Abstract
Consumption of pathogenic contaminated water has claimed the lives of many people. Hence, this scenario has emphasized the urgent need for research methods to avoid, treat and eliminate harmful pathogens in wastewater. Therefore, effective water treatment has become a matter of utmost importance. Membrane technology offers purer, cleaner, and pathogen-free water through the water separation method via a permeable membrane. Advanced membrane technology such as nanocomposite membrane, membrane distillation, membrane bioreactor, and photocatalytic membrane reactor can offer synergistic effects in removing pathogen through the integration of additional functionality and filtration in a single chamber. This paper also comprehensively discussed the application, challenges, and future perspective of the advanced membrane technology as a promising alternative in battling pathogenic microbial contaminants, which will also be beneficial and valuable in managing pandemics in the future as well as protecting human health and the environment. In addition, the potential of membrane technology in battling the ongoing global pandemic of coronavirus disease 2019 (COVID-19) was also discussed briefly.
Keywords: Membrane technology, Pathogen, Virus, Wastewater, Advanced membrane technology
1. Introduction
Pathogenic contaminated water is responsible for a wide variety of waterborne diseases such as cholera, cryptosporidiosis, diarrhea, gastroenteritis, giardiasis, hepatitis, meningitis, encephalitis, poliomyelitis, and other serious health problems which are the leading cause of mortality [1]. Due to the severity of clean water limitations in certain countries, wastewater is currently being reused for hygienic purposes and consumption. Countries that face severe water scarcity include Qatar, Israel, Lebanon, Iran, Jordan, Libya, Kuwait, Saudi Arabia, Eritrea, United Arab Emirates, San Marino, Bahrain, India, Pakistan, Turkmenistan, Oman, and Botswana [2]. According to World Health Organization (WHO), direct measurement of pathogens and indicator organisms in specific sources of water is suggested since it offers the most accurate estimates of microbial concentrations [3]. However, in many cases, resource constraints inhibit the use of this method. With the absence of measured pathogen concentrations, an alternate interim strategy is developed based on available data such as findings of sanitary surveys which are paired with the indicator testing process. While relatively small dosages (one infectious virus particle) can induce infection and sickness, drinking water with very low virus concentrations (1 log unit) can certainly pose a health risk [4].
The coronavirus disease 2019 (COVID-19) pandemic has caught the entire world by surprise as it has become the fastest transmitted disease to date [5]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At this point, COVID-19 still shows signs of mutation with an expected 5000 mutations on the spike (S) protein [6]. Currently, the delta variant is said to have the highest infection rate compared to the other variants [7]. This deadly virus has claimed the lives of>4.3 million people worldwide, hence emphasizing the urgent need for research methods to avoid, treat and eliminate the virus [8]. Although the key transmission routes of the virus are through respiratory droplets and contact with contaminated surfaces [9], [10], the transmission of the virus via water cannot be ignored as there is evidence that suggests the presence of genetic materials of this virus in wastewater [11], [12], [13], [14].
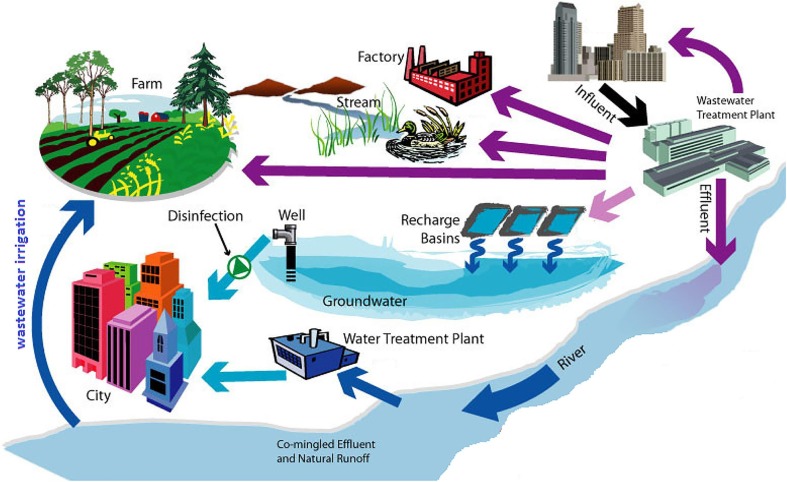
Briefly, researchers revealed harmful pathogens might enter the water system from urine, faecal, or stool of the infected people or animals [15], [16]. These wastes are then sourced through plumbing systems, which are mainly from hospitals, quarantine facilities, residential buildings, sewage, landfill, and drainage water thus, contaminating the wastewater treatment system (Fig. 1 ). The water is then cycled and reused for farming and domestic reuse. According to the WHO and United States Environmental Protection Agency (US-EPA),φ Australia, Singapore, Namibia, South Africa, Kuwait, Belgium, the United Kingdom and some regions in the United States are among the countries that reuse treated wastewater for drinking water production and distribution [3], [17]. However, the persistence of high loads of pathogens in the wastewater could pose a great risk to human health and the environment via leaking or broken underground pipes, which might contaminate the clean and treated water supply. Therefore, the possible transmission of diseases through the water should not be overlooked.
Fig. 1.
Transmission route of the virus through faecal matter into main water streams [15].
Currently, several conventional methods are implemented in order to remove pathogens in wastewater, which include coagulation [18], filtration [19], [20], chlorination [21], [22], activated sludge treatment process [23], and anaerobic digestion [24]. Repeated experimental analysis of pathogen removal has been conducted by multiple researchers. Previous studies highlighted a 1.5–3.5 log removal, 3.0 log removal, and 2.0–3.2 log removal of Giardia cyst, Coxsackie virus, and poliovirus respectively from a series of combined conventional treatments which include coagulation, flocculation, and sedimentation [25]. Furthermore, current disinfection methods have several environmental difficulties such as the utilization of harmful chemicals and the generation of toxic by-products. For example, pathogen disinfection via coagulation and sedimentation methods requires the utilization of harmful chemical coagulants such as aluminium and iron salts [26]. An excessive dosage of those nondegradable metal-based coagulants in water systems can initiate a negative impact upon the environment as they can be trapped in food chains [27]. Pathogen disinfection via coagulation and sedimentation methods can generate harmful by-products through the formation of sludge that may contain living and active pathogens. Therefore, WHO highlighted that wastewater should be treated in well-designed and well-managed centralized wastewater treatment stations [28]. In order to battle the transmission of waterborne diseases efficiently, the existing wastewater treatment may require essential upgrading and additional pre-treatment or post-treatment steps.
Membrane technology has gained global attraction in wastewater treatment due to its high separation efficiency, economical, environmental friendliness, ease of operation and maintenance, and relatively small footprint [29], [30], [31]. Membrane is a selective layer that allows selected constituents to pass through and inhibits undesirable constituents based on the size exclusion principle [32]. Apart from conventional membrane filtration techniques, the removal of pathogens via advanced membrane technologies such as nanocomposite membranes, membrane distillations, membrane bioreactors, and photocatalytic membrane reactors have attracted considerable attention of researchers [33], [34]. The advanced membrane technologies offer the integration of filtration properties with additional functionalities which can improve the properties of the original membrane [35], [36].
There are several published review papers on pathogen removal via membrane technology [37], [38], [39]. However, these reviews do not cover the development and application of advanced membrane technology in pathogen removal from water. Therefore, this study aims to review the capability of conventional and advanced membrane technology in pathogen removal, especially virus removal in wastewater. This review also covers the possible challenges and prospects of advanced membrane technology as a promising solution in combating pathogenic microbial pollutants, which will be beneficial and valuable in managing pandemics in the future as well as protecting human health and the environment.
2. Pathogen removal using conventional membrane filtration
Porous membranes are made up of a solid matrix with defined holes or pores varying in size from less than 2 nm to>20 μm in diameter. Porous membrane separation is mostly based on particle size. Pores on the membrane must be smaller than particles in the mixture to obtain good selectivity. The porous membranes are mainly used for microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF) processes.
Conventional membrane filtration can be classified into three processes, namely MF, UF, and NF. These processes are mainly categorized according to their porosity and their nominal molecular weight cut off [40]. For pathogen removal, membrane pore size is an important factor since size exclusion is the prevailing removal mechanism [41], [42]. Table 1 depicts the efficiency of conventional and membrane filtration methods in removing waterborne pathogens. According to the result shown in Table 1, viruses have low-efficiency removal ranging from 20 to 40% in MF because the size of membrane pores is smaller. Through comparing with the conventional methods, membrane filtration has proven to give better microbial removal efficiency while maintaining lower operating time and smaller footprint [43].
Table 1.
Type and efficiency of unit processes in removing waterborne pathogen [16]
| Type of pathogen | Coagulation + sedimentation + flocculation (%) | Microfiltration (%) | Ultrafiltration (%) | Nanofiltration / reverse osmosis (%) |
|---|---|---|---|---|
| Protozoa | 60–100 | 80–100 | 90–100 | >99.9 |
| Virus | 60–100 | 20–40 | 60–90 | >99.9 |
| Bacteria | 60–100 | 90–100 | 90–100 | >99.9 |
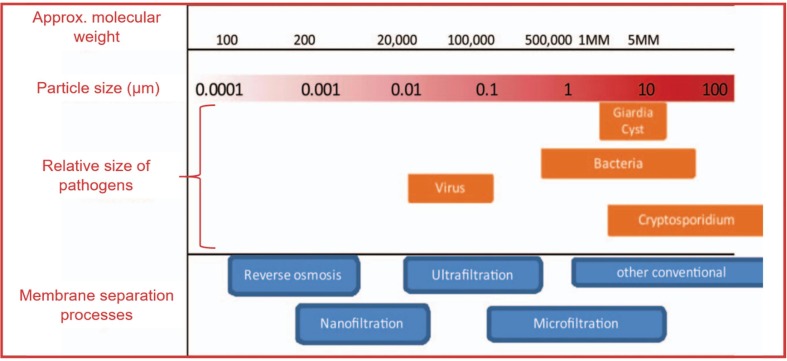
As demonstrated in Fig. 2 , the smallest size of pathogens are viruses, which are around 0.01 to 0.1 μm. The pore size of MF and UF membranes is around 0.1 to 10 μm and 0.01 to 0.15 μm respectively. The NF membranes have molecular weight cut off (MWCO) from 200 to 1000 Da and pore size in the range of 0.2–2 nm [4]. The NF membranes have molecular weight cut off (MWCO) from 200 to 1000 Da and pore size in the range of 0.2–2 nm [44]. The UF and NF processes seem to be the most effective method for removing pathogens in contaminated wastewater with the recorded removal of 0.5–5.9 log removal values (LRVs) and 4.1–7.0 LRVs respectively compared to the MF process which recorded pathogen removal of 0.7–4.6 LRVs [38]. However, MF offers better performance in terms of water permeability and fouling mitigation due to their bigger pore size [45]. In order to understand the effectiveness and limitation of each membrane category, further discussion was revealed in the following sections.
Fig. 2.
Membrane separation processes according to the particle size [46]
2.1. Microfiltration (MF)
Microfiltration (MF) is a low pressure-driven membrane process in which suspended colloids and particles in the approximate size range of 0.1 μm to 20 μm are retained by microporous membranes. Because of the vast range of pore sizes, MF membranes may be utilized in a variety of applications that require the separation of virus, bacteria, aerosols, and macromolecules from fluids [47]. Basically, the main mechanism of the MF process is physical separation. This low pressure-driven membrane process is a tempting disinfection alternative because it does not involve the use of harmful chemicals such as chlorine and ozone which are utilized in chlorination and ozonation techniques for pathogen disinfection [16].
MF process requires low pressure of 0.5 to 5 bar, and produces high solvent flux (65 to 85 LMH/bar) and comparable somatic coliphage rejection (up to 3.4 log) [45]. Madeini et al. reported that the rejection of poliovirus via MF membrane exceeded 99.5% with the presence of Escherichia coli (E.Coli) in water [48]. The higher virus removal via MF membrane might be due to the plugging or obstruction of larger pores by the bacteria. Previous study showed that hydrophobic MF membranes with pore sizes of 0.20 to 0.22 μm achieved 91% to 100% waterborne virus rejection of poliovirus [49]. Results showed that poliovirus is retained and accumulated on the surface of the membranes. This finding suggests that MF membranes are capable to remove pathogens under optimum experimental conditions which are at lower pH and low operating pressure [50], [51].
Wang et al. fabricated a new type of microfiltration membrane based on a two-layer nanoscale polyacrylonitrile (PAN)/microscale polyethylene terephthalate (PET) fibrous scaffold which is filled with ultrafine functional cellulose nanofibers (diameter about 5 nm) via electrospinning [52]. These membranes can totally remove E. coli (via pore exclusion) while retaining a high permeation rate (0.19 L/m2h/Pa) and an LRV of 4 for eliminating MS2 virus. The fabrication of a web-like structure with a high charge density and large surface area per unit volume for the adsorption of contaminated molecules is the basis for the development of these membranes.
Modification of MF membrane can efficiently enhance the removal of the pathogen from water. For example, Sinclair et al. modified the MF membrane surface by dip-coating it with a cationic polyethyleneimine (PEI) solution to remove MS2 bacteriophages [53]. A negatively charged PEI thin film was coated on a 0.45 μm MF membrane to induce the adsorption of the positively charged virus. They found this membrane could achieve LRV>3 log for MS2 bacteriophages removal. Interestingly, this method can also be operated under gravity filtration, hence reducing the need for additional operating costs for pressurised systems [53]. In 2019, the study of hybrid ultraviolet C/ microfiltration (UV-C/MF) membrane for the removal of E. coli, Enterococcus faecalis and Candida albican was conducted by a group of researchers from Spain [54]. When operating at low TMP 0.5 bar and low contact time (8 s), the hybrid membrane could achieve LRV of 4 for E. coli, thus proving a synergy and better intensification process.
Besides that, alteration of surface charge of the MF membrane could improve the virus removal. According to Hou et al., 0.22 μm cellulose nitrate membranes removed only 54% of poliovirus from tap water [55]. However, the removal efficiency of poliovirus was boosted to 99% by using an electropositive (Zeta-plus) membrane, whereas an electronegative membrane reduced it to 35%. This is because the isoelectric point of poliovirus is 6.6, the surface should be negatively charged at pH 8.0.
In conclusion, the traditional MF process alone is suitable to remove larger pathogens like protozoa or bacteria but is not suitable to be used in removing viruses because the size of membranes pores is larger. However, the modification of the MF membrane could efficiently enhance the removal of the pathogen from water. Removal of the pathogen via MF membrane does not involve any chemical disinfection as it operates based on size exclusion, therefore preventing the formation of disinfection by-product. Furthermore, gravity-driven membrane processes would reduce energy consumption and operating costs. However, the harmful pathogens are separated and removed rather than being destroyed or deactivated, thus resulting in high pathogen concentration in the retentate which can become harmful waste. Consequently, there is a higher risk of infection, sickness, and death. In order to produce the highest quality of drinking water, a combination of MF techniques with additional treatment should be utilized. Development of composite MF with hybrid functionality could ensure process dependability and high pathogen removal or deactivation efficiency.
