progenitor cells exposed to HIV. J. Neurovirol. 2017;23:808–824. doi: 10.1007/s13365-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.M. Pino, S. Paganini, C. Deleage, K. Padhan, J.L. Harper, C.T. King, L. Micci, B. Cervasi, J.C. Mudd, K.P. Gill, and S.M. Jean, Fingolimod retains cytolytic T cells and limits T follicular helper cell infection in lymphoid sites of SIV persistence, PLoS. pathog., 15(10), pp.1008081, 10.1371/journal.ppat.1008081. [DOI] [PMC free article] [PubMed]
- 125.Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 infection in a patient with multiple sclerosis treated with Fingolimod. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barnawi J., Tran H.B., Roscioli E., Hodge G., Jersmann H., Haberberger R., Hodge S. Pro-phagocytic effects of thymoquinone on cigarette smoke-exposed macrophages occur by modulation of the sphingosine-1-phosphate signalling system. COPD. 2016;13:653–661. doi: 10.3109/15412555.2016.1153614. [DOI] [PubMed] [Google Scholar]
- 127.Amara A., Mercer J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015;13:461–469. doi: 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morizono K., Xie Y., Olafsen T., Lee B., Dasgupta A., Wu A.M., Chen I.S. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Awl to mediate viral entry. Cell. Host. Microbe. 2011;9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meertens L., Carnec X., Lecoin M.P., Ramdasi R., Guivel-Benhassine F., Lew E., Lemke G., Schwartz O., Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell. Host. Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S., Qiu Z., Hou Y., Deng X., Zheng T., Yan R., Wu P., Xie S., Zhou Q., Huang J., Li X. AXL promotes SARS-CoV-2 infection of pulmonary and bronchial epithelial cells. Cell. Res. 2021;31:126–140. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bouhaddou M., Memon D., Meyer B., White K.M., Rezelj V.V., Marrero M.C., Polacco B.J., Melnyk J.E., Ulferts S., Kaake R.M., Batra J. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182:685–712. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Q., Parekh D., D’Souza V.K., Dancer R., Patel J.M., Bartis D., Gao F., Lian Q., Jin S., Thickett D.R. S102 Lipoxin A4 improves efferocytosis via inhibition of the HMGB1 in human alveolar macrophages. Thorax. 2014;69 doi: 10.1136/thoraxjnl-2014-206260.108. [DOI] [Google Scholar]
- 133.Street M.E. HMGB1: a possible crucial therapeutic target for COVID-19? Horm. Res. Paediatr. 2020;93:73–75. doi: 10.1159/000508291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qi Y.F., Zhang J., Wang L., Shenoy V., Krause E., Oh S.P., Pepine C.J., Katovich M.J., Raizada M.K. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. Int. J. Mol. Med. 2016;94:37–49. doi: 10.1007/s00109-015-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ascierto P.A., Fox B.A., Urba W.J., Anderson A.C., Atkins M.B., Borden E.C., Brahmer J.R., Butterfield L.H., Cesano A., Chen D.S., Gruijl T.D.De. Insights from immuno-oncology: the Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buonaguro F.M., Puzanov I., Ascierto P.A. Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med. 2020;14:165. doi: 10.1186/s12967-020-02333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Michot J.M., Albiges L., Chaput N., Said V., Pommeret F., Griscelli F., Balleyguier C., Besse B., Marabelle A., Netzer F., Merad M. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., Wang J., Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood. Adv. 2020;4:1307. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ucciferri C., Auricchio A., Di Nicola M., Potere N., Abbate A., Cipollone F., Vecchiet J., Falasca K. Canakinumab in a subgroup of patients with COVID-19. Lancet. Rheumatol. 2020;2:e457–ee458. doi: 10.1016/S2665-9913(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Steed P.M., Tansey M.G., Zalevsky J., Zhukovsky E.A., Desjarlais J.R., Szymkowski D.E., Abbott C., Carmichael D., Chan C., Cherry L., Cheung P. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 145.Rabies A.M., Tak P.P., Reedquist K.A. Histone deacetylase inhibitors suppress IL-6 production by rheumatoid arthritis firoblast-like synoviocytes and macrophages via modulation of mRNA stability rather than blockade of NF-κB signalling. Ann. Rheum. Dis. 2011;70:A30–A31. doi: 10.1136/ard.2011.154211. [DOI] [Google Scholar]
- 146.Han S.B., Lee J.K. Anti-inflammatory effect of Trichostatin-A on murine bone marrow-derived macrophages. Arch. Pharm. Res. 2009;32:613–624. doi: 10.1007/s12272-009-1418-4. [DOI] [PubMed] [Google Scholar]
- 147.Cui S.N., Chen Z.Y., Yang X.B., Chen L., Yang Y.Y., Pan S.W., Wang Y.X., Xu J.Q., Zhou T., Xiao H.R., Qin L. Trichostatin A modulates the macrophage phenotype by enhancing autophagy to reduce inflammation during polymicrobial sepsis. Int. Immunopharmacol. 2019;77 doi: 10.1016/j.intimp.2019.105973. [DOI] [PubMed] [Google Scholar]
- 148.Sloka J.S., Stefanelli M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult. Scler. J. 2005;11:425–432. doi: 10.1191/1352458505ms1190oa. [DOI] [PubMed] [Google Scholar]