2.2. Ultrafiltration (UF)
UF is a low-pressure membrane. The average pore size of the UF membrane is in the range of 1 to 100 nm [56]. Due to its ease of operation and relatively low cost, low-pressure membrane methods appear to be an appealing choice to remove pathogens in water [52]. Ultrafiltration membranes are an excellent barrier at the nanoscale region and are increasingly employed in wastewater treatment to remove pathogens [48], [57]. There are two main mechanisms involved in pathogen removal by UF which include size exclusion and electrostatic interaction.
Size exclusion occurs when the particles with a diameter greater than the membrane pore diameter are retained on the surface of the UF membrane. According to ElHadidy et al., a commercial UF membrane was used to evaluate the removal of two viral surrogates of similar size (MS2 and φX174 bacteriophage) [56]. Despite its lesser size, MS2 was easier to be removed than φX174 bacteriophage. The elimination of φX174 bacteriophage was shown to decrease dramatically when the pH of the feed solution increased from 6.5 to 9.4. Size exclusion was demonstrated to be the primary elimination mechanism for both bacteriophages using mathematical modeling. According to these findings, MS2 removal via size exclusion might result in 1.5–1.8 log rejection, but the greater experimental removal value of 3.7 log was most likely related to electrostatic repulsion. Solution extrusion and phase inversion were used to create polyvinylidene fluoride (PVDF) and unique polyphenylsulfone (PPSu) hollow fiber ultrafiltration membranes for the treatment of contaminated surface water. At 1 bar pressure, both PVDF and PPSu HF membranes provided 5 log reduction in E. coli bacteria.
Electrostatic interaction causes the adhesion of viruses onto the UF membrane surface. Removal of pathogens via UF membrane is more prone to electrostatic repulsion due to charge similarity for both pathogen and membrane surface. Gentile et al. investigated the effect of electrostatic interaction in bacteriophage PP7 virus removal via UF membrane through Derjaguin-Landau-Verwey-Overbeek (DLVO) and extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theories [20]. They identified that the composition of the aqueous matrix is important as it affects the efficiency of virus removal via the UF process. By using monovalent cation (Na+) with amphoteric behavior species, bicarbonate (HCO3–) results in better effectiveness with LRV value of 2.82 log compared to the divalent cations (Mg2+ and Ca2+) with a value of 1.53–1.5 log. This finding confirmed DLVO predictions that electrostatic forces reduce the filtration performance with the presence of divalent cations. Since both the membrane and bacteriophage PP7 were negatively charged, electrostatic repulsion would predict high removal rates. Viruses would not reach and adhere to the membrane surface instead of remaining in the retentate. Another study conducted by Lu et al. reported the improvement of repulsive virus-membrane interaction forces, which prevents the virus from approaching the membrane surface, hence leading to higher virus removal efficiency [58]. The zwitterionic hydrogel was grafted onto a UF membrane to introduce virus-membrane repulsion forces on the surface of the membrane. Modified UF membranes showed LRV value of > 6.0 log for both small bacteriophage (MS2) (∼30 nm) and human adenovirus 2 (HAdV-2) (∼170 nm) compared with the normal UF membranes that revealed > 2.5 log removal for MS2 and > 3 log removal for HAdV-2 [58]. The importance of electrostatic repulsion in improving virus elimination via membrane filtration was underlined by these findings.
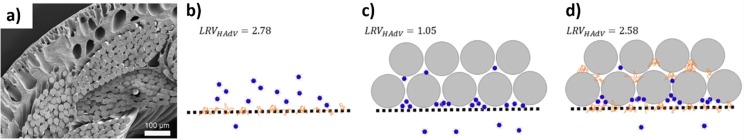
Although membrane fouling is widely recognized as a major obstacle to membrane filtration, some researchers discovered that fouling can considerably increase virus removal from wastewater via membrane filtration [36], [59]. Yin et al. studied the effect of membrane fouling by suspending and dissolving matter in the removal of human adenovirus 40 via hollow fiber UF membranes (0.04 μm) as shown in Fig. 3 (a–d) [59]. The average removal of adenovirus via UF membrane from DI water was 2.3 log. They found the presence of dissolved foulants, which was humic acid that increased the adenovirus removal due to the pore blockage in the UF membranes. The pore blockage in the membranes could decrease the membrane pore size and retain the penetration of the virus. With the presence of suspended particles such as silica microspheres, the adenovirus removal was observed to be decreased which attributed to the cake formation at the membrane surface for UF membranes. Some other studies also supported that membrane fouling and cake formation on the membrane surface such as MF and UF contributed to higher virus removal [45], [60]. Farahbakhsh and Smith claimed that the overall coliphage removal via the MF pilot plant ranged from 0.2 to 3.4 logs. The degree of membrane fouling and initial coliphage concentrations in the feed were noted by researchers as factors that impact coliphage removal [45]. Since the use of UF membranes was limited to the larger pore size, the higher removal of the virus via fouling could be described by these two mechanisms: (1) the formation of cake on the membrane surface via suspended foulants that acted as a secondary membrane barrier to retain the virus from passing through the membranes, and (2) the virus was excluded from the membrane due to pore blockage by foulants that presented as dissolved species which might decrease the size of the membrane pore and prevent the virus from penetrating the membranes.
Fig. 3.
a) SEM image of a cross-section of UF membrane with pore size 0.04 μm and a schematic diagram on the effect of fouling by (b) dissolved species (orange scribble), (c) suspended particles, (d) both dissolved species and suspended particles (blue dots represent adenovirus, orange scribbles represent dissolved species, grey spheres represent suspended particles) [59].
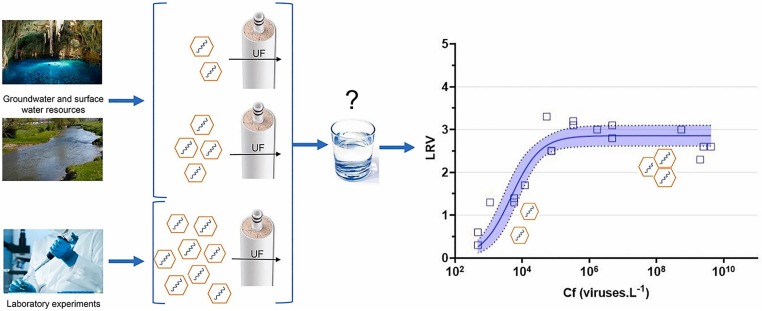
Most recently, the effect of virus feed concentration on UF efficiency has been investigated [61]. At lower concentrations of the three viruses coxsackievirus-B5 (CV-B5) (102 – 103), MS2 phages (103 – 104), and adenovirus type 41 (ADV 41) (102–103), removal efficiency of about 1 log was observed. At higher concentrations (for all viruses are 108), the removal efficiency increased up to 3.0 log for CV-B5 and MS2 phages and 3.5 log for ADV 41. These findings highlight the potential overestimation of UF efficiency during laboratory experiments conducted at high concentrations compared to low concentrations which were found in environmental resources and were used for drinking water production (Fig. 4 ).
Fig. 4.
UF efficiency on high concentrations of virus that were conducted in the laboratory compared to low concentrations of virus that found in groundwater and surface water [61].
In conclusion, UF has moderate potential in virus removal treatment. Pathogen removal by UF membranes is environmentally friendly because they do not require the use of additional harmful chemicals. However, the small size of viruses and relatively large pore size of the membranes limit the use of ultrafiltration in removing viruses from water and wastewater. Imperfections on the membrane surface can increase the possibility of virus penetration during filtration. Without specific pre-treatment or post-treatment procedures, virus removal via UF membranes is rarely successful. Therefore, modification of existing UF technology like incorporating nanomaterials that have antimicrobial properties in the UF membrane can improve the performance of UF.
2.3. Nanofiltration (NF)
NF is a membrane separation process that uses membranes with pore sizes ranging from 0.2 to 2.0 nm. NF membranes have a typical operating pressure of 3 to 30 bar. The separation mechanism of NF membranes includes both size sieving and electrostatic effects as most NF membranes are slightly charged [62]. Dielectric exclusion is triggered by the interactions of ions with the bound electric charges that are induced by ions at interfaces between media of different dielectric constants [63]. The NF membranes may be used to remove pathogens from drinking water as well as organic and inorganic contaminants that are commonly found in drinking water and wastewater [62], [64].
A pilot plant study conducted by Yahya et al. displayed successful removal of 4–6 log units of test virus bacteriophages MS-2 (28 nm) and PRD-l (65 nm) via NF membrane. The removal was successfully achieved due to the addition of pre-treatment steps such as sand filtration prior to NF [19]. These pre-treatment steps are crucial to maximise membrane recovery and lifetime. Previous finding also indicated that 99% of bacteriophages were removed by slow sand filter which was placed before the NF membrane and this figure reached 100% after passing through the NF membranes [65]. Thus, it could be concluded that integration of pre-treatment methods such as sand filtration and MF could enhance the NF performance in pathogen removal.
The efficiency of membrane NF under field conditions was demonstrated in a study that involved the removal of the MS2 virus from lake water [43]. For the MS2 bacteriophage, the NF membranes ES404 and AFC30 in recycle mode obtained the mean log removal values of 4.6 and 4.3 respectively, which met US-EPA requirements. The molecular weight cut-off (MWCO) of the ES404 membrane was approximately 4,000 Daltons, while the MWCO of the AFC30 membrane was approximately 1,000 Daltons. For the MS2 virus, the AFC30 membrane was unable to achieve superior log removal performance due to their tighter pore size. In the other similar study, the efficiency of the NF technique in removing the MS2 virus in the field and in the laboratory was established and the process was suggested to be used in small-scale drinking water systems [66]. The ES404 (MWCO = 4000 Da) and AFC30 (MWCO = 350 Da) NF membrane systems were used to eliminate MS2 virus. During three cycles in recycle mode, the planned membrane system ES404 achieved virtually 100% elimination (about 6 log removal) which showed that ES404 performed better.
In the study of Hamaguchi, bacteriophage Qβ virus rejection levels was recorded to have LRV > 4 with water fluxes ranging from 19 to 61 L m−2h−1 (operation pressure: 0.3 MPa) [67]. Photopolymerization of a fan-shaped diol molecule and imidazolium ionic liquid mixture which was followed by the removal of ionic liquid was used to fabricate these membranes. Hamaguchi et al. claimed that this is the first example of developing membrane based on two-component liquid crystals. The finding demonstrated that this approach would be beneficial in the development of functional and selective membrane via high water flow to remove pathogens and pollutants from wastewater.
To conclude, the pore size of the membrane and the size of the virus particle affect the efficiency of NF process. NF does not pose negative impact to environment because it does not involve the use of hazardous and toxic chemicals. However, there is a main challenge of using NF to remove pathogens from wastewater, which is membrane fouling. Membrane fouling occurs when there are adhesion and growth of microorganisms along with agglomeration of materials on the surface of the membrane [68]. This can reduce the effectiveness of the pore size of the membrane and decline the membrane performance. Under real situations, NF technology may not provide a complete barrier against human diseases such as bacteria and viruses. Therefore, it is necessary to explore structurally stable and fouling resistant NF membranes for real applications as well as research improved NF membranes with high permeability and selectivity.
3. Pathogen removal by advanced membrane technology
Advanced membrane technology is defined as an integration of filtration properties with additional functionalities while improving the properties of the original membrane. The removal of viruses and microbes via emerging advanced membrane technologies has attracted global attention [11], [33], [69], [70]. Initially, the concept of developing advanced membrane was fostered in the 1990 s to overcome the limitation of existing membranes, which was the trade-off relationship between permeability and selectivity [71]. In this section, the scope of advanced membrane technology is limited to nanocomposite membrane, membrane distillation, membrane bioreactor and photocatalytic membrane reactor. These technologies not only reject viruses and bacteria through size exclusion filtration, but also involve the deactivation mechanisms owing to the chemical and physical interactions.
3.1. Nanocomposite membrane
The nanocomposite membrane is known as one of the most commonly used membrane approaches that combine the adsorption process and membrane filtration in a single step. The nanocomposite membranes are known for the removal of charged contaminants from aqueous solutions. The efficacy of these membranes is due to the presence of reactive functional groups on their surface that are capable of forming bonds with charged impurities via a variety of interactions such as ion exchange and complexation [72]. Compared to the conventional membrane filtering methods such as MF, UF, and NF, the nanocomposite membrane improved pollutant removal efficiency while consuming less energy and allowing higher permeation flux [73].
The inclusion of fillers and additives in the membrane formulation will increase the permeability of the membrane due to their hydrophilicity qualities, hence assisting in the enhancement of water permeability in the membrane [74], [75], [76]. The fillers are typically constituted of inorganic materials particles (metals and metal oxides) such as silver (Ag) [77], copper (Cu), nickel (Ni) [78], selenium (Se) [79], zinc oxide (ZnO) [80], TiO2 [69] and SiO2 [81] may also exhibit antibacterial and antimicrobial properties.
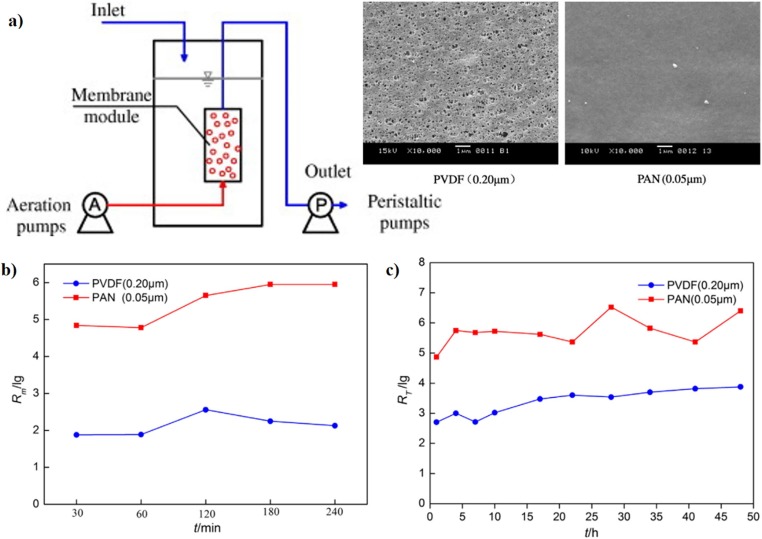
Coupling TiO2 into membrane adsorption reactor (MAR) system could improve the removal efficiency of F2 phage virus [69]. In the MAR system, two distinct flat membranes, PVDF (0.20 μm) and PAN (0.05 μm) reported removal efficiency of phage F2 was 1.88–2.56 log and 4.78–5.95 log respectively (Fig. 5 (a-b). The removal effectiveness of phage F2 was enhanced by coupling TiO2 nanoparticles and membranes, and the interception efficiency of the MAR systems with membrane PVDF and PAN was 3.88 log and 6.40 log, respectively (Fig. 5(c)). The coupling system had a sustained and effective effect on phage F2 elimination due to the adsorption of nanomaterials and the cake layer generated on the membrane surface during operation. The nano-TiO2 MAR system achieved not only the high removal efficiency of the virus but also the effective separation and recycling of nanoparticles.
Fig. 5.
a) Schematic diagram of MRA with SEM images of PVDF and PAN membranes, b) interception efficiency of phage F2 by different membrane modules, c) removal efficiency of phage F2 by the nano-TiO2 MAR coupling in the continuous operation system.
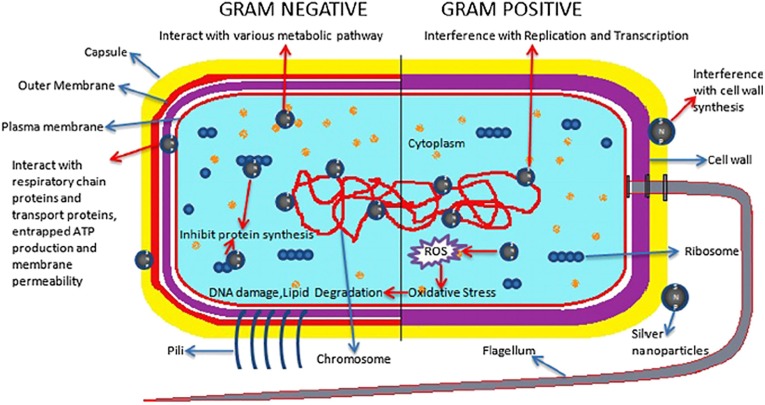
Ag nanoparticles (AgNPs) are a well-known disinfectant due to their excellent antibacterial properties [82]. The positive surface charge of metal nanoparticles enhances their attachment to bacteria with a negative surface charge thus, potentially enhancing their bactericidal activity [83]. Fig. 6 depicts the bacteria being disinfected by Ag particles. Apart from the influence of the smaller particle size, which particularly increased its surface area for bacteria attachment, the shape of the Ag also affects the efficacy of the Ag particles performance. It was reported that Ag with a truncated triangular-shaped triangular shape has a better performance over the rod and spherical-shaped AgNPs in disinfecting E. coli bacteria [84]. A dose of 10 μg of truncated triangular Ag particles completely prevented the growth of the E. coli bacteria after 24 h, whereas 100 μg of spherical nanoparticles only delay the growth of the bacteria for 8 to 10 h.
Fig. 6.
The mechanism of cell disinfectant by Ag nanoparticles [85].
Ag with a diameter of less than 10 nm can create pores in the cell wall resulting in cytoplasmic discharge from the cell leading to cell death [85]. The interaction of the NPs with the cell also can cause the cell to undergo programmed cell death called apoptosis [77]. The process by which Ag ions suppress bacteria is still poorly understood. On Ag+ treatment, it is hypothesized that deoxyribonucleic acid (DNA) loses its replication ability causing the cellular proteins to become inactive [86]. Additionally, it was demonstrated that Ag+ attaches to the functional groups of proteins resulting in denaturation of the proteins. According to published reports, electrostatic interaction between negatively charged bacterial cells and positively charged nanoparticles is critical for the bactericidal activity of nanoparticles [87]. The Ag particles utilized in this study are negatively charged. While the mechanism by which these particles interact with the constituents of the outer membrane of E.coli is still unknown. It was stipulated that although they possess a negatively charged surface, they interact with the “building elements” of the membrane of the bacteria leading to structural reforms and degradation, and eventually cell death.
The core substance and/or the ligands shell of nanoparticles have been considered as antiviral agents [88]. The core substance of nanoparticles has a significant impact on antibacterial activity due to their nano-sized properties that are more easily absorbed by the cell membranes of pathogens. For example, the Ag nanoparticles also have a greater surface area to interact with microbes that could increase the production of reactive oxygen species (ROS) in the process, which causes damage to the cellular structure lipids, DNA, ribonucleic acid (RNA) and proteins of the microbes. Ag atoms bind to thiol groups of enzymes, producing S–Ag stable bonds with molecules containing thiols, according to another study, deactivating enzymes in the cell membrane. Bacterial cell lysis occurs as a result, and this could be one of the reasons for its antibacterial activities [89].
The ligand shell of nanoparticles can be an antimicrobial agent due to the formation of metal complexes that can adopt three-dimensional structures made possible by metal coordination chemistry, which allows for the development of various antimicrobials. Furthermore, metal-based complexes may have distinct modes of action, such as ligand exchange or release, redox activation and catalytic generation of toxic species, as well as depletion of essential substrates allowing them to inhibit enzyme activity, disrupt membrane function, or damage DNA [90]. For instance, Melaiye et al. evaluated the antimicrobial properties of synthesized two pincer Ag(I)-carbene complex which are silver(I)-2,6-bis(ethanolimidazolemethyl)pyridine hydroxide (Compound A) and silver(I)-2,6-bis(pro- panolimidazolemethyl)pyridine hydroxide (Compound B) against E.coli, S. aureus and P. aeruginosa [91]. Compounds A and B showed better bacteriostatic activity than AgNO3, even at much lower concentrations. The colonies were visually counted, with the end point of the minimum bactericidal concentration as no growth on the agar plate. The diversity of the types of the ligand shell of nanoparticles is extremely valuable in gaining access to a newly unexplored chemical space for drug development, particularly for the design of novel antimicrobials.
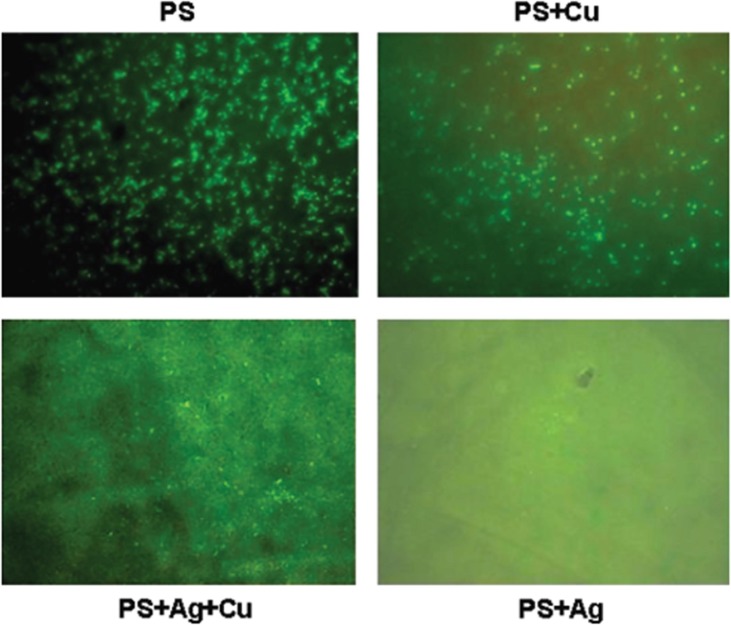
Copper (Cu) nanoparticles are highly toxic to a wide range of bacteria and fungi, owing to their high surface-to-volume ratio which was 0.25 and 0.05 that synthesized by electrolysis and chemical reduction respectively with antibacterial activities of Cu nanoparticles on E. coli was 15 mm as expressed in diameter inhibition zone [92]. Due to its unique inactivation mechanism mediated by direct contact, solid-state Cu offers greater effectiveness against pathogens [93]. Sunada et al. evaluated antiviral activity under conditions that would inhibit the direct physical contact of Cu compounds with viruses by placing a filter and chemically modifying the Cu surface with 1H-benzotriazole [94]. These two experiments demonstrated only a minor drop in viral titers (log10(N/N0) = 1.2 in the filter, which believed is due to interaction with the surface of cuprous compounds, and 0.6 in the BTA modification, respectively. According to these findings, direct contact on the Cu surface results the denaturation or destruction of biomolecules in viruses, leading to their inactivation. Cu is also inexpensive, safe sustainable, and frequently used as a surface coating agent for a variety of materials and air-cleaning filters [95]. For example, Kar et al found there was a decrease in adherence of bacteria E. coli on the polysulfone (PSf)/Cu, PSf/Ag/Cu and PSf/Ag nanocomposite membranes compared to the neat PSf membrane as observed under Carlzeiss Axioplus fluorescent microscope with blue excitation (488 nm) as shown in Fig. 7 [96]. This result evidenced incorporation of copper into PSf membrane retains the biocidal properties of copper. The PSf/Ag showed minimum adherence of bacterial due to the physicochemical properties of Ag that resulted in increased binding capability with bacteria's sulfur and phosphorous functionalized biomolecules for cell death [97].
Fig. 7.
Bacterial adherence studies on membrane surfaces of neat polysulfone and PSf/Cu, PSf/Ag/Cu and PSf/Ag nanocomposite membranes [96].
Akar et al. compared the antibacterial properties of polyethersulfone (PES) membrane containing Se and Cu [79]. They revealed adhesive interaction between copper blended membrane and microorganism was stronger than Se because of better electric property of Cu. The relative flux reduction (RFR) of a neat PES membrane was 93.8 % indicating that a large amount of biological suspension or their soluble microbial products such as protein and carbohydrate were deposited on the membrane surface, whereas the RFR of 0.05 Se and Cu blended membranes was 52.7% and 76.2% respectively. These findings imply that the anti-fouling characteristic of the Se and Cu nanoparticles blended membranes can be protected during the filtration process.
In conclusion, the application of nanocomposite membrane in pathogen removal from wastewater is a promising solution that synergizes the benefit of disinfectant and filtration techniques. However, the agglomeration of nanoparticles within the structure of the membrane reduces the performance of the resultant membrane. Agglomeration of nanoparticles within the membrane could form large voids in membrane structure, resulting in poor filtration. Other than that, the leaching of nanoparticles that bind with the harmful pathogen from the membrane substrate into the water system could initiate more serious water pollution. Therefore, the chemical functionality of nanoparticles can be improved by functionalizing them with organic ligands, as well as the compatibility of nanoparticles with polymeric matrixes. Functionalization on the surface of nanoparticles has been shown to reduce the tendency for agglomeration and facilitate self-organization. Hence, improve the pathogen removal from wastewater.
3.2. Membrane distillation
Membrane distillation (MD) is a thermally driven membrane process that transports water vapour from a contaminated feed stream to a distillate stream using a temperature/vapor pressure differential across a porous hydrophobic membrane as the driving mechanism [98]. The required operating temperature of MD is far lower than that of a typical distillation column, as the process liquids are not heated over their boiling points [99], [100]. In MD, the hydrophobic membrane is employed to separate the hot saline stream (feed) from the cold distillate stream. The vapour pressure differential is formed across the membrane as a result of the transmembrane temperature difference, which enables water vapour passage from the feed side to the distillate side while preventing nonvolatile species such as salt [101]. As a result, liquid/vapour interactions occur at membrane pore openings [102]. The high operating temperature in MD is the main mechanism to disinfect the pathogen in the feed while also acting as a secondary barrier in the event of membrane damage [70]. MD process is capable of producing pure water from water containing biological contaminants.
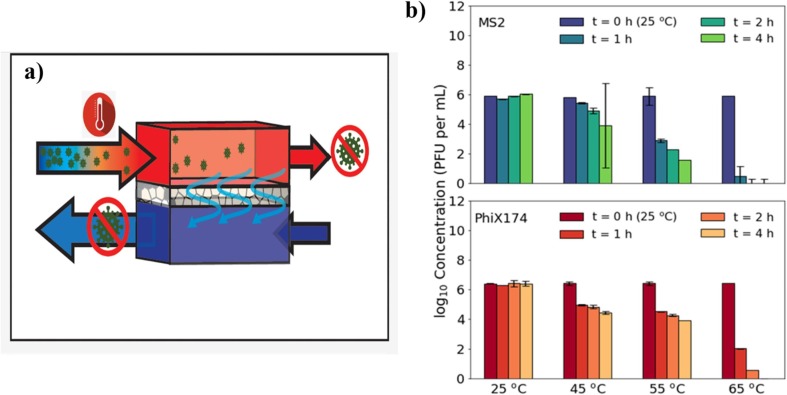
Commonly, pathogen removal by MD utilized direct contact membrane distillation (DCMD) configuration [70], [103], [104], [105]. DCMD occurs when an aqueous solution cooler and the feed solution are kept in direct contact with the permeate side of the membrane. Induced by transmembrane temperature differential, the volatile molecules evaporate at the heated liquid/vapour interface, crossing the membrane in the vapour phase, and condense inside the membrane module. For instance, Hardikar et al. studied the effects of feed temperature on the pathogen (MS2 and PhiX174 bacteriophage) removal by bench-scale DCMD as shown in Fig. 8 (a) [70]. Both viruses demonstrated a decrease in activity as the temperature increased as shown in Fig. 8(b). At 45 °C, both virus concentrations decreased by approximately 2 log in three hours following the initial decrease in the first hour. At 55 °C, viable MS2 concentrations decreased by almost 4 log, whereas PhiX174 concentrations decreased by>2 log. Temperature sensitivity experiments reveal that, for the surrogate viruses studied, MD operation temperatures (>65 °C) alone significantly reduce infectious viral levels in the feed solution by>6 log within four hours. Thus, the high operating temperature on the feed side acts as the initial barrier for virus elimination in the concentrated stream of the MD stream.
Fig. 8.
(a) Schematic diagram of the bench-scale DCMD for pathogen removal from water, (b) Effect of feed temperature on the concentration of MS2 and PhiX174 at temperature 25, 45, 55, and 65 °C [70].
MD biofouling had previously received little attention since MD acts at temperatures that are too high for most bacteria to thrive [106]. However, biofouling impacts on flux and salt separation in MD are increasingly becoming more common. The existence of thermophilic cells (bacteria that thrives at relatively high temperatures, between 41 and 122 °C) that emit endotoxins (lipopolysaccharides which are essential components of cell membranes of bacteria) also has become the major problem in executing the MD system [107]. The high operating temperature of MD can limit the growth of most bacteria, but not these thermophilic cells [108]. Therefore, several approaches have been done to enhance the membrane performance in reducing or eliminating the activity of these thermophilic cells such as impregnating the biocidal nanoparticles into the hydrophobic MD membrane [103], [109] and coating the MD membrane with polymer containing biocidal nanoparticles [105]. For instance, superhydrophobic SiO2NPs-embedded PVDF nanofiber membranes coated with a hydrophilic thin layer impregnated with carboxylated multiwalled carbon nanotubes (f-MWCNTs) and AgNPs showed remarkable biocidal properties towards thermophilic bacteria by a decrease in fouling (47% flux decline) and recorded high salt rejection (99.8%) [105].
A study reported on the use of carbon nanotubes (CNTs) and graphene oxide (GO) compounds in the polymeric membrane fabrication for the MD process [103]. The biocidal and endotoxin-removing properties of CNT and GO immobilized on the polytetrafluoroethylene (PTFE) supported on polypropylene composite membrane are reported for the direct contact membrane distillation of thermophilic bacteria (G. Stearothermophilus) and mesophilic bacteria (E. coli). They also compared the removal capabilities of the bacteria using neat PTFE, carbon nanotube immobilized membrane (CNIM), carboxylate functionalized CNTs (CNIM-COOH), and graphene oxide immobilized membrane (GOIM). The thermophilic cell reduction for neat PTFE was 68%, CNIM (96.2%), CNIM-COOH (84.3%), and GOIM (81.7%). Whereas the mesophilic cell reduction for neat PTFE was 24.4%, CNIM was 83.9%, CNIM-COOH (49.8%), and GOIM (47.8%). This study also indicated that the immobilization of the CNTs and GO has achieved 99.9 % endotoxins removal efficiency compared to the neat PTFE membrane.
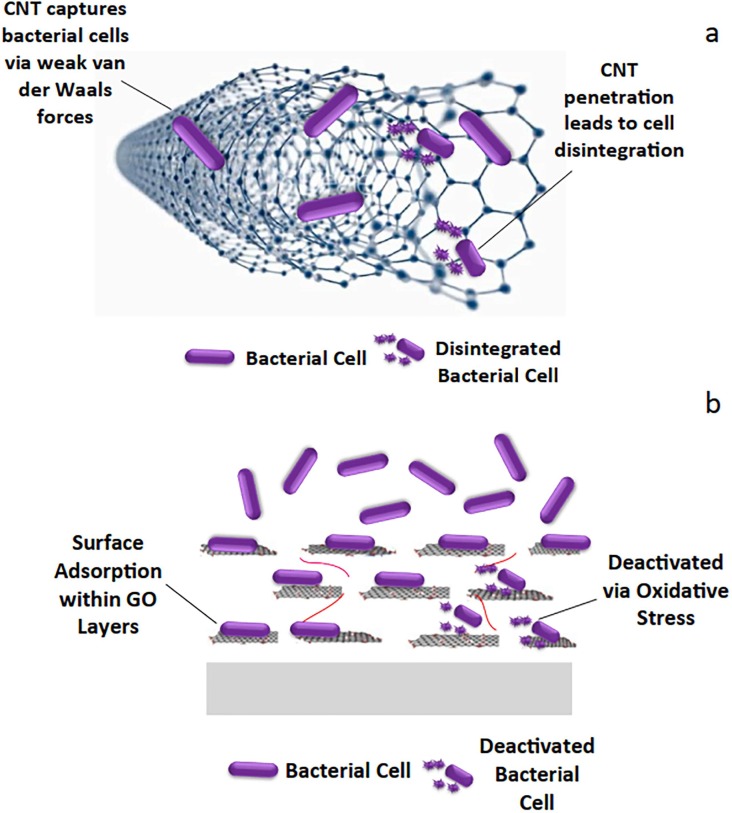
The proposed main mechanism of the biocidal activities of the immobilized CNTs and GO contained in the membrane during the MD process are piercing and wrapping and oxidative stress respectively as shown in Fig. 9 . It is hypothesized that the CNTs served as a net to capture the bacterial cells due to their extremely small size. Additionally, it is widely established that CNTs are bound together by weak van der Waals forces. The presence of these forces is critical for bacterial cells to adhere to the surface of carbon nanotubes. Once the CNTs attach to the bacterial cell membrane, they accelerate the uptake of ions from the surrounding environment, resulting in an ion accumulation within the cell cytoplasm. The build-up then lowers the mobility of the cell membrane, resulting in intracellular damage and ultimately cell death. Therefore, the main approach of antibacterial activity of CNT in MD application was attributed to the diameter- and length-dependent piercing and wrapping of CNTs, which destroyed gut microbial walls and membranes (Chen et al., 2013).
Fig. 9.
The biocidal activity mechanism of the immobilized (a) CNTs and (b) GO in the membrane for MD application [103].
Meanwhile, the pathogen disinfection by GO is mainly based on oxidation stress [110]. This can be hypothesized that GO is composed of layers of nanosheets, and we assume that bacteria are first confined within the nanosheets. Bacterial cell death is assumed to occur as a result of oxidative stress in the case of GO. The presence of GO leads to the formation of ROS within bacterial cells that could damage cellular components such as lipids, protein, and nucleic acid of the bacteria, reduction in membrane fluidity, and ultimately results in cell death.
To conclude, the MD process is capable of removing bacteria and viruses through the thermal activities of the MD process. The thermophilic pathogens can be destroyed by nanoparticles like AgNPs, CNTs, and GO which possessed unique biocidal activities against these harmful pathogens. However, the utilization of high operating temperatures could increase the operational cost. Therefore, utilization of natural heat sources such as geothermal energy, solar energy or even industrial waste can be performed to remove pathogens from wastewater via the MD process.
3.3. Membrane bioreactor
Membrane bioreactor (MBR) is a new type of wastewater treatment technology that combines membrane filtration with biological wastewater treatment process, activated sludge treatment. A dense microbial culture in suspension is used in activated sludge to biodegrade the pathogen under aerobic conditions and generate a biological floc for separation by a membrane. Membrane filtration guarantees that microorganisms are completely confined within the bioreactor, allowing for greater control of biological reactions and the modification of microorganism conditions in the aerated tank. The main principle that differentiates the MBR process from that of conventional biological treatments is the deployment of the membrane that acts as the barrier for the effective sludge separation process. This barrier can also serve as a filter of waterborne pathogens via the size exclusion principle. Due to the fact that viruses prefer solid surfaces, majority of the viruses that survive wastewater treatment are likely connected with waste-activated sludge and may be found in biosolids [111]. Thus, the removal of the biosolids from the influent in the MBR system will ultimately remove the virus. The MBR efficacy is primarily owed to the three major approaches which are (1) adsorption of virus particles to the biomass, (2) adsorption and rejection by the cleansed membrane, and (3) inactivation and degradation in the mixed liquid phase.
First of all, the adsorption of enteric virus particles to the biomass (mixed liquid suspended solids which contain bacteria and organic compounds that are bigger than membrane pores), makes it more difficult for them to pass through to the membrane layer. For example, Miura et al reported a 1.5 log removal of norovirus GI was achieved after 60 min of mixing with the mixed liquor suspended liquids [112]. As the biomass in the reactor expands, the gel/cake layer adhered to the surface of the membrane also increases. The adsorption of viral particles to the biomass is known for its role in virus clearance [113]. Phage or enteric virus particles adsorbing to the mixed liquor suspended solids, MLSS (bacteria and organic substances larger than membrane holes) impede their transit. Hirani et al. (2010) in a study have found that native MS-2 coliphage removed more particles than the seeded MS-2 coliphage in several AeMBR systems, which are attributed to particle association [114].
The second approach involves adsorption and rejection by the cleansed membrane. The biofilm adhered to the surface membrane aids in the removal by adsorption into the membrane pores or blocking them. The smaller effective pore size and fewer accessible pores increase the resistance of the membrane, making viral particle passage more difficult [115]. A study was conducted to determine the effect of biofilm growth on virus removal performance. The study discovered that removal efficiency increased significantly as more biofilm was attached to the membrane surface hence increasing the filtration resistance. The study also showed that a 21-day-biofilm achieved a 2.1 log removal while a 9-hr-biofilm achieved only a 0.3 log removal [116].
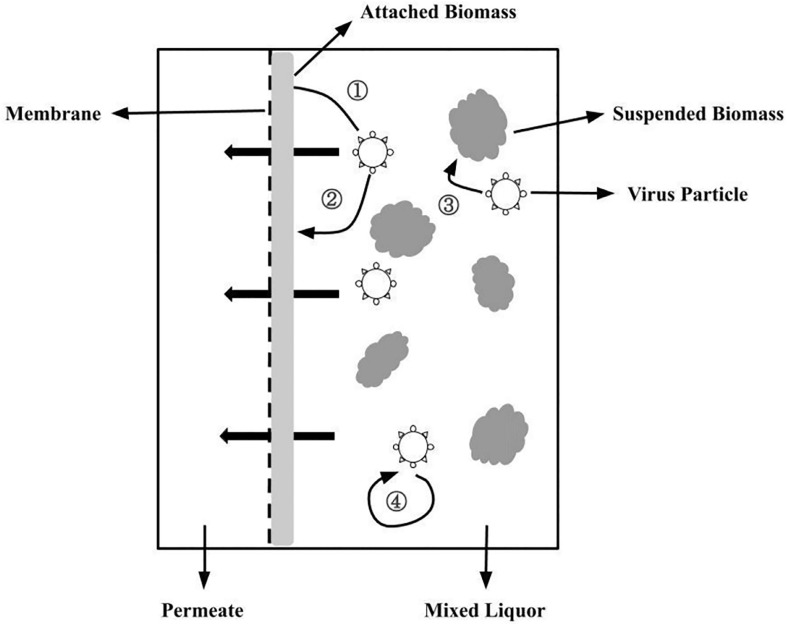
The third approach is known as inactivation and degradation in the mixed liquid phase. The principal processes responsible for the influence of mixed liquor on virus destruction and inactivation are thought to be the predation by other microbes and enzymatic breakdown [35]. A study has reported that the phage concentration in the anaerobic MBR (AnMBR) mixed liquor phase decreased by approximately 2 log over a two-week period, which is quicker than the predictable washout rate under the same hydraulic conditions. This implies that the reactor's anaerobic environment may aid in the virus inactivation [117]. Though all virus particles discarded by the membrane and attached biofilm are theoretically subjected to biodegradation, given the typical hydraulic retention time (HRT) of several hours to days in the MBR systems and the predominance of membrane/biofilm rejection, the effect of biodegradation on effluent virus concentration may be less distinct. Fig. 10 depicts the schematic mechanism of the MBR process for virus elimination using the three previously reported methodologies.
Fig. 10.
The mechanism of the virus removal process via MBR. (1) Electrostatic repulsion, sorption, and size rejection onto the membrane; (2) attachment onto the biomass layer attached on the membrane surface and pore-blocking effect; (3) predation and adsorption of suspended biomass; and (4) spontaneous inactivation and decay process [118].
Table 2 lists the previous MBR process studies for virus removal including Norovirus GI, T4 Phage, MS-2 Phage, Enterovirus, Somatic coliphage, Adenovirus, and Sapovirus. The membrane efficacy in terms of LRV has shown that the MBR scale and membrane pore size as well as the studied pathogens have significantly influenced the membrane performance.
Table 2.
The virus removal system using MBR process.
| Reactor scale | Nominal membrane pore size (µm) | Virus type | Hydraulic retention time, (h) | Log removal viable (LRV) | Ref. |
|---|---|---|---|---|---|
| Pilot-scale AeMBR | 0.4 | Norovirus GI | 35 | 1.82 | [119] |
| Bench-scale AnMBR | 0.4 | T4 Phage | 12 | 5.00–7.00 | [117] |
| Bench-scale AeMBR | 0.04 | MS-2 Phage | 10 | 1.70 | [35] |
| Pilot-scale AeMBR | 0.4 | Enterovirus | 7.2 | 0.30–3.20 | [120] |
| Full-scale AeMBR | 0.4 | Somatic coliphage | – | 3.24 | [121] |
| Full-scale AeMBR | 0.04 | Norovirus GI/GII | 0.18 | 2.30 | [33] |
| Full-scale AeMBR | 0.04 | Adenovirus | 11–12 | 4.10–6.30 | [122] |
| Full-scale AeMBR | 0.4 | Sapovirus | 36 | >3.00 | [112] |
In conclusion, the MBR can be successfully utilized for virus removal from the treated water. The selection of the membrane best features including membrane type, pore size, and surface charge may subsequently determine a better performance of the MBR system. By using membrane separation instead of gravity sedimentation, MBR offers substantial benefits over traditional activated sludge treatments [123]. Advantages offered by the MBR include high permeate quality, high nutrient recovery, and reduced environmental footprint [124], [125]. Besides that, the MBR system is known to have outstanding performance and advantages such as low energy consumption, no chemical required during the treatment, has high potential to reuse the effluent water, has a higher rate of nitrification and denitrification as well as constant effluent quality that is independent of the influent [126]. However, this system has some drawbacks such as high operational cost and investment, membrane fouling problem, and membrane lifetime replacement [127]. Furthermore, the biological activity present in MBR as well as the role of biomass could not be overlooked during the study of virus removal by MBR. For obtaining high pathogen elimination by the MBR system in the future, high inoculation sludge concentration, extended HRT, moderate foulant layer, and non-frequent chemical cleaning were advised.
3.4. Photocatalytic membrane reactor
A photocatalytic membrane reactor (PMR) is a hybrid system combining membrane separation and photocatalysis in a single process [128]. In PMR, the main mechanisms involved in pathogen removal are photocatalysis and membrane separation. Photocatalysis reaction is an advanced oxidation process in which a semiconductor is bombarded with light energy larger than its bandgap energy causing the creation of an excited electron and a positive hole that undergo subsequent reduction and oxidation, respectively [129]. The hydroxyl radicals (•OH) are known as non-selective oxidizers and are potent to oxidize the organic matter in the water. The photocatalyst itself refers to the semiconductor material that can produce chemical energy of electron-hole pairs from light energy. Photocatalysis also has been acknowledged as a green technique in water remediation due to the complete degradation of pollutants achieved using light irradiation [130].
The application for disinfection was first reported in 1985 whereby TiO2 loaded with platinum was used for catalytic inactivation of pathogens like Saccharomyces cerevisiae, E. coli, and Lactobacillus acidophilus [131]. The obtained results have shown that the number of cells was reduced up to 54% within 60 min of metal halide lamp irradiation and were further killed after 120 min of exposure. Since then, the application of photocatalysis for water disinfection has grown [132], [133], [134], [135], [136]. The use of photocatalyst for various applications including for water treatment process and combatting bacteria and virus including avian influenza H1N1 [136] and SARS-CoV-1 [137] in wastewater have proven that utilization of photocatalyst has been well developed. Many photocatalysts have been studied and reported such as TiO2, ZnO, WO3, Fe2O3, CuS, BiOBr, BaTiO3, and many more [135], [138], [139], [140], [141]. These multiple photocatalysts were utilized in various applications including antimicrobial activity, photocatalytic degradation of organic pollutants, superior antifouling agent as well as used for the nanocomposite sensor with high selectivity.
The exposure of TiO2 to UV-A light with a wavelength shorter than 385 nm has caused the TiO2 to produce significant oxidizing power. The bactericidal capabilities of TiO2 are ascribed to the high redox potential of the ROS generated by photo-excitation such as •OH, superoxide radical (O2∙-), and hydrogen peroxide (H2O2). Photo-oxidation mediated by TiO2 shows potential for eradicating bacteria in areas where chemical cleaning agents or biocides are inefficient or are regulated such as in the pharmaceutical and food industries. The utilization of this compound has been widely reported for the inactivation of microorganisms such as bacteria and viruses [142], [143]. The presence of TiO2 has further increased the inactivation of phage MS2 from 90% to 99.9% with the aid of 2 μM ferrous sulphate that caused hydroxyl radical oxidation with Fenton reaction enhancement, which is responsible for the viral degradation observed in the study. Table 3 summarises the microbial disinfection studies using TiO2 with UV irradiation. The effect of the exposure towards the removal efficiency of the bacteria varied due to the types of bacteria used. Some pathogens such as Coliphage required less than an hour to be removed up to 98% [144] while Lactobacillus casei and Phage PL-1 [145] required up to 24 h of exposure to be removed from the feed water. In general, most of the studies have shown>98 % removal efficiencies after irradiation under UV light.
Table 3.
The microbial disinfection studies using TiO2 with UV irradiation.
| Type of TiO2 immobilization | Bacteria | UV wavelength (nm) | UV exposure (h) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| TiO2 suspension | E. coli, Bacteriophage MS-2 | less than300 | 2 | ∼90 > 99 | [146] |
| TiO2 layer | Coliphage | 254 | 0.03 | 98 ∼ 100 | [144] |
| TiO2 suspension | E. coli K12 PHL849, E. coli K12 PHL1273 | 100–280 | 5 | ∼100 | [129] |
| TiO2-coated glass | Bacteriophage T4, E. coli | 300–400 | 3 | 100 | [147] |
| TiO2 film | Lactobacillus casei Phage PL-1 | 300–400 | 24 | 99.9 | [145] |
| TiO2 layer | Bacteriophage kNM1149 | 300–400 | 6 | 99.6 | [148] |
In some other studies, the PMRs were reported utilizing TiO2 film, carbon nanofibers decorated with Ag, TiO2 entrapped PVDF membrane, and TiO2 deposited thin-film-composite have shown antibacterial activities [149], [150], [151]. The presence of the photocatalysts in the membrane has eventually killed all bacteria that are present in the treated water with the assistance of a photocatalytic reaction and the aid of a UV light source. The killing mechanism has resulted in less attachment of the bacteria on the membrane and thus, reducing the biofouling occurrence. These studies have shown their efficacies (∼100% of bacteria were killed) were due to the physical activity (filtration) and chemical properties via photocatalytic activities. Additionally, the suspended catalyst configurations were used in most of the reported studies. Therefore, the immobilized catalyst PMRs for water disinfection is a way forward to be explored.
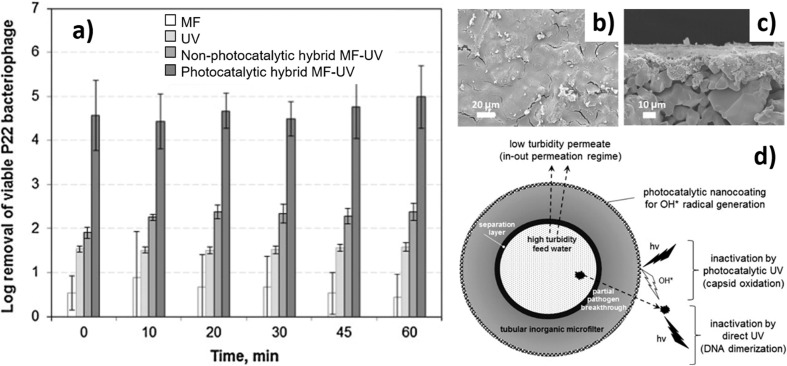
Nevertheless, the application of photocatalytic membranes for virus removal in the water was first documented by Guo et al. who employed P22 bacteriophage as a model virus [152]. In their work, the researcher compared the performance of virus removal by three different treatment processes of stand-alone MF, stand-alone UV disinfection, and hybrid photocatalytic MF-UV. They found that hybrid photocatalytic MF-UV recorded higher virus removal efficiency as follow: (1) stand-alone MF (LRV = 0.5 ± 0.5), (2) stand-alone UV disinfection (LRV = 2.3 ± 0.2), and (3) hybrid photocatalytic MF-UV (LRV = 5.0 ± 0.7) within 60 min of filtration as shown in Fig. 11 (a). The morphology of the surface and cross-section of the hybrid photocatalytic membrane is shown in Fig. 11 (b–c). The higher virus removal by this membrane is due to the generation of ROS by photocatalyst on the coated membrane played important role in the deterioration of protein capsid of viruses via oxidation process as illustrated in Fig. 11 (d).
Fig. 11.
a) Removal of P22 bacteriophage by (1) stand-alone MF, (2) stand-alone UV, (3) nonphotocatalytic hybrid MF-UV, (4) photocatalytic hybrid MF-UV. (b-c) SEM images of photocatalytic hybrid ceramic membrane (d) schematic illustration of the virus inactivation by hybrid photocatalytic MF-UV membrane [152].
Another study by Zheng et al. reported PMR has eliminated>5 log of bacteriophage f2 after 24 h of continuous operation [153]. The disinfection of the f2 resulted by the three kinds of scavengers which are •OH, h+ and e¯. Although adsorption and filter cake were present in this PMR system, a comparatively low TiO2 dose might mitigate these two effects correlating with the fact that phage f2 was mostly inactivated by the photocatalysis process of PMR [154].
As conclusion, PMR is a promising method for pathogen removal from wastewater. Compared to the suspended photocatalysis process, PMRs also offered a better option as this approach hybridized photocatalysis with the membrane process in a single unit. The advantages of the PMRs over the photoreactors include catalysts retaining by membrane barrier, enables the control of residence time of the pollutant in the reactor, facilitates simultaneous separation and photocatalysis reactions as well as reduction of energy consumption due to the unnecessary additional treatment process such as separation of the catalyst [155], [156]. However, the PMRs also have significant drawbacks such as damage on the polymer membrane structure due to exposure to light irradiation and reactive oxygen species for a long period and the agglomeration of photocatalysts on the membrane surface could lower the effective catalytic region for pathogen disinfection [157]. Therefore, developing a photocatalytic membrane with flexibility and self-standing qualities is a critical and urgent challenge to efficiently remove harmful pathogens from wastewater in the future.
4. Potential of membrane technology for wastewater epidemiology work especially in battling COVID-19
Advanced membrane technology has successfully disinfected viruses by combining size exclusion with additional functions such as adsorption, distillation, biological reaction and photocatalytic. SARS-CoV-2 viruses are related to SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [158]. However, there is a lack of discussion on the removal of those specific viruses from water. Thus, researchers can now develop novel and advanced water system treatments in order to treat these viruses.
SARS-CoV-2 originally falls under the zoonotic category and is later identified as an airborne and waterborne virus [159]. According to the current discovery of the genetic material of this virus in wastewater, it can give a warning of an epidemic outbreak. Therefore, SARS-CoV-2 can be categorized as both non-waterborne and waterborne virus [160]. Recent water quality results which were obtained from various parts of the world showed positive signs on the existence of ribonucleic acid (RNA) of SARS-CoV-2 in the wastewater [159]. To date, the virus has been found in wastewater treatment plants that are located in Australia, China, the Czech Republic, Ecuador, France, Japan, Italy, Pakistan, Spain, Netherlands, USA, Turkey, and other countries [12], [159], [161], [162], [163], [164], [165]). There were also published works that documented the presence of SARS-CoV-2 viruses in the treated wastewater [166], [167]. There was also a study that confirmed that surrogate coronaviruses remained contagious for days to weeks in water and sewage [168]. The presence of viruses in the treated wastewater increases the risk of diseases outbreaks [160]. The surveillance of wastewater for disease alert has gained growing interest and attention to remove viruses from wastewater efficiently [18], [153], [169]. Several works comprehensively discussed the occurrence and possible transmission of the SARS-CoV-2 virus through the water system [12], [159], [160], [163], [164], [165]. Briefly, the researchers revelated that the viruses enter the water system from the urine or stool of infected people and animals through various ways which include wastewater discharge from the hospitals, quarantine facilities, residential buildings, sewage, landfill, and drainage water. The presence of the SARS-CoV-2 was detected in the urine, stool, and feces, and these viruses remained infectious for days to weeks [22]. The rapid increase in the number of COVID-19 patients worldwide is expected to increase the concentration of viruses in sewage or wastewater, thus causing serious concerns globally. Previous study reported that surrogate coronaviruses like transmissible gastroenteritis (TGEV) and mouse hepatitis (MHV) remain alive and infectious in reagent-grade water at 25 °C in 22 days and 17 days respectively [168]. The persistence of high loads of coronaviruses in wastewater can pose a great risk to human health and the environment via leaking or broken underground pipes, which may contaminate the clean and treated water supply. Therefore, the possible transmission of SARS-CoV-2 through the water should not be overlooked.
However, the main challenge in dealing with the COVID-19 virus is the mutation of the virus. Mutation of the virus is defined as the transformation of the structure of the genes which results in a variant form that may be transmitted to future generations of the virus. Mutation can occur through alteration of single-based units in their RNA through deletion, insertion, or rearrangement of larger sections of the genes. Most recent studies reported one mutation that stood out in SARS-CoV-2, which was known as the D614G mutation [170]. Those studies reported that the amino acid aspartate (D, in biochemical shorthand) was being replaced regularly by glycine (G) at the 614th amino-acid position of the spike protein because of a copying fault that altered a single nucleotide in the virus’s 29,903-letter RNA code [171]. Surprisingly, in the beginning of February 2021, the world was shocked by the second and third waves of the pandemic which drove a drastic increase in daily infections and deaths due to mutation in the spike protein of the SARS-CoV-2 virus called the SARS-CoV-2 Delta variant [7]. Delta variant is a variant of lineage B.1.617 of SARS-CoV-2 that has been initially detected in India in late 2020. It has mutations in the SARS-CoV-2 spike protein gene that causes the substitution of T478K, P681R, and L452R. WHO warned that the Delta variant is highly contagious because of its higher transmissibility and less susceptibility to neutralization [172]. The Delta variant also becomes the dominant strain globally.
Advanced membrane technology offers a promising solution in battling COVID-19 variants through hybrid and synergistic functionalities such as photocatalytic oxidation, distillation, and filtration. The COVID-19 variants mainly involve mutations in the gene encoding the spike protein of SARS-CoV-2, which is the major surface glycoprotein that forms the cell wall or capsid of the viruses [173]. For this reason, the ongoing membrane technology can be designed and developed by considering the features of the spike protein of the SARS-CoV-2 virus. Advanced membrane technology like photocatalytic membrane is expected to be able to deactivate COVID-19 variants through the oxidation process via ROS, which is generated by binding the photocatalyst onto membrane surface or incorporating it into the membrane structure. When the COVID-19 variants are exposed to the photocatalytic membrane, the generated ROS will first oxidize the glycoprotein that makes the cell wall of the virus. When the cell wall is damaged, the cytoplasmic membrane and intracellular components containing RNA of the virus will be easily attacked by the ROS [132], [174]. As a consequence, the viruses will be deactivated permanently before being filtered by the membrane. In contrast, the efficiency of conventional membranes in the COVID-19 variants removal is doubted due to the mechanism of virus removal that mainly involves physical separation, which retains the pathogen concentrating in the retentate and the pathogen is not degraded or destroyed completely.
Besides that, the development of a smart membrane with stimuli-responsive material that is inspired by natural cell membrane can serve as a promising alternative solution to deal with those new COVID-19 variants. Since the mutation commonly occurs at the outer layer of the virus which targets the spike protein of the virus [7], [170], the new COVID-19 variants can be destroyed by a smart membrane by installing a sensor or protein-responsive detector to detect the spike protein of the virus. Stimuli-responsive materials have been explored in the water treatment field to respond under certain conditions such as electric, light, magnetic field, pH, and temperature-responsive [175], [176]. The protein stimuli-responsive smart membrane can overcome the bottlenecks of conventional and existing membrane technologies that function as automatic gates through flexible adjustment of pore sizes and surface properties in response to the variants of the targeted viruses. It is crucial to explore appropriate materials in order to develop protein stimuli-responsive smart membranes that are economical, high proficiency, and have long-term durability. Hence, it is critical to conduct more research studies to investigate the potential of membrane technology in battling COVID-19 and handling similar pandemics in the future.
5. Limitations and future perspectives
Membrane technology has been widely employed as a sustainable method for virus disinfection in wastewater. In this review, membrane technology was separated into conventional and advanced membranes. From this point of view, it can be predicted that advanced membrane technology offers a better and promising solution for removing pathogens compared to conventional membrane technology. This is due to the synergistic effect of filtration and additional functions of advanced membrane technology (adsorption, photocatalytic, distillation, and biological treatment).
However, numerous challenges are identified in the application of both membrane technologies for disinfecting pathogens from an aqueous system. The MF and UF processes mainly utilize the method of physical straining (sieve mechanism) in retaining particulate from passing through the membrane barrier. As a consequence, the viruses are not fully deactivated and destroyed. The viruses are only separated and excluded from the water, thus resulting in the formation of concentrated living virus retentate which can later become the most harmful wastes.
Besides that, membrane fouling like pore blocking and scaling can affect the adhesion of the virus on the membranes, hence reducing the performance of the membranes. This can increase the probability of the viruses passing through the membrane during filtration. Thus, the pressure-driven membrane process will rarely experience efficient virus removal without appropriate pre-treatment or post-treatment steps. Therefore, there is an urgent need in improving the development of a highly durable membrane that is furnished with resistance towards foulants for virus disinfection in the real wastewater system. Advanced membrane technologies including MBR, MD, PMR, and nanocomposite membranes have hybrid and synergistic properties that offer not only filtration properties but also the deactivation and destruction of the virus.
Nevertheless, there are different challenges faced by each advanced and nanocomposite membrane. Commonly, nanocomposite membranes and photocatalytic membranes are fabricated by embedding nanoparticles that have adsorption [75], [177] and photocatalytic [134] functionality. However, the high loading of nanoparticles can result in the agglomeration of nanoparticles within the membrane matrix, which can reduce the performance of virus removal and filtration.
The excess loading of nanoparticles can cause leaching or depletion, especially during long-run operations. To overcome these challenges, it is proposed to focus on the pre-treatment of nanoparticles or the membrane matrix through modification or functionalization before being embedded in the membrane matrix. It has been well acknowledged that modification on nanoparticles surfaces can lower the agglomeration and leaching issue [128], [178]. This is due to the abundant functional groups on the surface that can provide an active potential site for virus removal and improve the compatibility between nanoparticles and membrane matrix.
As for MD and MBR, the performance of the system is limited by membrane biofouling. The MD utilizes high energy usage in generating high-temperature conditions to deactivate the viruses, while MBR employs biological activity and membrane filtration in the removal of viruses from wastewater. Biofouling results from the proliferation of the living microbes or foulants that are present in the water system on the surface of the membranes. Since MBR deals with activated sludges that consist of living microbes as the main component [123], the tendency of biofouling is high. Even though the biofouling effect is minimal in MD due to the driving force of heat, the favorable condition for bacterial growth is within 20 to 40˚C. Furthermore, there are thermophilic microbes that have thermal resistance to grow at a higher temperature and form biofilm on the membrane [179]. To overcome the biofouling issue, modification of membrane with biocidal particles such as Ag, titania, zinc oxide, GO and copper oxide can be a promising alternative.
6. Conclusion
In conclusion, there has been a major increase in evidence that indicates the existence of pathogenic contaminants in the wastewater. Since prevention is the best remedy to break the chain and the spread of disease transmission, membrane technology is proposed to deactivate pathogens that are present in the wastewater. The perspective on the conventional and advanced membrane technology in the disinfection of pathogens can provide useful understanding and information in managing wastewater that is contaminated with harmful pathogens. The progress of pathogen removal through membrane technology will also be beneficial and valuable in managing similar pandemics in the future as well as protecting human health and the environment. In comparison to traditional membrane technology, advanced membrane technology offers a superior and more promising pathogen removal from water and wastewater. This is owing to the synergistic impact of filtration and additional functionalities of the advanced membrane technology such as adsorption, photocatalytic, distillation, and biological treatment. In the case of battling COVID-19, the ongoing membrane technology must be well designed and developed by considering the features of the spike protein of the SARS-CoV-2 virus.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This workwas funded by the Ministry of Higher Education, Malaysia under HICoE grant, R.J090301.7851.4 J433 and Ministry of Science, Technology and Innovation, Malaysia under the International Collaboration Fund (IF0120I1176). The authors also would like to acknowledge the financial support provided by Universiti Teknologi Malaysia under Professional Development Research Management grant, R.J130000.7113.04E66,
References
- 1.Gerardi M.H., Zimmerman M.C. John Wiley & Sons; 2004. Wastewater pathogens. [Google Scholar]
- 2.R.W. Hofste, P. Reig, S. Leah, 17 Countries , Home to One- Quarter of the World ’ s Population , Face Extremely High Water Stress, World Resour. Inst. (2019) 1–7. https://www.wri.org/insights/17-countries-home-one-quarter-worlds-population-face-extremely-high-water-stress (accessed October 21, 2021).
- 3.Who . 4th ed., 2017. Guidelines for Drinking-water Quality. [Google Scholar]
- 4.Lodder W.J., de Roda Husman A.M. Presence of Noroviruses and Other Enteric Viruses in Sewage and Surface Waters in The Netherlands. Appl. Environ. Microbiol. 2005;71(3):1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swayamsiddha S., Mohanty C. Application of cognitive Internet of Medical Things for COVID-19 pandemic, Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(5):911–915. doi: 10.1016/j.dsx.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Gao K., Wang R., Wei G.-W. Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies. Chem. Sci. 2021;12(20):6929–6948. doi: 10.1039/d1sc01203g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 15, Public Heal. Engl. (2021). https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings (accessed October 23, 2021).
- 8.Worldometers, COVID-19 Coronavirus Pandemic, Worldometer. (2021). doi:10.26439/iusetpraxis2020.n50-51.5049.
- 9.Romano-Bertrand S., Aho Glele L.-S., Grandbastien B., Lepelletier D. Preventing SARS-CoV-2 transmission in rehabilitation pools and therapeutic water environments. J. Hosp. Infect. 2020;105(4):625–627. doi: 10.1016/j.jhin.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M., Kumar A., Pittman C.U., Mlsna T., Mohan D. Coronavirus (SARS-CoV-2) in the environment : Occurrence, persistence, analysis in aquatic systems and possible management. Sci. Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui R., Khamis M., Ibrahim T., Khan N.A. SARS-CoV-2: The Increasing Importance of Water Filtration against Highly Pathogenic Microbes. ACS Chem. Neurosci. 2020;11(17):2482–2484. doi: 10.1021/acschemneuro.0c00468. [DOI] [PubMed] [Google Scholar]
- 12.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c0035710.1021/acs.estlett.0c00357.s001. [DOI] [PubMed] [Google Scholar]
- 15.Lahrich S., Laghrib F., Farahi A., Bakasse M., Saqrane S., El Mhammedi M.A. Review on the contamination of wastewater by COVID-19 virus : Impact and treatment. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwarciak-Kozlowska A., Wlodarczyk R. Waterborne Pathogens. Elsevier; 2020. pp. 81–103. [DOI] [Google Scholar]
- 17.USEPA, Guidelines for Water Reuse, 2012.
- 18.Shirasaki N., Matsushita T., Matsui Y., Murai K., Aochi A. Elimination of representative contaminant candidate list viruses, coxsackievirus, echovirus, hepatitis A virus, and norovirus, from water by coagulation processes. J. Hazard. Mater. 2017;326:110–119. doi: 10.1016/j.jhazmat.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Yahya M.T., Cluff C.B., Gerba C.P. Virus removal by slow sand filtration and nanofiltration. Water Sci. Technol. 1993;27(3-4):445–448. [Google Scholar]
- 20.Gentile G.J., Cruz M.C., Rajal V.B., Fidalgo de Cortalezzi M.M. Electrostatic interactions in virus removal by ultrafiltration membranes. J. Environ. Chem. Eng. 2018;6(1):1314–1321. [Google Scholar]
- 21.Zhang T., Hu Y., Jiang L., Yao S., Lin K., Zhou Y., Cui C. Removal of antibiotic resistance genes and control of horizontal transfer risk by UV, chlorination and UV/chlorination treatments of drinking water. Chem. Eng. J. 2019;358:589–597. doi: 10.1016/j.cej.2018.09.218. [DOI] [Google Scholar]
- 22.Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-Y., Guo B.-Z., Liu C., Ou G.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1-2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616–617:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- 24.Sassi H.P., Ikner L.A., Abd-Elmaksoud S., Gerba C.P., Pepper I.L. Comparative survival of viruses during thermophilic and mesophilic anaerobic digestion. Sci. Total Environ. 2018;615:15–19. doi: 10.1016/j.scitotenv.2017.09.205. [DOI] [PubMed] [Google Scholar]
- 25.Xagoraraki I., Harrington G.W., Assavasilavasukul P., Standridge J.H. Removal of emerging waterborne pathogens and pathogen indicators by pilot-scale conventional treatment. Journal-American Water Work. Assoc. 2004;96(5):102–113. doi: 10.1002/j.1551-8833.2003.tb10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Deng Y., Dong S., Wang H., Li P., Zhang H. The occurrence and control of waterborne viruses in drinking water treatment : A review. Chemosphere. 2021;281 doi: 10.1016/j.chemosphere.2021.130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurniawan S., Abdullah S., Imron M., Said N., Ismail N., Hasan H., Othman A., Purwanti I. Challenges and Opportunities of Biocoagulant / Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health. 2020;17(24):9312. doi: 10.3390/ijerph17249312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO, Water , sanitation , hygiene , and waste management for the COVID-19 virus: Interim guidance, (2020) 1–9. https://apps.who.int/iris/bitstream/handle/10665/331846/WHO-2019-nCoV-IPC_WASH-2020.3-eng.pdf?sequence=1&isAllowed=y (accessed January 14, 2021.
- 29.Zularisam A.W., Ismail A.F., Salim R. Behaviours of natural organic matter in membrane filtration for surface water treatment — a review. Desalination. 2006;194(1-3):211–231. doi: 10.1016/j.desal.2005.10.030. [DOI] [Google Scholar]
- 30.Liao Y., Loh C., Tian M., Wang R., Fane A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment : Fabrication, modification and applications. Prog. Polym. Sci. 2018;77:69–94. doi: 10.1016/j.progpolymsci.2017.10.003. [DOI] [Google Scholar]
- 31.Rabbani M., Aghapour S., Dabaghian Z., Dadashi M., Rahimpour A., Eke J., Escobar I.C., Abolhassani M., Greenlee L.F., Esfahani A.R., Sadmani A., Koutahzadeh N. Nanocomposite membranes for water separation and purification : Fabrication, modification, and applications. Sep. Purif. Technol. 2019;213:465–499. doi: 10.1016/j.seppur.2018.12.050. [DOI] [Google Scholar]
- 32.Nasir A.M., Goh P.S., Ismail A.F. Synthesis route for the fabrication of nanocomposite membranes, in. Micro Nano Technol., Elsevier. 2020:69–89. doi: 10.1016/B978-0-12-816710-6.00003-1. [DOI] [Google Scholar]
- 33.Purnell S., Ebdon J., Buck A., Tupper M., Taylor H. Removal of phages and viral pathogens in a full-scale MBR: Implications for wastewater reuse and potable water. Water Res. 2016;100:20–27. doi: 10.1016/j.watres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Al-Attabi R., Rodriguez-Andres J., Schütz J.A., Bechelany M., des Ligneris E., Chen X., Kong L., Morsi Y.S., Dumée L.F. Catalytic electrospun nano-composite membranes for virus capture and remediation. Sep. Purif. Technol. 2019;229:115806. doi: 10.1016/j.seppur.2019.115806. [DOI] [Google Scholar]
- 35.Chaudhry R.M., Holloway R.W., Cath T.Y., Nelson K.L. Impact of virus surface characteristics on removal mechanisms within membrane bioreactors. Water Res. 2015;84:144–152. doi: 10.1016/j.watres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Zodrow K., Brunet L., Mahendra S., Li D., Zhang A., Li Q., Alvarez P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43(3):715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Goswami K.P., Pugazhenthi G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manage. 2020;268:110583. doi: 10.1016/j.jenvman.2020.110583. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Guo L., Yang Y., Oguma K., an Hou L. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahid M.K., Kashif A., Fuwad A., Choi Y. Current advances in treatment technologies for removal of emerging contaminants from water – A critical review. Coord. Chem. Rev. 2021;442:213993. doi: 10.1016/j.ccr.2021.213993. [DOI] [Google Scholar]
- 40.Fadel M., Wyart Y., Moulin P. An efficient method to determine membrane molecular weight cut-off using fluorescent silica nanoparticles. Membranes (Basel). 2020;10:1–14. doi: 10.3390/membranes10100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: Source, fate and potential risks. Environ. Int. 2020;143:105962. doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Science E., Article R., Angeles L., A, Review of Water Treatment Membrane Nanotechnologies. 2010 [Google Scholar]
- 43.R. Sinha, N. Muhammad, R. Krishnan, A. Anderson, C. Patterson, D. Pearson, Laboratory and field evaluation of a nanofilter membrane to remove disinfection byproduct precursors and microorganisms from lake water sources used for drinking water, World Environ. Water Resour. Congr. 2010 Challenges Chang. - Proc. World Environ. Water Resour. Congr. 2010. (2010) 270–278. doi:10.1061/41114(371)31.
- 44.P. Chuntanalerg, S. Bureekaew, C. Klaysom, W.J. Lau, K. Faungnawakij, Nanomaterial-incorporated nanofiltration membranes for organic solvent recovery, in: Adv. Nanomater. Membr. Synth. Its Appl., Elsevier Inc., 2018: pp. 159–181. doi:10.1016/B978-0-12-814503-6.00007-0.
- 45.Farahbakhsh K., Smith D.W. Removal of coliphages in secondary effluent by microfiltration - Mechanisms of removal and impact of operating parameters. Water Res. 2004;38(3):585–592. doi: 10.1016/j.watres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Antony A., Blackbeard J., Leslie G. Removal efficiency and integrity monitoring techniques for virus removal by membrane processes. Crit. Rev. Environ. Sci. Technol. 2012;42(9):891–933. doi: 10.1080/10643389.2011.556539. [DOI] [Google Scholar]
- 47.C. Charcosset, Microfiltration, in: C.B.T.-M.P. in B. and P. Charcosset (Ed.), Membr. Process. Biotechnol. Pharm., Elsevier, Amsterdam, 2012: pp. 101–141. doi:https://doi.org/10.1016/B978-0-444-56334-7.00003-4.
- 48.Madaeni S.S., Fane A.G., Grohmann G.S. Virus removal from water and wastewater using membranes. J. Memb. Sci. 1995;102:65–75. [Google Scholar]
- 49.Kostenbader K.D., Cliver D.O. Membrane filter evaluations using poliovirus. J. Virol. Methods. 1983;7(5-6):253–257. doi: 10.1016/0166-0934(83)90076-9. [DOI] [PubMed] [Google Scholar]
- 50.Herath G., Yamamoto K., Urase T. Removal of viruses by microfiltration membranes at different solution environments. Water Sci. Technol. 1999;40(4-5):331–338. [Google Scholar]
- 51.Hu J.Y., Ong S.L., Song L.F., Feng Y.Y., Liu W.T., Tan T.W., Lee L.Y., Ng W.J. Removal of MS2 bacteriophage using membrane technologies. Water Sci. Technol. 2003;47(12):163–168. [PubMed] [Google Scholar]
- 52.Wang R., Guan S., Sato A., Wang X., Wang Z., Yang R., Hsiao B.S., Chu B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Memb. Sci. 2013;446:376–382. doi: 10.1016/j.memsci.2013.06.020. [DOI] [Google Scholar]
- 53.Sinclair T.R., Robles D., Raza B., van den Hengel S., Rutjes S.A., de Roda Husman A.M., de Grooth J., de Vos W.M., Roesink H.D.W. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surfaces A. 2018;551:33–41. doi: 10.1016/j.colsurfa.2018.04.056. [DOI] [Google Scholar]
- 54.Rodríguez-Chueca J., Mesones S., Marugán J. Hybrid UV-C/microfiltration process in membrane photoreactor for wastewater disinfection. Environ. Sci. Pollut. Res. 2019;26(36):36080–36087. doi: 10.1007/s11356-018-3262-x. [DOI] [PubMed] [Google Scholar]
- 55.Hou K., Gerba C.P., Goyal S.M., Zerda K.S. Capture of latex beads, bacteria, endotoxin, and viruses by charge-modified filters. Appl. Environ. Microbiol. 1980;40(5):892–896. doi: 10.1128/aem.40.5.892-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elhadidy A.M., Peldszus S., Van Dyke M.I. An evaluation of virus removal mechanisms by ultrafiltration membranes using MS2 and φx174 bacteriophage. Sep. Purif. Technol. 2013;120:215–223. doi: 10.1016/j.seppur.2013.09.026. [DOI] [Google Scholar]
- 57.Hagen K. Removal of particles, bacteria and parasites with ultrafiltration for drinking water treatment. Desalination. 1998;119(1-3):85–91. doi: 10.1016/S0011-9164(98)00117-9. [DOI] [Google Scholar]
- 58.Lu R., Zhang C., Piatkovsky M., Ulbricht M., Herzberg M., Nguyen T.H. Improvement of virus removal using ultrafiltration membranes modified with grafted zwitterionic polymer hydrogels. Water Res. 2017;116:86–94. doi: 10.1016/j.watres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 59.Yin Z., Tarabara V.V., Xagoraraki I. Human adenovirus removal by hollow fiber membranes: Effect of membrane fouling by suspended and dissolved matter. J. Memb. Sci. 2015;482:120–127. doi: 10.1016/j.memsci.2015.02.028. [DOI] [Google Scholar]
- 60.Lu R., Mosiman D., Nguyen T.H. Mechanisms of MS2 bacteriophage removal by fouled ultrafiltration membrane subjected to different cleaning methods. Environ. Sci. Technol. 2013;47(23):13422–13429. doi: 10.1021/es403426t. [DOI] [PubMed] [Google Scholar]
- 61.Jacquet N., Wurtzer S., Darracq G., Wyart Y., Moulin L., Moulin P. Effect of concentration on virus removal for ultrafiltration membrane in drinking water production. J. Memb. Sci. 2021;634:119417. doi: 10.1016/j.memsci.2021.119417. [DOI] [Google Scholar]
- 62.Mohammad A.W., Teow Y.H., Ang W.L., Chung Y.T., Oatley-Radcliffe D.L., Hilal N. Nanofiltration membranes review: Recent advances and future prospects. Desalination. 2015;356:226–254. [Google Scholar]
- 63.Yaroshchuk A.E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 2000;85(2-3):193–230. doi: 10.1016/s0001-8686(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 64.Beisl S., Monteiro S., Santos R., Figueiredo A.S., Sánchez-Loredo M.G., Lemos M.A., Lemos F., Minhalma M., de Pinho M.N. Synthesis and bactericide activity of nanofiltration composite membranes – Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019;149:225–231. doi: 10.1016/j.watres.2018.10.096. [DOI] [PubMed] [Google Scholar]
- 65.Otaki M., Yano K., Ohgaki S. Virus removal in a membrane separation process. Water Sci. Technol. 1998;37(10):107–116. [Google Scholar]
- 66.Patterson C., Anderson A., Sinha R., Muhammad N., Pearson D. Nanofiltration membranes for removal of color and pathogens in small public drinking water sources. J. Environ. Eng. 2012;138(1):48–57. [Google Scholar]
- 67.Hamaguchi K., Kuo D., Liu M., Sakamoto T., Yoshio M., Katayama H., Kato T. Nanostructured Virus Filtration Membranes Based on Two-Component Columnar Liquid Crystals. ACS Macro Lett. 2019;8(1):24–30. doi: 10.1021/acsmacrolett.8b0082110.1021/acsmacrolett.8b00821.s001. [DOI] [PubMed] [Google Scholar]
- 68.Ivnitsky H., Katz I., Minz D., Shimoni E., Chen Y., Tarchitzky J., Semiat R., Dosoretz C.G. Characterization of membrane biofouling in nanofiltration processes of wastewater treatment. Desalination. 2005;185(1-3):255–268. doi: 10.1016/j.desal.2005.03.081. [DOI] [Google Scholar]
- 69.Zheng X., Chen D.i., Wang Z., Lei Y., Cheng R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 2013;230:180–187. doi: 10.1016/j.cej.2013.06.069. [DOI] [Google Scholar]
- 70.Hardikar M., Ikner L.A., Felix V., Presson L.K., Rabe A.B., Hickenbottom K.L., Achilli A. Membrane Distillation Provides a Dual Barrier for Coronavirus and Bacteriophage Removal. Environ. Sci. Technol. Lett. 2021;8(8):713–718. doi: 10.1021/acs.estlett.1c0048310.1021/acs.estlett.1c00483.s001. [DOI] [PubMed] [Google Scholar]
- 71.Robeson L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991;62(2):165–185. doi: 10.1016/0376-7388(91)80060-J. [DOI] [Google Scholar]
- 72.Salleh M.A.M., Mahmoud D.K., Karim W.A.W.A., Idris A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination. 2011;280(1-3):1–13. doi: 10.1016/j.desal.2011.07.019. [DOI] [Google Scholar]
- 73.Warsinger D.M., Chakraborty S., Tow E.W., Plumlee M.H., Bellona C., Loutatidou S., Karimi L., Mikelonis A.M., Achilli A., Ghassemi A., Padhye L.P., Snyder S.A., Curcio S., Vecitis C.D., Arafat H.A., Lienhard J.H. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018;81:209–237. doi: 10.1016/j.progpolymsci.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdullah N., Gohari R.J., Yusof N., Ismail A.F., Juhana J., Lau W.J., Matsuura T. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: preparation, characterization and its adsorptive removal of lead (II) from aqueous solution. Chem. Eng. J. 2016;289:28–37. [Google Scholar]
- 75.Nasir A.M., Goh P.S., Ismail A.F. Highly adsorptive polysulfone/hydrous iron-nickel-manganese (PSF/HINM) nanocomposite hollow fiber membrane for synergistic arsenic removal. Sep. Purif. Technol. 2019;213:162–175. doi: 10.1016/j.seppur.2018.12.040. [DOI] [Google Scholar]
- 76.Yin J., Deng B. Polymer-matrix nanocomposite membranes for water treatment. J. Memb. Sci. 2015;479:256–275. doi: 10.1016/j.memsci.2014.11.019. [DOI] [Google Scholar]
- 77.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 78.Argueta-Figueroa L., Morales-Luckie R.A., Scougall-Vilchis R.J., Olea-Mejía O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu – Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014;24(4):321–328. doi: 10.1016/j.pnsc.2014.07.002. [DOI] [Google Scholar]
- 79.Akar N., Asar B., Dizge N., Koyuncu I. Investigation of characterization and biofouling properties of PES membrane containing selenium and copper nanoparticles. J. Memb. Sci. 2013;437:216–226. doi: 10.1016/j.memsci.2013.02.012. [DOI] [Google Scholar]
- 80.Kim J.H., Mahesh Kumar J., Joshua L., Chan Hee P., K. Cheol Sang, Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018;513:566–574. doi: 10.1016/j.jcis.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 81.Besinis A., De Peralta T., Handy R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8(1):1–16. doi: 10.3109/17435390.2012.742935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong C., Wang Z., Wu J., Wang Y., Wang J., Wang S. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination. 2017;401:32–41. doi: 10.1016/j.desal.2016.06.034. [DOI] [Google Scholar]
- 83.Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomedicine. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal S., Tak Y.K., Song J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium <em>Escherichia coli</em>. Appl. Environ. Microbiol. 2007;73:1712. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng Q.L., Wu J., Chen G.Q., Cui F.Z., Kim T.N., Kim J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 87.Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir. 2002;18(17):6679–6686. doi: 10.1021/la0202374. [DOI] [Google Scholar]
- 88.Di Gianvincenzo P., Marradi M., Martínez-Ávila O.M., Bedoya L.M., Alcamí J., Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg. Med. Chem. Lett. 2010;20(9):2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 89.Jalab J., Abdelwahed W., Kitaz A., Al-Kayali R. Green synthesis of silver nanoparticles using aqueous extract of Acacia cyanophylla and its antibacterial activity. Heliyon. 2021;7(9):e08033. doi: 10.1016/j.heliyon.2021.e08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Claudel M., Schwarte J.V., Fromm K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry (Easton). 2020;2:849–899. doi: 10.3390/chemistry2040056. [DOI] [Google Scholar]
- 91.Melaiye A., Simons R.S., Milsted A., Pingitore F., Wesdemiotis C., Tessier C.A., Youngs W.J. Formation of Water-Soluble Pincer Silver(I)-Carbene Complexes: A Novel Antimicrobial Agent. J. Med. Chem. 2004;47:973–977. doi: 10.1021/jm030262m. [DOI] [PubMed] [Google Scholar]
- 92.Theivasanthi T., Alagar M. Nano sized copper particles by electrolytic synthesis and characterizations. Int. J. Phys. Sci. 2011;6:3726–3735. doi: 10.5897/IJPS10.116. [DOI] [Google Scholar]
- 93.Minoshima M., Lu Y., Kimura T., Nakano R., Ishiguro H., Kubota Y., Hashimoto K., Sunada K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sunada K., Minoshima M., Hashimoto K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012;235–236:265–270. doi: 10.1016/j.jhazmat.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 95.Thurman R.B., Gerba C.P., Bitton G. Critical Reviews in Environmental Control The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses, Environ. Control. 1989;184:295–315. doi: 10.1080/10643388909388351. [DOI] [Google Scholar]
- 96.Kar S., Subramanian M., Ghosh A.K., Bindal R.C., Prabhakar S., Pillai C.G.S., Chattopadhyay S., Tewari P.K. Treatment Potential of nanoparticles for water purification : a case-study on anti-biofouling behaviour of metal based polymeric nanocomposite membrane behaviour of metal based polymeric nanocomposite membrane. Desalin. Water Treat. 2011;27:224–230. [Google Scholar]
- 97.Pandey J.K., Swarnkar R.K., Soumya K.K., Dwivedi P., Singh M.K., Sundaram S., Gopal R. Silver Nanoparticles Synthesized by Pulsed Laser Ablation: as a Potent Antibacterial Agent for Human Enteropathogenic Gram-Positive and Gram-Negative Bacterial Strains. Appl. Biochem. Biotechnol. 2014;174(3):1021–1031. doi: 10.1007/s12010-014-0934-y. [DOI] [PubMed] [Google Scholar]
- 98.Elimelech M., Phillip W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science. 2011;333(6043):712–717. doi: 10.1126/science.1200488. [DOI] [PubMed] [Google Scholar]
- 99.P. Le Goff, H. Matsuda, R. Rivero, Advances in Chemical Heat Pumps and Heat Transformers, in: S. Takamoto (Ed.), Heat Pumps, Pergamon, Oxford, 1990: pp. 117–126. doi:https://doi.org/10.1016/B978-0-08-040193-5.50025-0.
- 100.Drioli E., Ali A., Macedonio F. Membrane distillation: Recent developments and perspectives. Desalination. 2015;356:56–84. doi: 10.1016/j.desal.2014.10.028. [DOI] [Google Scholar]
- 101.Shaulsky E., Wang Z., Deshmukh A., Karanikola V., Elimelech M. Membrane distillation assisted by heat pump for improved desalination energy efficiency. Desalination. 2020;496:114694. doi: 10.1016/j.desal.2020.114694. [DOI] [Google Scholar]
- 102.El-Bourawi M.S., Ding Z., Ma R., Khayet M. A framework for better understanding membrane distillation separation process. J. Memb. Sci. 2006;285(1-2):4–29. doi: 10.1016/j.memsci.2006.08.002. [DOI] [Google Scholar]
- 103.Gupta I., Chakraborty J., Roy S., Farinas E.T., Mitra S. Nanocarbon immobilized membranes for generating bacteria and endotoxin free water via membrane distillation. Sep. Purif. Technol. 2021;259 [Google Scholar]
- 104.Ruiz-Aguirre A., Polo-López M.I., Fernández-Ibáñez P., Zaragoza G. Integration of Membrane Distillation with solar photo-Fenton for purification of water contaminated with Bacillus sp. and Clostridium sp. spores. Sci. Total Environ. 2017;595:110–118. doi: 10.1016/j.scitotenv.2017.03.238. [DOI] [PubMed] [Google Scholar]
- 105.Nthunya L.N., Gutierrez L., Khumalo N., Derese S., Mamba B.B., Verliefde A.R., Mhlanga S.D. Superhydrophobic PVDF nanofibre membranes coated with an organic fouling resistant hydrophilic active layer for direct-contact membrane distillation. Colloids Surfaces A Physicochem. Eng. Asp. 2019;575:363–372. doi: 10.1016/j.colsurfa.2019.05.031. [DOI] [Google Scholar]
- 106.Costa F.C.R., Ricci B.C., Teodoro B., Koch K., Drewes J.E., Amaral M.C.S. Biofouling in membrane distillation applications - a review. Desalination. 2021;516:115241. doi: 10.1016/j.desal.2021.115241. [DOI] [Google Scholar]
- 107.Zodrow K.R., Bar-Zeev E., Giannetto M.J., Elimelech M. Biofouling and microbial communities in membrane distillation and reverse osmosis. Environ. Sci. Technol. 2014;48(22):13155–13164. doi: 10.1021/es503051t. [DOI] [PubMed] [Google Scholar]
- 108.Frock A.D., Robert M.K. Extreme Thermophiles: Moving beyond single-enzyme biocatalysis. Curr. Opin. Chem. Eng. 2013;1:363–372. doi: 10.1016/j.coche.2012.07.003.Extreme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roy S., Bhadra M., Mitra S. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Sep. Purif. Technol. 2014;136:58–65. doi: 10.1016/j.seppur.2014.08.009. [DOI] [Google Scholar]
- 110.Liu S., Zeng T.H., Hofmann M., Burcombe E., Wei J., Jiang R., Kong J., Chen Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano. 2011;5(9):6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- 111.Xagoraraki I., Yin Z., Svambayev Z. Fate of Viruses in Water Systems. J. Environ. Eng. 2014;140(7):04014020. doi: 10.1061/(ASCE)EE.1943-7870.0000827. [DOI] [Google Scholar]
- 112.Miura T., Schaeffer J., Le Saux J.-C., Le Mehaute P., Le Guyader F.S. Virus Type-Specific Removal in a Full-Scale Membrane Bioreactor Treatment Process. Food Environ. Virol. 2018;10(2):176–186. doi: 10.1007/s12560-017-9330-4. [DOI] [PubMed] [Google Scholar]
- 113.Huang H., Young T.A., Schwab K.J., Jacangelo J.G. Mechanisms of virus removal from secondary wastewater effluent by low pressure membrane filtration. J. Memb. Sci. 2012;409–410:1–8. doi: 10.1016/j.memsci.2011.12.050. [DOI] [Google Scholar]
- 114.Hirani Z.M., DeCarolis J.F., Adham S.S., Jacangelo J.G. Peak flux performance and microbial removal by selected membrane bioreactor systems. Water Res. 2010;44(8):2431–2440. doi: 10.1016/j.watres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Noshadi I., Salahi A., Hemmati M., Rekabdar F., Mohammadi T. Experimental and ANFIS modeling for fouling analysis of oily wastewater treatment using ultrafiltration, Asia-Pacific. J Chem. Eng. 2013;8(4):527–538. doi: 10.1002/apj.1691. [DOI] [Google Scholar]
- 116.Ueda T., Horan N.J. Fate of indigenous bacteriophage in a membrane bioreactor. Water Res. 2000;34:2151–2159. doi: 10.1016/S0043-1354(99)00382-6. [DOI] [Google Scholar]
- 117.Fox R., Stuckey D. MS-2 and T4 phage removal in an anaerobic membrane bioreactor (AnMBR): effect of gas sparging rate. J. Chem. Technol. Biotechnol. 2015;90(3):384–390. doi: 10.1002/jctb.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y., Chen R., Li Y., Sano D. Virus removal by membrane bioreactors : A review of mechanism investigation and modeling effort s. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116522. [DOI] [PubMed] [Google Scholar]
- 119.Gurung K., Ncibi M.C., Sillanpää M. Assessing membrane fouling and the performance of pilot-scale membrane bioreactor (MBR) to treat real municipal wastewater during winter season in Nordic regions. Sci. Total Environ. 2017;579:1289–1297. doi: 10.1016/j.scitotenv.2016.11.122. [DOI] [PubMed] [Google Scholar]
- 120.Miura T., Okabe S., Nakahara Y., Sano D. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res. 2015;75:282–291. doi: 10.1016/j.watres.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 121.Francy D.S., Stelzer E.A., Bushon R.N., Brady A.M.G., Williston A.G., Riddell K.R., Borchardt M.A., Spencer S.K., Gellner T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 2012;46(13):4164–4178. doi: 10.1016/j.watres.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 122.Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 123.Wu J., Li H., Huang X. Indigenous somatic coliphage removal from a real municipal wastewater by a submerged membrane bioreactor. Water Res. 2010;44(6):1853–1862. doi: 10.1016/j.watres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 124.Iorhemen O., Hamza R., Tay J. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: membrane fouling. Membranes (Basel). 2016;6:33. doi: 10.3390/membranes6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao K., Liang S., Wang X., Chen C., Huang X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019;271:473–481. doi: 10.1016/j.biortech.2018.09.061. [DOI] [PubMed] [Google Scholar]
- 126.L.K. Wang, R. Menon, Membrane bioreactors, in: Adv. Biol. Treat. Process., Springer, 2009: pp. 129–156.
- 127.Ng A.N.L., Kim A.S. A mini-review of modeling studies on membrane bioreactor (MBR) treatment for municipal wastewaters. Desalination. 2007;212(1-3):261–281. doi: 10.1016/j.desal.2006.10.013. [DOI] [Google Scholar]
- 128.Argurio P., Fontananova E., Molinari R., Drioli E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes. 2018;6:1–27. doi: 10.3390/pr6090162. [DOI] [Google Scholar]
- 129.Guillard C., Bui T.-H., Felix C., Moules V., Lina B., Lejeune P. Microbiological disinfection of water and air by photocatalysis. Comptes Rendus Chim. 2008;11(1-2):107–113. doi: 10.1016/j.crci.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panthi G., Park M., Kim H., Lee S., Park S. Electrospun ZnO hybrid nanofibers for photodegradation of wastewater containing organic dyes : A review. J. Ind. Eng. Chem. 2015;21:26–35. doi: 10.1016/j.jiec.2014.03.044. [DOI] [Google Scholar]
- 131.Matsunaga T., Tomoda R., Nakajima T., Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985;29:211–214. http://www.sciencedirect.com/science/article/pii/0378109785903003 [Google Scholar]
- 132.Nasir A.M., Awang N., Hubadillah S.K., Jaafar J., Othman M.H.D., Wan Salleh W.N., Ismail A.F. A review on the potential of photocatalysis in combatting SARS-CoV-2 in wastewater. J. Water Process Eng. 2021;42:102111. doi: 10.1016/j.jwpe.2021.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nasir A.M., Jaafar J., Aziz F., Yusof N., Norhayati W., Salleh W., Ismail A.F., Aziz M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020;36 doi: 10.1016/j.jwpe.2020.101300. [DOI] [Google Scholar]
- 134.Nasir A.M., Awang N., Jaafar J., Ismail A.F., Othman M.H.D., A. Rahman M., Aziz F., Mat Yajid M.A. Recent progress on fabrication and application of electrospun nanofibrous photocatalytic membranes for wastewater treatment: A review. J. Water Process Eng. 2021;40:101878. doi: 10.1016/j.jwpe.2020.101878. [DOI] [Google Scholar]
- 135.N. Yahya, A.M. Nasir, N.A. Daub, F. Aziz, A. Aizat, J. Jaafar, W.J. Lau, N. Yusof, W.N.W. Salleh, A.F. Ismail, M. Aziz, Visible light–driven perovskite-based photocatalyst for wastewater treatment, in: Handb. Smart Photocatalytic Mater., Elsevier, 2020: pp. 265–302. doi:https://doi.org/10.1016/B978-0-12-819051-7.00009-9.
- 136.Takehara K., Yamazaki K., Miyazaki M., Yamada Y.u., Ruenphet S., Jahangir A., Shoham D., Okamura M., Nakamura M. Inactivation of avian influenza virus H1N1 by photocatalyst under visible light irradiation. Virus Res. 2010;151(1):102–103. doi: 10.1016/j.virusres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 137.Han W., Zhang P.H., Cao W.C., Yang D.L., Taira S., Okamoto Y., Arai J.I., Yan X.Y. The inactivation effect of photocatalytic titanium apatite filter on SARS virus. Prog. Biochem. Biophys. 2004;31:982–985. [Google Scholar]
- 138.Manna J., Goswami S., Shilpa N., Sahu N., Rana R.K. Biomimetic Method To Assemble Nanostructured Ag@ZnO on Cotton Fabrics: Application as Self-Cleaning Flexible Materials with Visible-Light Photocatalysis and Antibacterial Activities. ACS Appl. Mater. Interfaces. 2015;7(15):8076–8082. doi: 10.1021/acsami.5b00633. [DOI] [PubMed] [Google Scholar]
- 139.Vatanpour V., Madaeni S.S., Khataee A.R., Salehi E., Zinadini S., Monfared H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination. 2012;292:19–29. [Google Scholar]
- 140.Bandgar D.K., Navale S.T., Naushad M., Mane R.S., Stadler F.J., Patil V.B. Ultra-sensitive polyaniline–iron oxide nanocomposite room temperature flexible ammonia sensor. RSC Adv. 2015;5(84):68964–68971. doi: 10.1039/C5RA11512D. [DOI] [Google Scholar]
- 141.Daghrir R., Drogui P., Robert D. Modified TiO2 for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res. 2013;52(10):3581–3599. [Google Scholar]
- 142.Blake D.M., Maness P.-C., Huang Z., Wolfrum E.J., Huang J., Jacoby W.A. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep. Purif. Methods. 1999;28(1):1–50. [Google Scholar]
- 143.Sjogren J.C., Sierka R.A. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 1994;60(1):344–347. doi: 10.1128/aem.60.1.344-347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guimarães J.R., Barretto A.S. Photocatalytic inactivation of Clostridium perfringens and coliphages in water, Brazilian. J Chem. Eng. 2003;20(4):403–411. [Google Scholar]
- 145.Kashige N., Kakita Y., Nakashima Y., Miake F., Watanabe K. Mechanism of the photocatalytic inactivation of Lactobacillus casei phage PL-1 by titania thin film. Curr. Microbiol. 2001;42(3):184–189. doi: 10.1007/s002840010201. [DOI] [PubMed] [Google Scholar]
- 146.Cho M., Chung H., Choi W., Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005;71(1):270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ditta I.B., Steele A., Liptrot C., Tobin J., Tyler H., Yates H.M., Sheel D.W., Foster H.A. Photocatalytic antimicrobial activity of thin surface films of TiO 2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008;79(1):127–133. doi: 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- 148.Belháčová L., Krýsa J., Geryk J., Jirkovský J. Inactivation of microorganisms in a flow-through photoreactor with an immobilized TiO2 layer. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 1999;74:149–154. [Google Scholar]
- 149.Rahimpour A., Jahanshahi M., Rajaeian B., Rahimnejad M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination. 2011;278(1-3):343–353. doi: 10.1016/j.desal.2011.05.049. [DOI] [Google Scholar]
- 150.Kim S.H., Kwak S.-Y., Sohn B.-H., Park T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Memb. Sci. 2003;211(1):157–165. [Google Scholar]
- 151.Taha A.A. Direct synthesis of mesostructured carbon nanofibers decorated with silver-nanoparticles as a multifunctional membrane for water treatment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015;6:45003. [Google Scholar]
- 152.Guo B., Pasco E.V., Xagoraraki I., Tarabara V.V. Virus removal and inactivation in a hybrid microfiltration-UV process with a photocatalytic membrane. Sep. Purif. Technol. 2015;149:245–254. doi: 10.1016/j.seppur.2015.05.039. [DOI] [Google Scholar]
- 153.Zheng X., Wang Q., Chen L., Wang J., Cheng R. Photocatalytic membrane reactor (PMR) for virus removal in water: Performance and mechanisms. Chem. Eng. J. 2015;277:124–129. doi: 10.1016/j.cej.2015.04.117. [DOI] [Google Scholar]
- 154.Sarasidis V.C., Plakas K.V., Patsios S.I., Karabelas A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014;239:299–311. doi: 10.1016/j.cej.2013.11.026. [DOI] [Google Scholar]
- 155.Molinari R., Pirillo F., Falco M., Loddo V., Palmisano L. Photocatalytic degradation of dyes by using a membrane reactor. Chem. Eng. Process. Process Intensif. 2004;43(9):1103–1114. [Google Scholar]
- 156.Zhang C., Li Y.i., Li J. Improved disinfection performance towards human adenoviruses using an efficient metal-free heterojunction in a vis-LED photocatalytic membrane reactor: Operation analysis and optimization. Chem. Eng. J. 2020;392:123687. doi: 10.1016/j.cej.2019.123687. [DOI] [Google Scholar]
- 157.Shi Y., Huang J., Zeng G., Cheng W., Hu J. Photocatalytic membrane in water puri fi cation : is it stepping closer to be driven by visible light? J. Memb. Sci. 2019;584:364–392. doi: 10.1016/j.memsci.2019.04.078. [DOI] [Google Scholar]
- 158.Zeidler A., Karpinski T.M. SARS-COV, MERS-COV, SARS-COV-2 comparison of three emerging coronaviruses, Jundishapur. J. Microbiol. 2020;13:1–8. doi: 10.5812/jjm.103744. [DOI] [Google Scholar]
- 159.Ihsanullah I., Bilal M., Naushad M.u. Coronavirus 2 (SARS-CoV-2) in water environments: Current status, challenges and research opportunities. J. Water Process Eng. 2021;39:101735. doi: 10.1016/j.jwpe.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: A review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020;8(5):104429. doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lapolla P., Lee R., Mingoli A. Wastewater as a red flag in COVID-19 spread. Public Health. 2020;185:26. doi: 10.1016/j.puhe.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elsamadony M., Fujii M., Miura T., Watanabe T. Possible transmission of viruses from contaminated human feces and sewage: Implications for SARS-CoV-2. Sci. Total Environ. 2021;755:142575. doi: 10.1016/j.scitotenv.2020.142575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tran H.N., Le G.T., Nguyen D.T., Juang R.-S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.-P. SARS-CoV-2 coronavirus in water and wastewater: A critical review about presence and concern. Environ. Res. 2021;193:110265. doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhavana V., Thakor P., Singh S.B., Mehra N.K. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020;261:118336. doi: 10.1016/j.lfs.2020.118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mohapatra S., Menon N.G., Mohapatra G., Pisharody L., Pattnaik A., Menon N.G., Bhukya P.L., Srivastava M., Singh M., Barman M.K., Gin K.-H., Mukherji S. The novel SARS-CoV-2 pandemic: Possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci. Total Environ. 2021;765:142746. doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. MedRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Habibi-Yangjeh A., Asadzadeh-Khaneghah S., Feizpoor S., Rouhi A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020;580:503–514. doi: 10.1016/j.jcis.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu J., He C.L., Gao Q., Zhang G.J., Cao X.X., Long Q.X., Deng H.J., Huang L.Y., Chen J., Wang K. The D614G mutation of SARS-CoV-2 spike protein enhances viral infectivity. BioRxiv. 2020 [Google Scholar]
- 171.Callaway E. The coronavirus is mutating - does it matter? Nature. 2020;585(7824):174–177. doi: 10.1038/d41586-020-02544-6. [DOI] [PubMed] [Google Scholar]
- 172.W.H.O. Who, WHO Director-General ’ s opening remarks at the 8th meeting of the IHR Emergency Committee on COVID-19 – 14 World Heal. Organ. July 2021;2021:21–23. [Google Scholar]
- 173.Kumar V.G., Ogden D.S., Isu U.H., Polasa A., Losey J., Moradi M. Differential dynamic behavior of prefusion spike proteins of SARS coronaviruses 1 and 2. BioRxiv. 2019;2020:1–42. doi: 10.1101/2020.12.25.424008. [DOI] [Google Scholar]
- 174.Byrne J.A., Dunlop P.S.M., Hamilton J.W.J., Fernández-Ibáñez P., Polo-López I., Sharma P.K., Vennard A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20:5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suwaileh W., Pathak N., Shon H., Hilal N. Forward osmosis membranes and processes : A comprehensive review of research trends and future outlook. Desalination. 2020;485 doi: 10.1016/j.desal.2020.114455. [DOI] [Google Scholar]
- 176.Zou S., Qin M., He Z. Tackle reverse solute flux in forward osmosis towards sustainable water recovery: reduction and perspectives. Water Res. 2019;149:362–374. doi: 10.1016/j.watres.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 177.Nasir A.M., Goh P.S., Abdullah M.S., Cheer N.B., Ismail A.F. Adsorptive Nanocomposite Membranes for Heavy Metal Remediation: Recent Progresses and Challenges. Chemosphere. 2019;232:96–112. doi: 10.1016/j.chemosphere.2019.05.174. [DOI] [PubMed] [Google Scholar]
- 178.Arshad F., Selvaraj M., Zain J., Banat F., M. Abu Haija, Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2018;209:870–880. doi: 10.1016/j.seppur.2018.06.035. [DOI] [Google Scholar]
- 179.Bogler A., Lin S., Bar-Zeev E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Memb. Sci. 2017;542:378–398. doi: 10.1016/j.memsci.2017.08.001. [DOI] [Google Scholar]